PUBLIC HEALTH COMMUNINTY BASED SERVICES SERVICE SPECIFICATIONS
|
|
|
- Antonia Morrison
- 5 years ago
- Views:
Transcription
1 PUBLIC HEALTH COMMUNINTY BASED SERVICES SERVICE SPECIFICATIONS Service Specification No: Service: Authority Lead: CBS18-19(03) Provision of intrauterine device (IUD), intrauterine system (IUS) and sub-dermal implants (SDI) Fitting and Removal Service for contraceptive purposes and gynaecological (noncontraceptive) purposes including management of menorrhagia and hormone replacement therapy (HRT). Janet Hutchins Period: 1 st April 2018 to 31 st March 2019 Commissioner: The commissioner detailed as The County Councils in this service specification and refers to Leicestershire County Council and Rutland County Council Leicestershire County Council, County Hall, Glenfield, Leicester, LE3 8RA Rutland County Council, Catmose, Oakham, Rutland, LE15 6HP 1. Purpose This document represents the agreement between the provider and Leicestershire County Council and Rutland County Council for the before mentioned community based service and is an appendix to the Contract for the Provision of Public Health Services. 2. Contract Price and Payment Methods 2.1 The provider will be funded for this service, based on the evidence of competency completion, service aims and criteria, on a cost per case basis. 2.2 Payment will be at the rate of: SDI Fitting 40 Removal 35 Device Claim 80 IUD Fitting Post-fitting review IUS Fitting Post-fitting review Payment for the service will be based on the submission of activity at the end of each quarter and will be paid at the end of the following month after quarter end. Claims submitted for IUS services must categorise the reason for fitting. i.e. contraceptive purposes OR gynaecological purposes, such as menorrhagia OR both contraceptive
2 and gynaecological purposes. 2.4 If the provider wishes to purchase the implant device in bulk, claims can be made for the cost of individual devices as they are fitted via the current quarterly Community Based Services claim form. Alternatively Providers can obtain devices for patients via the prescribing route, in which case a claim for the device will not be necessary. All IUD and IUS devices will be obtained via the prescribing route. The cost of prescribed devices will be reimbursed by the Local Authority under arrangements with the relevant Clinical Commissioning Group. 2.5 If a practice is unable to provide the service according to the criteria set out below, patients at the practice should have the opportunity to receive his service from an alternative provider locally, e.g. Interpractice referrals across Federations. Providers wishing to provide the service for other practice populations in this way should, together with the referring practice, seek written permission from the Local Authority. This would entitle the approved Provider to the payment associated with this Community Based Service. See 3.2 below. 3. Service Delivery 3.1 This document represents the agreement between the Local Authority and the Provider for: a) the provision, monitoring, fitting and removal of intrauterine devices (IUDs), intrauterine systems (IUSs) and/or sub-dermal implants (SDIs) to a practice population as part of a wider range of contraceptive options provided, and b) the provision, review and subsequent removal, of Levonorgestrel Intrauterine System (LNG-IUS) for gynaecological (non-contraceptive) purposes including management of menorrhagia and hormone replacement therapy (HRT) commissioned on behalf of the Clinical Commissioning Group (CCG). Ongoing management of gynaecological issues which will not be included in this agreement. (The element of care covered by this specification in relation to use of LNG-IUS for management of gynaecological conditions such as menorrhagia is detailed in Appendix D.) 3.2 This service will be provided by the Provider for all registered patients only, unless otherwise agreed by the Local Authorities. See section 2.5. If approval is given, the provider would then be entitled to payment associated with services delivered to patient s resident within the County Councils boundaries. 3.3 The aims of this Community Based Service are: To increase the availability of intrauterine device (IUD), intrauterine system (IUS) and sub-dermal implants (SDI) for contraceptive purposes to a practice population as part of a range of contraceptive options. To raise awareness of the benefits of long-acting reversible contraception by providing high quality advice, support and information on the full range of contraception methods to all women on or seeking contraception. To ensure that the availability of post-coital IUD fitting for emergency contraception should be more adequately provided as another means of reducing unwanted pregnancies To provide the fitting and removal element of LNG-IUS for women requiring Levonorgestrel intrauterine system LNG-IUS fitting as management of menorrhagia or other gynaecological purpose such as HRT, endometriosis etc, where clinically relevant, thus reducing the requirement for hysterectomy. (As per Section 75 agreement between Leicestershire County Council, Rutland County Council, WLCCG and EL&R CCG) 4. Service Criteria
3 4.1 To ensure that patients are provided with information as to the full range of contraceptive options available. Written & verbal information should be provided by Providers to patients to enable informed choice of all options available prior to the acceptance of fitting an IUD/IUS or SDI for contraceptive purposes. Where appropriate patients are signposted to alternative service provision. 4.2 Provide fitting, monitoring, checking and removal of IUD/IUS or SDI licensed for use in the UK, as appropriate and in accordance with the most current clinical guidance. (e.g. NICE guidance on Long Acting Reversible Contraception, NICE Contraception Quality Standard QS129 and Faculty of Sexual & Reproductive Healthcare clinical guidance and standards and relevant guidance and standards relating to use of IUS for gynaecological purposes,(see Appendix D). 4.3 Maintain an up-to-date register of patients fitted with an IUD/IUS or SDI detailing for contraceptive purposes, gynaecological purpose or for both contraception and gynaecological purposes. This should include the details of all patients fitted with an IUD/IUS or SDI, type of device fitted, the batch number and expiry date and the name and designation of the person completing the procedure. This is to be used for audit purposes, call and recall, and to enable the Provider to target patients for health care checks. 4.4 Ensure all Practitioners performing this service are appropriately qualified and undertake regular continual professional development (CPD). Evidence of which must be provided to the commissioner to deliver the service. 4.5 Ensure the provision of adequate equipment. Certain special equipment is required. For IUD/IUS or SDI fitting and removal this includes an appropriate room fitted with a couch and with adequate space and equipment including decontamination equipment and basic resuscitation (For example: oxygen, suction and defibrillation) equipment as appropriate to minimise risks and ensure health and safety for patients. For SDI service provision, a variety of removal forceps and the facility for local anaesthesia provision needs to be available. For IUD/IUS service provision vaginal specula, cervical dilators, and equipment for cervical anaesthesia needs to be available. This specification includes the provision of sterile surgical instruments and other consumables. An appropriately trained assistant needs to be present in the building to support the patient and assist the clinician during the procedure if required. 4.6 Provide advice on the use of condoms to prevent infection, public health information on safer sex practices. (Clinical Guidance Intrauterine device, Faculty of Sexual & Reproductive Healthcare (FSRH) clinical guidance, April 2015 (Updated October 2015) p27) 4.7 Undertake a clinical history, including sexual history of all to ensure that the contraceptive choice is the most appropriate method of contraception based on medical evidence, clinical guidelines, sexual history and provider, and risk assessment. 4.8 Undertake a risk assessment. This is to assess the need for pregnancy, STI or HIV testing prior to recommending the IUD/IUS/SDI. The criteria for excluding pregnancy detailed in the FSRH Clinical Guidance for intrauterine contraception, with pregnancy testing undertaken as appropriate. As a minimum all women aged years are to be offered Chlamydia screening. Where diagnosis for Chlamydia is positive the Provider will treat and refer for screening for other STIs. This should be in
