Sample Research Protocol. The pages that follow contain a sample research protocol, including and informed consent document and other appendices.
|
|
|
- Noah Matthews
- 6 years ago
- Views:
Transcription
1 Sample Research Protocol The pages that follow contain a sample research protocol, including and informed consent document and other appendices. This document is provided as guidance to investigators for the preparation of protocols and consent documents. It cannot be slavishly followed as all protocols are different and not all possible variations can be captured in a single sample. Questions may be addressed to: Irv Freeman, Ph.D., J.D. Chair of the LECOM Institutional Review Board Phone: (724) , or ifreeman@lecom.edu.
2 Request for Approval of Human Subjects Research Title: Demographic and Other Factors Affecting Incidence of Singultus Among the Hot-and-Sour Soup Eating Population Principal Investigator: G. I. Specialist, D.O., MPH Clinical Professor of Internal Medicine Lake Erie College of Osteopathic Medicine Dr. Specialist is board certified in internal medicine with subspecialty certification in gastroenterology by both the American Osteopathic Board of Internal Medicine and the American Board of Internal Medicine and has five years of practice experience since completion of her fellowship. Via her MPH program, she has substantial training in research design and statistical analysis. She is the sole author or a co-author of four peer-reviewed papers and of at least seventeen published abstracts or poster presentations and has served as Principal Investigator of three prior studies involving human subjects. She most recently completed CITI human subjects protection training for Principal Investigators within the past month (documentation attached). Research Question: The primary question of this research is to establish the incidence of singultus following the ingestion of Hot and Sour Soup by regular consumers of Chinese Food (as prepared in Chinese restaurants in the United States). An additional research question is whether there are differences in the incidence based on demographic factors (i.e. age, race, gender) and/or comorbidity factors. Brief Review of the Literature: Since the publication in 1898 of E. E. Kellogg s monumental and pathbreaking Science in the Kitchen: A Scientific Treatise on Food Substances and Their Dietetic Properties, Together with Practical Explanation of the Principles of Healthful Cookery, and a Large Number of Original Palatable, and Wholesome Recipes, there is a huge literature concerning the impact of various foods on overall health and on specific disease states. While there is general information published on the nutritional characteristics of Hot and Sour Soup (see, for example, Jessica Bruso, Nutritional Facts for Hot & Sour Soup, at nutritional-facts-for-hot-sour-soup/) and on its potential health benefits (see, for example, M. Eileen Brown, Soupalooza: Hot and Sour Soup Cures What Ails You., at there does not
3 appear to be any published prior research on the anecdotally observed phenomenon of hiccoughs following consumption of hot and sour soup. This study will fill that gap in the scientific literature. Methodology: Subject Recruitment. Subjects will be recruited from among the customers who order Hot and Sour Soup at five cooperating Chinese restaurants (of the Chinese Buffet variety). The restaurants will be provided with copies of the recruitment leaflet (copy attached) and will be asked to provide a copy of the leaflet to each customer who orders Hot and Sour Soup. The restaurant staff s role will be solely to distribute the leaflets, they will not answer questions about the research or participate in the informed consent process. Prospective subjects will be asked to contact the investigator by telephone. They will be provided with a brief verbal overview of the study and, if interested will then be verbally screened for exclusion factors (known dietary allergies to Hot and Sour Soup ingredients, diabetes, current treatment for GERD, history of gastric ulcers, status as a minor). No written record will be made or maintained of the screening. If eligible, the prospective subject will be sent a copy of the informed consent document (copy attached). The PI will then schedule a telephone call for after the subject has had an opportunity to read the consent form and will answer any questions at that time. Then, if the prospective subject agrees to participate, he or she will be asked to sign the consent form and either to send it to the investigator or to bring it to the first experimental session. Experimental Sessions. Experimental sessions will occur on five consecutive Wednesday evenings, one at each of the five cooperating Chinese restaurants. Subjects will each be provided with a pint of that restaurant s Hot and Sour Soup and asked to consume the entire portion in one sitting. The subjects will be under observation during consumption and for a period of fifteen minutes post-consumption. Any episodes of hiccoughs during the period of observation will be noted. Subjects who are still hiccoughing at the end of the initial observation period will be requested to remain for additional observation. Following the observation, subjects will be offered a complimentary meal at the buffet. If subjects are still hiccoughing at the restaurants closing time, the PI will contact them by phone twice on each of the next two days (or until the hiccoughing resolves). If hiccoughing does not resolve within 48 hours (the common criterion for persistent singultus ), the subject will be referred for medical treatment and will be removed from the study. Other Data Collection. At the first experimental session, subjects will complete a questionnaire collecting demographic data and data on their prior medical history. The form utilized will be a standard, commercially available form
4 used in primary care physicians offices. Prior to the first intervention (i.e. the first bowl of Hot and Sour Soup) the history form will be reviewed by the PI for exclusion criteria and additional subjects may be excluded at this time (subjects excluded at the restaurant will be provided with the complimentary buffet). Subjects will also be asked for the contact information of their primary care physicians, if any. With appropriate HIPAA authorization embedded in the Consent Form, the PCPs will be asked to disclose any prior incidents of singultus. Risks. Potential physical risks of participating in the study include transient burning sensation in the lips, mouth, and esophagus from the potentially spicy nature of the soup, aggravation of pre-existing G.I. conditions, allergic reaction, weight gain, elevation of blood glucose. Procedures to minimize risk include the screening for exclusion criteria and the exclusion of prospective subjects reporting those criteria; the presence of the PI, a board-certified internist, at all experimental sessions; and the up to two-day follow-up as described above. In addition, any subjects who are observed to have an adverse reaction or who later report an adverse reaction will be discharged from the study before participating in additional experimental sessions. There is also a potential risk of breach of confidentiality. To minimize this risk, each subject will be assigned a sequential identifying number. This number, rather than the name, will be placed onto the data collection form at each experimental session and onto the questionnaire. The subject will be provided a card with the number and will be asked to bring the card to each session. The PI will maintain a separate list linking identities to names, but this list will be stored in a separate location from other study data and will remain in a locked cabinet except when in use. The linking list will be destroyed when data collection is complete. If PHI is obtained from the subject s PCP, response (i.e. either Yes or No on prior incidents of singultus) will be recorded onto the subject s questionnaire and the report received from the PCP will be destroyed. Benefits. No direct benefits to the particular subjects are anticipated. The benefit to society is the contribution to knowledge that will be obtained by either documenting or debunking the anecdotal association between Hot and Sour Soup and hiccoughs and, if an association is found, by the subsequent analysis of the demographic differences or differences based on medical history. Analysis. The overall incidence of hiccoughs and the incidence at each of the participating restaurants will be calculated. Demographic factors and medical history factors will be tested statistically to determine if the incidence varies based on those factors. Consent Process. The consent process was described above in the subject recruitment section. The investigator also requests that the IRB approve partial
5 waiver of the informed consent requirement, specifically that the element of informed consent that involves disclosing the purpose of the research be partially waived such that the consent process describes the purpose as to determine the impact of hot and sour soup on physiological processes in general, rather than specifically identifying hiccoughing. It is believed that knowledge on the subjects part of the focus on hiccoughing could influence the occurrence of that outcome.. The criteria for approval of the partial waiver are addressed below: 1. The research involves no more than minimal risk to the subjects. According to the Federal government, A risk is minimal where the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests. The risk-producing intervention of this study is the consumption of hot and sour soup. The subjects are limited to individuals who have been observed ordering hot and sour soup in their ordinary daily lives. Thus, the risk is exactly the same as the risk that they ordinarily assume themselves when not involved in research. 2. The waiver will not adversely affect the rights and welfare of the subjects. The subjects will still receive a complete explanation of the experimental intervention and of all other data collection. The subjects will also be generally aware that the purpose is to assess any physiological effects of consuming the soup. They will simply not know the specific effect that is of interest to the investigator. 3. The research could not practicably be carried out without the waiver or alternation. Psychological factors such as excitement and emotional stress have been cited as a cause of hiccoughs (see, for example, To control for the impact of psychological factors, it is requested that subjects be blinded to the specific interest in hiccoughs lest hiccoughs induced by the stress of desire-to-please not confound the results. 4. Whenever appropriate, the subjects will be provided with additional pertinent information after participation. Following the fifth experimental session (or earlier if a subject withdraws or is discharged), the subject will be provided with a written explanation (copy attached) of the true purpose of the study and with an explanation of the reason for the deception.
