Intensity Modulated Radiation Therapy (IMRT)
|
|
|
- Christal French
- 6 years ago
- Views:
Transcription
1 Intensity Modulated Radiation Therapy (IMRT) [For the list of services and procedures that need preauthorization, please refer to go to Comunicados a Proveedores, and click Cartas Circulares.] Medical Policy: MP-RA Original Effective Date: January 17, 2008 Revised: November 23, 2016 Next Revision: November, 2017 Related Policies: MP-RA MCS Image-Guided Radiation Therapy (IGRT) This policy applies to products subscribed by the following corporations, MCS Life Insurance Company (Commercial), and MCS Advantage, Inc. (Classicare) and, provider s contract; unless specific contract limitations, exclusions or exceptions apply. Please refer to the member s benefit certification language for benefit availability. Managed care guidelines related to referral authorization, and precertification of inpatient hospitalization, home health, home infusion and hospice services apply subject to the aforementioned exceptions. DESCRIPTION Intensity Modulated Radiation Therapy (IMRT) is a computer-based method of planning for, and delivery of patient specific, spatially and often temporally modulated beams of radiation to solid tumors within a patient. IMRT planning and delivery uses an approach for obtaining the highly conformal dose distributions needed to irradiate complex targets positioned near, or invaginated by, sensitive normal tissues, thus improving the therapeutic ratios. IMRT delivers a more precise radiation dose to the tumor while sparing the surrounding normal tissues by using non-uniform radiation beam intensities determined by various computer-based optimization techniques. The computer based optimization process is referred to as inverse planning. Inverse planning develops a dose distribution based on the input of specific dose constraints for the planned treatment volume (PTV) and nearby clinical structures and is the beginning of the IMRT treatment planning process. The gross tumor volume (GTV), the PTV and surrounding normal tissues must be identified by a contouring procedure and the optimization must sample the dose with a grid spacing of 1 centimeter or less. COVERAGE Benefits may vary between groups and contracts. Please refer to the appropriate member certificate and subscriber agreement contract for applicable diagnostic imaging, DME, laboratory, machine tests, benefits and coverage. INDICATIONS (MCS) considers Intensity Modulated Radiation Therapy (IMRT) medically necessary in instances where sparing of the surrounding normal tissue is essential, a highly conformal 1
2 dose planning is required and the Member meets at least one or more of the following conditions: (IMRT is NOT a replacement therapy for conventional and 3D conformal radiation therapy methods.) 1. An immediately adjacent area has been previously irradiated and abutting portals must be established with high precision. 2. Dose escalation is planned to deliver radiation doses in excess of those commonly utilized for similar tumors with conventional treatment. 3. The target volume is concave or convex, and critical normal tissues are within or around that that convexity or concavity. 4. The target volume is in close proximity to critical structures that must be protected. 5. The volume of interest must be covered with narrow margins to adequately protect immediately adjacent structures. On the basis of the above conditions demonstrating medical necessity, disease sites that may support the use of IMRT include the following: 1. Primary, metastatic or benign tumors of the central nervous system (CNS), including the brain, brain stem and spinal cord 2. Primary or metastatic tumors of the spine where spinal cord tolerance may be exceeded by conventional treatment or where the spinal cord has previously been irradiated 3. Primary, metastatic, benign or recurrent head and neck malignancies, including: orbits, sinuses, skull base, aerodigestive tract and salivary glands 4. Thoracic malignancies 5. Abdominal malignancies when dose constraints to small bowel or other normal abdominal tissue are exceeded and present administration of a therapeutic does 6. Pelvic malignancies including: prostatic, gynecological and anal carcinoma 7. Other pelvic and retroperitoneal malignancies Note 1 : Other malignancies not delineated in the above can be supported with submission of documentation for medical necessity should a denial occur. The determination of appropriateness and medical necessity for IMRT for any site shall be found in the documentation from the radiation oncologist and must be available when requested or submitted in the appeals process. 2
3 LIMITATIONS 1. IMRT will not be considered reasonable and medically necessary when at least one of the criteria listed in the "Coverage Section and one of the diagnoses listed in the "ICD-10 Codes section of this policy are not present. Clinical scenarios that would not typically support the use of IMRT include: Where IMRT does not offer an advantage over conventional or three-dimensional conformal radiation therapy techniques that deliver good clinical outcomes and low toxicity. Clinical urgency, such as spinal cord compression, superior vena cave syndrome or airway obstruction. Palliative treatment of metastatic disease where the prescribed dose does not approach normal tissue tolerances. Inability to accommodate for organ motion, such as for a mobile lung tumor. Inability of the patient to cooperate and tolerate immobilization to permit accurate and reproducible dose delivery. 2. There must be documented rationale of the advantage of IMRT versus the use of other radiation therapy methods in the medical record of each patient for whom IMRT is provided. 3. MRT services will be considered reasonable and necessary only when performed by appropriately trained providers. These personnel include the radiation oncologist or other qualified physician radiation/medical physicist, radiation technologist and radiation assistant. 4. Free standing facilities (office or clinic), hospital based practices, and mobile delivery units affiliated with a place of service (POS) must meet Federal and local (state) radiation protection guidelines in regard to patient safety and quality assurance as well as the physician supervision requirements. 5. Experimental or investigational Services with the exception of routine costs of qualifying clinical trial services with dates of service on or after September 19, 2000, which meet the requirements of the Clinical Trials NCD are considered reasonable and necessary. 6. Duration and frequency that is considered appropriate for the service must meet the correct requirements. 7. Re-determination process may be utilized for consideration of services performed outside of the reasonable and necessary requirements in this Policy. 3
4 DOCUMENTATION REQUIREMENTS The following documentation requirements must be met if not, it could lead to denials services: 1. The treatment plan/prescription must define the goals and requirements of the treatment, including the specific dose constraints for the target(s) and nearby critical structures. 2. A statement by the treating physician documenting the special need for performing IMRT on the patient in question, rather than performing conventional or three dimensional treatment planning and delivery. The physician must address the other organs at risk and/or adjacent critical structures. 3. Review (signed and dated) by the radiation oncologist of the CT or MRI based images of the target and all critical structures with representative isodose distributions that characterize the three-dimensional dose. 4. Radiation oncologist review of dose-volume histograms for all targets and critical structures. 5. Description of the number and location of each treatment step/rotation or portal to accomplish the treatment plan. 6. Documentation of dosimetric verification of treatment setup and delivery, signed by both the radiation oncologist and the medical physicist. 7. For compensator-based IMRT, the unique compensator design should be documented for east step or portal. Documentation of fluence distributions recomputed in a phantom, or an equivalent methodology consistent with patient specific IMRT treatment verification. Target verification methodology documentation to include documentation of the clinical treatment volume (CTV) and the planning target volume (PTV); documentation of immobilization and patient positioning, and means of dose verification and secondary means of verification. Note 2 : Other procedures performed during the episode of care must have documentation that supports the professional and technical components as applicable by identifying the place of service, the date of service, the supervising physician, and proof of work provided. 4
5 CODING INFORMATION CPT Codes (List may not be all inclusive) CPT Codes DESCRIPTION Respiratory motion management simulation Intensity modulated radiotherapy plan, including dose-volume histograms for target and critical structure partial tolerance specifications Multi Leaf Collimator (MLC) device(s) for Intensity modulated radiation therapy (IMRT), design and construction per IMRT plan Intensity modulated radiation treatment delivery (IMRT), includes guidance and tracking, when performed; simple Intensity modulated radiation treatment delivery (IMRT), includes guidance and tracking, when performed; complex Current Procedural Terminology (CPT ) 2016 American Medical Association. HCPCS CODES (List may not be all inclusive) HCPCS CODES DESCRIPTION G6015 Intensity modulated treatment delivery, single or multiple fields/arcs, via narrow spatially and temporally modulated beams, binary, dynamic MLC, per treatment session G6016 Compensator-based beam modulation treatment delivery of inverse planned treatment using 3 or more high resolution (milled or cast) compensator, convergent beam modulated fields, per treatment session 2016 HCPCS LEVEL II Professional Edition (American Medical Association). ICD-10 Codes (List may not be all inclusive) ICD-10-Codes C00.0 Malignant neoplasm of external upper lip C00.1 Malignant neoplasm of external lower lip DESCRIPTION C00.3 Malignant neoplasm of upper lip, inner aspect C00.4 Malignant neoplasm of lower lip, inner aspect C00.6 Malignant neoplasm of commissure of lip, unspecified C00.8 Malignant neoplasm of overlapping sites of lip C01 Malignant neoplasm of base of tongue C02.0 Malignant neoplasm of dorsal surface of tongue 5
