PANEL QUESTIONS FOR REGULATORY CONSIDERATION
|
|
|
- Jared Harrington
- 5 years ago
- Views:
Transcription
1 PANEL QUESTIONS FOR REGULATORY CONSIDERATION Biometric Monitoring Devices (BMDs) to Quantitatively Assess Quality-of-Life (QoL) Domains: Mobility/Frailty, Sleep, & Cognition
2 QUESTIONS FOR THE PANEL CAMD and its members have framed some questions to gain a perspective on how BMDs could be used as potential Drug Development Tools and Future Outcome Assessments in Clinical Drug Trials. The questions are group into the categories of: General Use Data Collection Clinical Validation/ Regulatory Path 2
3 3 GENERAL USE Do you agree that input from patients on which functional aspects of mobility/frailty, sleep, cognition, and mood should drive where focus should be given in validating BMD assessments? Should the data be derived from caregivers too? Given that both neurologic and psychiatric diseases have symptoms of altered mobility/frailty, sleep and cognition, would an assessment based on BMD measurements be faced with pseudo-specificity issues? What regulatory conversations are encouraged to ensure that that use is developed for a specific context-of-use? What are the considerations between deciding to use a commercial grade device and a medical device for a regulatory submission? - Would this be influenced by use in a label claim?
4 4 DATA COLLECTION What practices must be put into place that ensures data collected is from the intended research subject? - Fingerprint; Facial recognition; Behavioral phenotyping; Voice recognition As more technologies enter the clinical arena, comparative effectiveness studies should consider the impact of user-centric dimensions that have historically not been prominent considerations (e.g., easy user interface and user accessibility). - What is FDA s perspective about defining metrics to capture usage patterns, learnability, and other crucial traits of consumer-grade technologies?
5 5 DATA COLLECTION (continued) How critical is it to collect contextual metadata to integrate into the BMD assessment for interpretation? Case 1: Recent knee replacement ; Case 2: Hiking the Appalachian trail; Case 3: -40 o F outside in Minneapolis/ +100 o F outside in Phoenix; Case 4: Wheelchair bound; Case 5: Depression Many mhealth technologies have started incorporating more active components such as notifications and reminders that inevitably affect data collection and, potentially, outcomes. How can study reproducibility be improved in cases where prompting is not standardized? Is this a concern? Will use the type of assessment influence whether this is important?
6 6 CLINICAL VALIDATION How should the reliability of a BMD be established? How should test, re-test, and analytical reliability be documented? Should this be done across all intended patient populations? Are longitudinal studies required? In making assessment with BMDs, is it more important to compare results to normal populations, or is it more important to analyze the rate of change for an individual? Case in Point: If an individual normally resides 1.5 to 2 standard deviations above the norm for cognition, but deteriorates to the mean within 3 months, would this be a potential signal for acute brain dysfunction?
7 7 CLINICAL VALIDATION (continued) For clinical validation of a BMD would patients at each stage of disease need to be specifically explored? - e.g., pre-symptomatic, MCI, mild-to-moderate How are individual and patient population differences handled in guiding the assessment of normal? Case in Point: An algorithm may work well in non-obese, non-frail, etc. Will each patient population need to be studied for validity, or would reliability for a range of normals be sufficient to warrant immediate use?
8 8 CLINICAL VALIDATION (continued) Given the multi-dimensional impact of psychiatric and neurologic disease on the physiology supporting mobility, sleep, mood, and cognition, should future work focus on individual measures, or should composite assessments that probe the constellation of pathophysiologic dimensions most important to those affected by a disease drive where the field goes? For BMD tests that have already received FDA approval for assessing cognitive impairment for one type of brain dysfunction, what general considerations would need to be addressed to advance the use of this device for another brain disease that affects cognitive function? By what regulatory endorsement pathway should these assessments best progress: Fit-for-Purpose (e.g., Physiologically Based Model of Disease Progression)? Biomarker Qualification? COA Qualification? Individual Review Divisions within CDER (e.g., Psychiatry or Neurology)?
