The New England Journal of Medicine MATERNAL LEVELS OF PLASMA HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 RNA AND THE RISK OF PERINATAL TRANSMISSION
|
|
|
- Emory Bryant
- 5 years ago
- Views:
Transcription
1 MATERNAL LEVELS OF PLASMA HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 RNA AND THE RISK OF PERINATAL TRANSMISSION PATRICIA M. GARCIA, M.D., M.P.H., LESLIE A. KALISH, D.SC., JANE PITT, M.D., HOWARD MINKOFF, M.D., THOMAS C. QUINN, M.D., SANDRA K. BURCHETT, M.D., JANET KORNEGAY, PH.D., BROOKS JACKSON, M.D., JOHN MOYE, M.D., CELINE HANSON, M.D., CARMEN ZORRILLA, M.D., AND JUDY F. LEW, M.D., FOR THE WOMEN AND INFANTS TRANSMISSION STUDY GROUP* ABSTRACT Background The importance of plasma levels of human immunodeficiency virus type 1 (HIV-1) RNA in pregnant women in relation to the other factors known to influence the risk of transmission of infection to their infants is incompletely defined. We studied the relation of maternal plasma HIV-1 RNA levels to the risk of perinatal transmission and the timing of transmission. Methods We measured plasma HIV-1 RNA serially in 552 women with HIV-1 infection who had singleton pregnancies. The status of infection in their infants was assessed by culture of blood and further classified as early (if a culture of blood obtained within the first two days of life was positive) or late (if a culture of blood obtained in the first seven days of life was negative but subsequent cultures were positive). The rates of transmission at various levels of maternal plasma HIV-1 RNA were analyzed by tests for trend, with adjustment for covariates by stratification and logistic regression. Results Increasing geometric mean levels of plasma HIV-1 RNA were associated with increasing rates of transmission: the rate was 0 percent among women with less than 1000 copies per milliliter (0 of 57), 16.6 percent among women with 1000 to 10,000 copies per milliliter (32 of 193), 21.3 percent among women with 10,001 to 50,000 copies per milliliter (39 of 183), 30.9 percent among women with 50,001 to 100,000 copies per milliliter (17 of 54), and 40.6 percent among women with more than 100,000 copies per milliliter (26 of 64, P). The treatment status of one woman was unknown. The highest rate of transmission was among women whose plasma HIV-1 RNA levels exceeded 100,000 copies per milliliter and who had not received zidovudine (19 of 30 women, 63.3 percent). Neither higher HIV-1 RNA levels early in pregnancy nor higher levels late in pregnancy were associated with the timing of infection in the infants. Conclusions In pregnant women with HIV-1 infection, the level of plasma HIV-1 RNA predicts the risk but not the timing of transmission of HIV-1 to their infants. (N Engl J Med 1999;341: ) 1999, Massachusetts Medical Society. LITTLE was known of the biology of perinatal transmission of human immunodeficiency virus type 1 (HIV-1) when zidovudine treatment was proposed and subsequently proved effective for reducing the risk of neonatal infection. 1 Clarification of the relative contribution of maternal plasma HIV-1 RNA levels and other factors known to influence the risk of transmission might provide insight into the timing of transmission of the virus to the infant. We undertook this study to determine the relation between maternal plasma HIV-1 RNA levels and the risk and timing of perinatal transmission of HIV-1 infection, using data from one of the largest prospectively assembled and well-characterized cohorts of infected women and their infants in the United States. We sought to test the hypothesis that higher maternal plasma HIV-1 RNA levels independently increase the risk of transmission. We also assessed whether the risk of transmission was correlated more closely with the presence of higher levels earlier in pregnancy rather than later. Study Population METHODS The Women and Infants Study is an ongoing multicenter, prospective study of the perinatal transmission of HIV-1 and the natural history of HIV-1 infection in pregnant women and their infants. 2 Pregnant women with HIV-1 infection and their infants have been studied at eight centers in four states and Puerto Rico since The women are enrolled at any time during pregnancy, and their infants are enrolled at birth or shortly thereafter. Our study was approved by the institutional review committees at each center, and informed consent was obtained from each woman. The study subjects consisted of women who gave birth to sin- From the Department of Obstetrics and Gynecology, Northwestern University, Chicago (P.M.G.); New England Research Institutes, Watertown, Mass. (L.A.K.); the Department of Pediatrics, Columbia College of Physicians and Surgeons, New York (J.P.); the Department of Obstetrics and Gynecology, State University of New York Health Science Center, Brooklyn (H.M.); Johns Hopkins University, Baltimore (T.C.Q., B.J.); the National Institute of Allergy and Infectious Diseases, Bethesda, Md. (T.C.Q., J.F.L.); the Department of Pediatrics, Harvard Medical School, Boston (S.K.B.); Roche Molecular Systems, Alameda, Calif. (J.K.); the National Institute of Child Health and Human Development, Bethesda, Md. (J.M.); the Section of Allergy and Immunology, Baylor College of Medicine, Houston (C.H.); and the Department of Obstetrics and Gynecology, University of Puerto Rico, San Juan (C.Z.). Address reprint requests to Dr. Garcia at Rm. 410, Prentice Women s Hospital, 333 E. Superior St., Chicago, IL 60611, or at p-garcia@nwu.edu. *Other participants in the study are listed in the Appendix. 394 August 5, 1999
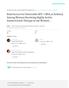
2 MATERNAL LEVELS OF PLASMA HIV-1 RNA AND THE RISK OF PERINATAL TRANSMISSION gleton infants between January 14, 1990, and October 15, 1995, and whose infants HIV-1 infection status was determined before December 1, In addition, plasma HIV-1 RNA levels had to have been measured at least once in the women during pregnancy or at delivery. Among 800 mother infant pairs potentially eligible for the analysis, 248 pairs were excluded because of the unavailability of data on HIV-1 RNA levels. The women in the excluded pairs had been enrolled more recently than the women in the included pairs, and the rate of transmission was therefore lower in this group (14 of 248 infants; 6 percent), but the excluded pairs were otherwise similar to the included pairs with respect to maternal and obstetrical covariates. Thus, a total of 552 pairs were studied. Study Protocol and Definitions The women were evaluated three times during pregnancy and once at delivery. At each visit, detailed medical and behavioral questionnaires were administered, a physical examination was performed, and venous blood was obtained. Obstetrical data were abstracted from medical records. Antiviral-drug therapy was left to the discretion of the clinicians caring for the women and the women themselves. The prevalence of zidovudine therapy increased and the rates of perinatal transmission declined in the cohort over time. 3 Women were classified as treated if they received any zidovudine therapy during pregnancy or labor and delivery. We further classified the women according to whether they were in the active-treatment group of the AIDS Clinical Trials Group (ACTG) Protocol 076 or were treated with zidovudine before the March 1994 release of the results of that study. Women who received zidovudine after March 1994 or before March 1994 if they were enrolled in the treated group of ACTG Protocol 076 were classified separately from women who reported receiving zidovudine therapy for specific indications during pregnancy but who delivered their babies before March 1994 and who were not in the treated group of ACTG Protocol 076. Maternal use of illicit drugs during pregnancy such as heroin, opiates, and cocaine was based on self-report or toxicologic confirmation on testing of urine samples. 4 All mothers were counseled regarding the risk of transmission associated with breastfeeding, and only four infants (none of whom became infected with HIV-1) were breast-fed. The infants were examined and peripheral-blood mononuclear cells were obtained for culture during the first 6 days of life; at the ages of 1, 2, 4, 6, 9, 12, and 18 months; and every 6 months thereafter. After April 1994, blood was obtained within the first 48 hours after birth and on days 6 to 10 after birth. Infants with no positive cultures and at least two negative cultures at or after the age of one month (with one negative culture obtained when the child was at least six months of age) were classified as uninfected. Infants with two or more positive cultures were defined as infected (there were no infants with only one positive culture). Infection was further classified as early or late according to the timing of the positive culture. 5 If the result of a culture of blood obtained within the first 48 hours after birth was positive, the infant was considered to have acquired the infection early (presumably in utero). If cultures of blood obtained within the first seven days of life were negative, but later cultures were positive, the infant was considered to have acquired the infection late (presumably during labor and delivery). Laboratory Studies Heparin-treated or EDTA-treated blood samples were separated into plasma and peripheral-blood mononuclear cells within 24 hours after collection at each center. CD4 counts were determined in fresh whole blood by flow cytometry. The laboratories followed the cytometry protocols of the Division of AIDS of the National Institute of Allergy and Infectious Diseases and participated in the division s flow-cytometric quality-assurance program. 6 A qualitative approach was used for cocultures of peripheral-blood mononuclear cells until October 1991; thereafter, a quantitative approach was used, according to the ACTG consensus protocols, with standard modifications for samples from infants. 7 Plasma HIV-1 RNA was measured in five laboratories from repository samples (stored at 70 C) according to the recommendations of the Virology Quality Assurance Program of the Division of AIDS. 8 RNA was extracted from the plasma samples with the use of silica particles. 9 The number of HIV-1 RNA copies per milliliter of maternal plasma was measured by a quantitative RNA polymerase-chain-reaction assay (Amplicor HIV-1 Monitor Test, Roche Diagnostic Systems, Branchburg, N.J.) as previously described. 