Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit
|
|
|
- Samson Sparks
- 6 years ago
- Views:
Transcription
1 URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number: ISRC Type of Action: Product Removal March 9, 2017 Dear Business Partner: CareFusion a subsidiary of BD is voluntarily performing a Medical Device Recall to remove affected devices subject to the below described potential risk. DETAILS ON THE AFFECTED DEVICES: Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit This recall applies to the following: Attachment A - Affected Product Lot Code List Attachment B - Graphics on where to locate the Product Code; Lot Code DESCRIPTION OF THE PROBLEM: CareFusion a subsidiary of BD has identified a potential risk associated with the packaging integrity - open seal of the affected products. If this failure is not recognized prior to a procedure it has the potential to result in an inflammatory / infectious response locally and/or systemically. Six (6) customer complaints have been received to date in the time frame of 01- NOV-2014 to 31-DEC-2016 with the issue of sterile packaging integrity. In all 6 reports the failure was recognized on visual inspection prior to patient use. No cases have been reported of patient(s) experiencing a serious injury or illness.
2 ACTION TO BE TAKEN BY THE CUSTOMER / USER: Check your inventory for the affected devices and place them in quarantine Complete the Customer Response Form certifying that all affected product in your inventory has been placed in quarantine and mail/ a copy of the response form to: CareFusion France 309 S.A.S 8 bis rue de la Renaissance Châteaubriant France Beatrice.Lambert@bd.com Please promptly return the enclosed Customer Response Form to expedite the correction process and acknowledge receipt of this notification. Transmission of this Field Safety Notice This notice needs to be passed on all those who need to be aware within your organisation or to any organisation where the potentially affected devices have been transferred. Please transfer this notice to other organisations on which this action has an impact. ACTIONS TO BE TAKEN BY CareFusion a subsidiary of BD: At receipt of your customer response form, Carefusion will inform you about further action to be taken of your quarantined products and a credit will be applied to your account for the affected product after certification and documentation of the required actions. We appreciate your prompt return of the enclosed Customer Response Form to expedite the correction process and acknowledge receipt of this Notification. This Field Safety Notice has been notified to the appropriate Regulatory Agency.
3 We recognize the inconvenience this issue may cause your facility and thank you for your support in this important matter. Sincerely, Enclosure(s): Customer Response Form Affected Product Code List (Attachment A ) Graphics on where to locate the Product Code; Lot Code (Attachment B )
4 URGENT FIELD SAFETY NOTICE CUSTOMER RESPONSE FORM Acknowledgment and Verification Form Product Reference: 4330; SN1015X; SN1016X; SN1017X; SN7016X; TIN3015; TIN3018; TJC3513; TJC4008; TJC4011; TJC6008; TJC6011; TJM3513: TJM4008;TJM4011; TJM6008; TJM6011; TPK1001 Refer to attachment A for Product - Lot Code list FSCA #: ISRC Name of Hospital / Facility Distributor Hospital / Facility Distributor Address Name Telephone number address Signature Date I have read and understand the contents of this Field Safety Notice and confirm that our inventory has been checked, and we no longer have the affected product in our inventory I have read and understood the contents of this Field Safety Notice and confirm that our medical equipment inventory has been checked, and we have the following affected product in our inventory
5 Model Number Lot Number QTY Note: If more space is needed for the above table please attach a separate document. Wholesaler/Distributor only: I have identified and notified all customers that purchased any affected product. Check below which method of notification was used (Include date and method): Date of Notification: Mail ; ; Fax ; Phone
6 The following person shall be contacted to coordinate the action (please complete if different than above) Name Telephone Number Please return a copy of the Customer Response Form to: CareFusion France 309 S.A.S 8 bis rue de la Renaissance Châteaubriant France Beatrice.Lambert@bd.com Once the form has been returned to CFN we will be contacting you regarding the disposition on any affected products.