6 C02.1 Malignant neoplasm of border of tongue C02.2 Malignant neoplasm of ventral surface of tongue C02.3 Malignant neoplasm of anterior two-thirds of tongue, part unspecified C02.4 Malignant neoplasm of lingual tonsil C02.8 Malignant neoplasm of overlapping sites of tongue C03.0 Malignant neoplasm of upper gum C03.1 Malignant neoplasm of lower gum C04.0 Malignant neoplasm of anterior floor of mouth C04.1 Malignant neoplasm of lateral floor of mouth C04.8 Malignant neoplasm of overlapping sites of floor of mouth C05.0 Malignant neoplasm of hard palate C05.1 Malignant neoplasm of soft palate C05.2 Malignant neoplasm of uvula C05.8 Malignant neoplasm of overlapping sites of palate C06.0 Malignant neoplasm of cheek mucosa C06.1 Malignant neoplasm of vestibule of mouth C06.2 Malignant neoplasm of retromolar area C06.80 Malignant neoplasm of overlapping sites of unspecified parts of mouth C06.89 Malignant neoplasm of overlapping sites of other parts of mouth C07 Malignant neoplasm of parotid gland C08.0 Malignant neoplasm of submandibular gland C08.1 Malignant neoplasm of sublingual gland C09.0 Malignant neoplasm of tonsillar fossa 6
7 C09.1 Malignant neoplasm of tonsillar pillar (anterior) (posterior) C09.8 Malignant neoplasm of overlapping sites of tonsil C10.0 Malignant neoplasm of vallecula C10.1 Malignant neoplasm of anterior surface of epiglottis C10.2 Malignant neoplasm of lateral wall of oropharynx C10.3 Malignant neoplasm of posterior wall of oropharynx C10.4 Malignant neoplasm of branchial cleft C10.8 Malignant neoplasm of overlapping sites of oropharynx C10.8 Malignant neoplasm of overlapping sites of oropharynx C11.0 Malignant neoplasm of superior wall of nasopharynx C11.1 Malignant neoplasm of posterior wall of nasopharynx C11.2 Malignant neoplasm of lateral wall of nasopharynx C11.3 Malignant neoplasm of anterior wall of nasopharynx C11.8 Malignant neoplasm of overlapping sites of nasopharynx C12 Malignant neoplasm of pyriform sinus C13.0 Malignant neoplasm of postcricoid region C13.1 Malignant neoplasm of aryepiglottic fold, hypopharyngeal aspect C13.2 Malignant neoplasm of posterior wall of hypopharynx C13.8 Malignant neoplasm of overlapping sites of hypopharynx C14.0 Malignant neoplasm of pharynx, unspecified C14.2 Malignant neoplasm of Waldeyer's ring C14.8 Malignant neoplasm of overlapping sites of lip, oral cavity and pharynx C15.3 Malignant neoplasm of upper third of esophagus 7
8 C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant neoplasm of overlapping sites of esophagus C16.0 Malignant neoplasm of cardia C16.1 Malignant neoplasm of fundus of stomach C16.2 Malignant neoplasm of body of stomach C16.3 Malignant neoplasm of pyloric antrum C16.4 Malignant neoplasm of pylorus C16.5 Malignant neoplasm of lesser curvature of stomach, unspecified C16.6 Malignant neoplasm of greater curvature of stomach, unspecified C16.8 Malignant neoplasm of overlapping sites of stomach C17.0 Malignant neoplasm of duodenum C17.1 Malignant neoplasm of jejunum C17.2 Malignant neoplasm of ileum C17.3 Meckel's diverticulum, malignant C17.8 Malignant neoplasm of overlapping sites of small intestine C18.0 Malignant neoplasm of cecum C18.1 Malignant neoplasm of appendix C18.2 Malignant neoplasm of ascending colon C18.3 Malignant neoplasm of hepatic flexure C18.4 Malignant neoplasm of transverse colon C18.5 Malignant neoplasm of splenic flexure C18.6 Malignant neoplasm of descending colon 8
9 C18.7 Malignant neoplasm of sigmoid colon C18.8 Malignant neoplasm of overlapping sites of colon C19 C20 Malignant neoplasm of rectosigmoid junction Malignant neoplasm of rectum C21.0 Malignant neoplasm of anus, unspecified C21.1 Malignant neoplasm of anal canal C21.2 Malignant neoplasm of cloacogenic zone C21.8 Malignant neoplasm of overlapping sites of rectum, anus and anal canal C22.0 Liver cell carcinoma C22.1 Intrahepatic bile duct carcinoma C22.2 Hepatoblastoma C22.3 Angiosarcoma of liver C22.4 Other sarcomas of liver C22.7 Other specified carcinomas of liver C22.8 Malignant neoplasm of liver, primary, unspecified as to type C22.9 Malignant neoplasm of liver, not specified as primary or secondary C23 Malignant neoplasm of gallbladder C24.0 Malignant neoplasm of extrahepatic bile duct C24.1 Malignant neoplasm of ampulla of Vater C24.8 Malignant neoplasm of overlapping sites of biliary tract C25.0 Malignant neoplasm of head of pancreas C25.1 Malignant neoplasm of body of pancreas C25.2 Malignant neoplasm of tail of pancreas 9
10 C25.3 Malignant neoplasm of pancreatic duct C25.4 Malignant neoplasm of endocrine pancreas C25.7 Malignant neoplasm of other parts of pancreas C25.8 Malignant neoplasm of overlapping sites of pancreas C30.0 Malignant neoplasm of nasal cavity C30.1 Malignant neoplasm of middle ear C31.0 Malignant neoplasm of maxillary sinus C31.1 Malignant neoplasm of ethmoidal sinus C31.2 Malignant neoplasm of frontal sinus C31.3 Malignant neoplasm of sphenoid sinus C31.8 Malignant neoplasm of overlapping sites of accessory sinuses C32.0 Malignant neoplasm of glottis C32.1 Malignant neoplasm of supraglottis C32.2 Malignant neoplasm of subglottis C32.3 Malignant neoplasm of laryngeal cartilage C32.8 Malignant neoplasm of overlapping sites of larynx C33 Malignant neoplasm of trachea C34.01 Malignant neoplasm of right main bronchus C34.02 Malignant neoplasm of left main bronchus C34.11 Malignant neoplasm of upper lobe, right bronchus or lung C34.12 Malignant neoplasm of upper lobe, left bronchus or lung C34.2 Malignant neoplasm of middle lobe, bronchus or lung C34.31 Malignant neoplasm of lower lobe, right bronchus or lung 10
11 C34.32 Malignant neoplasm of lower lobe, left bronchus or lung C34.81 Malignant neoplasm of overlapping sites of right bronchus and lung C34.82 Malignant neoplasm of overlapping sites of left bronchus and lung C37 Malignant neoplasm of thymus C38.0 Malignant neoplasm of heart C38.1 Malignant neoplasm of anterior mediastinum C38.2 Malignant neoplasm of posterior mediastinum C38.4 Malignant neoplasm of pleura C38.8 Malignant neoplasm of overlapping sites of heart, mediastinum and pleura C40.01 Malignant neoplasm of scapula and long bones of right upper limb C40.02 Malignant neoplasm of scapula and long bones of left upper limb C40.11 Malignant neoplasm of short bones of right upper limb C40.12 Malignant neoplasm of short bones of left upper limb C40.21 Malignant neoplasm of long bones of right lower limb C40.22 Malignant neoplasm of long bones of left lower limb C40.31 Malignant neoplasm of short bones of right lower limb C40.32 Malignant neoplasm of short bones of left lower limb C40.81 Malignant neoplasm of overlapping sites of bone and articular cartilage of right limb C40.82 Malignant neoplasm of overlapping sites of bone and articular cartilage of left limb C41.0 Malignant neoplasm of bones of skull and face C41.1 Malignant neoplasm of mandible C41.2 Malignant neoplasm of vertebral column C41.3 Malignant neoplasm of ribs, sternum and clavicle C41.4 Malignant neoplasm of pelvic bones, sacrum and coccyx 11
12 C43.0 Malignant melanoma of lip C43.11 Malignant melanoma of right eyelid, including canthus C43.12 Malignant melanoma of left eyelid, including canthus C43.21 Malignant melanoma of right ear and external auricular canal C43.22 Malignant melanoma of left ear and external auricular canal C43.31 Malignant melanoma of nose C43.39 Malignant melanoma of other parts of face C43.4 Malignant melanoma of scalp and neck C43.51 Malignant melanoma of anal skin C43.52 Malignant melanoma of skin of breast C4A.0 C4A.11 C4A.12 C4A.21 C4A.22 C4A.31 C4A.39 C4A.4 C4A.51 C4A.52 Merkel cell carcinoma of lip Merkel cell carcinoma of right eyelid, including canthus Merkel cell carcinoma of left eyelid, including canthus Merkel cell carcinoma of right ear and external auricular canal Merkel cell carcinoma of left ear and external auricular canal Merkel cell carcinoma of nose Merkel cell carcinoma of other parts of face Merkel cell carcinoma of scalp and neck Merkel cell carcinoma of anal skin Merkel cell carcinoma of skin of breast C44.01 Basal cell carcinoma of skin of lip C44.02 Squamous cell carcinoma of skin of lip C Basal cell carcinoma of skin of right eyelid, including canthus 12
13 C Basal cell carcinoma of skin of left eyelid, including canthus C Squamous cell carcinoma of skin of right eyelid, including canthus C Squamous cell carcinoma of skin of left eyelid, including canthus C Other specified malignant neoplasm of skin of right eyelid, including canthus C Other specified malignant neoplasm of skin of left eyelid, including canthus C Basal cell carcinoma of skin of right ear and external auricular canal C Basal cell carcinoma of skin of left ear and external auricular canal C Squamous cell carcinoma of skin of right ear and external auricular canal C Squamous cell carcinoma of skin of left ear and external auricular canal C Other specified malignant neoplasm of skin of right ear and external auricular canal C Other specified malignant neoplasm of skin of left ear and external auricular canal C Basal cell carcinoma of skin of nose C Basal cell carcinoma of skin of other parts of face C Squamous cell carcinoma of skin of nose C Squamous cell carcinoma of skin of other parts of face C Other specified malignant neoplasm of skin of nose C Other specified malignant neoplasm of skin of other parts of face C44.41 Basal cell carcinoma of skin of scalp and neck C44.42 Squamous cell carcinoma of skin of scalp and neck C44.49 Other specified malignant neoplasm of skin of scalp and neck C Basal cell carcinoma of anal skin C Basal cell carcinoma of skin of breast C Squamous cell carcinoma of anal skin 13