6 Appendix I Recruitment Brochure Hot & Sour Soup Research Study Dr. G.I. Specialist is conducting research on the physiological impact of eating Hot and Sour Soup. Participants will provide information about themselves, will eat five large bowls of Hot and Sour Soup (one each on five different days), and will be observed for at least fifteen minutes after each bowl. Participants will receive free buffet dinner each time. If interested, call Dr. Specialist at
7 Appendix II Informed Consent Document Consent to Participate as a Subject in a Research Study Physiologic Effects of Eating Hot & Sour Soup Introduction You are being invited to participate in a research study. The purpose of the research is to determine whether eating hot & sour soup has any immediate short-term effects on the human body that may be observed immediately following eating the soup. You are being invited to participate because you were observed ordering hot & sour soup in one of the participating restaurants. It is estimated that approximately 50 people will participate in the research. Voluntary Participation Whether or not you wish to participate is entirely up to you. Participation is voluntary and refusal to participate will not involve any penalty or loss of benefits to which you are otherwise entitled. Similarly, if you agree to participate, you may withdraw or discontinue your participation at any time without penalty or loss of benefits to which you are otherwise entitled. What Your Participation Will Involve If you agree to participate in this study, you will participate in five experimental sessions. The sessions will occur on five consecutive Wednesday evenings, one at each of five cooperating restaurants. So, the overall duration of your participation will be five weeks. At the first session, you will be asked to complete a brief form providing demographic information (i.e. gender, race, age) and medical history information. The form will be similar to medical history forms that you have probably completed at your doctor s office. The Researcher anticipates that completion of the form will take most participants 20 minutes or less. After the completion of the form at the first visit, and immediately upon arrival at the other four visits, you will be seated at a restaurant table and served a very large bowl of Hot & Sour soup. You will be asked to eat the entire bowl, or as much of it as you can eat if you do not care to eat the whole bowl. After you have stopped eating the Hot & Sour soup, you will remain at your table for fifteen minutes so that the Researcher may observe any effects that it has on you. If the Researcher observes any effect, she might ask you to stay for additional observation, but you are free to leave if you wish. It is estimated that participants will be able eat the soup in under 15 minutes and so, with the normal period of observation, will spend a total of approximately 30 minutes at each of second through fifth visits and will spend less than an hour at the first visit.
8 After your participation at each visit is completed, you will be offered a complimentary (free) dinner at that restaurant s buffet. You may accept the buffet dinner or may decline it as you choose. If you choose to accept the buffet dinner, it must be eaten at that time and cannot be saved until later. Risks and Benefits Potential risks of participating in this research study include a burning sensation in your lips, mouth, and esophagus (the tube connecting the throat to the stomach) or heartburn, due to the spicy nature of the soup. It is believed that this risk is minimal for you as you are a consumer of hot & sour soup anyway, outside of the research. There is also a risk that you might be allergic to one or more of the ingredients in the soup. Again, this is believed to be minimal because you eat the soup anyway. There may be other risks associated with your particular health (i.e. if you are diabetic or have certain other diseases). The Researcher has minimized this risk by excluding potential subjects during the initial interview and by dismissing any subjects who disclose such conditions on the medical history form. If the Researcher believes you experienced a problematic reaction, she will contact you over the next few days to monitor your condition. There might also be a risk of breach of confidentiality. To minimize this risk, you will be assigned an ID number at the first visit. You will be provided with a card with the ID number on it and asked to bring the card with you to each visit, so that you have the number available to you at each visit. Only your ID number, and not your name, will be placed on the medical history form and on the Researcher s form to record her observations each visit. The Researcher will have a list of participants linking names to ID numbers. This list will be used to match information received from your doctor, if any, as described below, and to contact you if follow-up is necessary, as described above. This list will be stored separately from research data in a locked location and will be destroyed after data collection is completed. It is not expected that participants will experience any direct benefits from participation in this research. It is hoped that society at large will benefit in the long run from the addition to scientific knowledge contributed by this research. Involuntary Withdrawal It is possible that the Researcher will withdraw you from further participation in the study whether or not you wish to withdraw. After you fill out the medical history form at the first visit, you will be withdrawn if you disclose any prior conditions that make your participation risky or might confound the results. If that occurs, you will still be offered the complimentary buffet for that evening. If the Researcher observes adverse physical effect on you during any visit or if you report adverse health effects to the Researcher after a session, you will be removed from the study. If you withdraw your authorization for your doctor to disclose information to Dr. Specialist (as described immediately below), you will be removed from the study.
9 Authorization to Use or Disclose Protected Health Information for Research Signing this informed consent to participate in the research study that it describes, also authorizes my primary care doctor (whose name and contact information I will separately provide to Dr. Specialist) to disclose any prior history I may have of the particular problems for which Dr. Specialist is looking to Dr. Specialist in order to enable Dr. Specialist to distinguish between reactions to the soup and prior medical problems. This authorization will expire three days after I complete the last experimental eating session for the research study. This authorization may be revoked at any time by contacting the Investigator at at , or via to gispecialist@lecom.edu, but that the revocation will not affect any information that may already have been disclosed before then. If you do not authorize the use or disclosure of your health information, you will be unable to participate in this research study, but it will have no other effect on you. The Federal Privacy Rule may no longer protect the health information that is disclosed to the recipient if the recipient is not itself covered by the Rule. For More Information If you have questions about this research or if you believe you have experienced a research-related injury, please contact Dr. Specialist at , or via to gispecialist@lecom.edu. If you have questions about your rights as a research subject, please contact Irv Freeman, Ph.D., J.D., at , or via to ifreeman@lecom.edu.