7 URGENT Field Safety Notice AFFECTED PRODUCT / LOT CODES ATTACHMENT A Product Code Lot Code Product Description MANOMETER TRAY STERILE 10/BX MANOMETER TRAY STERILE 10/BX MANOMETER TRAY STERILE 10/BX MANOMETER TRAY STERILE 10/BX MANOMETER TRAY STERILE 10/BX MANOMETER TRAY STERILE 10/BX SN1015X JAMSHIDI NEEDLE BIOPSY 15G X 100MM DISP SN1016X JAMSHIDI NEEDLE BIOPSY 16G X 100MM DISP SN1017X JAMSHIDI NEEDLE BIOPSY 17G X 100MM DISP SN7016X JAMSHIDI NEEDLE BIOPSY 16G X 70MM DISP TIN ILLINOIS (TJ) NEEDLE ASPIRATION
8 Product Code Lot Code Product Description TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION TIN ILLINOIS (TJ) NEEDLE ASPIRATION 18GA TIN ILLINOIS (TJ) NEEDLE ASPIRATION 18GA TIN ILLINOIS (TJ) NEEDLE ASPIRATION 18GA
9 Product Code Lot Code Product Description TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 13GX3.5 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 13GX3.5 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 13GX3.5 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X4
10 Product Code Lot Code Product Description TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 8G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X6 TJC JAMSHIDI (TJ) NEEDLE BONE MARROW 11G X6 TJM JAMSHIDI (TJ) NEEDLE BM 13G TJM JAMSHIDI (TJ) NEEDLE BM 13G TJM JAMSHIDI (TJ) NEEDLE BM 13G TJM JAMSHIDI (TJ) NEEDLE BM 13G TJM JAMSHIDI (TJ) NEEDLE BM 13G TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4
11 Product Code Lot Code Product Description TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4 TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4 TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4 TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4 TJM JAMSHIDI (TJ) NEEDLE BM 8G X 4 TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 11G
12 Product Code Lot Code Product Description TJM JAMSHIDI (TJ) NEEDLE BM 11G TJM JAMSHIDI (TJ) NEEDLE BM 8G X 6 TJM JAMSHIDI (TJ) NEEDLE BM 8G X 6 TJM JAMSHIDI (TJ) NEEDLE BM 11G X6 TJM JAMSHIDI (TJ) NEEDLE BM 11G X6 TJM JAMSHIDI (TJ) NEEDLE BM 11G X6 TPK THORACENTESIS/PARACENTESIS KIT 10/CS TPK THORACENTESIS/PARACENTESIS KIT 10/CS TPK THORACENTESIS/PARACENTESIS KIT 10/CS TPK THORACENTESIS/PARACENTESIS KIT 10/CS
13 URGENT: Medical Device Recall PACKAGING INTEGRITY OPEN SEAL Graphics on where to locate the Product Code / Lot Code ATTACHMENT B
14
15
16
17
URGENT FIELD SAFETY NOTICE
 AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL
 URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT: VOLUNTARY DRUG RECALL 07/05/2016
 9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
If you have any questions, please contact Jerry Webb at ext or
 1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
URGENT FIELD SAFETY NOTICE
 August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
Please find enclosed Dosimetry Service Licence No which replaces Dosimetry Service Licence No
 Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Urgent Medical Device Recall
 PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
URGENT MEDICAL DEVICE CORRECTION. Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps
 URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Urgent Notice September 16, 2014
 Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER
 URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV REV.8 Guideline Vigilance)
 FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
URGENT MEDICINE RECALL
 Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL
 January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
URGENT FIELD SAFETY NOTICE FSCA3193. Etest Ceftriaxone TXL32 (Ref , ) SPB Packaging
 16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193. ETEST Ceftriaxone TXL32 (Ref , ) FOAM packaging
 16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
NovoPen Echo URGENT MEDICAL DEVICE RECALL
 NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
URGENT: FIELD SAFETY NOTICE MSS A-FA / MSS B-FA. Medical Device Safety Advisory Notice
 URGENT: FIELD SAFETY NOTICE MSS-16-837A-FA / MSS-16-837B-FA Medical Device Safety Advisory Notice Date: Friday 13th January BD Eclipse Injection Needle, BD Eclipse Injection Needle with BD Luer-Lok Syringe
URGENT: FIELD SAFETY NOTICE MSS-16-837A-FA / MSS-16-837B-FA Medical Device Safety Advisory Notice Date: Friday 13th January BD Eclipse Injection Needle, BD Eclipse Injection Needle with BD Luer-Lok Syringe
Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS
 Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Limited, Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due
Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Limited, Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due
URGENT: DIETARY SUPPLEMENT RECALL
 URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
URGENT FIELD SAFETY NOTICE - EXPANSION
 URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA Telephone: 510/
 Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP)
 May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
AKA Good Manufacturing Practice (GMP) Certification Program
 AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
AKA Good Manufacturing Practice (GMP) Certification Program Preamble The American Kratom Association (AKA) is establishing this program to assure the safety and integrity of kratom dietary supplements
Urgent Field Corrective Action
 Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
Important Information about your 2.0 ml Animas Insulin Pump Cartridges
 URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Urgent Patient Notification
 Urgent Patient Notification Comfort, Comfort Short, and Contact Detach Infusion Sets - Tubing May Detach From the Connect/Disconnect Location November 24, 2014 Dear Valued Customer: At Animas, we hold
Urgent Patient Notification Comfort, Comfort Short, and Contact Detach Infusion Sets - Tubing May Detach From the Connect/Disconnect Location November 24, 2014 Dear Valued Customer: At Animas, we hold
Contacts Consumers:
 Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
Responding to a Food Recall
 Responding to a Food Recall Time: 4 hours National Food Service Management Institute The University of Mississippi 2013 Table of Contents Objectives... ii Lesson 1: Introduction to the Food Recall Process...
Responding to a Food Recall Time: 4 hours National Food Service Management Institute The University of Mississippi 2013 Table of Contents Objectives... ii Lesson 1: Introduction to the Food Recall Process...
PRODUCT RECALL NOTICE URGENT
 PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
PATIENTS (DEAF, HEARING IMPAIRED, BLIND, HANDICAPPED, LIMITED / NON- ENGLISH SPEAKING, LANGUAGE OR COMMUNICATION BARRIERS)
 Hospital-Wide Manual Department-Specific Manual POLICY & PROCEDURE: Policy # Manual Patient Care Services Category 01 Admitting Procedures INTERPRETERS / SERVICES FOR PATIENTS (DEAF, HEARING IMPAIRED,
Hospital-Wide Manual Department-Specific Manual POLICY & PROCEDURE: Policy # Manual Patient Care Services Category 01 Admitting Procedures INTERPRETERS / SERVICES FOR PATIENTS (DEAF, HEARING IMPAIRED,
Product NDC Code Lot Number Expiry Dates Distribution Date VALSARTAN TABLETS 40MG 30CT
 Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodimethylamine (NDMA) in the Products
Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodimethylamine (NDMA) in the Products
Urgent Field Safety Notice
 ADVIA Centaur Systems Dimension Vista Systems IMMULITE Systems Urgent Field Safety Notice CC 17-06.A.OUS Elevated Results in Patient s Due to Cross of with Our records indicate that your facility may have
ADVIA Centaur Systems Dimension Vista Systems IMMULITE Systems Urgent Field Safety Notice CC 17-06.A.OUS Elevated Results in Patient s Due to Cross of with Our records indicate that your facility may have
Carrie Havens. Dear Candidate,
 Public Safety Training Center Police Agility Program WESTERN CAMPUS 11000 W. PLEASANT VALLEY ROAD PSTC BLDG., STE. 221 PARMA, OH 44130 PHONE: 2169873033 Dear Candidate, Congratulations on your choice of
Public Safety Training Center Police Agility Program WESTERN CAMPUS 11000 W. PLEASANT VALLEY ROAD PSTC BLDG., STE. 221 PARMA, OH 44130 PHONE: 2169873033 Dear Candidate, Congratulations on your choice of
2010 Sharing Hope Program for men
 2010 Sharing Hope Program for men Criteria and Application Made possible by participating sperm banks and fertility centers Program Overview Goal Cancer patients have little opportunity to save for the
2010 Sharing Hope Program for men Criteria and Application Made possible by participating sperm banks and fertility centers Program Overview Goal Cancer patients have little opportunity to save for the
Infection Prevention & Control Resources for York Region Long-Term Care Homes
 Infection Prevention & Control Resources for York Region Long-Term Care Homes September 2017 CONTENTS INTRODUCTION 1 PUBLIC HEALTH INSPECTIONS 1 POLICY REVIEW 1 OUTBREAK MANAGEMENT 2 EDUCATION AND TRAINING
Infection Prevention & Control Resources for York Region Long-Term Care Homes September 2017 CONTENTS INTRODUCTION 1 PUBLIC HEALTH INSPECTIONS 1 POLICY REVIEW 1 OUTBREAK MANAGEMENT 2 EDUCATION AND TRAINING
ISO 13485:2016 MEDICAL DEVICES QMS TRANSITION GUIDE
 ISO 13485:2016 MEDICAL DEVICES QMS TRANSITION GUIDE ISO 13485, OVERVIEW ISO 13485 sets regulatory requirements or, when specified, customer requirements for a management system for medical devices or services.