14 C Squamous cell carcinoma of skin of breast C Other specified malignant neoplasm of anal skin C Other specified malignant neoplasm of skin of breast C45.0 Mesothelioma of pleura C45.1 Mesothelioma of peritoneum C45.2 Mesothelioma of pericardium C47.0 Malignant neoplasm of peripheral nerves of head, face and neck C47.11 Malignant neoplasm of peripheral nerves of right upper limb, including shoulder C47.12 Malignant neoplasm of peripheral nerves of left upper limb, including shoulder C47.21 Malignant neoplasm of peripheral nerves of right lower limb, including hip C47.22 Malignant neoplasm of peripheral nerves of left lower limb, including hip C47.3 Malignant neoplasm of peripheral nerves of thorax C47.4 Malignant neoplasm of peripheral nerves of abdomen C47.5 Malignant neoplasm of peripheral nerves of pelvis C47.8 Malignant neoplasm of overlapping sites of peripheral nerves and autonomic nervous system C48.0 Malignant neoplasm of retroperitoneum C48.1 Malignant neoplasm of specified parts of peritoneum C48.8 Malignant neoplasm of overlapping sites of retroperitoneum and peritoneum C49.0 Malignant neoplasm of connective and soft tissue of head, face and neck C49.11 Malignant neoplasm of connective and soft tissue of right upper limb, including shoulder C49.12 Malignant neoplasm of connective and soft tissue of left upper limb, including shoulder C49.21 Malignant neoplasm of connective and soft tissue of right lower limb, including hip C49.22 Malignant neoplasm of connective and soft tissue of left lower limb, including hip 14
15 C49.3 Malignant neoplasm of connective and soft tissue of thorax C49.4 Malignant neoplasm of connective and soft tissue of abdomen C49.5 Malignant neoplasm of connective and soft tissue of pelvis C49.6 Malignant neoplasm of connective and soft tissue of trunk, unspecified C49.8 Malignant neoplasm of overlapping sites of connective and soft tissue C Malignant neoplasm of nipple and areola, right female breast C Malignant neoplasm of nipple and areola, left female breast C Malignant neoplasm of nipple and areola, right male breast C Malignant neoplasm of nipple and areola, left male breast C Malignant neoplasm of central portion of right female breast C Malignant neoplasm of central portion of left female breast C Malignant neoplasm of central portion of right male breast C Malignant neoplasm of central portion of left male breast C Malignant neoplasm of upper-inner quadrant of right female breast C Malignant neoplasm of upper-inner quadrant of left female breast C Malignant neoplasm of upper-inner quadrant of right male breast C Malignant neoplasm of upper-inner quadrant of left male breast C Malignant neoplasm of lower-inner quadrant of right female breast C Malignant neoplasm of lower-inner quadrant of left female breast C Malignant neoplasm of lower-inner quadrant of right male breast C Malignant neoplasm of lower-inner quadrant of left male breast C Malignant neoplasm of upper-outer quadrant of right female breast C Malignant neoplasm of upper-outer quadrant of left female breast 15
16 C Malignant neoplasm of upper-outer quadrant of right male breast C Malignant neoplasm of upper-outer quadrant of left male breast C Malignant neoplasm of lower-outer quadrant of right female breast C Malignant neoplasm of lower-outer quadrant of left female breast C Malignant neoplasm of lower-outer quadrant of right male breast C Malignant neoplasm of lower-outer quadrant of left male breast C Malignant neoplasm of axillary tail of right female breast C Malignant neoplasm of axillary tail of left female breast C Malignant neoplasm of axillary tail of right male breast C Malignant neoplasm of axillary tail of left male breast C Malignant neoplasm of overlapping sites of right female breast C Malignant neoplasm of overlapping sites of left female breast C Malignant neoplasm of overlapping sites of right male breast C Malignant neoplasm of overlapping sites of left male breast C51.0 Malignant neoplasm of labium majus C51.1 Malignant neoplasm of labium minus C51.2 Malignant neoplasm of clitoris C51.8 Malignant neoplasm of overlapping sites of vulva C51.9 Malignant neoplasm of vulva, unspecified C52 Malignant neoplasm of vagina C53.0 Malignant neoplasm of endocervix C53.1 Malignant neoplasm of exocervix C53.8 Malignant neoplasm of overlapping sites of cervix uteri 16
17 C54.0 Malignant neoplasm of isthmus uteri C54.1 Malignant neoplasm of endometrium C54.2 Malignant neoplasm of myometrium C54.3 Malignant neoplasm of fundus uteri C54.8 Malignant neoplasm of overlapping sites of corpus uteri C56.1 Malignant neoplasm of right ovary C56.2 Malignant neoplasm of left ovary C57.01 Malignant neoplasm of right fallopian tube C57.02 Malignant neoplasm of left fallopian tube C57.11 Malignant neoplasm of right broad ligament C57.12 Malignant neoplasm of left broad ligament C57.21 Malignant neoplasm of right round ligament C57.22 Malignant neoplasm of left round ligament C57.3 Malignant neoplasm of parametrium C57.4 Malignant neoplasm of uterine adnexa, unspecified C57.7 Malignant neoplasm of other specified female genital organs C57.8 Malignant neoplasm of overlapping sites of female genital organs C57.9 Malignant neoplasm of female genital organ, unspecified C60.0 Malignant neoplasm of prepuce C60.1 Malignant neoplasm of glans penis C60.2 Malignant neoplasm of body of penis C60.8 Malignant neoplasm of overlapping sites of penis C61 Malignant neoplasm of prostate 17
18 C62.01 Malignant neoplasm of undescended right testis C62.02 Malignant neoplasm of undescended left testis C62.11 Malignant neoplasm of descended right testis C62.12 Malignant neoplasm of descended left testis C64.1 Malignant neoplasm of right kidney, except renal pelvis C64.2 Malignant neoplasm of left kidney, except renal pelvis C65.1 Malignant neoplasm of right renal pelvis C65.2 Malignant neoplasm of left renal pelvis C66.1 Malignant neoplasm of right ureter C66.2 Malignant neoplasm of left ureter C67.0 Malignant neoplasm of trigone of bladder C67.1 Malignant neoplasm of dome of bladder C67.2 Malignant neoplasm of lateral wall of bladder C67.3 Malignant neoplasm of anterior wall of bladder C67.4 Malignant neoplasm of posterior wall of bladder C67.5 Malignant neoplasm of bladder neck C67.6 Malignant neoplasm of ureteric orifice C67.7 Malignant neoplasm of urachus C67.8 Malignant neoplasm of overlapping sites of bladder C68.0 Malignant neoplasm of urethra C68.1 Malignant neoplasm of paraurethral glands C68.8 Malignant neoplasm of overlapping sites of urinary organs C69.01 Malignant neoplasm of right conjunctiva 18
19 C69.02 Malignant neoplasm of left conjunctiva C69.11 Malignant neoplasm of right cornea C69.12 Malignant neoplasm of left cornea C69.21 Malignant neoplasm of right retina C69.22 Malignant neoplasm of left retina C69.31 Malignant neoplasm of right choroid C69.32 Malignant neoplasm of left choroid C69.41 Malignant neoplasm of right ciliary body C69.42 Malignant neoplasm of left ciliary body C69.51 Malignant neoplasm of right lacrimal gland and duct C69.52 Malignant neoplasm of left lacrimal gland and duct C69.61 Malignant neoplasm of right orbit C69.62 Malignant neoplasm of left orbit C69.81 Malignant neoplasm of overlapping sites of right eye and adnexa C69.82 Malignant neoplasm of overlapping sites of left eye and adnexa C70.0 Malignant neoplasm of cerebral meninges C70.1 Malignant neoplasm of spinal meninges C71.0 Malignant neoplasm of cerebrum, except lobes and ventricles C71.1 Malignant neoplasm of frontal lobe C71.2 Malignant neoplasm of temporal lobe C71.3 Malignant neoplasm of parietal lobe C71.4 Malignant neoplasm of occipital lobe C71.5 Malignant neoplasm of cerebral ventricle 19
20 C71.6 Malignant neoplasm of cerebellum C71.7 Malignant neoplasm of brain stem C71.8 Malignant neoplasm of overlapping sites of brain C72.0 Malignant neoplasm of spinal cord C72.1 Malignant neoplasm of cauda equina C72.21 Malignant neoplasm of right olfactory nerve C72.22 Malignant neoplasm of left olfactory nerve C72.31 Malignant neoplasm of right optic nerve C72.32 Malignant neoplasm of left optic nerve C72.41 Malignant neoplasm of right acoustic nerve C72.42 Malignant neoplasm of left acoustic nerve C72.59 Malignant neoplasm of other cranial nerves C73 Malignant neoplasm of thyroid gland C74.01 Malignant neoplasm of cortex of right adrenal gland C74.02 Malignant neoplasm of cortex of left adrenal gland C74.11 Malignant neoplasm of medulla of right adrenal gland C74.12 Malignant neoplasm of medulla of left adrenal gland C75.0 Malignant neoplasm of parathyroid gland C75.4 Malignant neoplasm of carotid body C75.5 Malignant neoplasm of aortic body and other paraganglia C77.0 Secondary and unspecified malignant neoplasm of lymph nodes of head, face and neck C79.31 Secondary malignant neoplasm of brain C79.32 Secondary malignant neoplasm of cerebral meninges 20
21 C79.51 Secondary malignant neoplasm of bone C79.52 Secondary malignant neoplasm of bone marrow C79.71 Secondary malignant neoplasm of right adrenal gland C79.72 Secondary malignant neoplasm of left adrenal gland C81.01 Nodular lymphocyte predominant Hodgkin lymphoma, lymph nodes of head, face, and neck C81.02 Nodular lymphocyte predominant Hodgkin lymphoma, intrathoracic lymph nodes C81.03 Nodular lymphocyte predominant Hodgkin lymphoma, intra-abdominal lymph nodes C81.04 Nodular lymphocyte predominant Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.05 Nodular lymphocyte predominant Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.06 Nodular lymphocyte predominant Hodgkin lymphoma, intrapelvic lymph nodes C81.07 Nodular lymphocyte predominant Hodgkin lymphoma, spleen C81.08 Nodular lymphocyte predominant Hodgkin lymphoma, lymph nodes of multiple sites C81.09 Nodular lymphocyte predominant Hodgkin lymphoma, extranodal and solid organ sites C81.11 Nodular sclerosis classical Hodgkin lymphoma, lymph nodes of head, face, and neck C81.12 Nodular sclerosis classical Hodgkin lymphoma, intrathoracic lymph nodes C81.13 Nodular sclerosis classical Hodgkin lymphoma, intra-abdominal lymph nodes C81.14 Nodular sclerosis classical Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.15 Nodular sclerosis classical Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.16 Nodular sclerosis classical Hodgkin lymphoma, intrapelvic lymph nodes C81.17 Nodular sclerosis classical Hodgkin lymphoma, spleen C81.18 Nodular sclerosis classical Hodgkin lymphoma, lymph nodes of multiple sites C81.19 Nodular sclerosis classical Hodgkin lymphoma, extranodal and solid organ sites C81.21 Mixed cellularity classical Hodgkin lymphoma, lymph nodes of head, face, and neck 21