10 Samples below the limit of quantification of the assay (400 copies per milliliter) were assigned a value of 400 copies per milliliter for the purpose of statistical analysis. Statistical Analysis The primary quantitative viral measure used in analyses for each woman was the geometric mean of all available plasma HIV-1 RNA values for that woman. A priori categories of these levels were determined on the basis of clinically relevant cutoff points. For women with at least two RNA values, the within-person rate of change in plasma HIV-1 RNA levels (measured on a logarithmic [base 10] scale) per week was calculated by linear least-squares regression analysis. Cutoff points were chosen to represent a change of log copies per week (corresponding to a fivefold increase during a 40-week pregnancy). The remaining women were categorized as having stable plasma HIV-1 RNA levels. Women who were taking zidovudine during pregnancy whose plasma HIV-1 RNA level declined by at least log copies per week were classified as having a response to zidovudine. The primary association of interest was between maternal plasma HIV-1 RNA levels at various points during pregnancy and the overall risk of transmission. The Mantel extension test for trend was used to analyze transmission rates in ordered categories of variables, and a stratified version of the test for trend was used to control for categorical covariates. 11 The Wilcoxon or Kruskal Wallis test was used for univariate comparisons of the association between plasma HIV-1 RNA levels and covariates. 12 Logistic-regression analysis was used to adjust for covariates and determine which factors independently predicted the risk of perinatal transmission. 13 All statistical tests were two-sided. RESULTS Characteristics of the Women The 552 women included in the analysis were demographically similar to the entire cohort as well as to all childbearing women with HIV-1 infection in the United States. The mean age at delivery was 28 years, 81 percent of the women were black or Hispanic, and 18 percent had used injection drugs during pregnancy. The overall rate of perinatal transmission of HIV-1 infection in the cohort was 20.6 percent, which is similar to rates reported for other cohorts in North America. The rate of transmission declined during the period of data collection. Before March 1994 it was 24 percent; thereafter it was 9 percent. Univariate analysis was performed with selected measures of obstetrical outcome, disease status, and use of zidovudine therapy in order to identify covariates that were related to the risk of transmission of HIV-1 (Table 1). The resulting covariates were used in a logistic-regression model. Among the 552 pregnant women, plasma HIV-1 RNA was measured a total of 1396 times at four visits during pregnancy and delivery (Fig. 1). The number Volume 341 Number 6 395
3 TABLE 1. UNIVARIATE ANALYSIS OF THE RELATION OF VARIOUS MATERNAL AND OBSTETRICAL COVARIATES TO THE RISK OF PERINATAL TRANSMISSION OF HIV-1. CHARACTERISTIC WOMEN INFANTS INFECTED (%) P VALUE CHARACTERISTIC WOMEN INFANTS INFECTED (%) P VALUE Maternal age <30 yr»30 yr Race or ethnic group White Black Hispanic Other Site of enrollment Illinois Massachusetts New York Puerto Rico Texas Gestational age of infant»37 wk <37 wk Birth weight of infant»2500 g <2500 g Mode of delivery Cesarean section Elective cesarean section Vaginal Time from rupture of membranes to delivery «4 hr >4 hr (16.8) 55 (27.5) 21 (24.1) 44 (18.8) 43 (20.5) 6 (35.3) 31 (27.4) 24 (19.0) 34 (20.6) 23 (18.3) 2 (9.1) 84 (18.5) 30 (30.3) 73 (17.0) 34 (37.4) 21 (20.4) 2 (9.5) 87 (19.9) 43 (14.4) 58 (26.7) * Mean percentage of CD4 cells during pregnancy <29%»29% Positive HIV-1 cultures during pregnancy Not always Always Hepatitis C coinfection Yes No Zidovudine therapy during pregnancy Any According to ACTG Protocol 076 Treatment for specific indications None Cigarette use during pregnancy Yes No Hard-drug use during pregnancy Yes No Total (26.6) 37 (14.3) 33 (14.7) 81 (24.8) 38 (31.7) 52 (18.9) 35 (15.2) 11 (8.5) 24 (23.8) 79 (24.6) 70 (24.3) 44 (16.7) 59 (27.8) 55 (16.2) 114 (20.7) *The P value is for the comparison with all other modes of delivery. ACTG denotes AIDS Clinical Trials Group. The P value is for the comparison with the other three groups. of measurements was similar in the group of women with perinatal transmission of HIV-1 infection and those without perinatal transmission (data not shown). The individual maternal plasma HIV-1 RNA values ranged from less than 400 copies per milliliter to 3,101,258 copies per milliliter. Only 16 of 279 women (5.7 percent) with at least three values had persistently undetectable levels. The median plasma HIV-1 RNA values for each visit were similar and varied by less than 0.2 log, and the values were also relatively stable during pregnancy in individual women, as reflected by the median SD of 0.2 log within persons. For this reason, except where noted, the geometric mean of all available values from all visits was used in subsequent analyses. Maternal Plasma HIV-1 RNA Levels and the Risk of The median plasma HIV-1 RNA levels were higher among women who transmitted the infection to their infants than among those who did not transmit the infection (geometric mean, 29,235 vs. 10,049 copies per milliliter) (Fig. 1). The women who transmitted the infection had higher values throughout pregnancy, by 0.3 to 0.4 log at each visit. of HIV-1 infection occurred in a broad range of maternal plasma HIV-1 RNA levels (Fig. 1 and Table 2). In general, the risk of transmission increased with increasing maternal viral loads. However, no threshold could be defined above which the risk of transmission was 100 percent. Women who were not treated with zidovudine and whose plasma HIV-1 RNA levels exceeded 100,000 copies per milliliter had the highest risk of transmission (19 of 30 infants infected; 63.3 percent; P). Regardless of whether they received zidovudine therapy during pregnancy, none of the 57 women with RNA levels below 1000 copies per milliliter transmitted HIV-1 to their offspring (upper limit of the 95 percent confidence interval, 5.1 percent). To examine the pattern of change in plasma HIV-1 RNA levels, we stratified the women according to 396 August 5, 1999
4 MATERNAL LEVELS OF PLASMA HIV-1 RNA AND THE RISK OF PERINATAL TRANSMISSION whether they had increasing, decreasing, or stable levels throughout pregnancy. Although women whose plasma HIV-1 RNA levels increased throughout pregnancy had the highest rate of transmission overall, there was no statistically significant difference in the risk of transmission among these three groups, even after adjustment for the use of zidovudine therapy. Potential Cofactors The association between maternal plasma HIV-1 RNA levels and the risk of perinatal transmission of HIV-1 infection was assessed for confounding by other potential cofactors. The level was not associated with the time from the rupture of membranes to delivery, birth weight of the infant, illicit drug use or smoking during pregnancy, site of enrollment, or date of delivery. As expected, the level was strongly inversely associated with the percentage of CD4 cells and the absolute CD4 count (data not shown). After adjustment for these two covariates (Table 3), the association between maternal plasma HIV-1 RNA levels and the risk of perinatal transmission remained significant (P). Maternal Zidovudine Therapy Treatment with zidovudine was not associated with maternal plasma HIV-1 RNA levels (P=0.26), but the receipt of any type of zidovudine therapy in general was significantly associated with a lower rate of transmission (15.2 percent, as compared with 24.6 percent among women who did not receive zidovudine therapy; P). Among the women who were treated with zidovudine, plasma HIV-1 RNA levels were significantly higher in those who transmitted the infection than in those who did not (Table 4). However, when the women were stratified according to the type of zidovudine therapy they received, plasma HIV-1 RNA levels were not significantly different between the women who transmitted the infection and those who did not in the subgroup treated for specific indications rather than as part of ACTG Protocol 076. This group of women tended to have higher plasma HIV-1 RNA levels despite treatment with zidovudine, and the rate of perinatal transmission in this group was essentially the same as the rate in the untreated women and significantly higher than the rate in the women who were treated as part of or according to ACTG Protocol 076. Multivariate Analysis Logistic-regression analysis confirmed that maternal plasma HIV-1 RNA levels were strongly associated with the risk of transmission even after adjustment for other factors (Table 5). There was a progressively higher risk of transmission with higher plasma HIV- 1 RNA levels, even after the exclusion of the group of women with values below 1000 copies per milliliter, for which the transmission rate was zero. Maternal plasma HIV-1 RNA values of more than 50,000 copies per milliliter were associated with the greatest risk of transmission. The absence of zidovudine therapy during pregnancy, an interval of more than four hours between the rupture of membranes and delivery, and delivery of an infant who weighed less than 2500 g at birth were also independently associated with a higher risk of transmission in this analysis. Maternal Plasma HIV-1 RNA Levels and the Timing of In 88 of the 114 infected infants, the time at which the infection was diagnosed could be determined on the basis of HIV-1 culture results obtained during the first week of life. Sixty-three of the 88 infants (72 percent) were considered to have late, or peripartum, infection. Twenty-five infants had a positive HIV-1 culture within the first week of life. Of these 25, 16 had a culture during the first 48 hours, all of which were positive. These 16 infants were classified as having early presumably in utero infection. Despite the strong correlation between maternal plasma HIV-1 RNA levels and the risk of transmission overall, there was no significant difference in the median levels between the mothers of infants with early infection and the mothers of infants with late infection. There was no significant relation between the rates of early or late transmission and maternal plasma HIV-1 RNA levels measured throughout the pregnancy or in early or late pregnancy. Stratification of the groups according to the use of zidovudine therapy during pregnancy did not change the results. These results indicate that there was no relation between maternal plasma HIV-1 RNA levels at any time during pregnancy and the risk of transmission early or late in pregnancy. DISCUSSION Measurements of plasma HIV-1 RNA levels have considerable value as a marker of the response to antiretroviral therapy as well as for predicting the risk of disease progression and death. 14 Our findings demonstrate the importance of maternal plasma HIV-1 RNA measurements in predicting the risk of perinatal transmission of the infection. Higher plasma HIV-1 RNA values were associated with a significant risk of transmitting HIV-1 from mother to infant. A very strong correlation between maternal plasma HIV-1 RNA levels and the risk of perinatal transmission has been found in several small studies, 15,16 whereas larger studies have found little or no association Although the levels were not measured in all the women in our study at all four times, the number of measurements and the median plasma HIV-1 RNA levels at each visit were similar in the group of women with perinatal transmission of infection and the group without perinatal transmission. The re- Volume 341 Number 6 397
