Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis
|
|
|
- Rudolph Glenn
- 6 years ago
- Views:
Transcription
1 Regular Article From by guest on April 30, For personal use only. CLINICAL TRIALS AND OBSERVATIONS Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis Donna E. Reece, 1 Ute Hegenbart, 2 Vaishali Sanchorawala, 3 Giampaolo Merlini, 4 Giovanni Palladini, 4 Joan Bladé, 5 Jean-Paul Fermand, 6 Hani Hassoun, 7 Leonard Heffner, 8 Vishal Kukreti, 1 Robert A. Vescio, 9 Lixia Pei, 10 Christopher Enny, 10 Dixie-Lee Esseltine, 11 Helgi van de Velde, 12 Andrew Cakana, 13 and Raymond L. Comenzo 14 1 Princess Margaret Cancer Center, Toronto, ON, Canada; 2 Amyloidosis Center, University of Heidelberg, Heidelberg, Germany; 3 Boston University Medical Center, Boston, MA; 4 Amyloidosis Research and Treatment Center, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico, Policlinico San Matteo, Department of Molecular Medicine, University of Pavia, Pavia, Italy; 5 Hematology Department, Institute of Hematology and Oncology, Institut d Investigacions Biomèdiques August Pi i Sunyer, Hospital Clínic, University of Barcelona, Barcelona, Spain; 6 Hôpital Saint Louis, Paris, France; 7 Memorial Sloan-Kettering Cancer Center, New York, NY; 8 Hematology and Medical Oncology, Winship Cancer Institute of Emory University, Atlanta, GA; 9 Cedars- Sinai Medical Center, Los Angeles, CA; 10 Janssen Research and Development, LLC, Raritan, NJ; 11 Takeda Pharmaceuticals International Co., Cambridge, MA; 12 Janssen Research and Development, Division of Janssen Pharmaceutica NV, Beerse, Belgium; 13 Janssen Research and Development, High Wycombe, United Kingdom; and 14 Tufts Medical Center, Boston, MA Key Points Single-agent bortezomib produces durable hematologic responses and promising long-term overall survival in relapsed AL patients. Once-weekly bortezomib is better tolerated and produces similar responses to twiceweekly bortezomib in relapsed AL patients. CAN2007 was a phase 1/2 study of once- and twice-weekly single-agent bortezomib in relapsed primary systemic amyloid light chain amyloidosis (AL) amyloidosis. Seventy patients were treated, including 18 and 34 patients at the maximum planned doses on the once- and twice-weekly schedules. This prespecified final analysis provides mature response and long-term outcomes data after 3-year additional follow-up since the last report. In the once-weekly 1.6 mg/m 2 and twice-weekly 1.3 mg/m 2 bortezomib groups, final hematologic response rates were 68.8% and 66.7%; 80% of patients in each group sustained their response for 1 year. One-year progression-free rates were 72.2% and 76.8%. Median overall survival (OS) was 62.1 months and not reached; 4-year OS rates were 75.0% and 63.0%. Low baseline difference in k/l free light-chain level was associated with higher hematologic complete response rates and longer OS. At data cutoff, 40 (57%) patients had received subsequent therapy, including 19 (27%) retreated with bortezomib, 11 (58%) of whom achieved complete or partial hematologic responses. Four patients received prolonged bortezomib for between 3.5 and 5.6 years, with no new safety concerns, highlighting the feasibility of long-term therapy. Single-agent bortezomib produced durable hematologic responses and promising long-term OS in relapsed AL amyloidosis. This trial was registered at as #NCT (Blood. 2014;124(16): ) Introduction Primary systemic amyloid light chain amyloidosis (AL) is a protein misfolding disorder in which a clonal plasma cell dyscrasia results in excessive production of abnormal immunoglobulin light chains. 1-4 Extracellular deposition and toxicity of these fibril-forming abnormal light chains can lead to end-organ damage, particularly in the kidneys and heart, 1-4 and death. Suppression of the plasma cell dyscrasia and reduction or elimination of the production of amyloidogenic immunoglobulin light chains is the aim of AL treatment. 1 Amyloid deposits can be resorbed and organ function restored on elimination of plasma cells secreting the abnormal light chains. 1 Achievement of a hematologic response to therapy, particularly complete response (CR), is associated with improved organ function and prolonged survival. 5-8 The plasma cell dyscrasia in AL is similar to that in multiple myeloma (MM). 3,9 Thus, therapies that are effective in MM are typically used to treat AL. 1 High-dose melphalan followed by stem cell transplantation (HDM-SCT) is highly effective in AL; however, many patients may be transplantation ineligible 10,11 due to age, poor performance status, and multiple organ involvement. Despite improvements in the management of transplantation in AL patients in recent years, the observed transplantation-associated mortality is still higher in AL than MM. 12 Therefore, alternative therapies are needed for transplantation-ineligible AL patients; such therapies may also benefit transplantation-eligible patients. The proteasome inhibitor bortezomib is approved in the United States for the treatment of MM and for the treatment of mantle cell Submitted April 14, 2014; accepted August 11, Prepublished online as Blood First Edition paper, September 8, 2014; DOI /blood The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section Presented in poster format at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, The online version of this article contains a data supplement by The American Society of Hematology 2498 BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16
2 BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 BORTEZOMIB IN RELAPSED LIGHT CHAIN AMYLOIDOSIS 2499 lymphoma after $1 prior therapy. 13 It has been suggested that the pathology of AL makes cells highly sensitive to the effects of proteasome inhibition. 4 Several retrospective analyses 5,14-18 and case series studies have shown that bortezomib-based therapy is active in patients with previously untreated and relapsed AL. CAN2007 was the first prospective phase 1/2 study of single-agent bortezomib in relapsed AL. Phase 1 findings 23,24 and primary phase 2 efficacy and safety results from the study after to 37.7-month median follow-up across dose groups have been reported previously. 25 Here we report long-term outcome data at study closure after 51.8-month median follow-up (median, months across dose groups), and highlight a small subset of patients who received prolonged bortezomib treatment of up to 66.8 months (5.6 years). measurements. Best confirmed hematologic response was defined as the best hematologic response (CR or PR) achieved between cycle 1 and the time of first documented disease progression or, in the absence of progressive disease, the last response assessment confirmed by evaluation of the same category of response or better at the next visit. The overall hematologic response rate was defined as the rate of CR1PR (including unconfirmed responses) among patients with any postbaseline hematologic response assessment. Serum FLC assay and determination of the absolute value of the difference in k and l FLC levels (dflc) were performed at screening, prior to each bortezomib administration within and during the rest period of cycle 1, during the rest period of each subsequent cycle, at the end-of-treatment (EOT) visit, and every 6 weeks prior to disease progression. Adverse events (AEs) were monitored throughout the study and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v3.0. Statistical analysis Patients and methods Patients Eligibility criteria have been reported previously 23,25 and are summarized in the supplemental Appendix available on the Blood Web site. In brief, patients $18 years of age with confirmed AL diagnosis, who had previously been treated and required further treatment of AL due to persistent clonal disease, were eligible. Review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided informed consent. Study design CAN2007 was a nonrandomized, noncomparative, phase 1/2 study of weekly (QW) and twice-weekly (BIW) single-agent bortezomib in patients with relapsed AL (NCT ). The study was conducted at 9 sites in Canada, France, Germany, Italy, Spain, and the United States between July 25, 2005, and March 9, ,25 Study design has been reported previously. 23,25 During phase 1, patients sequentially received single-agent bortezomib at 0.7, 1.0, 1.3, or 1.6 mg/m 2 QW and then at 0.7, 1.0, or 1.3 mg/m 2 BIW. 23 Patients on the QW schedule received bortezomib on days 1, 8, 15, and 22 in 35-day cycles. Patients on the BIW schedule received bortezomib on days 1, 4, 8, and 11 in 21-day cycles. As the maximum tolerated dose of bortezomib was not reached on either schedule, the prespecified maximum planned dose levels of 1.6 mg/m 2 QW and 1.3 mg/m 2 BIW were expanded during phase The planned treatment duration was 8 cycles; prolonged treatment beyond 8 cycles was permitted for patients showing ongoing clinical benefit. Patients were followed every 6 weeks until disease progression (defined per criteria reported in Gertz et al 2005) 26 and every 3 months for survival during longterm follow-up. Objectives The primary phase 2 objective was to evaluate the safety of QW and BIW single-agent bortezomib in relapsed AL. Secondary objectives included determining hematologic response rate (CR plus partial response [PR]) and duration of response (DOR). Exploratory objectives included assessing the rate of organ response, overall survival (OS), and the value of frequent free light chain (FLC) measurements during cycle 1 and their relationship to hematologic response. The specific objectivesofthis final analysis, conducted after data cutoff for study closure, were to determine final hematologic response rates, final DOR, time to hematologic disease progression, OS, and subsequent AL therapy. Assessments Hematologic responses were determined during the rest period of each cycle according to established consensus criteria, 26 as previously reported. 25 Investigator-assessed responses were confirmed by an Independent Data Monitoring Committee and were based on central laboratory efficacy Definitions of analysis populations and details of statistical analyses performed in this study are provided in the supplemental Appendix. Results Patients As previously reported, patients were enrolled across 7 dose groups: 18 received bortezomib 1.6 mg/m 2 QW, 34 received bortezomib 1.3 mg/m 2 BIW, and 18 received bortezomib at lower doses QW or BIW. Patient demographics and baseline disease characteristics were reported previously. 25 Among all 70 patients, median age was 60.5 years (range, years), 27 (39%) patients were $65 years of age, 39 (56%) were men, and 63 (90%) were white. Thirty-one (44%) patients had $3 organs involved, with 51 (73%), 39 (56%), and 20 (29%) having renal, cardiac, and gastrointestinal involvement, respectively. Forty-one (59%) patients had a neurologic history, and 24 (34%) had reported cardiac history or baseline cardiac condition, including cardiac arrhythmias (n 5 14), heart failure (n 5 13), angina pectoris, and prior myocardial infarction (each n 5 1). Forty (57%), 20 (29%), and 10 (14%) patients had received 1, 2, and $3 prior lines of therapy for amyloidosis, respectively. Overall, 67 (96%) patients had received prior melphalan, with 40 (57%) having previously undergone HDM-SCT; 57 (81%) had received prior glucocorticoids, and 26 (37%) had received prior lenalidomide or thalidomide. Patient characteristics were generally similar across dose groups, with the exception of a higher median age and higher proportion of patients $65 years of age in the 1.3 mg/m 2 BIW group 25 and a higher proportion of patients with $3 organs involved, gastrointestinal involvement, cardiac history, neurologic history, and prior HDM-SCT in the 1.6 mg/m 2 QW group. 25 Disposition, treatment exposure, and safety Table 1 summarizes patient disposition and treatment exposure at the final analysis (August 28, 2012). Patients in the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lower-dose groups received a median (range) of 8 (1-39), 6 (1-57), and 8 (3-57) cycles of bortezomib, respectively. Nine (50%), 9 (26%), and 10 (56%) patients, respectively, had completed the planned 8 cycles of treatment, and 9 (50%), 25 (74%), and 8 (44%) patients, respectively, had discontinued treatment prior to completing the planned 8 cycles. Four (6%) patients were receiving ongoing treatment after having received 39 to 57 cycles. Patient flowthroughthestudyissummarizedinsupplemental Figure 1.