10 Consent I certify that I have read this consent document, that I have discussed the research with Dr. Specialist, and that any questions I had have been answered. I consent to participate in this research study. Signature of Subject Date
11 Appendix III Debriefing Thank You and Additional Information Thank you for participating in this research study. I hope you found your participation to be interesting and enjoyable. The purpose of this memorandum is to provide you with one piece of information that was withheld from you until now. During the informed consent process, you were advised that the purpose of this research project is to determine whether eating hot & sour soup has any immediate short-term effects on the human body that may be observed immediately following eating the soup. That purpose statement is true, but it is incomplete and non-specific. The actual purpose is to determine whether eating hot & sour soup has the effect of inducing hiccoughs while eating or immediately following eating. That is the only effect for which we were looking. You were not previously told that we were looking for hiccoughs because we were afraid that knowledge could have an impact on whether or not you would develop hiccoughs. Now that the data gathering is complete, the full information is being provided to you. Our procedure in providing you with less than complete information about the research s purpose during the informed consent process was approved by the LECOM Institutional Review Board (IRB). In approving this waiver from the normal requirements for informed consent, the IRB followed the requirements laid out by the Federal government. Specifically, the IRB determined that (1) the research involved no more than minimal risk to you, (2) the waiver would not adversely affect your rights and welfare, and (3) the research could not practicably be carried out without the waiver. Finally, the IRB made sure that I would be providing you with pertinent information (in the form of this memorandum) after your participation. If you have any questions or concerns about this, please contact Irv Freeman, Ph.D., J.D., Chair of the LECOM Institutional Review Board, at , ifreeman@lecom.edu. Thank you, Dr. G. I. Specialist
12
PROVIDENCE CHRISTIAN COLLEGE INSTITUTIONAL REVIEW BOARD APPLICATION TO USE HUMAN PARTICIPANTS IN RESEARCH
 PROVIDENCE CHRISTIAN COLLEGE INSTITUTIONAL REVIEW BOARD APPLICATION TO USE HUMAN PARTICIPANTS IN RESEARCH Before completing this application, please review Procedures for Obtaining Institutional Approval
PROVIDENCE CHRISTIAN COLLEGE INSTITUTIONAL REVIEW BOARD APPLICATION TO USE HUMAN PARTICIPANTS IN RESEARCH Before completing this application, please review Procedures for Obtaining Institutional Approval
VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION
 VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION Study Title: Cytokine Production and Lymphoproliferation With and Without Co-inhibitory Signaling Blockade: An Assessment of Functional
VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION Study Title: Cytokine Production and Lymphoproliferation With and Without Co-inhibitory Signaling Blockade: An Assessment of Functional
Flexibility and Informed Consent Process
 Flexibility and Informed Consent Process April 30, 2014 Regulatory & Ethical Requirements for Informed Consent Megan Kasimatis Singleton, JD, MBE, CIP Associate Director, IRB Dave Heagerty IRB 8 Coordinator
Flexibility and Informed Consent Process April 30, 2014 Regulatory & Ethical Requirements for Informed Consent Megan Kasimatis Singleton, JD, MBE, CIP Associate Director, IRB Dave Heagerty IRB 8 Coordinator
Type of Review Requested:
 Type of Review Requested: FOR OFFICE USE ONLY IRB Protocol # Exempt [Status (see RR 101)] Expedited Full Board For details regarding types of review, please see Levels of Review under FAQ at www.seu.edu/irb
Type of Review Requested: FOR OFFICE USE ONLY IRB Protocol # Exempt [Status (see RR 101)] Expedited Full Board For details regarding types of review, please see Levels of Review under FAQ at www.seu.edu/irb
RESEARCH INVOLVING HUMAN PARTICIPANTS EXPEDITED/FULL APPLICATION
 This information listed below should be submitted to Florida Tech s IRB if the proposed research has more than minimal risk (none of the exempt conditions apply) or if the research utilizes a special population
This information listed below should be submitted to Florida Tech s IRB if the proposed research has more than minimal risk (none of the exempt conditions apply) or if the research utilizes a special population
IRB EXPEDITED REVIEW
 IRB EXPEDITED REVIEW Research activities that (1) present no more than minimal risk* to human research participants, and (2) involve only procedures listed in one or more of the following categories may
IRB EXPEDITED REVIEW Research activities that (1) present no more than minimal risk* to human research participants, and (2) involve only procedures listed in one or more of the following categories may
Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH
 Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH Project Title: Genetics of Prostate Cancer Principal Investigator or Faculty Advisor: William J. Catalona, M.D.
Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH Project Title: Genetics of Prostate Cancer Principal Investigator or Faculty Advisor: William J. Catalona, M.D.
IRB Approval From: 3/8/2010 To: 10/28/2010
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy
 A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts
A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts
Northwestern University. Consent Form and HIPAA Authorization for Research
 Northwestern University Consent Form and HIPAA Authorization for Research PROTOCOL TITLE: Yoga Versus Resistance Training in Parkinson s Disease: A Randomized, Controlled Pilot Study of Feasibility & Efficacy
Northwestern University Consent Form and HIPAA Authorization for Research PROTOCOL TITLE: Yoga Versus Resistance Training in Parkinson s Disease: A Randomized, Controlled Pilot Study of Feasibility & Efficacy
Phone Numbers: (work) (cell/home) Phone Numbers: (work) (cell/home)
 CLARKSON COLLEGE Institutional Review Board (IRB) Application INSTRUCTIONS: Applicants, please complete the following sections accordingly Section 1 and Section IV completed by ALL applicants; Section
CLARKSON COLLEGE Institutional Review Board (IRB) Application INSTRUCTIONS: Applicants, please complete the following sections accordingly Section 1 and Section IV completed by ALL applicants; Section
IRB policy and procedures 1. Institutional Review Board: Revised Policy and Procedures Elmhurst College
 IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
Elements of Informed Consent. Lu Pai, Ph.D. Associate Professor, Taipei Medical University IRB member, Tri-service General Hospital
 Elements of Informed Consent Lu Pai, Ph.D. Associate Professor, Taipei Medical University IRB member, Tri-service General Hospital Informed Consent Informed consent is process of ensuring that subjects
Elements of Informed Consent Lu Pai, Ph.D. Associate Professor, Taipei Medical University IRB member, Tri-service General Hospital Informed Consent Informed consent is process of ensuring that subjects
Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus
 Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE
 TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
Language for Consent Forms
 New York University University Committee on Activities Involving Human Subjects 665 Broadway, Suite 804, New York, NY 10012 VOICE: 212-998-4808 FAX: 212-995-4304 www.nyu.edu/ucaihs/ Language for Consent
New York University University Committee on Activities Involving Human Subjects 665 Broadway, Suite 804, New York, NY 10012 VOICE: 212-998-4808 FAX: 212-995-4304 www.nyu.edu/ucaihs/ Language for Consent
3/6/2017-6/15/2017 Permission to Take Part in a Human Research Study Page 1 of 6
 Permission to Take Part in a Human Research Study Page 1 of 6 University at Buffalo Institutional Review Board (UBIRB) Office of Research Compliance Clinical and Translational Research Center Room 5018
Permission to Take Part in a Human Research Study Page 1 of 6 University at Buffalo Institutional Review Board (UBIRB) Office of Research Compliance Clinical and Translational Research Center Room 5018
MC IRB Protocol No.:
 APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
We are inviting you to participate in a research study/project that has two components.
 Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
CONSENT FORM. The Full Study Title Should Be Placed Here. Principal Investigator: Dr. John Smith Sub-Investigator: Dr. Jane Smith
 CONSENT FORM The Full Study Title Should Be Placed Here. Principal Investigator: Dr. John Smith Sub-Investigator: Dr. Jane Smith Queen Elizabeth Hospital Queen Elizabeth Hospital 60 Riverside Drive 60
CONSENT FORM The Full Study Title Should Be Placed Here. Principal Investigator: Dr. John Smith Sub-Investigator: Dr. Jane Smith Queen Elizabeth Hospital Queen Elizabeth Hospital 60 Riverside Drive 60
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
Waiver of Consent. March 29, 2008 Stephanie derijke
 Waiver of Consent March 29, 2008 Stephanie derijke Waiver of Documentation of IC Waiver of Documentation of IC May be granted in 2 situations for research that is not regulated by the FDA: 1. When the
Waiver of Consent March 29, 2008 Stephanie derijke Waiver of Documentation of IC Waiver of Documentation of IC May be granted in 2 situations for research that is not regulated by the FDA: 1. When the
KAISER PERMANENTE OF COLORADO CONSENT TO PARTICIPATE IN A MEDICAL RESEARCH STUDY
 KAISER PERMANENTE OF COLORADO CONSENT TO PARTICIPATE IN A MEDICAL RESEARCH STUDY STUDY TITLE: Strategies for Overcoming Depression Symptoms Study (SOAR) PRINCIPAL INVESTIGATOR: Arne Beck You are being
KAISER PERMANENTE OF COLORADO CONSENT TO PARTICIPATE IN A MEDICAL RESEARCH STUDY STUDY TITLE: Strategies for Overcoming Depression Symptoms Study (SOAR) PRINCIPAL INVESTIGATOR: Arne Beck You are being
MDCH IRB REVIEW APPLICATION Authority: Code of Federal Regulations Title 45 Part 46
 The Michigan Department of Community Health Institutional Review Board for the Protection of Human Research Subjects Capitol View Building, 7 th Floor, 201 Townsend Street, Lansing, MI 48913 Phone: 517/241-1928
The Michigan Department of Community Health Institutional Review Board for the Protection of Human Research Subjects Capitol View Building, 7 th Floor, 201 Townsend Street, Lansing, MI 48913 Phone: 517/241-1928
DE LA SALLE UNIVERSITY. Checklist A Research Ethics Checklist for Investigations involving Human Participants
 DE LA SALLE UNIVERSITY Checklist A Research Ethics Checklist for Investigations involving Human Participants This checklist must be completed AFTER the De La Salle University Code of Research Ethics and
DE LA SALLE UNIVERSITY Checklist A Research Ethics Checklist for Investigations involving Human Participants This checklist must be completed AFTER the De La Salle University Code of Research Ethics and
INFORMED CONSENT REQUIREMENTS AND EXAMPLES
 Office of Research Compliance INFORMED CONSENT REQUIREMENTS AND EXAMPLES No investigator may involve a human being as a subject in research unless the investigator has obtained the legally effective informed
Office of Research Compliance INFORMED CONSENT REQUIREMENTS AND EXAMPLES No investigator may involve a human being as a subject in research unless the investigator has obtained the legally effective informed
*Explain the purpose & role of the IRB *Explain the IRB Review Categories *Discuss the potential risks to research participants
 Explain the purpose & role of the IRB Explain the IRB Review Categories Discuss the potential risks to research participants Discuss the informed consent process Review the IRB Submission Process The Institutional
Explain the purpose & role of the IRB Explain the IRB Review Categories Discuss the potential risks to research participants Discuss the informed consent process Review the IRB Submission Process The Institutional
THE UNIVERSITY OF HONG KONG Human Research Ethics Committee for Non-Clinical Faculties Application Form for Ethical Approval
 50/1011 amended THE UNIVERSITY OF HONG KONG Human Research Ethics Committee for n-clinical Faculties Application Form for Ethical Approval For official use: Ref..: Received date: tes: (1) Please read carefully
50/1011 amended THE UNIVERSITY OF HONG KONG Human Research Ethics Committee for n-clinical Faculties Application Form for Ethical Approval For official use: Ref..: Received date: tes: (1) Please read carefully
We are inviting you to participate in a research study/project that has two components.
 Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
HSPC/IRB Description of Research Form (For research projects involving human participants)
 HSPC/IRB Description of Research Form (For research projects involving human participants) This form is to be completed by the Principal Investigator (P.I.) of the research project being submitted to the
HSPC/IRB Description of Research Form (For research projects involving human participants) This form is to be completed by the Principal Investigator (P.I.) of the research project being submitted to the
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1)
 INTER AMERICAN UNIVERSITY OF PUERTO RICO INSTITUTIONAL REVIEW BOARD APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1) TITLE*: PRINCIPAL INVESTIGATOR S NAME, TELEPHONE AND POSTAL ADDRESS*:
INTER AMERICAN UNIVERSITY OF PUERTO RICO INSTITUTIONAL REVIEW BOARD APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1) TITLE*: PRINCIPAL INVESTIGATOR S NAME, TELEPHONE AND POSTAL ADDRESS*:
IRB FREQUENTLY ASKED QUESTIONS. 1. Who must apply for human subjects review through the IRB (Institutional Research Board)?
