PRODUCT INFORMATION MIRENA NAME OF THE MEDICINE
|
|
|
- Martin Campbell
- 6 years ago
- Views:
Transcription
1 PRODUCT INFORMATION MIRENA NAME OF THE MEDICINE MIRENA 20 microgram per 24 hours intrauterine delivery system contains 52 mg levonorgestrel, a progestogen, as the active ingredient. MIRENA is also an intrauterine contraceptive device. The levonorgestrel initial release rate is 20 microgram per 24 hours. Levonorgestrel is a white or almost white, odourless or almost odourless, crystalline powder. It is insoluble in water or hexane, slightly soluble in ethanol or acetone, and sparingly soluble in methylene chloride. The chemical name for levonorgestrel is 13β-ethyl-17β-hydroxy-18, 19- dinor-17α-pregn-4-en-20-yn-3-one. The CAS registry number for levonorgestrel is OH H H C CH H H O Chemical Formula: C 21H 28O 2 Molecular Weight: Melting Point: C DESCRIPTION MIRENA is an intrauterine delivery system (IUS) based on a T-shaped polyethylene frame, with a cylinder containing a mixture of dimethylsiloxane/methylvinylsiloxane (cross-linked) elastomer and levonorgestrel around its vertical arm. The cylinder is covered by a dimethylsiloxane/methylvinylsiloxane (cross-linked) elastomer membrane, which regulates the release of levonorgestrel. It contains a total of 52 mg levonorgestrel. The levonorgestrel IUS consists of a white or almost white drug core covered with an opaque membrane, which is mounted on the vertical stem of a T-body. The white T-body has a loop at one end of the vertical stem and two horizontal arms at the other end. Brown removal threads are attached to the loop. The T-body of MIRENA contains barium sulfate, which makes it visible in X-ray examination. The vertical stem of the IUS is loaded in the insertion tube at the tip of the inserter. The IUS and inserter are essentially free of visible impurities MIRENA PI 1
2 Excipients Dimethylsiloxane/methylvinylsiloxane (cross-linked) elastomer, Silica - colloidal anhydrous, polyethylene, barium sulfate, iron oxide black CI PHARMACOLOGY Levonorgestrel is a potent progestin of the 19-nortestosterone class which possesses characteristic gestagenic properties such as endometrial transformation (development of a secretory endometrium), antigonadotropic action and antiestrogenic effects. The antiestrogenic activity of levonorgestrel is not the result of direct estrogen antagonism, since levonorgestrel does not bind to the estrogen receptor in vitro, but the result of modification of peripheral estrogenic effects. Levonorgestrel does not possess antiandrogenic or glucocorticoid properties, but does have marked partial androgenic activity. Levonorgestrel is used in gynaecology as the progestogenic component in combined oral contraceptives and for contraception in progestogen-only pills. Levonorgestrel can also be administered into the uterine cavity with an intrauterine delivery system such as MIRENA. This allows a very low daily dosage, as the hormone is released directly into the uterine cavity. MIRENA, when inserted according to the instructions, has a failure rate of approximately 0.2% at 1 year and a cumulative failure rate of approximately 0.7% at 5 years. MIRENA has mainly local progestogenic effects in the uterine cavity. The high levonorgestrel concentrations in the endometrium inhibits the endometrial synthesis of estrogen receptors, making the endometrium insensitive to the circulating estradiol, and a strong antiproliferative effect is seen. Thickening of the cervical mucus prevents passage of sperm through the cervical canal. The local milieu of the uterus and of the ovarian tubes inhibits sperm motility and function, preventing fertilisation. Ovulation is inhibited in some women. Morphological changes of the endometrium and a weak foreign body reaction, due to the presence of an intrauterine contraceptive device, are also observed during use of MIRENA. Pharmacokinetics Absorption Following insertion, levonorgestrel is released from the IUS into the uterine cavity without delay based on serum concentration measurements MIRENA PI 2
3 After insertion of MIRENA, levonorgestrel is detectable in serum after 1 hour. The maximum concentration is reached within 2 weeks after insertion. In correspondence with the declining release rate, the median serum concentration of levonorgestrel declines from 206 picogram/ml (25th to 75th percentiles: 151 picogram/ml to 264 picogram/ml) at 6 months to 194 picogram/ml (146 mg/ml to 266 picogram/ml) at 12 months, and to 131 picogram/ml (113 picogram/ml to 161 picogram/ml) at 60 months in women of reproductive age weighing above 55 kg. In postmenopausal women using MIRENA together with non-oral estrogen treatment, the median serum concentration of levonorgestrel declines from 257 picogram/ml (25th to 75th percentiles: 186 picogram/ml to 326 picogram/ml) at 12 months, to 149 picogram/ml (122 picogram/ml to 180 picogram/ml at 60 months. When MIRENA is used together with oral estrogen treatment, the serum levonorgestrel concentration at 12 months is increased to approximately 478 picogram/ml (25th to 75th percentiles: 341 picogram/ml to 655 picogram/ml) due to the induction of SHBG by oral estrogen treatment. Distribution Levonorgestrel is bound non-specifically to serum albumin and specifically to sex hormone binding globulin (SHBG). About 1-2% of the circulating levonorgestrel is present as free steroid and 42-62% is specifically bound to SHBG. During the use of MIRENA, the concentration of SHBG declines. Accordingly, the fraction bound to SHBG decreases during the treatment and the free fraction increases. The mean apparent volume of distribution of levonorgestrel is about 106 L. Body weight and serum SHBG concentration have been shown to affect systemic levonorgestrel concentration i.e. low body weight and/or a high SHBG level increase levonorgestrel concentration. In women of reproductive age with a low body weight (37 to 55 kg) the median serum concentration of levonorgestrel is about 1.5 fold higher. Metabolism Levonorgestrel is extensively metabolised. The major metabolites in the plasma are the unconjugated and conjugated forms of 3α, 5β-tetrahydrolevonorgestrel. Based on in vitro and in vivo studies, CYP3A4 is the main enzyme involved in the metabolism of levonorgestrel. CYP2E1, CYP2C19 and CYP2C9 may also be involved, but to a smaller extent. Elimination The total clearance of levonorgestrel from plasma is approximately 1.0 ml/min/kg. Only trace amounts of levonorgestrel are excreted in unchanged form. The metabolites are excreted with the faeces and urine at an excretion ratio of about 1. The excretion half-life which is mainly represented by metabolites is about 1 day. CLINICAL TRIALS Contraception The contraceptive efficacy of MIRENA has been studied in 5 major clinical studies with 3330 women using MIRENA. Two of the 5 studies were 5-year studies and 3 were 1-year studies. The failure rate (Pearl Index) was 0.21% at 1 year (based on data from 3330 women) and 0.14% at 5 years (based on data from 2245 women). This Pearl Index also includes pregnancies due to undetected expulsions and perforations. The cumulative failure rate was 0.2% at 1 years and 0.71% at 5 years MIRENA PI 3
4 In a large, prospective, comparative, non-interventional cohort study with an observation period of one year including more than 43,000 MIRENA users, the Pearl Index of MIRENA was 0.06 (95% CI: ). The use of MIRENA does not alter the course of future fertility. About 80% of the women wishing to become pregnant conceived within 12 months following the removal of MIRENA. Menstrual blood loss is generally reduced during use of MIRENA. Scanty blood flow frequently develops into oligomenorrhoea or amenorrhoea. Amenorrhoea is due to the local effect of levonorgestrel on the endometrium, which under strong local suppression, does not proliferate in response to estrogen. The menstrual pattern does not reflect the ovarian cycle. In the process of inactivation of the proliferation of the endometrium there can be an initial increase of spotting during the first months of use. Thereafter, the strong suppression of the endometrium results in the reduction of the duration and volume of menstrual bleeding during use of MIRENA. The production of the ovarian hormones, progesterone and estradiol, remain within normal limits, even when the users of MIRENA are amenorrhoeic. In clinical studies during the first year of use, 17% of women experienced amenorrhoea of at least three months duration, but the cumulative gross discontinuation rate for amenorrhoea was very low. The most common adverse event with the use of MIRENA is the change in menstrual bleeding pattern. These changes may include spotting, shorter or longer menstrual periods, or oligo/amenorrhoea. However spotting decreases gradually and the number of days spotting after six months was less than four, which was comparable to the experience with copper IUDs. During the first month of use, 20% of users experienced prolonged bleeding (more than eight days). For many women, periods became shorter and during the third month of use, only 3% of users had prolonged bleeding. Although bleeding patterns may vary from regular scanty menstruation in some women to oligo/amenorrhoea in others, there is no clear difference in follicle development, ovulation