ISO 13485:2016 MEDICAL DEVICES QMS TRANSITION GUIDE ISO 13485, OVERVIEW ISO 13485 sets regulatory requirements or, when specified, customer requirements for a management system for medical devices or services.
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17
 TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
OPERATOR MEMBERSHIP APPLICATION
 NORTH CAROLINA MOTORCOACH ASSOCIATION Mailing Address: 106 Main Street, Brookneal, VA 24528 P.O. Box 31, Randleman, NC 27317 336-495-4970 FAX 336-495-5833 OPERATOR MEMBERSHIP APPLICATION Name of Company:
NORTH CAROLINA MOTORCOACH ASSOCIATION Mailing Address: 106 Main Street, Brookneal, VA 24528 P.O. Box 31, Randleman, NC 27317 336-495-4970 FAX 336-495-5833 OPERATOR MEMBERSHIP APPLICATION Name of Company:
The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues
 The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues On December 7, 2015, while the CBI was conducting standard quality checks, an unexplained variability of results in
The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues On December 7, 2015, while the CBI was conducting standard quality checks, an unexplained variability of results in
URGENT FIELD SAFETY NOTICE IMMEDIATE ACTION REQUIRED
 Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Organisational reporting of completion fees apply
 Self reporting of completion free service Proof of completion of all courses is provided to all learners in the form of a printable and emailable Certificate of Completion. Learners can use these certificates
Self reporting of completion free service Proof of completion of all courses is provided to all learners in the form of a printable and emailable Certificate of Completion. Learners can use these certificates
Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA.
 Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA (Laser Notice 51) Document issued on: May 27, 2001 U.S. Department of Health
Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA (Laser Notice 51) Document issued on: May 27, 2001 U.S. Department of Health
This license is required for any businesses offering tobacco products for sale.
 Guidelines for City of Moorhead 500 Center Avenue, PO Box 779 Moorhead, MN 56560-0799 Phone: 218.299.5304 Fax: 218.299.5306 cityclerk@ci.moorhead.mn.us Moorhead City Code, 2-5A OVERVIEW This license is
Guidelines for City of Moorhead 500 Center Avenue, PO Box 779 Moorhead, MN 56560-0799 Phone: 218.299.5304 Fax: 218.299.5306 cityclerk@ci.moorhead.mn.us Moorhead City Code, 2-5A OVERVIEW This license is
MC IRB Protocol No.:
 APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
URGENT: Important Safety Information
 October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
PURCHASING MEMORANDUM
 NUMBER: CL-602 DATE: March 3, 2005 Pricing Clarification MMCAP has received several calls concerning the prices invoiced for vaccine received during the current flu season. All orders filled by Aventis
NUMBER: CL-602 DATE: March 3, 2005 Pricing Clarification MMCAP has received several calls concerning the prices invoiced for vaccine received during the current flu season. All orders filled by Aventis
A13. MISCELLANEOUS SERVICE ARRANGEMENTS
 GENERAL SUBSCRIBER SERVICE TARIFF Eleventh Revised Page 1 TELECOMMUNICATIONS, INC. Cancels Tenth Revised Page 1 CONTENTS A13.1 through A13.79 (DELETED) 1 A13.80 711 Dialing Code for Telephone Relay Service
GENERAL SUBSCRIBER SERVICE TARIFF Eleventh Revised Page 1 TELECOMMUNICATIONS, INC. Cancels Tenth Revised Page 1 CONTENTS A13.1 through A13.79 (DELETED) 1 A13.80 711 Dialing Code for Telephone Relay Service
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies
 ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
CRYOPRESERVATION OF SEMEN FROM TESTICULAR TISSUE
 INFERTILITY & IVF MEDICAL ASSOCIATES OF WESTERN NEW YORK CRYOPRESERVATION OF SEMEN FROM TESTICULAR TISSUE BUFFALOIVF.COM When you have scheduled your appointment with Dr Crickard or Dr Sullivan to sign
INFERTILITY & IVF MEDICAL ASSOCIATES OF WESTERN NEW YORK CRYOPRESERVATION OF SEMEN FROM TESTICULAR TISSUE BUFFALOIVF.COM When you have scheduled your appointment with Dr Crickard or Dr Sullivan to sign
Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively.