3 2500 REECE et al BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 Table 1. Patient disposition and treatment exposure Bortezomib dose groups Parameter 1.6 mg/m 2 QW (n 5 18) 1.3 mg/m 2 BIW (n 5 34) Lower doses QW/BIW (n 5 18) Median number of cycles received, n (range) 8 (1-39) 6 (1-57) 8 (3-57) Mean actual dose received/planned dose of bortezomib, % Received 8 cycles, n (%) 10 (56) 11 (32) 11 (61) Completed 8 cycles, n (%) 9 (50) 9 (26) 10 (56) Discontinued treatment, n (%) 9 (50) 25 (74) 8 (44) Adverse events 5 (28) 10 (29) 3 (17) Patient choice 3 (17) 4 (12) 2 (11) Disease progression 1 (6) 3 (9) 0 Clinical deterioration* 0 2 (6) 3 (17) Death 0 2 (6) 0 Other causes 0 4 (12) 0 Ongoing on treatment, n Alive, n (%) 11 (61) 22 (65) 9 (50) KPS, Karnofsky performance status. *Clinical deterioration includes deterioration in KPS and organ function and deterioration in patients overall condition. Other causes of discontinuation were attainment of CR after cycle 2, study drug intolerance, clinical progression on maintenance (treatment cycle 11), and plateaued hematologic response in cycle 4 (each n 5 1). One patient has progressed since data cutoff and discontinued treatment. Twenty-eight (40%) patients had died at the final analysis compared with 11 (16%) in the previous report. 25 Cause of death among the 17 additional patients who died since the previous report was progressive disease (n 5 13), and pneumonia, infection, respiratory failure leading to cardiorespiratory arrest, and sudden cardiac death (each n 5 1). Detailed phase 2 safety information was reported previously. 25 Nervous system disorder AEs were comparable between the 41 patients with a neurologic history and the 29 without a history (supplemental Table 1). No substantive changes in the previously reported safety profile had occurred, as all but 4 patients receiving long-term bortezomib had completed therapy at the time of the prior report. Grade 3/4 AEs in these long-term exposure patients are detailed below. Response to treatment Table 2 summarizes hematologic response and outcomes in the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lower-dose groups. Overall best confirmed hematologic response rates (CR1PR) were 68.8%, 66.7%, and 38.9% for the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lowerdose groups, respectively. Overall response rates in the long-term extension phase did not change compared with the previously published results 25 ; however, 1 patient in the lower-dose group who had previously achieved PR upgraded to CR. Eighty percent, 80.0%, and 83.3% of responders in the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lower-dose groups, respectively, sustained their response for $1 year. The relationship between baseline dflc and hematologic response was explored in response-evaluable patients. Overall response rates/cr rates in patients with high vs low baseline dflc ( vs #124.2 mg/l, cutoff per the median baseline dflc) after cycles 1, 2, and 4, and at EOT were 39%/3% vs 40%/9%, 50%/ 8% vs 52%/18%, 70%/5% vs 54%/21%, and 43%/18% vs 58%/32%, respectively (Table 3). Similar results were obtained using baseline dflc cutoffs of 180 (previously validated to be of prognostic importance in AL) 27 and 50 mg/l (per the definition of measurable disease in AL) 28 (supplemental Table 2). Table 2. Hematologic response, disease progression, and overall survival Bortezomib dose groups Parameter 1.6 mg/m 2 QW 1.3 mg/m 2 BIW Lower doses QW/BIW Hematologic response n 5 16 n 5 33 n 5 18 Best confirmed hematologic response rate (CR1PR), n (%) 11 (68.8) 22 (66.7) 7 (38.9) CR rate, n (%) 6 (37.5) 8 (24.2) 3 (16.7) Median time to hematologic response, months (range) n 5 11 n 5 22 n 5 7 To first response 2.1 ( ) 0.7 ( ) 1.2 ( ) To best confirmed response 3.2 ( ) 1.2 ( ) 1.2 ( ) 1-year DOR rate, %* Hematologic disease progression Median follow-up, months (range) 21.6 (0-35) 11.3 (0-18) 9.9 (2-49) Patients progressing, n year hematologic disease progression-free rate, % OS Median follow-up, months (range) 51.8 (1-68) 46.1 (1-61) 66.1 (2-80) Deaths, n Median OS, months (95% CI) 62.1 (50.1-NE) NE (30.2-NE) 63.2 (45.4-NE) 4-year OS rate, % NE, not estimable. *Based on Kaplan-Meier analysis.
4 BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 BORTEZOMIB IN RELAPSED LIGHT CHAIN AMYLOIDOSIS 2501 Table 3. Relationship between baseline dflc and change in dflc during cycle 1 and hematologic response rates after 1, 2, and 4 cycles and at the end of treatment Hematologic response rates High baseline dflc* (>124.2 mg/l) Low baseline dflc* ( mg/l) High dflc decrease during cycle 1 (>59%) Low dflc decrease during cycle 1 ( 59%) After cycle 1 Patients evaluable, n { Hematologic response rate, n (%) 12 (39) 14 (40) 22 (69) 4 (12) CR 1 (3) 3 (9) 3 (9) 1 (3) PR 11 (35) 11 (31) 19 (59) 3 (9) After cycle 2 Patients evaluable, n { Hematologic response rate, n (%) 13 (50) 17 (52) 23 (79) 7 (23) CR 2 (8) 6 (18) 6 (21) 2 (7) PR 11 (42) 11 (33) 17 (59) 5 (17) After cycle 4 Patients evaluable, n { Hematologic response rate, n (%) 14 (70) 15 (54) 20 (87) 9 (36) CR 1 (5) 6 (21) 4 (17) 3 (12) PR 13 (65) 9 (32) 16 (70) 6 (24) End of treatment Patients evaluable, n { Hematologic response rate, n (%) 12 (43) 18 (58) 21 (78) 9 (29) CR 5 (18) 10 (32) 11 (41) 4 (13) PR 7 (25) 8 (26) 10 (37) 5 (16) *Groups were defined based on a median baseline dflc of mg/l. Groups were defined based on a median percentage decrease in dflc from baseline to the last visit of cycle 1 of 59%. For the high vs low baseline dflc comparison, evaluable responses available for 66 patients after cycle 1, 59 patients after cycle 2, 48 patients after cycle 4, and 59 patients at the end of treatment. {For the high vs low dflc decrease comparison, evaluable responses available for 65 patients after cycle 1, 59 patients after cycle 2, 48 patients after cycle 4, and 58 patients at the end of treatment. The relationship between percentage dflc change from baseline to the last visit of cycle 1 and hematologic response was also assessed. Overall response rates/cr rates in patients with high vs low percentage decreases in dflc during cycle 1 (.59% vs #59%, cutoff per the median dflc decrease during cycle 1) after cycles 1, 2, and 4 and at EOT were 69%/9% vs 12%/3%, 79%/21% vs 23%/7%, 87%/17% vs 36%/12%, and 78%/41% vs 29%/13%, respectively (Table 3). Similar results were obtained when a cutoff of 50% decrease in dflc during cycle 1 was applied (50% dflc reduction being the definition of a PR according to recently updated AL response criteria) 29 (supplemental Table 3). Hematologic disease progression and overall survival Time to hematologic disease progression and OS are summarized in Table 2. One-year hematologic disease progression-free rates in the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lower-dose groups were 72.2%, 76.8%, and 88.5%, respectively. After a 51.8-month median follow-up for all patients, median OS was 62.7 months, and the 4-year OS rate was 67.3%. Median OS was 62.1 months, not reached, and 63.2 months in the 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lowerdose groups, respectively. Respective 4-year OS rates were 75.0%, 63.0%, and 69.7%. Kaplan-Meier distribution curves for OS by dose group are shown in Figure 1. As with hematologic response, the relationship between baseline dflc and change in dflc from baseline to cycle 1 with OS was explored. In patients subgrouped according to a median baseline dflc of mg/l, high baseline dflc was associated with inferior OS (median 50.1 vs 63.2 months with low baseline dflc # mg/l, P ; Figure 2A). Significantly shorter OS was also observed in patients with high baseline dflc according to a cutoff of either 180 (median 45.4 vs 63.2 months with low baseline dflc # 180 mg/l, P ; supplemental Figure 2A) or 50 mg/l (median 50.1 months vs not reached with low baseline dflc # 50 mg/l, P ; supplemental Figure 2B). In contrast, the magnitude of the change in dflc from baseline to end of cycle 1 did not appear to correlate with OS (median 56.6 vs 62.7 months with high [.59%] vs low [#59%] dflc decrease, P ; Figure 2B). Similarly, no correlation with OS was observed when a cutoff of 50% decrease in dflc from baseline to end of cycle 1 was applied (median 56.6 vs 62.7 months for patients with.50% vs #50% change in dflc, respectively, P ; supplemental Figure 3). As baseline dflc level appeared to predict for both hematologic CR rate and OS, the relationship between achievement of hematologic CR and OS was explored in a landmark analysis from EOT. There was a trend for longer OS in patients who achieved a hematologic CR vs,cr by EOT (median not reached vs 54.5 months; P 5.134; supplemental Figure 4). Subsequent therapy for AL At data cutoff, 40 (57%) patients had received subsequent therapy for AL, and 6 (9%) had died due to disease progression prior to receiving subsequent therapy. Twenty-four (34%) patients remained alive and had not received subsequent therapy. In the 1.6 mg/m 2 QW group, 7 (39%) patients had received subsequent therapy, 2 (11%) had died prior to subsequent therapy, and the median time to subsequent therapy/death was 50.1 months (95% confidence interval [CI], not estimable). In the 1.3 mg/m 2 BIW group, 21 (62%) patients had received subsequent therapy, 2 (6%) had died prior to subsequent therapy, and the median time to subsequent therapy/death was 17.2 months (95% CI, ). In the lower doses group, 12 (67%) patients had received subsequent therapy, 2 (11%) had died prior to subsequent therapy, and the median time to subsequent therapy/ death was 29.0 months (95% CI, ).