22 C81.22 Mixed cellularity classical Hodgkin lymphoma, intrathoracic lymph nodes C81.23 Mixed cellularity classical Hodgkin lymphoma, intra-abdominal lymph nodes C81.24 Mixed cellularity classical Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.25 Mixed cellularity classical Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.26 Mixed cellularity classical Hodgkin lymphoma, intrapelvic lymph nodes C81.27 Mixed cellularity classical Hodgkin lymphoma, spleen C81.28 Mixed cellularity classical Hodgkin lymphoma, lymph nodes of multiple sites C81.29 Mixed cellularity classical Hodgkin lymphoma, extranodal and solid organ sites C81.31 Lymphocyte depleted classical Hodgkin lymphoma, lymph nodes of head, face, and neck C81.32 Lymphocyte depleted classical Hodgkin lymphoma, intrathoracic lymph nodes C81.33 Lymphocyte depleted classical Hodgkin lymphoma, intra-abdominal lymph nodes C81.34 Lymphocyte depleted classical Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.35 Lymphocyte depleted classical Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.36 Lymphocyte depleted classical Hodgkin lymphoma, intrapelvic lymph nodes C81.37 Lymphocyte depleted classical Hodgkin lymphoma, spleen C81.38 Lymphocyte depleted classical Hodgkin lymphoma, lymph nodes of multiple sites C81.39 Lymphocyte depleted classical Hodgkin lymphoma, extranodal and solid organ sites C81.41 Lymphocyte-rich classical Hodgkin lymphoma, lymph nodes of head, face, and neck C81.42 Lymphocyte-rich classical Hodgkin lymphoma, intrathoracic lymph nodes C81.43 Lymphocyte-rich classical Hodgkin lymphoma, intra-abdominal lymph nodes C81.44 Lymphocyte-rich classical Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.45 Lymphocyte-rich classical Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.46 Lymphocyte-rich classical Hodgkin lymphoma, intrapelvic lymph nodes 22
23 C81.47 Lymphocyte-rich classical Hodgkin lymphoma, spleen C81.48 Lymphocyte-rich classical Hodgkin lymphoma, lymph nodes of multiple sites C81.49 Lymphocyte-rich classical Hodgkin lymphoma, extranodal and solid organ sites C81.71 Other classical Hodgkin lymphoma, lymph nodes of head, face, and neck C81.72 Other classical Hodgkin lymphoma, intrathoracic lymph nodes C81.73 Other classical Hodgkin lymphoma, intra-abdominal lymph nodes C81.74 Other classical Hodgkin lymphoma, lymph nodes of axilla and upper limb C81.75 Other classical Hodgkin lymphoma, lymph nodes of inguinal region and lower limb C81.76 Other classical Hodgkin lymphoma, intrapelvic lymph nodes C81.77 Other classical Hodgkin lymphoma, spleen C81.78 Other classical Hodgkin lymphoma, lymph nodes of multiple sites C81.79 Other classical Hodgkin lymphoma, extranodal and solid organ sites C82.01 Follicular lymphoma grade I, lymph nodes of head, face, and neck C82.02 Follicular lymphoma grade I, intrathoracic lymph nodes C82.03 Follicular lymphoma grade I, intra-abdominal lymph nodes C82.04 Follicular lymphoma grade I, lymph nodes of axilla and upper limb C82.05 Follicular lymphoma grade I, lymph nodes of inguinal region and lower limb C82.06 Follicular lymphoma grade I, intrapelvic lymph nodes C82.07 Follicular lymphoma grade I, spleen C82.08 Follicular lymphoma grade I, lymph nodes of multiple sites C82.09 Follicular lymphoma grade I, extranodal and solid organ sites C82.11 Follicular lymphoma grade II, lymph nodes of head, face, and neck C82.12 Follicular lymphoma grade II, intrathoracic lymph nodes 23
24 C82.13 Follicular lymphoma grade II, intra-abdominal lymph nodes C82.14 Follicular lymphoma grade II, lymph nodes of axilla and upper limb C82.15 Follicular lymphoma grade II, lymph nodes of inguinal region and lower limb C82.16 Follicular lymphoma grade II, intrapelvic lymph nodes C82.17 Follicular lymphoma grade II, spleen C82.18 Follicular lymphoma grade II, lymph nodes of multiple sites C82.19 Follicular lymphoma grade II, extranodal and solid organ sites C82.21 Follicular lymphoma grade III, unspecified, lymph nodes of head, face, and neck C82.22 Follicular lymphoma grade III, unspecified, intrathoracic lymph nodes C82.23 Follicular lymphoma grade III, unspecified, intra-abdominal lymph nodes C82.24 Follicular lymphoma grade III, unspecified, lymph nodes of axilla and upper limb C82.25 Follicular lymphoma grade III, unspecified, lymph nodes of inguinal region and lower limb C82.26 Follicular lymphoma grade III, unspecified, intrapelvic lymph nodes C82.27 Follicular lymphoma grade III, unspecified, spleen C82.28 Follicular lymphoma grade III, unspecified, lymph nodes of multiple sites C82.29 Follicular lymphoma grade III, unspecified, extranodal and solid organ sites C82.31 Follicular lymphoma grade IIIa, lymph nodes of head, face, and neck C82.32 Follicular lymphoma grade IIIa, intrathoracic lymph nodes C82.33 Follicular lymphoma grade IIIa, intra-abdominal lymph nodes C82.34 Follicular lymphoma grade IIIa, lymph nodes of axilla and upper limb C82.35 Follicular lymphoma grade IIIa, lymph nodes of inguinal region and lower limb C82.36 Follicular lymphoma grade IIIa, intrapelvic lymph nodes C82.37 Follicular lymphoma grade IIIa, spleen 24
25 C82.38 Follicular lymphoma grade IIIa, lymph nodes of multiple sites C82.39 Follicular lymphoma grade IIIa, extranodal and solid organ sites C82.41 Follicular lymphoma grade IIIb, lymph nodes of head, face, and neck C82.42 Follicular lymphoma grade IIIb, intrathoracic lymph nodes C82.43 Follicular lymphoma grade IIIb, intra-abdominal lymph nodes C82.44 Follicular lymphoma grade IIIb, lymph nodes of axilla and upper limb C82.45 Follicular lymphoma grade IIIb, lymph nodes of inguinal region and lower limb C82.46 Follicular lymphoma grade IIIb, intrapelvic lymph nodes C82.47 Follicular lymphoma grade IIIb, spleen C82.48 Follicular lymphoma grade IIIb, lymph nodes of multiple sites C82.49 Follicular lymphoma grade IIIb, extranodal and solid organ sites C82.51 Diffuse follicle center lymphoma, lymph nodes of head, face, and neck C82.52 Diffuse follicle center lymphoma, intrathoracic lymph nodes C82.53 Diffuse follicle center lymphoma, intra-abdominal lymph nodes C82.54 Diffuse follicle center lymphoma, lymph nodes of axilla and upper limb C82.55 Diffuse follicle center lymphoma, lymph nodes of inguinal region and lower limb C82.56 Diffuse follicle center lymphoma, intrapelvic lymph nodes C82.57 Diffuse follicle center lymphoma, spleen C82.58 Diffuse follicle center lymphoma, lymph nodes of multiple sites C82.59 Diffuse follicle center lymphoma, extranodal and solid organ sites C82.61 Cutaneous follicle center lymphoma, lymph nodes of head, face, and neck C82.62 Cutaneous follicle center lymphoma, intrathoracic lymph nodes C82.63 Cutaneous follicle center lymphoma, intra-abdominal lymph nodes 25
26 C82.64 Cutaneous follicle center lymphoma, lymph nodes of axilla and upper limb C82.65 Cutaneous follicle center lymphoma, lymph nodes of inguinal region and lower limb C82.66 Cutaneous follicle center lymphoma, intrapelvic lymph nodes C82.67 Cutaneous follicle center lymphoma, spleen C82.68 Cutaneous follicle center lymphoma, lymph nodes of multiple sites C82.69 Cutaneous follicle center lymphoma, extranodal and solid organ sites C82.81 Other types of follicular lymphoma, lymph nodes of head, face, and neck C82.82 Other types of follicular lymphoma, intrathoracic lymph nodes C82.83 Other types of follicular lymphoma, intra-abdominal lymph nodes C82.84 Other types of follicular lymphoma, lymph nodes of axilla and upper limb C82.85 Other types of follicular lymphoma, lymph nodes of inguinal region and lower limb C82.86 Other types of follicular lymphoma, intrapelvic lymph nodes C82.87 Other types of follicular lymphoma, spleen C82.88 Other types of follicular lymphoma, lymph nodes of multiple sites C82.89 Other types of follicular lymphoma, extranodal and solid organ sites C82.91 Follicular lymphoma, unspecified, lymph nodes of head, face, and neck C82.92 Follicular lymphoma, unspecified, intrathoracic lymph nodes C82.93 Follicular lymphoma, unspecified, intra-abdominal lymph nodes C82.94 Follicular lymphoma, unspecified, lymph nodes of axilla and upper limb C82.95 Follicular lymphoma, unspecified, lymph nodes of inguinal region and lower limb C82.96 Follicular lymphoma, unspecified, intrapelvic lymph nodes C82.97 Follicular lymphoma, unspecified, spleen C82.98 Follicular lymphoma, unspecified, lymph nodes of multiple sites 26
27 C82.99 Follicular lymphoma, unspecified, extranodal and solid organ sites C83.01 Small cell B-cell lymphoma, lymph nodes of head, face, and neck C83.02 Small cell B-cell lymphoma, intrathoracic lymph nodes C83.03 Small cell B-cell lymphoma, intra-abdominal lymph nodes C83.04 Small cell B-cell lymphoma, lymph nodes of axilla and upper limb C83.05 Small cell B-cell lymphoma, lymph nodes of inguinal region and lower limb C83.06 Small cell B-cell lymphoma, intrapelvic lymph nodes C83.07 Small cell B-cell lymphoma, spleen C83.08 Small cell B-cell lymphoma, lymph nodes of multiple sites C83.11 Mantle cell lymphoma, lymph nodes of head, face, and neck C83.12 Mantle cell lymphoma, intrathoracic lymph nodes C83.13 Mantle cell lymphoma, intra-abdominal lymph nodes C83.14 Mantle cell lymphoma, lymph nodes of axilla and upper limb C83.15 Mantle cell lymphoma, lymph nodes of inguinal region and lower limb C83.16 Mantle cell lymphoma, intrapelvic lymph nodes C83.17 Mantle cell lymphoma, spleen C83.18 Mantle cell lymphoma, lymph nodes of multiple sites C83.31 Diffuse large B-cell lymphoma, lymph nodes of head, face, and neck C83.32 Diffuse large B-cell lymphoma, intrathoracic lymph nodes C83.33 Diffuse large B-cell lymphoma, intra-abdominal lymph nodes C83.34 Diffuse large B-cell lymphoma, lymph nodes of axilla and upper limb C83.35 Diffuse large B-cell lymphoma, lymph nodes of inguinal region and lower limb C83.36 Diffuse large B-cell lymphoma, intrapelvic lymph nodes 27