 IRB FREQUENTLY ASKED QUESTIONS 1. Who must apply for human subjects review through the IRB (Institutional Research Board)? All Regis University faculty, students (graduate and undergraduate), and staff
IRB FREQUENTLY ASKED QUESTIONS 1. Who must apply for human subjects review through the IRB (Institutional Research Board)? All Regis University faculty, students (graduate and undergraduate), and staff
by at Study Roles and Responsibilities
 Full Study DHA Non-Exempt Human Subject Research Protocol Submission (You may need to click Enable Editing on the yellow bar above in order to complete this template) 1.0 Study Contacts Name/Rank/Degree:
Full Study DHA Non-Exempt Human Subject Research Protocol Submission (You may need to click Enable Editing on the yellow bar above in order to complete this template) 1.0 Study Contacts Name/Rank/Degree:
IRB for Humanists. Naomi E. Coll, MPH, CPH, CIP Manager of Research Integrity
 IRB for Humanists Naomi E. Coll, MPH, CPH, CIP Manager of Research Integrity Grace Caskie, Ph.D. Associate Professor Counseling Psychology IRB co-chair Patti Manz, Ph.D. Associate Professor School Psychology
IRB for Humanists Naomi E. Coll, MPH, CPH, CIP Manager of Research Integrity Grace Caskie, Ph.D. Associate Professor Counseling Psychology IRB co-chair Patti Manz, Ph.D. Associate Professor School Psychology
Human Subjects Research: Overview. Colleen Kohashi and Tani Prestage Office for the Protection of Human Subjects (OPHS) February 26, 2016
 Human Subjects Research: Overview Colleen Kohashi and Tani Prestage Office for the Protection of Human Subjects (OPHS) February 26, 2016 Topics to be Covered Am I doing human subjects research?; Risk determinations
Human Subjects Research: Overview Colleen Kohashi and Tani Prestage Office for the Protection of Human Subjects (OPHS) February 26, 2016 Topics to be Covered Am I doing human subjects research?; Risk determinations
Eliada Assessment Center Application for Services
 Student s Name: Record # Date of Birth: Race: Biological Sex: Male Female Gender Identity: Male Female Transgender/Non-Binary Date Placement Needed: SSN: - - Legal Custodian: Name, Address, Phone, Email
Student s Name: Record # Date of Birth: Race: Biological Sex: Male Female Gender Identity: Male Female Transgender/Non-Binary Date Placement Needed: SSN: - - Legal Custodian: Name, Address, Phone, Email
Human Subject Institutional Review Board Proposal Form
 FOR IRB USE ONLY Protocol Number: IRB- Human Subject Institutional Review Board Proposal Form Activity Title: PRINCIPAL INVESTIGATOR ASSURANCE I agree to use procedures with respect to safeguarding human
FOR IRB USE ONLY Protocol Number: IRB- Human Subject Institutional Review Board Proposal Form Activity Title: PRINCIPAL INVESTIGATOR ASSURANCE I agree to use procedures with respect to safeguarding human
About this consent form. Why is this research study being done? Partners HealthCare System Research Consent Form
 Protocol Title: Gene Sequence Variants in Fibroid Biology Principal Investigator: Cynthia C. Morton, Ph.D. Site Principal Investigator: Cynthia C. Morton, Ph.D. Description of About this consent form Please
Protocol Title: Gene Sequence Variants in Fibroid Biology Principal Investigator: Cynthia C. Morton, Ph.D. Site Principal Investigator: Cynthia C. Morton, Ph.D. Description of About this consent form Please
Vanderbilt University Institutional Review Board Informed Consent Document for Research. Name of participant: Age:
 This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Epileptic Encephalopathies: Clinical and Genetic Predictors of Outcomes and Therapeutic Insights This is a research study.
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Epileptic Encephalopathies: Clinical and Genetic Predictors of Outcomes and Therapeutic Insights This is a research study.
CONSENT TO GENETIC TESTING THROUGH A RESEARCH STUDY
 CONSENT TO GENETIC TESTING THROUGH A RESEARCH STUDY Title Principal Investigator Genetic Counsellor Prevent Ovarian Cancer Program: Systematic identification of high-risk women for ovarian cancer prevention
CONSENT TO GENETIC TESTING THROUGH A RESEARCH STUDY Title Principal Investigator Genetic Counsellor Prevent Ovarian Cancer Program: Systematic identification of high-risk women for ovarian cancer prevention
Deborah Watkins-Bruner, Ph.D. Fox Chase Cancer Center Philadelphia, Pennsylvania 19111
 AD Award Number: DAMD17-02-1-0055 TITLE: Preference and Utilities for Prostate Cancer Screening & Treatment: Assessment of the Underlying Decision Making Process PRINCIPAL INVESTIGATOR: Deborah Watkins-Bruner,
AD Award Number: DAMD17-02-1-0055 TITLE: Preference and Utilities for Prostate Cancer Screening & Treatment: Assessment of the Underlying Decision Making Process PRINCIPAL INVESTIGATOR: Deborah Watkins-Bruner,
Human Subjects Application for Full IRB and Expedited Exempt Review
 Human Subjects Application for Full IRB and Expedited Exempt Review 1. Project Title and Identification As Principal Investigator of this study, I assure the IRB that the following statements are true:
Human Subjects Application for Full IRB and Expedited Exempt Review 1. Project Title and Identification As Principal Investigator of this study, I assure the IRB that the following statements are true:
APPLICATION/RESEARCH PROTOCOL REVIEW FORM
 APPLICATION/RESEARCH PROTOCOL REVIEW FORM For Research Involving Human Participants Institutional Review Board (IRB) Contact Information: The Office of Research and Sponsored Programs (Billy C. Black Building,
APPLICATION/RESEARCH PROTOCOL REVIEW FORM For Research Involving Human Participants Institutional Review Board (IRB) Contact Information: The Office of Research and Sponsored Programs (Billy C. Black Building,
Guidelines for Ethical Conduct of Behavioral Projects Involving Human Participants by High School Students
 Guidelines for Ethical Conduct of Behavioral Projects Involving Human Participants by High School Students Introduction Improving science literacy in the United States requires strengthening science, technology,
Guidelines for Ethical Conduct of Behavioral Projects Involving Human Participants by High School Students Introduction Improving science literacy in the United States requires strengthening science, technology,
INSTITUTIONAL REVIEW BOARD (IRB) PROCESS AND GUIDELINES FOR CONDUCTING RESEARCH AT ORANGE COAST COLLEGE
 1 INSTITUTIONAL REVIEW BOARD (IRB) PROCESS AND GUIDELINES FOR CONDUCTING RESEARCH AT ORANGE COAST COLLEGE Developed by: Dr. Eduardo Jesús Arismendi-Pardi Department of Mathematics Sheri Sterner Office
1 INSTITUTIONAL REVIEW BOARD (IRB) PROCESS AND GUIDELINES FOR CONDUCTING RESEARCH AT ORANGE COAST COLLEGE Developed by: Dr. Eduardo Jesús Arismendi-Pardi Department of Mathematics Sheri Sterner Office
CAPE PENINSULA UNIVERSITY OF TECHNOLOGY HEALTH AND WELLNESS SCIENCES RESEARCH ETHICS COMMITTEE
 CAPE PENINSULA UNIVERSITY OF TECHNOLOGY HEALTH AND WELLNESS SCIENCES RESEARCH ETHICS COMMITTEE HUMAN PARTICIPANTS' REVIEW APPLICATION External Researchers wanting CPUT as a data collection site This application
CAPE PENINSULA UNIVERSITY OF TECHNOLOGY HEALTH AND WELLNESS SCIENCES RESEARCH ETHICS COMMITTEE HUMAN PARTICIPANTS' REVIEW APPLICATION External Researchers wanting CPUT as a data collection site This application
BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research
 BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research Project Title Principal Investigator Name and highest earned degree: Office Phone: Facsimile Phone Number: Department:
BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research Project Title Principal Investigator Name and highest earned degree: Office Phone: Facsimile Phone Number: Department:
Institutional Review Boards and Human Subjects Protection
 Institutional Review Boards and Human Subjects Protection Professor Ron Fricker! Naval Postgraduate School! Monterey, California! 6/25/12 1 Goals for this Lecture! A nasty little bit of history! The Belmont
Institutional Review Boards and Human Subjects Protection Professor Ron Fricker! Naval Postgraduate School! Monterey, California! 6/25/12 1 Goals for this Lecture! A nasty little bit of history! The Belmont
Protecting Human Subjects In Social-Behavioral-Educational Research:
 Protecting Human Subjects In Social-Behavioral-Educational Research: Working with the IRB Lloyd Byrd, MS Chair, VCU IRB Panel E Member, VCU IRB Panel B Monika S. Markowitz, Ph.D. Director, Office of Research
Protecting Human Subjects In Social-Behavioral-Educational Research: Working with the IRB Lloyd Byrd, MS Chair, VCU IRB Panel E Member, VCU IRB Panel B Monika S. Markowitz, Ph.D. Director, Office of Research
[Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example
![[Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example [Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example](/thumbs/76/73422439.jpg) [YOUR INSTITUTIONAL LETTERHEAD] [Name of Please Principle do not Investigator] submit consent forms on the WHO letter head [Informed Consent Form for ] Name the group of individuals for whom this consent
[YOUR INSTITUTIONAL LETTERHEAD] [Name of Please Principle do not Investigator] submit consent forms on the WHO letter head [Informed Consent Form for ] Name the group of individuals for whom this consent
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE ON ELECTRONIC INFORMED CONSENT
 COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE ON ELECTRONIC INFORMED CONSENT I. BACKGROUND Electronic consenting ( e-consenting ) is the use of electronic systems and processes, whether in person
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE ON ELECTRONIC INFORMED CONSENT I. BACKGROUND Electronic consenting ( e-consenting ) is the use of electronic systems and processes, whether in person
Office of Research Compliance. Research Involving Human Subjects
 Office of Research Compliance Research Involving Human Subjects Office of Research Compliance Three FTE and one student worker Facilitate the IACUC We are not the IRB or the IACUC Best way to contact us
Office of Research Compliance Research Involving Human Subjects Office of Research Compliance Three FTE and one student worker Facilitate the IACUC We are not the IRB or the IACUC Best way to contact us
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
About this consent form
 Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
Subject Name: Sponsor Consent Tmplt Date: Version 2 (17-Jul-2014) Unit Number:
 SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v )
 UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v. 11.2012) Project Title: The Impact of Achilles Tightness on Lower Etremity Injuries in Adolescent Athletes Principal Investigator:
UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v. 11.2012) Project Title: The Impact of Achilles Tightness on Lower Etremity Injuries in Adolescent Athletes Principal Investigator:
TRANSITION OF CARE APPLICATION
 Dear Member: Thank you for enrolling in MedStar Medicare Choice. You may currently be receiving services from healthcare providers that are not part of the MedStar Medicare Choice Provider Network. An
Dear Member: Thank you for enrolling in MedStar Medicare Choice. You may currently be receiving services from healthcare providers that are not part of the MedStar Medicare Choice Provider Network. An
CONSENT TO PARTICIPATE IN A RESEARCH STUDY. Why are you being invited to take part in a research study?
 HRP-502 (6/1/2016) LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER in Shreveport Institutional Review Board (IRB) for the Protection of Human Research Subjects Remove all instructional text and red color-coding
HRP-502 (6/1/2016) LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER in Shreveport Institutional Review Board (IRB) for the Protection of Human Research Subjects Remove all instructional text and red color-coding
You are the parent or guardian granting consent for a minor in this study.
 Please check one of the following: You are an adult subject in this study. You are the parent or guardian granting consent for a minor in this study. Print minor's name here: The following information
Please check one of the following: You are an adult subject in this study. You are the parent or guardian granting consent for a minor in this study. Print minor's name here: The following information
PROTECTION OF HUMAN SUBJECTS
 PROTECTION OF HUMAN SUBJECTS Human Subjects Policy Statement Lesley University is committed to the ethical principles for the protection of human subjects in research set forth in the Belmont Report of
PROTECTION OF HUMAN SUBJECTS Human Subjects Policy Statement Lesley University is committed to the ethical principles for the protection of human subjects in research set forth in the Belmont Report of
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
IRB Reviewer Worksheet for Expedited Reviews
 IRB Reviewer Worksheet for Expedited Reviews This reviewer worksheet is copied with modifications from Khan and Kornetsky s Overview of Initial Protocol Review printed in IRB Management and Function (2006).
IRB Reviewer Worksheet for Expedited Reviews This reviewer worksheet is copied with modifications from Khan and Kornetsky s Overview of Initial Protocol Review printed in IRB Management and Function (2006).
Institutional Review Board Application
 Institutional Review Board Application Ashoka University values research that is conducted with high standards of scholarship and ethics. The Institutional Review Board (IRB) ensures that the University
Institutional Review Board Application Ashoka University values research that is conducted with high standards of scholarship and ethics. The Institutional Review Board (IRB) ensures that the University
You are the parent or guardian granting permission for a child in this study.
 Please check one of the following: You are an adult participant in this study. You are the parent or guardian granting permission for a child in this study. Print child s name here: The following information
Please check one of the following: You are an adult participant in this study. You are the parent or guardian granting permission for a child in this study. Print child s name here: The following information
Last Name First name Academic degrees. Professional titles and/or work position within your home institution
 Date received stamp ETHICS AND RESEARCH APPLICATION FORM Kenyatta National Hospital/University of Nairobi KEMRI CENTERS ETHICS RESEARCH COMMITTEE Application Number Submit three copies of this form (including
Date received stamp ETHICS AND RESEARCH APPLICATION FORM Kenyatta National Hospital/University of Nairobi KEMRI CENTERS ETHICS RESEARCH COMMITTEE Application Number Submit three copies of this form (including
IRB, IDEATE, AND HSR. February, 2018 Manuel Gonzalez
 IRB, IDEATE, AND HSR February, 2018 Manuel Gonzalez Outline What is the IRB? Human Subjects Research and Researcher Responsibilities Citi Certification Ethical Principles Revisions etc. Submitting Studies