5 2502 REECE et al BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 Figure 1. Kaplan-Meier analysis of overall survival in the bortezomib 1.6 mg/m 2 QW, 1.3 mg/m 2 BIW, and lower-dose QW/BIW groups. Subsequent AL therapies received are summarized in Table 4. The most common agents included dexamethasone (n 5 28, 40%), bortezomib (n 5 19, 27%), lenalidomide (n 5 18, 26%), cyclophosphamide (n 5 9, 13%), melphalan (n 5 8, 11%), and thalidomide (n 5 7, 10%). Of 19 patients who received bortezomib-based retreatment, 11 achieved a hematologic CR (n 5 6) or PR (n 5 5), giving an overall hematologic response rate to bortezomib retreatment of 58%. Responses to initial bortezomib and to bortezomib retreatment are summarized in supplemental Table 4. Relationship between organ response and time to subsequent therapy and OS The relationship between hematologic response and organ response was previously demonstrated in the primary analysis of the CAN2007 study. 25 The proportion of patients achieving an organ response was higher in patients who achieved a hematologic response vs those who did not (32% vs 13%). In the present longterm follow-up analysis of CAN2007, the relationship between organ response and time to subsequent therapy and OS was explored. As shown in supplemental Figure 5A-B, median time to subsequent therapy (36 vs 26 months, P ) and median OS (not reached vs 46 months, P 5.04) appeared longer for patients who achieved an organ response compared with those who did not. Long-term bortezomib exposure In1center,4patients(3withclinicalbenefit but no CR at cycle 8) received long-term bortezomib for between 3.5 and 5.6 years, including 1 patient each in the 1.6 mg/m 2 QW and 1.3 mg/m 2 BIW groups and 2 patients in the lower-dose group, who were treated at 1.3 mg/m 2 QW and 0.7 mg/m 2 BIW, respectively. Disease/treatment information for these patients is summarized in Table 5. At the final analysis, all 4 patients remained progression-free and were ongoing with bortezomib. Patient 2 progressed after final data cutoff and is now in remission on alternative therapy. There were no new safety concerns with extended bortezomib treatment, and no grade 3/4 peripheral neuropathy or second primary malignancies. Discussion This final analysis of CAN2007 provides mature data on response and outcomes in relapsed AL patients treated with single-agent bortezomib after an additional 3-year follow-up since the last publication. 25 Our data indicate that bortezomib can produce durable hematologic responses and promising long-term OS in this patient population. Consistent with the previous report, 25 single-agent bortezomib produced overall hematologic response rates of 68.8% and 66.7% in the 1.6 mg/m 2 QW and 1.3 mg/m 2 BIW groups, respectively. Responses were durable, with $80% of responding patients in all treatment groups sustaining hematologic response for a year or more. These response rates with single-agent bortezomib appear comparable or favorable in the context of other combination regimens in relapsed AL, including cyclophosphamide-thalidomidedexamethasone, 30 cyclophosphamide-lenalidomide-dexamethasone, 31 and lenalidomide-dexamethasone, 32 which produced hematologic response rates in the range of 62% to 74%; however, cross-study comparisons should be interpreted with caution due to the heterogeneous nature of the AL patient population. After a 51.8-month median follow-up, the median OS for all patients was 62.7 months. OS with single-agent bortezomib in this study is encouraging with respect to that reported in studies of other combination regimens 20,30,31 ; however, data exclusively in the relapsed AL setting are generally lacking. Forty (57%) patients received subsequent AL therapy, most commonly with dexamethasone, bortezomib, lenalidomide, cyclophosphamide, melphalan, or thalidomide. The notable OS reported here may therefore be due, in part, to additional AL therapies based on novel agents such as bortezomib, lenalidomide, and thalidomide, available to patients in more recent years, compared with the more limited treatment options available at the time of older studies that reported considerably shorter OS The long-term follow-up of CAN2007 also allowed for analysis of time to subsequent AL therapy and OS in patients stratified by achievement of organ response. Extending on our previously published findings, 25 achievement of organ response was found to positively impact time to subsequent therapy and OS in this study.
6 BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 BORTEZOMIB IN RELAPSED LIGHT CHAIN AMYLOIDOSIS 2503 Figure 2. Kaplan-Meier analysis of OS. Overall survival was analyzed in patients grouped according to (A) high ( mg/l) vs low (#124.2 mg/l) baseline dflc or (B) high (.59%) vs low (#59%) percentage decrease in dflc during cycle 1. Cutoffs based on median baseline dflc and median percentage dflc decrease from baseline to the last visit of cycle 1, respectively. In light of previous studies demonstrating an association between FLC levels and hematologic response or long-term outcomes, 29,37-40 measuring dflc parameters during cycle 1 and assessing the relationship to hematologic response was included as an exploratory objective in this study. Higher overall and complete response rates at EOT were observed in patients with low vs high baseline dflc (using 3 different cutoffs for patient dichotomization: [median], 180 [validated as of prognostic importance], 27 and50mg/l[definition of measurable disease] 28 ) and high vs low percentage dflc decrease at the end of cycle 1 (using cutoffs for patient dichotomization of 59% [median] and 50% [validated cutoff defining dflc PR] 29 ). The former association may reflect a deeper impact of treatment in patients with lower disease burden at baseline. Notably, a low baseline dflc, but not a.50% or. 59% decrease in dflc at the end of cycle 1, was predictive of improved OS. The latter result may be explained, in part, by the post-cycle 1 percentage dflc reduction being too premature a measurement to predict for longterm survival. It is of note, however, that per recently updated and validated response criteria in AL, a 50% reduction in dflc (corresponding to achievement of a PR) at 3 and/or 6 months after treatment initiation has been shown to confer an OS benefit. 29 Although these efficacy data with single-agent bortezomib are promising, our findings may not be generalizable to the entire AL patient population. As previously reported, 25 patients with the poorest prognosis and cardiac status (New York Heart Association classification III or IV, grade 2/3 atrioventricular block) were excluded to ensure a comprehensive characterization of the safety profile of bortezomib in AL. The relatively low median age, the high proportion of patients with prior HDM-SCT, and the long median time from AL diagnosis, plus the fact that all patients were at first or later relapse, 41 are suggestive of a more robust patient population compared with the general AL patient population. Nevertheless, these data add to a growing body of evidence supporting the activity of bortezomib in AL treatment. Since commencement of CAN2007, bortezomib has been studied in various combination regimens in relapsed and previously untreated AL and demonstrated
7 2504 REECE et al BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 Table 4. Subsequent therapies for AL amyloidosis Bortezomib dose groups Subsequent therapy for AL, n (%)* 1.6 mg/m 2 QW (n 5 18) 1.3 mg/m 2 BIW (n 5 34) Lower doses QW/BIW (n 5 18) Total (n 5 70) Any subsequent therapy for AL 7 (39) 21 (62) 12 (67) 40 (57) Median (95% CI) time to subsequent therapy, months 50.1 (11.4-NE) 17.2 ( ) 29.0 ( ) 22.7 ( ) Agents received in any subsequent line Dexamethasone 6 (33) 13 (38) 9 (50) 28 (40) Bortezomib 5 (28) 10 (29) 4 (22) 19 (27) Lenalidomide 2 (11) 9 (26) 7 (39) 18 (26) Cyclophosphamide 4 (22) 3 (9) 2 (11) 9 (13) Melphalan 2 (11) 3 (9) 3 (17) 8 (11) Thalidomide 3 (17) 1 (3) 3 (17) 7 (10) Prednisone 1 (6) 3 (9) 0 4 (6) Investigational drug 0 2 (6) 1 (6) 3 (4) Bendamustine 1 (6) 1 (3) 0 2 (3) Cisplatin 1 (6) 1 (3) 0 2 (3) Doxorubicin 1 (6) 1 (3) 0 2 (3) Etoposide 1 (6) 1 (3) 0 2 (3) HDM-SCT (11) 2 (3) *Therapies listed are not mutually exclusive; patients could have received.1 agent or combination of agents. 27 patients with subsequent therapy had discontinued initial bortezomib treatment in CAN2007 prior to disease progression. Patients received their first HDM-SCT as a subsequent therapy. substantial activity, with hematologic response rates in the range of 54% to 94%. 5,15-17,19,20,42 Based on data from this and other studies, bortezomib 6 dexamethasone, bortezomib-melphalan-dexamethasone, and bortezomib-cyclophosphamide-dexamethasone are currently recommended (category 2A) as primary treatment options for AL in the US National Comprehensive Cancer Network guidelines. Further, local treatment guidelines in several European countries, including Holland and France, recognize bortezomib-based regimens as treatment options for AL. 43,44 The optimal duration of bortezomib therapy for patients with relapsed AL, or with relapsed MM, has not been established in prospective trials. Although the phase 3 APEX trial, which demonstrated the superiority of bortezomib over high-dose dexamethasone for relapsed MM, mandated a fixed duration of therapy, 45 the phase 2 SUMMIT and CREST trials allowed continuation of bortezomib in patients who, in the investigator s opinion, would benefit from extended treatment. 46 In addition, the subsequent phase 3 DOXIL- MMY-3001 study, which compared the combination of bortezomib and pegylated liposomal doxorubicin with bortezomib alone in relapsed or refractory MM, permitted continued bortezomib administration under similar circumstances. 47 Although prolonged bortezomib therapy was shown to be tolerable, these trials were not designed to evaluate the efficacy of continued therapy. Four AL patients in this study received long-term bortezomib treatment of between 3.5 and 5.6 years, with no new safety concerns; of these, 3 remained progression-free and on bortezomib treatment at the time of manuscript writing. It is of note that, although all 4 long-term exposure patients showed clinical stability or improvement to initial therapy, 3 had only stable disease or PR as their best hematologic response. These data suggest the feasibility of single-agent bortezomib for long-term disease control in relapsed AL, particularly in patients who do not initially achieve a hematologic CR. In patients responding to treatment but with residual disease, continuation of therapy may lead to suppression of the culprit plasma cell clone and longer disease control. Indications for patients who might benefit most from prolonged bortezomib therapy and the optimal schedule on which long-term bortezomib should be administered cannot be surmised from this analysis. However, it should be noted that the 4 long-term patients were of relatively young age (53-55 years) at study entry and had all previously undergone HDM-SCT, perhaps reflecting their relative fitness and ability to tolerate prolonged bortezomib. In addition, all had experienced improvement or disease stabilization in $1 affected organ. Additional follow-up since the previous report of the study 25 allowed for collection of subsequent therapy data in patients with progressive disease following initial bortezomib treatment. Of 19 patients who received bortezomib retreatment, 11 (58%) achieved a hematologic response (CR or PR). These data suggest that bortezomib-based therapy may be feasible and active at.1 stage in the AL treatment paradigm. In CAN2007, the potential utility of QW and BIW bortezomib administration was explored. Although the study was not designed to directly compare the 2 administration schedules, our data suggest that bortezomib 1.6 mg/m 2 QW was well tolerated with fewer discontinuations (50% vs 74% for BIW), allowing for a higher proportion of patients to complete $8 treatment cycles than with bortezomib 1.3 mg/m 2 BIW. These findings are in accordance with prior studies of QW vs BIW bortezomib administration in patients with MM or lymphoma, in which less intensive QW dosing in the context of combination therapy improved treatment tolerability without compromising efficacy The feasibility of less intensive bortezomib dosing for relapsed AL is also indicated by long-term disease control in 2 lower-dose group patients who received prolonged bortezomib therapy in CAN2007. QW bortezomib administration may also offer a potential advantage over BIW dosing in terms of patient convenience. However, response was achieved more quickly with BIW bortezomib, suggesting that this could be the initial schedule for patients with a high disease burden who require rapid FLC reduction. On the other hand, QW bortezomib may be a preferred schedule in patients with less rapidly progressing disease or after an initial response is obtained. Other factors, such as the tolerability of the dose and planned duration of therapy, should also be taken into account when determining the optimal dose for a given patient. In conclusion, single-agent bortezomib produced durable hematologic responses and promising long-term OS in relapsed AL patients. Prolonged therapy is feasible and can provide long-term disease control, potentially in patients who do not initially achieve a