9 Thank you!
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016
 Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Collaborating to Develop Digital Biomarkers with Passive Data Collection
 Collaborating to Develop Digital Biomarkers with Passive Data Collection Iain Simpson IXICO June 2018 1 Setting of Data Collection Market evolution: biosensors and digital biomarkers Clinic Home Digital
Collaborating to Develop Digital Biomarkers with Passive Data Collection Iain Simpson IXICO June 2018 1 Setting of Data Collection Market evolution: biosensors and digital biomarkers Clinic Home Digital
Measuring Functional Change in Outpatient Therapy Claims-Based Data Collection Reqs. for Outpatient Therapy Services
 Measuring Functional Change in Outpatient Therapy Paulette Niewczyk, MPH, PhD Director of Research Manager of Research, Development, and Analytical Services Manager of CFAR Uniform Data System for Medical
Measuring Functional Change in Outpatient Therapy Paulette Niewczyk, MPH, PhD Director of Research Manager of Research, Development, and Analytical Services Manager of CFAR Uniform Data System for Medical
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database
 Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
Critical Path Institute Coalition Against Major Diseases (CAMD) Alzheimer s Clinical Trial Database Dr. Carolyn Compton, M.D., Ph.D. Chief Executive Officer Critical Path Institute 1 What We Do DEVELOP
In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review
 In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review Cardiac Safety Research Consortium December 3, 2015 Lea Carrington Division of Immunology and Hematology Devices Office of In
In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review Cardiac Safety Research Consortium December 3, 2015 Lea Carrington Division of Immunology and Hematology Devices Office of In
Medical Device Regulatory Decision Points
 Medical Device Regulatory Decision Points Medical Device Classification Benefit Risk Regulatory Path Predicate Devices Valid Scientific Evidence Least Burdensome Approach Medical Devices Classified by
Medical Device Regulatory Decision Points Medical Device Classification Benefit Risk Regulatory Path Predicate Devices Valid Scientific Evidence Least Burdensome Approach Medical Devices Classified by
Patient-Reported Outcome Measures
 Medication Safety in Older People Network & Citizen Senate Event: Working together for Medication Safety - 6 th March 2018 Patient-Reported Outcome Measures Sam Salek School of Life & Medical Sciences
Medication Safety in Older People Network & Citizen Senate Event: Working together for Medication Safety - 6 th March 2018 Patient-Reported Outcome Measures Sam Salek School of Life & Medical Sciences
Electronic Capture of PROs in NCI Clinical Trials
 Electronic Capture of PROs in NCI Clinical Trials Lori Minasian, MD, FACP Deputy Director, Division of Cancer Prevention, NCI July 27, 2017 Vision for PROs in NCI Clinical Trials Incorporate patient reported
Electronic Capture of PROs in NCI Clinical Trials Lori Minasian, MD, FACP Deputy Director, Division of Cancer Prevention, NCI July 27, 2017 Vision for PROs in NCI Clinical Trials Incorporate patient reported
RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process. Disclaimer:
 RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational
RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Outline. Outline
 Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
NGS ONCOPANELS: FDA S PERSPECTIVE
 NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
Patient Reported Outcomes
 Date Patient Reported Outcomes Presented by: Ari Gnanasakthy RTI Health Solutions 9 th Feb 2015 RTI Health Solutions Research Triangle Park, NC, USA Ann Arbor, MI, USA Barcelona, Spain Ljungskile, Sweden
Date Patient Reported Outcomes Presented by: Ari Gnanasakthy RTI Health Solutions 9 th Feb 2015 RTI Health Solutions Research Triangle Park, NC, USA Ann Arbor, MI, USA Barcelona, Spain Ljungskile, Sweden
Tactile Internet and Edge Computing: Emerging Technologies for Mobile Health
 Tactile Internet and Edge Computing: Emerging Technologies for Mobile Health Zaher Dawy, PhD Department of Electrical and Computer Engineering American University of Beirut http://www.aub.edu.lb/~zd03