 a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
URGENT: Important Safety Information
 July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
2013 AUXILIARY AIDS AND SERVICE PLAN FOR PERSONS WITH DISABILITIES AND PERSONS WITH LIMITED ENGLISH PROFICIENCY
 Legal Aid Society of Palm Beach County, Inc. Domestic Violence Project 423 Fern Street, Ste. 200 West Palm Beach, FL 33401 (561) 655-8944 ext. 238 Toll-free: (800) 403-9353, ext. 238 Fax: (561) 655-5269
Legal Aid Society of Palm Beach County, Inc. Domestic Violence Project 423 Fern Street, Ste. 200 West Palm Beach, FL 33401 (561) 655-8944 ext. 238 Toll-free: (800) 403-9353, ext. 238 Fax: (561) 655-5269
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 9 Inspections, Compliance, Enforcement, and Criminal Investigations Phoenix Medical Devices, LLC 9/29/2009 Department of Health and Human Services Public Health Service Food and Drug Administration
Page 1 of 9 Inspections, Compliance, Enforcement, and Criminal Investigations Phoenix Medical Devices, LLC 9/29/2009 Department of Health and Human Services Public Health Service Food and Drug Administration
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
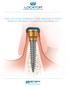 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
Criteria and Application for Men
 Criteria and Application for Men Return completed form via fax or email to LIVESTRONG Foundation attn LIVESTRONG Fertility Fax 512.309.5515 email Cancer.Navigation@LIVESTRONG.org Made possible by participating
Criteria and Application for Men Return completed form via fax or email to LIVESTRONG Foundation attn LIVESTRONG Fertility Fax 512.309.5515 email Cancer.Navigation@LIVESTRONG.org Made possible by participating
Permit to Import Quarantine Material
 Quarantine Act 1908 Section 13(2AA) Permit to Import Quarantine Material Phone: 02 6272 4578 Fax: 02 6249 1798 File Ref: Permit: IP14011730 Valid From: 9 Jul 2014 Valid To: 9 Jul 2016 Page 1 of 7 Importer
Quarantine Act 1908 Section 13(2AA) Permit to Import Quarantine Material Phone: 02 6272 4578 Fax: 02 6249 1798 File Ref: Permit: IP14011730 Valid From: 9 Jul 2014 Valid To: 9 Jul 2016 Page 1 of 7 Importer
Lopez Gonzalez Santana Corporation dba Domel and dba Dermixx 8/28/17
 Lopez Gonzalez Santana Corporation dba Domel and dba Dermixx 8/28/17 Office of Human and Animal Food Operations East Division IV Compliance Branch 466 Avenida Fernández Juncos San Juan, Puerto Rico 00901-3223
Lopez Gonzalez Santana Corporation dba Domel and dba Dermixx 8/28/17 Office of Human and Animal Food Operations East Division IV Compliance Branch 466 Avenida Fernández Juncos San Juan, Puerto Rico 00901-3223
New York State Department of Health. Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine
 New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
Part I is supported by a letter from a medical professional attesting to the presence of the condition.
