Medical Policy. MP Parenteral, Enteral and Oral Nutrition in The Home. Related Policies None
|
|
|
- Kristopher Logan
- 5 years ago
- Views:
Transcription
1 Medical Policy MP Parenteral, Enteral and Oral Nutrition in The Home BCBSA Ref. Policy: Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Durable Medical Equipment Related Policies None DISCLAIMER Our medical policies are designed for informational purposes only and are not an authorization, explanation of benefits or a contract. Receipt of benefits is subject to satisfaction of all terms and conditions of the coverage. Medical technology is constantly changing, and we reserve the right to review and update our policies periodically. POLICY Parenteral Nutrition (PN) (prescribed by an MD, DO, NP, or PA) may be considered medically necessary for patients who are unable to maintain adequate nutrition intake via oral or tube feedings due to: 1) severe pathology of the alimentary tract that does not allow absorption of sufficient nutrients; and 2) the inability to receive no more than 30% of their caloric needs orally; or 3) the patient cannot benefit from tube feedings as a result of a malabsorptive disorder, which may include but is not limited to the following conditions: 1. inflammatory bowel disease (Crohn s disease or ulcerative colitis); or 2. obstruction secondary to stricture or neoplasm of the esophagus or stomach; or 3. loss of the swallowing mechanism due to a central nervous system disorder, where the risk of aspiration is great; or 4. short bowel syndrome secondary to massive small bowel resection; or 5. malabsorption due to enterocolic, enterovesical, or enterocutaneous fistulas (PN being temporary until the fistula is repaired); or 6. intractable motility disorder (i.e., intestinal pseudo-obstruction and gastroparesis); or 7. newborn infants with severe gastrointestinal anomalies (i.e., tracheoesophageal fistula, gastroschisis, omphalocele, or intestinal atresia); or 8. infants and young children who fail to thrive due to systemic disease or secondarily to intestinal insufficiency (associated with short bowel syndrome, malabsorption, or chronic idiopathic diarrhea); or 9. patients with prolonged paralytic ileus following major surgery or multiple injuries; or 10. hyperemesis gravidarum after medical management has been tried and been unsuccessful in maintaining adequate nutritional intake. Enteral nutrition (EN) formula (prescribed by an MD, DO, NP, or PA) may be considered medically necessary when administered via a surgically placed feeding tube when one of the following criteria is met: 1. the presence of a non-functional proximal gastrointestinal tract or disease of the of the structures that normally allows food to reach the small bowel (e.g., head and neck cancer a tumor that obstructs the esophagus or stomach) where the tube feedings are needed to provide
2 adequate nutrition to maintain the patient's overall health status; or 2. central nervous system disease leading to sufficient interference with the neuromuscular coordination of chewing and swallowing that a risk of aspiration exists (i.e., dysphagia secondary to Cerebral Vascular Accident [CVA]). Enteral Nutrition (EN) formula (prescribed by an MD, DO, NP, or PA) may be considered medically necessary when administered via a nasogastric feeding tube when the following criterion is met: 1. Presence of inadequate nutritional oral intake, related to medical condition (less than 50% of the caloric needs are being met by oral intake) requiring supplementation for a limited time period (e.g., 4-6-weeks). Digestive enzyme cartridges (e.g. Relizorb, Alcresta Pharmaceuticals) are considered investigational for all indications, including but not limited to, patients receiving enteral tube feedings. Oral Nutrition (ON) formula (prescribed by an MD, DO, NP, or PA) when used as a supplement or for dietary replacement may be considered medically necessary for the treatment of inborn errors of metabolism when: 1. Used to prevent illness resulting from a by-product of metabolism or amino acid accumulation; or 2. Required to restore an essential nutrient that is lacking because of an inborn error of metabolism. Generally, a daily caloric intake of calories is sufficient to maintain body weight. If 750 calories per day or less are being administered by EN or PN, it is considered not medically necessary and is considered supplemental because it is not the primary source of caloric intake for the patient. POLICY GUIDELINES Nutrients and their manner of administration for both PN and EN must be specifically ordered by a physician and be preapproved on an individual consideration basis. Approved PN, EN, and ON services (those meeting criteria) may include, but are not limited to, the following: cost of nutritional solutions cost of rental or purchase of an infusion pump cost of supplies and/or equipment required for effective delivery of PN or EN home visits by a medical practitioner administering skilled care BENEFIT APPLICATION BLUECARD /NATIONAL ACCOUNT ISSUES State or federal mandates (e.g., FEP) may dictate that certain U.S. Food and Drug Administration approved devices, drugs, or biologics may not be considered investigational, and thus these devices may be assessed only on the basis of their medical necessity. Plans may wish to establish global allowances for PN services to include nursing services, cost of supplies, clinical pharmacy service, supply delivery, and administrative overhead. Prior authorization is recommended. Benefits may be provided for placement of central venous catheters and gastrostomy or jejunostomy feeding tubes when policy guidelines have been met for PN or EN. Original Policy Date: June 2017 Page: 2