or estradiol and progesterone production in women with different bleeding patterns. Menorrhagia MIRENA can be successfully used in the treatment of idiopathic menorrhagia, where no organic reasons for excessive bleeding can be found (see CONTRAINDICATIONS). Menorrhagia caused by submucosal fibroids may respond less favourably to treatment with MIRENA. Results from three comparative studies indicate that in menorrhagic women, menstrual blood loss decreased by 62-94% at the end of three months and by 71-95% at the end of six months of use. MIRENA appears to have similar effects to endometrial ablation/resection in reducing the menstrual blood loss up to two years. Reduced bleeding may increase ferritin and haemoglobin levels. MIRENA may also alleviate dysmenorrhoea. The following table (see Table 1) indicates the effectiveness of MIRENA in the treatment of idiopathic menorrhagia from the clinical trials. The results indicate that menstrual blood loss was reduced by approximately 93% in women using MIRENA while the reduction in blood loss in the reference treatments was % depending upon the treatment. Another clinical trial (Study No ) compared the use of MIRENA with various standard oral treatments prior to a planned hysterectomy. More patients in the MIRENA group (67% compared with 15% in the reference group) decided to continue with MIRENA rather than proceed with the hysterectomy MIRENA PI 4
5 Table 1: Comparison between MIRENA (LNG IUS) and reference treatments on the extent of menstrual blood loss in women with idiopathic menorrhagia. Study No. Treatment (no. pts) Duration Study of Baseline values (range) End of study values Method Analysis of LNG IUS (28) 6 cycles 168 ml (80-348) Tranexamic 140 ml Acid (30) 5 (81-569) LNG IUS (22) 3 cycles 106 ml (82-780) NET 1,6 (22) 120 ml (82-336) 11 ml (0-151) 53 ml (21-528) 6 ml (0-284) 21 ml (0-137) Objective 3 Objective LNG IUS (30) 12 months PBAC 4 score ( ) (0-463) TCRE 2 (29) 271 ( ) 0 (0-61) 1 NET = Norethisterone, 2 TCRE = transcervical endometrial resection, 3 Objective = menstrual blood loss, 4 PBAC = pictorial blood loss assessment chart, 5 Dosage: two 500mg tablets, 4 times a day for maximum 5 days during bleeding, 6 Dosage: 15mg/day for 21 days in 28 day cycle. Hormone Replacement Therapy (HRT) MIRENA provides the progestogenic component of continuous HRT. Due to the local administration, the systemic levonorgestrel concentration is very low. The efficacy of MIRENA in preventing endometrial hyperplasia during continuous estrogen treatment has been equally efficacious when administering estrogen either orally or transdermally. The observed hyperplasia rate under estrogen therapy alone is as high as 20% after one year of continuous treatment. In clinical studies with a total of 634 perimenopausal and postmenopausal users of MIRENA, no endometrial hyperplasias were reported during the observation period varying from one up to five years. To date, clinical data presented on the use of MIRENA for the prevention of endometrial hyperplasia has been in study trials of 24 months duration or less. In clinical studies with MIRENA and copper IUDs used in contraception, no significant differences were found between the groups in serum levels of triglycerides, HDL cholesterol and total cholesterol after two and five years of treatment. The effect of MIRENA on lipid levels has been shown to be neutral. The concomitant estrogens used in the HRT studies were oral continuous estradiol valerate 2 mg/24 h, continuous transdermal estradiol 50 microgram /24 h, oral conjugated equine estrogen 0.625, 1.25 mg/day, estradiol implants 36 microgram/24 h and estradiol gel 1.5 mg/24 h. MIRENA was effective in preventing endometrial hyperplasia in association with these regimens. INDICATIONS MIRENA is indicated for: MIRENA PI 5
6 Contraception Treatment of idiopathic menorrhagia Prevention of endometrial hyperplasia during estrogen replacement therapy. CONTRAINDICATIONS Known or suspected pregnancy current or recurrent pelvic inflammatory disease lower genital tract infection postpartum endometritis infected abortion during the past three months cervicitis cervical dysplasia uterine or cervical malignancy confirmed or suspected hormone dependent tumours including breast cancer undiagnosed abnormal uterine bleeding congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity conditions associated with increased susceptibility to infections acute liver disease or liver tumour hypersensitivity to the active substance or to any of the excipients PRECAUTIONS General MIRENA may be used with caution after specialist consultation, or removal of the system should be considered if any of the following conditions exist or arise for the first time: migraine, focal migraine with asymmetrical visual loss or other symptoms indicating transient cerebral ischaemia exceptionally severe headache jaundice marked increase in blood pressure severe arterial disease such as stroke or myocardial infarction acute venous thromboembolism MIRENA is not the method of first choice for young nulligravid women. Previous studies indicate that an increased number of sexual partners may increase susceptibility to sexually transmitted infections (see Pelvic Infections). MIRENA is not the first choice for postmenopausal women with advanced uterine atrophy as the cervical canal is likely to be narrow, making the insertion more difficult. Tumours A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs), mainly using estrogen-progestogen preparations. The excess risk gradually disappears during the course of the 10 years after cessation of COC use. Because breast cancer is rare in women under 40 years of age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer. The risk of having breast cancer diagnosed in progestogen-only pill users is possibly of similar magnitude to that associated with COC. However, for progestogen-only MIRENA PI 6
7 preparations, the evidence is based on much smaller populations of users and so is less conclusive than that for COCs. Due to the limited exposure in MIRENA trials in the indication prevention of endometrial hyperplasia during estrogen replacement therapy, the available data is not sufficient to confirm or refute a risk for breast cancer when MIRENA is used in this indication. The product information of the estrogen replacement therapy should also be consulted for additional information. Since a biological effect cannot be excluded, an individual benefit-risk assessment should be made in women in whom breast cancer is diagnosed while using MIRENA. Removal of MIRENA should be considered. Irregular bleedings may mask some symptoms and signs of endometrial polyps or cancer. Endometrial pathology should therefore be excluded before using MIRENA. Irregular bleeding/spotting is common during the first few months of treatment, however if irregular bleeding develops during prolonged treatment, appropriate diagnostic measures should be taken. Heart Disease MIRENA may be used with caution in women who have congenital heart disease or valvular heart disease, at risk of infective endocarditis. Antibiotic prophylaxis should be administered to these patients when inserting or removing MIRENA. Diabetes Low-dose levonorgestrel may affect glucose tolerance, and the blood glucose concentration should be monitored in diabetic users of MIRENA. However there is generally no need to alter the therapeutic regimen in Type 1 diabetics using MIRENA. Oligo/amenorrhoea In a study with women of fertile age using MIRENA, oligomenorrhoea and amenorrhoea developed gradually in about 57% and 16% of women, respectively, at the end of the first year of use. The possibility of pregnancy should be considered if menstruation does not occur within six weeks of the onset of the previous menstruation. A repeated pregnancy test is not necessary in amenorrhoeic subjects unless indicated by other signs of pregnancy. When MIRENA is used in combination with continuous estrogen replacement therapy, a nonbleeding pattern gradually develops in most women during the first year. The rate of total amenorrhoea for at least 90 days is about 30% when MIRENA is used in perimenopausal women, and 50% in postmenopausal women after 1 year. During prolonged use of MIRENA the amount of amenorrhoea increases. Pelvic infection The insertion tube helps to prevent MIRENA from contamination with microorganisms during the insertion and the MIRENA inserter has been designed to minimise the risk of infections. In users of copper intrauterine devices, the highest rate of pelvic infections occurs during the first month after insertion and decreases later. Some studies suggest that the rate of pelvic infection in users of MIRENA is lower than with the copper-releasing intrauterine devices. Known risk factors for pelvic inflammatory disease are multiple sexual partners. Pelvic infection may have serious consequences and it may impair fertility and increase the risk of ectopic pregnancy MIRENA PI 7