8 BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 BORTEZOMIB IN RELAPSED LIGHT CHAIN AMYLOIDOSIS 2505 Table 5. Disease and treatment information for the 4 patients who received long-term treatment with bortezomib in CAN2007 Characteristic/parameter Patient 1 Patient 2 Patient 3 Patient 4 Gender Male Male Female Female Age at study entry, years Dose level, mg/m QW 0.7 BIW 1.6 QW 1.3 BIW KPS AL type IgA l IgG k IgG l l light chain Time since diagnosis, months Organ involvement 2: cardiac, renal 1: GI* 2: GI, other sites 2: cardiac, GI Prior therapy (best response achieved) Melphalan plus ASCT (PR) ASCT (PR) ASCT (SD) Cardiac allograft then ASCT (PR) Cycles of bortezomib Months/years on bortezomib 66.8/ / / /3.5 Planned cumulative dose, mg/m Cumulative dose received, mg/m % cumulative/planned dose Dose intensity, mg/mg 2 /cycle Grade 3/4 AEs, dose No grade 3/4 AEs No grade 3/4 AEs Grade 3 paralytic ileus, Grade 3 vasculitis, cycle 2 modifications Adjusted to 1.3 mg/m 2 Q2W, cycle 18, due to other reason Adjusted to 0.7 mg/m 2 QW, cycle 10, due to grade 2 pain in extremity cycle 8, dose reduced to 1.3 then 1.0 mg/m 2 (dose reduced to 1.0 mg/m 2, cycle 3); grade 3 volvulus, cycle 16 (dose reduced to 0.7 mg/m 2 ); other grade 3 AEs: pneumonia, hemoptysis, and C difficile diarrhea Hematologic response CR SD PR PR Organ responses Renal: response Renal: response Renal: NC Renal: response Cardiac: NC Cardiac: NC Cardiac: NC Cardiac: NC Status Ongoing, progression-free Progressed, in remission on lenalidomide/dexamethasone Ongoing, progression-free Ongoing, progression-free ASCT, autologous stem cell transplantation; GI, gastrointestinal; NC, no change; Q2W, every two weeks; SD, stable disease. *Biopsy-confirmed GI involvement in the ascending transverse and descending colons and stomach. hematologic CR. Favorable hematologic response rates can also be achieved with bortezomib retreatment in this setting. Previous studies have demonstrated the feasibility and activity of bortezomib in combination regimens. In the future, bortezomib might prove useful as part of initial AL treatment and possibly as part of extended therapy after HDM-SCT or alternative nontransplantation therapies. Acknowledgments The authors thank all the patients who participated in this study and their families, as well as the investigators and staff at all CAN2007 clinical sites for their valuable contribution to the study. The authors acknowledge Emma Landers, a medical writer with FireKite, part of KnowledgePoint360 group, an Ashfield Company, for writing support during the development of this manuscript, which was funded by Millennium: The Takeda Oncology Company and Janssen Global Services. This study was supported by research funding from Millennium: The Takeda Oncology Company and Janssen Research and Development. G.M. and G.P. are supported by a grant from Associazione Italiana per la Ricerca sul Cancro Special Program Molecular Clinical Oncology 5 per mille n Authorship Contribution: D.E.R., V.S., H.v.d.V., A.C., D.-L.E., and R.L.C. designed the trial; D.E.R., U.H., G.M., G.P., J.B., J.-P.F., H.H., L.H., V.K., R.A.V., C.E., D.-L.E., H.v.d.V., and A.C. interpreted the data; U.H., V.S., H.H., L.H., and R.L.C. collected the data; U.H., L.P., and H.v.d.V. analyzed the data; L.P. and H.v.d.V. performed the statistical analysis; and all authors contributed to the writing/review of the manuscript and approved the final version for publication. Conflict-of-interest disclosure: D.E.R. receives honoraria from Janssen, Celgene, Otsuka, and Merck and research funding from Janssen, Millennium: The Takeda Oncology Company, Celgene, Merck, Novartis, Otsuka, and Bristol-Myers Squibb. U.H. receives honoraria from Janssen. V.S. receives research support from Celgene and Millennium: The Takeda Oncology Company. G.M. receives honoraria from Millennium: The Takeda Oncology Company. J.B. receives honoraria from, and has membership on an advisory board for, Janssen and Celgene and receives research funding from Janssen. H.H. receives research support from Celgene and Millennium: The Takeda Oncology Company. L.H. receives research support from Janssen and Millennium: The Takeda Oncology Company. V.K. receives honoraria from Celgene and Janssen. R.A.V. participates in a speakers bureau for Millennium: The Takeda Oncology Company. L.P. is employed by Janssen. C.E. and H.v.d.V. are employed by Janssen R&D and receive equity ownership in Johnson & Johnson. D.-L.E. is employed by Takeda Pharmaceuticals International Co. and receives equity ownership in Takeda and Johnson & Johnson. A.C. is a former employee of, formerly provided expert testimony for, and has equity ownership in Johnson & Johnson. R.L.C. receives research support from Millennium: The Takeda Oncology Company, Prothena, and Teva. G.P. and J.-P.F. declare no competing financial interests. Correspondence: Donna E. Reece, Princess Margaret Cancer Center, 610 University Ave, Suite 5-207, Toronto, ON, Canada M5G 2M9; donna.reece@uhn.ca.
9 2506 REECE et al BLOOD, 16 OCTOBER 2014 x VOLUME 124, NUMBER 16 References 1. Comenzo RL. Managing systemic light-chain amyloidosis. J Natl Compr Canc Netw. 2007;5(2): Desport E, Bridoux F, Sirac C, et al. Al amyloidosis. Orphanet J Rare Dis. 2012;7(Aug 21): Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13): Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 2007;92(10): Lamm W, Willenbacher W, Lang A, et al. Efficacy of the combination of bortezomib and dexamethasone in systemic AL amyloidosis. Ann Hematol. 2011;90(2): Gertz MA, Lacy MQ, Dispenzieri A, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92(10): Palladini G, Russo P, Nuvolone M, et al. Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. Blood. 2007;110(2): Sanchorawala V, Skinner M, Quillen K, Finn KT, Doros G, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with highdose melphalan and stem-cell transplantation. Blood. 2007;110(10): Suzuki K. Diagnosis and treatment of multiple myeloma and AL amyloidosis with focus on improvement of renal lesion. Clin Exp Nephrol. 2012;16(5): Gertz MA, Lacy MQ, Dispenzieri A, et al. Autologous stem cell transplant for immunoglobulin light chain amyloidosis: a status report. Leuk Lymphoma. 2010;51(12): Rosenzweig M, Landau H. Light chain (AL) amyloidosis: update on diagnosis and management. J Hematol Oncol. 2011;4(Nov 18): Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1(6): Millennium Pharmaceuticals Inc. VELCADE Ò Prescribing Information. October 2012 (Revision 15). 14. Chari A, Barley K, Jagannath S, Osman K. Safety and efficacy of triplet regimens in newly diagnosed light chain amyloidosis. Clin Lymphoma Myeloma Leuk. 2013;13(1): Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. Cyclophosphamide-bortezomibdexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119(19): Venner CP, Lane T, Foard D, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012; 119(19): Wechalekar AD, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Efficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal disease. Haematologica. 2008;93(2): Shah GL, Kaul E, Fallo S, et al. Subcutaneous bortezomib in combination regimens in newly diagnosed patients with myeloma or systemic AL amyloidosis: high response rates and minimal toxicity. Blood. 2012;120(21):Abstract Kastritis E, Anagnostopoulos A, Roussou M, et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92(10): Kastritis E, Wechalekar AD, Dimopoulos MA, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28(6): Palladini G, Foli A, Russo P, et al. Treatment of IgM-associated AL amyloidosis with the combination of rituximab, bortezomib, and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11(1): Zhai YP, Liu HN, Yu YP, et al. [Treatment of primary systemic amyloidosis with the combination of bortezomib and dexamethasone]. Zhonghua Xue Ye Xue Za Zhi. 2010;31(5): Reece DE, Sanchorawala V, Hegenbart U, et al; VELCADE CAN2007 Study Group. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 doseescalation study. Blood. 2009;114(8): Dubrey SW, Reece DE, Sanchorawala V, et al; Velcade Can2007 Study Group. Bortezomib in a phase 1 trial for patients with relapsed AL amyloidosis: cardiac responses and overall effects. QJM. 2011;104(11): Reece DE, Hegenbart U, Sanchorawala V, et al. Efficacy and safety of once-weekly and twiceweekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4): Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, April Am J Hematol. 2005; 79(4): Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9): Mollee P, Tate J, Pretorius CJ. Evaluation of the N Latex free light chain assay in the diagnosis and monitoring of AL amyloidosis. Clin Chem Lab Med. 2013;51(12): Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36): Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109(2): Palladini G, Russo P, Milani P, et al. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica. 2013;98(3): Sanchorawala V, Wright DG, Rosenzweig M, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109(2): Dhodapkar MV, Hussein MA, Rasmussen E, et al; United States Intergroup Trial Southwest Oncology Group. Clinical efficacy of high-dose dexamethasone with maintenance dexamethasone/alpha interferon in patients with primary systemic amyloidosis: results of United States Intergroup Trial Southwest Oncology Group (SWOG) S9628. Blood. 2004;104(12): Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997; 336(17): Skinner M, Anderson J, Simms R, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. 1996;100(3): Palladini G, Anesi E, Perfetti V, et al. A modified high-dose dexamethasone regimen for primary systemic (AL) amyloidosis. Br J Haematol. 2001; 113(4): Dispenzieri A, Lacy MQ, Katzmann JA, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107(8): Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122(1): Kumar SK, Dispenzieri A, Lacy MQ, et al. Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. Am J Hematol. 2011;86(3): Kumar S, Dispenzieri A, Katzmann JA, et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24): Raval GG, Gertz MA, Lacy MQ, et al. Survival after second, third, and fourth line therapy better than expected in patients with previously treated AL amyloidosis who were not transplant candidates at diagnosis. Blood. 2012;120(21):Abstract Gasparetto C, Sanchorawala V, Synder RM, et al. Use of melphalan/dexamethasone/bortezomib in AL amyloidosis. J Clin Oncol. 2010;28(Suppl 15): Abstract Arnaud J, Mohty D, Desport E, Bridoux F. Actualites dans le traitement de l amylose AL. Hematologie. 2012;18(2): Minnema M, Hazenberg B, Croockewit A, et al. De behandeling van AL-amyloidose in Nederland anno Ned Tijdschr Hematol. 2013;10(5): Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352(24): Berenson JR, Jagannath S, Barlogie B, et al. Safety of prolonged therapy with bortezomib in relapsed or refractory multiple myeloma. Cancer. 2005;104(10): Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25): Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23): Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115(16): de Vos S, Goy A, Dakhil SR, et al. Multicenter randomized phase II study of weekly or twiceweekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27(30):
10 : doi: /blood originally published online September 8, 2014 Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis Donna E. Reece, Ute Hegenbart, Vaishali Sanchorawala, Giampaolo Merlini, Giovanni Palladini, Joan Bladé, Jean-Paul Fermand, Hani Hassoun, Leonard Heffner, Vishal Kukreti, Robert A. Vescio, Lixia Pei, Christopher Enny, Dixie-Lee Esseltine, Helgi van de Velde, Andrew Cakana and Raymond L. Comenzo Updated information and services can be found at: Articles on similar topics can be found in the following Blood collections Clinical Trials and Observations (4744 articles) Free Research Articles (5003 articles) Lymphoid Neoplasia (2773 articles) Multiple Myeloma (395 articles) Information about reproducing this article in parts or in its entirety may be found online at: Information about ordering reprints may be found online at: Information about subscriptions and ASH membership may be found online at: Blood (print ISSN , online ISSN ), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC Copyright 2011 by The American Society of Hematology; all rights reserved.