Tactile Internet and Edge Computing: Emerging Technologies for Mobile Health Zaher Dawy, PhD Department of Electrical and Computer Engineering American University of Beirut http://www.aub.edu.lb/~zd03
NGS IN ONCOLOGY: FDA S PERSPECTIVE
 NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
WHO s code of conduct for open and timely sharing of pathogen genetic sequence data during outbreaks of infectious disease
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 WHO s code of conduct for open and timely sharing of pathogen genetic sequence data during outbreaks of infectious
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 WHO s code of conduct for open and timely sharing of pathogen genetic sequence data during outbreaks of infectious
REVIEW MANAGEMENT. Clinical Review of Drugs to Reduce the Risk of Cancer CONTENTS
 REVIEW MANAGEMENT Clinical Review of Drugs to Reduce the Risk of Cancer CONTENTS PURPOSE BACKGROUND REFERENCES DEFINITIONS AND ACRONYMS POLICY PROCEDURES EFFECTIVE DATE PURPOSE This MAPP describes: The
REVIEW MANAGEMENT Clinical Review of Drugs to Reduce the Risk of Cancer CONTENTS PURPOSE BACKGROUND REFERENCES DEFINITIONS AND ACRONYMS POLICY PROCEDURES EFFECTIVE DATE PURPOSE This MAPP describes: The
Challenges in Developing Learning Algorithms to Personalize mhealth Treatments
 Challenges in Developing Learning Algorithms to Personalize mhealth Treatments JOOLHEALTH Bar-Fit Susan A Murphy 01.16.18 HeartSteps SARA Sense 2 Stop Continually Learning Mobile Health Intervention 1)
Challenges in Developing Learning Algorithms to Personalize mhealth Treatments JOOLHEALTH Bar-Fit Susan A Murphy 01.16.18 HeartSteps SARA Sense 2 Stop Continually Learning Mobile Health Intervention 1)
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: AZ.CP.PHAR.10.11.8 Effective Date: 07.2016 Last Review Date: 09.12.18 Line of Business: Arizona Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: Reference Number: AZ.CP.PHAR.10.11.8 Effective Date: 07.2016 Last Review Date: 09.12.18 Line of Business: Arizona Medicaid Revision Log See Important Reminder at the end of this policy
Update on the Clinical Outcome Assessment Qualification Program and COA Compendium
 Update on the Clinical Outcome Assessment Qualification Program and COA Compendium Seventh Annual PRO Consortium Workshop April 27-28, 2016 Michelle Campbell, PhD Clinical Outcomes Assessment Staff (COA
Update on the Clinical Outcome Assessment Qualification Program and COA Compendium Seventh Annual PRO Consortium Workshop April 27-28, 2016 Michelle Campbell, PhD Clinical Outcomes Assessment Staff (COA
Study Endpoint Considerations: Final PRO Guidance and Beyond
 Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
Frailty: Are we able to identify the older adult who is frail? A discussion on methods and limitations. Neil Pendleton University of Manchester
 Frailty: Are we able to identify the older adult who is frail? A discussion on methods and limitations Neil Pendleton University of Manchester Frailty Foundation in observation by clinicians dealing with
Frailty: Are we able to identify the older adult who is frail? A discussion on methods and limitations Neil Pendleton University of Manchester Frailty Foundation in observation by clinicians dealing with
Flex Program Guide: Developing MBQIP Peer Mentoring Programs
 Flex Program Guide: Developing MBQIP Peer Mentoring Programs One of the most pressing barriers to MBQIP success is the frequent turnover of critical access hospital quality improvement staff. MBQIP data
Flex Program Guide: Developing MBQIP Peer Mentoring Programs One of the most pressing barriers to MBQIP success is the frequent turnover of critical access hospital quality improvement staff. MBQIP data
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE. Yuan Xu 18- JAN- 2018
 BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
BEST PRACTICE CHANGE CONTROL : DECIDING WHEN TO SUBMIT A 510(K) FOR A DEVICE CHANGE Yuan Xu 18- JAN- 2018 Background AGENDA What is Medical Device? What is 510(k)? Principles of best practices of device
An assistive application identifying emotional state and executing a methodical healing process for depressive individuals.