5 »1,000,000 Visit 1 (N=177) Visit 2 (N=385) Visit 3 (N=387) 500, ,000 Plasma HIV-1 RNA (copies/ml) 50,000 10,000 5,000 1, Median plasma HIV-1 RNA copies/ml (log copies) transmission No perinatal transmission All women P value 24,603 (4.39) 11,508 (4.06) 13,740 (4.14) ,227 (4.37) 9,462 (3.98) 12,246 (4.09) 17,021 (4.21) 9,204 (3.96) 9,772 (3.99) Figure 1. Maternal Plasma HIV-1 RNA Levels at Each Visit and Geometric Mean Values during Pregnancy, According to Status. The limit of detection of the assay was 400 HIV-1 RNA copies per milliliter. Visit 1 occurred during the first 18 weeks of gestation, visit 2 between 19 and 31 weeks of gestation, and visit 3 from 32 weeks of gestation to delivery. The geometric mean values include the values obtained throughout pregnancy and at delivery. Bars indicate the median plasma HIV-1 RNA levels. P values are for the comparison between women who transmitted the infection to their infants and those who did not transmit the infection. 398 August 5, 1999
6 MATERNAL LEVELS OF PLASMA HIV-1 RNA AND THE RISK OF PERINATAL TRANSMISSION»1,000,000 At Delivery (N=445) Geometric Mean (N=552) 500, ,000 Plasma HIV-1 RNA (copies/ml) 50,000 10,000 5,000 1, Median plasma HIV-1 RNA copies/ml (log copies) transmission No perinatal transmission All women P value 26,997 (4.43) 11,350 (4.06) 14,488 (4.16) 29,235 (4.47) 10,049 (4.0) 12,971 (4.11) Volume 341 Number 6 399
7 TABLE 2. RATES OF PERINATAL TRANSMISSION OF HIV-1 ACCORDING TO MATERNAL PLASMA HIV-1 RNA LEVELS AND THE USE OF ZIDOVUDINE THERAPY DURING PREGNANCY.* ZIDOVUDINE THERAPY MATERNAL PLASMA HIV-1 RNA COPIES/ml P VALUE < ,000 >10,000 50,000 >50, ,000 >100,000 no. of infants infected/total no. (%) Yes 0/22 10/83 (12.0) 13/75 (17.3) 5/16 (31.2) 7/34 (20.6) 0.02 No 0/35 22/110 (20.0) 26/108 (24.1) 12/38 (31.6) 19/30 (63.3) Total 0/57 32/193 (16.6) 39/183 (21.3) 17/54 (30.9) 26/64 (40.6) *Values are the geometric means of measurements obtained throughout pregnancy. For each woman, levels were measured up to three times during pregnancy and once at delivery. The treatment status of one woman was not known. The P values were calculated with use of the Mantel extension test for trend. TABLE 3. RATES OF PERINATAL TRANSMISSION OF HIV-1 ACCORDING TO MATERNAL PLASMA HIV-1 RNA LEVELS, PERCENTAGE OF CD4 CELLS, AND CD4 CELL COUNT. VARIABLE MATERNAL PLASMA HIV-1 RNA COPIES/ml* < ,000 >10,000 50,000 >50,000 no. of infants infected/total no. (%) Percentage of CD4 cells <14% 14 28%»29% CD4 cell count <200/mm /mm 3»500/mm 3 0/1 0/7 0/48 0/1 0/12 0/43 2/4 (50.0) 11/71 (15.5) 19/116 (16.4) 2/10 (20.0) 11/71 (15.5) 17/108 (15.7) 2/21 (9.5) 25/96 (26.0) 12/66 (18.2) 4/26 (15.4) 18/90 (20.0) 17/67 (25.4) 7/21 (33.3) 30/68 (44.1) 6/29 (20.7) 12/33 (36.4) 26/64 (40.6) 5/21 (23.8) *The median values for all visits are shown. P by tests for trend for rates of transmission and maternal plasma HIV-1 RNA levels, with adjustment for the percentage of CD4 cells and CD4 cell count. Data were missing for four women. Data were missing for six women. sults of our analyses were unchanged whether geometric mean values for all visits or values from each of the four visits were used. In addition, the risk of transmission was more closely related to the absolute maternal plasma HIV-1 RNA level than to a change in the level. Previous studies have suggested that there is an upper threshold of maternal viremia, above which transmission is unavoidable, and a lower threshold, below which transmission is rare. 15,20 Despite the high rate of transmission (63.3 percent) among women who had not received zidovudine and whose plasma HIV-1 RNA levels exceeded 100,000 copies per milliliter, we found no threshold level above which the rate of transmission was 100 percent. transmission did not occur among women with plasma HIV-1 RNA levels of less than 1000 copies per milliliter, but there are at least three reports of transmission occurring at low levels, including one in which 16 of 132 women (12 percent) who had HIV-1 RNA levels of less than 1000 copies per milliliter transmitted HIV-1 to their infants. 17,18,21 Treatment with zidovudine during pregnancy reduces the risk of perinatal transmission. 1,3,22,23 In our study, treatment with zidovudine during pregnancy was associated with a lower rate of transmission among women with plasma HIV-1 RNA levels of 1000 copies per milliliter or more, especially among those who were treated according to ACTG Protocol 076. There was no significant difference in trans- 400 August 5, 1999
8 MATERNAL LEVELS OF PLASMA HIV-1 RNA AND THE RISK OF PERINATAL TRANSMISSION TABLE 4. MATERNAL PLASMA HIV-1 RNA LEVELS THROUGHOUT PREGNANCY, ACCORDING TO TRANSMISSION STATUS AND THE TYPE OF ZIDOVUDINE THERAPY. ZIDOVUDINE THERAPY* PERINATAL TRANSMISSION NO PERINATAL TRANSMISSION P VALUE WOMEN MEDIAN HIV-1 RNA LEVEL TRANSMISSION RATE WOMEN MEDIAN HIV-1 RNA LEVEL copies/ml % copies/ml None 79 28, ,691 Any According to ACTG Protocol 076 Treatment for specific indications ,227 31,352 26, *Data were missing for one woman. ACTG denotes AIDS Clinical Trials Group. Values are the geometric means of all measurements obtained throughout pregnancy and at delivery. P values were calculated by the two-sample Wilcoxon test for the comparison of women who transmitted HIV-1 infection with those who did not ,625 7,587 18, Total , , mission rates between the women with a response to zidovudine therapy and those with no response. Taken together, these data support the conclusion of Sperling et al. 17 that treatment with zidovudine during pregnancy reduces the risk of perinatal transmission, but not solely as a result of a reduction in maternal plasma HIV-1 RNA levels. The same may not be true for combinations of potent antiretroviral drugs that are capable of reducing maternal plasma HIV-1 RNA to undetectable levels. In our study, perinatal transmission occurred predominantly in the peripartum period. Although maternal plasma HIV-1 RNA levels were closely associated with the risk of perinatal transmission of HIV-1 infection overall, there was no discernible relation between the levels and the timing of neonatal infection. The relation between maternal plasma HIV-1 RNA levels and the risk of perinatal transmission may relate more to the mechanism of transmission (i.e., transplacental transfer of virus or exposure to virus in the genital tract) than to the timing of transmission. How, then, should measurements of viral load be used in the care of HIV-infected pregnant women? One answer is to use antiretroviral therapy to improve the woman s health, as advised in treatment guidelines developed by the Public Health Service. 24 Although there is uncertainty about the degree to which viral replication must be suppressed to prevent disease progression, preserve immune function, and inhibit the development of resistant strains of HIV-1, there is general agreement that decreasing plasma HIV-1 RNA levels to below the limits of detection of currently available assays is the most appropriate treatment goal. Achieving that goal should TABLE 5. RESULTS OF MULTIPLE LOGISTIC-REGRESSION ANALYSIS OF THE RISK OF PERINATAL TRANSMISSION OF HIV-1.* COVARIATE Maternal plasma HIV-1 RNA level ,000 copies/ml >10,000 50,000 copies/ml >50,000 copies/ml Zidovudine therapy None Any Treatment for specific indications According to ACTG Protocol 076 Time from rupture of membranes to delivery >4 hr ODDS RATIO (95% CI) ( ) 3.7 ( ) P VALUE *The analysis excluded women with plasma HIV-1 RNA levels of less than 1000 copies per milliliter, since there were no cases of transmission in this group. CI denotes confidence interval, and ACTG AIDS Clinical Trials Group. Plasma HIV-1 RNA levels (geometric mean values for all measurements made throughout pregnancy and at delivery) were modeled as a categorical variable (data were available for 437 women). Results were very similar when plasma HIV-1 RNA levels were modeled as a single ordered categorical variable (with equally spaced categories) that included women with values of less than 1000 copies per milliliter (data were available for 491 women). The P value is for the test for trend. also help prevent perinatal transmission, given the absence of transmission among women with low plasma HIV-1 RNA levels in our study. Validating this treatment goal is important, since a treatment-induced reduction in the viral load may not be equivalent to a spontaneously low level with respect to the risk of perinatal transmission ( ) ( ) 2.0 ( ) Low birth weight of infant (<2500 g) 2.8 ( ) Volume 341 Number 6 401
9 Because the causes of perinatal transmission are multifactorial, attention must be paid to other potentially modifiable risk factors. Of all the independent factors related to the risk of transmission in this cohort of women, maternal plasma HIV-1 RNA levels and the use of antiretroviral-drug therapy are perhaps the most easily modifiable risk factors. Yet, women with low or undetectable plasma HIV-1 RNA levels should not be falsely reassured but, instead, should be offered zidovudine therapy because of its demonstrated efficacy in reducing the risk of transmission regardless of the maternal HIV-1 RNA levels. Whether there is an additional benefit of elective cesarean delivery in decreasing the already low risk of perinatal transmission among pregnant women with low levels of plasma HIV-1 RNA has yet to be determined Supported by grants from the National Institute of Allergy and Infectious Diseases (U01 AI 34840, U01 AI 34841, U01 AI 34842, U01 AI 34856, U01 AI 34858, and N01 AI 35161), the National Institute of Child Health and Human Development (HD and RO-1-HD ), and the National Institute on Drug Abuse. Presented in part at the meeting of the Society of Obstetricians, Miami, February 5, We are indebted to the patients and dedicated study personnel (data-entry persons, nurses, coordinators, laboratory technicians, and clinicians) at all sites for their efforts; to Ms. Tracy Antonelli for assistance with statistical analysis; to Dr. Mike Socol for his support and review of the manuscript; and to Roche Molecular Systems for their generous contribution of Amplicor HIV-1 Monitor Test kits. APPENDIX The study s principal investigators, coordinators, and program officers were as follows: University of Puerto Rico, San Juan C. Diaz, E. Pacheco- Acosta; Boston Worcester site, Boston R. Tuomala, E. Cooper, D. Mesthene; Columbia Presbyterian Hospital, New York J. Pitt, A. Higgins; State University of New York, Brooklyn S. Landesman, H. Mendez, G. Moroso; University of Illinois at Chicago, Chicago K. Rich, D. Turpin; Baylor College of Medicine, Houston W. Shearer, N. Cooper; National Institute of Allergy and Infectious Diseases, Bethesda, Md. M. Fowler; National Institute of Child Health and Human Development, Bethesda, Md. R. Nugent; National Institute on Drug Abuse, Rockville, Md. V. Smeriglio; New England Research Institute, Watertown, Mass. S. McKinlay, L. Kalish, S. Ellis. REFERENCES 1. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994;331: Sheon AR, Fox HE, Rich KC, et al. The Women and Infants Study (WITS) of maternal-infant HIV transmission: study design, methods, and baseline data. J Womens Health 1996;5: Cooper ER, Nugent RP, Diaz C, et al. After AIDS clinical trial 076: the changing pattern of zidovudine use during pregnancy, and the subsequent reduction in the vertical transmission of human immunodeficiency virus in a cohort of infected women and their infants. J Infect Dis 1996; 174: Rodriguez EM, Mofenson LM, Chang B-H, et al. Association of maternal drug use during pregnancy with maternal HIV culture positivity and perinatal HIV transmission. AIDS 1996;10: Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med 1992;327: Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping; a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry 1993;14: Hollinger FB, ed. ACTG virology manual for HIV laboratories: version 2.1. Bethesda, Md.: National Institutes of Allergy and Infectious Diseases, September Hollinger FB, Bremer JW, Myers LE, Gold JWM, McQuay L, NIH/NIAID/DAIDS/ACTG Virology Laboratories. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. J Clin Microbiol 1992;30: Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990;28: Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N Engl J Med 1997;336: Mantel N. Chi-square tests with one degree of freedom: extensions of the Mantel-Haenszel procedure. J Am Stat Assoc 1963;58: Rosner B. Fundamentals of biostatistics. 3rd ed. Boston: PWS-Kent Publishing, Hosmer DW Jr, Lemeshow S. Applied logistic regression. New York: John Wiley, Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126: Dickover RE, Garratty EM, Herman SA, et al. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission: effect of maternal zidovudine treatment on viral load. JAMA 1996;275: Weiser B, Nachman S, Tropper P, et al. Quantitation of human immunodeficiency virus type 1 during pregnancy: relationship of viral titer to mother-to-child transmission and stability of viral load. Proc Natl Acad Sci U S A 1994;91: Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med 1996;335: Cao Y, Krogstad P, Korber BT, et al. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med 1997;3: Jackson JB, Hom D, Piwowar E, et al. Maternal HIV-1 RNA serum levels at delivery and vertical transmission in Uganda. Pediatr AIDS HIV Infect 1996;7: Fang G, Burger H, Grimson R, et al. Maternal plasma human immunodeficiency virus type 1 RNA level: a determinant and projected threshold for mother-to-child transmission. Proc Natl Acad Sci U S A 1995;92: Mayaux MJ, Dussaix E, Isopet J, et al. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. J Infect Dis 1997;175: Administration of zidovudine during late pregnancy and delivery to prevent perinatal HIV transmission Thailand, MMWR Morb Mortal Wkly Rep 1998;47: Fiscus SA, Adimora AA, Schoenbach VJ, et al. HIV infection and the effect of zidovudine therapy on transmission in rural and urban counties. JAMA 1996;275: Public Health Service Task Force recommendations for the use of antiretroviral drugs in pregnant women infected with HIV-1 for maternal health and for reducing perinatal HIV-1 transmission in the United States. MMWR Morb Mortal Wkly Rep 1998;47(RR-2):1-30. [Errata, MMWR Morb Mortal Wkly Rep 1998;47:287, 315.] 25. Mandelbrot L, Le Chenadec J, Berrebi A, et al. HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Cohort. JAMA 1998;280: The European Mode of Delivery Collaboration. Elective caesareansection versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet 1999;353: The International HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1: a metaanalysis of 15 prospective cohort studies. N Engl J Med 1999;340: August 5, 1999
Decline of CD3-positive T-cell counts by 6 months of age is associated with rapid disease progression in HIV-1 infected infants
 Decline of CD3-positive T-cell counts by 6 months of age is associated with rapid disease progression in HIV-1 infected infants Javier Chinen, Baylor College of Medicine Kirk Easley, Emory University Herman
Decline of CD3-positive T-cell counts by 6 months of age is associated with rapid disease progression in HIV-1 infected infants Javier Chinen, Baylor College of Medicine Kirk Easley, Emory University Herman
TB/HIV/STD Epidemiology and Surveillance Branch. First Annual Report, Dated 12/31/2009
 TB/HIV/STD Epidemiology and Surveillance Branch First Annual Report, Dated 12/31/29 This Enhanced Perinatal Surveillance Report is the first annual report generated by the Texas Department of State Health
TB/HIV/STD Epidemiology and Surveillance Branch First Annual Report, Dated 12/31/29 This Enhanced Perinatal Surveillance Report is the first annual report generated by the Texas Department of State Health
Human immunodeficiency virus (HIV) can be HJOG. HIV infection in pregnancy: Analysis of twenty cases. Research. Abstract
 HJOG An Obstetrics and Gynecology International Journal Research HIV infection in pregnancy: Analysis of twenty cases Kasioni Spiridoula 1, Pappas Stefanos 2, Vlachadis Nikolaos 3, Valsamidi Irene 1, Stournaras
HJOG An Obstetrics and Gynecology International Journal Research HIV infection in pregnancy: Analysis of twenty cases Kasioni Spiridoula 1, Pappas Stefanos 2, Vlachadis Nikolaos 3, Valsamidi Irene 1, Stournaras
CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA. Date
 CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA 350 300 250 Number 200 150 100 50 0 1/01/1997 1/01/1998 1/01/1999 1/01/2000 31/12/2000 31/12/2001 31/12/2002 Date July 2004 Reported number of perinatally exposed
CUMULATIVE PERINATAL HIV EXPOSURE, AUSTRALIA 350 300 250 Number 200 150 100 50 0 1/01/1997 1/01/1998 1/01/1999 1/01/2000 31/12/2000 31/12/2001 31/12/2002 Date July 2004 Reported number of perinatally exposed
QUANTITATIVE HIV RNA (VIRAL LOAD)
 CLINICAL GUIDELINES For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS QUANTITATIVE HIV RNA (VIRAL LOAD) Policy Number: PDS - 008 Effective Date: October
CLINICAL GUIDELINES For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS QUANTITATIVE HIV RNA (VIRAL LOAD) Policy Number: PDS - 008 Effective Date: October
Prevention of Mother to Child Transmission of HIV: Our Experience in South India
 pg 62-66 Original Article Prevention of Mother to Child Transmission of HIV: Our Experience in South India Karthekeyani Vijaya 1, Alexander Glory 2, Solomon Eileen 3, Rao Sarita 4, Rao P.S.S.Sunder 5 1
pg 62-66 Original Article Prevention of Mother to Child Transmission of HIV: Our Experience in South India Karthekeyani Vijaya 1, Alexander Glory 2, Solomon Eileen 3, Rao Sarita 4, Rao P.S.S.Sunder 5 1
Review Article Perinatal HIV infection Raj AY
 Perinatal HIV infection Raj AY The ORION Medical Journal 2003 Sep;16:117-118 Human immunodeficiency virus (HIV) infection causes a broad spectrum of disease and a varied clinical course. Acquired Immunodeficiency
Perinatal HIV infection Raj AY The ORION Medical Journal 2003 Sep;16:117-118 Human immunodeficiency virus (HIV) infection causes a broad spectrum of disease and a varied clinical course. Acquired Immunodeficiency
ANTIRETROVIRAL THERAPY DURING PREGNANCY ANTIRETROVIRAL THERAPY DURING PREGNANCY AND THE RISK OF AN ADVERSE OUTCOME
 ANTIRETROVIRAL THERAPY DURING PREGNANCY ANTIRETROVIRAL THERAPY DURING PREGNANCY AND THE RISK OF AN ADVERSE OUTCOME RUTH E. TUOMALA, M.D., DAVID E. SHAPIRO, PH.D., LYNNE M. MOFENSON, M.D., YVONNE BRYSON,
ANTIRETROVIRAL THERAPY DURING PREGNANCY ANTIRETROVIRAL THERAPY DURING PREGNANCY AND THE RISK OF AN ADVERSE OUTCOME RUTH E. TUOMALA, M.D., DAVID E. SHAPIRO, PH.D., LYNNE M. MOFENSON, M.D., YVONNE BRYSON,
A Descriptive Study of Outcomes of Interventions to Prevent Mother to Child Transmission of HIV in Lusaka, Zambia
 ORIGINAL PAPER A Descriptive Study of Outcomes of Interventions to Prevent Mother to Child Transmission of HIV in Lusaka, Zambia Chibesa Shichitamba W, National Malaria Control Centre, Lusaka-Zambia ABSTRACT
ORIGINAL PAPER A Descriptive Study of Outcomes of Interventions to Prevent Mother to Child Transmission of HIV in Lusaka, Zambia Chibesa Shichitamba W, National Malaria Control Centre, Lusaka-Zambia ABSTRACT
Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006
 DOI 10.1007/s10995-015-1853-4 Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006 L. Ghazaryan 1 L. Smith 2 M. Parker 3 C. Flanigan 4 W. Pulver 2 T. Sullivan 5 A.
DOI 10.1007/s10995-015-1853-4 Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006 L. Ghazaryan 1 L. Smith 2 M. Parker 3 C. Flanigan 4 W. Pulver 2 T. Sullivan 5 A.
Virus Levels in Untreated African Infants Infected with Human Immunodeficiency Virus Type 1
 1838 Virus Levels in Untreated African Infants Infected with Human Immunodeficiency Virus Type 1 Robert J. Biggar, 1 Michelle Janes, 4 Richard Pilon, 4 Paolo Miotti, 2 Taha E. T. Taha, 3 Robin Broadhead,
1838 Virus Levels in Untreated African Infants Infected with Human Immunodeficiency Virus Type 1 Robert J. Biggar, 1 Michelle Janes, 4 Richard Pilon, 4 Paolo Miotti, 2 Taha E. T. Taha, 3 Robin Broadhead,
Prevention of Perinatal HIV Transmission
 Prevention of Perinatal HIV Transmission Emily Adhikari, MD Division of Maternal-Fetal Medicine Obstetrics and Gynecology University of Texas Southwestern Medical Center February 20, 2018 None Understand
Prevention of Perinatal HIV Transmission Emily Adhikari, MD Division of Maternal-Fetal Medicine Obstetrics and Gynecology University of Texas Southwestern Medical Center February 20, 2018 None Understand
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States
Study population The patient population comprised HIV-positive pregnant women whose HIV status was known.
 Prevention of mother-to-child transmission of HIV-1 infection: alternative strategies and their cost-effectiveness Ratcliffe J, Ades A E, Gibb D, Sculpher M J, Briggs A H Record Status This is a critical
Prevention of mother-to-child transmission of HIV-1 infection: alternative strategies and their cost-effectiveness Ratcliffe J, Ades A E, Gibb D, Sculpher M J, Briggs A H Record Status This is a critical
Overview of role of immunologic markers in HIV diagnosis
 Overview of role of immunologic markers in HIV diagnosis Savita Pahwa, M.D. Departments of Microbiology & Immunology and Pediatrics University of Miami, Miller School of Medicine, Miami, Florida Background:
Overview of role of immunologic markers in HIV diagnosis Savita Pahwa, M.D. Departments of Microbiology & Immunology and Pediatrics University of Miami, Miller School of Medicine, Miami, Florida Background:
Risk Factors for Detectable HIV-1 RNA at Delivery Among Women Receiving Highly Active Antiretroviral Therapy in the Women...