28 C83.37 Diffuse large B-cell lymphoma, spleen C83.38 Diffuse large B-cell lymphoma, lymph nodes of multiple sites C83.51 Lymphoblastic (diffuse) lymphoma, lymph nodes of head, face, and neck C83.52 Lymphoblastic (diffuse) lymphoma, intrathoracic lymph nodes C83.53 Lymphoblastic (diffuse) lymphoma, intra-abdominal lymph nodes C83.54 Lymphoblastic (diffuse) lymphoma, lymph nodes of axilla and upper limb C83.55 Lymphoblastic (diffuse) lymphoma, lymph nodes of inguinal region and lower limb C83.56 Lymphoblastic (diffuse) lymphoma, intrapelvic lymph nodes C83.57 Lymphoblastic (diffuse) lymphoma, spleen C83.58 Lymphoblastic (diffuse) lymphoma, lymph nodes of multiple sites C83.71 Burkitt lymphoma, lymph nodes of head, face, and neck C83.72 Burkitt lymphoma, intrathoracic lymph nodes C83.73 Burkitt lymphoma, intra-abdominal lymph nodes C83.74 Burkitt lymphoma, lymph nodes of axilla and upper limb C83.75 Burkitt lymphoma, lymph nodes of inguinal region and lower limb C83.76 Burkitt lymphoma, intrapelvic lymph nodes C83.77 Burkitt lymphoma, spleen C83.78 Burkitt lymphoma, lymph nodes of multiple sites C83.81 Other non-follicular lymphoma, lymph nodes of head, face, and neck C83.82 Other non-follicular lymphoma, intrathoracic lymph nodes C83.83 Other non-follicular lymphoma, intra-abdominal lymph nodes C83.84 Other non-follicular lymphoma, lymph nodes of axilla and upper limb C83.85 Other non-follicular lymphoma, lymph nodes of inguinal region and lower limb 28
29 C83.86 Other non-follicular lymphoma, intrapelvic lymph nodes C83.87 Other non-follicular lymphoma, spleen C83.88 Other non-follicular lymphoma, lymph nodes of multiple sites C83.89 Other non-follicular lymphoma, extranodal and solid organ sites C84.01 Mycosis fungoides, lymph nodes of head, face, and neck C84.02 Mycosis fungoides, intrathoracic lymph nodes C84.03 Mycosis fungoides, intra-abdominal lymph nodes C84.04 Mycosis fungoides, lymph nodes of axilla and upper limb C84.05 Mycosis fungoides, lymph nodes of inguinal region and lower limb C84.06 Mycosis fungoides, intrapelvic lymph nodes C84.07 Mycosis fungoides, spleen C84.08 Mycosis fungoides, lymph nodes of multiple sites C84.09 Mycosis fungoides, extranodal and solid organ sites C84.11 Sezary disease, lymph nodes of head, face, and neck C84.12 Sezary disease, intrathoracic lymph nodes C84.13 Sezary disease, intra-abdominal lymph nodes C84.14 Sezary disease, lymph nodes of axilla and upper limb C84.15 Sezary disease, lymph nodes of inguinal region and lower limb C84.16 Sezary disease, intrapelvic lymph nodes C84.17 Sezary disease, spleen C84.18 Sezary disease, lymph nodes of multiple sites C84.19 Sezary disease, extranodal and solid organ sites C84.41 Peripheral T-cell lymphoma, not classified, lymph nodes of head, face, and neck 29
30 C84.42 Peripheral T-cell lymphoma, not classified, intrathoracic lymph nodes C84.43 Peripheral T-cell lymphoma, not classified, intra-abdominal lymph nodes C84.44 Peripheral T-cell lymphoma, not classified, lymph nodes of axilla and upper limb C84.45 Peripheral T-cell lymphoma, not classified, lymph nodes of inguinal region and lower limb C84.46 Peripheral T-cell lymphoma, not classified, intrapelvic lymph nodes C84.47 Peripheral T-cell lymphoma, not classified, spleen C84.48 Peripheral T-cell lymphoma, not classified, lymph nodes of multiple sites C84.49 Peripheral T-cell lymphoma, not classified, extranodal and solid organ sites C84.61 Anaplastic large cell lymphoma, ALK-positive, lymph nodes of head, face, and neck C84.62 Anaplastic large cell lymphoma, ALK-positive, intrathoracic lymph nodes C84.63 Anaplastic large cell lymphoma, ALK-positive, intra-abdominal lymph nodes C84.64 Anaplastic large cell lymphoma, ALK-positive, lymph nodes of axilla and upper limb C84.65 Anaplastic large cell lymphoma, ALK-positive, lymph nodes of inguinal region and lower limb C84.66 Anaplastic large cell lymphoma, ALK-positive, intrapelvic lymph nodes C84.67 Anaplastic large cell lymphoma, ALK-positive, spleen C84.68 Anaplastic large cell lymphoma, ALK-positive, lymph nodes of multiple sites C84.69 Anaplastic large cell lymphoma, ALK-positive, extranodal and solid organ sites C84.71 Anaplastic large cell lymphoma, ALK-negative, lymph nodes of head, face, and neck C84.72 Anaplastic large cell lymphoma, ALK-negative, intrathoracic lymph nodes C84.73 Anaplastic large cell lymphoma, ALK-negative, intra-abdominal lymph nodes C84.74 Anaplastic large cell lymphoma, ALK-negative, lymph nodes of axilla and upper limb C84.75 Anaplastic large cell lymphoma, ALK-negative, lymph nodes of inguinal region and lower limb C84.76 Anaplastic large cell lymphoma, ALK-negative, intrapelvic lymph nodes 30
31 C84.77 Anaplastic large cell lymphoma, ALK-negative, spleen C84.78 Anaplastic large cell lymphoma, ALK-negative, lymph nodes of multiple sites C84.79 Anaplastic large cell lymphoma, ALK-negative, extranodal and solid organ sites C84.A1 C84.A2 C84.A3 C84.A4 C84.A5 C84.A6 C84.A7 C84.A8 C84.A9 C84.Z1 C84.Z2 C84.Z3 C84.Z4 C84.Z5 C84.Z6 C84.Z7 C84.Z8 C84.Z9 Cutaneous T-cell lymphoma, unspecified lymph nodes of head, face, and neck Cutaneous T-cell lymphoma, unspecified, intrathoracic lymph nodes Cutaneous T-cell lymphoma, unspecified, intra-abdominal lymph nodes Cutaneous T-cell lymphoma, unspecified, lymph nodes of axilla and upper limb Cutaneous T-cell lymphoma, unspecified, lymph nodes of inguinal region and lower limb Cutaneous T-cell lymphoma, unspecified, intrapelvic lymph nodes Cutaneous T-cell lymphoma, unspecified, spleen Cutaneous T-cell lymphoma, unspecified, lymph nodes of multiple sites Cutaneous T-cell lymphoma, unspecified, extranodal and solid organ sites Other mature T/NK-cell lymphomas, lymph nodes of head, face, and neck Other mature T/NK-cell lymphomas, intrathoracic lymph nodes Other mature T/NK-cell lymphomas, intra-abdominal lymph nodes Other mature T/NK-cell lymphomas, lymph nodes of axilla and upper limb Other mature T/NK-cell lymphomas, lymph nodes of inguinal region and lower limb Other mature T/NK-cell lymphomas, intrapelvic lymph nodes Other mature T/NK-cell lymphomas, spleen Other mature T/NK-cell lymphomas, lymph nodes of multiple sites Other mature T/NK-cell lymphomas, extranodal and solid organ sites C84.91 Mature T/NK-cell lymphomas, unspecified, lymph nodes of head, face, and neck C84.92 Mature T/NK-cell lymphomas, unspecified, intrathoracic lymph nodes 31
32 C84.93 Mature T/NK-cell lymphomas, unspecified, intra-abdominal lymph nodes C84.94 Mature T/NK-cell lymphomas, unspecified, lymph nodes of axilla and upper limb C84.95 Mature T/NK-cell lymphomas, unspecified, lymph nodes of inguinal region and lower limb C84.96 Mature T/NK-cell lymphomas, unspecified, intrapelvic lymph nodes C84.97 Mature T/NK-cell lymphomas, unspecified, spleen C84.98 Mature T/NK-cell lymphomas, unspecified, lymph nodes of multiple sites C84.99 Mature T/NK-cell lymphomas, unspecified, extranodal and solid organ sites C85.11 Unspecified B-cell lymphoma, lymph nodes of head, face, and neck C85.12 Unspecified B-cell lymphoma, intrathoracic lymph nodes C85.13 Unspecified B-cell lymphoma, intra-abdominal lymph nodes C85.14 Unspecified B-cell lymphoma, lymph nodes of axilla and upper limb C85.15 Unspecified B-cell lymphoma, lymph nodes of inguinal region and lower limb C85.16 Unspecified B-cell lymphoma, intrapelvic lymph nodes C85.17 Unspecified B-cell lymphoma, spleen C85.18 Unspecified B-cell lymphoma, lymph nodes of multiple sites C85.19 Unspecified B-cell lymphoma, extranodal and solid organ sites C86.0 Extranodal NK/T-cell lymphoma, nasal type C86.1 Hepatosplenic T-cell lymphoma C86.2 Enteropathy-type (intestinal) T-cell lymphoma C86.3 Subcutaneous panniculitis-like T-cell lymphoma C86.4 Blastic NK-cell lymphoma C90.30 Solitary plasmacytoma not having achieved remission C91.40 Hairy cell leukemia not having achieved remission 32
33 C91.41 Hairy cell leukemia, in remission C91.42 Hairy cell leukemia, in relapse C96.0 Multifocal and multisystemic (disseminated) Langerhans-cell histiocytosis C96.2 Malignant mast cell tumor C96.4 Sarcoma of dendritic cells (accessory cells) C96.9 Malignant neoplasm of lymphoid, hematopoietic and related tissue, unspecified C96.A C96.Z Histiocytic sarcoma Other specified malignant neoplasms of lymphoid, hematopoietic and related tissue D05.01 Lobular carcinoma in situ of right breast D05.02 Lobular carcinoma in situ of left breast D05.11 Intraductal carcinoma in situ of right breast D05.12 Intraductal carcinoma in situ of left breast D05.81 Other specified type of carcinoma in situ of right breast D05.82 Other specified type of carcinoma in situ of left breast D05.91 Unspecified type of carcinoma in situ of right breast D05.92 Unspecified type of carcinoma in situ of left breast D32.0 Benign neoplasm of cerebral meninges D32.1 Benign neoplasm of spinal meninges D33.3 Benign neoplasm of cranial nerves D35.2 Benign neoplasm of pituitary gland D35.3 Benign neoplasm of craniopharyngeal duct D35.4 Benign neoplasm of pineal gland D35.6 Benign neoplasm of aortic body and other paraganglia 33
82330 CALCIUM; IONIZED. ICD-10 Codes that Support Medical Necessity. ICD-10 Code. Description. A15.0 Tuberculosis of lung
 82330 CALCIUM; IONIZED ICD-10 Codes that Support Medical Necessity ICD-10 Code Description A15.0 Tuberculosis of lung A15.4 Tuberculosis of intrathoracic lymph nodes A15.5 Tuberculosis of larynx, trachea
82330 CALCIUM; IONIZED ICD-10 Codes that Support Medical Necessity ICD-10 Code Description A15.0 Tuberculosis of lung A15.4 Tuberculosis of intrathoracic lymph nodes A15.5 Tuberculosis of larynx, trachea
Cancer Association of South Africa (CANSA)
 Cancer Association of South Africa (CANSA) Fact Sheet on ICD-10 Coding of Neoplasms Introduction The International Statistical Classification of Diseases and Related Health Problems, 10 th Revision (ICD-10)
Cancer Association of South Africa (CANSA) Fact Sheet on ICD-10 Coding of Neoplasms Introduction The International Statistical Classification of Diseases and Related Health Problems, 10 th Revision (ICD-10)
Model Policy. Coverage of Proton Therapy
 Model Policy Coverage of Proton Therapy Last Revised - February 2019 INTRODUCTION Proton therapy is a technologically advanced method to deliver curative radiation doses to cancerous tumors. The unique
Model Policy Coverage of Proton Therapy Last Revised - February 2019 INTRODUCTION Proton therapy is a technologically advanced method to deliver curative radiation doses to cancerous tumors. The unique
CLINICAL MEDICATION POLICY
 CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