 An assistive application identifying emotional state and executing a methodical healing process for depressive individuals. Bandara G.M.M.B.O bhanukab@gmail.com Godawita B.M.D.T tharu9363@gmail.com Gunathilaka
An assistive application identifying emotional state and executing a methodical healing process for depressive individuals. Bandara G.M.M.B.O bhanukab@gmail.com Godawita B.M.D.T tharu9363@gmail.com Gunathilaka
Psyllium Products and Blood Cholesterol Lowering
 Psyllium Products and Blood Cholesterol Lowering Summary of Health Canada s Assessment of a Health Claim about Food Products Containing Psyllium and Blood Cholesterol Lowering December 2011 Bureau of Nutritional
Psyllium Products and Blood Cholesterol Lowering Summary of Health Canada s Assessment of a Health Claim about Food Products Containing Psyllium and Blood Cholesterol Lowering December 2011 Bureau of Nutritional
Measure #106 (NQF 0103): Adult Major Depressive Disorder (MDD): Comprehensive Depression Evaluation: Diagnosis and Severity
 Measure #106 (NQF 0103): Adult Major Depressive Disorder (MDD): Comprehensive Depression Evaluation: Diagnosis and Severity 2014 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: Percentage
Measure #106 (NQF 0103): Adult Major Depressive Disorder (MDD): Comprehensive Depression Evaluation: Diagnosis and Severity 2014 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: Percentage
FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs Hyman, Phelps & McNamara, P.C. Washington, DC
 AIPLA Annual Meeting Joint Biotechnology Committee/ Special Committee on FDA Law Program October 21, 2010 Marriott Wardman Park Hotel Washington, DC FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs
AIPLA Annual Meeting Joint Biotechnology Committee/ Special Committee on FDA Law Program October 21, 2010 Marriott Wardman Park Hotel Washington, DC FDA Regulation of Diagnostic Tests Jeffrey N. Gibbs
THE ESSENTIAL BRAIN INJURY GUIDE
 THE ESSENTIAL BRAIN INJURY GUIDE Outcomes Section 9 Measurements & Participation Presented by: Rene Carfi, LCSW, CBIST Senior Brain Injury Specialist Brain Injury Alliance of Connecticut Contributors Kimberly
THE ESSENTIAL BRAIN INJURY GUIDE Outcomes Section 9 Measurements & Participation Presented by: Rene Carfi, LCSW, CBIST Senior Brain Injury Specialist Brain Injury Alliance of Connecticut Contributors Kimberly
Integrating Pain Metrics into Oncologic Clinical and Regulatory Decision-Making. Charles Cleeland MD Anderson Cancer Center
 Integrating Pain Metrics into Oncologic Clinical and Regulatory Decision-Making Charles Cleeland MD Anderson Cancer Center Panelists Charles Cleeland, Department Chair, Department of Symptom Research,
Integrating Pain Metrics into Oncologic Clinical and Regulatory Decision-Making Charles Cleeland MD Anderson Cancer Center Panelists Charles Cleeland, Department Chair, Department of Symptom Research,
Pediatrics Milestones and Meaningful Assessment Translating the Pediatrics Milestones into Assessment Items for use in the Clinical Setting
 Pediatrics Milestones and Meaningful Assessment Translating the Pediatrics Milestones into Assessment Items for use in the Clinical Setting Ann Burke Susan Guralnick Patty Hicks Jeanine Ronan Dan Schumacher
Pediatrics Milestones and Meaningful Assessment Translating the Pediatrics Milestones into Assessment Items for use in the Clinical Setting Ann Burke Susan Guralnick Patty Hicks Jeanine Ronan Dan Schumacher
Enhanced Asthma Management with Mobile Communication
 Enhanced Asthma Management with Mobile Communication P.S. Ngai, S. Chan, C.T. Lau, K.M. Lau Abstract In this paper, we propose a prototype system to enhance the management of asthma condition in patients
Enhanced Asthma Management with Mobile Communication P.S. Ngai, S. Chan, C.T. Lau, K.M. Lau Abstract In this paper, we propose a prototype system to enhance the management of asthma condition in patients
Measure #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care
 Measure #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
Measure #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
DESCRIPTION: Percentage of patients with dementia for whom an assessment of functional status was performed at least once in the last 12 months
 Quality ID #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention, Treatment, and Management of Mental Health 2019 COLLECTION
Quality ID #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention, Treatment, and Management of Mental Health 2019 COLLECTION
2) Percentage of adult patients (aged 18 years or older) with a diagnosis of major depression or dysthymia and an
 Quality ID #370 (NQF 0710): Depression Remission at Twelve Months National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention, Treatment, and Management of Mental Health
Quality ID #370 (NQF 0710): Depression Remission at Twelve Months National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention, Treatment, and Management of Mental Health
Clinical and Regulatory Challenges in Developing New Treatments for Rare Diseases
 Clinical and Regulatory Challenges in Developing New Treatments for Rare Diseases R. Anand, M.D. APC AG, Switzerland ISCTM, Washington DC February 20 th, 2018 Conflict of Interests A - Z Too many to mention
Clinical and Regulatory Challenges in Developing New Treatments for Rare Diseases R. Anand, M.D. APC AG, Switzerland ISCTM, Washington DC February 20 th, 2018 Conflict of Interests A - Z Too many to mention
Agenda. 15 Dec
 Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Sarcopenia - a Regulatory Perspective
 Sarcopenia - a Regulatory Perspective Dragos Roman, M.D. Team Leader Division of Metabolism and Endocrinology Products, Office of New Drugs, Center for Drug Evaluation and Research, FDA May 11, 2012 1
Sarcopenia - a Regulatory Perspective Dragos Roman, M.D. Team Leader Division of Metabolism and Endocrinology Products, Office of New Drugs, Center for Drug Evaluation and Research, FDA May 11, 2012 1
May 16, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org May 16, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org May 16, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration
Patient-Reported Outcomes: A Critical Insight into the Impact of Therapy
 Patient-Reported Outcomes: A Critical Insight into the Impact of Therapy John Spertus MD MPH Missouri/Lauer Endowed Chair and Professor, UMKC Saint Luke s Mid America Heart Institute Presentation Overview
Patient-Reported Outcomes: A Critical Insight into the Impact of Therapy John Spertus MD MPH Missouri/Lauer Endowed Chair and Professor, UMKC Saint Luke s Mid America Heart Institute Presentation Overview
A Physician/Patient Hybrid Perspective on Biometric Monitoring Devices (BMD)
 A Physician/Patient Hybrid Perspective on Biometric Monitoring Devices (BMD) David W. Levine, MD, MPH Biometric Monitoring Device Workshop May 9-10,2017 Outline Objectives Background Demographic As Physician/Clinical
A Physician/Patient Hybrid Perspective on Biometric Monitoring Devices (BMD) David W. Levine, MD, MPH Biometric Monitoring Device Workshop May 9-10,2017 Outline Objectives Background Demographic As Physician/Clinical
Measure #402: Tobacco Use and Help with Quitting Among Adolescents National Quality Strategy Domain: Community / Population Health
 Measure #402: Tobacco Use and Help with Quitting Among Adolescents National Quality Strategy Domain: Community / Population Health 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION:
Measure #402: Tobacco Use and Help with Quitting Among Adolescents National Quality Strategy Domain: Community / Population Health 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION:
GLOOKO DREAMED FOR ANDROID USER GUIDE
 GLOOKO DREAMED FOR ANDROID USER GUIDE November 2018 IFU-0017 02 TABLE OF CONTENTS TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Glooko Intended Use... 2 Dreamed Intended Use... 2
GLOOKO DREAMED FOR ANDROID USER GUIDE November 2018 IFU-0017 02 TABLE OF CONTENTS TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Glooko Intended Use... 2 Dreamed Intended Use... 2
Quality ID #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care
 Quality ID #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Quality ID #282: Dementia: Functional Status Assessment National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff Document issued on: July 29, 2016. The draft of this document was issued on January 20, 2015.
