Take-home messages from Monday 15th January 2018
|
|
|
- Randell Shaw
- 5 years ago
- Views:
Transcription
1 Take-home messages from Monday 15th January 2018 Marc Bourliere, MD 11th PHC Hôpital Saint Joseph Paris 15-16th January 2018 PHC
2 Disclosures Board member for : MSD, Janssen, Gilead, Boehringer Ingelheim, BMS, vartis, Roche, AbbVie, GSK, Vertex, Idenix, Intercept Speaker for : MSD, Janssen, Gilead, BMS, Abbvie, Intercept
3 Hepatitis C : first session Treatment of HCV: 100% cure? Difficult to treat patients Results in real life
4 Treatment of HCV: 100% cure? O N F N F O O H N SOF O LDV SOF + RBV weeks VEL SOF/LDV ± RBV 8 24 weeks Jan 2014 May 2014 SOF + SMV ± RBV weeks SMV Sept 2014 v 2014 Jan 2015 SOF + DCV ± RBV weeks OMV/PTV/R TV ± DSV ± RBV weeks PTV DCV O O N H O F SOF/VEL/V OX July 2016 July 2017 GRZ/EB V ± RBV weeks GLE/PIB 8 12 weeks F O O S VOX 12 weeks GRZ DSV N SOF/VEL ± RBV 12 weeks EBV OMV H N GLE PIBPIB Asselah, Marcellin & Schinazi. Liver Int 2018, in press. Tarik Asselah PHC 2018
5 Treatment of HCV: 100% cure? HCV elimination PREVENTION Harm reduction Infection control Blood safety TEST AND TREAT HCV screening (universal) Linkage to care : Treat with optimal DAAs AWARENESS Increase awareness Fights barriers & stigma Advocacy Tarik Asselah PHC 2018
6 Difficult to treat patients Broad treatment indications in patients with HCV and (de)compensated cirrhosis, pre- and post-transplant Decompensated cirrhosis: Sofosbuvir +NS5A-inhibitor Protease and non-nucleosidic polymerase inhibitors are contraindicated in patients with decompensated liver cirrhosis Safety of DAAs in these populations not yet fully defined thorough surveillance during therapy Consider drug-drug interactions, in particular immunosuppressants in transplanted patients Timing of DAA treatment under discussion in patients with chronic hepatitis C and HCC treated with curative intention Patients with HCV-associated liver disease should disappear in the transplant setting Stefan Zeuzem PHC 2018
7 Results in real Life Depression Depression Substance Substance misuse misuse Cognitive Cognitive impairment impairment Psychosis Psychosis Cardiovascular Cardiovascular disease disease Cirrhosis Cirrhosis 92% HCV patients had co-morbidities Lung Lung disease disease 40% had 5 or more co-morbidities Portal Portal Hypertension Hypertension Diabetes Diabetes DAAs combinations are highly effective and well tolerated in the real-world setting and globally, data from the real-life cohorts confirm those observed in clinical trials Dyslipidaemia Dyslipidaemia Transplant Transplant Renal Renal disease disease HIV HIV Co-infection Co-infection However, in some subgroups of patients it remains difficult to define optimal regimens (treatment duration, use of RBV, ) based on real-life cohorts In real-life, the majority of HCV patients has co-morbidities and multiple medications leading to potential DDIs Real-life cohorts are useful to highlight safety concerns ( bradycardia with amiodarone or HBV reactivation) Christophe Hezode PHC 2018
8 Clinical case: Management of patients with HCV related vasculitis Greater than 70% of mixed (II, III) cryoglogbulinemic patients are associated with HCV RNA HCV triggers an immune response but most cases are asymptomatic Clinical relevant disease include neuropathies, cutaneous ulcers, arthropathies, renal failure, and vasculitis DAAs are highly likely to achieve SVR and restoration of immune system Advanced stages of the MC-vasculitis require additional pharmacotherapy, e.g. Rituximab, to achieve remission of the vasculitis in spite of SVR SVR Eugene Schiff PHC 2018
9 Hepatitis C : second session Cost benefit of treatment in F1 patients Special population The next waves of HCV : the epidemic of intravenous drug use
10 Cost-benefit of treatment in F1 patients What happens if you don t treat early HCV? Age distribution of newly reported confirmed cases of hepatitis C virus infection --- Massachusetts, 2002 and 2009 Graham Foster PHC 2018
11 Cost-benefit of treatment in F1 patients Cost-benefits in mild disease Cost-benefits in mild disease Avoidance of liver complications PROVEN (and avoids long term follow up costs) Costs of therapy (drugs /admin) MINUS costs of follow up Avoidance of non-liver complications PROVEN Costs vary by country but in ALL countries drug price should no longer be rate limiting Prevention of transmission DATA SUPPORTED Graham Foster PHC 2018
12 Treatment of «special populations» CKD HCV/HIV co-infection DAA failures GT3 Hemoglobin Pangenotypic DAAs removed Patients with experienced Specialdiseases populations»cancer cirrhotic «patients failures Organ donor Addict patients Decompensated cirrhosis Safety of DAAs in those populations not yet fully defined thorough surveillance during therapy Stanislas Pol PHC 2018
13 The next wave of HCV : the epidemic of intravenous drug use ( USA) PREVALENCE OF HCV IS CHANGING TWO WAVES OF PATIENTS Column >60 First Wave Second Wave Number of persons Millions Thousands Description Baby Boomers Millennials Age (years) s Mode of infection Medical care Drug use Drug use Alcohol abuse Moderate Low HCV testing NA for decades Readily available Curative treatment NA for decades Readily available At risk for cirrhosis 33-50% t significant Mitchell Shiffman PHC 2018
14 Is Global elimination of HCV realistic? Disease Eradication vs Elimination vs Control Many HCV patients unknown to the health care system TREAT AND CURE 100% OF INFECTED DAAs providing SVR near, but not, 100% 100% SVR animal reservoir Large carrier pool as reservoir Therapy as cure and prevention HCV eradication achieved PREVENT ALL NEW ACUTE AND CHRONIC NFECTIONS Reinfection likely in high risk groups Without screenings and HCV vaccine HCV can be contained, maybe eliminated > 2030, but not eradicated Antonio Craxi PHC 2018
15 Is Global elimination of HCV realistic? The 'Anna Karenina principle' All happy families look alike; each unhappy family is unhappy in its own way Patients with chronic HCV present patterns consistent with Anna Karenina effect Treated or on treatment They all showed willingness to be treated, link to care, adherence to treatments Difficult-to-treat Heterogeneous group, with poor willingness to be treated and difficult link to care Antonio Craxi PHC 2018
16 NASH Worldwide epidemiology of NAFLD Prognosis of NASH Management of patients with NASH in real life Future therapies in NASH