3 The total cost of renting equipment should not exceed the purchase cost of the equipment. Benefits are usually not provided for nutritional substances when used: to increase protein or caloric intake in addition to the patient s daily diet; in patients with a stable nutritional status, in whom only short-term parenteral nutrition might be required, (i.e., for less than 2 weeks); for routine pre- and/or postoperative care; for over-the-counter enteral nutrition. Blenderized baby food and regular shelf food used with an enteral system are not eligible for benefits. BACKGROUND Parenteral nutrition (PN), also known as parenteral hyper-alimentation, provides nutritional requirements through an intravenous route. PN is used for patients with medical conditions that impair gastrointestinal absorption to a level incompatible with life. A nutritionally adequate hypertonic solution consisting of glucose (sugar), amino acids (protein), electrolytes (sodium, potassium), vitamins, minerals, and lipids (fats) is administered daily. An infusion pump is generally used to assure a steady flow of the solution either on a continuous (24-hour), intermittent, or cyclic schedule. If intermittent, a heparin lock device and diluted heparin are used to prevent clotting inside the catheter. 7 Enteral nutrition (EN) is used for treating patients with severe malabsorption or patients with a functioning intestinal tract, but with disorders of the pharynx, esophagus, or stomach that prevent nutrients from reaching the absorbing surfaces in the small intestine. EN involves administering special nutritional liquids directly into the gastrointestinal tract through nasogastric, gastrostomy, or jejunostomy tubes. An infusion pump may be used to assist the flow of liquids. Feedings may be either intermittent or continuous (infused 24 hours a day). 7 Relizorb (Alcresta Pharmaceuticals) enzyme cartridge was approved by U.S. Food and Drug Administration (FDA) in 2015 under the De Novo product classification. Relizorb is a single use digestive enzyme cartridge indicated for use in adults to break down enteral formula. The device fits in line with enteral feeding systems and consists of an outer casing containing an inert polymer with a covalently bound enzyme through which nutritional formula is directed. It is designed to hydrolyze fat present in the enteral formula from triglycerides into fatty acids and monoglycerides to allow for their absorption by the body. This breakdown of fats is intended to mimic the function of the enzyme lipase in patients who do not excrete sufficient levels of pancreatic lipase. Oral nutrition therapy is formula or medical food taken orally to replace or supplement a diet. Inborn errors of metabolism are a group of disorders resulting in the excessive accumulation of an amino acid or other product along the metabolic pathway. Manifestations may include: central nervous system dysfunction; developmental delay; seizures; and liver dysfunction. If diagnosis is achieved early and appropriate treatment with dietary protein or amino acid restriction is initiated immediately, clinical manifestations in many of these disorders can be prevented. These disorders are named for the accumulating amino acid and include but are not limited to: phenylketonuria (PKU); citrullinemia; cystinosis; homocystinuria; and methylmalonic acidemia. RATIONALE PARENTERAL NUTRITION (PN) Original Policy Date: June 2017 Page: 3
4 A search of peer reviewed literature on parenteral nutrition identified the following medical position and guideline: The American Gastroenterological Association (AGA) published a medical position on parenteral nutrition in 2001 that states: In general, parenteral nutrition is indicated to prevent the adverse effects of malnutrition in patients who are unable to obtain adequate nutrients by oral or enteral routes. The decision to use parenteral nutrition requires an understanding of the patient s clinical condition and anticipated outcome, judgment as to the patient s ability to tolerate undernutrition, knowledge of the clinical efficacy of parenteral nutrition and an appreciation of the patient s desires and needs. 1 The American Society for Parenteral and Enteral Nutrition (ASPEN) (2002) published clinical guidelines as an update to the first published guidelines in 1993 as a result of new evidence. The ASPEN update included the following recommendations: When specialized nutrition support (SNS) is indicated, PN should be used when gastrointestinal tract is not functional or cannot be accessed and in patients who cannot be adequately nourished by oral diets or EN. This was a grade B recommendation which provides fair research-based evidence to support the guideline (well-designed studies without randomization). The nutritional requirements for adults who need SNS should be based on the results of the formal individualized nutrition assessment. The requirements for each nutrient may vary with nutrition status, disease, organ function, metabolic condition, medication use, and duration of nutrition support. 4 Per the ASPEN guidelines, common indications for home PN include: inflammatory bowel disease, nonterminal cancer, ischemic bowel, and radiation enteritis, motility disorders of the bowel, bowel obstruction, high-output intestinal or pancreatic fistulae, celiac disease, hyperemesis gravidarum, and protein-losing enteropathy. ENTERAL NUTRITION (EN) Enteral nutrition, oral nutrition, and parenteral nutrition therapies are seen as valuable treatment in the management of select patients requiring nutritional support to prevent the adverse effects of malnutrition. To determine the type of nutritional support and accurately calculate the patient's nutritional needs review of the following data is necessary: patient age and gender; review of preexisting medical conditions and history; and body mass index determination. The National Institutes of Health (NIH) and the American Society for Parenteral and Enteral Nutrition (ASPEN) published a joint conference report that reviewed current literature concerning the importance of nutritional support. 5 The review included prospective, randomized, controlled trials where nutritional therapy was administered for a minimum of five days and provided satisfactory nutrients to meet daily requirements. In total, more than 2500 patients were included in the studies reviewed. With one final statement, the report concluded that: The usage of nutritional therapy requires careful integration of data from pertinent clinical trials; Clinical expertise is required in the illness or injury being treated; Clinical input from nutritional therapy clinicians; Input from the patient and family. No human studies were identified with the use of Relizorb. The FDA approval is based on well-established pre-clinical porcine models that mimic the inability to digest and absorb fat. 3 A search of ClinicalTrials.gov identified one multicenter safety, tolerability, and fat absorption study. This clinical trial anticipated enrolling 35 subjects (pediatric and adult) with cystic fibrosis. Subjects with confirmed exocrine pancreatic insufficiency used an enteral Original Policy Date: June 2017 Page: 4