September 30, Eric BASTINGS, MD. Acting Director Division of Neurology Products (DNP) Center for Drug Evaluation and Research (CDER)
 ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
Sit to Stand. The sit to stand maneuver assisted by the Up n Free accomplishes three important functions:
 Sit to Stand The sit to stand maneuver assisted by the Up n Free accomplishes three important functions: 1. Raising the user by supplementing their lower extremity strength 2. Learning/reinforcing the
Sit to Stand The sit to stand maneuver assisted by the Up n Free accomplishes three important functions: 1. Raising the user by supplementing their lower extremity strength 2. Learning/reinforcing the
Best practices for a successful wellness screening program
 Health & Wellness Best practices for a successful wellness screening program Identify risk. Increase engagement. Incite change. Identifying health risks in your employee population People at risk for chronic
Health & Wellness Best practices for a successful wellness screening program Identify risk. Increase engagement. Incite change. Identifying health risks in your employee population People at risk for chronic
Sanofi Press Breakfast on Behavioral Science. Friday 22 September 2017
 Sanofi Press Breakfast on Behavioral Science Friday 22 September 2017 Agenda WHY UNDERSTANDING BEHAVIORS CAN UNLOCK CARE SUCCESS 8.45-9.00 Welcome coffee 9.00 Kick-off 9.00-9.10 Introduction by Alexandra
Sanofi Press Breakfast on Behavioral Science Friday 22 September 2017 Agenda WHY UNDERSTANDING BEHAVIORS CAN UNLOCK CARE SUCCESS 8.45-9.00 Welcome coffee 9.00 Kick-off 9.00-9.10 Introduction by Alexandra
Quality ID #293: Parkinson s Disease: Rehabilitative Therapy Options National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #293: Parkinson s Disease: Rehabilitative Therapy Options National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
Quality ID #293: Parkinson s Disease: Rehabilitative Therapy Options National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Measure #109: Osteoarthritis (OA): Function and Pain Assessment National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #109: Osteoarthritis (OA): Function and Pain Assessment National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #283: Dementia Associated Behavioral and Psychiatric Symptoms Screening and Management National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Quality ID #283: Dementia Associated Behavioral and Psychiatric Symptoms Screening and Management National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
The PhenX Toolkit: Standard Measures for Collaborative Research
 The PhenX Toolkit: Standard Measures for Collaborative Research Clinical Common Data Elements Task Force (CDETF) November 4, 2016 Carol M. Hamilton, PhD RTI International is a trade name of Research Triangle
The PhenX Toolkit: Standard Measures for Collaborative Research Clinical Common Data Elements Task Force (CDETF) November 4, 2016 Carol M. Hamilton, PhD RTI International is a trade name of Research Triangle
FAO SPECIFICATIONS FOR PLANT PROTECTION PRODUCTS DNOC. 4,6-dinitro-o-cresol WITH PETROLEUM OIL PRODUCTS
 AGP : CP / 79 FAO SPECIFICATIONS FOR PLANT PROTECTION PRODUCTS DNOC 4,6-dinitro-o-cresol WITH PETROLEUM OIL PRODUCTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1979 1 DISCLAIMER 1 FAO
AGP : CP / 79 FAO SPECIFICATIONS FOR PLANT PROTECTION PRODUCTS DNOC 4,6-dinitro-o-cresol WITH PETROLEUM OIL PRODUCTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1979 1 DISCLAIMER 1 FAO
Your Path Starts Here
 spring fall 2014 2014 Your Path Starts Here Our Wellness Portal is the perfect place to begin living healthier - Is sleep apnea hurting your health? - Call for 24/7 advice from a nurse - Optional dental
spring fall 2014 2014 Your Path Starts Here Our Wellness Portal is the perfect place to begin living healthier - Is sleep apnea hurting your health? - Call for 24/7 advice from a nurse - Optional dental
NHC Webinar Series on Clinical Outcome Assessments. Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference?
 NHC Webinar Series on Clinical Outcome Assessments Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference? NOVEMBER 7, 2018 Outline Why are we having this webinar? Introduction
NHC Webinar Series on Clinical Outcome Assessments Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference? NOVEMBER 7, 2018 Outline Why are we having this webinar? Introduction
OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks
 OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks Michael C. Nevitt, PhD Dept of Epidemiology and Biostatistics University of California, San Francisco OAI
OA Biomarkers: What is required for validation and qualification? Part I. Evaluation Frameworks Michael C. Nevitt, PhD Dept of Epidemiology and Biostatistics University of California, San Francisco OAI
Lecture 1: Introduction to Personalized Medicine. Donglin Zeng, Department of Biostatistics, University of North Carolina
 Lecture 1: Introduction to Personalized Medicine Personalized Medicine A Quick View Personalized Medicine is a general medical paradigm referring to systematic use of individual patient information to
Lecture 1: Introduction to Personalized Medicine Personalized Medicine A Quick View Personalized Medicine is a general medical paradigm referring to systematic use of individual patient information to
The Industry s Views on Older Old Patients
 The Industry s Views on Older Old Patients Susanna Del Signore and Philippe Guillet Global Regulatory Policy and Ageing Therapeutic Strategic Unit SANOFI R&D 1 Outline Introduction EFPIA Survey: Overview
The Industry s Views on Older Old Patients Susanna Del Signore and Philippe Guillet Global Regulatory Policy and Ageing Therapeutic Strategic Unit SANOFI R&D 1 Outline Introduction EFPIA Survey: Overview
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Vraylar) Reference Number: CP.PMN.91 Effective Date: 11.16.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Vraylar) Reference Number: CP.PMN.91 Effective Date: 11.16.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Richard Watson, Chief Transformation Officer. Dr P Holloway, GP Clinical Lead for Cancer Lisa Parrish, Senior Transformation Lead
 GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
PRO ASSESSMENT IN CANCER TRIALS. Emiliano Calvo, Anita Margulies, Eric Raymond, Ian Tannock, Nadia Harbeck and Lonneke van de Poll-Franse
 PRO ASSESSMENT IN CANCER TRIALS Emiliano Calvo, Anita Margulies, Eric Raymond, Ian Tannock, Nadia Harbeck and Lonneke van de Poll-Franse DISCLOSURES Emiliano Calvo has reported no conflicts of interest
PRO ASSESSMENT IN CANCER TRIALS Emiliano Calvo, Anita Margulies, Eric Raymond, Ian Tannock, Nadia Harbeck and Lonneke van de Poll-Franse DISCLOSURES Emiliano Calvo has reported no conflicts of interest
Emergency Monitoring and Prevention (EMERGE)
 Emergency Monitoring and Prevention (EMERGE) Results EU Specific Targeted Research Project (STREP) 6. Call of IST-Programme 6. Framework Programme Funded by IST-2005-2.6.2 Project No. 045056 Dr. Thomas
Emergency Monitoring and Prevention (EMERGE) Results EU Specific Targeted Research Project (STREP) 6. Call of IST-Programme 6. Framework Programme Funded by IST-2005-2.6.2 Project No. 045056 Dr. Thomas
Top: Healthy Vertebrae Above: Osteoporotic bone
 Top: Healthy Vertebrae Above: Osteoporotic bone 2 OSTEOPOROSIS IS A DISEASE OF THE BONES, WHICH LEADS TO AN INCREASED RISK OF FRACTURE. IN OSTEOPOROSIS, THE DENSITY AND QUALITY OF BONE ARE REDUCED. THE
Top: Healthy Vertebrae Above: Osteoporotic bone 2 OSTEOPOROSIS IS A DISEASE OF THE BONES, WHICH LEADS TO AN INCREASED RISK OF FRACTURE. IN OSTEOPOROSIS, THE DENSITY AND QUALITY OF BONE ARE REDUCED. THE
American Board of Psychiatry and Neurology, Inc. Geriatric Psychiatry Core Competencies Outline
 American Board of Psychiatry and Neurology, Inc. Geriatric Psychiatry Core Competencies Outline I. Geriatric Psychiatry Patient Care and Procedural Skills Core Competencies A. Geriatric psychiatrists shall
American Board of Psychiatry and Neurology, Inc. Geriatric Psychiatry Core Competencies Outline I. Geriatric Psychiatry Patient Care and Procedural Skills Core Competencies A. Geriatric psychiatrists shall
Emil D. Kakkis, M.D., Ph.D. President Kakkis EveryLife Foundation
 Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the EveryLife Foundation for Rare Diseases for educational purposes only. They are for use by drug development professionals
Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the EveryLife Foundation for Rare Diseases for educational purposes only. They are for use by drug development professionals
Measure #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety
 Measure #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety 2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE: Process This is a two-part measure which
Measure #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety 2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE: Process This is a two-part measure which
Collaborative Approaches for Developing Kidney Safety Biomarkers
 Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Quality ID #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety
 Quality ID #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage of
Quality ID #154 (NQF: 0101): Falls: Risk Assessment National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: Percentage of
iviewdose Confidence and assurance in dose delivery
 iviewdose Confidence and assurance in dose delivery As radiotherapy techniques advance and treatment plans become more complex, involving escalated doses and increasingly conformal deliveries, the need
iviewdose Confidence and assurance in dose delivery As radiotherapy techniques advance and treatment plans become more complex, involving escalated doses and increasingly conformal deliveries, the need
Smart NDT Tools: A New Generation of NDT Devices
 ECNDT 2006 - Th.2.1.2 Smart NDT Tools: A New Generation of NDT Devices Sébastien ROLET, EADS Corporate Research Center, Colomiers, France Abstract. EADS CRC is designing specific NDT solutions for EADS
ECNDT 2006 - Th.2.1.2 Smart NDT Tools: A New Generation of NDT Devices Sébastien ROLET, EADS Corporate Research Center, Colomiers, France Abstract. EADS CRC is designing specific NDT solutions for EADS
PCWP meeting with all eligible patient & consumer organisations 26 Nov 2015 Swedish Medicines Agency proposals for patient engagement
 PCWP meeting with all eligible patient & consumer organisations 26 Nov 2015 Swedish Medicines Agency proposals for patient engagement Lena Ring Uppdaterat 1 januari 2015 Our vision A leading force in collaboration
PCWP meeting with all eligible patient & consumer organisations 26 Nov 2015 Swedish Medicines Agency proposals for patient engagement Lena Ring Uppdaterat 1 januari 2015 Our vision A leading force in collaboration
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Language Biomarkers of Brain Health. Rhoda Au, Ph.D. 2 nd International Symposium on Language Resources and Intelligence
 Language Biomarkers of Brain Health Rhoda Au, Ph.D. 2 nd International Symposium on Language Resources and Intelligence 12-17-18 Global Burden of Dementia Can We Prevent the Tsunami Wave of Dementia? So
Language Biomarkers of Brain Health Rhoda Au, Ph.D. 2 nd International Symposium on Language Resources and Intelligence 12-17-18 Global Burden of Dementia Can We Prevent the Tsunami Wave of Dementia? So
APHL-CDC Influenza Cost Estimation Models
 APHL-CDC Influenza Cost Estimation Models Introduction An optimal influenza surveillance system requires adequate resources to support all elements of the system. Sustaining the national, state and local
APHL-CDC Influenza Cost Estimation Models Introduction An optimal influenza surveillance system requires adequate resources to support all elements of the system. Sustaining the national, state and local
Total agree 14.6% 14.1% 15.2% 14.5% 15.5% 13.7% 16.3% 12.7% 14.5% 16.6% Total disagree 70.0% 68.9% 70.8% 70.1% 69.6% 70.4% 71.2% 71.8% 68.7% 67.