17 Worldwide Epidemiology of NAFLD NAFLD is a chronic liver condition characterized by hepatic fat accumulation in the absence of ethanol abuse (<20g/day) & other identifiable causes NAFLD is associated to insulin resistance NAFLD is considered the hepatic manifestation of Metabolic Syndrome n-alcoholic fatty liver disease NAFLD : a multi-system disease Extrahepatic complications of NAFLD Type 2 Diabetes Hepatic cirrhosis, HCC Metabolic Central obesity, Insulin resistance, Type 2 diabetes Chronic Kidney Disease OSAS (Sleep Apnea) Cardiovascular Dyslipidemia, Hypertension Cardiovascular Disease (CVD) Cardiovascular Disease NAFLD PCOS Colorectal cancer Hypothiyoidism Osteoporosis Stefano Bellentani PHC 2018
18 Worldwide Epidemiology of NAFLD Global prevalence of NAFLD: 25% Global prevalence of overweight and obesity: 39% Obesity: 1 billion subjects overweight or obese around the world Diabetes: over 380-milion cases, but 550 in 2030 Younossi et al., Hepatology 2016 Stefano Bellentani PHC 2018
19 ARE WE READY TO CHANGE FROM A NEGATIVE DEFINITION (=NAFLD/ NASH) TO A POSITIVE ONE? Moving to a positive definition of NASH: MAFL (Metabolic Associated Fatty Liver) and MASH (Metabolic Associated SteatoHepatitis) thus revising the old definition and classification Stefano Bellentani PHC 2018
20 Prognosis of NASH NAFLD patients have increased overall mortality compared to matched controls without NAFLD. The most common cause of death in NAFLD patients is cardiovascular disease followed by cancers. Fibrosis is associated with overall and liver-related mortality. n-invasive markers of fibrosis predict mortality. Jean François Dufour PHC 2018
21 Management of patient with NASH in real life NAFLD /NASH remains under diagnosed in general and specialist practices Pattern of practice for the screening, diagnosis or therapeutic management of NAFLD /NASH are quite heterogeneous according to practitioners profile and country of origin, with poor adherence to guidelines This highlight the need for spreading NAFLD/NASH educational in the medical community and to promote the use of simple tools for patients screening. Lawrence Serfaty PHC 2018
22 Future Therapy in NASH Who needs intervention Those at risk for progression: multiple features of MetS (obesity + T2DM or HTN) Elevated ALT Steatohepatitis with some fibrosis Targets for NASH treatment PPARs FXR GLP-1 FABAC FGF21 Those who have progressed (bridging fibrosis or cirrhosis) Vitamin E ASK1 Metabolism (steatosis) identified by non-invasive methods further risk stratification with MELD or HVPG CCR2-CCR5 (Cencriviroc blocks this target) Cell stress apoptosis Anti-fibrotics inflammation Fibrogenic remodeling CIRRHOSIS Arun Sanyal PHC 2018
23 THERAPY IN NASH Rational approach to therapeutics for NASH Disease stage 4 Targets: Metabolic Inflammation Fibrosis Mainly anti-fibrotic Mainly metabolic + Inflammatory Lifestyle Metabolic targets 0 4 Disease activity (NAS) 8 Arun Sanyal PHC 2018
24 Diagnosis of NASH NIT Triage in large unselected populations Liver biopsy Assessment in selected populations or in clinical trials Best used as an integrated system to allow more efficient evaluation of patients with NAFLD Pierre Bedossa & Laurent Castera PHC 2018
25 Around the world table : Access to therapy Western countries Others countries
26 Access to HCV therapy in western countries Germany Spain Italy France UK National Plan Yes (soon) ( soon) Screening program Yes Soon Program to improve access to Trt (local) Yes (Prison, PWID) Treatment restrictions (2017) (2017) Nb HCV Patients 200, ,000 >300, , ,000 Nb patients treated 55,000 so far with DAAs 81, ,408 91,764 25,000 Nb patients treated 13,200 in 2016/17 29,732 45, (2016) 18,800 (2017) Untreated Pts 110, ,000 Thomas Berg & Maria Buti & Massimo Colombo & Victor de Ledinghen & Graham Foster PHC 2018
27 Access to HCV therapy in other countries Morocco Egypt Russia Brazil Poland Romania National Plan Yes (2017) Yes Yes Yes (2018) Screening program Yes (2017) Yes 3,300,000 (PWID..) Yes (2018) Yes Program to improve access to Trt Yes (soon) Yes (2018) Yes Treatment restrictions (2017) Yes Yes (2015 Yes Nb HCV Patients 450,000 6,000,000 Nb patients treated so far with DAAs 11,000 Nb patients treated in 2016/17 5,000 ) 5,000, ,500, , ,000 1,344,496 35, ,114 56,997 (DAAs) 25, ,000 36,627 (2016) 12,911 (2017) 12,000 12,000 20,000 Untreated Pts Mustapha Benazzouz & Gamal Esmat & Vasily Isakov & Raymundo Parana & Robert Flisiak Adriana Popescu PHC 2018
28 Message from Syria Treating chronic viral hepatitis is not an easy task during war However Nabil and his college did their best to treat liver patients adequately Sanctions penalize the population dan the patients and has no positive impact on the events or to bring peace back Nabil and his colleges ask us to put pressure on the leaders of our countries to lift the sanctions against Syrian population Nabil Antaki PHC 2018
29 Conclusions of the day The Hepatologist Menu The Hepatologist Menu HEREDITARY NAFLD/NASH HCV HCC AUTOIMMUNE HEREDITARY HBV NAFLD ALCOHOL HCC ALCOHOL AUTOIMMUNE HBV HCV NEW THERAPY!!! HCV Control : feasible HCV elimination : a goal HCV eradication : a dream that we need to fight hard in order to be achieve
30 Thank you for your attention A great thanks to all speakers who provide me their presentation
15 & 16 January International Conference on the Management of Liver Diseases. Organised by Pr Patrick Marcellin
 15 & 16 January 2018 PARIS - Palais des Congrès International Conference on the Management of Liver Diseases Organised by Pr Patrick Marcellin Organising Committee Blaise Kutala, Monelle Muntlak Hôpital
15 & 16 January 2018 PARIS - Palais des Congrès International Conference on the Management of Liver Diseases Organised by Pr Patrick Marcellin Organising Committee Blaise Kutala, Monelle Muntlak Hôpital
WORLDWIDE EPIDEMIOLOGY OF NASH
 WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
WORLDWIDE EPIDEMIOLOGY OF NASH Stefano Bellentani, M.D., Ph.D. Chief of Gastroenterology and Hepatology Service Clinica Santa Chiara Locarno Switzerland & Fondazione Italiana Fegato (FIF), Bassovizza (Trieste),
HCV therapy : Clinical case
 HCV therapy : Clinical case PHC 2018 Paris January 14th, 2018 Tarik Asselah (MD, PhD) Professor of Medicine Hepatology, Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France. Disclosures Professor Asselah