5 feeding digestive enzyme cartridge (Relizorb) connected to enteral pump set. This study is ongoing, not actively recruiting participants, and is supported by the product manufacturer, Alcresta Pharmaceuticals (NCT ). The estimated completion date was June There is insufficient published literature for the use of digestive enzyme cartridges. Larger randomized controlled trials are needed to support the safety, efficacy, and the health impact on humans. SECTION SUMMARY There is insufficient published literature for the use of digestive enzyme cartridges. Larger randomized controlled trials are needed to support the safety, efficacy, and the health impact on humans. SUPPLEMENTAL INFORMATION PRACTICE GUIDELINES AND POSITION STATEMENTS American Society for Parenteral and Enteral Nutrition The 2002 ASPEN Practice Guidelines: Indications for Specialized Nutrition Support (SNS) state the following for home specialized nutritional support: 1. Home SNS (HSNS) should be used in patients who cannot meet their nutrient requirements by oral intake and who are able to receive therapy outside of an acute care setting. (B) 2. When HSNS is required, HEN is the preferred route of administration when feasible. (B) 3. When HSNS is indicated, HPN should be used when the gastrointestinal tract is not functional and in patients who cannot be adequately supported with HEN. (B) Please refer to the full article for disease specific practice guidelines. U.S. PREVENTIVE SERVICES TASK FORCE RECOMMENDATIONS Not applicable. MEDICARE NATIONAL COVERAGE On July 11, 1984, Centers for Medicare and Medicaid Services published a National Coverage Determination: # Enteral and Parenteral Nutritional Therapy. 2 Please refer to the National Coverage Determination (NCD) for Enteral and Parenteral Nutritional Therapy (180.2). Also, see the DME MAC Local Coverage Determinations (LCDs) for Enteral Nutrition and the DME MAC LCDs for Parenteral Nutrition at REFERENCES 1. American Gastroenterological Association. American Gastroenterological Association medical position statement: Parenteral nutrition. Gastroenterology. 2001;121(4): Centers for Medicare and Medicaid Services. National Coverage Determination: Enteral and Parenteral Nutritional Therapy. NCD # Effective July 11, Available at: Accessed on April 18, FDA De Novo classification: Relizorb. Available at: < (accessed April 20, 2017). 4. Guidelines for the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients. Journal of Parenteral and Enteral Nutrition, American Society of Parenteral and Enteral Nutrition (2002 Jan/Feb) 26(1 Supplement): Klein, S., Kinney, J., Jeejeebhoy, K., Alpers, D., Hellerstein, M., Murray, M., & Twomey, P. (1997). Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, Original Policy Date: June 2017 Page: 5
6 CODES American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. The American journal of clinical nutrition, 66(3), Safety, Tolerability and Fat Absorption Using Enteral Feeding In-line Enzyme Cartridge (Relizorb). Available at: < (accessed April 20, 2017). NCT Total Parenteral Nutrition and Enteral Nutrition in the Home. (Archived) Chicago, Illinois: Blue Cross Blue Shield Association Consortium Health Plan Medical Policy Reference Manual (1996 July 31) Durable Medical Equipment: Codes Number Description CPT 36555, 36556, Placement of central venous catheter code range 36557, 36558, 36560, 36561, 36563, 36565, 36566, 36568, 36569, 36570, , 36581, Replacement of central venous catheter code range 36582, 36583, 36584, Esophagogastroduodenoscopy, flexible, transoral; with directed placement of percutaneous gastrostomy tube Insertion of gastrostomy tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report Tube of needle catheter jejunostomy for enteral alimentation, intra-operative, any method HCPCS B4102, B4103, Enteral formulae code range B4104, B4149, B4150, B4152, B4153, B4154, B4155, B4157, B4158, B4159, B4160, B4161, B4162 B4034, B4035, Enteral feeding supply kit B4036 B4164, B4168, Parenteral solution code range B4172, B4176, B4178, B4180, B4185, B4189, B4193, B4197, B4199, B4216, B4220, B4222, B4224, B5000, B5100, B5200 B9002 Enteral nutrition infusion pump code Original Policy Date: June 2017 Page: 6
7 ICD-10-CM ICD-10-PCS Type of service Place of service B9004-B9006 E0781 S9364-S9368 S9340-S9343 S9433-S9435 DME and Supplies Home Parenteral nutrition infusion pump code range Ambulatory infusion pump Home infusion therapy, total parenteral nutrition, per diem code range Home therapy, enteral nutrition, per diem code range Medical food nutritionally complete POLICY HISTORY Date Action Reason 06/01/17 Add policy to Durable Medical Equipment section Blue Cross of Idaho reviewed policy through April 20, /25/17 Replace policy Policy statement changed from "available by prescription only" to "prescribed by an MD, DO, NP, or PA." No other changes. 03/29/18 Update only Medical policy renumbered from to /25/18 Replace policy Blue Cross of Idaho annual review; no change to policy. Original Policy Date: June 2017 Page: 7
Total Parenteral Nutrition and Enteral Nutrition in the Home. Original Policy Date 12:2013
 MP 1.02.01 Total Parenteral Nutrition and Enteral Nutrition in the Home Medical Policy Section Durable Medical Equipment Issue Original Policy Date Last Review Status/Date Return to Medical Policy Index
MP 1.02.01 Total Parenteral Nutrition and Enteral Nutrition in the Home Medical Policy Section Durable Medical Equipment Issue Original Policy Date Last Review Status/Date Return to Medical Policy Index
Nutrition Therapy. Medical Coverage Policy Enteral/Parenteral EFFECTIVE DATE: POLICY LAST UPDATED: 11/20/2018 OVERVIEW
 Medical Coverage Policy Enteral/Parenteral Nutrition Therapy EFFECTIVE DATE: 01 20 2007 POLICY LAST UPDATED: 11/20/2018 OVERVIEW This policy describes the reimbursement for enteral and parenteral nutrition
Medical Coverage Policy Enteral/Parenteral Nutrition Therapy EFFECTIVE DATE: 01 20 2007 POLICY LAST UPDATED: 11/20/2018 OVERVIEW This policy describes the reimbursement for enteral and parenteral nutrition
Original Effective Date: 9/10/09
 Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
Original Effective Date: 3/8/2018. Subject: Digestive Enzymes For Use With Enteral Tube Feedings [e.g. RELiZORB immobilized lipase cartridge]
![Original Effective Date: 3/8/2018. Subject: Digestive Enzymes For Use With Enteral Tube Feedings [e.g. RELiZORB immobilized lipase cartridge] Original Effective Date: 3/8/2018. Subject: Digestive Enzymes For Use With Enteral Tube Feedings [e.g. RELiZORB immobilized lipase cartridge]](/thumbs/94/122259654.jpg) Subject: Digestive Enzymes For Use With Enteral Tube Feedings [e.g. RELiZORB immobilized lipase cartridge] Policy Number: MCP-304 Original Effective Date: 3/8/2018 Revision Date(s): Review Date: 3/8/2018
Subject: Digestive Enzymes For Use With Enteral Tube Feedings [e.g. RELiZORB immobilized lipase cartridge] Policy Number: MCP-304 Original Effective Date: 3/8/2018 Revision Date(s): Review Date: 3/8/2018
MEDICAL POLICY: Enteral and Parenteral Nutrition