8 As with other gynaecological or surgical procedures, severe infection or sepsis (including group A streptococcal sepsis) can occur following IUD insertion. If the woman experiences recurrent endometritis or pelvic infections or if an acute infection is severe or does not respond to treatment (antibiotics) within a few days, MIRENA must be removed. Even when clinical symptoms indicate an infection, bacteriological examinations (to test for organisms such as chlamydia) are indicated and further gynaecological monitoring over the subsequent days is recommended in order to ensure proper diagnosis of the underlying infection. Expulsion From the clinical studies with MIRENA, the 5-year gross cumulative expulsion rate was 2.2 to 5.8 per 100. Expulsion is reported as common or 1% and < 10% in the table which lists Adverse Drug Reactions. Symptoms of the partial or complete expulsion of any intrauterine device may include bleeding or pain. Other indications of a partial expulsion include an increase in the length of the removal threads or if the stem of the intrauterine system is visible in the cervix. An ultrasonographic examination may be needed to ensure the proper fundal position of MIRENA. However, the system can be expelled from the uterine cavity without the woman noticing it leading to a loss of contraceptive protection. Partial expulsion may decrease the effectiveness of MIRENA. As MIRENA decreases menstrual flow, increase of menstrual flow may be indicative of an expulsion. A displaced MIRENA should be removed and a new system can be inserted at that time. The woman should be advised how to check for the threads of MIRENA. Perforation Perforation or penetration of the uterine corpus or cervix by an intrauterine device may occur, most often during insertion, although it may not be detected until sometime later, and may decrease the effectiveness of MIRENA. Excessive pain or bleeding during insertion or while MIRENA is in situ may be indicative of a perforation. Such occurrences and/or lost threads should be further investigated. The frequency of perforations is similar to that with a copper intrauterine device ( 0.01% to <0.1%). Should a perforation occur, the system must be removed as soon as possible; surgery may be required. In a large, prospective, comparative, non-interventional cohort study in IUD users (n=61,448 women), the incidence of perforation was 1.3 (95% CI: ) per 1000 insertions in the entire study cohort; 1.4 (95% CI: ) per 1000 insertions in the MIRENA cohort and 1.1 (95% CI: ) per 1000 insertions in the copper IUD cohort. The study showed that both breastfeeding at the time of insertion and insertion up to 36 weeks after giving birth were associated with an increased risk of perforation (see Table 2). These risk factors were independent of the type of IUD inserted. Table 2: Incidence of perforation per 1000 insertions for the entire study cohort, stratified by breastfeeding and time since delivery at insertion (parous women) Breastfeeding at time of insertion Not breastfeeding at time of insertion MIRENA PI 8
9 Insertion 36 weeks after delivery Insertion 36 weeks after delivery 5.6 (95% CI: ; n=6,047 insertions) 1.6 (95% CI: ; n=608 insertions) 1.7 (95% CI: ; n=5,927 insertions) 0.7 (95% CI: ; n=41,910 insertions) The risk of perforations may be increased in women with fixed retroverted uterus. Re-examination after insertion should follow the guidance given under the heading Medical Examination (see DOSAGE AND ADMINISTRATION), which may be adapted as clinically indicated in women with risk factors for perforation. Ectopic Pregnancy Women with a previous history of ectopic pregnancy, tubal surgery or pelvic infection carry a higher risk of ectopic pregnancy. The possibility of an ectopic pregnancy should be considered in the case of lower abdomen pain especially in connection with missed periods or if an amenorrhoeic woman starts bleeding. In clinical trials, the ectopic pregnancy rate with MIRENA was approximately 0.1% per year. In a large, prospective, comparative, non-interventional cohort study with an observation period of one year, the ectopic pregnancy rate with MIRENA was 0.02%. This rate is lower than in women not using any contraception ( % per year). The absolute risk of ectopic pregnancy in MIRENA users is low. However, when a woman becomes pregnant with MIRENA in situ, the relative likelihood of this pregnancy being ectopic is increased and urgent assessment is required (see ADVERSE EFFECTS). Sexually Transmitted Infections MIRENA does not protect against HIV infection (AIDS) and other sexually transmitted infections (STIs). The woman should be advised that additional measures, e.g. condoms, are needed to prevent the transmission of STIs. Lost Threads If the retrieval threads are not visible at the cervix on follow-up examinations, pregnancy must be excluded. The threads may have been drawn up into the uterus or cervical canal and may appear during the next menstrual period. If pregnancy has been excluded, the threads may usually be located by gently probing with a suitable instrument. If they cannot be found, the possibility of expulsion or perforation should be considered. Ultrasound diagnosis may be used to ascertain the correct position of the system. If ultrasound is not available or successful, X-ray may be used to locate MIRENA. Ovarian Cysts Since the contraceptive effect of MIRENA is mainly due to its local effect, ovulatory cycles with follicular rupture occur in women of fertile age. Sometimes atresia of the follicle is delayed and folliculogenesis may continue. These enlarged follicles cannot be distinguished clinically from ovarian cysts. Ovarian cysts have been reported as adverse drug reactions in approximately 7% of women using MIRENA. Most of these follicles are asymptomatic, although some may be accompanied by pelvic pain or dyspareunia. In most cases, the ovarian cysts disappear spontaneously during two to three months observation. Should this not happen, continued ultrasound monitoring and other diagnostic/therapeutic measures are recommended. Rarely, surgical intervention may be required. Carcinogenicity No studies of the carcinogenic potential of MIRENA have been performed. Some studies suggest that combination oral contraceptive use has been associated with an increase in the MIRENA PI 9
10 risk of cervical intraepithelial neoplasia in some populations of women but there continues to be controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as human papilloma virus (HPV). Irregular bleeding patterns associated with the use of MIRENA could mask symptoms of cervical or endometrial cancer. Close clinical surveillance is essential in all women using MIRENA and in all cases of persistent or recurrent abnormal vaginal bleeding, appropriate diagnostic measures should be taken to eliminate the possibility of malignancy. Benign hepatic adenomas have been found to be associated with the use of oral contraceptives containing levonorgestrel. Although benign, hepatic adenomas may rupture and cause death through intra-abdominal haemorrhage. The contribution of the progestin component of oral contraceptives to the development of hepatic adenomas is not known. Genotoxicity Saline, ethanol and DMSO extracts of MIRENA were without mutagenic activity when tested in histidine-dependent auxotrophs of Salmonella typhimurium and tryptophan-dependent auxotrophs of Escherichia coli. Saline and DMSO extracts of the drug-releasing core of MIRENA were not mutagenic in mouse lymphoma cells or clastogenic in Chinese hamster ovary cells in vitro and they did not induce bone marrow micronuclei in mice in vivo. Saline and DMSO extracts of the polyethylene T-body of MIRENA were not mutagenic in bacteria or mouse lymphoma cells or clastogenic in human lymphocytes in vitro and neither saline or sesame oil extracts induced bone marrow nuclei in mice in vivo. Effects on Fertility Studies have suggested that in women who discontinue MIRENA for planned pregnancy, the pregnancy rate at one year is similar to those who do not use contraception. Use in Pregnancy: Category B3 MIRENA is not to be used during an existing or suspected pregnancy. If the woman becomes pregnant when using MIRENA, removal of the system is recommended, since any intrauterine contraceptive device left in situ may increase the risk of abortion and preterm labour. Removal of MIRENA or probing of the uterus may result in spontaneous abortion. Ectopic pregnancy should be excluded. If the intrauterine contraceptive device cannot be gently removed, termination of the pregnancy may be considered. If the woman wishes to continue the pregnancy and the system cannot be withdrawn, she should be informed about the risks and the possible consequences of premature birth to the infant. The course of such a pregnancy should be monitored closely. The woman should be instructed to report all symptoms that suggest complications of the pregnancy, like cramping abdominal pain with fever. Because of the intrauterine administration and the local exposure to the hormone, the possible occurrence of virilising effects in the fetus should be taken into consideration. Clinical experience of the outcomes of pregnancies under MIRENA is limited due to the high contraceptive efficacy, but the woman should be informed that, to date, there is no evidence of birth defects caused by MIRENA use in cases where pregnancy continues to term with MIRENA in place. When levonorgestrel-impregnated silastic devices were introduced into the uteri of pregnant rabbits, the incidence of late foetal resorption was increased when compared to sham-operated controls. There were no other effects on the foetuses that could be attributed specifically to the device or to levonorgestrel MIRENA PI 10