 See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/40908390 Risk Factors for Detectable HIV-1 RNA at Delivery Among Women Receiving Highly Active
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/40908390 Risk Factors for Detectable HIV-1 RNA at Delivery Among Women Receiving Highly Active
QUANTITATIVE HIV RNA (VIRAL LOAD)
 CLINICAL GUIDELINES For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS QUANTITATIVE HIV RNA (VIRAL LOAD) Policy Number: PDS - 008 Effective Date: January
CLINICAL GUIDELINES For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS QUANTITATIVE HIV RNA (VIRAL LOAD) Policy Number: PDS - 008 Effective Date: January
Obstetrics and HIV An Update. Jennifer Van Horn MD University of Utah
 Obstetrics and HIV An Update Jennifer Van Horn MD University of Utah Obstetrics and HIV Perinatal transmission Testing Antiretroviral therapy Antepartum management Intrapartum management Postpartum management
Obstetrics and HIV An Update Jennifer Van Horn MD University of Utah Obstetrics and HIV Perinatal transmission Testing Antiretroviral therapy Antepartum management Intrapartum management Postpartum management
Neurological and developmental signs are often
 Early Cognitive and Motor Development Among Infants Born to Women Infected With Human Immunodeficiency Virus Cynthia Chase, PhD*; Janice Ware, PhD ; Joan Hittelman, PhD ; Ileana Blasini, MD MPH ; Renee
Early Cognitive and Motor Development Among Infants Born to Women Infected With Human Immunodeficiency Virus Cynthia Chase, PhD*; Janice Ware, PhD ; Joan Hittelman, PhD ; Ileana Blasini, MD MPH ; Renee
Labor & Delivery Management for Women Living with HIV. Pooja Mittal, DO Lisa Rahangdale, MD
 Labor & Delivery Management for Women Living with HIV Pooja Mittal, DO Lisa Rahangdale, MD Statistics for Perinatally Acquired HIV Timing of Perinatal HIV Transmission Most transmission occurs close to
Labor & Delivery Management for Women Living with HIV Pooja Mittal, DO Lisa Rahangdale, MD Statistics for Perinatally Acquired HIV Timing of Perinatal HIV Transmission Most transmission occurs close to
Early Immunological Predictors of Neurodevelopmental Outcomes in HIV-Infected Children
 HIV/AIDS MAJOR ARTICLE Early Immunological Predictors of Neurodevelopmental Outcomes in HIV-Infected Children Jutarat Mekmullica, 1,9 Pim Brouwers, 2,3 Manhattan Charurat, 4 Mary Paul, 2 William Shearer,
HIV/AIDS MAJOR ARTICLE Early Immunological Predictors of Neurodevelopmental Outcomes in HIV-Infected Children Jutarat Mekmullica, 1,9 Pim Brouwers, 2,3 Manhattan Charurat, 4 Mary Paul, 2 William Shearer,
California Perinatal Quality Care Collaborative 2013 Standards of Care for the Prevention of Perinatal HIV Transmission
 2013 Revised Edition Authors: David K Hong, MD, Nivedita Srinivas, MD Original Authors: Mary Campbell Bliss, RN, MS, CNS, William Gilbert, MD, Jan Lanouette, MD, Courtney Nisbet, RN, MS, David Wirtschafter,
2013 Revised Edition Authors: David K Hong, MD, Nivedita Srinivas, MD Original Authors: Mary Campbell Bliss, RN, MS, CNS, William Gilbert, MD, Jan Lanouette, MD, Courtney Nisbet, RN, MS, David Wirtschafter,
Pediatric HIV Cure Research
 Pediatric HIV Cure Research HIV Cure Research Training Curriculum Pediatric HIV Cure Research Presented by: Priyanka Uprety,MSPH, PhD Laboratory of Deborah Persaud, MD Johns Hopkins University July 2016
Pediatric HIV Cure Research HIV Cure Research Training Curriculum Pediatric HIV Cure Research Presented by: Priyanka Uprety,MSPH, PhD Laboratory of Deborah Persaud, MD Johns Hopkins University July 2016
Journal Club 3/4/2011
 Journal Club 3/4/2011 Maternal HIV Infection and Antibody Responses Against Vaccine-Preventable Diseases in Uninfected Infants JAMA. 2011 Feb 9;305(6):576-84. Jones et al Dept of Pediatrics, Imperial College,
Journal Club 3/4/2011 Maternal HIV Infection and Antibody Responses Against Vaccine-Preventable Diseases in Uninfected Infants JAMA. 2011 Feb 9;305(6):576-84. Jones et al Dept of Pediatrics, Imperial College,
Mother-to-Child transmission of hiv and neonatal hiv ManageMent
 Mother-to-Child transmission of hiv and neonatal hiv ManageMent Perinatal transmission of the human immunodeficiency virus (HIV), or mother-to-child transmission (MTCT), occurs when a mother living with
Mother-to-Child transmission of hiv and neonatal hiv ManageMent Perinatal transmission of the human immunodeficiency virus (HIV), or mother-to-child transmission (MTCT), occurs when a mother living with
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 999, by the Massachusetts Medical Society VOLUME 34 A UGUST 5, 999 NUMBER 6 RISK FACTORS FOR PERINATAL OF HUMAN IMMUNODEFICIENCY VIRUS TYPE IN WOMEN TREATED
The New England Journal of Medicine Copyright, 999, by the Massachusetts Medical Society VOLUME 34 A UGUST 5, 999 NUMBER 6 RISK FACTORS FOR PERINATAL OF HUMAN IMMUNODEFICIENCY VIRUS TYPE IN WOMEN TREATED
Use of a Multidisciplinary Care Model for Pregnant Women Living with HIV & Their Infants Sarah McBeth, MD MPH
 Use of a Multidisciplinary Care Model for Pregnant Women Living with HIV & Their Infants Sarah McBeth, MD MPH University of Pittsburgh Medical Center Disclosures Presenter has no financial interests to
Use of a Multidisciplinary Care Model for Pregnant Women Living with HIV & Their Infants Sarah McBeth, MD MPH University of Pittsburgh Medical Center Disclosures Presenter has no financial interests to
What s New. In The 2016 Perinatal HIV Treatment Guidelines? Provided by CDC s Elimination of Perinatal HIV Transmission Stakeholders Group
 What s New In The 2016 Perinatal HIV Treatment Guidelines? Provided by CDC s Elimination of Perinatal HIV Transmission Stakeholders Group Guidelines for our Online Meeting Room You will be listening to
What s New In The 2016 Perinatal HIV Treatment Guidelines? Provided by CDC s Elimination of Perinatal HIV Transmission Stakeholders Group Guidelines for our Online Meeting Room You will be listening to
Women are the fastest-growing group of persons with
 Clinical Guidelines Prenatal Screening for HIV: A Review of the Evidence for the U.S. Preventive Services Task Force Roger Chou, MD; Ariel K. Smits, MD, MPH; Laurie Hoyt Huffman, MS; Rongwei Fu, PhD; and
Clinical Guidelines Prenatal Screening for HIV: A Review of the Evidence for the U.S. Preventive Services Task Force Roger Chou, MD; Ariel K. Smits, MD, MPH; Laurie Hoyt Huffman, MS; Rongwei Fu, PhD; and
Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update
 Frontier AIDS Education and Training Center Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update Brian R. Wood, MD Assistant Professor of Medicine, University of Washington Medical Director,
Frontier AIDS Education and Training Center Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update Brian R. Wood, MD Assistant Professor of Medicine, University of Washington Medical Director,
Research Article The Use of Protease Inhibitors in Pregnancy: Maternal and Fetal Considerations
 Infectious Diseases in Obstetrics and Gynecology Volume 2015, Article ID 563727, 5 pages http://dx.doi.org/10.1155/2015/563727 Research Article The Use of Protease Inhibitors in Pregnancy: Maternal and
Infectious Diseases in Obstetrics and Gynecology Volume 2015, Article ID 563727, 5 pages http://dx.doi.org/10.1155/2015/563727 Research Article The Use of Protease Inhibitors in Pregnancy: Maternal and
Infertility Treatment and HIV
 Infertility Treatment and HIV Infertility Treatment by IVF Or Intra-cytoplasmic Sperm Injections (ICSC) In Chronic HIV-1 Sero- discordant Couples (Poster 670) Retrospective study of outcome of IVF or ICSC
Infertility Treatment and HIV Infertility Treatment by IVF Or Intra-cytoplasmic Sperm Injections (ICSC) In Chronic HIV-1 Sero- discordant Couples (Poster 670) Retrospective study of outcome of IVF or ICSC
Downloaded from by guest on 31 October 2018
 328 Vitamin A Deficiency and Other Nutritional Indices During Pregnancy in Human Immunodeficiency Virus Infection: Prevalence, Clinical Correlates, and Outcome David N. Burns, Gordon FitzGerald, Richard
328 Vitamin A Deficiency and Other Nutritional Indices During Pregnancy in Human Immunodeficiency Virus Infection: Prevalence, Clinical Correlates, and Outcome David N. Burns, Gordon FitzGerald, Richard
HIV in Pregnancy. In Practice. Cheryl Roth Pauline F. Hrenchir Christine J. Pacheco
 In Practice Photo istock Collection / thinkstockphotos.com IImagine this scenario: You are working on a Saturday afternoon when a woman comes into the obstetrics triage unit. She is pregnant for the fourth
In Practice Photo istock Collection / thinkstockphotos.com IImagine this scenario: You are working on a Saturday afternoon when a woman comes into the obstetrics triage unit. She is pregnant for the fourth
AMCHP Annual Meeting March 9, 2010 Shannon Weber, MSW
 Making the Call to Improve Pregnancy Outcomes: A Focus on Tobacco Cessation Quitlines and HIV Hotlines: The National Perinatal HIV Hotline AMCHP Annual Meeting March 9, 2010 Shannon Weber, MSW sweber@nccc.ucsf.edu
Making the Call to Improve Pregnancy Outcomes: A Focus on Tobacco Cessation Quitlines and HIV Hotlines: The National Perinatal HIV Hotline AMCHP Annual Meeting March 9, 2010 Shannon Weber, MSW sweber@nccc.ucsf.edu
Repeat Pregnancies and HIV Care Engagement among Postpartum HIV-infected Women in Atlanta, Georgia,
 Repeat Pregnancies and HIV Care Engagement among Postpartum HIV-infected Women in Atlanta, Georgia, 2011-2015 Anandi N. Sheth, Christina M. Meade, Martina Badell, Susan A. Davis, Stephanie Hackett, Joy
Repeat Pregnancies and HIV Care Engagement among Postpartum HIV-infected Women in Atlanta, Georgia, 2011-2015 Anandi N. Sheth, Christina M. Meade, Martina Badell, Susan A. Davis, Stephanie Hackett, Joy
ABSTRACT Background It is unclear whether there are differences
 INITIAL PLASMA HIV-1 RNA LEVELS AND PROGRESSION TO AIDS IN WOMEN AND MEN TIMOTHY R. STERLING, M.D., DAVID VLAHOV, PH.D., JACQUIE ASTEMBORSKI, M.H.S., DONALD R. HOOVER, PH.D., M.P.H., JOSEPH B. MARGOLICK,
INITIAL PLASMA HIV-1 RNA LEVELS AND PROGRESSION TO AIDS IN WOMEN AND MEN TIMOTHY R. STERLING, M.D., DAVID VLAHOV, PH.D., JACQUIE ASTEMBORSKI, M.H.S., DONALD R. HOOVER, PH.D., M.P.H., JOSEPH B. MARGOLICK,
Pregnancy and HIV Reviewing the Vertical Transmission Risks 2015 OCN Education Day V Logan Kennedy RN, MN Research Associate and Clinical Nursing
 Pregnancy and HIV Reviewing the Vertical Transmission Risks 2015 OCN Education Day V Logan Kennedy RN, MN Research Associate and Clinical Nursing Specialist Women s College Research Institute, Women s
Pregnancy and HIV Reviewing the Vertical Transmission Risks 2015 OCN Education Day V Logan Kennedy RN, MN Research Associate and Clinical Nursing Specialist Women s College Research Institute, Women s
NIH Public Access Author Manuscript J Acquir Immune Defic Syndr. Author manuscript; available in PMC 2010 June 1.