 POLICY: PG0114 ORIGINAL EFFECTIVE: 02/15/07 LAST REVIEW: 08/14/18 MEDICAL POLICY: Enteral and Parenteral Nutrition GUIDELINES This policy does not certify benefits or authorization of benefits, which is
POLICY: PG0114 ORIGINAL EFFECTIVE: 02/15/07 LAST REVIEW: 08/14/18 MEDICAL POLICY: Enteral and Parenteral Nutrition GUIDELINES This policy does not certify benefits or authorization of benefits, which is
Clinical Policy: Total Parenteral Nutrition and Intradialytic Parenteral Nutrition Reference Number: CP.PHAR.205
 Clinical Policy: Reference Number: CP.PHAR.205 Effective Date: 05.16 Last Review Date: 02.18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: Reference Number: CP.PHAR.205 Effective Date: 05.16 Last Review Date: 02.18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Medical Policy Enteral Nutrition Formulas and Supplements
 Document Number: 017 Medical Policy Enteral Nutrition Formulas and Supplements Commercial and Qualified Health Plans MassHealth Authorization required X X No notification or authorization Not covered Overview
Document Number: 017 Medical Policy Enteral Nutrition Formulas and Supplements Commercial and Qualified Health Plans MassHealth Authorization required X X No notification or authorization Not covered Overview
Enteral Nutrition Corporate Medical Policy
 Enteral Nutrition Corporate Medical Policy File name: Enteral Nutrition File code: UM.DME.05 Origination: 10/2004 as part of BCBSVT Medical Policy on Total Parenteral Nutrition and Enteral Nutrition Last
Enteral Nutrition Corporate Medical Policy File name: Enteral Nutrition File code: UM.DME.05 Origination: 10/2004 as part of BCBSVT Medical Policy on Total Parenteral Nutrition and Enteral Nutrition Last
Clinical Policy: Total Parenteral Nutrition and Intradialytic Parenteral Nutrition
 Clinical Policy: Reference Number: CP.MP.163 Last Review Date: 04/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Parenteral
Clinical Policy: Reference Number: CP.MP.163 Last Review Date: 04/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Parenteral
Medical Policy Enteral Nutrition Formulas and Supplements
 Medical Policy Enteral Nutrition Formulas and Supplements Document Number: 017 Commercial and MassHealth Connector/Qualified Health Plans Authorization required X X Notification within 24 hours of service
Medical Policy Enteral Nutrition Formulas and Supplements Document Number: 017 Commercial and MassHealth Connector/Qualified Health Plans Authorization required X X Notification within 24 hours of service
Enteral Nutrition Corporate Medical Policy
 Enteral Nutrition Corporate Medical Policy File Name: Enteral Nutrition File Code: UM.DME.05 Origination: 10/2004 as part of BCBSVT Medical Policy on Total Parenteral Nutrition and Enteral Nutrition Last
Enteral Nutrition Corporate Medical Policy File Name: Enteral Nutrition File Code: UM.DME.05 Origination: 10/2004 as part of BCBSVT Medical Policy on Total Parenteral Nutrition and Enteral Nutrition Last
Subject: Enteral Formulas
 09-J0000-61 Original Effective Date: 07/15/02 Reviewed: 04/26/18 Revised: 11/15/18 Subject: Enteral Formulas THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
09-J0000-61 Original Effective Date: 07/15/02 Reviewed: 04/26/18 Revised: 11/15/18 Subject: Enteral Formulas THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
Claim Filing Information INSIDE THIS ISSUE: FEP Point-of-Service identification numbers
 May 30, 2000 INSIDE THIS ISSUE: CLAIMS FILING INFORMATION Pages 1-4 FEP Point-Of-Service Identification Numbers Guidelines For Newborn Care Claims Mailing Address for Claims and Customer Service Inquiries
May 30, 2000 INSIDE THIS ISSUE: CLAIMS FILING INFORMATION Pages 1-4 FEP Point-Of-Service Identification Numbers Guidelines For Newborn Care Claims Mailing Address for Claims and Customer Service Inquiries
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
PARENTERAL NUTRITION THERAPY
 UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare West BENEFIT INTERPRETATION POLICY
WHEN To Initiate Parenteral Nutrition A Frequent Question With New Answers
 WHEN To Initiate Parenteral Nutrition A Frequent Question With New Answers Ainsley Malone, MS, RD, LD, CNSC, FAND, FASPEN Dubai International Nutrition Conference 2018 Disclosures No commercial relationship
WHEN To Initiate Parenteral Nutrition A Frequent Question With New Answers Ainsley Malone, MS, RD, LD, CNSC, FAND, FASPEN Dubai International Nutrition Conference 2018 Disclosures No commercial relationship
Enteral Nutrition. The Health Plan ENTERAL NUTRITION
 Enteral Nutrition Adopted from the National Government Services website. For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit category
Enteral Nutrition Adopted from the National Government Services website. For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit category
Clinical Medical Policy Department Clinical Affairs Division DESCRIPTION
 Total Parenteral Nutrition (TPN) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.] Medical
Total Parenteral Nutrition (TPN) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.] Medical
The revision date appears in the footer of the document. Links within the document are updated as changes occur throughout the year.
 An Independent Licensee of the Blue Cross Blue Shield Association. APPENDIX C HOME INFUSION THERAPY MANUAL This appendix to the Business Procedure Manual briefly describes home infusion therapy benefits
An Independent Licensee of the Blue Cross Blue Shield Association. APPENDIX C HOME INFUSION THERAPY MANUAL This appendix to the Business Procedure Manual briefly describes home infusion therapy benefits
Who Needs Parenteral Nutrition? Is Parenteral Nutrition An Appropriate Intervention?
 Who Needs Parenteral Nutrition? 1 Is Parenteral Nutrition An Appropriate Intervention? Key questions to ask with initial consultation Can the gastrointestinal (GI) tract be utilized? Can the GI tract be
Who Needs Parenteral Nutrition? 1 Is Parenteral Nutrition An Appropriate Intervention? Key questions to ask with initial consultation Can the gastrointestinal (GI) tract be utilized? Can the GI tract be
Nutritional Management in Enterocutaneous fistula Dr Deepak Govil
 Nutritional Management in Enterocutaneous fistula Dr Deepak Govil MS, PhD (GI Surgery) Senior Consultant Surgical Gastroenterology Indraprastha Apollo Hospital New Delhi What is enterocutaneous fistula
Nutritional Management in Enterocutaneous fistula Dr Deepak Govil MS, PhD (GI Surgery) Senior Consultant Surgical Gastroenterology Indraprastha Apollo Hospital New Delhi What is enterocutaneous fistula
MEDICAL POLICY I. POLICY POLICY TITLE PARENTERAL HOME INFUSION THERAPY (INCLUDING TOTAL PARENTERAL NUTRITION) MP-3.