IRB, IDEATE, AND HSR February, 2018 Manuel Gonzalez Outline What is the IRB? Human Subjects Research and Researcher Responsibilities Citi Certification Ethical Principles Revisions etc. Submitting Studies
Protection of Human Subjects Policies and Procedures
 Protection of Human Subjects Policies and Procedures Introduction Wilmington University has a functioning committee and procedures to review and approve all research involving human subjects. All human
Protection of Human Subjects Policies and Procedures Introduction Wilmington University has a functioning committee and procedures to review and approve all research involving human subjects. All human
Navigating the Regulatory Requirements of Pediatric Research Nathan Lee, CIP Vice Chair, Schulman IRB
 December 15, 2016 Navigating the Regulatory Requirements of Pediatric Research Nathan Lee, CIP Vice Chair, Schulman IRB About Schulman IRB Established in 1983 Superior audit history with FDA five consecutive
December 15, 2016 Navigating the Regulatory Requirements of Pediatric Research Nathan Lee, CIP Vice Chair, Schulman IRB About Schulman IRB Established in 1983 Superior audit history with FDA five consecutive
NCI Community Oncology Research Program Kansas City (NCORP-KC)
 NCI Community Oncology Research Program Kansas City (NCORP-KC) Consent Form Study Title for Study Participants: Comparing Two Dose Levels of Bupropion Versus Placebo for Sexual Desire Official Study Title
NCI Community Oncology Research Program Kansas City (NCORP-KC) Consent Form Study Title for Study Participants: Comparing Two Dose Levels of Bupropion Versus Placebo for Sexual Desire Official Study Title
Marie Stopes International Informed Consent Guidelines for Research
 Marie Stopes International Informed Consent Guidelines for Research Developed by: Research, Monitoring, and Evaluation Team Health System Department, MSI Marie Stopes International Informed Consent Guidelines
Marie Stopes International Informed Consent Guidelines for Research Developed by: Research, Monitoring, and Evaluation Team Health System Department, MSI Marie Stopes International Informed Consent Guidelines
Life After Prostate Cancer Diagnosis Research Study
 Life After Prostate Cancer Diagnosis Research Study If you are looking at this information sheet this means you have read the covering letter and therefore have had a diagnosis of prostate cancer. If you
Life After Prostate Cancer Diagnosis Research Study If you are looking at this information sheet this means you have read the covering letter and therefore have had a diagnosis of prostate cancer. If you
PREPARING FOR REFLUX TESTING. Digitrapper Reflux Testing System
 PREPARING FOR REFLUX TESTING Digitrapper Reflux Testing System An innovative solution to evaluate your gastroesophageal reflux symptoms on or off anti-reflux therapy WHY TEST FOR GERD? Do you have frequent
PREPARING FOR REFLUX TESTING Digitrapper Reflux Testing System An innovative solution to evaluate your gastroesophageal reflux symptoms on or off anti-reflux therapy WHY TEST FOR GERD? Do you have frequent
Home Sleep Test (HST) Instructions
 Home Sleep Test (HST) Instructions 1. Your physician has ordered an unattended home sleep test (HST) to diagnose or rule out sleep apnea. This test cannot diagnose any other sleep disorders. 2. This device
Home Sleep Test (HST) Instructions 1. Your physician has ordered an unattended home sleep test (HST) to diagnose or rule out sleep apnea. This test cannot diagnose any other sleep disorders. 2. This device
National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment
 National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
PROTECTING COMMUNITY STAFF FROM EXPOSURE TO SECONDHAND SMOKE
 BREATHING SPACE YOUR HOME-OUR WORKPLACE PROTECTING COMMUNITY STAFF FROM EXPOSURE TO SECONDHAND SMOKE GUIDANCE DOCUMENT HEALTH EQUALITIES GROUP CONTENTS Introduction... 3 Secondhand smoke... 3 Protecting
BREATHING SPACE YOUR HOME-OUR WORKPLACE PROTECTING COMMUNITY STAFF FROM EXPOSURE TO SECONDHAND SMOKE GUIDANCE DOCUMENT HEALTH EQUALITIES GROUP CONTENTS Introduction... 3 Secondhand smoke... 3 Protecting
Research Involving Human Subjects: Ethics, Process, and Guidance for Streamlining IRB Review
 Research Involving Human Subjects: Ethics, Process, and Guidance for Streamlining IRB Review Graduate Medical Education October 9, 2013 Cortni Romaine Education and Outreach Coordinator Office of Human
Research Involving Human Subjects: Ethics, Process, and Guidance for Streamlining IRB Review Graduate Medical Education October 9, 2013 Cortni Romaine Education and Outreach Coordinator Office of Human
Consent and Authorization Document
 Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
STUDIES INVOLVING THE NON-INVASIVE MEASUREMENT OF BLOOD PRESSURE IN THE ARM
 STUDIES INVOLVING THE NON-INVASIVE MEASUREMENT OF BLOOD PRESSURE IN THE ARM 1. SCOPE Blood pressure is a vital sign that is used in many clinical situations, and assesses the pressure of blood as it flows
STUDIES INVOLVING THE NON-INVASIVE MEASUREMENT OF BLOOD PRESSURE IN THE ARM 1. SCOPE Blood pressure is a vital sign that is used in many clinical situations, and assesses the pressure of blood as it flows
LETTER OF INFORMATION AND CONSENT FORM
 Page 1 of 7 LETTER OF INFORMATION AND CONSENT FORM Functional neuroimaging of intrinsic hemodynamic networks in bipolar disorder, unipolar depressive disorder, and healthy controls: Finding a biomarker
Page 1 of 7 LETTER OF INFORMATION AND CONSENT FORM Functional neuroimaging of intrinsic hemodynamic networks in bipolar disorder, unipolar depressive disorder, and healthy controls: Finding a biomarker
Barrett s Oesophagus
 PATIENT INFORMATION Barrett s Oesophagus Radiofrequency Ablation of Barrett s Columnar Lined Oesophagus and Squamous Dysplasia MulticentreTrial with Long Term Follow Up Using a Central Database Project
PATIENT INFORMATION Barrett s Oesophagus Radiofrequency Ablation of Barrett s Columnar Lined Oesophagus and Squamous Dysplasia MulticentreTrial with Long Term Follow Up Using a Central Database Project
Effects of a Mediterranean-style diet and fish oil supplements on mood and health Chief Investigators INFORMATION SHEET Background and aim
 Effects of a Mediterranean-style diet and fish oil supplements on mood and health Chief Investigators University of South Australia: Dr Natalie Parletta, Dr Dorota Zarnowiecki, Ji Hyun (Catherine) Cho,
Effects of a Mediterranean-style diet and fish oil supplements on mood and health Chief Investigators University of South Australia: Dr Natalie Parletta, Dr Dorota Zarnowiecki, Ji Hyun (Catherine) Cho,
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
POLICY. Institutional Research Projects/Data Requests #7220
 POLICY 1. This policy is intended to ensure that data requests and research projects conducted by any college office, employee, student, or affiliate are sound and that they do not violate board policy,
POLICY 1. This policy is intended to ensure that data requests and research projects conducted by any college office, employee, student, or affiliate are sound and that they do not violate board policy,
Preliminary Research Considerations. Lecture Overview. Stephen E. Brock, Ph.D., NCSP
 Preliminary Research Considerations Stephen E. Brock, Ph.D., NCSP California State University, Sacramento 1 Lecture Overview Research Hypotheses Research Resources Exemptions from Human Subjects Committee
Preliminary Research Considerations Stephen E. Brock, Ph.D., NCSP California State University, Sacramento 1 Lecture Overview Research Hypotheses Research Resources Exemptions from Human Subjects Committee