HCV therapy : Clinical case PHC 2018 Paris January 14th, 2018 Tarik Asselah (MD, PhD) Professor of Medicine Hepatology, Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France. Disclosures Professor Asselah
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany
 Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
Hepatitis C Update: What s New in 2017
 Hepatitis C Update: What s New in 2017 Cody A. Chastain, MD Assistant Professor of Medicine Viral Hepatitis Program Division of Infectious Diseases Vanderbilt University Medical Center Cody.a.Chastain@Vanderbilt.edu
Hepatitis C Update: What s New in 2017 Cody A. Chastain, MD Assistant Professor of Medicine Viral Hepatitis Program Division of Infectious Diseases Vanderbilt University Medical Center Cody.a.Chastain@Vanderbilt.edu
Current trends in CHC 1st genotype treatment
 Current trends in CHC 1st genotype treatment Tarik Asselah MD, PhD Professor of Medicine Hepatology, Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France Disclosures Employee of Paris Public University
Current trends in CHC 1st genotype treatment Tarik Asselah MD, PhD Professor of Medicine Hepatology, Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France Disclosures Employee of Paris Public University
30 & 31 January 2017
 1 0 t h A N N I V E R S A R Y 30 & 31 January 2017 PARIS - Palais des Congrès International Conference on the Management of Liver Diseases Organised by Pr Patrick Marcellin, APHC Organising Committee Michelle
1 0 t h A N N I V E R S A R Y 30 & 31 January 2017 PARIS - Palais des Congrès International Conference on the Management of Liver Diseases Organised by Pr Patrick Marcellin, APHC Organising Committee Michelle
Position Paper of the Italian Association for the Study of the Liver for the rational use of anti-hcv drugs available in Italy
 Position Paper of the Italian Association for the Study of the Liver for the rational use of anti-hcv drugs available in Italy Advisory committee on new drugs for Hepatitis C : Alessio Aghemo (Coordinator)
Position Paper of the Italian Association for the Study of the Liver for the rational use of anti-hcv drugs available in Italy Advisory committee on new drugs for Hepatitis C : Alessio Aghemo (Coordinator)
Hepatitis C and HIV. Stanislas Pol
 Hepatitis C and HIV Stanislas Pol Unité d Hépatologie, Hôpital Cochin Inserm U1223 & USM20 Institut Pasteur Université Paris Descartes Paris, France stanislas.pol@cch.aphp.fr Lisboa, 30 January 2017 Disclosures
Hepatitis C and HIV Stanislas Pol Unité d Hépatologie, Hôpital Cochin Inserm U1223 & USM20 Institut Pasteur Université Paris Descartes Paris, France stanislas.pol@cch.aphp.fr Lisboa, 30 January 2017 Disclosures
HCV Treatment in 2016: is there still a role for IFNa and ribavirin?
 HCV Treatment in 2016: is there still a role for IFNa and ribavirin? Heiner Wedemeyer Hannover Medical School Germany 1 Disclosures Honoraria for consulting or speaking (last 5 years): Abbott, AbbVie,
HCV Treatment in 2016: is there still a role for IFNa and ribavirin? Heiner Wedemeyer Hannover Medical School Germany 1 Disclosures Honoraria for consulting or speaking (last 5 years): Abbott, AbbVie,
11 & 12 January 2016
 11 & 12 January 2016 PARIS - Palais des Congrès International Conference on the Management of Patients with Viral Hepatitis Organised by Pr Patrick Marcellin Organising Committee Emilie Estrabaud, Michelle
11 & 12 January 2016 PARIS - Palais des Congrès International Conference on the Management of Patients with Viral Hepatitis Organised by Pr Patrick Marcellin Organising Committee Emilie Estrabaud, Michelle
Universal HCV treatment: Strategies for simplification
 Universal HCV treatment: Strategies for simplification PARIS HEPATOLOGY CONFERENCE 3 January 217 Tarik Asselah (MD, PhD) Hepatology & Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France. Disclosures
Universal HCV treatment: Strategies for simplification PARIS HEPATOLOGY CONFERENCE 3 January 217 Tarik Asselah (MD, PhD) Hepatology & Chief INSERM UMR 1149, Hôpital Beaujon, Clichy, France. Disclosures
Failure after treatment with DAAs: What to do? Marseille France 2-3 th June 2016
 Failure after treatment with DAAs: What to do? Marc Bourliere, MD White Nights of Hepatology Hôpital Saint Joseph Saint Petersburg Marseille France 2-3 th June 16 Disclosures Board member for : Schering-Plough,
Failure after treatment with DAAs: What to do? Marc Bourliere, MD White Nights of Hepatology Hôpital Saint Joseph Saint Petersburg Marseille France 2-3 th June 16 Disclosures Board member for : Schering-Plough,
Treatment of HCV : 100 % cure?
 Treatment of HCV : % cure? PHC 8 PARIS January 5th, 8 Tarik Asselah (MD, PhD) Professor of Medicine Hepatology, Chief ISERM UMR 49, Hôpital Beaujon, Clichy, France. PHC 8 - www.aphc.info Disclosures Employee
Treatment of HCV : % cure? PHC 8 PARIS January 5th, 8 Tarik Asselah (MD, PhD) Professor of Medicine Hepatology, Chief ISERM UMR 49, Hôpital Beaujon, Clichy, France. PHC 8 - www.aphc.info Disclosures Employee
Hepatitis C in Correctional Facilities: Big Problem, Bigger Opportunity. Cody A. Chastain, MD
 Hepatitis C in Correctional Facilities: Big Problem, Bigger Opportunity Cody A. Chastain, MD Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study
Hepatitis C in Correctional Facilities: Big Problem, Bigger Opportunity Cody A. Chastain, MD Disclosures Research supported by Gilead Sciences Inc.: Site investigator for HIV/HCV SWITCH Registry Study
Management of Chronic HCV 2017 and Beyond
 Management of Chronic HCV 2017 and Beyond Blaire E Burman, MD Virginia Mason Gastroenterology & Hepatology Relevant Disclosures No financial disclosures to report Leaning Objectives Burden of HCV Prevalence
Management of Chronic HCV 2017 and Beyond Blaire E Burman, MD Virginia Mason Gastroenterology & Hepatology Relevant Disclosures No financial disclosures to report Leaning Objectives Burden of HCV Prevalence
Hepatitis C: The New World of Treatment
 Hepatitis C: The New World of Treatment Aban 1395, NIOC Hospital Shahin Merat, M.D. Professor of Medicine Digestive Disease Research Institute Tehran University of Medical Sciences 1 Drugs NS5B polymerase
Hepatitis C: The New World of Treatment Aban 1395, NIOC Hospital Shahin Merat, M.D. Professor of Medicine Digestive Disease Research Institute Tehran University of Medical Sciences 1 Drugs NS5B polymerase