 Chronically Homeless Qualification Checklist Instructions: This suggested checklist may be used as a guide for staff of a program serving chronically homeless persons to assure that participants meet program
Chronically Homeless Qualification Checklist Instructions: This suggested checklist may be used as a guide for staff of a program serving chronically homeless persons to assure that participants meet program
OKLAHOMA STATE UNIVERSITY X-RAY MACHINE PROGRAM AND PI HANDBOOK For easy navigation, please use Adobe s bookmark feature.
 OKLAHOMA STATE UNIVERSITY X-RAY MACHINE PROGRAM AND PI HANDBOOK For easy navigation, please use Adobe s bookmark feature. Table of Contents Overview... 2 Section 1: X-ray Program Oversight... 2 1.a: Institutional
OKLAHOMA STATE UNIVERSITY X-RAY MACHINE PROGRAM AND PI HANDBOOK For easy navigation, please use Adobe s bookmark feature. Table of Contents Overview... 2 Section 1: X-ray Program Oversight... 2 1.a: Institutional
Canadian Grain Commission
 Canadian Grain Commission Licensing feed mills Discussion paper February 9, 2015 Ce document est aussi disponible en français. 1 About the Canadian Grain Commission The Canadian Grain Commission is a federal
Canadian Grain Commission Licensing feed mills Discussion paper February 9, 2015 Ce document est aussi disponible en français. 1 About the Canadian Grain Commission The Canadian Grain Commission is a federal
HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3
 URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
Recruited by: Information for Payment by Credit Card
 NORTH CAROLINA MOTORCOACH ASSOCIATION Mailing Address: 106 Main Street, Brookneal, VA 24528 P.O. Box 31, Randleman, NC 27317 336-495-4970 FAX 336-495-5833 APPLICATION FOR TOUR OPERATOR MEMBERSHIP Please
NORTH CAROLINA MOTORCOACH ASSOCIATION Mailing Address: 106 Main Street, Brookneal, VA 24528 P.O. Box 31, Randleman, NC 27317 336-495-4970 FAX 336-495-5833 APPLICATION FOR TOUR OPERATOR MEMBERSHIP Please
Important Safety Notice!!! March 2018 Dräger Jaundice Meter JM-103 Out of Range Indication
 To all customers of the JM-103 Jaundice Meters Important Safety Notice!!! March 2018 Dräger Jaundice Meter JM-103 Out of Range Indication Dear Ladies and Gentlemen, Our continuous post market surveillance
To all customers of the JM-103 Jaundice Meters Important Safety Notice!!! March 2018 Dräger Jaundice Meter JM-103 Out of Range Indication Dear Ladies and Gentlemen, Our continuous post market surveillance
Initial Clinical History and Physical Form
 601 E FM 544, Suite 400, Murphy, TX, 75094 TEL: 972-442-4700 Initial Clinical History and Physical Form Patient Information Name: Age: of Birth: / / Sex: Male / Female Marital Status: Single Married Divorced
601 E FM 544, Suite 400, Murphy, TX, 75094 TEL: 972-442-4700 Initial Clinical History and Physical Form Patient Information Name: Age: of Birth: / / Sex: Male / Female Marital Status: Single Married Divorced
ARAPAHOE COMMUNITY COLLEGE PHYSICAL THERAPIST ASSISTANT PROGRAM 2018 Application for Admission
 ARAPAHOE COMMUNITY COLLEGE PHYSICAL THERAPIST ASSISTANT PROGRAM 2018 Application for Admission Please make sure all written information is legible. The PTA program is not responsible for mailing errors
ARAPAHOE COMMUNITY COLLEGE PHYSICAL THERAPIST ASSISTANT PROGRAM 2018 Application for Admission Please make sure all written information is legible. The PTA program is not responsible for mailing errors
Blood borne Pathogen Exposure Policy for Students
 Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
PATIENT CARE PROGRAM
 PATIENT CARE PROGRAM OVERVIEW Does someone in your community need cataract surgery but not have the means to pay for it? Do you know of a deaf person that hasn t been able to use the telephone because
PATIENT CARE PROGRAM OVERVIEW Does someone in your community need cataract surgery but not have the means to pay for it? Do you know of a deaf person that hasn t been able to use the telephone because
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION. (Moscow, 23 December 2014)
 AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
XOSERVE LIMITED SERVICES SCHEDULE FOR THE PROVISION OF NON-CODE USER PAYS SERVICES (REFERENCE NUMBER XNCUP(SS)06) DATED 20 INTRODUCTION
 XOSERVE LIMITED SERVICES SCHEDULE FOR THE PROVISION OF NON-CODE USER PAYS SERVICES (REFERENCE NUMBER XNCUP(SS)06) DATED 20 INTRODUCTION 1 This services schedule forms part of the framework contract for
XOSERVE LIMITED SERVICES SCHEDULE FOR THE PROVISION OF NON-CODE USER PAYS SERVICES (REFERENCE NUMBER XNCUP(SS)06) DATED 20 INTRODUCTION 1 This services schedule forms part of the framework contract for
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Approval Signature: Original signed by B. Postl. June 2007
 POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
POLICY REGIONAL Applicable to all WRHA governed sites and facilities (including hospitals and personal care homes), and all funded hospitals and personal care homes. All other funded entities are excluded
Quality Assurance Standard. Implemented 1991 Revised Version 3.0
 Implemented 1991 Revised 2006 Version 3.0 IQPP Contents I. Introduction... 3 II. Definitions... 4 III. PPTA Source Quality Assurance Principles... 4 IV. Audits and Compliance Verification... 5 Page 2 IQPP
Implemented 1991 Revised 2006 Version 3.0 IQPP Contents I. Introduction... 3 II. Definitions... 4 III. PPTA Source Quality Assurance Principles... 4 IV. Audits and Compliance Verification... 5 Page 2 IQPP
Pro Active Physical Therapy & Sports Medicine
 Pro Active Physical Therapy & Sports Medicine Consent and Statement of Financial Responsibility 1. CONSENT FOR TREATMENT: I consent to and authorize my physical therapist, occupational therapist and other
Pro Active Physical Therapy & Sports Medicine Consent and Statement of Financial Responsibility 1. CONSENT FOR TREATMENT: I consent to and authorize my physical therapist, occupational therapist and other
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
WHO Prequalification of In Vitro Diagnostics Programme
 WHO Prequalification of In Vitro Diagnostics Programme International HIV/Viral Hepatitis Co-Infection Satellite Meeting 19 July 2014, Melbourne Anita Sands Prequalification Diagnostics Team Department
WHO Prequalification of In Vitro Diagnostics Programme International HIV/Viral Hepatitis Co-Infection Satellite Meeting 19 July 2014, Melbourne Anita Sands Prequalification Diagnostics Team Department
Information Regarding Immunizations
 Information Regarding Immunizations Dear Staff Member / Volunteer The state of Massachusetts require our staff members and volunteers aged 17 and under to have and provide evidence of the following immunizations:
Information Regarding Immunizations Dear Staff Member / Volunteer The state of Massachusetts require our staff members and volunteers aged 17 and under to have and provide evidence of the following immunizations:
Noninvasive Glucose Monitors to 2022
 Noninvasive Glucose Monitors to 2022 Devices, Technologies, Players and Prospects Report Prospectus Greystone Research associates N o n i n v a s i v e G l u c o s e M o n i t o r s t o 2 0 2 2 Greystone
Noninvasive Glucose Monitors to 2022 Devices, Technologies, Players and Prospects Report Prospectus Greystone Research associates N o n i n v a s i v e G l u c o s e M o n i t o r s t o 2 0 2 2 Greystone
Quality Management System Certification. Understanding Quality Management System (QMS) certification
 Quality Management System Certification Understanding Quality Management System (QMS) certification The medical device manufacturing sector is one of the most regulated sectors in which significant quality
Quality Management System Certification Understanding Quality Management System (QMS) certification The medical device manufacturing sector is one of the most regulated sectors in which significant quality
Hazard Communication Program. Santa Clara University (SCU) 500 El Camino Real Santa Clara, CA 95053
 Hazard Communication Program Santa Clara University (SCU) 500 El Camino Real Santa Clara, CA 95053 February 2018 Program Review Record Revision 1 - January 2011 Name Title Department Jeff Charles Director