At Fox Chase Cancer Centre during study participation
 Long-Term Outcome of a Phase 1 Study of the Investigational Oral Proteasome Inhibitor Ixazomib at the Recommended Phase 3 Dose in Patients with Relapsed or Refractory Systemic Light-Chain (AL) Amyloidosis
Long-Term Outcome of a Phase 1 Study of the Investigational Oral Proteasome Inhibitor Ixazomib at the Recommended Phase 3 Dose in Patients with Relapsed or Refractory Systemic Light-Chain (AL) Amyloidosis
Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012
 Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012 Support AL Amyloidosis (Light Chain Amyloidosis) Effective Date: 01/01/2013 Document: ARB0413:01 Revision Date: 10/24/2012 Code(s):
Medical Policy Title: HDC Progenitor Cell ARBenefits Approval: 02/08/2012 Support AL Amyloidosis (Light Chain Amyloidosis) Effective Date: 01/01/2013 Document: ARB0413:01 Revision Date: 10/24/2012 Code(s):
Review Article Autologous Stem Cell Transplant for AL Amyloidosis
 Bone Marrow Research Volume 2012, Article ID 238961, 5 pages doi:10.1155/2012/238961 Review Article Autologous Stem Cell Transplant for AL Amyloidosis Vivek Roy Division of Hematology/Oncology, Mayo Clinic,
Bone Marrow Research Volume 2012, Article ID 238961, 5 pages doi:10.1155/2012/238961 Review Article Autologous Stem Cell Transplant for AL Amyloidosis Vivek Roy Division of Hematology/Oncology, Mayo Clinic,
Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study
 2009 114: 1489-1497 Prepublished online Jun 4, 2009; doi:10.1182/blood-2009-02-203398 Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study
2009 114: 1489-1497 Prepublished online Jun 4, 2009; doi:10.1182/blood-2009-02-203398 Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study
Protocol. Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Mayo Clinic, Rochester, Minnesota; 2 Tufts Medical Center, Boston, Massachusetts; 3
 NEOD001 Demonstrates Organ Biomarker Responses in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction: Final Results From a Phase 1/2 Study Morie A. Gertz, 1 Raymond L. Comenzo, 2 Heather
NEOD001 Demonstrates Organ Biomarker Responses in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction: Final Results From a Phase 1/2 Study Morie A. Gertz, 1 Raymond L. Comenzo, 2 Heather
A.M.W. van Marion. H.M. Lokhorst. N.W.C.J. van de Donk. J.G. van den Tweel. Histopathology 2002, 41 (suppl 2):77-92 (modified)
 chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
chapter 4 The significance of monoclonal plasma cells in the bone marrow biopsies of patients with multiple myeloma following allogeneic or autologous stem cell transplantation A.M.W. van Marion H.M. Lokhorst
Disclosures. Consultancy, Research Funding and Speakers Bureau: Celgene Corporation, Millennium, Onyx, Cephalon
 Pomalidomide With or Without Low-dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma: Outcomes in Patients Refractory to Lenalidomide and Bortezomib Ravi Vij 1, Paul G. Richardson
Pomalidomide With or Without Low-dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma: Outcomes in Patients Refractory to Lenalidomide and Bortezomib Ravi Vij 1, Paul G. Richardson
Disclosures. Membership of Advisory Committees: Research Support/ PI: Celgene Corporation Millennium Pharmaceuticals Johnson & Johnson
 Randomized, Open-Label Phase 1/2 Study of Pomalidomide Alone or in Combination With Low-Dose Dexamethasone in Patients With Relapsed and Refractory Multiple Myeloma Who Have Received Prior Treatment That
Randomized, Open-Label Phase 1/2 Study of Pomalidomide Alone or in Combination With Low-Dose Dexamethasone in Patients With Relapsed and Refractory Multiple Myeloma Who Have Received Prior Treatment That
Methods: Studies included in the analysis
 Efficacy and safety of long-term ixazomib maintenance therapy in patients with newly diagnosed multiple myeloma not undergoing transplant: An integrated analysis of four phase 1/2 studies Meletios A. Dimopoulos,
Efficacy and safety of long-term ixazomib maintenance therapy in patients with newly diagnosed multiple myeloma not undergoing transplant: An integrated analysis of four phase 1/2 studies Meletios A. Dimopoulos,
Cyclophosphamide, bortezomib and dexamethasone (CyBorD) combination in Waldenstrom Macroglobulinemia
 Cyclophosphamide, bortezomib and dexamethasone (CyBorD) combination in Waldenstrom Macroglobulinemia Houry Leblebjian, PharmD, Kimberly Noonan, NP, Claudia Paba-Prada, MD, Steven P. Treon, MD, Jorge J.
Cyclophosphamide, bortezomib and dexamethasone (CyBorD) combination in Waldenstrom Macroglobulinemia Houry Leblebjian, PharmD, Kimberly Noonan, NP, Claudia Paba-Prada, MD, Steven P. Treon, MD, Jorge J.
Sheena Surindran Grand Rounds 2/15/11
 Sheena Surindran Grand Rounds 2/15/11 Affects 5 12 person per million / year 5 10% associated with myeloma Median survival without treatment is 12 40 months Most commonly affected organs are kidney, heart
Sheena Surindran Grand Rounds 2/15/11 Affects 5 12 person per million / year 5 10% associated with myeloma Median survival without treatment is 12 40 months Most commonly affected organs are kidney, heart
To Maintain or Not to Maintain? Immunomodulators vs PIs Yes: Proteasome Inhibitors
 To Maintain or Not to Maintain? Immunomodulators vs PIs Yes: Proteasome Inhibitors James Berenson, MD Institute for Myeloma and Bone Cancer Research West Hollywood, CA Financial Disclosures Takeda, Celgene
To Maintain or Not to Maintain? Immunomodulators vs PIs Yes: Proteasome Inhibitors James Berenson, MD Institute for Myeloma and Bone Cancer Research West Hollywood, CA Financial Disclosures Takeda, Celgene
Treatment of elderly multiple myeloma patients
 SAMO Interdisciplinary Workshop on Myeloma March 30 th -31 st 2012, Seehotel Hermitage, Lucerne Treatment of elderly multiple myeloma patients Federica Cavallo, MD, PhD Federica Cavallo, MD, PhD Division
SAMO Interdisciplinary Workshop on Myeloma March 30 th -31 st 2012, Seehotel Hermitage, Lucerne Treatment of elderly multiple myeloma patients Federica Cavallo, MD, PhD Federica Cavallo, MD, PhD Division
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Systemic Light Chain Amyloidosis
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2016 NCCN.org Continue Version 1.2016, 09/04/15 National Comprehensive Cancer Network, Inc. 2015,
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2016 NCCN.org Continue Version 1.2016, 09/04/15 National Comprehensive Cancer Network, Inc. 2015,
EBMT2008_22_44:EBMT :26 Pagina 424 CHAPTER 27. HSCT for primary amyloidosis in adults. J. Esteve
 EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 424 * CHAPTER 27 HSCT for primary amyloidosis in adults J. Esteve EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 425 CHAPTER 27 Amyloidosis in adults 1. Introduction
EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 424 * CHAPTER 27 HSCT for primary amyloidosis in adults J. Esteve EBMT2008_22_44:EBMT2008 6-11-2008 9:26 Pagina 425 CHAPTER 27 Amyloidosis in adults 1. Introduction
Primary Amyloidosis. Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center
 Primary Amyloidosis Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center Systemic Amyloidosis A group of complex diseases caused by tissue
Primary Amyloidosis Kihyun Kim Div. of Hematology/Oncology, Dept. of Medicine, Sungkyunkwan Univ. School of Medidine Samsung Medical Center Systemic Amyloidosis A group of complex diseases caused by tissue
Disclosures for Palumbo Antonio, MD
 Disclosures for Palumbo Antonio, MD Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau Honoraria Scientific Advisory Board o relevant conflicts of interest to declare o relevant
Disclosures for Palumbo Antonio, MD Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau Honoraria Scientific Advisory Board o relevant conflicts of interest to declare o relevant
Experience with bortezomib (Velcade) in multiple myeloma. Peter Černelč Clinical center Ljubljana Department of Haematology
 Experience with bortezomib (Velcade) in multiple myeloma Peter Černelč Clinical center Ljubljana Department of Haematology Our experience with bortezomib (Velcade) in multiple myeloma 1. Our first experience
Experience with bortezomib (Velcade) in multiple myeloma Peter Černelč Clinical center Ljubljana Department of Haematology Our experience with bortezomib (Velcade) in multiple myeloma 1. Our first experience
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: , 2015
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1895-1900, 2015 Clinical characteristics of a group of patients with multiple myeloma who had two different λ light chains by immunofixation electrophoresis: A
EXPERIMENTAL AND THERAPEUTIC MEDICINE 9: 1895-1900, 2015 Clinical characteristics of a group of patients with multiple myeloma who had two different λ light chains by immunofixation electrophoresis: A
Effective response with bortezomib retreatment in relapsed multiple myeloma
 Published 27 April 2012, doi:10.4414/smw.2012.13562 Cite this as: Effective response with bortezomib retreatment in relapsed multiple myeloma A multicentre retrospective survey in Switzerland 1 Christian
Published 27 April 2012, doi:10.4414/smw.2012.13562 Cite this as: Effective response with bortezomib retreatment in relapsed multiple myeloma A multicentre retrospective survey in Switzerland 1 Christian
Standard of care for patients with newly diagnosed multiple myeloma who are not eligible for a transplant
 Standard of care for patients with newly diagnosed multiple myeloma who are not eligible for a transplant Pr Philippe Moreau University Hospital, Nantes, France MP: Standard of care until 2007 J Clin Oncol
Standard of care for patients with newly diagnosed multiple myeloma who are not eligible for a transplant Pr Philippe Moreau University Hospital, Nantes, France MP: Standard of care until 2007 J Clin Oncol
Bortezomib (Velcade)
 Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Amyloidosis: What to do and how to diagnose: An Update 2017
 Amyloidosis: What to do and how to diagnose: An Update 2017 Jonathan L. Kaufman, MD Associate Professor Hematology & Oncology Winship Cancer Institute of Emory University Amyloidosis Protein Conformation/Deposition
Amyloidosis: What to do and how to diagnose: An Update 2017 Jonathan L. Kaufman, MD Associate Professor Hematology & Oncology Winship Cancer Institute of Emory University Amyloidosis Protein Conformation/Deposition
Guidelines on the management of AL amyloidosis
 guideline Guidelines on the management of AL amyloidosis Ashutosh D. Wechalekar, 1 Julian D. Gillmore, 1 Jenny Bird, 2 Jamie Cavenagh, 3 Stephen Hawkins, 4 Majid Kazmi, 5 Helen J. Lachmann, 1 Philip N.
guideline Guidelines on the management of AL amyloidosis Ashutosh D. Wechalekar, 1 Julian D. Gillmore, 1 Jenny Bird, 2 Jamie Cavenagh, 3 Stephen Hawkins, 4 Majid Kazmi, 5 Helen J. Lachmann, 1 Philip N.