THE THERAPEUTIC REVOLUTION THAT TRANSFORMED CHRONIC HEPATITIS C TO A CURABLE DISEASE
 THE THERAPEUTIC REVOLUTION THAT TRANSFORMED CHRONIC HEPATITIS C TO A CURABLE DISEASE MARIA SCHINA CONSULTANT PHYSICIAN INTERNAL MEDICINE AND HEPATOLOGY ATHENS EUROCLINIC 10 th INTERNATIONAL CONGRESS OF
THE THERAPEUTIC REVOLUTION THAT TRANSFORMED CHRONIC HEPATITIS C TO A CURABLE DISEASE MARIA SCHINA CONSULTANT PHYSICIAN INTERNAL MEDICINE AND HEPATOLOGY ATHENS EUROCLINIC 10 th INTERNATIONAL CONGRESS OF
IFN-free therapy in naïve HCV GT1 patients
 IFN-free therapy in naïve HCV GT1 patients Paris Hepatitis Conference Paris, 12th January, 2015 Pr Tarik Asselah MD, PhD; Service d Hépatologie & INSERM U773 University Paris Diderot, Hôpital Beaujon,
IFN-free therapy in naïve HCV GT1 patients Paris Hepatitis Conference Paris, 12th January, 2015 Pr Tarik Asselah MD, PhD; Service d Hépatologie & INSERM U773 University Paris Diderot, Hôpital Beaujon,
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD)
 Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
Disease Burden of Non Alcoholic Fatty Liver Disease (NAFLD) Dr. H. Razavi May 31, 2017 First European NASH NAFLD Summit Disclosure: This work with funded by a multi-sponsored research grant from Intercept,
Hepatitis C - results in real life
 Hepatitis C - results in real life Robert Flisiak Department of Infectious Diseases and Hepatology Medical University of Białystok, Poland 10th PHC Paris, 30-31 January 2017 Disclosures Advisor and/or
Hepatitis C - results in real life Robert Flisiak Department of Infectious Diseases and Hepatology Medical University of Białystok, Poland 10th PHC Paris, 30-31 January 2017 Disclosures Advisor and/or
HEPATITIS C: UPDATE AND MANAGEMENT
 HEPATITIS C: UPDATE AND MANAGEMENT José Franco, MD Professor of Medicine Associate Dean for Educational Improvement Associate Director, Kern Institute STAR Center Director José Franco, MD Disclosures I
HEPATITIS C: UPDATE AND MANAGEMENT José Franco, MD Professor of Medicine Associate Dean for Educational Improvement Associate Director, Kern Institute STAR Center Director José Franco, MD Disclosures I
2017 Bruce Lucas Hepatology and Liver Transplant Symposium October 13th 2017 Management of Hepatitis C in Pre- and Post-Transplant Patients
 2017 Bruce Lucas Hepatology and Liver Transplant Symposium October 13th 2017 Management of Hepatitis C in Pre- and Post-Transplant Patients Jens Rosenau, MD Associate Professor of Medicine Acting Director
2017 Bruce Lucas Hepatology and Liver Transplant Symposium October 13th 2017 Management of Hepatitis C in Pre- and Post-Transplant Patients Jens Rosenau, MD Associate Professor of Medicine Acting Director
Baseline and acquired viral resistance to DAAs: how to test and manage
 Baseline and acquired viral resistance to DAAs: how to test and manage Round table discussion by Marc Bourliere, Robert Flisiak, Vasily Isakov, Mark Sulkowsky & Konstantin Zhdanov Prevalence of baseline
Baseline and acquired viral resistance to DAAs: how to test and manage Round table discussion by Marc Bourliere, Robert Flisiak, Vasily Isakov, Mark Sulkowsky & Konstantin Zhdanov Prevalence of baseline
47 th Annual Meeting AISF
 47 th Annual Meeting AISF Rome, 21 February 2014 Present and future treatment strategies for patients with HCV infection: chronic hepatitis and special populations (HCV/HIV coinfection, advanced cirrhosis,
47 th Annual Meeting AISF Rome, 21 February 2014 Present and future treatment strategies for patients with HCV infection: chronic hepatitis and special populations (HCV/HIV coinfection, advanced cirrhosis,
How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France
 How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France Paris Hepatitis Conference, January 12, 2016 Disclosures I have received funding
How to optimize treatment in G3 patients? Jérôme GOURNAY, MD Hépatologie Centre Hospitalier Universitaire de Nantes France Paris Hepatitis Conference, January 12, 2016 Disclosures I have received funding
HCV Infection: EASL Clinical Practice Guidelines Francesco Negro University Hospital Geneva Switzerland
 HCV Infection: EASL Clinical Practice Guidelines 2016 Francesco Negro University Hospital Geneva Switzerland Panel Codinat: Jean-Michel Pawlotsky Panel: Alessio Aghemo David Back Geoffrey Dusheiko Xavier
HCV Infection: EASL Clinical Practice Guidelines 2016 Francesco Negro University Hospital Geneva Switzerland Panel Codinat: Jean-Michel Pawlotsky Panel: Alessio Aghemo David Back Geoffrey Dusheiko Xavier
Special developments in the management of Hepatitis C. Disclosures
 Special developments in the management of Hepatitis C Sandeep Mukherjee,MD Division of Gastroenterology CHI Health and Creighton University Medical Center Omaha, NE 68154 Sandeep.Mukherjee@alegent.org
Special developments in the management of Hepatitis C Sandeep Mukherjee,MD Division of Gastroenterology CHI Health and Creighton University Medical Center Omaha, NE 68154 Sandeep.Mukherjee@alegent.org
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis B and Hepatitis C Virus in non-liver Transplant Recipients. Karim Qumosani MD, FRCPC, ABIM, MdMEd Multi-organ Transplant Unit, London
 Hepatitis B and Hepatitis C Virus in non-liver Transplant Recipients Karim Qumosani MD, FRCPC, ABIM, MdMEd Multi-organ Transplant Unit, London Financial Disclosures Research Grants Merck, Gilead, Abbvie,
Hepatitis B and Hepatitis C Virus in non-liver Transplant Recipients Karim Qumosani MD, FRCPC, ABIM, MdMEd Multi-organ Transplant Unit, London Financial Disclosures Research Grants Merck, Gilead, Abbvie,
HCV care after cure. This program is supported by educational grants from
 HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
HCV care after cure This program is supported by educational grants from Raffaele Bruno,MD Department of Infectious Diseases, Hepatology Outpatients Unit University of Pavia Fondazione IRCCS Policlinico
HIV/HCV Coinfection: Why It Matters and What To Do About It. Cody A. Chastain, MD 10/26/16
 HIV/HCV Coinfection: Why It Matters and What To Do About It Cody A. Chastain, MD 10/26/16 Disclosures I have no relevant financial disclosures. Objectives At the end of this lecture, the learner will be
HIV/HCV Coinfection: Why It Matters and What To Do About It Cody A. Chastain, MD 10/26/16 Disclosures I have no relevant financial disclosures. Objectives At the end of this lecture, the learner will be
Why make this statement?