4 accordance with national policy or local policy if there is no relevant national policy. 4.9 Undertake assessment and follow up in accordance with FSRH guidance. SDI: Routine annual checks are not required; however arrangements should be in place to review patients experiencing problems in a timely fashion. Arrangements should be in place to ensure timely access for patients requesting removal of the implant for any reason including problems or at expiry of device. The implant should be removed or replaced within three years. IUD/IUS: A routine follow-up visit can be advised after the first menses following insertion of IUC or 3-6 weeks later. However, it is not essential and it may be more important to advise women as to signs and symptoms of infection, perforation and expulsion, returning if they have any problems relating to their intrauterine method. Follow up that does not relate specifically to the insertion of the LNG-IUS i.e. related to the overall gynaecological condition is not included in this agreement. This remains part of the general GMS/PMS contract Provide information. Appropriate verbal and written information should be provided at the time of counselling and reinforced at fitting with information on effectiveness, duration of use, possible side effects, follow-up and those symptoms that require urgent assessment. Patient information is available from Produce an appropriate clinical record. Adequate recording should be made regarding the patient s clinical, reproductive and sexual history, the counselling process, the results of any STI screening, the pelvic examination if applicable, problems with insertion, the type and batch number of the device, expiry date of the device and follow up arrangements If the patient is not registered with the Provider providing this community based service, the Provider, with the consent of the patient, must ensure that the patient s registered Practice is given all appropriate clinical details for inclusion into the patient s notes Submit annual audit for each individual practitioner, including locums, delivering the service during the contract period (Appendices A & B) Contractors are advised to design their data collection to reflect the requirements of the audit for this particular community based service. Audits for the period 1 April March 2018 should be submitted electronically via the Public Health Community Based Services On-line Claim System ( with a return date of 1 May Audits for the period 1 April March 2019 should be submitted electronically via the Public Health Community Based Services On-line Claim System ( with a return date of 1 May Audits for the period 1 April March 2020 should be submitted electronically via the Public Health Community Based Services On-line Claim System ( with a return date of 1 May The annual audit is a key component of demonstrating competency to deliver the service. Payment will be withheld to providers that do not submit their annual audits within the timescales above. If audits are continually not returned, the commissioner has the right to terminate contracts In addition to terms set out in Section 1 contractors must at all times meet the most recent relevant standards set out in FSRH Guidance for intrauterine contraception and sub dermal implants; NICE Clinical Guidance on Long Acting Reversible Contraceptives Ref CG30 October 2005,NICE guidance for one to one interventions to reduce the transmission of STIs including HIV and reduce the rate of under 18 conceptions especially amongst the most vulnerable and at risk groups PH Feb 2007, NICE guidance on Contraceptive Services with a focus on young people
5 up to the age of 25 Ref PH51, NICE Contraception Quality Standard QS129 and guidance specific to IUS provision for gynaecological purposes(see Appendix D) The Provider will not ordinarily refer a patient to specialist contraceptive/sexual health services for an IUS/IUD or SDI insertion, removal or review procedure. Exceptions to this would include patient choice for a female practitioner if unavailable at the provider, following a failed IUD/S insertion, inability to palpate an implant, inability to remove an implant or concerns about patients long term medical conditions or other disorders, in which case patients should be referred to the Leicester, Leicestershire & Rutland Integrated Sexual Health Service or other appropriate service A robust system for referral of adverse pathology must be in place and demonstrated if required by the Local Authority. 5. Accreditation 5.1 The Provider must satisfy The Local Authority that all health care professionals are appropriately accredited and trained to provide the services detailed in this Service Specification and can meet the Service Criteria as detailed in section 4. (See Appendix B) Providers must ensure that: 5.3 Health care professionals providing the service hold membership of an approved professional body and are approved and eligible to practice in a setting that is appropriate to deliver the service detailed in the specification. 5.4 Health care professionals have a regular appraisal and maintain professional development generally 5.5 The Provider will procure appropriate training for all relevant staff to ensure safe and competent delivery of this Service Specification 5.6 Up-to-date certifications of practitioner competency must be maintained and will be requested by the Local Authority. If this evidence cannot be demonstrated to the Local Authority when requested, the practitioner cannot deliver the service under this contract. Providers must notify the Local Authority of any changes in practitioners delivering the service and provide up-to-date certifications. 5.7 Provider Protocols: - The Provider will ensure all health care professionals are compliant with the Provider protocols for the clinical management of all patients in receipt of services commissioned. These protocols must be in line with best practice clinical guidelines and be reviewed on a regular basis. The Provider must ensure that all protocols reflect up-to-date national and local guidance and are amended in the light of any changes. 5.8 Provider Recording Systems: - As relevant to the Service Specification, a register of all patients will be maintained at all times and include the patient s name, identification number and other relevant information to the service. This information must also be included within the patient s lifelong record. 5.9 All health care professionals inserting, monitoring and removing IUD/IUS or SDIs must notify themselves to The Local Authority and provide the necessary evidence to confirm eligibility before delivering the service from 1 st April Practitioner contact details will be shared with the Leicester, Leicestershire & Rutland Integrated Sexual Health Service to enable the service to offer support to fitters and to cascade details of relevant training and clinical update information.
6 5.10 It is essential that all practitioners undertaking these procedures hold either: An up to date Faculty of Sexual & Reproductive Healthcare letter of competency in sub-dermal contraceptive implant insertion and removal (LoC SDI) and/or Faculty of Sexual & Reproductive Healthcare letter of competency in intrauterine techniques (LoC IUT) in accordance with services provided by that practitioner. This requirement applies to all practitioners newly delivering this service in LLR. OR Hold an up to date local LoC recertification IUT/SDI following training as detailed in Appendix B. (Existing LLR Practitioners only) In addition: Each practitioner providing IUD/S should fit at least 12 IUD/S devices in a twelve month period within twenty-four months prior to recertification (minimum of two types of device) and keep an audit of their practice. Each practitioner providing SDIs should complete a minimum of 6 procedures in a twelve month period within twenty four months prior to recertification (at least 1 insertion and 1 removal) and keep an audit of their practice. It is considered desirable that practitioners deliver a minimum of 12 IUD/S devices and/or 6 SDI procedures in each audit 12 month period. Lower fitting rates will be considered on an individual basis in conjunction with the annual audit information for the practitioner Appendix A). Important Note: From 1 April 2020, all practitioners will be required to hold and evidence the FSRH IUT Loc and/or LoC SDI to deliver these services in future contracts with Leicestershire and Rutland County Councils Re-certification All health professionals providing IUD/IUS or SDI services must apply for recertification every 5 years or earlier if required and should provide evidence of:- Re-certification for LoC SDI /IUT from FSRHC or RCN as appropriate Or Local recertification as detailed in Appendix C All practitioners must be able to evidence the FSRH IUT LoC and/or LoC SDI and completion of FSRH or local recertification to the commissioner to deliver the service. 6. Excluded Services 6.1 Some services may be specifically excluded from this agreement. These are listed below. Gynaecological care outside of IUS fitting pathway as described in this agreement.
7 7. Appendices APPENDIX A IUD / IUS AUDIT The annual audit form has been digitalised for online submission alongside the claim process and is accessible at for annual completion.