Light chain type predicts organ involvement and survival in AL amyloidosis patients receiving stem cell transplantation
 REGULAR ARTICLE Light chain type predicts organ involvement and survival in AL amyloidosis patients receiving stem cell transplantation M Hasib Sidiqi, Mohammed A. Aljama, Eli Muchtar, Francis K. Buadi,
REGULAR ARTICLE Light chain type predicts organ involvement and survival in AL amyloidosis patients receiving stem cell transplantation M Hasib Sidiqi, Mohammed A. Aljama, Eli Muchtar, Francis K. Buadi,
MYBORPRE. Protocol Code. Lymphoma, Leukemia/BMT. Tumour Group. Dr. Kevin Song. Contact Physician
 BC Cancer Protocol Summary for the Treatment of Multiple Myeloma Using Bortezomib, Dexamethasone With or Without Cyclophosphamide as Induction Pre-Stem Cell Transplant Protocol Code Tumour Group Contact
BC Cancer Protocol Summary for the Treatment of Multiple Myeloma Using Bortezomib, Dexamethasone With or Without Cyclophosphamide as Induction Pre-Stem Cell Transplant Protocol Code Tumour Group Contact
Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naïve patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III)
 Plasma Cell Disorders Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naïve patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III) ARTICLES Arnaud Jaccard, 1 Raymond
Plasma Cell Disorders Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naïve patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III) ARTICLES Arnaud Jaccard, 1 Raymond
A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma
 A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma Jatin J. Shah, MD 1, Edward A. Stadtmauer, MD 2, Rafat
A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma Jatin J. Shah, MD 1, Edward A. Stadtmauer, MD 2, Rafat
& 2004 Nature Publishing Group All rights reserved /04 $
 (2004) 33, 381 388 & 2004 Nature Publishing Group All rights reserved 0268-3369/04 $25.00 www.nature.com/bmt Amyloidosis High-dose intravenous melphalan and autologous stem cell transplantation as initial
(2004) 33, 381 388 & 2004 Nature Publishing Group All rights reserved 0268-3369/04 $25.00 www.nature.com/bmt Amyloidosis High-dose intravenous melphalan and autologous stem cell transplantation as initial
Traditional Therapies for Waldenstrom s Macroglobulinemia. Christine Chen Princess Margaret Cancer Centre Toronto, Canada May 2014
 Traditional Therapies for Waldenstrom s Macroglobulinemia Christine Chen Princess Margaret Cancer Centre Toronto, Canada May 2014 Jeff Atlin (1953-2014) Standard treatment options Single drug therapies
Traditional Therapies for Waldenstrom s Macroglobulinemia Christine Chen Princess Margaret Cancer Centre Toronto, Canada May 2014 Jeff Atlin (1953-2014) Standard treatment options Single drug therapies
Myeloma update ASH 2014
 Myeloma update ASH 2014 Updates in Newly Diagnosed Multiple Myeloma FIRST: effect of age on lenalidomide/dexamethasone vs MPT in transplantation-ineligible pts Phase III: MPT-T vs MPR-R in transplantation-ineligible
Myeloma update ASH 2014 Updates in Newly Diagnosed Multiple Myeloma FIRST: effect of age on lenalidomide/dexamethasone vs MPT in transplantation-ineligible pts Phase III: MPT-T vs MPR-R in transplantation-ineligible
A Phase 1 Trial of Lenalidomide (REVLIMID ) With Bortezomib (VELCADE ) in Relapsed and Refractory Multiple Myeloma
 A Phase 1 Trial of Lenalidomide (REVLIMID ) With Bortezomib (VELCADE ) in Relapsed and Refractory Multiple Myeloma P.G. Richardson, 1 R. Schlossman, 1 N. Munshi, 1 D. Avigan, 2 S. Jagannath, 3 M. Alsina,
A Phase 1 Trial of Lenalidomide (REVLIMID ) With Bortezomib (VELCADE ) in Relapsed and Refractory Multiple Myeloma P.G. Richardson, 1 R. Schlossman, 1 N. Munshi, 1 D. Avigan, 2 S. Jagannath, 3 M. Alsina,
Consolidation and maintenance therapy for transplant eligible myeloma patients
 Consolidation and maintenance therapy for transplant eligible myeloma patients Teeraya Puavilai, M.D. Division of Hematology, Department of Medicine Faculty of Medicine Ramathibodi Hospital Mahidol University
Consolidation and maintenance therapy for transplant eligible myeloma patients Teeraya Puavilai, M.D. Division of Hematology, Department of Medicine Faculty of Medicine Ramathibodi Hospital Mahidol University
Published Ahead of Print on May 23, 2014, as doi: /haematol Copyright 2014 Ferrata Storti Foundation.
 Published Ahead of Print on May 23, 2014, as doi:10.3324/haematol.2014.104109. Copyright 2014 Ferrata Storti Foundation. Efficacy of Bortezomib, Cyclophosphamide and Dexamethasone in treatment of naive
Published Ahead of Print on May 23, 2014, as doi:10.3324/haematol.2014.104109. Copyright 2014 Ferrata Storti Foundation. Efficacy of Bortezomib, Cyclophosphamide and Dexamethasone in treatment of naive
Smoldering Myeloma: Leave them alone!
 Smoldering Myeloma: Leave them alone! David H. Vesole, MD, PhD Co-Director, Myeloma Division Director, Myeloma Research John Theurer Cancer Center Hackensack University Medical Center Prevalence 1960 2002
Smoldering Myeloma: Leave them alone! David H. Vesole, MD, PhD Co-Director, Myeloma Division Director, Myeloma Research John Theurer Cancer Center Hackensack University Medical Center Prevalence 1960 2002
Dana-Farber Cancer Institute, Boston, MA, USA; 2. H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA; 3
 The investigational agent MLN9708, an oral proteasome inhibitor, in patients with relapsed and/or refractory multiple myeloma (MM): results from the expansion cohorts of a phase 1 dose-escalation study
The investigational agent MLN9708, an oral proteasome inhibitor, in patients with relapsed and/or refractory multiple myeloma (MM): results from the expansion cohorts of a phase 1 dose-escalation study
Multiple Myeloma Updates 2007
 Multiple Myeloma Updates 2007 Brian Berryman, M.D. Multiple Myeloma Updates 2007 Goals for today: Understand the staging systems for myeloma Understand prognostic factors in myeloma Review updates from
Multiple Myeloma Updates 2007 Brian Berryman, M.D. Multiple Myeloma Updates 2007 Goals for today: Understand the staging systems for myeloma Understand prognostic factors in myeloma Review updates from
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis. Original Policy Date
 MP 7.03.29 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
MP 7.03.29 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning
 Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning Challenge Question: Role of Autologous Stem Cell Transplant Which of the following is true about eligibility for high-dose
Module 3: Multiple Myeloma Induction and Transplant Strategies Treatment Planning Challenge Question: Role of Autologous Stem Cell Transplant Which of the following is true about eligibility for high-dose
Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes before and after 2006
 (2011) 46, 970 975 & 2011 Macmillan Publishers Limited All rights reserved 0268-3369/11 www.nature.com/bmt ORIGINAL ARTICLE Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes
(2011) 46, 970 975 & 2011 Macmillan Publishers Limited All rights reserved 0268-3369/11 www.nature.com/bmt ORIGINAL ARTICLE Trends in day 100 and 2-year survival after auto-sct for AL amyloidosis: outcomes
A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma
 A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma Jatin J. Shah, MD 1, Edward A. Stadtmauer, MD 2, Rafat
A Multi-Center Phase I/II Trial of Carfilzomib and Pomalidomide with Dexamethasone (Car- Pom-d) in Patients with Relapsed/Refractory Multiple Myeloma Jatin J. Shah, MD 1, Edward A. Stadtmauer, MD 2, Rafat
STUDY DESIGN. VMP 6-week cycles, total of 9 cycles. Figure 2. Alcyone study design. Countries housing study sites are shaded in gold.