10. Has your child ever been diagnosed with an unexplained seizure disorder or exercise-induced asthma?
 PLAYING IT SAFE Cardiac Screening Intake Form Patient Information: First Name: MI Last Name: Date of Birth Month Day Year Address: City State Zip Telephone: Second Phone Parent/Guardian Name: Primary Physician:
PLAYING IT SAFE Cardiac Screening Intake Form Patient Information: First Name: MI Last Name: Date of Birth Month Day Year Address: City State Zip Telephone: Second Phone Parent/Guardian Name: Primary Physician:
The AAA statement on Ethnography and Institutional Review Boards (2004) provides a useful working definition:
 Ethnographic Research and IRB-SBS Protocols INTRODUCTION The Department of Anthropology has drafted the following guidelines intended to help inform the process of IRB oversight of ethnographic research
Ethnographic Research and IRB-SBS Protocols INTRODUCTION The Department of Anthropology has drafted the following guidelines intended to help inform the process of IRB oversight of ethnographic research
Standards for Professional Conduct In The Practice of Dentistry
 Standards for Professional Conduct In The Practice of Dentistry Preamble The Standards for Professional Conduct for licensees of the Virginia Board of Dentistry establishes a set of principles to govern
Standards for Professional Conduct In The Practice of Dentistry Preamble The Standards for Professional Conduct for licensees of the Virginia Board of Dentistry establishes a set of principles to govern
POLICY NO Adopted: Final approval 10/25/06 Revised 10/13/06 Revised: 10/30/06 Revised: 5/27/08 DRUG TESTING PROGRAM
 POLICY NO. 5600 Adopted: Final approval 10/25/06 Revised 10/13/06 Revised: 10/30/06 Revised: 5/27/08 DRUG TESTING PROGRAM 1 DRUG TESTING PROGRAM POLICY NO. 5600 Adopted: 9/06 Revised 10/13/06 Revised:
POLICY NO. 5600 Adopted: Final approval 10/25/06 Revised 10/13/06 Revised: 10/30/06 Revised: 5/27/08 DRUG TESTING PROGRAM 1 DRUG TESTING PROGRAM POLICY NO. 5600 Adopted: 9/06 Revised 10/13/06 Revised:
WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)?
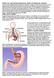 WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)? The term gastroesophageal reflux describes the movement (or reflux) of stomach contents back up into the esophagus, the muscular tube that extends from the
WHAT IS GASTROESOPHAGEAL REFLUX DISEASE (GERD)? The term gastroesophageal reflux describes the movement (or reflux) of stomach contents back up into the esophagus, the muscular tube that extends from the
Ohio SNAP-Ed Adult & Teen Programs Foods to Decrease
 Page 1 Ohio SNAP-Ed Adult & Teen Programs Foods to Decrease Task Topic: Task Title: Teaching Message(s): Resources: MyPlate Foods to Decrease Use MyPlate to make food choices for a healthy lifestyle. Use
Page 1 Ohio SNAP-Ed Adult & Teen Programs Foods to Decrease Task Topic: Task Title: Teaching Message(s): Resources: MyPlate Foods to Decrease Use MyPlate to make food choices for a healthy lifestyle. Use
IRB Review Points to Consider September 2016
 POINTS TO CONSIDER Principal investigators 1. Does the principal investigator have the appropriate qualifications, experience, and facilities to ensure that all aspects of the project and follow-up will
POINTS TO CONSIDER Principal investigators 1. Does the principal investigator have the appropriate qualifications, experience, and facilities to ensure that all aspects of the project and follow-up will
IRB Approved: 09-Jan-2015 To: 08-Jan-2016
 Developing Assays to Evaluate Immunologic Response to HIV and HIV Vaccines CONSENT FORM/HIPAA AUTHORIZATION FOR VENIPUNCTURE Investigators: Pablo Tebas, MD Phone Number: (215) 349-8092 Study Staff: Joseph
Developing Assays to Evaluate Immunologic Response to HIV and HIV Vaccines CONSENT FORM/HIPAA AUTHORIZATION FOR VENIPUNCTURE Investigators: Pablo Tebas, MD Phone Number: (215) 349-8092 Study Staff: Joseph
Novel processed carrot-based products to supplement vegetable intake
 HEALTH AND BIOSECURITY Novel processed carrot-based products to supplement vegetable intake INFORMATION SHEET HUMAN RESEARCH ETHICS COMMITTEE NUMBER: 06/2017 INTRODUCTION Each year CSIRO performs a number
HEALTH AND BIOSECURITY Novel processed carrot-based products to supplement vegetable intake INFORMATION SHEET HUMAN RESEARCH ETHICS COMMITTEE NUMBER: 06/2017 INTRODUCTION Each year CSIRO performs a number
POLICIES GOVERNING PROCEDURES FOR THE USE OF ANIMALS IN RESEARCH AND TEACHING AT WESTERN WASHINGTON UNIVERSITY and REVIEW OF HUMAN SUBJECT RESEARCH
 Appendix 9 POLICIES GOVERNING PROCEDURES FOR THE USE OF ANIMALS IN RESEARCH AND TEACHING AT WESTERN WASHINGTON UNIVERSITY and REVIEW OF HUMAN SUBJECT RESEARCH INTRODUCTION It is the policy of Western Washington
Appendix 9 POLICIES GOVERNING PROCEDURES FOR THE USE OF ANIMALS IN RESEARCH AND TEACHING AT WESTERN WASHINGTON UNIVERSITY and REVIEW OF HUMAN SUBJECT RESEARCH INTRODUCTION It is the policy of Western Washington
Ethical Conduct for Research Involving Humans
 PROCEDURES Policy No. F.1.01 Title Ethical Conduct for Research Involving Humans Approval Body Board of Governors Policy Sponsor Vice-President Academic, Students & Research Last Revised/Replaces April
PROCEDURES Policy No. F.1.01 Title Ethical Conduct for Research Involving Humans Approval Body Board of Governors Policy Sponsor Vice-President Academic, Students & Research Last Revised/Replaces April
IDENTIFYING, ASSESSING, AND RESOLVING ISSUES IN SOCIAL, BEHAVIORAL, AND EDUCATIONAL RESEARCH (SBER)
 IDENTIFYING, ASSESSING, AND RESOLVING ISSUES IN SOCIAL, BEHAVIORAL, AND EDUCATIONAL RESEARCH (SBER) Cynthia Monahan, MBA, CIP IRB Director Boston University Charles River Campus Objectives Overview of
IDENTIFYING, ASSESSING, AND RESOLVING ISSUES IN SOCIAL, BEHAVIORAL, AND EDUCATIONAL RESEARCH (SBER) Cynthia Monahan, MBA, CIP IRB Director Boston University Charles River Campus Objectives Overview of
P.O. Box Austin, Texas Phone: Fax: Texas Society of Infection Control & Prevention
 Texas Society of Infection Control & Prevention P.O. Box 341357 Austin, Texas 78734 Phone: 512-722-3717 Fax: 512-722-3608 The Texas Society of Infection Control & Prevention (TSICP) is looking forward
Texas Society of Infection Control & Prevention P.O. Box 341357 Austin, Texas 78734 Phone: 512-722-3717 Fax: 512-722-3608 The Texas Society of Infection Control & Prevention (TSICP) is looking forward
HREC/17/RCHM/334 RCH HREC 37278A. ANZ CLARITY Establishment of a National Juvenile Idiopathic Arthritis Biobank.
 HREC Project Number: Research Project Title: Principal Researchers: HREC/17/RCHM/334 RCH HREC 37278A ANZ CLARITY Establishment of a National Juvenile Idiopathic Arthritis Biobank. Associate Professor Justine
HREC Project Number: Research Project Title: Principal Researchers: HREC/17/RCHM/334 RCH HREC 37278A ANZ CLARITY Establishment of a National Juvenile Idiopathic Arthritis Biobank. Associate Professor Justine