11 Use in Lactation About 0.1% of the levonorgestrel dose is transferred to the infant during breastfeeding. However it is not likely that there will be a risk for the infant with the dose released from MIRENA when it is inserted in the uterine cavity. Uterine bleeding has rarely been reported in women using MIRENA during lactation. There appears to be no deleterious effects on infant growth or development when using MIRENA after six weeks postpartum. Progestogen-only methods do not appear to affect the quantity or quality of breast milk (see Perforation section under PRECAUTIONS). Paediatric Use Safety and efficacy have been established in women of reproductive age. There is no relevant indication for the use of MIRENA before menarche. Use in the Elderly MIRENA has not been studied in women over the age of 65 years. Patients with Hepatic Impairment MIRENA is contraindicated in women with acute liver disease or liver tumour. Patients with Renal Impairment MIRENA has not been studied in women with renal impairment. Effects on Ability to Drive or Use Machines Not known INTERACTIONS WITH OTHER MEDICINES Interactions can occur with drugs that induce or inhibit microsomal enzymes, which can result in increased or decreased clearance of sex hormones. Substances increasing the clearance of levonorgestrel, e.g.: Phenytoin, barbiturates, primidone, carbamazepine, rifampicin, rifabutin, and possibly also oxcarbazepine, topiramate, felbamate, griseofulvin, and products containing St. John s wort. The influence of these drugs on the contraceptive efficacy of MIRENA is not known, but it is not believed to be of major importance due to the local mechanism of action. Substances with variable effects on the clearance of levonorgestrel: When co-administered with sex hormones, many HIV/HCV protease inhibitors and nonnucleoside reverse transcriptase inhibitors can increase or decrease plasma concentrations of the progestogen. Substances decreasing the clearance of levonorgestrel (enzyme inhibitors), e.g.: Strong and moderate CYP3A4 inhibitors such as azole antifungals (e.g. fluconazole, itraconazole, ketoconazole, voriconazole), verapamil, macrolides (e.g. clarithromycin, erythromycin), diltiazem and grapefruit juice can increase plasma concentrations of the progestin. ADVERSE EFFECTS MIRENA PI 11
12 Adverse events are more common during the first months after the insertion, and subside during prolonged use. In addition to the adverse effects listed in PRECAUTIONS, the following undesirable effects have been reported in users of MIRENA. Menstrual problems are the most often reported adverse events as described below. The most common adverse effect of MIRENA is a change in menstrual bleeding patterns. The changes may include spotting, shorter or longer menstrual periods, irregular bleeding, oligomenorrhoea, amenorrhoea, heavy flow, back pain and period pain/dysmenorrhoea (see CLINICAL STUDIES). The majority of women experience changes in menstrual bleeding pattern after insertion of MIRENA. In a study with women of fertile age using MIRENA, prolonged bleeding was experienced by 22% and irregular bleeding by 67% of women during the first 90 days after postmenstrual insertion of MIRENA, decreasing to 3% and 19% at the end of the first year of use, respectively. Concomitantly, amenorrhoea is experienced by 0% and infrequent bleeding by 11% during the first 90 days, increasing to 16% and 57% at the end of the first year of use, respectively. When MIRENA is used in combination with continuous estrogen replacement therapy, a nonbleeding pattern gradually develops in most women during the first year. The table below reports adverse reactions by MedDRA system organ classes with the frequencies based on clinical trial data. Frequencies are defined as: Very common ( 1/10) Common ( 1/100 to <1/10) Uncommon ( 1/1000 to <1/100) Rare ( 1/10000 to <1/1000) The frequencies are crude incidences of the events observed in clinical trials for the indications contraception and idiopathic menorrhagia (with 5,091 women and 12,101 woman-years). Adverse reactions in clinical trials in the indication prevention of endometrial hyperplasia during estrogen replacement therapy (with 514 women and 1,218.9 woman-years) were observed at a similar frequency unless specified by footnotes. Table 3: Adverse reactions reported in clinical trials System Organ Very common Common Uncommon Rare Class Psychiatric Depressed mood/ Altered mood disorders Depression Nervousness Decreased libido Nervous system Headache Migraine disorders Gastrointestinal disorders Skin and subcutaneous disorders Abdominal /pelvic pain Nausea Acne Hirsutism Abdominal distension Alopecia Pruritis Eczema Rash Urticaria MIRENA PI 12
13 System Organ Class Musculoskeletal, connective tissue and bone disorders Reproductive system and breast disorders General disorders and administration site conditions Very common Common Uncommon Rare Bleeding changes including increased and decreased menstrual bleeding, spotting, oligomenorrhoea and amenorrhoea Vulvovaginitis* Genital discharge* Back pain** Upper genital tract infection Ovarian cyst Dysmenorrhoea Breast tenderness Breast pain** Intra-uterine contraceptive device expelled (complete and partial) Cervicitis/Papanicolaou smear normal, class II Oedema Uterine perforation*** Investigations Weight increased The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and related condition. * Endometrial prevention trials: common ** Endometrial prevention trials: very common *** This frequency is based on clinical trials that excluded breastfeeding women. In a large, prospective, comparative, non-interventional cohort study in IUD users, the frequency of perforation in women who were breastfeeding or had an insertion up to 36 weeks after delivery was uncommon (see PRECAUTIONS). Description of Post Market Adverse Reactions Pregnancy, puerperium and perinatal conditions: When a woman becomes pregnant with MIRENA in situ, the relative risk of ectopic pregnancy is increased. The ectopic pregnancy rate with MIRENA is approximately 0.1% per year (see Precautions). Reproductive system and breast disorders The system or parts of it may perforate the uterine wall (see PRECAUTIONS). Functional ovarian cysts may develop, and have been diagnosed in about 7% of women using MIRENA. Most of these cysts are asymptomatic (see PRECAUTIONS). Breast Disorders Cases of breast cancer have been reported (frequency unknown), (see PRECAUTIONS). The risk of breast cancer is unknown when MIRENA is used in the indication prevention of endometrial hyperplasia during estrogen replacement therapy. Immune System Disorders: Hypersensitivity including rash, urticaria and angioedema MIRENA PI 13
14 Reproductive system disorders: The removal threads may be felt by the partner during intercourse. Injury, poisoning and procedural complications: The following ADRs have been reported in connection with the insertion or removal procedure of MIRENA: Procedural pain, procedural bleeding, insertion-related vasovagal reaction with dizziness or syncope. The procedure may precipitate a seizure in an epileptic patient. Infections and infestations: Cases of sepsis (including group A streptococcal sepsis) have been reported following IUD insertion (see PRECAUTIONS). Investigations: Blood pressure increased DOSAGE AND ADMINISTRATION The in vivo dissolution rate is approximately 20 microgram/24 h and is gradually reduced to approximately 10 microgram/24 h after five years. The mean dissolution rate of levonorgestrel is about 14 microgram/24 h over the time up to five years. Special instructions for insertion are in the package. The insertion technique differs to that for other intrauterine devices. Care must therefore be given to adequate training in the correct insertion technique and the availability of appropriate instruments for the insertion of MIRENA. Medical Examination Before insertion, the woman must be informed of the efficacy, risks and side effects of MIRENA. A physical examination including pelvic examination, examination of the breasts, and a cervical smear should be performed. Standard testing procedures should be used to exclude pregnancy and sexually transmitted diseases, and genital infections must have been successfully treated. The position of the uterus and the size of the uterine cavity should be determined. Fundal positioning of MIRENA is particularly important in order to ensure uniform exposure of the endometrium to the progestogen, prevent expulsion and maximise efficacy. Therefore, the instructions for insertion should be followed carefully. Insertion and removal may be associated with some pain and bleeding. The procedure may precipitate fainting as a vasovagal reaction, or a seizure in an epileptic patient. The woman should be re-examined 4 to 12 weeks after insertion and once a year thereafter, or more frequently if clinically indicated. Insertion and Removal of MIRENA In women of fertile age, MIRENA is to be inserted into the uterine cavity within seven days of the onset of menstruation. MIRENA can be replaced by a new system at any time in the cycle. The system can also be inserted immediately after first trimester abortion. Postpartum insertions should be postponed until the uterus is fully involuted, however not earlier than six weeks after delivery. If involution is substantially delayed, consider waiting until 12 weeks postpartum. In case of a difficult insertion and/or exceptional pain or bleeding during insertion, physical examination and ultrasound should be performed immediately to exclude perforation. MIRENA is not suitable for use as a post-coital contraceptive MIRENA PI 14