Contractor Information. LCD Information. Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416)
 Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Serum Iron Studies
 190.18 - Serum Iron Studies Serum iron studies are useful in the evaluation of disorders of iron metabolism, particularly iron deficiency and iron excess. Iron studies are best performed when the patient
190.18 - Serum Iron Studies Serum iron studies are useful in the evaluation of disorders of iron metabolism, particularly iron deficiency and iron excess. Iron studies are best performed when the patient
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Keytruda (pembrolizumab) MP-014-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 01/15/2018; 08/01/2017; 06/01/2016
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Keytruda (pembrolizumab) MP-014-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 01/15/2018; 08/01/2017; 06/01/2016
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICATION POLICY Granulocyte Colony Stimulating Factors (G-CSFs) MP-016-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date:
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICATION POLICY Granulocyte Colony Stimulating Factors (G-CSFs) MP-016-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date:
Clinical Coding for CRS Standards
 Clinical Coding for CRS Standards The following appendices set out the amended PRIMARY DIAGNOSIS coding structure to be used for the monitoring of cancer waiting times following the implementation of 4th
Clinical Coding for CRS Standards The following appendices set out the amended PRIMARY DIAGNOSIS coding structure to be used for the monitoring of cancer waiting times following the implementation of 4th
WLH Tumor Frequencies between cohort enrollment and 31-Dec Below the Women Lifestyle and Health tumor frequencies are tabulated according to:
 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not recorded =191 (non-melanoma skin cancer) treated as not recorded
DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not recorded =191 (non-melanoma skin cancer) treated as not recorded
WLH Tumor Frequencies between cohort enrollment and 31-Dec Below the Women Lifestyle and Health tumor frequencies are tabulated according to:
 WLH Tumor Frequencies between cohort enrollment and 31-Dec 2012 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not
WLH Tumor Frequencies between cohort enrollment and 31-Dec 2012 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not
SCCA REFERENCE MANUAL ICD-10
 SCCA REFERENCE MANUAL ICD-10 NORTHWEST HOSPITAL 1 BREAST CANCER BREAST (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer
SCCA REFERENCE MANUAL ICD-10 NORTHWEST HOSPITAL 1 BREAST CANCER BREAST (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer
155.2 Malignant neoplasm of liver not specified as primary or secondary. C22.9 Malignant neoplasm of liver, not specified as primary or secondary
 ICD-9 TO ICD-10 Reference ICD-9 150.9 Malignant neoplasm of esophagus unspecified site C15.9 Malignant neoplasm of esophagus, unspecified 151.9 Malignant neoplasm of stomach unspecified site C16.9 Malignant
ICD-9 TO ICD-10 Reference ICD-9 150.9 Malignant neoplasm of esophagus unspecified site C15.9 Malignant neoplasm of esophagus, unspecified 151.9 Malignant neoplasm of stomach unspecified site C16.9 Malignant
Pembrolizumab (Keytruda )
 Last Review Date: March 14, 2017 Number: MG.MM.PH.10f Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: March 14, 2017 Number: MG.MM.PH.10f Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO 06/24/2011 Section: Radiology Place(s) of
Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO 06/24/2011 Section: Radiology Place(s) of
Peripheral Nerve Blocks
 Last Review Date: April 21, 2017 Number: MG.MM.ME.64v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.ME.64v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
ICD-10 and Radiation Oncology
 ICD-10 and Radiation Oncology Steven M. Verno, CEMCS ICD-10 and Radiation Oncology Steven M. Verno, CEMCS September 23, 2008 Note: ICD-9-CM and ICD-10 are owned and copyrighted by the World Health Organization.
ICD-10 and Radiation Oncology Steven M. Verno, CEMCS ICD-10 and Radiation Oncology Steven M. Verno, CEMCS September 23, 2008 Note: ICD-9-CM and ICD-10 are owned and copyrighted by the World Health Organization.
DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY
 Date: 10 th April 2018 DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY Request: 1. Utilization Data of Overseas Beam Therapy and Brachytherapy 2. Diagnoses Data of Overseas Claims for Beam Therapy and Brachytherapy
Date: 10 th April 2018 DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY Request: 1. Utilization Data of Overseas Beam Therapy and Brachytherapy 2. Diagnoses Data of Overseas Claims for Beam Therapy and Brachytherapy
CEA (CARCINOEMBRYONIC ANTIGEN)
 (CARCINOEMBRYONIC ANTIGEN) 428 C15.3 Malignant neoplasm of upper third of esophagus C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant
(CARCINOEMBRYONIC ANTIGEN) 428 C15.3 Malignant neoplasm of upper third of esophagus C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant
Genetic Testing for Cancer Susceptibility
 Last Review Date: March 10, 2017 Number: MG.MM.AD.17v3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: March 10, 2017 Number: MG.MM.AD.17v3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Contractor Information. LCD Information. Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Document Information
 Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Opdivo (nivolumab) MP-004-MC-PA Medical Management; Clinical Pharmacy Provider Notice Date: 09/01/2018; 06/15/2018; 04/01/2017
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Opdivo (nivolumab) MP-004-MC-PA Medical Management; Clinical Pharmacy Provider Notice Date: 09/01/2018; 06/15/2018; 04/01/2017
MALIGNANT NEOPLASMS OF THE BREAST MALIGNANT NEOPLASMS OF FEMALE GENITAL ORGANS
 MALIGNANT NEOPLASMS OF THE (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer quadrant 5 - Lower-outer quadrant 6 - Axillary
MALIGNANT NEOPLASMS OF THE (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer quadrant 5 - Lower-outer quadrant 6 - Axillary
Contractor Information. LCD Information. Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Document Information
 Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR
 SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR Anus: Anal Canal; Anus, NOS; Other Parts of Rectum C21.0-C21.2, C21.8 C21.0 Anus, NOS (excludes skin of anus and perianal skin C44.5) C21.1 Anal canal C21.2
SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR Anus: Anal Canal; Anus, NOS; Other Parts of Rectum C21.0-C21.2, C21.8 C21.0 Anus, NOS (excludes skin of anus and perianal skin C44.5) C21.1 Anal canal C21.2
Contractor Information. LCD Information. Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Document Information
 Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Neoplasms/Lymphoma/Leukemia
 Neoplasms/Lymphoma/Leukemia Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and
Neoplasms/Lymphoma/Leukemia Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 03/01/2015 Section: Radiology
Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 03/01/2015 Section: Radiology
Gamma Glutamyl Transferase
 Other Names/Abbreviations GGT 190.32 - Gamma Glutamyl Transferase Gamma glutamyl transferase (GGT) is an intracellular enzyme that appears in blood following leakage from cells. Renal tubules, liver, and
Other Names/Abbreviations GGT 190.32 - Gamma Glutamyl Transferase Gamma glutamyl transferase (GGT) is an intracellular enzyme that appears in blood following leakage from cells. Renal tubules, liver, and
Colony Stimulating Factors: Neupogen (filgrastim), Neulasta (pegfilgrastim), Leukine (sargramostim), Granix (tbo-filgrastim)
 Neupogen (filgrastim), Neulasta (sargramostim), Granix (tbo-filgrastim) Date of Origin: 10/17/2008 Dates Reviewed: 6/17/2009, 12/22/2009, 06/15/2010/ 7/20/2010, 09/2010, 12/2010, 03/2011, 06/2011, 09/2011,
Neupogen (filgrastim), Neulasta (sargramostim), Granix (tbo-filgrastim) Date of Origin: 10/17/2008 Dates Reviewed: 6/17/2009, 12/22/2009, 06/15/2010/ 7/20/2010, 09/2010, 12/2010, 03/2011, 06/2011, 09/2011,
Anatomical Considerations for Lab Practical II
 Anatomical Considerations for Lab Practical II For each of the following please be prepared to provide: Identification System Organ(s) or ducts to Function(s) location which it is attached Use your lecture
Anatomical Considerations for Lab Practical II For each of the following please be prepared to provide: Identification System Organ(s) or ducts to Function(s) location which it is attached Use your lecture
Cancer in Estonia 2014
 Cancer in Estonia 2014 Estonian Cancer Registry (ECR) is a population-based registry that collects data on all cancer cases in Estonia. More information about ECR is available at the webpage of National
Cancer in Estonia 2014 Estonian Cancer Registry (ECR) is a population-based registry that collects data on all cancer cases in Estonia. More information about ECR is available at the webpage of National
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2017 Section: Radiology
Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/01/2017 Section: Radiology
Khapzory (levoleucovorin) (Intravenous)
 Khapzory (levoleucovorin) (Intravenous) Last Review Date: 12/04/2018 Date of Origin: 12/04/2018 Dates Reviewed: 12/2018 Document Number: IC-0408 I. Length of Authorization Coverage will be provided for
Khapzory (levoleucovorin) (Intravenous) Last Review Date: 12/04/2018 Date of Origin: 12/04/2018 Dates Reviewed: 12/2018 Document Number: IC-0408 I. Length of Authorization Coverage will be provided for
Carcinoembryonic Antigen
 Other Names/Abbreviations CEA 190.26 - Carcinoembryonic Antigen Carcinoembryonic antigen (CEA) is a protein polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring
Other Names/Abbreviations CEA 190.26 - Carcinoembryonic Antigen Carcinoembryonic antigen (CEA) is a protein polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring
Annual Report. Cape Cod Hospital and Falmouth Hospital Regional Cancer Network Expert physicians. Quality hospitals. Superior care.
 Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
BRAF Mutation Analysis
 Last Review Date: October 13, 2017 Number: MG.MM.LA.38aC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: October 13, 2017 Number: MG.MM.LA.38aC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
2011 to 2015 New Cancer Incidence Truman Medical Center - Hospital Hill
 Number of New Cancers Truman Medical Center Hospital Hill Cancer Registry 2015 Statistical Summary Incidence In 2015, Truman Medical Center diagnosed and/or treated 406 new cancer cases. Four patients
Number of New Cancers Truman Medical Center Hospital Hill Cancer Registry 2015 Statistical Summary Incidence In 2015, Truman Medical Center diagnosed and/or treated 406 new cancer cases. Four patients
Fusilev (levoleucovorin) Document Number: IC-0183
 Fusilev (levoleucovorin) Document Number: IC-0183 Last Review Date: 2/1/2018 Date of Origin: 01/02/2014 Dates Reviewed: 08/2014, 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016,
Fusilev (levoleucovorin) Document Number: IC-0183 Last Review Date: 2/1/2018 Date of Origin: 01/02/2014 Dates Reviewed: 08/2014, 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016,
MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site
 POLICY: PG0364 ORIGINAL EFFECTIVE: 04/22/16 LAST REVIEW: 07/26/18 MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0364 ORIGINAL EFFECTIVE: 04/22/16 LAST REVIEW: 07/26/18 MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site GUIDELINES This policy does not certify benefits or authorization
Table of Contents. Last updated by CLO: 5/8/2013 1
 Table of Contents Drug-induced liver injury algorithm - Cases... 2 Drug-induced liver injury algorithm - Controls... 3 1. Summary of drug-induced liver injury algorithm... 4 2. Terminology... 4 3. Threshold
Table of Contents Drug-induced liver injury algorithm - Cases... 2 Drug-induced liver injury algorithm - Controls... 3 1. Summary of drug-induced liver injury algorithm... 4 2. Terminology... 4 3. Threshold
Keytruda (pembrolizumab) (Intravenous)
 Keytruda (pembrolizumab) (Intravenous) Last Review Date: 02/06/2018 Date of Origin: 09/30/2014 Document Number: IC-0209 Dates Reviewed: 9/2014, 3/2015, 5/2015, 8/2015, 10/2015, 11/2015, 2/2016, 5/2016,
Keytruda (pembrolizumab) (Intravenous) Last Review Date: 02/06/2018 Date of Origin: 09/30/2014 Document Number: IC-0209 Dates Reviewed: 9/2014, 3/2015, 5/2015, 8/2015, 10/2015, 11/2015, 2/2016, 5/2016,
Anatomy. Contents Brain (Questions)
 Anatomy 12 Contents 12.1 Brain (Questions).................................................... 683 12.2 Head and Neck (Questions)............................................. 685 12.3 Thorax (Questions)...................................................
Anatomy 12 Contents 12.1 Brain (Questions).................................................... 683 12.2 Head and Neck (Questions)............................................. 685 12.3 Thorax (Questions)...................................................
ANNUAL CANCER REGISTRY REPORT-2005
 ANNUAL CANCER REGISTRY REPORT-25 CANCER STATISTICS Distribution of neoplasms Of a total of 3,115 new neoplasms diagnosed or treated at the Hospital from January 25 to December, 25, 1,473 were seen in males
ANNUAL CANCER REGISTRY REPORT-25 CANCER STATISTICS Distribution of neoplasms Of a total of 3,115 new neoplasms diagnosed or treated at the Hospital from January 25 to December, 25, 1,473 were seen in males
Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence
 Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence In 2014, there were 452 new cancer cases diagnosed and or treated at Truman Medical Center- Hospital Hill and an additional
Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence In 2014, there were 452 new cancer cases diagnosed and or treated at Truman Medical Center- Hospital Hill and an additional
SEER Advanced Topic 2018 Presentation. EOD 2018 and SS2018 Jennifer Ruhl
 SEER Advanced Topic 2018 Presentation EOD 2018 and SS2018 Jennifer Ruhl May 25, 2018 Outline General overview of EOD Schemas Basic review of what is needed to collect Primary Tumor, Regional Nodes and
SEER Advanced Topic 2018 Presentation EOD 2018 and SS2018 Jennifer Ruhl May 25, 2018 Outline General overview of EOD Schemas Basic review of what is needed to collect Primary Tumor, Regional Nodes and
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 PROPOSED/DRAFT Local Coverage Determination (LCD): CT of the Head (DL34417) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: This is a Proposed/Draft
PROPOSED/DRAFT Local Coverage Determination (LCD): CT of the Head (DL34417) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: This is a Proposed/Draft
Radiation Oncology Study Guide
 Radiation Oncology Study Guide For the Initial CertificationQualifying (Computer-Based) Examination General and Radiation Oncology This examination is designed to assess your understanding of the entire
Radiation Oncology Study Guide For the Initial CertificationQualifying (Computer-Based) Examination General and Radiation Oncology This examination is designed to assess your understanding of the entire
Crosswalk File of ICD9 Diagnosis Codes to Risk Group Assignment 1-Apr-15
 1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
Introduction to ICD-O-3 coding rules
 Introduction to ICD-O-3 coding rules Weena Laddachayaporn, MD National Cancer Institute, Bangkok, Thailand ICD-O-3 The International Classification of Diseases for Oncology Is a coding system for primary
Introduction to ICD-O-3 coding rules Weena Laddachayaporn, MD National Cancer Institute, Bangkok, Thailand ICD-O-3 The International Classification of Diseases for Oncology Is a coding system for primary
Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green
 Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green Digestive system 1. Teeth Main points: external and internal structure of a tooth, fixation of a tooth
Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green Digestive system 1. Teeth Main points: external and internal structure of a tooth, fixation of a tooth
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS RESPIRATORY SYSTEM
 ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Identify structures
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Identify structures
AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE
 Targeted Drug Delivery AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE 2018 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Targeted Drug Delivery AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE 2018 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
S2 File. Clinical Classifications Software (CCS). The CCS is a
 S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
Morphine Equivalent Dosing
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by the Texas Medicaid Vendor Drug Program to ensure appropriate and safe utilization. Clinical
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by the Texas Medicaid Vendor Drug Program to ensure appropriate and safe utilization. Clinical
Fentora (Fentanyl Buccal)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Fentora (Fentanyl Buccal) Clinical Edit Information Included in this Document Fentora 100mcg Drugs requiring prior authorization:
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Fentora (Fentanyl Buccal) Clinical Edit Information Included in this Document Fentora 100mcg Drugs requiring prior authorization:
This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors
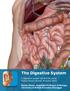 1 This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors 2 A. Digestive System Overview To Start: Go to the Views menu and scroll down to the
1 This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors 2 A. Digestive System Overview To Start: Go to the Views menu and scroll down to the
incidence rate x 100,000/year
 Tier R=rare C=common Cancer Entity European crude and age adjusted incidence by cancer, years of diagnosis 2000 and 2007 Analisys based on 83 population-based cancer registries * applying the European
Tier R=rare C=common Cancer Entity European crude and age adjusted incidence by cancer, years of diagnosis 2000 and 2007 Analisys based on 83 population-based cancer registries * applying the European
RESPIRATORY SYSTEM. described: pp. 744,746 fig. 25.1, described: p. 746 fig described: p. 776 fig. 26.3
 ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Identify structures
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Identify structures
Contractor Information. LCD Information. Local Coverage Determination (LCD): Flow Cytometry (L34513) Document Information
 Local Coverage Determination (LCD): Flow Cytometry (L34513) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Local Coverage Determination (LCD): Flow Cytometry (L34513) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
The original MED criteria can be referenced at the Texas Vendor Drug Program website located at
 Morphine Equivalent Dosing (MED) Clinical Edit Criteria Drug/Drug Class Morphine Equivalent Dosing (MED) Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical
Morphine Equivalent Dosing (MED) Clinical Edit Criteria Drug/Drug Class Morphine Equivalent Dosing (MED) Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Flow Cytometry (L34215) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Local Coverage Determination (LCD): Flow Cytometry (L34215) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Florida Cancer Data System STAT File Documentation Version 2019
 Florida Cancer Data System STAT File Documentation Version 2019 Field Description NAACCR Item Recoded Patient ID Number 20 Addr at DX - State 80 X County at DX 90 Addr at DX Country 102 X Marital Status
Florida Cancer Data System STAT File Documentation Version 2019 Field Description NAACCR Item Recoded Patient ID Number 20 Addr at DX - State 80 X County at DX 90 Addr at DX Country 102 X Marital Status
NEW/REVISED MATERIAL: Presented in italicized text EFFECTIVE DATE: January 1, 2008 GUIDELINES FOR REPORTING ADMINISTRATION OF EPOGEN MEDICARE
 DISCLAIMER: Please be advised that while every effort has been made to ensure the accuracy of the information provided according to the most current LCD pertaining to the subject, periodic change to rules
DISCLAIMER: Please be advised that while every effort has been made to ensure the accuracy of the information provided according to the most current LCD pertaining to the subject, periodic change to rules
2016 Cancer Registry Annual Report
 2016 Cancer Registry Annual Report Cancer Committee Chairman s Report The Cancer Committee at Cancer Treatment Centers of America (CTCA) at Eastern Regional Medical Center (Eastern), established in 2006,
2016 Cancer Registry Annual Report Cancer Committee Chairman s Report The Cancer Committee at Cancer Treatment Centers of America (CTCA) at Eastern Regional Medical Center (Eastern), established in 2006,
END-SEMESTER EXAM 2018 ANATOMY, HISTOLOGY AND EMBRYOLOGY FACULTY OF MEDICINE, 2 ND SEMESTER
 University of Szeged, Faculty of Medicine Department of Anatomy, Histology and Embryology Chairman: Prof. Antal Nógrádi MD, PhD, DSc Kossuth L. sgt. 40., H-6724 Szeged, Hungary Tel.: +36-62-545-665 P.
University of Szeged, Faculty of Medicine Department of Anatomy, Histology and Embryology Chairman: Prof. Antal Nógrádi MD, PhD, DSc Kossuth L. sgt. 40., H-6724 Szeged, Hungary Tel.: +36-62-545-665 P.
2012 Cancer Report 2011 Registry Data
 2012 Cancer Report 2011 Registry Data Contents Goals and Objectives 1 2012 Cancer Committee Members 2 Total Cancer Cases 1981-2011 3 Cancer Registry Frequency Report 1981-2011 4-5 Cancer Registry Frequency
2012 Cancer Report 2011 Registry Data Contents Goals and Objectives 1 2012 Cancer Committee Members 2 Total Cancer Cases 1981-2011 3 Cancer Registry Frequency Report 1981-2011 4-5 Cancer Registry Frequency
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Oncologic Genetic Testing Panels MP-074-MD-PA Medical Management Provider Notice Date: 11/15/2018 Issue Date: 12/15/2018 Effective
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Oncologic Genetic Testing Panels MP-074-MD-PA Medical Management Provider Notice Date: 11/15/2018 Issue Date: 12/15/2018 Effective
John R. Marsh Cancer Center
 John R. Marsh Cancer Center Lung Program Overview: 2014-2015 Initiatives Lung CT Screening Dr. Gregory Zimmerman In cooperation with The Lung Cancer Steering Committee, Diagnostic Imaging Services at the
John R. Marsh Cancer Center Lung Program Overview: 2014-2015 Initiatives Lung CT Screening Dr. Gregory Zimmerman In cooperation with The Lung Cancer Steering Committee, Diagnostic Imaging Services at the
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.138.MH Oral Maxillofacial Prosthesis This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.138.MH Oral Maxillofacial Prosthesis This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute
 Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute Paul R. Yarnold, Ph.D. and Robert C. Soltysik, M.S. Optimal Data Analysis, LLC Imagine a random sample
Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute Paul R. Yarnold, Ph.D. and Robert C. Soltysik, M.S. Optimal Data Analysis, LLC Imagine a random sample
General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p.