8
9
10 APPENDIX A IMPLANT AUDIT
11
12 APPENDIX B: SUMMARY OF COMPETENCY/TRAINING REQUIREMENT OPTIONS FOR PRACTITIONERS PROVIDING IUD/S AND SDI SERVICES. SDI: FSRH LoC SDI with FSRH recertification every 5 yearsevidence: FSRH certificates Applies to all new practitioners in LLR Or FSRH LoC SDI with option of LLR recertification (Appendix C). N.B. This is only valid until 31 March 2020 Evidence: FSRH LoC certificate and LLR recertification approval certificate Minimum fit/removal activity: Desirable: Minimum of 6 procedures in 12 month audit period. (At least 1 insertion and 1 removal) Essential for recertification: At least 6 procedures in a twelve month period within twenty four months prior to recertification. (At least 1 insertion and 1 removal) Annual audit submitted See Appendix A Or LLR LoC SDI via East Midlands Scheme undertaken in the period with LLR recertification (Appendix C) evidenced and achieved, valid until 31 March Evidence: training attendance certificate and LLR recertification approval certificate Evidence of each practitioner s competency must be provided to commissioners on request..
13 IUD/S: Or Or FSRH LoC IUT with FSRH recertification every 5 years Evidence: FSRH certificates Applies to all new practitioners in llr FSRH LoC IUT with option of LLR recertification (Appendix C). N.B. This is only valid until 31 March 2020 Evidence: FSRH LoC certificate and LLR recertification approval certificate For experienced fitters prior to 2009 who completed LLR Local theory training undertaken in the period with LLR recertification (Appendix C) evidenced and achieved by 1 st October 2016 valid until 31 March 2020 Minimum fit/removal activity: Desirable: Minimum of 12 IUD/S device fits in 12 month audit period. (Minimum of 2 types of device) Essential for recertification: At least 12 IUD/S device fits in a twelve month period within twenty four months prior to recertification. (Minimum of 2 types of device) Annual audit submitted See Appendix A Evidence: training attendance certificate and LLR recertification approval certificate Or LLR LoC IUT via East Midlands Scheme undertaken in the period with LLR recertification (Appendix C) evidenced and achieved valid until 31 March 2020 Evidence of each practitioner s competency must be provided to commissioners on request. Evidence: LLR Local certificate and LLR recertification approval certificate
14 Appendix C: Pathway to recertify LOCAL Letters of Competence (LoC) for Intrauterine Techniques (IUT) and Sub Dermal Implant (SDI). For existing fitters only (Criteria: those who submitted an audit in 2014/15) Must demonstrate holding previous FRSH or local LoC, with evidence of completion of theory & practical training. IUT SDI Minimum of 2 CPD credits per LoC Relevant to IUT Evidence required as detailed in Relevant to SDI Evidence required as detailed in Attendance course/meeting (e.g. Fitter forum held locally) Reading. Peer reviewed publications with reflective evidence. Reading FSRH CEU guidance & completion of self-assessment questions. Peer observation. IUT/SDI audit ( personal or local) Recent esrh module 18 Evidence: certificate Recent esrh module 17 Evidence: certificate Basic life support and Anaphylaxis training completed in last 18 months Evidence: certificates Audit of procedures covering 12 months within 24 months of recertification: Showing minimum of: 12 insertions of at least 2 types of IUT 6 procedures SDI (at least 1 removal) Evidence: submission of practitioner audit as required by the CBS Contract, reviewed as satisfactory by consultant in SRH Recertification until 31 March 2020 Evidence of each practitioner s competency must be provided to commissioners on request.
15 APPENDIX D: OVERVIEW OF LNG-IUS SERVICE FOR GYNAECOLOGICAL PURPOSES (a) Provision, review and subsequent removal, of Levonorgestrel Intrauterine System (LNG-IUS) for gynaecological (non-contraceptive) purposes including management of menorrhagia and hormone replacement therapy (HRT) (b) Local authority commissioned provision of LNG-IUS fitting, review and removal on behalf of the Clinical Commissioning Group (CCG). AIMS AND OUTCOMES To provide the fitting and removal element of LNG-IUS for women requiring Levonorgestrel intrauterine system LNG-IUS fitting as management of menorrhagia or other gynaecological purpose such as HRT, endometriosis etc, where clinically relevant, thus reducing the requirement for hysterectomy. BACKGROUND The (LNG-IUS) is an intrauterine, long-term progestogen-only method of contraception licensed for 5 years of use. The effects of the LNG-IUS are local and hormonal, including prevention of endometrial proliferation and thickening of cervical mucus and suppression of ovulation in a small minority of women. The system has to be fitted and removed by a qualified practitioner. As well as being licensed as a contraceptive device, the LNG-IUS is also licensed for the management of idiopathic menorrhagia. Menorrhagia / Heavy menstrual bleeding is defined as excessive menstrual blood loss which interferes with the woman s physical, emotional, social and material quality of life, and which can occur alone or in combination with other symptoms. Any intervention should aim to improve quality of life measures. The Levonorgestrel-releasing intrauterine system (LNG-IUS) is recommended as first line treatment for women with heavy menstrual bleeding and no underlying pathology (dysfunctional uterine bleeding) and in some women with heavy menstrual bleeding and identified benign pathology such as small fibroids (less than 3 cm in diameter which are causing no distortion of the uterine cavity) provided that long-term use is anticipated (at least 12 months). The LNG-IUS may also be recommended, following gynaecological investigation, for the management of conditions such as endometriosis. 1 Evidence from two systematic reviews and one subsequent publication shows that LNG-IUS produces a clinically relevant reduction in menstrual blood loss in women complaining of heavy menstrual bleeding. jul-2010/ Local defined outcomes Reduction in secondary care referrals to gynaecology (in particular for menorrhagia) Reduction in number of hysterectomies Improved uptake of long-acting reversible contraception (LARC) Reduction in unplanned pregnancies Improved quality of life for women receiving the LNG-IUS Locally convenient service with improved access to care and reduced waiting times for LNG- IUS fitting Improved quality of care 1 Heavy menstrual bleeding: assessment and management. Clinical guideline. National Institute for Health and Care Excellence Last updated August 2016.
16 Algorithm for use of LNG-IUS for management of gynaecological conditions such as menorrhagia. Applicable national standards NICE clinical guideline 44 (January Last updated August 2016) Heavy Menstrual Bleeding: assessment and management. Referral guidelines for suspected cancer gynaecological cancers (Implemented Oct 2000 DOH) NICE (2005a) Referral guidelines for suspected cancer: quick reference guide. Clinical guideline 27. National Institute for Health and Clinical Excellence. NICE (2005b) Long-acting reversible contraception (NICE guideline). National Institute for Health and Clinical Excellence. NICE (2007a) Heavy menstrual bleeding: understanding NICE guidance. National Institute for Health and Clinical Excellence. NICE (2007b) Audit criteria: heavy menstrual bleeding. National Institute for Health and Clinical Excellence. RCOG (1998) The initial management of menorrhagia. Evidence-based clinical guidelines no.1. Royal College of Obstetricians and Gynaecologists. FSRH (2009) UK medical eligibility criteria for contraceptive use [Superseded]. Faculty of Family Planning and Reproductive Health Care. NICE support for commissioning for heavy menstrual bleeding. (September 2013) FSRH CEU Clinical Guidance Intrauterine Contraception. (2015) NB: These standards may be updated during the contract period.