15 When used for endometrial protection during estrogen replacement therapy, MIRENA can be inserted at any time in an amenorrhoeic woman, or during the last days of menstruation or withdrawal bleeding. It is recommended that MIRENA should only be inserted by physicians/health care professionals who are experienced in MIRENA insertions and/or have undergone sufficient training for MIRENA insertion. Because irregular bleeding/spotting is common during the first months of therapy, endometrial pathology should be excluded before insertion of MIRENA. If the woman continues the use of MIRENA inserted earlier for contraception, endometrial pathology has to be excluded in the case of bleeding disturbances that appear after commencing estrogen replacement therapy. If bleeding irregularities develop during prolonged treatment, appropriate diagnostic measures should also be taken. MIRENA is removed by gently pulling on the threads with forceps. If the threads are not visible and the system is in the uterine cavity, it may be removed using either ovum forceps or an IUD hook. This may require dilatation of the cervical canal or other surgical intervention. The system should be removed after five years. If the user wishes to continue using the same method, a new system can be inserted at the same time. If pregnancy is not desired, the removal should be carried out during menstruation in women of reproductive age, provided there appears to be a menstrual cycle. If the system is removed in the mid-cycle and the woman has had intercourse within a week, she is at risk of pregnancy unless a new system is inserted immediately following removal. After removal of MIRENA, the system should be checked to see if it is intact. During difficult removals, single cases have been reported of the hormone cylinder sliding over the horizontal arms hiding them inside the cylinder. Further intervention is not required once completeness of the IUS has been ascertained. The knobs of the horizontal arms usually prevent complete detachment of the cylinder from the T-body. Concomitant Dosage Instructions for Use in HRT The concomitant estrogens used in the HRT studies were oral continuous estradiol valerate 2 mg/day, continuous transdermal estradiol 50 microgram/24 h, oral conjugated equine estrogen 0.625, 1.25 mg/day, estradiol implants 36 microgram/24 h and estradiol gel 1.5 mg/day. MIRENA was effective in preventing endometrial hyperplasia in association with these regimens. OVERDOSAGE Not applicable for this product. PRESENTATION AND STORAGE CONDITIONS MIRENA is supplied in a sterile pack, which should not be opened until required for insertion by a professional experienced in the insertion of intrauterine systems. The exposed product should be handled with aseptic precautions. If the seam of the sterile package is broken, the product should be discarded. A discarded or removed IUS should be treated as medicinal waste, since it may contain hormone remnants. Each pack contains one intrauterine system MIRENA PI 15
16 Special instructions for insertion are in the package. MIRENA is packaged in a polyethylene terephthalate glycol (PETG) sachet sealed with a peelable polyethylene film. Store below 25 C. NAME AND ADDRESS OF THE SPONSOR Bayer Australia Ltd ABN Pacific Highway Pymble NSW 2073 POISON SCHEDULE OF THE MEDICINE S4 DATE OF FIRST INCLUSION IN THE AUSTRALIAN REGISTER OF THERAPEUTIC GOODS (THE ARTG) 24 July 2000 DATE OF MOST RECENT AMENDMENT 21 July 2017 Registered Trademark of the Bayer Group, Germany MIRENA PI 16
Emergency contraception is an occasional method. It should in no instance replace a regular contraceptive method.
 1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
MIRENA (Mi RAY na) Consumer Medicine Information WHAT IS IN THIS LEAFLET WHAT MIRENA IS USED FOR BEFORE YOU USE MIRENA
 MIRENA (Mi RAY na) levonorgestrel intrauterine delivery system Consumer Medicine Information WHAT IS IN THIS LEAFLET This leaflet answers some common questions about Mirena. It does not contain all the
MIRENA (Mi RAY na) levonorgestrel intrauterine delivery system Consumer Medicine Information WHAT IS IN THIS LEAFLET This leaflet answers some common questions about Mirena. It does not contain all the
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system)
 FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
Levosert levonorgestrel 20mcg/24hour intrauterine device
 Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
Product Information. Confidence that lasts
 Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
PRODUCT INFORMATION LEVONELLE- 1. LEVONELLE-1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel.
 PRODUCT INFORMATION LEVONELLE 1 NAME OF THE DRUG LEVONELLE1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel. Levonorgestrel is a progestogen. The chemical
PRODUCT INFORMATION LEVONELLE 1 NAME OF THE DRUG LEVONELLE1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel. Levonorgestrel is a progestogen. The chemical
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME POSTINOR-1 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Levonorgestrel 1.5 mg 3 PHARMACEUTICAL FORM Each round white tablet contains 1.5 mg of levonorgestrel. The tablet
New Zealand Datasheet 1 PRODUCT NAME POSTINOR-1 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Levonorgestrel 1.5 mg 3 PHARMACEUTICAL FORM Each round white tablet contains 1.5 mg of levonorgestrel. The tablet
Instruction for the patient
 WS 4 Case 3 STI and IUD Your situation Instruction for the patient You are 32 years old, divorced and have one child; you have just started a new relationship You underwent surgical resection of the left
WS 4 Case 3 STI and IUD Your situation Instruction for the patient You are 32 years old, divorced and have one child; you have just started a new relationship You underwent surgical resection of the left
PRODUCT MONOGRAPH. Pr MIRENA. Levonorgestrel-releasing Intrauterine System (52 mg) to deliver. up to 20 mcg levonorgestrel per day.
 PRODUCT MONOGRAPH Pr MIRENA Levonorgestrel-releasing Intrauterine System (52 mg) to deliver up to 20 mcg levonorgestrel per day Progestogen Bayer Inc. 2920 Matheson Blvd East Mississauga, Ontario L4W 5R6
PRODUCT MONOGRAPH Pr MIRENA Levonorgestrel-releasing Intrauterine System (52 mg) to deliver up to 20 mcg levonorgestrel per day Progestogen Bayer Inc. 2920 Matheson Blvd East Mississauga, Ontario L4W 5R6
POSTINOR-1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel.
 PRODUCT INFORMATION POSTINOR 1 NAME OF THE MEDICINE POSTINOR1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel. Levonorgestrel is a progestogen. The chemical
PRODUCT INFORMATION POSTINOR 1 NAME OF THE MEDICINE POSTINOR1 is an emergency oral contraceptive tablet containing the synthetic progestogen, levonorgestrel. Levonorgestrel is a progestogen. The chemical
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines
 Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
The tablet is white, round, biconvex and 5 mm in diameter. On one side it is coded KV above 2 and on
 1. NAME OF THE MEDICINAL PRODUCT Cerazette 75 microgram film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 75 microgram desogestrel. Excipient (s) with known effect:
1. NAME OF THE MEDICINAL PRODUCT Cerazette 75 microgram film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 75 microgram desogestrel. Excipient (s) with known effect:
LONG-ACTING REVERSIBLE CONTRACEPTION. Summary Tables
 LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
Family Planning UNMET NEED. The Nurse Mildred Radio Talk Shows
 Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
Welcome to Mirena. The Mirena Handbook: A Personal Guide to Your New Mirena. mirena.com. Mirena is the #1 prescribed IUD * in the U.S.
 Mirena is the #1 prescribed IUD * in the U.S. Welcome to Mirena The Mirena Handbook: A Personal Guide to Your New Mirena *Intrauterine Device Supported by 2015-2016 SHS data INDICATIONS FOR MIRENA Mirena
Mirena is the #1 prescribed IUD * in the U.S. Welcome to Mirena The Mirena Handbook: A Personal Guide to Your New Mirena *Intrauterine Device Supported by 2015-2016 SHS data INDICATIONS FOR MIRENA Mirena
Contraception. Objectives. Unintended Pregnancy. Unintended Pregnancy in the US. What s the Impact? 10/7/2014
 Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
MODUS TABLETS. Medroxyprogesterone Tablets IP
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory MODUS TABLETS Medroxyprogesterone Tablets IP QUALITATIVE AND QUANTITATIVE COMPOSITION MODUS TABLET 2.5 mg Each uncoated
PRODUCT INFORMATION MICROLUT
 1 PRODUCT INFORMATION MICROLUT NAME OF THE MEDICINE Each Microlut tablet contains 0.03 mg levonorgestrel. The chemical name for levonorgestrel is 13β-ethyl-17β-hydroxy-18,19-dinor-17α-pregn- 4-en-20-yn-3-one.