 NIH Public Access Author Manuscript Published in final edited form as: J Acquir Immune Defic Syndr. 2009 June 1; 51(2): 209 215. Pediatric HIV-1 in Kenya: Pattern and Correlates of Viral Load and Association
NIH Public Access Author Manuscript Published in final edited form as: J Acquir Immune Defic Syndr. 2009 June 1; 51(2): 209 215. Pediatric HIV-1 in Kenya: Pattern and Correlates of Viral Load and Association
Recommended Clinical Guidelines on the Prevention of Perinatal HIV Transmission
 Recommended Clinical Guidelines on the Prevention of Perinatal HIV Transmission Scientific Committee of the Advisory Council on AIDS, Hong Kong April 2001 Preamble Recommended clinical guidelines on the
Recommended Clinical Guidelines on the Prevention of Perinatal HIV Transmission Scientific Committee of the Advisory Council on AIDS, Hong Kong April 2001 Preamble Recommended clinical guidelines on the
Deborah Kacanek, Konstantia Angelidou, Paige L. Williams, Miriam Chernoff, Kenneth Gadow, Sharon Nachman, The IMPAACT P1055 Study Team
 Psychiatric disorder symptoms are associated with longitudinal changes in antiretroviral (ARV) non-adherence in perinatally HIV-infected youth in the US: Results from IMPAACT P1055 Deborah Kacanek, Konstantia
Psychiatric disorder symptoms are associated with longitudinal changes in antiretroviral (ARV) non-adherence in perinatally HIV-infected youth in the US: Results from IMPAACT P1055 Deborah Kacanek, Konstantia
Cost-effectiveness of cesarean section delivery to prevent mother-to-child transmission of HIV-1 Halpern M T, Read J S, Ganoczy D A, Harris D R
 Cost-effectiveness of cesarean section delivery to prevent mother-to-child transmission of HIV-1 Halpern M T, Read J S, Ganoczy D A, Harris D R Record Status This is a critical abstract of an economic
Cost-effectiveness of cesarean section delivery to prevent mother-to-child transmission of HIV-1 Halpern M T, Read J S, Ganoczy D A, Harris D R Record Status This is a critical abstract of an economic
What will happen to these children?
 The AIDS Epidemic: An Issue for Maternal and Child Health and Nutrition The AIDS Epidemic: An Issue for Maternal and Child Health and Nutrition Kathleen Rasmussen, PhD Professor, Division of Nutritional
The AIDS Epidemic: An Issue for Maternal and Child Health and Nutrition The AIDS Epidemic: An Issue for Maternal and Child Health and Nutrition Kathleen Rasmussen, PhD Professor, Division of Nutritional
RNA PCR, Proviral DNA and Emerging Trends in Infant HIV Diagnosis
 RNA PCR, Proviral DNA and Emerging Trends in Infant HIV Diagnosis 1 B R E N D A N M C M U L L A N I N F E C T I O U S D I S E A S E S, S Y D N E Y C H I L D R E N S H O S P I T A L S C H O O L O F W O
RNA PCR, Proviral DNA and Emerging Trends in Infant HIV Diagnosis 1 B R E N D A N M C M U L L A N I N F E C T I O U S D I S E A S E S, S Y D N E Y C H I L D R E N S H O S P I T A L S C H O O L O F W O
PERINATAL TRANSMISSION OF
 ORIGINAL CONTRIBUTION Trends in Perinatal Transmission of HIV/AIDS in the United States Mary Lou Lindegren, MD Robert H. Byers, Jr, PhD Pauline Thomas, MD Susan F. Davis, MD Blake Caldwell, MD, MPH Martha
ORIGINAL CONTRIBUTION Trends in Perinatal Transmission of HIV/AIDS in the United States Mary Lou Lindegren, MD Robert H. Byers, Jr, PhD Pauline Thomas, MD Susan F. Davis, MD Blake Caldwell, MD, MPH Martha
DYNAMICS OF MATERNAL LYMPHOCYTE SUBSETS FROM 3 RD TRIMESTER TO POSTPARTUM AND THEIR IMPACT ON MOTHER-TO- CHILD HIV-1 TRANSMISSION
 DYNAMICS OF MATERNAL LYMPHOCYTE SUBSETS FROM 3 RD TRIMESTER TO POSTPARTUM AND THEIR IMPACT ON MOTHER-TO- CHILD HIV-1 TRANSMISSION By Chimwemwe Chitsulo DEDICATION To my parents for their untiring love
DYNAMICS OF MATERNAL LYMPHOCYTE SUBSETS FROM 3 RD TRIMESTER TO POSTPARTUM AND THEIR IMPACT ON MOTHER-TO- CHILD HIV-1 TRANSMISSION By Chimwemwe Chitsulo DEDICATION To my parents for their untiring love
Dr Graham P Taylor Reader in Communicable Diseases
 HIV in Pregnancy Joint RCOG/BHIVA Multidisciplinary Conference Dr Graham Taylor Imperial College London Friday 20 January 2012, Royal College of Obstetricians and Gynaecologist, London Prevention of post-partum
HIV in Pregnancy Joint RCOG/BHIVA Multidisciplinary Conference Dr Graham Taylor Imperial College London Friday 20 January 2012, Royal College of Obstetricians and Gynaecologist, London Prevention of post-partum
All HIV+ Women on Antiretroviral Therapy Should Breastfeed in Both Low and High Resource Settings VOTE NO!!
 All HIV+ Women on Antiretroviral Therapy Should Breastfeed in Both Low and High Resource Settings VOTE NO!! Lynne Mofenson MD Elizabeth Glaser Pediatric AIDS Foundation My Esteemed Opponent Will Likely
All HIV+ Women on Antiretroviral Therapy Should Breastfeed in Both Low and High Resource Settings VOTE NO!! Lynne Mofenson MD Elizabeth Glaser Pediatric AIDS Foundation My Esteemed Opponent Will Likely
PREGNANCY OUTCOMES AMONG HIV-INFECTED WOMEN IN UGANDA AND ZIMBABWE
 PREGNANCY OUTCOMES AMONG HIV-INFECTED WOMEN IN UGANDA AND ZIMBABWE Kathryn Lancaster, MPH 3rd International Workshop on HIV & Women January 15, 2013 HIV among women of reproductive age Women of reproductive
PREGNANCY OUTCOMES AMONG HIV-INFECTED WOMEN IN UGANDA AND ZIMBABWE Kathryn Lancaster, MPH 3rd International Workshop on HIV & Women January 15, 2013 HIV among women of reproductive age Women of reproductive
Children infected with the human immunodeficiency. Cardiac Dysfunction and Mortality in HIV-Infected Children
 Cardiac Dysfunction and Mortality in HIV-Infected Children The Prospective P 2 C 2 HIV Multicenter Study Steven E. Lipshultz, MD; Kirk A. Easley, MS; E. John Orav, PhD; Samuel Kaplan, MD; Thomas J. Starc,
Cardiac Dysfunction and Mortality in HIV-Infected Children The Prospective P 2 C 2 HIV Multicenter Study Steven E. Lipshultz, MD; Kirk A. Easley, MS; E. John Orav, PhD; Samuel Kaplan, MD; Thomas J. Starc,
Effect of HAART on growth parameters and absolute CD4 count among HIV-infected children in a rural community of central Nigeria
 Niger J Paed 2014; 41 (1): 1-6 Ebonyi AO Oguche S Dablets E Sumi B Yakubu E Sagay AS ORIGINAL Effect of HAART on growth parameters and absolute CD4 count among HIV-infected children in a rural community
Niger J Paed 2014; 41 (1): 1-6 Ebonyi AO Oguche S Dablets E Sumi B Yakubu E Sagay AS ORIGINAL Effect of HAART on growth parameters and absolute CD4 count among HIV-infected children in a rural community
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Rough K, Seage GR III, Williams PL, et al. Birth outcomes for
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Rough K, Seage GR III, Williams PL, et al. Birth outcomes for
Obstetric Complications in HIV-Infected Women. Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School
 Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
Outline. Aim with PMTCT. How are children transmitted. Prevention of mother-to-child transmission of HIV. How does HIV transmit to children?
 Prevention of mother-to-child transmission of HIV Outline AimofPMTCT How HIV is transmitted to children Epidemiology of HIV in children How to reduce HIV transmission to children Guidelines Lars T. Fadnes
Prevention of mother-to-child transmission of HIV Outline AimofPMTCT How HIV is transmitted to children Epidemiology of HIV in children How to reduce HIV transmission to children Guidelines Lars T. Fadnes
HIV infection in pregnancy
 HIV infection in pregnancy Peter Brocklehurst National Perinatal Epidemiology Unit, University of Oxford What is the size of the problem? in the population as a whole? in women? in pregnant women? in children?
HIV infection in pregnancy Peter Brocklehurst National Perinatal Epidemiology Unit, University of Oxford What is the size of the problem? in the population as a whole? in women? in pregnant women? in children?
Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an open-label trial
 Infect Dis Obstet Gynecol 2001;9:75 80 Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an open-label trial L. Laurie Scott 1, Lisa M.