PUBLIC HEALTH LOCAL SERVICES AGREEMENTS 2016/17 SERVICE SPECIFICATION SIGN-UP. GP Practice Subdermal Contraceptive Implants
 PUBLIC HEALTH LOCAL SERVICES AGREEMENTS 2016/17 SERVICE SPECIFICATION SIGN-UP GP Practice Subdermal Contraceptive Implants Contract expiry date: 31 March 2017 Specific Training/Accreditation: Evidence
PUBLIC HEALTH LOCAL SERVICES AGREEMENTS 2016/17 SERVICE SPECIFICATION SIGN-UP GP Practice Subdermal Contraceptive Implants Contract expiry date: 31 March 2017 Specific Training/Accreditation: Evidence
Levosert levonorgestrel 20mcg/24hour intrauterine device
 Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
Cuts, Closures and Contraception
 Cuts, Closures and Contraception An audit of local contraceptive services in England November 2017 1. Sexual health services are at a tipping point. Local Government Association I SRH provision as a whole
Cuts, Closures and Contraception An audit of local contraceptive services in England November 2017 1. Sexual health services are at a tipping point. Local Government Association I SRH provision as a whole
Management of Emergency Contraception (EC)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Management of Emergency Contraception (EC) The risks and benefits of an IUD or oral EC should be discussed and documented (see appendix). Reasonable measures
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Management of Emergency Contraception (EC) The risks and benefits of an IUD or oral EC should be discussed and documented (see appendix). Reasonable measures
Leicestershire & Rutland Sexual Health Strategies Year two progress report
 Leicestershire & Rutland Sexual Health Strategies Year two progress report Background The Leicestershire Sexual Health Strategy 2016-2019 (available at http://www.lsr-online.org/sexualhealth-joint-strategic-ne.html)
Leicestershire & Rutland Sexual Health Strategies Year two progress report Background The Leicestershire Sexual Health Strategy 2016-2019 (available at http://www.lsr-online.org/sexualhealth-joint-strategic-ne.html)
PATIENT GROUP DIRECTION SUPPLY of Emergency Hormonal Contraception To females under 25 years Levonelle 1500 (Levonorgestrel 1500 micrograms)
 PATIENT GROUP DIRECTION SUPPLY of Emergency Hormonal Contraception To females under 25 years Levonelle 1500 (Levonorgestrel 1500 micrograms) VALID FROM: 1 st July 2016 REVIEWED DATE: 30 th June 2019 This
PATIENT GROUP DIRECTION SUPPLY of Emergency Hormonal Contraception To females under 25 years Levonelle 1500 (Levonorgestrel 1500 micrograms) VALID FROM: 1 st July 2016 REVIEWED DATE: 30 th June 2019 This
30th Sexual Health & BBV MCN Education Day, Gannochy Trust Lecture Theatre, Ninewells Hospital, Dundee 9.00am-4.30pm. Cross, Dundee am-1.
 April 2017-March 2018 Dates for 2017-2018 April 2017 May 2017 June 2017 30th Education Day, Gannochy Trust Lecture Theatre, Ninewells Hospital, Dundee 9.00am-4.30pm 6 th Safe Relationships & The Law on
April 2017-March 2018 Dates for 2017-2018 April 2017 May 2017 June 2017 30th Education Day, Gannochy Trust Lecture Theatre, Ninewells Hospital, Dundee 9.00am-4.30pm 6 th Safe Relationships & The Law on
Sexual Health Services (Emergency Hormonal Contraception, Chlamydia Screening, Condom Distribution & Pregnancy Testing) in Pharmacies.
 Local Enhanced Service (LES) Specification for: Sexual Health Services (Emergency Hormonal Contraception, Chlamydia Screening, Condom Distribution & Pregnancy Testing) in Pharmacies. 1. Introduction 2.
Local Enhanced Service (LES) Specification for: Sexual Health Services (Emergency Hormonal Contraception, Chlamydia Screening, Condom Distribution & Pregnancy Testing) in Pharmacies. 1. Introduction 2.
Women & Children's Business Unit Maternity Contraception and Sexual Health
 Women & Children's Business Unit Maternity Contraception and Sexual Health Author/s Contact name Approval process Obstetric Guidelines Group/Associate Medical Director First Issue Date Trust intranet ref:
Women & Children's Business Unit Maternity Contraception and Sexual Health Author/s Contact name Approval process Obstetric Guidelines Group/Associate Medical Director First Issue Date Trust intranet ref:
ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE
 ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE Version 1.0 Page 1 of 11 MARCH 2014 POLICY DOCUMENT VERSION CONTROL CERTIFICATE TITLE Title: Assisted Conception
ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE Version 1.0 Page 1 of 11 MARCH 2014 POLICY DOCUMENT VERSION CONTROL CERTIFICATE TITLE Title: Assisted Conception
Contraception Update: what s new in 2016? Felicity Young MA BSc (Hons) RMN RGN RM NDFSRH A08 Consultant Nurse for Sexual and Reproductive Healthcare
 Contraception Update: what s new in 2016? Felicity Young MA BSc (Hons) RMN RGN RM NDFSRH A08 Consultant Nurse for Sexual and Reproductive Healthcare Declaration of Competing Interests I have not received
Contraception Update: what s new in 2016? Felicity Young MA BSc (Hons) RMN RGN RM NDFSRH A08 Consultant Nurse for Sexual and Reproductive Healthcare Declaration of Competing Interests I have not received
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2018/19
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2017/18
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health and social care directorate. Quality standards and indicators.