1 PRODUCT INFORMATION MICROLUT NAME OF THE MEDICINE Each Microlut tablet contains 0.03 mg levonorgestrel. The chemical name for levonorgestrel is 13β-ethyl-17β-hydroxy-18,19-dinor-17α-pregn- 4-en-20-yn-3-one.
Menstrual Disorders & Ambulatory Gynaecology
 Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
Chapter 100 Gynecologic Disorders
 Chapter 100 Gynecologic Disorders Episode Overview: 1. Describe the presentation and RF for Adnexal torsion 2. List the imaging findings of adnexal torsion (US vs CT) 3. What is the management of adnexal
Chapter 100 Gynecologic Disorders Episode Overview: 1. Describe the presentation and RF for Adnexal torsion 2. List the imaging findings of adnexal torsion (US vs CT) 3. What is the management of adnexal
Primary Care Gynaecology Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
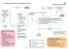 Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
The 28-day pack (Every-Day pack) contains 21 hormonal tablets, 6 tablets each with gestodene (17αethinyl-13-ethyl-17β-hydroxy-4,15-gonadiene-3-one)
 REGISTERED PACKAGE INSERT SCHEDULING STATUS S3 PROPRIETARY NAME AND DOSAGE FORM TRIODENE ED Tablets COMPOSITION The 28-day pack (Every-Day pack) contains 21 hormonal tablets, 6 tablets each with gestodene
REGISTERED PACKAGE INSERT SCHEDULING STATUS S3 PROPRIETARY NAME AND DOSAGE FORM TRIODENE ED Tablets COMPOSITION The 28-day pack (Every-Day pack) contains 21 hormonal tablets, 6 tablets each with gestodene
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
 SUMMARY TABLE SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION Cu-IUD = Copper-bearing intrauterine device; LNG-IUS = Levonorgestrel-releasing intrauterine system; IMP = Progestogen-only implant;
SUMMARY TABLE SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION Cu-IUD = Copper-bearing intrauterine device; LNG-IUS = Levonorgestrel-releasing intrauterine system; IMP = Progestogen-only implant;
World Health Organization Medical Eligibility for Contraceptive Use. Connie Kraus, PharmD, BCACP Professor (CHS) Director Office of Global Health
 World Health Organization Medical Eligibility for Contraceptive Use Connie Kraus, PharmD, BCACP Professor (CHS) Director Office of Global Health Objectives After this session, learners should be able to:
World Health Organization Medical Eligibility for Contraceptive Use Connie Kraus, PharmD, BCACP Professor (CHS) Director Office of Global Health Objectives After this session, learners should be able to:
International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage:
 Review Article ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com A REVIEW ON INTRAUTERINE DEVICES Boddu Venkata Komali* 1, M. Kalyani
Review Article ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com A REVIEW ON INTRAUTERINE DEVICES Boddu Venkata Komali* 1, M. Kalyani
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer
 levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
AROMASIN 25mg (Tablets)
 APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD your guide to
 your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
What s New in Adolescent Contraception?
 What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
Hyperandrogenism and polycystic ovary syndrome are clear casual factors (trends) which result in hirsuitism and acne.
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Indication: Treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhoea) and/or hirsutism,
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Indication: Treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhoea) and/or hirsutism,
Heavy Menstrual Bleeding. Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist
 Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
Topics. Periods Menopause & HRT Contraception Vulva problems
 Girls stuff Topics Periods Menopause & HRT Contraception Vulva problems Menorrhagia Excessive menstrual loss occurring with regular or irregular cycles Ovulatory Anovulatory Usual blood loss 30-40ml per
Girls stuff Topics Periods Menopause & HRT Contraception Vulva problems Menorrhagia Excessive menstrual loss occurring with regular or irregular cycles Ovulatory Anovulatory Usual blood loss 30-40ml per
100% Highly effective No cost No side effects
 effective? Advantages Disadvantages How do I get Cost Abstinence For some it can mean no sexual contact. For others it is no sexual intercourse or vaginal penetration. A permanent surgical procedure available
effective? Advantages Disadvantages How do I get Cost Abstinence For some it can mean no sexual contact. For others it is no sexual intercourse or vaginal penetration. A permanent surgical procedure available
Jadelle Contraceptive Implants up to 5 years Insertion and Removal
 Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Contraceptive Implants up to 5 years Bayer AG Global HealthCare Programs Family Planning 13342 Berlin, Germany www.bayer.com September 2017 Global HealthCare Programs Family Planning Contraceptive Independence»»
Package leaflet: Information for the user. Levonorgestrel 1.5 mg Tablet. levonorgestrel
 Package leaflet: Information for the user Levonorgestrel 1.5 mg Tablet levonorgestrel Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Levonorgestrel 1.5 mg Tablet levonorgestrel Read all of this leaflet carefully before you start taking this medicine because it contains important information
Core Safety Profile. Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU. Date of FAR:
 Core Safety Profile Active substance: Urofollitropin Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU P - RMS: UK/H/PSUR/0059/001 Date of FAR: 04.12.2009 4.2 Posology and method
Core Safety Profile Active substance: Urofollitropin Pharmaceutical form(s)/strength: Lyophilised powder for injection / 75 IU P - RMS: UK/H/PSUR/0059/001 Date of FAR: 04.12.2009 4.2 Posology and method
1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during : A systematic analysis
 1 2 3 1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980 2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet
1 2 3 1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980 2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet
CRISANTA LS Tablets (Drospirenone + Ethinylestradiol)
 Published on: 10 Jul 2014 CRISANTA LS Tablets (Drospirenone + Ethinylestradiol) Black Box Warning Cigarette Smoking and Serious Cardiovoscular Events: Cigarette smoking increases the risk of serious cardiovascular
Published on: 10 Jul 2014 CRISANTA LS Tablets (Drospirenone + Ethinylestradiol) Black Box Warning Cigarette Smoking and Serious Cardiovoscular Events: Cigarette smoking increases the risk of serious cardiovascular
PERSONAL CHARACTERISTICS AND REPRODUCTIVE HISTORY...3 Pregnancy...3 Age...3 Parity...3 Breastfeeding...3 Postpartum...3 Post-abortion...
 Table of contents Summary tables PERSONAL CHARACTERISTICS AND REPRODUCTIVE HISTORY...3 Pregnancy...3 Age...3 Parity...3 Breastfeeding...3 Postpartum...3 Post-abortion...3 Past ectopic pregnancy...3 History
Table of contents Summary tables PERSONAL CHARACTERISTICS AND REPRODUCTIVE HISTORY...3 Pregnancy...3 Age...3 Parity...3 Breastfeeding...3 Postpartum...3 Post-abortion...3 Past ectopic pregnancy...3 History
WHAT ARE CONTRACEPTIVES?
 CONTRACEPTION WHAT ARE CONTRACEPTIVES? Methods used to prevent fertilization *Also referred to as birth control methods With contraceptives, it is important to look at what works for you and your body.
CONTRACEPTION WHAT ARE CONTRACEPTIVES? Methods used to prevent fertilization *Also referred to as birth control methods With contraceptives, it is important to look at what works for you and your body.
Estrogens and progestogens
 Estrogens and progestogens Estradiol and Progesterone hormones produced by the gonads are necessary for: conception embryonic maturation development of primary and secondary sexual characteristics at puberty.
Estrogens and progestogens Estradiol and Progesterone hormones produced by the gonads are necessary for: conception embryonic maturation development of primary and secondary sexual characteristics at puberty.
GlaxoSmithKline. Renal impairment. Hepatic impairment
 RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
Concomitant antiretroviral therapy : Avifanz must be given in combination with other antiretroviral medications.