 Question 1: Agree or disagree? I would allow a mobile app to collect my biometric data, such as a fingerprint or face scan. Strongly agree 5.4% 5.7% 5.7% 4.6% 5.4% 5.4% 6.6% 4.8% 4.1% 6.7% Somewhat agree
Question 1: Agree or disagree? I would allow a mobile app to collect my biometric data, such as a fingerprint or face scan. Strongly agree 5.4% 5.7% 5.7% 4.6% 5.4% 5.4% 6.6% 4.8% 4.1% 6.7% Somewhat agree
Frailty in Older Adults
 Frailty in Older Adults John Puxty puxtyj@providencecare Geriatrics 20/20: Bringing Current Issues into Perspective Session Overview Definition of Frailty Strategies for identifying frail older adults
Frailty in Older Adults John Puxty puxtyj@providencecare Geriatrics 20/20: Bringing Current Issues into Perspective Session Overview Definition of Frailty Strategies for identifying frail older adults
Phase 2b/3 Topline Trial Results
 Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
Chapter 15 - Biometrics
 Chapter 15 - Biometrics Alex Slutsky Computer security seminar Spring 2014 University of Haifa What is Biometrics? Biometrics refers to the quantifiable data (or metrics) related to human characteristics
Chapter 15 - Biometrics Alex Slutsky Computer security seminar Spring 2014 University of Haifa What is Biometrics? Biometrics refers to the quantifiable data (or metrics) related to human characteristics
Measure #279: Sleep Apnea: Assessment of Adherence to Positive Airway Pressure Therapy National Quality Strategy Domain: Effective Clinical Care
 Measure #279: Sleep Apnea: Assessment of Adherence to Positive Airway Pressure Therapy National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Measure #279: Sleep Apnea: Assessment of Adherence to Positive Airway Pressure Therapy National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
What is Occupational Therapy? Introduction to Occupational Therapy. World Federation of Occupational Therapists 2012
 World Federation of Occupational Therapists 2012 Introduction to Occupational Therapy Suki HUI Occupational Therapist I Statement on Occupational Therapy Occupational therapy is a client-centred health
World Federation of Occupational Therapists 2012 Introduction to Occupational Therapy Suki HUI Occupational Therapist I Statement on Occupational Therapy Occupational therapy is a client-centred health
CYGNUS: A digital brain health platform for improving dementia outcomes
 CYGNUS: A digital brain health platform for improving dementia outcomes Léa Marais - IXICO Hakim Yadi - Northern Health Science Alliance www.ecygnus.com Background to Cygnus Many people with dementia are
CYGNUS: A digital brain health platform for improving dementia outcomes Léa Marais - IXICO Hakim Yadi - Northern Health Science Alliance www.ecygnus.com Background to Cygnus Many people with dementia are
The Use of Voice Recognition and Speech Command Technology as an Assistive Interface for ICT in Public Spaces.
 The Use of Voice Recognition and Speech Command Technology as an Assistive Interface for ICT in Public Spaces. A whitepaper published by Peter W Jarvis (Senior Executive VP, Storm Interface) and Nicky
The Use of Voice Recognition and Speech Command Technology as an Assistive Interface for ICT in Public Spaces. A whitepaper published by Peter W Jarvis (Senior Executive VP, Storm Interface) and Nicky
INTERQUAL BEHAVIORAL HEALTH CRITERIA ADOLESCENT PSYCHIATRY REVIEW PROCESS
 INTERQUAL BEHAVIORAL HEALTH CRITERIA ADOLESCENT PSYCHIATRY REVIEW PROCESS RP-1 RP-2 AGE PARAMETERS Adolescent Psychiatry Behavioral Health Criteria are for the review of patients who are ages 13 to 17
INTERQUAL BEHAVIORAL HEALTH CRITERIA ADOLESCENT PSYCHIATRY REVIEW PROCESS RP-1 RP-2 AGE PARAMETERS Adolescent Psychiatry Behavioral Health Criteria are for the review of patients who are ages 13 to 17
An open-source mobile device for outside-lab sound field research
 An open-source mobile device for outside-lab sound field research Institute of Hearing Technology and Audiology Jade University of Applied Sciences Inga Holube with contributions from Jörg Bitzer, Sven
An open-source mobile device for outside-lab sound field research Institute of Hearing Technology and Audiology Jade University of Applied Sciences Inga Holube with contributions from Jörg Bitzer, Sven
Cognition in the Canadian Longitudinal Study on Aging
 Cognition in the Canadian Longitudinal Study on Aging Holly Tuokko, Lauren Griffith, Helena Kadlec, Megan O'Connell, Martine Simard, Vanessa Taler, & Stacey Voll Cognition What is cognition? What are the
Cognition in the Canadian Longitudinal Study on Aging Holly Tuokko, Lauren Griffith, Helena Kadlec, Megan O'Connell, Martine Simard, Vanessa Taler, & Stacey Voll Cognition What is cognition? What are the
Actigraphy-based Clinical Study Endpoints: A Regulatory Perspective
 Actigraphy-based Clinical Study Endpoints: A Regulatory Perspective Ebony Dashiell-Aje, PhD Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation and Research U.S. Food and
Actigraphy-based Clinical Study Endpoints: A Regulatory Perspective Ebony Dashiell-Aje, PhD Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation and Research U.S. Food and
Assistive Technology for Senior Adults Facing Cognitive Impairments: Neuroscience Considerations. Roger P. Carrillo.