 HCV Council 2014 10 clinical practice statements were evaluated by the Council A review of the available literature was conducted The level of support and level of evidence for the statements were discussed
HCV Council 2014 10 clinical practice statements were evaluated by the Council A review of the available literature was conducted The level of support and level of evidence for the statements were discussed
HCV Therapies: The Last challenges 12th Paris Hepatology Conference 14th January Stefan Zeuzem, MD University Hospital, Frankfurt, Germany
 HCV Therapies: The Last challenges 2th Paris Hepatology Conference 4th January 209 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany Disclosures Advisory boards: AbbVie, Gilead Sciences, Intercept,
HCV Therapies: The Last challenges 2th Paris Hepatology Conference 4th January 209 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany Disclosures Advisory boards: AbbVie, Gilead Sciences, Intercept,
Viva La Revolución: Options to Combat Hepatitis C
 Viva La Revolución: Options to Combat Hepatitis C David L. Wyles, MD Professor of Medicine University of Colorado Chief, Division of Infectious Disease Denver Health Learning Objectives After attending
Viva La Revolución: Options to Combat Hepatitis C David L. Wyles, MD Professor of Medicine University of Colorado Chief, Division of Infectious Disease Denver Health Learning Objectives After attending
3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice
 3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice Rome, 13 December 2013 Management and monitoring of HCC in the future era of DAA s Prof. Massimo Colombo Chairman Department of Liver,
3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice Rome, 13 December 2013 Management and monitoring of HCC in the future era of DAA s Prof. Massimo Colombo Chairman Department of Liver,
Hepatitis C: Newest Treatment Options and What To Do When We Cure It!
 Hepatitis C: Newest Treatment Options and What To Do When We Cure It! Richard Kalman, MD Division of Hepatology Department of Transplantation Einstein Medical Center Learning Objectives Scope of HCV How
Hepatitis C: Newest Treatment Options and What To Do When We Cure It! Richard Kalman, MD Division of Hepatology Department of Transplantation Einstein Medical Center Learning Objectives Scope of HCV How
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR
 Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
Hepatitis Alert: Management of Patients With HCV Who Have Achieved SVR This program is supported by educational grants from AbbVie, Gilead Sciences, and Merck About These Slides Please feel free to use,
Disclosures. Advanced HCV management. Overview. Renal failure 1/10/2018. Research Grant support to UCSF from AbbVie Gilead Merck Proteus NIH
 Disclosures Advanced HCV management Annie Luetkemeyer, MD Division of HIV, ID and Global Medicine ZSFG, UCSF Research Grant support to UCSF from AbbVie Gilead Merck Proteus NIH Overview Renal failure Acute
Disclosures Advanced HCV management Annie Luetkemeyer, MD Division of HIV, ID and Global Medicine ZSFG, UCSF Research Grant support to UCSF from AbbVie Gilead Merck Proteus NIH Overview Renal failure Acute
Genotype 4, finally cured? Imam Waked Professor of Medicine National Liver Institute
 Genotype 4, finally cured? Imam Waked Professor of Medicine National Liver Institute Paris, January 12, 215 Disclosures Investigator, speaker, and advisory board member for: Roche, MSD, BMS, Gilead, Janssen,
Genotype 4, finally cured? Imam Waked Professor of Medicine National Liver Institute Paris, January 12, 215 Disclosures Investigator, speaker, and advisory board member for: Roche, MSD, BMS, Gilead, Janssen,
A One-day Scientific Conference: Updates on Hepatitis C Treatments along with Consensus on Management of Hepatitis C in Iran
 A One-day Scientific Conference: Updates on Hepatitis C Treatments along with Consensus on Management of Hepatitis C in Iran Teheran, 22 July 2016 Massimo Colombo Treatment of HCV genotype 1 & 4 with DAAs
A One-day Scientific Conference: Updates on Hepatitis C Treatments along with Consensus on Management of Hepatitis C in Iran Teheran, 22 July 2016 Massimo Colombo Treatment of HCV genotype 1 & 4 with DAAs
Ari Bunim, M.D. Director of Hepatology New York Hospital Queens Assistant Professor of Clinical Medicine Weill Cornell Medical College
 Ari Bunim, M.D. Director of Hepatology New York Hospital Queens Assistant Professor of Clinical Medicine Weill Cornell Medical College New York State Law Goes into Effect January 1, 2014 Hepatitis C Virus
Ari Bunim, M.D. Director of Hepatology New York Hospital Queens Assistant Professor of Clinical Medicine Weill Cornell Medical College New York State Law Goes into Effect January 1, 2014 Hepatitis C Virus
Antiviral treatment in HCV cirrhotic patients on waiting list
 Antiviral treatment in HCV cirrhotic patients on waiting list Krzysztof Tomasiewicz Department of Hepatology and Infectious Diseases Medical University of Lublin, Poland Disclosures Consultancy/Advisory
Antiviral treatment in HCV cirrhotic patients on waiting list Krzysztof Tomasiewicz Department of Hepatology and Infectious Diseases Medical University of Lublin, Poland Disclosures Consultancy/Advisory
All Hands on Deck: Taking on Hepatitis C in Tennessee
 All Hands on Deck: Taking on Hepatitis C in Tennessee Cody A. Chastain, MD Assistant Professor of Medicine Viral Hepatitis Program Division of Infectious Diseases Vanderbilt University Medical Center Cody.a.Chastain@Vanderbilt.edu
All Hands on Deck: Taking on Hepatitis C in Tennessee Cody A. Chastain, MD Assistant Professor of Medicine Viral Hepatitis Program Division of Infectious Diseases Vanderbilt University Medical Center Cody.a.Chastain@Vanderbilt.edu
HCV elimination : lessons from Scotland
 HCV elimination : lessons from Scotland Sharon Hutchinson Glasgow Caledonian University / Health Protection Scotland BHIVA Hepatology Highlights, Edinburgh, 17 th April 2018 Disclosures Honoraria from
HCV elimination : lessons from Scotland Sharon Hutchinson Glasgow Caledonian University / Health Protection Scotland BHIVA Hepatology Highlights, Edinburgh, 17 th April 2018 Disclosures Honoraria from
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Will difficult-to-treat patients remain difficultto-treat. generation of treatments?