 Avifanz Tablet Description Avifanz is the brand name for Efavirenz. Efavirenz, a synthetic antiretroviral agent, is a non-nucleoside reverse transcriptase inhibitor. While Efavirenz is pharmacologically
Avifanz Tablet Description Avifanz is the brand name for Efavirenz. Efavirenz, a synthetic antiretroviral agent, is a non-nucleoside reverse transcriptase inhibitor. While Efavirenz is pharmacologically
trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene
![trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene](/thumbs/95/124190064.jpg) Genox Tamoxifen citrate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Tamoxifen (as citrate) trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene Structural formula:
Genox Tamoxifen citrate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Tamoxifen (as citrate) trans-1-[4-(2-dimethylaminoethoxy)phenyl]1,2-diphenyl-1-butene Structural formula:
2
 1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
Unintended Pregnancy is Common LEARNING OBJECTIVES. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy And Contraceptive Use
 3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
Imvexxy (estradiol) NEW PRODUCT SLIDESHOW
 Imvexxy (estradiol) NEW PRODUCT SLIDESHOW Introduction Brand name: Imvexxy Generic name: Estradiol Pharmacological class: Estrogen Strength and Formulation: 4mcg, 10mcg; vaginal inserts Manufacturer: TherapeuticsMD,
Imvexxy (estradiol) NEW PRODUCT SLIDESHOW Introduction Brand name: Imvexxy Generic name: Estradiol Pharmacological class: Estrogen Strength and Formulation: 4mcg, 10mcg; vaginal inserts Manufacturer: TherapeuticsMD,
5. Summary of Data Reported and Evaluation
 168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ebateva 10 mg Orodispersible Tablets Ebateva 20 mg Orodispersible Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One orodispersible
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ebateva 10 mg Orodispersible Tablets Ebateva 20 mg Orodispersible Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One orodispersible
Ardhanu Kusumanto Oktober Contraception methods for gyne cancer survivors
 Ardhanu Kusumanto Oktober 2017 Contraception methods for gyne cancer survivors Background cancer treatment Care of gyn cancer survivor Promotion of sexual, cardiovascular, bone, and brain health management
Ardhanu Kusumanto Oktober 2017 Contraception methods for gyne cancer survivors Background cancer treatment Care of gyn cancer survivor Promotion of sexual, cardiovascular, bone, and brain health management
Fitting of an Intrauterine Device (IUD)
 PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is an IUD? An IUD is a small T-shaped plastic and copper device that is put into
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is an IUD? An IUD is a small T-shaped plastic and copper device that is put into
Data Sheet. BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other.
 BICALOX Data Sheet Bicalutamide 50 mg tablets Presentation BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other. Uses Actions
BICALOX Data Sheet Bicalutamide 50 mg tablets Presentation BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other. Uses Actions
Anatomic Therapeutic and Chemical (ATC) Classification and Distribution Category Anatomic Therapeutic and Chemical (ATC) Classification: G03AC03
 Name of the medicinal product FAMY POP (Levonorgestrel Tablets, 0.03 mg) Anatomic Therapeutic and Chemical (ATC) Classification and Distribution Category Anatomic Therapeutic and Chemical (ATC) Classification:
Name of the medicinal product FAMY POP (Levonorgestrel Tablets, 0.03 mg) Anatomic Therapeutic and Chemical (ATC) Classification and Distribution Category Anatomic Therapeutic and Chemical (ATC) Classification:
Dr Mary Birdsall. Fertility Associates Auckland
 Dr Mary Birdsall Fertility Associates Auckland Period Problems Mary Birdsall Medical Director Fertility Associates Auckland Period Problems Basic Physiology No Periods Irregular Periods Heavy Periods
Dr Mary Birdsall Fertility Associates Auckland Period Problems Mary Birdsall Medical Director Fertility Associates Auckland Period Problems Basic Physiology No Periods Irregular Periods Heavy Periods
White to off-white, round, flat-faced, bevelled-edge tablets with an embossed B on one side and a diameter of 7 mm.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Visanne 2 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg dienogest Excipient: each tablet contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Visanne 2 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg dienogest Excipient: each tablet contains
Endometriosis. *Chocolate cyst in the ovary
 Endometriosis What is endometriosis? Endometriosis is a common condition in young women. It's chronic, painful, and it often progressively gets worse over the time. *Chocolate cyst in the ovary Normally,
Endometriosis What is endometriosis? Endometriosis is a common condition in young women. It's chronic, painful, and it often progressively gets worse over the time. *Chocolate cyst in the ovary Normally,
LEARNING OBJECTIVES. Beyond the Pill: Long Acting Contraception. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy is Common
 4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
Contraceptives. Kim Dawson October 2010
 Contraceptives Kim Dawson October 2010 Objectives: You will learn about: The about the different methods of birth control. How to use each method of birth control. Emergency contraception What are they?
Contraceptives Kim Dawson October 2010 Objectives: You will learn about: The about the different methods of birth control. How to use each method of birth control. Emergency contraception What are they?
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ellaone 30 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30 mg ulipristal acetate. Excipients with known
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ellaone 30 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30 mg ulipristal acetate. Excipients with known
GLOBALTRAINING. IMPLANON : Reference Guide
 GLOBALTRAINING IMPLANON : Reference Guide IMPLANON : Clinical Information IMPLANON is a subdermal, long-acting, progestagen-only contraceptive effective for up to 3 years. The mechanisms of action of IMPLANON
GLOBALTRAINING IMPLANON : Reference Guide IMPLANON : Clinical Information IMPLANON is a subdermal, long-acting, progestagen-only contraceptive effective for up to 3 years. The mechanisms of action of IMPLANON
CLIMARA 25 CLIMARA 50 CLIMARA 75 CLIMARA 100
 REGISTERED PACKAGE INSERT SCHEDULING STATUS S4 PROPRIETARY NAMES AND DOSAGE FORMS CLIMARA 25 CLIMARA 50 CLIMARA 75 CLIMARA 100 Estradiol transdermal systems COMPOSITION Climara 25: Climara 50: Climara
REGISTERED PACKAGE INSERT SCHEDULING STATUS S4 PROPRIETARY NAMES AND DOSAGE FORMS CLIMARA 25 CLIMARA 50 CLIMARA 75 CLIMARA 100 Estradiol transdermal systems COMPOSITION Climara 25: Climara 50: Climara
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Orgalutran 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that
 The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that may be used in the future Abstinence Choosing not to
The following lesson on contraception (birth control) is not intended to infer that you will be sexually active as a teen. This is information that may be used in the future Abstinence Choosing not to
Menopause management NICE Implementation
 Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Menopause management NICE Implementation Dr Paula Briggs Consultant in Sexual & Reproductive Health Southport and Ormskirk NHS Hospital Trust Why a NICE guideline (NG 23) Media reports about HRT have not
Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.
 Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) Rx Only This product (like all oral contraceptives) is intended to prevent pregnancy.
Patient Guide Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) Rx Only This product (like all oral contraceptives) is intended to prevent pregnancy.
Medical Eligibility for Contraception Use
 Medical Eligibility for Contraception Use DIVISION OF REPRODUCTIVE HEALTH CENTERS FOR DISEASE CONTROL AND PREVENTION 2016 US Medical Eligibility Criteria for Contraceptive Use (US MEC) Purpose To assist
Medical Eligibility for Contraception Use DIVISION OF REPRODUCTIVE HEALTH CENTERS FOR DISEASE CONTROL AND PREVENTION 2016 US Medical Eligibility Criteria for Contraceptive Use (US MEC) Purpose To assist
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
Frequency of menses. Duration of menses 3 days to 7 days. Flow/amount of menses Average blood loss with menstruation is 60-80cc.
 Frequency of menses 24 days (0.5%) to 35 days (0.9%) Age 25, 40% are between 25 and 28 days Age 25-35, 60% are between 25 and 28 days Teens and women over 40 s cycles may be longer apart Duration of menses
Frequency of menses 24 days (0.5%) to 35 days (0.9%) Age 25, 40% are between 25 and 28 days Age 25-35, 60% are between 25 and 28 days Teens and women over 40 s cycles may be longer apart Duration of menses
SUMMARY OF PRODUCT CHARACTERISTICS
 MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
Contraception and gynecological pathologies
 1 Contraception and gynecological pathologies 18 years old, 2 CMI normal First menstruation at 14 years old Irregular (every 2/3 months), painful + She does not need contraception She is worried about
1 Contraception and gynecological pathologies 18 years old, 2 CMI normal First menstruation at 14 years old Irregular (every 2/3 months), painful + She does not need contraception She is worried about
Learning Objectives. Peri menopause. Menopause Overview. Recommendation grading categories
 Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene)
 EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
Management of Emergency Contraception (EC)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Management of Emergency Contraception (EC) The risks and benefits of an IUD or oral EC should be discussed and documented (see appendix). Reasonable measures
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Management of Emergency Contraception (EC) The risks and benefits of an IUD or oral EC should be discussed and documented (see appendix). Reasonable measures
Reproduction and Development. Female Reproductive System
 Reproduction and Development Female Reproductive System Outcomes 5. Identify the structures in the human female reproductive system and describe their functions. Ovaries, Fallopian tubes, Uterus, Endometrium,
Reproduction and Development Female Reproductive System Outcomes 5. Identify the structures in the human female reproductive system and describe their functions. Ovaries, Fallopian tubes, Uterus, Endometrium,
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
Bio 12- Ch. 21: Reproductive System
 Bio 12- Ch. 21: Reproductive System 21.1- Male Reproductive System o Male anatomy o Testes and how they relate to sperm production and male sex hormones o Hormone regulation in males 21.2- Female Reproductive
Bio 12- Ch. 21: Reproductive System 21.1- Male Reproductive System o Male anatomy o Testes and how they relate to sperm production and male sex hormones o Hormone regulation in males 21.2- Female Reproductive
PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG. (goserelin)
 ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S.
 1 2 1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S. The contraceptive action of all IUDs is mainly in the uterine cavity. The major effect
1 2 1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S. The contraceptive action of all IUDs is mainly in the uterine cavity. The major effect
5. Summary of Data Reported and Evaluation
 326 5. Summary of Data Reported and Evaluation 5.1 Exposure data Combined estrogen progestogen menopausal therapy involves the co-administration of an estrogen and a progestogen to peri- or postmenopausal
326 5. Summary of Data Reported and Evaluation 5.1 Exposure data Combined estrogen progestogen menopausal therapy involves the co-administration of an estrogen and a progestogen to peri- or postmenopausal
Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa
 Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa Objectives Distinguish the different types of IUDs Understand the mechanism of action and selection of candidates
Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa Objectives Distinguish the different types of IUDs Understand the mechanism of action and selection of candidates
VI.2. ELEMENTS FOR A PUBLIC SUMMARY
 VI.2. ELEMENTS FOR A PUBLIC SUMMARY VI.2.1 Overview of Disease Epidemiology COCs containing DRSP-EE are indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method
VI.2. ELEMENTS FOR A PUBLIC SUMMARY VI.2.1 Overview of Disease Epidemiology COCs containing DRSP-EE are indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method
Bursting Pelvic Inflammatory Disease.
 www.infertiltysolutionsng.info/blog Disclaimer The information in this book is provided for educational purposes only and is not intended to treat, diagnose or prevent any disease. The information in this
www.infertiltysolutionsng.info/blog Disclaimer The information in this book is provided for educational purposes only and is not intended to treat, diagnose or prevent any disease. The information in this
CYPROSTAT 100mg PRODUCT INFORMATION NAME OF THE MEDICINE. Cyprostat 100mg. Each pack contains tablets with 100mg cyproterone acetate.
 CYPROSTAT 100mg PRODUCT INFORMATION NAME OF THE MEDICINE Cyprostat 100mg Each pack contains tablets with 100mg cyproterone acetate. Cyproterone acetate: Chemical Name: 6-chloro-17 hydroxy-1,2-methylene-pregna-4,6-diene-3,20-dione
CYPROSTAT 100mg PRODUCT INFORMATION NAME OF THE MEDICINE Cyprostat 100mg Each pack contains tablets with 100mg cyproterone acetate. Cyproterone acetate: Chemical Name: 6-chloro-17 hydroxy-1,2-methylene-pregna-4,6-diene-3,20-dione
The 6 th Scientific Meeting of the Asia Pacific Menopause Federation
 Abnormal uterine bleeding in the perimenopause Perimenopausal menstrual problems are among the most common causes for family practitioner and specialist referral. Often it is due to the hormone changes
Abnormal uterine bleeding in the perimenopause Perimenopausal menstrual problems are among the most common causes for family practitioner and specialist referral. Often it is due to the hormone changes
New Zealand Datasheet. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Bicalutamide 50 mg film coated tablets
 New Zealand Datasheet 1 PRODUCT NAME Binarex 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Bicalutamide 50 mg film coated tablets 3 PHARMACEUTICAL FORM Binarex tablets are white to off-white, circular, biconvex,
New Zealand Datasheet 1 PRODUCT NAME Binarex 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Bicalutamide 50 mg film coated tablets 3 PHARMACEUTICAL FORM Binarex tablets are white to off-white, circular, biconvex,
Contraception Effective Methods of Birth Control
 Contraception Effective Methods of Birth Control Abstinence Means choosing NOT to have sex It is the ONLY method that is 100% effective It is your right to be in control of your body and say NO What are
Contraception Effective Methods of Birth Control Abstinence Means choosing NOT to have sex It is the ONLY method that is 100% effective It is your right to be in control of your body and say NO What are
Emergency Contraception THE FACTS
 Emergency Contraception Quick Facts What is it? Emergency contraception is birth control that you use after you have had unprotected sex--if you didn t use birth control or your regular birth control failed.
Emergency Contraception Quick Facts What is it? Emergency contraception is birth control that you use after you have had unprotected sex--if you didn t use birth control or your regular birth control failed.
Community Gynaecology. Top Tips for GPs
 Community Gynaecology Top Tips for GPs Top Tips for GPs Case Scenarios- common referral themes 6 topics What you can do in Primary Care to avoid or before referral. What we don t need to see Triage ensures
Community Gynaecology Top Tips for GPs Top Tips for GPs Case Scenarios- common referral themes 6 topics What you can do in Primary Care to avoid or before referral. What we don t need to see Triage ensures
Information for Informed Consent for Insertion of a Mirena IUD
 Information for Informed Consent for Insertion of a Mirena IUD What is an IUD (intrauterine Device)? An intrauterine device (IUD) is a plastic device that is placed into your uterus to prevent pregnancy.
Information for Informed Consent for Insertion of a Mirena IUD What is an IUD (intrauterine Device)? An intrauterine device (IUD) is a plastic device that is placed into your uterus to prevent pregnancy.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SAMPLE REPORT. Order Number: PATIENT. Age: 40 Sex: F MRN:
 Patient: Age: 40 Sex: F MRN: SAMPLE PATIENT Order Number: Completed: Received: Collected: SAMPLE REPORT Progesterone ng/ml 0.34 0.95 21.00 DHEA-S mcg/dl Testosterone ng/ml 48 35 0.10 0.54 0.80 430 Sex
Patient: Age: 40 Sex: F MRN: SAMPLE PATIENT Order Number: Completed: Received: Collected: SAMPLE REPORT Progesterone ng/ml 0.34 0.95 21.00 DHEA-S mcg/dl Testosterone ng/ml 48 35 0.10 0.54 0.80 430 Sex
Estrogens & Antiestrogens
 Estrogens & Antiestrogens Menstrual cycle... Changes and hormonal events Natural estrogens: Estadiol >> Estrone > Estriol Ineffective orally Synthesis: From cholesterol ; role of aromatase enzyme in converting
Estrogens & Antiestrogens Menstrual cycle... Changes and hormonal events Natural estrogens: Estadiol >> Estrone > Estriol Ineffective orally Synthesis: From cholesterol ; role of aromatase enzyme in converting
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS
 CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
VI.2. ELEMENTS FOR A PUBLIC SUMMARY
 VI.2. ELEMENTS FOR A PUBLIC SUMMARY VI.2.1 Overview of Disease Epidemiology COCs (Combined Oral Contraceptives) containing DRSP-EE (Drospirenone- Ethinylestradiol) are indicated for the prevention of pregnancy
VI.2. ELEMENTS FOR A PUBLIC SUMMARY VI.2.1 Overview of Disease Epidemiology COCs (Combined Oral Contraceptives) containing DRSP-EE (Drospirenone- Ethinylestradiol) are indicated for the prevention of pregnancy
HRT in Perimenopausal Women. Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College
 HRT in Perimenopausal Women Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College 1 This is the Change But the CHANGE is not a disease 2 Introduction With a marked increase in longevity, women now
HRT in Perimenopausal Women Dr. Rubina Yasmin Asst. Prof. Medicine Dhaka Dental College 1 This is the Change But the CHANGE is not a disease 2 Introduction With a marked increase in longevity, women now
ESSURE A RESOURCE FOR CODING
 ESSURE REIMBURSEMENT GUIDE A RESOURCE FOR CODING INDICATION Essure is indicated for women who desire permanent birth control (female sterilization) by bilateral occlusion of fallopian tubes. IMPORTANT
ESSURE REIMBURSEMENT GUIDE A RESOURCE FOR CODING INDICATION Essure is indicated for women who desire permanent birth control (female sterilization) by bilateral occlusion of fallopian tubes. IMPORTANT