 Assistive Technology for Senior Adults Facing Cognitive Impairments: Neuroscience Considerations Roger P. Carrillo Senior Advisor Camanio Care, Inc. 2 Assistive Technology for Senior Adults Facing Cognitive
Assistive Technology for Senior Adults Facing Cognitive Impairments: Neuroscience Considerations Roger P. Carrillo Senior Advisor Camanio Care, Inc. 2 Assistive Technology for Senior Adults Facing Cognitive
POLICY REF NO: SABP/SERVICE IMPROVEMENT/0024 POLICY
 POLICY REF NO: SABP/SERVICE IMPROVEMENT/0024 POLICY NAME OF POLICY: REASON FOR THE POLICY: WHAT THE POLICY WILL ACHIEVE: WHO NEEDS TO KNOW ABOUT IT: DATE APPROVED: VERSION NUMBER: APPROVING COMMITTEE:
POLICY REF NO: SABP/SERVICE IMPROVEMENT/0024 POLICY NAME OF POLICY: REASON FOR THE POLICY: WHAT THE POLICY WILL ACHIEVE: WHO NEEDS TO KNOW ABOUT IT: DATE APPROVED: VERSION NUMBER: APPROVING COMMITTEE:
A critical appraisal of: Canadian guideline fysisk aktivitet using the AGREE II Instrument
 A critical appraisal of: Canadian guideline fysisk aktivitet using the AGREE II Instrument Created with the AGREE II Online Guideline Appraisal Tool. No endorsement of the content of this document by the
A critical appraisal of: Canadian guideline fysisk aktivitet using the AGREE II Instrument Created with the AGREE II Online Guideline Appraisal Tool. No endorsement of the content of this document by the
PHYSICAL FUNCTION A brief guide to the PROMIS Physical Function instruments:
 PROMIS Bank v1.0 - Physical Function* PROMIS Short Form v1.0 Physical Function 4a* PROMIS Short Form v1.0-physical Function 6a* PROMIS Short Form v1.0-physical Function 8a* PROMIS Short Form v1.0 Physical
PROMIS Bank v1.0 - Physical Function* PROMIS Short Form v1.0 Physical Function 4a* PROMIS Short Form v1.0-physical Function 6a* PROMIS Short Form v1.0-physical Function 8a* PROMIS Short Form v1.0 Physical
CONNECTED HEALTH SUPPORTING HOME STAY IN DEMENTIA (CHESS)
 CONNECTED HEALTH SUPPORTING HOME STAY IN DEMENTIA (CHESS) Dr Estefanía Guisado Fernández Early Stage Researcher at Insight Data Analytics Centre (UCD) Physical Medicine and Rehabilitation Medical Doctor
CONNECTED HEALTH SUPPORTING HOME STAY IN DEMENTIA (CHESS) Dr Estefanía Guisado Fernández Early Stage Researcher at Insight Data Analytics Centre (UCD) Physical Medicine and Rehabilitation Medical Doctor
Clinical Policy: Digital Breast Tomosynthesis Reference Number: CP.MP.90
 Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Psychosocial Indicators via Hand Tremor
 Carnegie Mellon University From the SelectedWorks of Ted Selker Summer September 5, 2011 Psychosocial Indicators via Hand Tremor Ted Selker, PhD, Carnegie Mellon University Patricia Collins, PhD, Carnegie
Carnegie Mellon University From the SelectedWorks of Ted Selker Summer September 5, 2011 Psychosocial Indicators via Hand Tremor Ted Selker, PhD, Carnegie Mellon University Patricia Collins, PhD, Carnegie
Schizophrenia: New Concepts for Therapeutic Discovery
 Schizophrenia: New Concepts for Therapeutic Discovery William T. Carpenter, M.D. Professor of Psychiatry and Pharmacology University of Maryland School of Medicine Department of Psychiatry Maryland Psychiatric
Schizophrenia: New Concepts for Therapeutic Discovery William T. Carpenter, M.D. Professor of Psychiatry and Pharmacology University of Maryland School of Medicine Department of Psychiatry Maryland Psychiatric
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Process
 Quality ID #177: Rheumatoid Arthritis (RA): Periodic Assessment of Disease Activity National Quality Strategy Domain: Effective Clinical Care Measure Meaningful Measure Area: Management of Chronic Conditions
Quality ID #177: Rheumatoid Arthritis (RA): Periodic Assessment of Disease Activity National Quality Strategy Domain: Effective Clinical Care Measure Meaningful Measure Area: Management of Chronic Conditions
Clinical Policy: Multiple Sleep Latency Testing
 Clinical Policy: Reference Number: CP.MP.24 Last Review Date: 04/18 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.24 Last Review Date: 04/18 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
ACNP Annual Meeting Submission Site User Guide
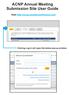 Visit http://acnp.societyconference.com Clicking Log In will open the below pop-up window. Select Start a New Abstract These are your current submissions. You can access the submission by clicking the
Visit http://acnp.societyconference.com Clicking Log In will open the below pop-up window. Select Start a New Abstract These are your current submissions. You can access the submission by clicking the
Clinical Policy: DNA Analysis of Stool to Screen for Colorectal Cancer
 Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
Open Range Software, LLC
 Comprehensive Tracking System CTS User Manual CTS IH/Medical Surveillance Interface Open Range Software, LLC Revised: 09/04/2013 CONTENTS Introduction...3 WHAT IS THE CTS IH/MEDICAL SURVEILLANCE INTERFACE?...3
Comprehensive Tracking System CTS User Manual CTS IH/Medical Surveillance Interface Open Range Software, LLC Revised: 09/04/2013 CONTENTS Introduction...3 WHAT IS THE CTS IH/MEDICAL SURVEILLANCE INTERFACE?...3