 General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p. 10 Principal Joints (Immovable) p. 12 Synovial Joints
General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p. 10 Principal Joints (Immovable) p. 12 Synovial Joints
Lab 5 Digestion and Hormones of Digestion. 7/16/2015 MDufilho 1
 Lab 5 Digestion and Hormones of Digestion 1 Figure 23.1 Alimentary canal and related accessory digestive organs. Mouth (oral cavity) Tongue* Parotid gland Sublingual gland Submandibular gland Salivary
Lab 5 Digestion and Hormones of Digestion 1 Figure 23.1 Alimentary canal and related accessory digestive organs. Mouth (oral cavity) Tongue* Parotid gland Sublingual gland Submandibular gland Salivary
Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies
 Web appendix 2: SEARCH STRATEGIES Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies MEDLINE 1. exp epidemiologic studies/
Web appendix 2: SEARCH STRATEGIES Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies MEDLINE 1. exp epidemiologic studies/
Clinical Policy: Intensity-Modulated Radiotherapy Reference Number: CP.MP.69 Coding Implications Last Review Date: 02/18 Revision Log
 Clinical Policy: Intensity-Modulated Radiotherapy Reference Number: CP.MP.69 Coding Implications Last Review Date: 02/18 Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Intensity-Modulated Radiotherapy Reference Number: CP.MP.69 Coding Implications Last Review Date: 02/18 Revision Log See Important Reminder at the end of this policy for important regulatory
SHN-1 Human Digestive Panel Test results
 SHN-1 Human Digestive Panel Test results HN-30 tongue HN-24 salivary gland HN-12 larynx HN-28 esophagus HN-29 stomach HN-20 pancreas HN-13 liver HN-14 gall bladder HN-27-1 duodenum HN-27-2 ileum HN-27-3
SHN-1 Human Digestive Panel Test results HN-30 tongue HN-24 salivary gland HN-12 larynx HN-28 esophagus HN-29 stomach HN-20 pancreas HN-13 liver HN-14 gall bladder HN-27-1 duodenum HN-27-2 ileum HN-27-3
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Abdomen and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_abdomen_and_pelvis
Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Abdomen and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_abdomen_and_pelvis
Jhia Anjela D. Rivera 1 1. BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines
 DIGESTIVE SYSTEM Jhia Anjela D. Rivera 1 1 BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines DIGESTIVE SYSTEM Consists of the digestive tract (gastrointestinal
DIGESTIVE SYSTEM Jhia Anjela D. Rivera 1 1 BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines DIGESTIVE SYSTEM Consists of the digestive tract (gastrointestinal
RAMATHIBODI CANCER REPORT
 RAMATHIBODI CANCER REPORT 2016 Ramathibodi Cancer Registry : A subsidiary of Ramathibodi Comprehensive Cancer Center Faculty of Medicine, Ramathibodi Hospital Mahidol University Table of content INTRODUCTION...III
RAMATHIBODI CANCER REPORT 2016 Ramathibodi Cancer Registry : A subsidiary of Ramathibodi Comprehensive Cancer Center Faculty of Medicine, Ramathibodi Hospital Mahidol University Table of content INTRODUCTION...III
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Intensity Modulated Radiation Therapy (IMRT) Page 1 of 19 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Intensity Modulated Radiation Therapy (IMRT)
Intensity Modulated Radiation Therapy (IMRT) Page 1 of 19 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Intensity Modulated Radiation Therapy (IMRT)
Lungs a. d. b. c. e.
 Lungs d. e. Lungs Right superior lobe Right middle lobe Right inferior lobe d. Left superior lobe e. Left inferior lobe Sinuses d. Nasal Cavity & Sinuses g. g. i. Nasal Cavity & Sinuses g. h. d. f. e.
Lungs d. e. Lungs Right superior lobe Right middle lobe Right inferior lobe d. Left superior lobe e. Left inferior lobe Sinuses d. Nasal Cavity & Sinuses g. g. i. Nasal Cavity & Sinuses g. h. d. f. e.
CODING PRIMARY SITE. Nadya Dimitrova
 CODING PRIMARY SITE Nadya Dimitrova OUTLINE What is coding and why do we need it? ICD-10 and ICD-O ICD-O-3 Topography coding rules ICD-O-3 online WHAT IS CODING AND WHY DO WE NEED IT? Coding: to assign
CODING PRIMARY SITE Nadya Dimitrova OUTLINE What is coding and why do we need it? ICD-10 and ICD-O ICD-O-3 Topography coding rules ICD-O-3 online WHAT IS CODING AND WHY DO WE NEED IT? Coding: to assign
Human Anatomy & Physiology. Introduction (Ch. 1)
 Human Anatomy & Physiology Introduction (Ch. 1) Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another Gross or macroscopic Microscopic
Human Anatomy & Physiology Introduction (Ch. 1) Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another Gross or macroscopic Microscopic
Digestive System. In one end and out the other.
 Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
APPENDIX ONE: ICD CODES
 APPENDIX ONE: ICD CODES ICD-10-AM ICD-9-CM Malignant neoplasms C00 C97 140 208, 238.6, 273.3 Lip, oral cavity and pharynx C00 C14 140 149 Digestive organs C15 C26 150 157, 159 Oesophagus 4 C15 150 excluding
APPENDIX ONE: ICD CODES ICD-10-AM ICD-9-CM Malignant neoplasms C00 C97 140 208, 238.6, 273.3 Lip, oral cavity and pharynx C00 C14 140 149 Digestive organs C15 C26 150 157, 159 Oesophagus 4 C15 150 excluding
American Cancer Society Estimated Cancer Deaths by Sex and Age (years), 2013
 American Cancer Society Estimated Cancer Deaths by Sex and Age (years), 2013 All ages Younger than 45 45 and Older Younger than 65 65 and Older All sites, men 306,920 9,370 297,550 95,980 210,940 All sites,
American Cancer Society Estimated Cancer Deaths by Sex and Age (years), 2013 All ages Younger than 45 45 and Older Younger than 65 65 and Older All sites, men 306,920 9,370 297,550 95,980 210,940 All sites,
Descriptive Histology
 Atlas of Descriptive Histology Michael H. Ross University of Florida College of Medicine Gainesville, Florida Wojciech Pawlina Mayo Medical School College of Medicine, Mayo Clinic Rochester, Minnesota
Atlas of Descriptive Histology Michael H. Ross University of Florida College of Medicine Gainesville, Florida Wojciech Pawlina Mayo Medical School College of Medicine, Mayo Clinic Rochester, Minnesota
*
 Introduction Cancer is complex, can have many possible causes, and is increasingly common. For the U.S. population, 1 in 2 males and 1 in 3 females is at risk of developing cancer in their lifetime. The
Introduction Cancer is complex, can have many possible causes, and is increasingly common. For the U.S. population, 1 in 2 males and 1 in 3 females is at risk of developing cancer in their lifetime. The
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (Brentuximab Vedotin) MP-035-MD-WV Provider Notice Date: 07/03/2017 Original Effective Date: 08/03/2017 Annual Approval Date:
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (Brentuximab Vedotin) MP-035-MD-WV Provider Notice Date: 07/03/2017 Original Effective Date: 08/03/2017 Annual Approval Date:
AJCC Staging of Head & Neck Cancer (7 th edition, 2010) -LIP & ORAL CAVITY-
 TX: primary tumor cannot be assessed T0: no evidence of primary tumor Tis: carcinoma in situ. T1: tumor is 2 cm or smaller AJCC Staging of Head & Neck Cancer (7 th edition, 2010) -LIP & ORAL CAVITY- T2:
TX: primary tumor cannot be assessed T0: no evidence of primary tumor Tis: carcinoma in situ. T1: tumor is 2 cm or smaller AJCC Staging of Head & Neck Cancer (7 th edition, 2010) -LIP & ORAL CAVITY- T2:
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (brentuximab vedotin) MP-035-MD-DE Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017 Annual Approval Date:
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (brentuximab vedotin) MP-035-MD-DE Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017 Annual Approval Date:
Anatomy: Know Your Abdomen
 Anatomy: Know Your Abdomen Glossary Abdomen - part of the body below the thorax (chest cavity); separated by the diaphragm. Anterior - towards the front of the body. For example, the umbilicus is anterior
Anatomy: Know Your Abdomen Glossary Abdomen - part of the body below the thorax (chest cavity); separated by the diaphragm. Anterior - towards the front of the body. For example, the umbilicus is anterior
Respiratory & Digestive Organs of the Head and Neck, Human;
 Name Date Lab Exercise 5: Lab Exercise 6: Lab Exercise 7: Lab Exercise 8: Respiratory & Digestive Organs of the Head and Neck, Human; Histology of the Respiratory System Digestive System Models, Human
Name Date Lab Exercise 5: Lab Exercise 6: Lab Exercise 7: Lab Exercise 8: Respiratory & Digestive Organs of the Head and Neck, Human; Histology of the Respiratory System Digestive System Models, Human
Date Lab Pd. Lecture Notes (57)
 Name SECTION OBJECTIVES Describe the locations of the major body cavities List the organs located in each major body cavity Name the membranes associated with the thoracic and abdominopelvic cavities Name
Name SECTION OBJECTIVES Describe the locations of the major body cavities List the organs located in each major body cavity Name the membranes associated with the thoracic and abdominopelvic cavities Name
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICAL POLICY ADCETRIS (Brentuximab Vedotin) MP-035-MD-DE Provider Notice Date: 11/1/2016 Original Effective Date: 12/1/2016 Medical Management Annual
Policy Name: Policy Number: Approved By: CLINICAL MEDICAL POLICY ADCETRIS (Brentuximab Vedotin) MP-035-MD-DE Provider Notice Date: 11/1/2016 Original Effective Date: 12/1/2016 Medical Management Annual
2018 Grade PEGGY ADAMO, RHIT, CTR OCTOBER 11, 2018
 1 2018 Grade PEGGY ADAMO, RHIT, CTR ADAMOM@MAIL.NIH.GOV OCTOBER 11, 2018 2 Acknowledgements Donna Hansen, CCR Jennifer Ruhl, NCI SEER Introduction 3 Histologic Type vs. Grade Credit: Dr. Kay Washington
1 2018 Grade PEGGY ADAMO, RHIT, CTR ADAMOM@MAIL.NIH.GOV OCTOBER 11, 2018 2 Acknowledgements Donna Hansen, CCR Jennifer Ruhl, NCI SEER Introduction 3 Histologic Type vs. Grade Credit: Dr. Kay Washington