 TPS8608 A Randomized Open-label Study of Bortezomib, Melphalan, And Prednisone (VMP) Versus Daratumumab () Plus VMP in Patients With Previously Untreated Multiple Myeloma (MM) Who Are Ineligible for High-dose
TPS8608 A Randomized Open-label Study of Bortezomib, Melphalan, And Prednisone (VMP) Versus Daratumumab () Plus VMP in Patients With Previously Untreated Multiple Myeloma (MM) Who Are Ineligible for High-dose
Mantle cell lymphoma An update on management
 Mantle cell lymphoma An update on management Dr Kim Linton Consultant Medical Oncologist The Christie NHS Foundation Trust 6 th October 2016 This educational meeting is organised and sponsored by Janssen-Cilag
Mantle cell lymphoma An update on management Dr Kim Linton Consultant Medical Oncologist The Christie NHS Foundation Trust 6 th October 2016 This educational meeting is organised and sponsored by Janssen-Cilag
Terapia del mieloma. La terapia di prima linea nel paziente giovane. Elena Zamagni
 Terapia del mieloma La terapia di prima linea nel paziente giovane Elena Zamagni Istituto di Ematologia ed Oncologia Medica Seràgnoli Università degli Studi di Bologna Newly diagnosed MM Candidate for
Terapia del mieloma La terapia di prima linea nel paziente giovane Elena Zamagni Istituto di Ematologia ed Oncologia Medica Seràgnoli Università degli Studi di Bologna Newly diagnosed MM Candidate for
Abstract. Tisch Cancer Institute, Mount Sinai School of Medicine, New York, NY, USA; 2 Hospital-12-de-Octubre, Madrid, Spain; 3
 Daratumumab, Carfilzomib, and Dexamethasone (D-Kd) in Lenalidomiderefractory Patients with Relapsed Multiple Myeloma (MM): Subgroup Analysis of MMY11 Chari A, 1* Martinez-Lopez J, 2 Mateos M-V, 3 Bladé
Daratumumab, Carfilzomib, and Dexamethasone (D-Kd) in Lenalidomiderefractory Patients with Relapsed Multiple Myeloma (MM): Subgroup Analysis of MMY11 Chari A, 1* Martinez-Lopez J, 2 Mateos M-V, 3 Bladé
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Comorbidities in Multiple Myeloma
 Comorbidities in Multiple Myeloma Michel Delforge, MD, PhD University Hospital Leuven Leuven, Belgium COMy, Bangkok 12 may 2014 Comy Meeting, Bangkok, 12 may 2014 Disclosures Advisory board: Janssen,
Comorbidities in Multiple Myeloma Michel Delforge, MD, PhD University Hospital Leuven Leuven, Belgium COMy, Bangkok 12 may 2014 Comy Meeting, Bangkok, 12 may 2014 Disclosures Advisory board: Janssen,
CME Information LEARNING OBJECTIVES
 CME Information LEARNING OBJECTIVES Identify patients with MM who have undergone autologous stem cell transplant and would benefit from maintenance lenalidomide. Counsel older patients (age 65 or older)
CME Information LEARNING OBJECTIVES Identify patients with MM who have undergone autologous stem cell transplant and would benefit from maintenance lenalidomide. Counsel older patients (age 65 or older)
Amiloidosis AL Tratamiento. Joan Bladé Asturias, 6 de Noviembre de 2012
 Amiloidosis AL Tratamiento Joan Bladé Asturias, 6 de Noviembre de 2012 AL Concept Incidence: 5-12 persons per million per year Clonal bone marrow plasma cells Amyloid precursor: variable region of LC (80%
Amiloidosis AL Tratamiento Joan Bladé Asturias, 6 de Noviembre de 2012 AL Concept Incidence: 5-12 persons per million per year Clonal bone marrow plasma cells Amyloid precursor: variable region of LC (80%
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/26/2013 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/26/2013 Section: Transplants
ClinicalTrials.gov Identifier: NCT
 Efficacy of Daratumumab, Lenalidomide, and Dexamethasone Versus Lenalidomide and Dexamethasone Alone for Relapsed or Refractory Multiple Myeloma Among Patients With to 3 Prior Lines of Therapy Based on
Efficacy of Daratumumab, Lenalidomide, and Dexamethasone Versus Lenalidomide and Dexamethasone Alone for Relapsed or Refractory Multiple Myeloma Among Patients With to 3 Prior Lines of Therapy Based on
TREATMENT FOR NON-TRANSPLANT ELIGIBLE MULTIPLE MYELOMA
 TREATMENT FOR NON-TRANSPLANT ELIGIBLE MULTIPLE MYELOMA Ekarat Rattarittamrong, MD Division of Hematology Department of Internal Medicine Faculty of Medicine Chiang Mai University OUTLINE Overview of treatment
TREATMENT FOR NON-TRANSPLANT ELIGIBLE MULTIPLE MYELOMA Ekarat Rattarittamrong, MD Division of Hematology Department of Internal Medicine Faculty of Medicine Chiang Mai University OUTLINE Overview of treatment
Elotuzumab is a humanized monoclonal antibody designed to treat multiple myeloma (MM)
 A Phase 2 Study of in Combination with Lenalidomide and Low-Dose Dexamethasone in Patients with Relapsed/ Refractory Multiple Myeloma: Updated Results Paul G. Richardson, 1,2 Sundar Jagannath, 2,3 Philippe
A Phase 2 Study of in Combination with Lenalidomide and Low-Dose Dexamethasone in Patients with Relapsed/ Refractory Multiple Myeloma: Updated Results Paul G. Richardson, 1,2 Sundar Jagannath, 2,3 Philippe
Multiple Myeloma: Induction, Consolidation and Maintenance Therapy
 Multiple Myeloma: Induction, Consolidation and Maintenance Therapy James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Establish the Goals of
Multiple Myeloma: Induction, Consolidation and Maintenance Therapy James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Establish the Goals of
Living Well with Myeloma Teleconference Series Thursday, March 24 th :00 PM Pacific/5:00 PM Mountain 6:00 PM Central/7:00 PM Eastern
 Living Well with Myeloma Teleconference Series Thursday, March 24 th 216 4: PM Pacific/5: PM Mountain 6: PM Central/7: PM Eastern Speakers Dr. Brian Durie, IMF Chairman Cedars Sinai Samuel Oschin Cancer
Living Well with Myeloma Teleconference Series Thursday, March 24 th 216 4: PM Pacific/5: PM Mountain 6: PM Central/7: PM Eastern Speakers Dr. Brian Durie, IMF Chairman Cedars Sinai Samuel Oschin Cancer
Therapeutic effects of autologous hematopoietic stem cell transplantation in multiple myeloma patients
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 6: 977-982, 2013 Therapeutic effects of autologous hematopoietic stem cell transplantation in multiple myeloma patients CHENGCHENG FU, JUAN WANG, XUE XIN, HUI LIU,
EXPERIMENTAL AND THERAPEUTIC MEDICINE 6: 977-982, 2013 Therapeutic effects of autologous hematopoietic stem cell transplantation in multiple myeloma patients CHENGCHENG FU, JUAN WANG, XUE XIN, HUI LIU,
Managing Myeloma Virtual Grand Rounds Newly Diagnosed, Transplant Eligible Patient. Case Study
 Managing Myeloma Virtual Grand Rounds Newly Diagnosed, Transplant Eligible Patient Case Study 2 2011 Newly Diagnosed Patient The patient is a 61-year-old Caucasian female History of high blood pressure
Managing Myeloma Virtual Grand Rounds Newly Diagnosed, Transplant Eligible Patient Case Study 2 2011 Newly Diagnosed Patient The patient is a 61-year-old Caucasian female History of high blood pressure
Highlights from EHA Mieloma Multiplo
 Highlights from EHA Mieloma Multiplo Michele Cavo Istituto di Ematologia L. e A. Seràgnoli Alma Mater Studiorum Università degli studi di Bologna Firenze, 22-23 Settembre 27 Myeloma XI TE pathway 7 R :
Highlights from EHA Mieloma Multiplo Michele Cavo Istituto di Ematologia L. e A. Seràgnoli Alma Mater Studiorum Università degli studi di Bologna Firenze, 22-23 Settembre 27 Myeloma XI TE pathway 7 R :
COMy Congress The case for IMids. Xavier Leleu. Hôpital la Milétrie, PRC, CHU, Poitiers, France
 Xavier Leleu Hôpital la Milétrie, PRC, CHU, Poitiers, France The case for IMids COMy Congress 21 Disclosures Grants/research support: Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium/Takeda, Novartis,
Xavier Leleu Hôpital la Milétrie, PRC, CHU, Poitiers, France The case for IMids COMy Congress 21 Disclosures Grants/research support: Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium/Takeda, Novartis,
Light Chain Amyloidosis 2014
 International Journal of Hematological Disorders, 2014, Vol. 1, No. 1A, 21-29 Available online at http://pubs.sciepub.com/ijhd/1/1a/4 Science and Education Publishing DOI:10.12691/ijhd-1-1A-4 Light Chain
International Journal of Hematological Disorders, 2014, Vol. 1, No. 1A, 21-29 Available online at http://pubs.sciepub.com/ijhd/1/1a/4 Science and Education Publishing DOI:10.12691/ijhd-1-1A-4 Light Chain
Debate: Is transplant a necessity or a choice? Focus on the necessity for CR and MRD. Answer: NO
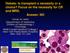 Debate: Is transplant a necessity or a choice? Focus on the necessity for CR and MRD. Answer: NO Tomer M. Mark Department of Medicine, Division of Hematology / Oncology Weill-Cornell Medical College /
Debate: Is transplant a necessity or a choice? Focus on the necessity for CR and MRD. Answer: NO Tomer M. Mark Department of Medicine, Division of Hematology / Oncology Weill-Cornell Medical College /
Systemic Light-Chain Amyloidosis: Advances in Diagnosis, Prognosis, and Therapy
 MULTIPLE MYELOMA Systemic Light-Chain Amyloidosis: Advances in Diagnosis, Prognosis, and Therapy Adam D. Cohen 1 and Raymond L. Comenzo 2 1 Fox Chase Cancer Center, Philadelphia, PA; 2 Tufts Medical Center,
MULTIPLE MYELOMA Systemic Light-Chain Amyloidosis: Advances in Diagnosis, Prognosis, and Therapy Adam D. Cohen 1 and Raymond L. Comenzo 2 1 Fox Chase Cancer Center, Philadelphia, PA; 2 Tufts Medical Center,
What are the hurdles to using cell of origin in classification to treat DLBCL?
 What are the hurdles to using cell of origin in classification to treat DLBCL? John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical
What are the hurdles to using cell of origin in classification to treat DLBCL? John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical
Post Transplant Maintenance- for everyone? Disclosures
 Post Transplant Maintenance- for everyone? NO Because of limited survival data, not all patients require maintenance April 2012 Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida Joseph Mikhael,
Post Transplant Maintenance- for everyone? NO Because of limited survival data, not all patients require maintenance April 2012 Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida Joseph Mikhael,
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
AHFS Final. Criteria Used in. Strength. Grade of. hydrochloride. per day on 12, and mg/m 2 IV. Strength. Grade of. Bortezomib 1.