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Home infusion services may be considered medically necessary
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY Home infusion services may be considered medically necessary
Methods of Nutrition Support KNH 406
 Methods of Nutrition Support KNH 406 Malnutrition 30 50% of hospitalized patients 95% of nursing home patients Resulting in reduced quality of life & increased health care costs May be remedied by providing
Methods of Nutrition Support KNH 406 Malnutrition 30 50% of hospitalized patients 95% of nursing home patients Resulting in reduced quality of life & increased health care costs May be remedied by providing
LOUISIANA MEDICAID PROGRAM ISSUED:
 37.12 TOTAL PARENTERAL NUTRITION Overview Introduction In This Section This Section explains the LMPBM program s Total Parenteral Nutrition (TPN) therapy coverage, limitations, prior authorization, reimbursement
37.12 TOTAL PARENTERAL NUTRITION Overview Introduction In This Section This Section explains the LMPBM program s Total Parenteral Nutrition (TPN) therapy coverage, limitations, prior authorization, reimbursement
Coverage Guidelines: Oral Formula: Rhode Island Products
 Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
Coverage Guidelines: Oral Formula: Rhode Island Products Effective: August 9, 2017 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization Tufts Health
Nutrition. By Dr. Ali Saleh 2/27/2014 1
 Nutrition By Dr. Ali Saleh 2/27/2014 1 Nutrition Functions of nutrients: Providing energy for body processes and movement. Providing structural material for body tissues. Regulating body processes. 2/27/2014
Nutrition By Dr. Ali Saleh 2/27/2014 1 Nutrition Functions of nutrients: Providing energy for body processes and movement. Providing structural material for body tissues. Regulating body processes. 2/27/2014
Parenteral Nutrition. The Health Plan PARENTERAL NUTRITION
 Parenteral Nutrition Adopted from National Government Services website For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit category
Parenteral Nutrition Adopted from National Government Services website For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit category
The Role of Parenteral Nutrition. in PEDIATRIC INTENSIVE CARE UNIT. Dzulfikar DLH. Pediatric Emergency and Intensive Care Unit
 The Role of Parenteral Nutrition in PEDIATRIC INTENSIVE CARE UNIT Dzulfikar DLH Pediatric Emergency and Intensive Care Unit Department of Child Health, Faculty of Medicine Universitas Padjajaran, Hasan
The Role of Parenteral Nutrition in PEDIATRIC INTENSIVE CARE UNIT Dzulfikar DLH Pediatric Emergency and Intensive Care Unit Department of Child Health, Faculty of Medicine Universitas Padjajaran, Hasan
01/10/17. Replaces Effective Policy Dated: Amino Acid Based Elemental Formula (AABF) 09/28/15 Reference #: MP/A003 Page: 1 of 3
 Reference #: MP/A003 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MP/A003 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Current Trends in Home Parenteral Nutrition
 Current Trends in Home Parenteral Nutrition Jeremy Nightingale Consultant Gastroenterologist St Mark s Hospital Parenteral Routes of drug administration, which do not involve the alimentary canal Includes:
Current Trends in Home Parenteral Nutrition Jeremy Nightingale Consultant Gastroenterologist St Mark s Hospital Parenteral Routes of drug administration, which do not involve the alimentary canal Includes:
Medical Necessity Guidelines: Oral Formula: Massachusetts Products
 Medical Necessity Guidelines: Oral Formula: Massachusetts Products Effective: January 1, 2018 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization
Medical Necessity Guidelines: Oral Formula: Massachusetts Products Effective: January 1, 2018 Clinical Documentation and Prior Authorization Required Applies to: Coverage Guideline, No Prior Authorization
IEHP Healthy Kids Benefit Manual 07/15 N-100.1
 Revised: August 2007 Approval: Newborn Child Coverage Benefit Coverage Newborn examinations and nursery care are covered while the mother is hospitalized. Examples of Covered Benefits 1. Medically necessary
Revised: August 2007 Approval: Newborn Child Coverage Benefit Coverage Newborn examinations and nursery care are covered while the mother is hospitalized. Examples of Covered Benefits 1. Medically necessary
Department of Origin: Integrated Healthcare Services. Approved by: Chief Medical Officer Department(s) Affected: Date approved: 01/10/17
 Reference #: MP/D005 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MP/D005 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
AC15 [RW] 23 F. Educating Your Referral Sources to Facilitate the Intake Process
![AC15 [RW] 23 F. Educating Your Referral Sources to Facilitate the Intake Process AC15 [RW] 23 F. Educating Your Referral Sources to Facilitate the Intake Process](/thumbs/89/99978458.jpg) 2015 NHIA Annual Conference & Exposition Dynamic Handout Session 23 F Rethinking the Intake Process AC15 [RW] 23 F Educating Your Referral Sources to Facilitate the Intake Process Group Exercise Break
2015 NHIA Annual Conference & Exposition Dynamic Handout Session 23 F Rethinking the Intake Process AC15 [RW] 23 F Educating Your Referral Sources to Facilitate the Intake Process Group Exercise Break
Effect of changing lipid formulation in Parenteral Nutrition in the Newborn Experimental Pathology BSc
 Effect of changing lipid formulation in Parenteral Nutrition in the Newborn Experimental Pathology BSc Word count: 6939 0 CONTENTS Abstract...2 Acknowledgements...3 Introduction...4 Materials and Methods...11
Effect of changing lipid formulation in Parenteral Nutrition in the Newborn Experimental Pathology BSc Word count: 6939 0 CONTENTS Abstract...2 Acknowledgements...3 Introduction...4 Materials and Methods...11
ESPEN Congress Florence 2008
 ESPEN Congress Florence 2008 PN Guidelines presentation PN Guidelines in gastroenterology A. van Gossum (Belgium) ESPEN-Parenteral Guidelines in Gastroenterology André Van Gossum, Eduard Cabre, Xavier
ESPEN Congress Florence 2008 PN Guidelines presentation PN Guidelines in gastroenterology A. van Gossum (Belgium) ESPEN-Parenteral Guidelines in Gastroenterology André Van Gossum, Eduard Cabre, Xavier
Nutrition Management in GI Diseases
 Nutrition Management in GI Diseases Aryono Hendarto MD Nutrition & Metabolic Diseases Division Department of Child Health Cipto Mangunkusumo Hospital University of Indonesia 1 Patient s Care 1. Drugs 2.
Nutrition Management in GI Diseases Aryono Hendarto MD Nutrition & Metabolic Diseases Division Department of Child Health Cipto Mangunkusumo Hospital University of Indonesia 1 Patient s Care 1. Drugs 2.
Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement
 Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement Line of Business: Medicaid P & T Approval Date: May 16,2018 Effective Date: July 1, 2018 This policy has been developed through
Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement Line of Business: Medicaid P & T Approval Date: May 16,2018 Effective Date: July 1, 2018 This policy has been developed through
Current concepts in Critical Care Nutrition
 Current concepts in Critical Care Nutrition Dr.N.Ramakrishnan AB (Int Med), AB (Crit Care), MMM, FACP, FCCP, FCCM Director, Critical Care Services Apollo Hospitals, Chennai Objectives Why? Enteral or Parenteral
Current concepts in Critical Care Nutrition Dr.N.Ramakrishnan AB (Int Med), AB (Crit Care), MMM, FACP, FCCP, FCCM Director, Critical Care Services Apollo Hospitals, Chennai Objectives Why? Enteral or Parenteral
Enteral Nutrition. Presented by Melanie Farwell RD, LD Keene Medical Products Dietitian
 Enteral Nutrition Presented by Melanie Farwell RD, LD Keene Medical Products Dietitian What is it? Liquid feeding provided to the gastrointestinal tract via nose, stomach or small intestine -Specifically
Enteral Nutrition Presented by Melanie Farwell RD, LD Keene Medical Products Dietitian What is it? Liquid feeding provided to the gastrointestinal tract via nose, stomach or small intestine -Specifically
Review/Revision Dates: 12/07, 09/2014, 09/15, 2/16
 Subject: HEALTH PLAN OF SAN JOAQUIN Nutritional Supplements for Medical Conditions Department: Medical Management / Pharmacy Policy #: PH19 Effective Date: 06/01/2007 Committee/Approval Date: P&T 02/16/16
Subject: HEALTH PLAN OF SAN JOAQUIN Nutritional Supplements for Medical Conditions Department: Medical Management / Pharmacy Policy #: PH19 Effective Date: 06/01/2007 Committee/Approval Date: P&T 02/16/16
MCT AND THE ROLES NUTRITION
 MCT AND THE ROLES NUTRITION Nguyen Hoang Nhut Hoa Department of Nutrition Children's Hospital 2 OBJECTIVES Structure Absorption and metabolic Effects of MCT in the treatment of certain diseases Demand
MCT AND THE ROLES NUTRITION Nguyen Hoang Nhut Hoa Department of Nutrition Children's Hospital 2 OBJECTIVES Structure Absorption and metabolic Effects of MCT in the treatment of certain diseases Demand
ENFit Compatible. Patient Guide
 Patient Guide ENFit Compatible 1 TABLE OF CONTENTS 1. Table of contents... inside front cover 2. Glossary of terms... 1 3. Introduction... 2 4. What is RELiZORB?... 2 5. How does RELiZORB connect into
Patient Guide ENFit Compatible 1 TABLE OF CONTENTS 1. Table of contents... inside front cover 2. Glossary of terms... 1 3. Introduction... 2 4. What is RELiZORB?... 2 5. How does RELiZORB connect into
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy
 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy File Name: Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy File Name: Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders
MEDICAL MANAGEMENT POLICY
 PAGE: 1of 8 This Medical policy is not a guarantee of benefits or coverage, nor should it be deemed as medical advice. In the event of any conflict concerning benefit coverage, the employer/member summary
PAGE: 1of 8 This Medical policy is not a guarantee of benefits or coverage, nor should it be deemed as medical advice. In the event of any conflict concerning benefit coverage, the employer/member summary
Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement
 Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement Line of Business: Medicaid P & T Approval Date: May 16,2018 Effective Date: July 1, 2018 This policy has been developed through
Drug Class Prior Authorization Criteria Adult Enteral Nutritional Supplement Line of Business: Medicaid P & T Approval Date: May 16,2018 Effective Date: July 1, 2018 This policy has been developed through
Chapter 20. Assisting With Nutrition and Fluids
 Chapter 20 Assisting With Nutrition and Fluids Food and water: Are physical needs Basics of Nutrition Are necessary for life A poor diet and poor eating habits: Increase the risk for diseases and infection
Chapter 20 Assisting With Nutrition and Fluids Food and water: Are physical needs Basics of Nutrition Are necessary for life A poor diet and poor eating habits: Increase the risk for diseases and infection
Before addressing the specific issues and citing case studies and clinical examples, the following points are of utmost importance:
 POSITION STATEMENT Inadequacy of Medicare Part B and Medicare Part D (Prescription Drug Coverage) To Meet the Needs of Patients Requiring Parenteral Nutrition The American Society for Parenteral and Enteral
POSITION STATEMENT Inadequacy of Medicare Part B and Medicare Part D (Prescription Drug Coverage) To Meet the Needs of Patients Requiring Parenteral Nutrition The American Society for Parenteral and Enteral
Nutrition Services at a glance
 Nutrition Services at a glance Ragini Raghuveer, MS, RD, LD/N Systems Clinical Nutrition Manager Linette De Armas, RD, LD/N Clinical Dietitian Melissa Lorenzo, RD, LD/N Clinical Dietitian 1 Learning Objectives
Nutrition Services at a glance Ragini Raghuveer, MS, RD, LD/N Systems Clinical Nutrition Manager Linette De Armas, RD, LD/N Clinical Dietitian Melissa Lorenzo, RD, LD/N Clinical Dietitian 1 Learning Objectives
LIST OF CLINICAL PRIVILEGES DIETITIAN
 LIST OF CLINICAL PRIVILEGES DIETITIAN AUTHORITY: Title 10, U.S.C. Chapter 55, Sections 1094 and 1102. PRINCIPAL PURPOSE: To define the scope and limits of practice for individual providers. Privileges
LIST OF CLINICAL PRIVILEGES DIETITIAN AUTHORITY: Title 10, U.S.C. Chapter 55, Sections 1094 and 1102. PRINCIPAL PURPOSE: To define the scope and limits of practice for individual providers. Privileges
Medical Policy. MP Ingestible ph and Pressure Capsule
 Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
10/28/2015. Disclaimer. Disclaimer
 Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
vedolizumab (Entyvio )
 vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
Adult Enteral Nutritional Supplement Drug Class Prior Authorization Protocol
 Adult Enteral Nutritional Supplement Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Adult Enteral Nutritional Supplement Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
PARENTERAL NUTRITION
 PARENTERAL NUTRITION DEFINITION Parenteral nutrition [(PN) or total parenteral nutrition (TPN)] is the intravenous infusion of some or all nutrients for tissue maintenance, metabolic requirements and growth
PARENTERAL NUTRITION DEFINITION Parenteral nutrition [(PN) or total parenteral nutrition (TPN)] is the intravenous infusion of some or all nutrients for tissue maintenance, metabolic requirements and growth
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 12/19/17 SECTION: MEDICINE LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
MEDICAL FOODS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Scott A. Lynch, MD, MPH,FAAFP Assistant Professor
 Scott A. Lynch, MD, MPH,FAAFP Assistant Professor Lynch.Scott@mayo.edu 2015 MFMER 3543652-1 Nutrition in the Hospital Mayo School of Continuous Professional Development 2nd Annual Inpatient Medicine for
Scott A. Lynch, MD, MPH,FAAFP Assistant Professor Lynch.Scott@mayo.edu 2015 MFMER 3543652-1 Nutrition in the Hospital Mayo School of Continuous Professional Development 2nd Annual Inpatient Medicine for
1 TABLE OF CONTENTS 2 GLOSSARY OF TERMS. 1. Table of contents...inside front cover. 2. Glossary of terms Introduction...
 Patient Guide TABLE OF CONTENTS GLOSSARY OF TERMS. Table of contents...inside front cover. Glossary of terms... 3. Introduction... 4. What is RELiZORB?... 5. How does RELiZORB connect into my feeding pump?...
Patient Guide TABLE OF CONTENTS GLOSSARY OF TERMS. Table of contents...inside front cover. Glossary of terms... 3. Introduction... 4. What is RELiZORB?... 5. How does RELiZORB connect into my feeding pump?...