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health and social care directorate Quality standards and indicators Briefing paper Quality standard topic: Heavy menstrual bleeding Output: Prioritised
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health and social care directorate Quality standards and indicators Briefing paper Quality standard topic: Heavy menstrual bleeding Output: Prioritised
APPENDIX A SERVICE SPECIFICATION
 Service Specification No. Service Authority Lead Provider Lead 2013/14 APPENDIX A SERVICE SPECIFICATION Participation in the Ruclear chlamydia and gonorrhoea screening programme (General practices) Period
Service Specification No. Service Authority Lead Provider Lead 2013/14 APPENDIX A SERVICE SPECIFICATION Participation in the Ruclear chlamydia and gonorrhoea screening programme (General practices) Period
MMPr001 EMERGENCY CONTRACEPTION PROTOCOL
 MMPr001 EMERGENCY CONTRACEPTION PROTOCOL MMPr001 Emergency Contraception - (Sept18-Sept20) Page 1 of 8 Table of Contents MMPr001 EMERGENCY CONTRACEPTION PROTOCOL... 1 Why we need this protocol... 3 What
MMPr001 EMERGENCY CONTRACEPTION PROTOCOL MMPr001 Emergency Contraception - (Sept18-Sept20) Page 1 of 8 Table of Contents MMPr001 EMERGENCY CONTRACEPTION PROTOCOL... 1 Why we need this protocol... 3 What
MMPr001 EMERGENCY CONTRACEPTION PROTOCOL
 MMPr001 EMERGENCY CONTRACEPTION PROTOCOL Table of Contents MMPr001 EMERGENCY CONTRACEPTION PROTOCOL... 1 Why we need this Protocol... 2 What the Protocol is trying to do... 2 Which stakeholders have been
MMPr001 EMERGENCY CONTRACEPTION PROTOCOL Table of Contents MMPr001 EMERGENCY CONTRACEPTION PROTOCOL... 1 Why we need this Protocol... 2 What the Protocol is trying to do... 2 Which stakeholders have been
NEW GENERAL MEDICAL SERVICES CONTRACT SPECIFICATION FOR THE PROVISION OF AN ENHANCED SERVICE SERVICE SPECIFICATION CHLAMYDIA SCREENING
 NEW GENERAL MEDICAL SERVICES CONTRACT SPECIFICATION FOR THE PROVISION OF AN ENHANCED SERVICE SERVICE SPECIFICATION CHLAMYDIA SCREENING April 1, 2013 March 31, 2014 SERVICE CHLAMYDIA SCREENING REFERENCE
NEW GENERAL MEDICAL SERVICES CONTRACT SPECIFICATION FOR THE PROVISION OF AN ENHANCED SERVICE SERVICE SPECIFICATION CHLAMYDIA SCREENING April 1, 2013 March 31, 2014 SERVICE CHLAMYDIA SCREENING REFERENCE
2
 1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
LONG-ACTING REVERSIBLE CONTRACEPTION. Summary Tables
 LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
Increasing the Proportion of Women using Long Acting Reversible Contraception (LARC) within a Geographical Area in Scotland
 Increasing the Proportion of Women using Long Acting Reversible Contraception (LARC) within a Geographical Area in Scotland Dr Audrey Brown Dr Alison Bigrigg Greater Glasgow and Clyde Long-acting reversible
Increasing the Proportion of Women using Long Acting Reversible Contraception (LARC) within a Geographical Area in Scotland Dr Audrey Brown Dr Alison Bigrigg Greater Glasgow and Clyde Long-acting reversible
CABINET PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND
 CABINET Report No: 105/2017 PUBLIC REPORT 16 May 2017 PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND Report of the Director of Public Health Strategic Aim: Safeguarding Key Decision:
CABINET Report No: 105/2017 PUBLIC REPORT 16 May 2017 PROCURING A SUBSTANCE MISUSE & COMMUNITY TREATMENT SERVICE IN RUTLAND Report of the Director of Public Health Strategic Aim: Safeguarding Key Decision:
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)
 E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
NICE guideline Published: 14 March 2018 nice.org.uk/guidance/ng88
 Heavy menstrual bleeding: assessment and management NICE guideline Published: 14 March 2018 nice.org.uk/guidance/ng88 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Heavy menstrual bleeding: assessment and management NICE guideline Published: 14 March 2018 nice.org.uk/guidance/ng88 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Leicestershire Sexual Health Strategy
 Leicestershire Sexual Health Strategy 2016-2019 1 Introduction The sexual health needs of the population are evolving. Over the past few decades there have been significant changes in relationships, and
Leicestershire Sexual Health Strategy 2016-2019 1 Introduction The sexual health needs of the population are evolving. Over the past few decades there have been significant changes in relationships, and
the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD your guide to
 your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
NHS public health functions agreement Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men
 NHS public health functions agreement 2018-19 Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men (HPV-MSM) 1 NHS public health functions agreement 2018-19
NHS public health functions agreement 2018-19 Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men (HPV-MSM) 1 NHS public health functions agreement 2018-19
Menstrual Disorders & Ambulatory Gynaecology
 Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer
 levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Specialist List in Special Care Dentistry
 Specialist List in Special Care Dentistry Definition of Special Care Dentistry Special Care Dentistry (SCD) is concerned with providing enabling the delivery of oral care for people with an impairment
Specialist List in Special Care Dentistry Definition of Special Care Dentistry Special Care Dentistry (SCD) is concerned with providing enabling the delivery of oral care for people with an impairment
CLINICAL CONTENT OF PATIENT GROUP DIRECTION FOR EMERGENCY HORMONAL CONTRACEPTION Levonorgestrel 1500mcg
 CLINICAL CONTENT OF PATIENT GROUP DIRECTION FOR EMERGENCY HORMONAL CONTRACEPTION Levonorgestrel 1500mcg Version Control This document is only valid on the day it was printed The current version of this
CLINICAL CONTENT OF PATIENT GROUP DIRECTION FOR EMERGENCY HORMONAL CONTRACEPTION Levonorgestrel 1500mcg Version Control This document is only valid on the day it was printed The current version of this
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641
 Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Blackpool CCG. Policies for the Commissioning of Healthcare. Assisted Conception
 1 Introduction Blackpool CCG Policies for the Commissioning of Healthcare Assisted Conception 1.1 This policy describes circumstances in which NHS Blackpool Clinical Commissioning Group (CCG) will fund
1 Introduction Blackpool CCG Policies for the Commissioning of Healthcare Assisted Conception 1.1 This policy describes circumstances in which NHS Blackpool Clinical Commissioning Group (CCG) will fund
Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle
 Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle Contents 1. Agreement period 2. Scope 3. Targets 4. Service outline 5. Support for clients using
Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle Contents 1. Agreement period 2. Scope 3. Targets 4. Service outline 5. Support for clients using
St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16
 St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16 1 Standard Operating Procedure St Helens CCG NHS Funded Treatment for Sub Fertility Policy Version 1 Implementation Date May 2015 Review
St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16 1 Standard Operating Procedure St Helens CCG NHS Funded Treatment for Sub Fertility Policy Version 1 Implementation Date May 2015 Review
A Suite of Enhanced Services for. Prudent Structured Care for Adults with Type 2 Diabetes
 An Enhanced Service for Prudent Structured Care for Adults with Type 2 Diabetes Page 1 A Suite of Enhanced Services for Prudent Structured Care for Adults with Type 2 Diabetes 1. Introduction All practices
An Enhanced Service for Prudent Structured Care for Adults with Type 2 Diabetes Page 1 A Suite of Enhanced Services for Prudent Structured Care for Adults with Type 2 Diabetes 1. Introduction All practices
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines
 Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY. CONTENTS Page
 HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO TREATMENT 3 4. DEFINITION
HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO TREATMENT 3 4. DEFINITION
Primary Care Gynaecology Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
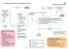 Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
Warrington Centre for Sexual Health WCSH Local Enhanced Service LES
 Warrington Centre for Sexual Health WCSH Local Enhanced Service LES 2015-19 NOTE: This LES is only for asymptomatic persons and does not cover testing and treatment of symptomatic persons Introduction
Warrington Centre for Sexual Health WCSH Local Enhanced Service LES 2015-19 NOTE: This LES is only for asymptomatic persons and does not cover testing and treatment of symptomatic persons Introduction
Assisted Conception Policy
 Assisted Conception Policy NHS Eligibility Criteria for assisted conception services (excluding In vitro fertilisation (IVF) Intracytoplasmic sperm injection (ICSI) treatment) for people with infertility
Assisted Conception Policy NHS Eligibility Criteria for assisted conception services (excluding In vitro fertilisation (IVF) Intracytoplasmic sperm injection (ICSI) treatment) for people with infertility
LASS Guidelines and Protocols for Community Based Point of Care HIV Testing
 LASS Guidelines and Protocols for Community Based Point of Care HIV Testing SCOPE AND CONTEXT This document addresses Point of Care HIV Testing (POCT) using Rapid Test Devices (RTD) for people at (high)
LASS Guidelines and Protocols for Community Based Point of Care HIV Testing SCOPE AND CONTEXT This document addresses Point of Care HIV Testing (POCT) using Rapid Test Devices (RTD) for people at (high)
Uterine artery embolisation for treating adenomyosis
 Uterine artery embolisation for treating Issued: December 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional procedures
Uterine artery embolisation for treating Issued: December 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional procedures
Leicester City, East Leicestershire and Rutland & West Leicestershire Collaborative Commissioning Policy Gamete/Embryo cryopreservation
 Leicester City Clinical Commissioning Group West Leicestershire Clinical Commissioning Group East Leicestershire and Rutland Clinical Commissioning Group Leicester City, East Leicestershire and Rutland
Leicester City Clinical Commissioning Group West Leicestershire Clinical Commissioning Group East Leicestershire and Rutland Clinical Commissioning Group Leicester City, East Leicestershire and Rutland
Camden Clinical Commissioning Group
 Locally Commissioned Service Clinical Lead Commissioner Reporting Mechanism/Frequency Payment Frequency Payment Contact Childhood Immunisation Dr Oliver Anglin Camden Clinical Commissioning Group Partially
Locally Commissioned Service Clinical Lead Commissioner Reporting Mechanism/Frequency Payment Frequency Payment Contact Childhood Immunisation Dr Oliver Anglin Camden Clinical Commissioning Group Partially
Heavy Menstrual Bleeding. Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist
 Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT
 SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
Pharmacy Needle and Syringe Programme. Enhanced Contract
 Pharmacy Needle and Syringe Programme Enhanced Contract 1 st April 2012 31 st March 2013 Signed on behalf of LPC Signed on behalf of CRI Signed on behalf of Turning Point Signed on behalf of KCA Signed
Pharmacy Needle and Syringe Programme Enhanced Contract 1 st April 2012 31 st March 2013 Signed on behalf of LPC Signed on behalf of CRI Signed on behalf of Turning Point Signed on behalf of KCA Signed
Service Specification
 Service Specification Chlamydia Screening Release: Final Date: 1 August 2010 Author: Steph Cook Head of Sexual Health Commissioning NHS Derby City Owner: Maureen Whittaker Associate Director of Public
Service Specification Chlamydia Screening Release: Final Date: 1 August 2010 Author: Steph Cook Head of Sexual Health Commissioning NHS Derby City Owner: Maureen Whittaker Associate Director of Public
Notes to Teacher continued Contraceptive Considerations
 Abstinence a conscious decision to refrain from sexual intercourse 100% pregnancy will not occur if close contact between the penis and vagina does not take place. The risk of a number of STDs, including
Abstinence a conscious decision to refrain from sexual intercourse 100% pregnancy will not occur if close contact between the penis and vagina does not take place. The risk of a number of STDs, including
Commissioning Policy Individual Funding Request
 Commissioning Policy Individual Funding Request Female Sterilisation Prior Approval Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team - A partnership between Bristol,
Commissioning Policy Individual Funding Request Female Sterilisation Prior Approval Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team - A partnership between Bristol,
GMS Contract in Wales Enhanced Service for Homeless Patients Specification
 Doc 4 GMS Contract in Wales 2008-09 Enhanced Service for Homeless Patients Specification Introduction The purpose of this paper is to provide an enhanced service specification for Local Health Boards when
Doc 4 GMS Contract in Wales 2008-09 Enhanced Service for Homeless Patients Specification Introduction The purpose of this paper is to provide an enhanced service specification for Local Health Boards when
Local Enhanced Service Specification. MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly
 Local Enhanced Service Specification MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly 1 st January 2016 to 31 st August 2016 1 Local Enhanced Service
Local Enhanced Service Specification MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly 1 st January 2016 to 31 st August 2016 1 Local Enhanced Service
Nikki Mallinder on behalf of Linda Honey. Karen Thorburn Date: 04/10/18
 Agenda item: 7 ii Paper no: PCCC ic 20-18 Title of Report: Status: Committee: Venue: Update to the Attention Deficit Hyperactivity Disorder (ADHD) in Childhood) 12 monthly physical medication monitoring
Agenda item: 7 ii Paper no: PCCC ic 20-18 Title of Report: Status: Committee: Venue: Update to the Attention Deficit Hyperactivity Disorder (ADHD) in Childhood) 12 monthly physical medication monitoring
One-day Essentials Contraception. Dr Paula Briggs, General Practitioner, Clinical Lead Community Sexual Health, Sefton and West Lancashire
 One-day Essentials Contraception { Dr Paula Briggs, General Practitioner, Clinical Lead Community Sexual Health, Sefton and West Lancashire 80% women access contraception from their GP Therefore it is
One-day Essentials Contraception { Dr Paula Briggs, General Practitioner, Clinical Lead Community Sexual Health, Sefton and West Lancashire 80% women access contraception from their GP Therefore it is
NHS Sheffield Community Pharmacy Seasonal Flu Vaccination Programme for hard to reach at risk groups (and catch up campaign for over 65s)
 NHS Sheffield Community Pharmacy Seasonal Flu Vaccination Programme for hard to reach at risk groups 2012-13 (and catch up campaign for over 65s) Service Evaluation! Supported by Sheffield!Local!Pharmaceutical!Committee!
NHS Sheffield Community Pharmacy Seasonal Flu Vaccination Programme for hard to reach at risk groups 2012-13 (and catch up campaign for over 65s) Service Evaluation! Supported by Sheffield!Local!Pharmaceutical!Committee!
Intrauterine (IUI) and Donor Insemination (DI) Policy (excluding In vitro fertilisation (IVF) & Intracytoplasmic sperm injection (ICSI) treatment)
 Leicester City Clinical Commissioning Group West Leicestershire Clinical Commissioning Group East Leicestershire and Rutland Clinical Commissioning Group POLICY DOCUMENT Intrauterine (IUI) and Donor Insemination
Leicester City Clinical Commissioning Group West Leicestershire Clinical Commissioning Group East Leicestershire and Rutland Clinical Commissioning Group POLICY DOCUMENT Intrauterine (IUI) and Donor Insemination
Patient Group Direction for LEVONELLE (Version 02) Valid From 1 October September 2019
 Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Unintended Pregnancy is Common LEARNING OBJECTIVES. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy And Contraceptive Use
 3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator
 Publication Report Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator Year ending March 2014 Publication date 30 September 2014 A National Statistics Publication for Scotland
Publication Report Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator Year ending March 2014 Publication date 30 September 2014 A National Statistics Publication for Scotland
Policy for Hysteroscopy Page 1 of 5. Policy for Hysteroscopy. V1.1 OPCS/ICD codes added. Version of: Version of: March 2018
 Policy for Hysteroscopy Version of: 16.03.2018 Version of: March 2018 Version Number: Changes Made: V1.1 OPCS/ICD codes added. V1 Ratified policy agreed by Healthier Lancashire and South Cumbria s Joint
Policy for Hysteroscopy Version of: 16.03.2018 Version of: March 2018 Version Number: Changes Made: V1.1 OPCS/ICD codes added. V1 Ratified policy agreed by Healthier Lancashire and South Cumbria s Joint
Locally Enhanced Service for Stopping Smoking
 NHS Devon Locally Enhanced Service for Stopping Smoking This Local Enhanced Service (LES) Specification details the agreement between Devon PCT (the commissioner) and community pharmacies (the service
NHS Devon Locally Enhanced Service for Stopping Smoking This Local Enhanced Service (LES) Specification details the agreement between Devon PCT (the commissioner) and community pharmacies (the service
STANDARD OPERATING PROCEDURE Chlamydia screening and treatment for year olds
 STANDARD OPERATING PROCEDURE Chlamydia screening and treatment for 15-24 year olds Service Commencement Date: 1 st October 2014 Contract Term: 17 months to 31 st March 2016 Produced by: Leicestershire
STANDARD OPERATING PROCEDURE Chlamydia screening and treatment for 15-24 year olds Service Commencement Date: 1 st October 2014 Contract Term: 17 months to 31 st March 2016 Produced by: Leicestershire
SERVICE SPECIFICATION FOR THE PROVISION OF PRIMARY CARE PRESCRIBING FOR DRUG MISUSE IN DORSET ONLY
 Revised for: 1 April 2014 Appendix 2.4 SERVICE SPECIFICATION FOR THE PROVISION OF PRIMARY CARE PRESCRIBING FOR DRUG MISUSE IN DORSET ONLY Dorset County Council Dorset Procurement, Level 3, North East Wing,
Revised for: 1 April 2014 Appendix 2.4 SERVICE SPECIFICATION FOR THE PROVISION OF PRIMARY CARE PRESCRIBING FOR DRUG MISUSE IN DORSET ONLY Dorset County Council Dorset Procurement, Level 3, North East Wing,
Provision of Stop Smoking Support in Pharmacy
 Public Health Agreement for the Provision of Stop Smoking Support in Pharmacy 1 st April 2017 to 31 st March 2018 BETWEEN Surrey County Council AND Pharmacy 1. Introduction 2. Aims 3. Service Outline 4.
Public Health Agreement for the Provision of Stop Smoking Support in Pharmacy 1 st April 2017 to 31 st March 2018 BETWEEN Surrey County Council AND Pharmacy 1. Introduction 2. Aims 3. Service Outline 4.
NHS FUNDED TREATMENT FOR SUBFERTILITY. ELIGIBILITY CRITERIA POLICY GUIDANCE/OPTIONS FOR CCGs
 NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA POLICY GUIDANCE/OPTIONS FOR CCGs CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO
NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA POLICY GUIDANCE/OPTIONS FOR CCGs CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO
Family Planning Eligibility Program
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER REFERENCE M ODULE Family Planning Eligibility Program L I B R A R Y R E F E R E N C E N U M B E R : P R O M O D 0 0 0 5 3 P U B L I S H E D : N O V E M B E R 2
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER REFERENCE M ODULE Family Planning Eligibility Program L I B R A R Y R E F E R E N C E N U M B E R : P R O M O D 0 0 0 5 3 P U B L I S H E D : N O V E M B E R 2
NHS FORTH VALLEY LOCAL ENHANCED SERVICE (2010) General Practitioner Prescribing Service (GPPS) Opiate Assisted Treatment Service Specification
 NHS FORTH VALLEY LOCAL ENHANCED SERVICE (2010) Rationale General Practitioner Prescribing Service (GPPS) Opiate Assisted Treatment Service Specification Aims It is the responsibility of general practitioners
NHS FORTH VALLEY LOCAL ENHANCED SERVICE (2010) Rationale General Practitioner Prescribing Service (GPPS) Opiate Assisted Treatment Service Specification Aims It is the responsibility of general practitioners
ACE Programme SOMERSET INTEGRATED LUNG CANCER PATHWAY. Phases One and Two Final Report
 ACE Programme SOMERSET INTEGRATED LUNG CANCER PATHWAY Phases One and Two Final Report July 2017 Introduction This paper presents the learning and actions that have been generated from phase One and Two
ACE Programme SOMERSET INTEGRATED LUNG CANCER PATHWAY Phases One and Two Final Report July 2017 Introduction This paper presents the learning and actions that have been generated from phase One and Two
Do you find this clinical policy or service specification clear and comprehensive?
 Service specification for HIV: British HIV Association response Version 3 25012013 Do you find this clinical policy or service specification clear and comprehensive? No. There are a number of areas that
Service specification for HIV: British HIV Association response Version 3 25012013 Do you find this clinical policy or service specification clear and comprehensive? No. There are a number of areas that
Long Acting Reversible Methods of Contraception (LARC) in Scotland
 Publication Report Long Acting Reversible Methods of Contraception (LARC) in Scotland Year ending March 2015 Publication date 3 November 2015 A National Statistics Publication for Scotland Contents Introduction...
Publication Report Long Acting Reversible Methods of Contraception (LARC) in Scotland Year ending March 2015 Publication date 3 November 2015 A National Statistics Publication for Scotland Contents Introduction...
Telford & Wrekin Pharmacy LES Chlamydia Screening Page 1 of 16
 NATIONAL CHLAMYDIA SCREENING PROGRAMME LOCAL ENHANCED SERVICE (LES) SPECIFICATION FOR OPPORTUNISTIC CHLAMYDIA SCREENING PILOT IN PHARMACY April 2010 Telford & Wrekin Pharmacy LES Chlamydia Screening 2010-11
NATIONAL CHLAMYDIA SCREENING PROGRAMME LOCAL ENHANCED SERVICE (LES) SPECIFICATION FOR OPPORTUNISTIC CHLAMYDIA SCREENING PILOT IN PHARMACY April 2010 Telford & Wrekin Pharmacy LES Chlamydia Screening 2010-11
CALDERDALE PRIMARY CARE TRUST
 CALDERDALE PRIMARY CARE TRUST PATIENT GROUP DIRECTION FOR THE SUPPLY AND ADMINISTRATION OF MEDICINES BY NON- MEDICAL PERSONNEL Progestogen-only emergency contraception for use in Community Pharmacies APPROVED
CALDERDALE PRIMARY CARE TRUST PATIENT GROUP DIRECTION FOR THE SUPPLY AND ADMINISTRATION OF MEDICINES BY NON- MEDICAL PERSONNEL Progestogen-only emergency contraception for use in Community Pharmacies APPROVED
Director of Commissioning, Telford and Wrekin CCG and Shropshire CCG. Version No. Approval Date August 2015 Review Date August 2017
 Commissioning Policy for In Vitro Fertilisation (IVF)/ Intracytoplasmic Sperm Injection (ICSI) within tertiary Infertility Services, in Shropshire and Telford and Wrekin Owner(s) Version No. Director of
Commissioning Policy for In Vitro Fertilisation (IVF)/ Intracytoplasmic Sperm Injection (ICSI) within tertiary Infertility Services, in Shropshire and Telford and Wrekin Owner(s) Version No. Director of
NHS Sheffield Community Pharmacy Catch Up Seasonal Flu Vaccination Programme for hard to reach at risk groups
 NHS Sheffield Community Pharmacy Catch Up Seasonal Flu Vaccination Programme for hard to reach at risk groups 2011-12 Service Evaluation Supported by Sheffield Local Pharmaceutical Committee Supporting
NHS Sheffield Community Pharmacy Catch Up Seasonal Flu Vaccination Programme for hard to reach at risk groups 2011-12 Service Evaluation Supported by Sheffield Local Pharmaceutical Committee Supporting
Community Sleep Studies Hub and Spoke service Community first attends (schedule 1) and Community follow-ups (schedule 2)
 Community Sleep Studies Hub and Spoke service 2018-2019 Community first attends (schedule 1) and Community follow-ups (schedule 2) 1. Purpose of Agreement This local service agreement is for patients with
Community Sleep Studies Hub and Spoke service 2018-2019 Community first attends (schedule 1) and Community follow-ups (schedule 2) 1. Purpose of Agreement This local service agreement is for patients with
What s New in Adolescent Contraception?
 What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
LEARNING OBJECTIVES. Beyond the Pill: Long Acting Contraception. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy is Common
 4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
FERTILITY SERVICE POLICY
 FERTILITY SERVICE POLICY Page 1 of 8 FERTILITY SERVICE POLICY Please note that all Clinical Commissioning policies are currently under review and elements within the individual policies may have been replaced
FERTILITY SERVICE POLICY Page 1 of 8 FERTILITY SERVICE POLICY Please note that all Clinical Commissioning policies are currently under review and elements within the individual policies may have been replaced
Investigating HMB- an evidence based approach
 BSGE Meeting: Contemporary management of heavy menstrual bleeding (HMB) in primary and secondary care: (7 th December 2018, RCOG) Investigating HMB- an evidence based approach T. Justin Clark MB ChB, MD(Hons),
BSGE Meeting: Contemporary management of heavy menstrual bleeding (HMB) in primary and secondary care: (7 th December 2018, RCOG) Investigating HMB- an evidence based approach T. Justin Clark MB ChB, MD(Hons),
Fertility treatment and referral criteria for tertiary level assisted conception
 Fertility treatment and referral criteria for tertiary level assisted conception Version Number Name of Originator/Author Cross Reference V2 East of England Consortium Commissioning Policy for Fertility
Fertility treatment and referral criteria for tertiary level assisted conception Version Number Name of Originator/Author Cross Reference V2 East of England Consortium Commissioning Policy for Fertility
NHS public health functions agreement Service specification No.11 Human papillomavirus (HPV) programme
 NHS public health functions agreement 2018-19 Service specification No.11 Human papillomavirus (HPV) programme 1 NHS public health functions agreement 2018-19 Service specification No.11 Human papillomavirus
NHS public health functions agreement 2018-19 Service specification No.11 Human papillomavirus (HPV) programme 1 NHS public health functions agreement 2018-19 Service specification No.11 Human papillomavirus
Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator
 Publication Report Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator Year ending March 2013 Publication date 24 September 2013 A National Statistics Publication for Scotland
Publication Report Long Acting Reversible Methods of Contraception (LARC) Key Clinical Indicator Year ending March 2013 Publication date 24 September 2013 A National Statistics Publication for Scotland
Our Moment of Truth 2013 Survey Women s Health Care Experiences & Perceptions: Spotlight on Family Planning & Contraception
 Our Moment of Truth 2013 Survey Women s Health Care Experiences & Perceptions: Spotlight on Family Planning & Contraception Thank you for taking part in this survey. We know your time is valuable. Through
Our Moment of Truth 2013 Survey Women s Health Care Experiences & Perceptions: Spotlight on Family Planning & Contraception Thank you for taking part in this survey. We know your time is valuable. Through
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system)
 FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
Nelly Mugo, MBChB, MMed, MPH Kenya Medical Research Institute. May 2015
 Nelly Mugo, MBChB, MMed, MPH Kenya Medical Research Institute May 2015 Outline Rationale for the trial Design and objectives Contraceptive methods to be evaluated Study population and follow-up Potential
Nelly Mugo, MBChB, MMed, MPH Kenya Medical Research Institute May 2015 Outline Rationale for the trial Design and objectives Contraceptive methods to be evaluated Study population and follow-up Potential
Enhanced CPD Programme Module 1. Introducing Starting Well
 Enhanced CPD Programme Module 1 Introducing Starting Well Contents Where did the need of the scheme come from? In summary, what is the scheme about? How will the scheme work? Preventive Practices Advanced
Enhanced CPD Programme Module 1 Introducing Starting Well Contents Where did the need of the scheme come from? In summary, what is the scheme about? How will the scheme work? Preventive Practices Advanced
Recommended Interim Policy Statement 150: Assisted Conception Services
 Southampton City Clinical Commissioning Group (CCG) took on commissioning responsibility for Assisted Conception Services from 1 April 2013 for its population and agreed to adopt the interim policy recommendations
Southampton City Clinical Commissioning Group (CCG) took on commissioning responsibility for Assisted Conception Services from 1 April 2013 for its population and agreed to adopt the interim policy recommendations
SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD
 AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
Fertility Policy. December Introduction
 Fertility Policy December 2015 Introduction Camden Clinical Commissioning Group (CCG) is responsible for commissioning a range of health services including hospital, mental health and community services
Fertility Policy December 2015 Introduction Camden Clinical Commissioning Group (CCG) is responsible for commissioning a range of health services including hospital, mental health and community services
PATIENT GROUP DIRECTION (PGD) FOR THE SUPPLY OR ADMINISTRATION OF LEVONORGESTREL 1500 MICROGRAM TABLET (e.g LEVONELLE 1500 ) PGD
 This Patient Group Direction () must only be used by registered pharmacists who have been named and authorized by their organization to practice under it. The most recent and in date final signed version
This Patient Group Direction () must only be used by registered pharmacists who have been named and authorized by their organization to practice under it. The most recent and in date final signed version
Postpartum LARC. (Long Acting Reversible Contraception) NURSING EDUCATION
 Postpartum LARC (Long Acting Reversible Contraception) NURSING EDUCATION What is LARC Long-acting reversible contraception (LARC) methods include the intrauterine device (IUD) and the birth control implant.
Postpartum LARC (Long Acting Reversible Contraception) NURSING EDUCATION What is LARC Long-acting reversible contraception (LARC) methods include the intrauterine device (IUD) and the birth control implant.
Product Information. Confidence that lasts
 Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
Prescriber and Pharmacy Guide for the Opsumit REMS Program
 Prescriber and Pharmacy Guide for the Opsumit REMS Program (Risk Evaluation and Mitigation Strategy) including BOXED WARNING for teratogenicity. Risk of teratogenicity Introduction to Opsumit (macitentan)
Prescriber and Pharmacy Guide for the Opsumit REMS Program (Risk Evaluation and Mitigation Strategy) including BOXED WARNING for teratogenicity. Risk of teratogenicity Introduction to Opsumit (macitentan)
Heavy menstrual bleeding: assessment and management
 Heavy menstrual bleeding: assessment and management NICE guideline Draft for consultation, August 0 This guideline covers assessing and treating heavy menstrual bleeding. It aims to help healthcare professionals
Heavy menstrual bleeding: assessment and management NICE guideline Draft for consultation, August 0 This guideline covers assessing and treating heavy menstrual bleeding. It aims to help healthcare professionals
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Postmenopausal bleeding (PMB) guidelines for women with abnormal vaginal bleeding
 Guideline Postmenopausal bleeding (PMB) guidelines for women with abnormal vaginal bleeding 1 Scope For local use. 2 Purpose To provide evidence based care relating to several areas of the service including
Guideline Postmenopausal bleeding (PMB) guidelines for women with abnormal vaginal bleeding 1 Scope For local use. 2 Purpose To provide evidence based care relating to several areas of the service including
A. Service Specification
 A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