Hazard Communication Program Santa Clara University (SCU) 500 El Camino Real Santa Clara, CA 95053 February 2018 Program Review Record Revision 1 - January 2011 Name Title Department Jeff Charles Director
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS. Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails
 STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
URGENT: MEDICAL DEVICE CORRECTION
 December 5, 2014 Dear Healthcare Professional, URGENT: MEDICAL DEVICE CORRECTION Alere INRatio PT/INR Monitor system This letter contains important information co ncerning the Alere IN Ratio PT/I NR Monitor
December 5, 2014 Dear Healthcare Professional, URGENT: MEDICAL DEVICE CORRECTION Alere INRatio PT/INR Monitor system This letter contains important information co ncerning the Alere IN Ratio PT/I NR Monitor
VITAL SIGNS EMS CONFERENCE 2017 EXHIBITOR APPLICATION FORM
 VITAL SIGNS EMS CONFERENCE 2017 EXHIBITOR APPLICATION FORM Company Name: Company Address: City: State: Zip Code: Contact Person: Telephone Number: Fax Number: Tax ID #: E-Mail Address: We will issue exhibitor
VITAL SIGNS EMS CONFERENCE 2017 EXHIBITOR APPLICATION FORM Company Name: Company Address: City: State: Zip Code: Contact Person: Telephone Number: Fax Number: Tax ID #: E-Mail Address: We will issue exhibitor
ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS
 c t ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to December
c t ANIMAL HEALTH AND PROTECTION ACT SWINE IMPORTATION REGULATIONS PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this regulation, current to December
STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION
 STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION STATE OF NEW JERSEY Division of Gaming Enforcement MERCHANDISING PERMIT APPLICATION FOR
STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION STATE OF NEW JERSEY Division of Gaming Enforcement MERCHANDISING PERMIT APPLICATION FOR
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
CTO Establishment Inspections. an Atlantic update - Tissues
 Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
The Kansas State Deaf-Blind Fund: Frequently Asked Questions and Answers
 The Kansas State Deaf-Blind Fund: Frequently Asked Questions and Answers Early Childhood, Special Education, and Title Services An Equal Employment/Educational Opportunity Agency The Kansas State Department
The Kansas State Deaf-Blind Fund: Frequently Asked Questions and Answers Early Childhood, Special Education, and Title Services An Equal Employment/Educational Opportunity Agency The Kansas State Department
Lake Charles Transit System (LCTS) Application for Para-Transit Service Program
 Lake Charles Transit System (LCTS) Application for Para-Transit Service Program Only original applications are accepted. No faxed copies allowed. If you have physical difficulties that prevent you from
Lake Charles Transit System (LCTS) Application for Para-Transit Service Program Only original applications are accepted. No faxed copies allowed. If you have physical difficulties that prevent you from
OHRP Guidance on the Involvement of Prisoners in Research
 NOTE: THIS GUIDANCE REPLACES THE FOLLOWING OHRP GUIDANCE: "OHRP Guidance on Approving Research Involving Prisoners" (May 19, 2000) found on the OHRP website at: http://ohrp.osophs.dhhs.gov/humansubjects/guidance/prison.htm
NOTE: THIS GUIDANCE REPLACES THE FOLLOWING OHRP GUIDANCE: "OHRP Guidance on Approving Research Involving Prisoners" (May 19, 2000) found on the OHRP website at: http://ohrp.osophs.dhhs.gov/humansubjects/guidance/prison.htm
Dear Prospective Volunteer:
 Dear Prospective Volunteer: Thank you for your interest in becoming an Aria Health volunteer. An exciting experience and special commitment may be about to begin. Volunteerism at Aria Health Hospitals
Dear Prospective Volunteer: Thank you for your interest in becoming an Aria Health volunteer. An exciting experience and special commitment may be about to begin. Volunteerism at Aria Health Hospitals