Infect Dis Obstet Gynecol 2001;9:75 80 Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an open-label trial L. Laurie Scott 1, Lisa M.
Fertility Desires/Management of Serodiscordant HIV + Couples
 Fertility Desires/Management of Serodiscordant HIV + Couples William R. Short, MD, MPH Assistant Professor of Medicine Division Of Infectious Diseases Jefferson Medical College of Thomas Jefferson University
Fertility Desires/Management of Serodiscordant HIV + Couples William R. Short, MD, MPH Assistant Professor of Medicine Division Of Infectious Diseases Jefferson Medical College of Thomas Jefferson University
CCC ARV Dosing Recommendations for HIV-exposed infants Updated
 USERS NOTE: Please note this document does not provide guidance on overall decisionmaking regarding what medication(s) to use for HIV-exposed infants. This document is meant to facilitate ARV dosing for
USERS NOTE: Please note this document does not provide guidance on overall decisionmaking regarding what medication(s) to use for HIV-exposed infants. This document is meant to facilitate ARV dosing for
ARTICLE. Trends From an HIV Seroprevalence Study Among Childbearing Women in New York State From 1988 Through 2000
 Trends From an HIV Seroprevalence Study Among Childbearing Women in New York State From 1988 Through 2000 A Valuable Epidemiologic Tool ARTICLE Wendy P. Pulver, MS; Donna Glebatis, MS; Nancy Wade, MD,
Trends From an HIV Seroprevalence Study Among Childbearing Women in New York State From 1988 Through 2000 A Valuable Epidemiologic Tool ARTICLE Wendy P. Pulver, MS; Donna Glebatis, MS; Nancy Wade, MD,
Management of the HIV-Exposed Infant
 Management of the HIV-Exposed Infant Katherine Knapp, MD Medical Director, Perinatal Program Department of Infectious Diseases St. Jude Children s Research Hospital Disclosures No conflicts of interest
Management of the HIV-Exposed Infant Katherine Knapp, MD Medical Director, Perinatal Program Department of Infectious Diseases St. Jude Children s Research Hospital Disclosures No conflicts of interest
Available online International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(2):
 Available online www.ijpras.com International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(2):407-411 Research Article ISSN : 2277-3657 CODEN(USA) : IJPRPM Investigating the Impacts of
Available online www.ijpras.com International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(2):407-411 Research Article ISSN : 2277-3657 CODEN(USA) : IJPRPM Investigating the Impacts of
WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES?
 WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES? Today s Webinar will be starting soon For the audio portion of this meeting: Dial 1-855-702-5382 Enter participant code 596-825-4701# Guidelines for online
WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES? Today s Webinar will be starting soon For the audio portion of this meeting: Dial 1-855-702-5382 Enter participant code 596-825-4701# Guidelines for online
Dr Laura Byrne. UCL Institute of Child Health, London
 Dr Laura Byrne UCL Institute of Child Health, London RCOG, London Elimination of vertical transmission in the UK: what is left to do? Results of the NSHPC audit of perinatal HIV since 2006 Laura Byrne,
Dr Laura Byrne UCL Institute of Child Health, London RCOG, London Elimination of vertical transmission in the UK: what is left to do? Results of the NSHPC audit of perinatal HIV since 2006 Laura Byrne,
What Women Need to Know: The HIV Treatment Guidelines for Pregnant Women
 : The HIV Treatment Guidelines for Pregnant Women : The HIV Treatment Guidelines for Pregnant Women What Women Need to Know: Prepared by Elaine Gross, RN, MS, CNS-C National Pediatric & Family HIV Resource
: The HIV Treatment Guidelines for Pregnant Women : The HIV Treatment Guidelines for Pregnant Women What Women Need to Know: Prepared by Elaine Gross, RN, MS, CNS-C National Pediatric & Family HIV Resource
HIV, Women, & Pregnancy
 HIV, Women, & Pregnancy Facts, Myths, and Management Nina K. Sublette, PhD, ACRN, AACRN, FNP University of Tennessee Department of Obstetrics and Gynecology St. Jude Children s Research Hospital Department
HIV, Women, & Pregnancy Facts, Myths, and Management Nina K. Sublette, PhD, ACRN, AACRN, FNP University of Tennessee Department of Obstetrics and Gynecology St. Jude Children s Research Hospital Department
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1996, by the Massachusetts Medical Society VOLUME 335 N OVEMBER 28, 1996 NUMBER 22 MATERNAL VIRAL LOAD, ZIDOVUDINE TREATMENT, AND THE RISK OF TRANSMISSION
The New England Journal of Medicine Copyright, 1996, by the Massachusetts Medical Society VOLUME 335 N OVEMBER 28, 1996 NUMBER 22 MATERNAL VIRAL LOAD, ZIDOVUDINE TREATMENT, AND THE RISK OF TRANSMISSION
Date of study period: April 12 May 7, 2010 and August September, 2010
 Classification of antiretroviral therapy failure using immunologic and clinical versus virologic monitoring in HIV-infected children and adolescents in Cambodia Date of study period: April 12 May 7, 2010
Classification of antiretroviral therapy failure using immunologic and clinical versus virologic monitoring in HIV-infected children and adolescents in Cambodia Date of study period: April 12 May 7, 2010
Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study
 DOI: 10.1111/1471-0528.13442 www.bjog.org General obstetrics Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study H Peters, a L Byrne,
DOI: 10.1111/1471-0528.13442 www.bjog.org General obstetrics Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study H Peters, a L Byrne,
0.14 ( 0.053%) UNAIDS 10% (94) ( ) (73-94/6 ) 8,920
 0.14 UNAIDS 0.053% 2 250 60 10% 94 73 20 73-94/6 8,920 12 43 Public Health Service Task Force Recommendations 5-10% for Use of Antiretroviral Drugs in 10-20% Pregnant HIV-1-Infected Women for Maternal
0.14 UNAIDS 0.053% 2 250 60 10% 94 73 20 73-94/6 8,920 12 43 Public Health Service Task Force Recommendations 5-10% for Use of Antiretroviral Drugs in 10-20% Pregnant HIV-1-Infected Women for Maternal
The Situation and the Progress of PMTC in Africa
 ,**1 The Japanese Society for AIDS Research The Journal of AIDS Research : The Situation and the Progress of PMTC in Africa Naomi WAKASUGI Graduate School of Political Science, Wasada University +,**0
,**1 The Japanese Society for AIDS Research The Journal of AIDS Research : The Situation and the Progress of PMTC in Africa Naomi WAKASUGI Graduate School of Political Science, Wasada University +,**0
GLOBAL AIDS MONITORING REPORT
 KINGDOM OF SAUDI ARABIA MINISTRY OF HEALTH GLOBAL AIDS MONITORING REPORT COUNTRY PROGRESS REPORT 2017 KINGDOM OF SAUDI ARABIA Submission date: March 29, 2018 1 Overview The Global AIDS Monitoring 2017
KINGDOM OF SAUDI ARABIA MINISTRY OF HEALTH GLOBAL AIDS MONITORING REPORT COUNTRY PROGRESS REPORT 2017 KINGDOM OF SAUDI ARABIA Submission date: March 29, 2018 1 Overview The Global AIDS Monitoring 2017
ROLE OF ANTIRETROVIRAL THERAPY IN HIV POSITIVE PREGNANT WOMEN
 Page1 Research Article Biological Sciences ROLE OF ANTIRETROVIRAL THERAPY IN HIV POSITIVE PREGNANT WOMEN P.S.Rashmi 1, Hegde Kiran 2, Patil Rajashri 3, Vijayanath.V 4* 1 Department of Obstetrics & Gyeanecology,
Page1 Research Article Biological Sciences ROLE OF ANTIRETROVIRAL THERAPY IN HIV POSITIVE PREGNANT WOMEN P.S.Rashmi 1, Hegde Kiran 2, Patil Rajashri 3, Vijayanath.V 4* 1 Department of Obstetrics & Gyeanecology,
The Cumulative Incidence of HIV Infection in HIVexposed Infants with a Birth Weight of 1500g Receiving Breast Milk and Daily Nevirapine
 The Cumulative Incidence of HIV Infection in HIVexposed Infants with a Birth Weight of 1500g Receiving Breast Milk and Daily Nevirapine A retrospective, descriptive study conducted at Kalafong hospital,
The Cumulative Incidence of HIV Infection in HIVexposed Infants with a Birth Weight of 1500g Receiving Breast Milk and Daily Nevirapine A retrospective, descriptive study conducted at Kalafong hospital,
Increased Vertical Transmission of Human Immunodeficiency Virus from Hepatitis C Virus Coinfected Mothers
 414 Increased Vertical Transmission of Human Immunodeficiency Virus from Hepatitis C Virus Coinfected Mothers Ronald C. Hershow, Katherine A. Riester, Judy Lew, Thomas C. Quinn, Lynne M. Mofenson, Katherine
414 Increased Vertical Transmission of Human Immunodeficiency Virus from Hepatitis C Virus Coinfected Mothers Ronald C. Hershow, Katherine A. Riester, Judy Lew, Thomas C. Quinn, Lynne M. Mofenson, Katherine
Mother-to-Child HIV Transmission: National and International Progress and Challenges
 Mother-to-Child HIV Transmission: National and International Progress and Challenges Elaine J. Abrams, md Associate Professor of Clinical Pediatrics and Clinical Epidemiology Columbia University College
Mother-to-Child HIV Transmission: National and International Progress and Challenges Elaine J. Abrams, md Associate Professor of Clinical Pediatrics and Clinical Epidemiology Columbia University College
Appendix 1: summary of the modified GRADE system (grades 1A 2D)
 Appendix 1: summary of the modified GRADE system (grades 1A 2D) 1A 1B 1C 1D 2A 2B 2C 2D Strong recommendation High-quality evidence Benefits clearly outweigh risk and burdens, or vice versa Consistent
Appendix 1: summary of the modified GRADE system (grades 1A 2D) 1A 1B 1C 1D 2A 2B 2C 2D Strong recommendation High-quality evidence Benefits clearly outweigh risk and burdens, or vice versa Consistent
PSI Health Impact Estimation Model: Use of Nevirapine for Prevention of Mother-to-Child Transmission of HIV
 PSI Health Impact Estimation Model: Use of Nevirapine for Prevention of Mother-to-Child Transmission of HIV Hongmei Yang Research & Metrics, Population Services International December 2010 This document
PSI Health Impact Estimation Model: Use of Nevirapine for Prevention of Mother-to-Child Transmission of HIV Hongmei Yang Research & Metrics, Population Services International December 2010 This document
Objectives. Outline. Section 1: Interaction between HIV and pregnancy. Effects of HIV on Pregnancy. Section 2: Mother-to-Child-Transmission (MTCT)
 Objectives Prevention of Mother-to-Child Transmission (PMTCT) Teen Club Community Partners Training Programme By the end of the session participants will be able to: 1. Identify factors affecting the transmission
Objectives Prevention of Mother-to-Child Transmission (PMTCT) Teen Club Community Partners Training Programme By the end of the session participants will be able to: 1. Identify factors affecting the transmission
EDMA HIV-AIDS TEAM Fact Sheet November 2007
 EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
All HIV-exposed Infants Should Receive Triple Drug Antiretroviral Prophylaxis. Against the motion
 All HIV-exposed Infants Should Receive Triple Drug Antiretroviral Prophylaxis Against the motion Hermione Lyall Imperial Healthcare NHS Trust, London, UK Acknowledgements: Pat Tookey, Ali Judd, Claire
All HIV-exposed Infants Should Receive Triple Drug Antiretroviral Prophylaxis Against the motion Hermione Lyall Imperial Healthcare NHS Trust, London, UK Acknowledgements: Pat Tookey, Ali Judd, Claire
Management of Viral Infection during Pregnancy
 Vaccination Management of Viral Infection during Pregnancy JMAJ 45(2): 69 74, 2002 Takashi KAWANA Professor of Obstetrics and Gynecology, Teikyo University Mizonokuchi Hospital Abstract: Viral infection
Vaccination Management of Viral Infection during Pregnancy JMAJ 45(2): 69 74, 2002 Takashi KAWANA Professor of Obstetrics and Gynecology, Teikyo University Mizonokuchi Hospital Abstract: Viral infection
Sterilization in HIV infected women in Thailand.