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN)
 Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Nutritional products (Special Foods) supplied by Nutricia
 2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
Dedication...iii Contributors...iv v Reviewers... vi vii Preface... viii x
 Table of Contents Dedication...iii Contributors...iv v Reviewers... vi vii Preface... viii x Part I. Parenteral Nutrition A. introduction to Parenteral Nutrition 1. What is Parenteral Nutrition? A.S.P.E.N.
Table of Contents Dedication...iii Contributors...iv v Reviewers... vi vii Preface... viii x Part I. Parenteral Nutrition A. introduction to Parenteral Nutrition 1. What is Parenteral Nutrition? A.S.P.E.N.
sebelipase alfa (Kanuma )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
ESPEN LLL PROGRAMME IN CLINICAL NUTRITION AND METABOLISM Summary of Topics 2018
 ESPEN LLL PROGRAMME IN CLINICAL NUTRITION AND METABOLISM Summary of Topics 2018 Topic 3 Nutritional Assessment and Techniques To learn nutritional screening and assessment; the methods used; To know the
ESPEN LLL PROGRAMME IN CLINICAL NUTRITION AND METABOLISM Summary of Topics 2018 Topic 3 Nutritional Assessment and Techniques To learn nutritional screening and assessment; the methods used; To know the
Medical Review Criteria. Formulas and Enteral Nutrition. Subject: Formulas and Enteral Nutrition
 Medical Review Criteria Subject: Policy: HPHC covers special medical formulas and enteral nutrition 1 that are ordered by a licensed health care provider, and medically necessary to prevent clinical deterioration
Medical Review Criteria Subject: Policy: HPHC covers special medical formulas and enteral nutrition 1 that are ordered by a licensed health care provider, and medically necessary to prevent clinical deterioration
Parenteral and Enteral Nutrition
 Parenteral and Enteral Nutrition Audis Bethea, Pharm.D. Assistant Professor Therapeutics I December 5 & 9, 2003 Parenteral Nutrition Definition process of supplying nutrients via the intravenous route
Parenteral and Enteral Nutrition Audis Bethea, Pharm.D. Assistant Professor Therapeutics I December 5 & 9, 2003 Parenteral Nutrition Definition process of supplying nutrients via the intravenous route
MEDICAL POLICY SUBJECT: NUTRITIONAL THERAPY
 MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Corporate Medical Policy Fecal Microbiota Transplantation
 Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Bariatric Surgery MM /11/2001. HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient; Inpatient
 Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
SARASOTA MEMORIAL HOSPITAL
 SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: PARENTERAL NUTRITION AND INTRAVENOUS FAT EMULSION (ADULT AND PEDIATRICS) Nursing DATE: REVIEWED: PAGES: RESPONSIBILITY: RN, LPN II 12/80
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: PARENTERAL NUTRITION AND INTRAVENOUS FAT EMULSION (ADULT AND PEDIATRICS) Nursing DATE: REVIEWED: PAGES: RESPONSIBILITY: RN, LPN II 12/80
Corporate Medical Policy
 Corporate Medical Policy Small Bowel, Small Bowel with Liver, or Multivisceral Transplant File Name: Origination: Last CAP Review: Next CAP Last Review: small_bowel_liver_and_multivisceral_transplant 2/1996
Corporate Medical Policy Small Bowel, Small Bowel with Liver, or Multivisceral Transplant File Name: Origination: Last CAP Review: Next CAP Last Review: small_bowel_liver_and_multivisceral_transplant 2/1996
Nutrition and Medicine, 2006 Tufts University School of Medicine Nutrition and Acute Illness: Learning Objectives
 Nutrition and Medicine, 2006 Tufts University School of Medicine Nutrition and Acute Illness: Learning Objectives Margo N. Woods, D.Sc. 1. Define protein-calorie, or protein-energy malnutrition (PEM) and
Nutrition and Medicine, 2006 Tufts University School of Medicine Nutrition and Acute Illness: Learning Objectives Margo N. Woods, D.Sc. 1. Define protein-calorie, or protein-energy malnutrition (PEM) and
Adult Trauma Feeding Access Guideline
 Adult Trauma Feeding Access Guideline Background: Enteral feeding access mode (NGT, NDT, PEG, PEG-J, Jejunostomy tube) dependent upon patient characteristics. Enteral feeding management guidelines aim
Adult Trauma Feeding Access Guideline Background: Enteral feeding access mode (NGT, NDT, PEG, PEG-J, Jejunostomy tube) dependent upon patient characteristics. Enteral feeding management guidelines aim
Clinical Policy: Nutritional Therapy Reference Number: CP.MP.254
 Clinical Policy: Reference Number: CP.MP.254 Effective Date: 02/06 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.254 Effective Date: 02/06 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Nutrition care plan for surgical patients. Objectives
 Slide 1 Nutrition care plan for surgical patients Surgical Nutrition Training Module Level 1 Philippine Society of General Surgeons Committee on Surgical Training In this session we will discuss the most
Slide 1 Nutrition care plan for surgical patients Surgical Nutrition Training Module Level 1 Philippine Society of General Surgeons Committee on Surgical Training In this session we will discuss the most
Nutritional Requirements in Intestinal Failure
 Nutritional Requirements in Intestinal Failure Christopher Duggan, MD, MPH Center for Nutrition Center for Advanced Intestinal Rehabilitation (CAIR) Division of Gastroenterology, Hepatology and Nutrition
Nutritional Requirements in Intestinal Failure Christopher Duggan, MD, MPH Center for Nutrition Center for Advanced Intestinal Rehabilitation (CAIR) Division of Gastroenterology, Hepatology and Nutrition
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abdominal pain, enteral therapy in acute pancreatitis and, 812 Abscess(es), pancreatic, nutritional support for, 814 815 Acute Physiology and
Note: Page numbers of article titles are in boldface type. A Abdominal pain, enteral therapy in acute pancreatitis and, 812 Abscess(es), pancreatic, nutritional support for, 814 815 Acute Physiology and
Why You Switched to the Tiger 2 Self-Advancing Nasal Jejunal Feeding Tube
 Why You Switched to the Tiger 2 Self-Advancing Nasal Jejunal Feeding Tube Ease of Use Self-advancing technology minimises hands-on time for clinicians. Requires no additional devices or costly capital
Why You Switched to the Tiger 2 Self-Advancing Nasal Jejunal Feeding Tube Ease of Use Self-advancing technology minimises hands-on time for clinicians. Requires no additional devices or costly capital
Diabetes Management, Equipment and Supplies
 Coverage Summary Diabetes Management, Equipment and Supplies Policy Number: D-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 11/01/2006 Approved by: UnitedHeatlhcare Medicare
Coverage Summary Diabetes Management, Equipment and Supplies Policy Number: D-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 11/01/2006 Approved by: UnitedHeatlhcare Medicare