 Will difficult-to-treat patients remain difficultto-treat with the new generation of treatments? Jordan J Feld MD MPH Toronto Centre for Liver Disease Sandra Rotman Centre for Global Health University
Will difficult-to-treat patients remain difficultto-treat with the new generation of treatments? Jordan J Feld MD MPH Toronto Centre for Liver Disease Sandra Rotman Centre for Global Health University
Current HCV Treatment by Genotype
 Current HCV Treatment by Genotype Ari Bunim, MD Assistant Professor Clinical Medicine Weill Cornell Medical College Clinical Director of Hepatology New York-Presbyterian/Queens Objectives To understand
Current HCV Treatment by Genotype Ari Bunim, MD Assistant Professor Clinical Medicine Weill Cornell Medical College Clinical Director of Hepatology New York-Presbyterian/Queens Objectives To understand
The Changing World of Hepatitis C
 The Changing World of Hepatitis C Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul s Hospital Site Disclosures
The Changing World of Hepatitis C Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul s Hospital Site Disclosures
HCV TREATMENT PRE- AND POST TRANSPLANTATION
 HCV TREATMENT PRE- AND POST TRANSPLANTATION Mitchell L. Shiffman, MD, FACG Medical Director Liver Institute of Virginia Bon Secours Health System Richmond and Newport News, VA, USA IVer Liver Institute
HCV TREATMENT PRE- AND POST TRANSPLANTATION Mitchell L. Shiffman, MD, FACG Medical Director Liver Institute of Virginia Bon Secours Health System Richmond and Newport News, VA, USA IVer Liver Institute
NAFLD & NASH: Russian perspective
 NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
NAFLD & NASH: Russian perspective Vasily Isakov, MD, PhD Professor, Chief, Department Gastroenterology & Hepatology, Federal Research Center of nutrition, biotechnology and food safety Disclosures Received
End Stage Liver Disease & Disease Specific Indications for Liver Transplant. Susan Kang, RN, MSN, ANP-BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
1/16/2019. Goals of HCV Therapy. Objectives. Treating Hepatitis C and HIV Co Infection. Cure Defined as sustained virologic response (SVR)
 HCV ECHO WESTERN STATES HCV ECHO WESTERN STATES Treating Hepatitis C and HIV Co Infection Paulina Deming, Pharm D Associate Professor, College of Pharmacy Assistant Director, Viral Hepatitis Programs,
HCV ECHO WESTERN STATES HCV ECHO WESTERN STATES Treating Hepatitis C and HIV Co Infection Paulina Deming, Pharm D Associate Professor, College of Pharmacy Assistant Director, Viral Hepatitis Programs,
A Nation-wide Investigation of Real-World Community Effectiveness in HCV Treatment for Policy-making Toward Elimination of HCV by 2030 in Taiwan
 A Nation-wide Investigation of Real-World Community Effectiveness in HCV Treatment for Policy-making Toward Elimination of HCV by 2030 in Taiwan MING-LUNG YU Distinguished Professor, Hepatobiliary Division,
A Nation-wide Investigation of Real-World Community Effectiveness in HCV Treatment for Policy-making Toward Elimination of HCV by 2030 in Taiwan MING-LUNG YU Distinguished Professor, Hepatobiliary Division,
Length of Authorization: 8-16 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure appropriate
HIV/hepatitis co-infection. Christoph Boesecke Department of Medicine I University Hospital Bonn Germany
 HIV/hepatitis co-infection Christoph Boesecke Department of Medicine I University Hospital Bonn Germany Clinical Management and Treatment of HBV and HCV Co-infection in HIVpositive Persons Hepatitis B
HIV/hepatitis co-infection Christoph Boesecke Department of Medicine I University Hospital Bonn Germany Clinical Management and Treatment of HBV and HCV Co-infection in HIVpositive Persons Hepatitis B
The Dawn of a New Era: Hepatitis C
 The Dawn of a New Era: Hepatitis C Naudia L. Jonassaint Assistant Professor of Medicine and Surgery University Pittsburgh School of Medicine December 1, 2015 Objectives After presentation the learner should
The Dawn of a New Era: Hepatitis C Naudia L. Jonassaint Assistant Professor of Medicine and Surgery University Pittsburgh School of Medicine December 1, 2015 Objectives After presentation the learner should
Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France
 9th Paris Hepatitis Conference, January 11-12, 2016 Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France Disclosures
9th Paris Hepatitis Conference, January 11-12, 2016 Follow-up of patients with SVR Lawrence Serfaty Service d Hépatologie, UMR_S 938 Hôpital Saint-Antoine Université Pierre&Marie Curie Paris, France Disclosures
Felizarta, Bo Fu, Teresa Ng, Chih-Wei Lin, Federico Mensa Abstract 253. Pibrentasvir (formerly ABT-530) pangenotypic NS3/4A protease inhibitor
 ENDURANCE-1: A Phase 3 Evaluation of the Efficacy and Safety of 8- versus 12-week Treatment with Glecaprevir/ Pibrentasvir (formerly ABT-493/ABT-53) in HCV Genotype 1 Infected Patients with or without
ENDURANCE-1: A Phase 3 Evaluation of the Efficacy and Safety of 8- versus 12-week Treatment with Glecaprevir/ Pibrentasvir (formerly ABT-493/ABT-53) in HCV Genotype 1 Infected Patients with or without
Prior Authorization Guideline
 Prior Authorization Guideline Guideline Name Olysio (simeprevir) Formulary UnitedHealthcare Community & State Formulary Note Approval Date 2/19/2014 Revision Date 7/9/2014 1. Indications Drug Name: Olysio
Prior Authorization Guideline Guideline Name Olysio (simeprevir) Formulary UnitedHealthcare Community & State Formulary Note Approval Date 2/19/2014 Revision Date 7/9/2014 1. Indications Drug Name: Olysio
The following page contains the final YODA Project review approving this proposal.
 The YODA Project Research Proposal Review The following page contains the final YODA Project review approving this proposal. The Yale University Open Data Access (YODA) Project Yale University Center for
The YODA Project Research Proposal Review The following page contains the final YODA Project review approving this proposal. The Yale University Open Data Access (YODA) Project Yale University Center for
HCV in 2017: New Therapies and New Opportunities. Presentation prepared by: Date prepared: OBJECTIVES
 Project ECHO HCV Collaborative HCV in 217: New Therapies and New Opportunities Paulina Deming, PharmD Assistant Director Hepatitis C Programs, ECHO Institute Associate Professor College of Pharmacy University
Project ECHO HCV Collaborative HCV in 217: New Therapies and New Opportunities Paulina Deming, PharmD Assistant Director Hepatitis C Programs, ECHO Institute Associate Professor College of Pharmacy University
Dr. Siddharth Srivastava
 Dr. Siddharth Srivastava MD, DM (Gastroenterology) Associate Professor GIPMER, New Delhi Rashtriya Gaurav Award 2013 for work on hepatitis B and C Set up Liver clinic at GIPMER and in charge EUS laboratory.
Dr. Siddharth Srivastava MD, DM (Gastroenterology) Associate Professor GIPMER, New Delhi Rashtriya Gaurav Award 2013 for work on hepatitis B and C Set up Liver clinic at GIPMER and in charge EUS laboratory.