 Sterilization in HIV infected women in Thailand. Camille Lallemant 1, Sophie Le Coeur 2, Nelly Briand 2, Marc Lallemant 3. 1. London School of Hygiene and Tropical Medicine 2. Institut National d'etudes
Sterilization in HIV infected women in Thailand. Camille Lallemant 1, Sophie Le Coeur 2, Nelly Briand 2, Marc Lallemant 3. 1. London School of Hygiene and Tropical Medicine 2. Institut National d'etudes
Original Article. Ezeanolue EE, Pharr JR 1, Hunt A, Patel D, Jackson D. Abstract. Introduction
 Original Article Why are Children Still Being Infected with HIV? Impact of an Integrated Public Health and Clinical Practice Intervention on Mother to Child HIV Transmission in Las Vegas, Nevada, 2007
Original Article Why are Children Still Being Infected with HIV? Impact of an Integrated Public Health and Clinical Practice Intervention on Mother to Child HIV Transmission in Las Vegas, Nevada, 2007
Group B Streptococcus
 Group B Streptococcus (Invasive Disease) Infants Younger than 90 Days Old DISEASE REPORTABLE WITHIN 24 HOURS OF DIAGNOSIS Per N.J.A.C. 8:57, healthcare providers and administrators shall report by mail
Group B Streptococcus (Invasive Disease) Infants Younger than 90 Days Old DISEASE REPORTABLE WITHIN 24 HOURS OF DIAGNOSIS Per N.J.A.C. 8:57, healthcare providers and administrators shall report by mail
VQA Control SOP Version 4.0 Roche Amplicor HIV-1 DNA Test, v August 2007
 1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
Breast-Milk Infectivity in Human Immunodeficiency Virus Type 1 Infected Mothers
 MAJOR ARTICLE Breast-Milk Infectivity in Human Immunodeficiency Virus Type 1 Infected Mothers Barbra A. Richardson, 1,3 Grace C. John-Stewart, 2 James P. Hughes, 1 Ruth Nduati, 5 Dorothy Mbori-Ngacha,
MAJOR ARTICLE Breast-Milk Infectivity in Human Immunodeficiency Virus Type 1 Infected Mothers Barbra A. Richardson, 1,3 Grace C. John-Stewart, 2 James P. Hughes, 1 Ruth Nduati, 5 Dorothy Mbori-Ngacha,
HIV Testing and Prophylaxis to Prevent Mother-to-Child Transmission in the United States
 POLICY STATEMENT HIV Testing and Prophylaxis to Prevent Mother-to-Child Transmission in the United States Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health
POLICY STATEMENT HIV Testing and Prophylaxis to Prevent Mother-to-Child Transmission in the United States Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health
HIV/AIDS CLINICAL CARE QUALITY MANAGEMENT CHART REVIEW CHARACTERISTICS OF PATIENTS FACTORS ASSOCIATED WITH IMPROVED IMMUNOLOGIC STATUS
 HIV/AIDS CLINICAL CARE QUALITY MANAGEMENT CHART REVIEW CHARACTERISTICS OF PATIENTS WITH LOW CD4 COUNTS IN 2008 AND FACTORS ASSOCIATED WITH IMPROVED IMMUNOLOGIC STATUS FROM 2004 THROUGH 2008 For the Boston
HIV/AIDS CLINICAL CARE QUALITY MANAGEMENT CHART REVIEW CHARACTERISTICS OF PATIENTS WITH LOW CD4 COUNTS IN 2008 AND FACTORS ASSOCIATED WITH IMPROVED IMMUNOLOGIC STATUS FROM 2004 THROUGH 2008 For the Boston
INTERPROFESSIONAL PROTOCOL - MUHC
 INTERPROFESSIONAL PROTOCOL - MUHC Medication included No Medication included THIS IS NOT A MEDICAL ORDER Title: PREVENTION OF MATERNAL TO INFANT HIV INFECTION Intrapartum, Peripartum, and Postpartum Antiretroviral
INTERPROFESSIONAL PROTOCOL - MUHC Medication included No Medication included THIS IS NOT A MEDICAL ORDER Title: PREVENTION OF MATERNAL TO INFANT HIV INFECTION Intrapartum, Peripartum, and Postpartum Antiretroviral
Understanding the Disparity: Predictors of Virologic Failure in Women using HAART vary by Race/Ethnicity
 Understanding the Disparity: Predictors of Virologic Failure in Women using HAART vary by Race/Ethnicity Allison M. McFall, David W. Dowdy, Carla E. Zelaya, Kerry Murphy, Tracey E. Wilson, Mary A. Young,
Understanding the Disparity: Predictors of Virologic Failure in Women using HAART vary by Race/Ethnicity Allison M. McFall, David W. Dowdy, Carla E. Zelaya, Kerry Murphy, Tracey E. Wilson, Mary A. Young,
Paediatrica Indonesiana. Outcomes of prevention of HIV mother-to-child transmission in Cipto Mangunkusumo Hospital
 Paediatrica Indonesiana VOLUME 52 September NUMBER 5 Original Article Outcomes of prevention of HIV mother-to-child transmission in Cipto Mangunkusumo Hospital Dina Muktiarti, Nia Kurniati, Arwin AP Akib,
Paediatrica Indonesiana VOLUME 52 September NUMBER 5 Original Article Outcomes of prevention of HIV mother-to-child transmission in Cipto Mangunkusumo Hospital Dina Muktiarti, Nia Kurniati, Arwin AP Akib,
Should we treat hepatitis B positive pregnant women to prevent mother to child transmission?
 Should we treat hepatitis B positive pregnant women to prevent mother to child transmission? Daniel Shouval Liver Unit Hadassah-Hebrew University Hospital Jerusalem, Israel VHPB Vienna June 1-2, 2017 Background
Should we treat hepatitis B positive pregnant women to prevent mother to child transmission? Daniel Shouval Liver Unit Hadassah-Hebrew University Hospital Jerusalem, Israel VHPB Vienna June 1-2, 2017 Background
Comparing Proportions between Two Independent Populations. John McGready Johns Hopkins University
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
Breast Feeding for Women with HIV?
 Breast Feeding for Women with HIV? CHIVA / BHIVA Hermione Lyall Imperial Healthcare NHS Trust 17.11.17 Acknowledgements: Nell Freeman-Romilly, Pat Tookey, Claire Townsend, Claire Thorne, Kate Francis,
Breast Feeding for Women with HIV? CHIVA / BHIVA Hermione Lyall Imperial Healthcare NHS Trust 17.11.17 Acknowledgements: Nell Freeman-Romilly, Pat Tookey, Claire Townsend, Claire Thorne, Kate Francis,
Structured Guidance for Postpartum Retention in HIV Care
 An Approach to Creating a Safety Net for Individual Patients and for Programmatic Improvements 1. Problem statement and background: Pregnant women living with HIV (WLH) are a vulnerable population that
An Approach to Creating a Safety Net for Individual Patients and for Programmatic Improvements 1. Problem statement and background: Pregnant women living with HIV (WLH) are a vulnerable population that
PERINATAL HEPATIDES AND HUMAN IMMUNODEFICIENCY VIRUS (HIV) Pamela Palasanthiran Staff Specialist, Paediatric Infectious Diseases
 PERINATAL HEPATIDES AND HUMAN IMMUNODEFICIENCY VIRUS (HIV) Pamela Palasanthiran Staff Specialist, Paediatric Infectious Diseases Viruses in July (ViJ), 2004 Overview Epidemiology Perinatal transmission
PERINATAL HEPATIDES AND HUMAN IMMUNODEFICIENCY VIRUS (HIV) Pamela Palasanthiran Staff Specialist, Paediatric Infectious Diseases Viruses in July (ViJ), 2004 Overview Epidemiology Perinatal transmission
TOWARDS ELIMINATION OF MOTHER TO CHILD TRANSMISSION OF HIV
 TOWARDS ELIMINATION OF MOTHER TO CHILD TRANSMISSION OF HIV Gladwel Muthoni KPA Conference 24 th April, 2018 OUTLINE Burden of HIV in PMTCT Mechanism and timing of Mother to Child Transmission (MTCT) Four
TOWARDS ELIMINATION OF MOTHER TO CHILD TRANSMISSION OF HIV Gladwel Muthoni KPA Conference 24 th April, 2018 OUTLINE Burden of HIV in PMTCT Mechanism and timing of Mother to Child Transmission (MTCT) Four
HIV/AIDS MEASURES GROUP OVERVIEW
 2014 PQRS OPTIONS F MEASURES GROUPS: HIV/AIDS MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN HIV/AIDS MEASURES GROUP: #159. HIV/AIDS: CD4+ Cell Count or CD4+ Percentage Performed #160. HIV/AIDS: Pneumocystis
2014 PQRS OPTIONS F MEASURES GROUPS: HIV/AIDS MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN HIV/AIDS MEASURES GROUP: #159. HIV/AIDS: CD4+ Cell Count or CD4+ Percentage Performed #160. HIV/AIDS: Pneumocystis