SUSTAIN BASELINE DATA COLLECTION FORM. Revised 2/4/2014 (both pediatric and adult data elements) 1. Gestational age at birth ( weeks gestation)
 SUSTAIN BASELINE DATA COLLECTION FORM Patient Demographics Revised 2/4/2014 (both pediatric and adult data elements) Did the patient begin Home PN over 90 days ago? Yes No Date began Home PN Patient Number
SUSTAIN BASELINE DATA COLLECTION FORM Patient Demographics Revised 2/4/2014 (both pediatric and adult data elements) Did the patient begin Home PN over 90 days ago? Yes No Date began Home PN Patient Number
Medical Policy. MP Dynamic Spinal Visualization
 Medical Policy MP 6.01.46 BCBSA Ref. Policy: 6.01.46 Last Review: 03/29/2018 Effective Date: 03/29/2018 Section: Radiology End Date: 12/14/2018 Related Policies 6.01.48 Positional Magnetic Resonance Imaging
Medical Policy MP 6.01.46 BCBSA Ref. Policy: 6.01.46 Last Review: 03/29/2018 Effective Date: 03/29/2018 Section: Radiology End Date: 12/14/2018 Related Policies 6.01.48 Positional Magnetic Resonance Imaging
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System
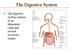 The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
THE AUTHOR OF THIS WHAT S NEW IN NUTRITION? OBJECTIVES & OUTLINE EVIDENCE-BASED MEDICINE: PARENTERAL NUTRITION (PN)
 WHAT S NEW IN NUTRITION? Alisha Mutch, Pharm.D., BCPS THE AUTHOR OF THIS PRESENTATION HAS NOTHING TO DISCLOSE. OBJECTIVES & OUTLINE MALNUTRITION OBJECTIVES Indicate when parenteral nutrition (PN) is warranted
WHAT S NEW IN NUTRITION? Alisha Mutch, Pharm.D., BCPS THE AUTHOR OF THIS PRESENTATION HAS NOTHING TO DISCLOSE. OBJECTIVES & OUTLINE MALNUTRITION OBJECTIVES Indicate when parenteral nutrition (PN) is warranted
Adult Enteral Tube Feeding Medical Directive
 Harmonized A printed copy of this document may not reflect the current, electronic version on Lakeridge Health s Intranet, The Wave. Any copies of this document appearing in paper form should ALWAYS be
Harmonized A printed copy of this document may not reflect the current, electronic version on Lakeridge Health s Intranet, The Wave. Any copies of this document appearing in paper form should ALWAYS be
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Urea Cycle Disorders Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Urea Cycle Disorders Prime Therapeutics will review Prior Authorization requests
Urea Cycle Disorders Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Urea Cycle Disorders Prime Therapeutics will review Prior Authorization requests
Guideline scope Neonatal parenteral nutrition
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Neonatal parenteral nutrition The Department of Health in England has asked NICE to develop a new guideline on parenteral nutrition in
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Neonatal parenteral nutrition The Department of Health in England has asked NICE to develop a new guideline on parenteral nutrition in
Gastroenterology Fellowship Program
 Gastroenterology Fellowship Program Outpatient Clinical Rotations I. Overview A. Three Year Continuity Clinic Experience All gastroenterology fellows will be required to have a ½ day continuity clinic
Gastroenterology Fellowship Program Outpatient Clinical Rotations I. Overview A. Three Year Continuity Clinic Experience All gastroenterology fellows will be required to have a ½ day continuity clinic
VELTASSA (patiromer) oral suspension
 VELTASSA (patiromer) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
VELTASSA (patiromer) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Clinical Policy: Fecal Calprotectin Assay Reference Number: CP.MP.135
 Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
tens_(transcutaneous_electrical_nerve_stimulator) 7/ / / /2014 This policy is NOT effective until January 13, 2015
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Parenteral Nutrition in Oncology
 Parenteral Nutrition in Oncology Presenter: Pam Wagner, RD, CNSC Learning Objectives List indications for initiating PN in oncology patients Describe considerations when determining an appropriate candidate
Parenteral Nutrition in Oncology Presenter: Pam Wagner, RD, CNSC Learning Objectives List indications for initiating PN in oncology patients Describe considerations when determining an appropriate candidate
Wireless Capsule Endoscopy
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
ENTYVIO (vedolizumab)
 ENTYVIO (vedolizumab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
ENTYVIO (vedolizumab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Nutritional Support For The Critical Patient Andrea Collins, BBA, LVT, VTS (ECC)
 Nutritional Support For The Critical Patient Andrea Collins, BBA, LVT, VTS (ECC) TOPICS PART 1 Importance Indications for support Nutritional assessment Energy requirements The plan Enteral/Parenteral
Nutritional Support For The Critical Patient Andrea Collins, BBA, LVT, VTS (ECC) TOPICS PART 1 Importance Indications for support Nutritional assessment Energy requirements The plan Enteral/Parenteral
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: esophageal_ph_monitoring 4/2011 5/2017 5/2018 5/2017 Description of Procedure or Service Acid reflux is the
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: esophageal_ph_monitoring 4/2011 5/2017 5/2018 5/2017 Description of Procedure or Service Acid reflux is the
Nutrition, part 2. Because 1 part isn t enough!
 Nutrition, part 2 Because 1 part isn t enough! 4. Calories and Caloric Intake Calories per gram of our Macro and Micro nutrients Macro Carbohydrates: 4 cal/g Fats: 9 cal/g Proteins: 4 cal/g Micro Vitamins:
Nutrition, part 2 Because 1 part isn t enough! 4. Calories and Caloric Intake Calories per gram of our Macro and Micro nutrients Macro Carbohydrates: 4 cal/g Fats: 9 cal/g Proteins: 4 cal/g Micro Vitamins:
Section 38-1 Food and Nutrition (pages )
 Name Class Date Section 38-1 Food and Nutrition (pages 971-977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy (page 971) 1. Cells convert
Name Class Date Section 38-1 Food and Nutrition (pages 971-977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy (page 971) 1. Cells convert
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter
 Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
HOMES AND SENIORS SERVICES. APPROVAL DATE: February 2011 REVISION DATE: January 2015; July 2018
 POLICY: Page 1 of 6 A resident requiring enteral (tube) feeding as a sole source or adjunctive nutrition support have access to a comprehensive enteral feeding program and receive appropriate support from
POLICY: Page 1 of 6 A resident requiring enteral (tube) feeding as a sole source or adjunctive nutrition support have access to a comprehensive enteral feeding program and receive appropriate support from