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18
 Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18 Overview Hepatitis C Virus Prevalence Effects of Hepatitis C Prevention Diagnosis
Hepatitis C Infection: Updated Information for Front Line Workers in Primary Care Settings MAMTA K. JAIN, MD, MPH 2/14/18 Overview Hepatitis C Virus Prevalence Effects of Hepatitis C Prevention Diagnosis
Hepatocellular carcinoma
 Hepatocellular carcinoma Mary Ann Y. Huang, M.D., M.S., FAASLD Transplant hepatologist Peak Gastroenterology Associates Porter Adventist Hospital Denver, Colorado Background - Worldwide Hepatocellular
Hepatocellular carcinoma Mary Ann Y. Huang, M.D., M.S., FAASLD Transplant hepatologist Peak Gastroenterology Associates Porter Adventist Hospital Denver, Colorado Background - Worldwide Hepatocellular
Initial Treatment of HCV G Hugo E. Vargas, MD Professor of Medicine Medical, Director Office of Clinical Research Mayo Clinic Arizona
 Initial Treatment of HCV G1 2016 Hugo E. Vargas, MD Professor of Medicine Medical, Director Office of Clinical Research Mayo Clinic Arizona Disclosure Information Disclosure Information Dr. Vargas receives
Initial Treatment of HCV G1 2016 Hugo E. Vargas, MD Professor of Medicine Medical, Director Office of Clinical Research Mayo Clinic Arizona Disclosure Information Disclosure Information Dr. Vargas receives
Primary Care Approach to Diagnosis and Management of Chronic Hepatitis C Brian Viviano, D.O.
 Primary Care Approach to Diagnosis and Management of Chronic Hepatitis C Brian Viviano, D.O. Objectives Epidemiology of chronic hepatitis C CDC guidelines on screening or hepatitis C Diagnosing hepatitis
Primary Care Approach to Diagnosis and Management of Chronic Hepatitis C Brian Viviano, D.O. Objectives Epidemiology of chronic hepatitis C CDC guidelines on screening or hepatitis C Diagnosing hepatitis
Selecting HCV Treatment
 Selecting HCV Treatment Caveats Focus on treatment selection for genotypes 1, 2, and 3. Majority of US population infected with GT 1, 2, or 3 GT 4 treatment closely reflects GT 1 treatment GT 5 and 6 are
Selecting HCV Treatment Caveats Focus on treatment selection for genotypes 1, 2, and 3. Majority of US population infected with GT 1, 2, or 3 GT 4 treatment closely reflects GT 1 treatment GT 5 and 6 are
HEPATITIS B: WHO AND WHEN TO TREAT?
 HEPATITIS B: WHO AND WHEN TO TREAT? George V. Papatheodoridis Professor in Medicine & Gastroenterology Medical School of National & Kapodistrian University of Athens Director of Academic Department of
HEPATITIS B: WHO AND WHEN TO TREAT? George V. Papatheodoridis Professor in Medicine & Gastroenterology Medical School of National & Kapodistrian University of Athens Director of Academic Department of
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Hepatits C Criteria Direct Acting Antiviral Medications
 Hepatits C Criteria Direct Acting Antiviral Medications Harvoni-Formulary PA required 1. Is the patient being treated for a funded condition by the Oregon Health Plan? 2. Does the member have a diagnosis
Hepatits C Criteria Direct Acting Antiviral Medications Harvoni-Formulary PA required 1. Is the patient being treated for a funded condition by the Oregon Health Plan? 2. Does the member have a diagnosis
Treating now vs. post transplant
 Resistance with treatment failure Treating now vs. post transplant Pros (for treating pre transplant) If SVR efficacy means Better quality of life Removal from waiting list No post transplant recurrence
Resistance with treatment failure Treating now vs. post transplant Pros (for treating pre transplant) If SVR efficacy means Better quality of life Removal from waiting list No post transplant recurrence
Treating HCV Prior to Liver Transplantation. What Are the Treatment Options? Xavier Forns Liver Unit Hospital Clinic, CIBEREHD, IDIBAPS Barcelona
 Treating HCV Prior to Liver Transplantation What Are the Treatment Options? Xavier Forns Liver Unit Hospital Clinic, CIBEREHD, IDIBAPS Barcelona Disclosures Unrestricted Grant Support: Janssen and Abbvie
Treating HCV Prior to Liver Transplantation What Are the Treatment Options? Xavier Forns Liver Unit Hospital Clinic, CIBEREHD, IDIBAPS Barcelona Disclosures Unrestricted Grant Support: Janssen and Abbvie
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st NAFLD/NASH : an expanding burden on liver health
 First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
First European NAFLD-NASH Summit European Parliament, Brussels, May 31 st 2017 NAFLD/NASH : an expanding burden on liver health Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière,
Hepatitis C Policy Discussion
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Hepatitis C Genotypes
 9/2/21 OBJECTIVES Project ECHO HCV Collaborative HCV in 21: New Therapies and New Opportunities Paulina Deming, PharmD Assistant Director Hepatitis C Programs, ECHO Institute Associate Professor College
9/2/21 OBJECTIVES Project ECHO HCV Collaborative HCV in 21: New Therapies and New Opportunities Paulina Deming, PharmD Assistant Director Hepatitis C Programs, ECHO Institute Associate Professor College
Prior Authorization Guideline
 Prior Authorization Guideline Guideline Name Sovaldi (sofosbuvir) Formulary UnitedHealthcare Community & State Formulary Note Approval Date 2/19/2014 Revision Date 7/8/2014 1. Indications Drug Name: Sovaldi
Prior Authorization Guideline Guideline Name Sovaldi (sofosbuvir) Formulary UnitedHealthcare Community & State Formulary Note Approval Date 2/19/2014 Revision Date 7/8/2014 1. Indications Drug Name: Sovaldi
Length of Authorization: 8-12 weeks. Requires PA: All direct-acting antivirals for treatment of Hepatitis C. Approval Criteria
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
HCV Treatment in 2016: Genotypes 1, 2, and 3. Cody A. Chastain, MD October 12, 2016
 HCV Treatment in 2016: Genotypes 1, 2, and 3 Cody A. Chastain, MD October 12, 2016 Disclosures I have no financial disclosures. Caveats I will only discuss treatment of GT 1-3. Majority of US population
HCV Treatment in 2016: Genotypes 1, 2, and 3 Cody A. Chastain, MD October 12, 2016 Disclosures I have no financial disclosures. Caveats I will only discuss treatment of GT 1-3. Majority of US population
HCV Elimination in France
 HCV Elimination in France Stanislas Pol, MD, PhD PHC 14 January 2017 Liver Department, Hôpital Cochin Inserm U-1223, Institut Pasteur Université Paris Descartes, Paris, France stanislas.pol@aphp.fr Disclosures
HCV Elimination in France Stanislas Pol, MD, PhD PHC 14 January 2017 Liver Department, Hôpital Cochin Inserm U-1223, Institut Pasteur Université Paris Descartes, Paris, France stanislas.pol@aphp.fr Disclosures
Liver Pathology and the Clinician in 2015: At the Crossroads. Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York
 Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Hepatitis C in Disclosures
 Hepatitis C in 2018 Sandeep Mukherjee, MD CHI Health and Creighton University Medical Center Division of Gastroenterology Grant support: Abbvie Disclosures Speaker: Abbvie, Gilead, Merck Section editor
Hepatitis C in 2018 Sandeep Mukherjee, MD CHI Health and Creighton University Medical Center Division of Gastroenterology Grant support: Abbvie Disclosures Speaker: Abbvie, Gilead, Merck Section editor
Hepatitis C Direct-Acting Antivirals
 Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Hepatitis C Direct-Acting Antivirals Goals: Approve use of cost-effective treatments supported by the medical evidence. Provide consistent patient evaluations across all hepatitis C treatments. Ensure
Update in hepatitis C virus infection
 Update in hepatitis C virus infection Eoin Feeney Consultant in Infectious Diseases St. Vincent s University Hospital Overview Natural history Diagnosis, screening, staging Management Barriers going forward
Update in hepatitis C virus infection Eoin Feeney Consultant in Infectious Diseases St. Vincent s University Hospital Overview Natural history Diagnosis, screening, staging Management Barriers going forward
Future strategies with new DAAs
 Future strategies with new DAAs Ola Weiland professor New direct antiviral drugs Case no 1 male with genotype 2b Male with gt 2b chronic HCV Male with gt 2b relapse afer peg-ifn + RBV during 24 weeks
Future strategies with new DAAs Ola Weiland professor New direct antiviral drugs Case no 1 male with genotype 2b Male with gt 2b chronic HCV Male with gt 2b relapse afer peg-ifn + RBV during 24 weeks
CHRONIC HCV TREATMENT: In Special Populations.
 CHRONIC HCV TREATMENT: In Special Populations. By Taher EL-ZANATY Prof. of Internal Medicine CAIRO UNIVERSITY Introduction: HCV is the major cause of chronic hepatitis in Egypt. Its end stage is liver
CHRONIC HCV TREATMENT: In Special Populations. By Taher EL-ZANATY Prof. of Internal Medicine CAIRO UNIVERSITY Introduction: HCV is the major cause of chronic hepatitis in Egypt. Its end stage is liver
ERADICATION, ELIMINATION, OR DISEASE CONTROL OF HEPATITIS C. Alfredo Alberti
 ERADICATION, ELIMINATION, OR DISEASE CONTROL OF HEPATITIS C 2017 Alfredo Alberti Department of Molecular Medicine University of Padova ITALY MY DISCLOSURES Research Grants : Roche, Gilead, Janssen, MSD,
ERADICATION, ELIMINATION, OR DISEASE CONTROL OF HEPATITIS C 2017 Alfredo Alberti Department of Molecular Medicine University of Padova ITALY MY DISCLOSURES Research Grants : Roche, Gilead, Janssen, MSD,
Ledipasvir-Sofosbuvir (Harvoni)
 HEPATITIS WEB STUDY HEPATITIS C ONLINE Ledipasvir-Sofosbuvir (Harvoni) Robert G. Gish MD Professor, Consultant, Stanford University Medical Center Senior Medical Director, St Josephs Hospital and Medical
HEPATITIS WEB STUDY HEPATITIS C ONLINE Ledipasvir-Sofosbuvir (Harvoni) Robert G. Gish MD Professor, Consultant, Stanford University Medical Center Senior Medical Director, St Josephs Hospital and Medical
Treatment of HCV in 2016
 5/1/16 Treatment of HCV in 16 Graham R Foster Professor of Hepatology QMUL Conflicts of Interest Speaker and consultancy fees received from AbbVie, BI, BMS, Gilead, Janssen, Roche, Merck, Novartis, Springbank,
5/1/16 Treatment of HCV in 16 Graham R Foster Professor of Hepatology QMUL Conflicts of Interest Speaker and consultancy fees received from AbbVie, BI, BMS, Gilead, Janssen, Roche, Merck, Novartis, Springbank,
Current HCV Treatment by Genotype Ira M. Jacobson, MD
 Current HCV Treatment by Genotype Ira M. Jacobson, MD Director of Hepatology NYU School of Medicine Objectives To understand the prevalence of HCV and distribution of HCV genotypes Describe the HCV lifecycle
Current HCV Treatment by Genotype Ira M. Jacobson, MD Director of Hepatology NYU School of Medicine Objectives To understand the prevalence of HCV and distribution of HCV genotypes Describe the HCV lifecycle
The impact of the treatment of HCV in developing Hepatocellular Carcinoma
 The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
The impact of the treatment of HCV in developing Hepatocellular Carcinoma Paul Y Kwo, MD Professor of Medicine Medical Director, Liver Transplantation Gastroenterology/Hepatology Division Indiana University
Learning Objective. After completing this educational activity, participants should be able to:
 Learning Objective After completing this educational activity, participants should be able to: Use patient characteristics and preferences to select HCV treatment strategies that maximize the potential
Learning Objective After completing this educational activity, participants should be able to: Use patient characteristics and preferences to select HCV treatment strategies that maximize the potential
Utility of Virological Assays at the DAA Era
 Utility of Virological Assays at the DAA Era Prof. Jean-Michel Pawlotsky, MD, PhD National Reference Center for Viral Hepatitis B, C and delta Department of Virology & INSERM U955 Henri Mondor Hospital
Utility of Virological Assays at the DAA Era Prof. Jean-Michel Pawlotsky, MD, PhD National Reference Center for Viral Hepatitis B, C and delta Department of Virology & INSERM U955 Henri Mondor Hospital
Liver transplant: what is left after the viruses
 Riunione Monotematica A.I.S.F. 2016 The Future of Liver Disease: Beyond HCV is there a Role for Hepatologist? Milan 15 th 2016 Liver transplant: what is left after the viruses Stefano Ginanni Corradini
Riunione Monotematica A.I.S.F. 2016 The Future of Liver Disease: Beyond HCV is there a Role for Hepatologist? Milan 15 th 2016 Liver transplant: what is left after the viruses Stefano Ginanni Corradini
Eliminating Hepatitis C from New Zealand
 Eliminating Hepatitis C from New Zealand Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital Disclosures I have the following
Eliminating Hepatitis C from New Zealand Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital Disclosures I have the following
Treatment of Hepatitis C Recurrence after Liver Transplantation. Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona
 Treatment of Hepatitis C Recurrence after Liver Transplantation Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Agenda 1. Introduction 2. Treatment options for hepatitis C recurrence after transplantation
Treatment of Hepatitis C Recurrence after Liver Transplantation Maria Carlota Londoño Liver Unit Hospital Clínic Barcelona Agenda 1. Introduction 2. Treatment options for hepatitis C recurrence after transplantation
Is prioritization the best way to treat hepatitis C? Vicente Soriano Infectious Diseases Unit La Paz University Hospital Madrid, Spain
 Is prioritization the best way to treat hepatitis C? Vicente Soriano Infectious Diseases Unit La Paz University Hospital Madrid, Spain Disclosures Advisory boards and speaker s bureau for: Gilead, Merck,
Is prioritization the best way to treat hepatitis C? Vicente Soriano Infectious Diseases Unit La Paz University Hospital Madrid, Spain Disclosures Advisory boards and speaker s bureau for: Gilead, Merck,
DIAGNOSIS OF NASH LIVER BIOPSY. PHC
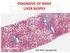 DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