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bortezomib in Combination with Doxorubicin and Dexamethasone as Inductionn Therapy for Newly Diagnosed Multiple Myeloma in Transplant-eligible
AHFS Final Determination of Medical Acceptance: Off-label Use of Bortezomib in Combination with Doxorubicin and Dexamethasone as Inductionn Therapy for Newly Diagnosed Multiple Myeloma in Transplant-eligible
Curing Myeloma So Close and Yet So Far! Luciano J. Costa, MD, PhD Associate Professor of Medicine University of Alabama at Birmingham
 Curing Myeloma So Close and Yet So Far! Luciano J. Costa, MD, PhD Associate Professor of Medicine University of Alabama at Birmingham What is cure after all? Getting rid of it? Stopping treatment without
Curing Myeloma So Close and Yet So Far! Luciano J. Costa, MD, PhD Associate Professor of Medicine University of Alabama at Birmingham What is cure after all? Getting rid of it? Stopping treatment without
Systemic Light Chain Amyloidosis
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2018 September 6, 2017 NCCN.org Continue Version 1.2018, 09/06/17 National Comprehensive Cancer
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Systemic Light Chain Amyloidosis Version 1.2018 September 6, 2017 NCCN.org Continue Version 1.2018, 09/06/17 National Comprehensive Cancer
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis Policy Number: Original Effective Date: MM.07.019 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section: Transplants
Titolo relazione. AMILOIDOSI AL L approccio terapeutico. Progetto Ematologia Romagna
 AMILOIDOSI AL L approccio terapeutico Elena Zamagni Istituto di Ematologia «Seragnoli» Università degli Studi di Bologna Treatment of AL amyloidosis n If the serum level of the amyloidogenic protein decreases,
AMILOIDOSI AL L approccio terapeutico Elena Zamagni Istituto di Ematologia «Seragnoli» Università degli Studi di Bologna Treatment of AL amyloidosis n If the serum level of the amyloidogenic protein decreases,
Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
 Original Article Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment Guangzhong Yang, Wenming Chen, Yin Wu Department
Original Article Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment Guangzhong Yang, Wenming Chen, Yin Wu Department
Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL
 New Evidence reports on presentations given at ASH 2009 Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL From ASH 2009: Non-Hodgkin
New Evidence reports on presentations given at ASH 2009 Strategies for the Treatment of Elderly DLBCL Patients, New Combination Therapy in NHL, and Maintenance Rituximab Therapy in FL From ASH 2009: Non-Hodgkin
Amyloidosis for Practicing Hematologists
 Amyloidosis for Practicing Hematologists Vishal Kukreti, MD Princess Margaret Cancer Centre October 2, 2015 Disclosures Honoraria Celgene, Janssen Ortho, Amgen, Lundbeck Objectives Case Approach subtyping,
Amyloidosis for Practicing Hematologists Vishal Kukreti, MD Princess Margaret Cancer Centre October 2, 2015 Disclosures Honoraria Celgene, Janssen Ortho, Amgen, Lundbeck Objectives Case Approach subtyping,
Role of consolidation therapy in Multiple Myeloma. Pieter Sonneveld. Erasmus MC Cancer Institute Rotterdam The Netherlands
 Role of consolidation therapy in Multiple Myeloma Pieter Sonneveld Erasmus MC Cancer Institute Rotterdam The Netherlands Disclosures Research support : Amgen, Celgene, Janssen, Karyopharm Advisory Boards/Honoraria:
Role of consolidation therapy in Multiple Myeloma Pieter Sonneveld Erasmus MC Cancer Institute Rotterdam The Netherlands Disclosures Research support : Amgen, Celgene, Janssen, Karyopharm Advisory Boards/Honoraria:
Center for Amyloidosis and Acute Phase Proteins, University College London Medical School, UK
 Use of plasma cell immunophenotype as prognostic markers in patients with systemic AL amyloidosis Sajitha Sachchithanantham 1*, Anna Baginska 1*, Dorota Rowczenio 1, Shameem A Mahmood 1, Rabya Sayed 1,
Use of plasma cell immunophenotype as prognostic markers in patients with systemic AL amyloidosis Sajitha Sachchithanantham 1*, Anna Baginska 1*, Dorota Rowczenio 1, Shameem A Mahmood 1, Rabya Sayed 1,
Phase 1 Study of ARRY-520 and Carfilzomib in Patients With Relapsed/Refractory Multiple Myeloma (RRMM)
 Phase 1 Study of ARRY-520 and Carfilzomib in Patients With Relapsed/Refractory Multiple Myeloma (RRMM) Jatin J Shah, MD, Sheeba Thomas, MD, Donna Weber, MD, Michael Wang, MD, Raymond Alexanian, MD, Robert
Phase 1 Study of ARRY-520 and Carfilzomib in Patients With Relapsed/Refractory Multiple Myeloma (RRMM) Jatin J Shah, MD, Sheeba Thomas, MD, Donna Weber, MD, Michael Wang, MD, Raymond Alexanian, MD, Robert
NEOD001 for amyloid light-chain (AL) amyloidosis
 NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
The impact of dialysis on the survival of patients with immunoglobulin light chain (AL) amyloidosis undergoing autologous stem cell transplantation
 Nephrol Dial Transplant (2016) 31: 1284 1289 doi: 10.1093/ndt/gfv328 Advance Access publication 30 November 2015 Original Article The impact of dialysis on the survival of patients with immunoglobulin
Nephrol Dial Transplant (2016) 31: 1284 1289 doi: 10.1093/ndt/gfv328 Advance Access publication 30 November 2015 Original Article The impact of dialysis on the survival of patients with immunoglobulin
Long-term ixazomib maintenance is tolerable and improves depth of response following ixazomiblenalidomide-dexamethasone
 Long-term ixazomib maintenance is tolerable and improves depth of response following ixazomiblenalidomide-dexamethasone induction in patients with previously untreated multiple myeloma (MM): Phase 2 study
Long-term ixazomib maintenance is tolerable and improves depth of response following ixazomiblenalidomide-dexamethasone induction in patients with previously untreated multiple myeloma (MM): Phase 2 study
Autologous Stem Cell Transplant in the Era of Bortezomib-Based Induction for AL Amyloidosis A Single Institution 11 Year Experience
 Citation: Scott E.C. et al. (2018) Autologous Stem Cell Transplant in the Era of Bortezomib-Based Induction. Science Publishing Group Journal 1(2). REVIEW ARTICLE Corresponding Author: Emma C. Scott, MD
Citation: Scott E.C. et al. (2018) Autologous Stem Cell Transplant in the Era of Bortezomib-Based Induction. Science Publishing Group Journal 1(2). REVIEW ARTICLE Corresponding Author: Emma C. Scott, MD
Introduction. Methods
 CLINICAL TRIALS AND OBSERVATIONS Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma
CLINICAL TRIALS AND OBSERVATIONS Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma
CREDIT DESIGNATION STATEMENT
 CME Information LEARNING OBJECTIVES Integrate emerging research information on the use of proteasome inhibitors and immunomodulatory agents to individualize induction treatment recommendations and maintenance
CME Information LEARNING OBJECTIVES Integrate emerging research information on the use of proteasome inhibitors and immunomodulatory agents to individualize induction treatment recommendations and maintenance
one patient advocacy group (Myeloma Canada) the submitter (Cancer Care Ontario Hematology Disease Site Group) the manufacturer (Janssen Inc.
 perc noted that the pcodr Economic Guidance Panel s estimates of the use of bortezomib in both pre- ASCT and post-asct settings when compared with standard induction therapy and observation-only maintenance
perc noted that the pcodr Economic Guidance Panel s estimates of the use of bortezomib in both pre- ASCT and post-asct settings when compared with standard induction therapy and observation-only maintenance
= Medical Policy. MP Hematopoietic Cell Transplantation for Primary Amyloidosis
 = Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
= Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
Novel Combination Therapies for Untreated Multiple Myeloma
 Novel Combination Therapies for Untreated Multiple Myeloma Andrzej J. Jakubowiak, MD, PhD Director, Myeloma Program New York, NY, October 27, 201 Disclosures 2 Employee Consultant Major Stockholder Speakers
Novel Combination Therapies for Untreated Multiple Myeloma Andrzej J. Jakubowiak, MD, PhD Director, Myeloma Program New York, NY, October 27, 201 Disclosures 2 Employee Consultant Major Stockholder Speakers
Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma
 Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma Kahl BS et al. Cancer 2010;116(1):106-14. Introduction > Bendamustine is a novel alkylating
Bendamustine is Effective Therapy in Patients with Rituximab-Refractory, Indolent B-Cell Non-Hodgkin Lymphoma Kahl BS et al. Cancer 2010;116(1):106-14. Introduction > Bendamustine is a novel alkylating
Daratumumab: Mechanism of Action
 Phase 3 Randomized Controlled Study of Daratumumab, Bortezomib and Dexamethasone (D) vs Bortezomib and Dexamethasone () in Patients with Relapsed or Refractory Multiple Myeloma (RRMM): CASTOR* Antonio
Phase 3 Randomized Controlled Study of Daratumumab, Bortezomib and Dexamethasone (D) vs Bortezomib and Dexamethasone () in Patients with Relapsed or Refractory Multiple Myeloma (RRMM): CASTOR* Antonio
Second Autologous Stem Cell Transplantation as Salvage Therapy for Multiple Myeloma: Impact on Progression-Free and Overall Survival
 Second Autologous Stem Cell Transplantation as Salvage Therapy for Multiple Myeloma: Impact on Progression-Free and Overall Survival Victor H. Jimenez-Zepeda, 1 Joseph Mikhael, 2 Andrew Winter, 1 Norman
Second Autologous Stem Cell Transplantation as Salvage Therapy for Multiple Myeloma: Impact on Progression-Free and Overall Survival Victor H. Jimenez-Zepeda, 1 Joseph Mikhael, 2 Andrew Winter, 1 Norman
Management of Multiple Myeloma: The Changing Paradigm
 Management of Multiple Myeloma: The Changing Paradigm High-Dose Chemotherapy and Stem Cell Transplantation Todd Zimmerman, MD University of Chicago Medical Center Case Presentation R.M. is a 64 year old
Management of Multiple Myeloma: The Changing Paradigm High-Dose Chemotherapy and Stem Cell Transplantation Todd Zimmerman, MD University of Chicago Medical Center Case Presentation R.M. is a 64 year old
Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma
 Multidisciplinary Cancer Investigation October 2017, Volume 1, Issue 4 DOI: 10.21859/mci-01041 Original Article Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma Hasan Jalaeikhoo
Multidisciplinary Cancer Investigation October 2017, Volume 1, Issue 4 DOI: 10.21859/mci-01041 Original Article Role of Suppressed Immunoglobulins in Outcome of Patients With Multiple Myeloma Hasan Jalaeikhoo
Michel Delforge Belgium. New treatment options for multiple myeloma
 Michel Delforge Belgium New treatment options for multiple myeloma Progress in the treatment of MM over the past 40 years 1962 Prednisone + melphalan 1990s Supportive care 1999 First report on thalidomide
Michel Delforge Belgium New treatment options for multiple myeloma Progress in the treatment of MM over the past 40 years 1962 Prednisone + melphalan 1990s Supportive care 1999 First report on thalidomide
Seok Jin Kim 1,, Kihyun Kim 1,, Young Rok Do 2, Sung Hwa Bae 3, Deok-Hwan Yang 4 and Je-Jung Lee 4,* INTRODUCTION
 Jpn J Clin Oncol 2011;41(3)353 357 doi:10.1093/jjco/hyq194 Advance Access Publication 14 October 2010 Low-dose Acyclovir is Effective for Prevention of Herpes Zoster in Myeloma Patients Treated with Bortezomib:
Jpn J Clin Oncol 2011;41(3)353 357 doi:10.1093/jjco/hyq194 Advance Access Publication 14 October 2010 Low-dose Acyclovir is Effective for Prevention of Herpes Zoster in Myeloma Patients Treated with Bortezomib:
AL AMYLOIDOSIS. SAMO November 2015 Lucerne.
 AL AMYLOIDOSIS SAMO November 2015 Lucerne bernhard.gerber@eoc.ch amyloidosis diagnosis pathophysiology prognosis therapy perspectives amyloidosis diagnosis pathophysiology prognosis therapy perspectives
AL AMYLOIDOSIS SAMO November 2015 Lucerne bernhard.gerber@eoc.ch amyloidosis diagnosis pathophysiology prognosis therapy perspectives amyloidosis diagnosis pathophysiology prognosis therapy perspectives
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Bortezomib as first line induction prior to melphalan and autologous stem cell transplantation (ASCT) in untreated symptomatic multiple myeloma patients suitable
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Bortezomib as first line induction prior to melphalan and autologous stem cell transplantation (ASCT) in untreated symptomatic multiple myeloma patients suitable
Epidemiology of AL amyloidosis: a real-world study using US claims data
 REGULAR ARTICLE Epidemiology of AL amyloidosis: a real-world study using US claims data Tiffany P. Quock, 1 Tingjian Yan, 2 Eunice Chang, 2 Spencer Guthrie, 3 and Michael S. Broder 2 1 Prothena Biosciences
REGULAR ARTICLE Epidemiology of AL amyloidosis: a real-world study using US claims data Tiffany P. Quock, 1 Tingjian Yan, 2 Eunice Chang, 2 Spencer Guthrie, 3 and Michael S. Broder 2 1 Prothena Biosciences
Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study
 Regular Article TRANSPLANTATION Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study Tilmann Bochtler, 1,2, * Ute Hegenbart, 1,2,
Regular Article TRANSPLANTATION Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study Tilmann Bochtler, 1,2, * Ute Hegenbart, 1,2,
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus
