Chapter 21 Treatment of Alopecia Areata
|
|
|
- Bruno Tucker
- 5 years ago
- Views:
Transcription
1 Chapter 21 Treatment of Alopecia Areata C. Perret and R. Happle 1 Introduction The patchy form of alopecia areata has a high rate of spontaneous remission. In severe forms, the chance of a spontaneous regrowth is much lower but still present. This fact must be taken into account when different therapeutic modalities are evaluated. For topical therapeutics, efficacy must be established by treating only parts of the scalp, the non treated areas serving as a control. Systemic drugs must be evaluated in placebo-controlled studies. Although alopecia areata is a disease that may cause major psychosocial problems, it is at any rate not life threatening. For this reason, the obtained results must be weighed against possible undesired effects. Maintenance treatment should be suitable for a long period of time, and possible long-term side effects must be taken into consideration. If the available modes of treatment are screened according to these criteria, the absence of a consistently effective and, at the same time, safe method is evident. This is furthermore reflected by the abundance of various doubtful therapeutic modalities advocated for alopecia areata. It is not the aim of this chapter to give a complete overview of all proposed modes of treatment. We discuss first some therapeutic approaches which, in our opinion, are of proven effectiveness. We then briefly mention some other therapeutic modalities which have, for various reasons, gained attention, but which cannot in our opinion be recommended because they are either ineffective or associated with unacceptable side effects. This selection may be criticized for being subjective, but we feel that most readers would prefer an evaluation of the available therapeutic methods instead of their simple enumeration. The most promising approach is topical immunotherapy, followed by photochemotherapy. Corticosteroids still seem to have, at least under certain circumstances, a limited place in the treatment of alopecia areata. Other therapeutic attempts, such as topically applied minoxidil, have gained much attention, but have not proven useful. Cyc1osporin, albeit theoretically promising, has serious side effects. Reports on a beneficial effect of inosiplex await confirmation. A summary at the end of this chapter is intended to provide the reader with advice in certain practical questions, such as how to treat children, and what to do with patchy forms of alopecia areata. C. E. Orfanos et al. (eds.), Hair and Hair Diseases Springer-Verlag Berlin Heidelberg 1990
2 572 C. Perret and R. Happle 2 Therapeutic Modalities with Proven Effectiveness 2.1 Topical Immunotherapy In this mode of treatment, a contact sensitization is induced and a mild contact eczema is maintained on the scalp by weekly applications of a potent contact allergen. By modulating the characteristic peribulbar and intrabulbar infiltrates, the allergic contact dermatitis stimulates hair regrowth that is confined to the area treated The Choice of Contact Allergens For obvious reasons, a strong contact allergen should be used in order to assure that the patient becomes sensitized easily Allergens no Longer Used: DNCB, Primula, Nickel In principle, any strong contact allergen may be used. There are test reports on hair regrowth in patients with alopecia areata in an area of contact eczema to nickel (Martin 1984) and primula obconica (Rhodes et al. 1981). For obvious reasons, the use of common antigens has disadvantages. Therefore, contact allergens were chosen which are not present in the natural or industrial environment of man. The first contact allergen used in treating alopecia areata was dinitrochlorobenzene (DNCB). In 1976 Rosenberg and Drake reported regrowth of hair in two patients following topical application of DNCB. In three studies on a total of 200 patients with alopecia areata, one of us (R. H.) could induce unilateral hair regrowth with weekly applications of DNCB on the scalp (Happle and Echternacht 1977; Happle et al. 1978; Happle 1979). To exclude a possible spontaneous regrowth, initially only one side of the scalp was treated, using the untreated side as a control Currently used Allergens: DCP, SADBE As DNCB has been shown to be mutagenic in the Ames test, DNCB was, at least in Europe, replaced by diphenylcyclopropenone (DCP) and squaric acid dibutylester (SAD BE) (Happle 1985a; Fig. 1). It should be borne in mind, however, that the possible toxicological effects of both substances are far from fully characterized (Happle 1986). Informed consent from the patient is therefore mandatory. For the same reason, wc do not treat patients younger than 16 years of age. The efficacy of topical immunotherapy with DCP or SADBE has been shown in numerous clinical studies (Case et al. 1984; Happle et al. 1980, 1983; Kietzmann Fig. 1 a, b. Contact allergens used for topical immunotherapy of alopecia areata. a Diphenylcyclopropenone. b Squaric acid dibutylester
3 Treatment of Alopecia Areata 573 et al. 1985; MacDonald-Hull and Norris 1988; Monk and Williams 1988; Ochsendorf et al. 1988; Orecchia and Douville 1988; Valsecchi et al. 1986) Therapeutic Regimen Currently, we prefer to use DCP, and as an alternative SADBE (Happle 1985b; Ochsendorf et al. 1988; Perret et al. (1990, in press). In the literature, various vehicles such as petrolatum, polyethylenglycol, and acetone are used (Kietzmann et al. 1985). We prefer acetone as a vehicle because it dries readily and allows the patient to put on his wig immediately after the application. Moreover, the risk of dissemination is rather low. In order to prevent a flare-up at contact sites we sensitize exclusively on the scalp. Sensitization is obtained with a 2% DCP solution in acetone in an area of approximately 50 cm 2 The sensitization is considered successful if a contact dermatitis appears or can be elicited 2 weeks after the sensitization. Although DCP and SADBE are potent contact allergens, we observed some patients in whom we could not elicit a contact allergy. Fortunately, such cases are very rare (1 %-2%). When the eczematous reaction has faded away, we begin to treat one side of the scalp with a % or 0.01 % DCP solution. Because hair regrowth seems to be dependent on a switch-on/switch-off mechanism of the immune response, we do not treat more often than once per week. The concentration is increased until a mild eczematous reaction is obtained, consisting of itching, erythema, and mild scaling. A vesicular reaction is not necessary and should be avoided by lowering the concentration. The necessary concentrations may vary between 10-7 % and 2%. Initially, we always treat one half of the scalp until we see hair regrowth confined to the treated area; DCP is then applied on the entire scalp. Visible hair usually appears within 8-12 weeks of treatment. Treatment of the eyebrows but not of the eyelashes is feasible. After hair regrowth is complete, we try to increase the intervals up to 4 weeks, then try a complete stop if hair growth remains stable. The treatment is merely symptomatic, and therefore relapses are possible. If the alopecia areata again shows activity we start the treatment again at weekly intervals. If no regrowth can be obtained within 6 months, the treatment is discontinued, or we shift over to SADBE treatment. The effectiveness of the topical immunotherapy is illustrated in Fig Side Effects of Topical Immunotherapy The side effects can be divided into desired and undesired consequences. Necessary and desired side effects are the signs and symptoms of a mild contact eczema: itching, erythema, and scaling. Especially in the beginning, a vesicular reaction cannot always be avoided. Enlargement of regional lymph nodes is frequently seen. Undesired effects are dissemination of the eczema and, very rarely, urticarial or erythema multiforme-like reactions. In our series of 400 patients we have seen three patients with an erythema multiforme-like reaction. In these patients, topical and systemic treatment with corticosteroids resulted in a complete remission of the rash.
4 574 C. Perret and R. Happle b c d Fig.2a--d. Topical immunotherapy of alopecia areata totalis. a Prior to Therapy. b Unilateral hair growth after 16-week unilateral treatment. c Regrowth after subsequent 8-week bilateral treatment. d Complete regrowth after 34 weeks
5 Treatment of Alopecia Areata Immunohistological Findings: Comparison of Untreated Versus Treated Alopecia Areata Lymphocytic Infiltrate The histological hallmark of alopecia areata consists of a peri bulbar accumulation of lymphocytes. This infiltrate is most pronounced in the active phase of the disease. Although less dense, lymphocytic infiltrates may still be found in longstanding alopecia areata. The characterization of this infiltrate by means of monoclonal antibodies showed that 90% of the lymphocytes are T cells (Perret et al. 1982). Our group could further show that this T-cell infiltrate consists predominantly of CD: T -cells with a CD 4/CDs ratio of approximately 4: 1 (Perret et al. 1984). Most likely, B cells do not playa role in the pathogenesis of alopecia areata. These findings have been confirmed by other groups (Messenger and Bleehan 1985; Peereboom-Wynia et al. 1986; Todes-Taylor et al. 1984). The presence of lymphocytic infiltrates in active alopecia areata offers two possible therapeutic approaches. On the one hand, immunosuppression should result in a reduction of the peri- and intra bulbar infiltrate and thus stimulate hair regrowth. In theory, this can be achieved by systemic application of corticosteroids. (For the practical shortcomings of this treatment see Sect ). Another approach is the modulation of the inflammatory infiltrate according to the hypothesis of antigenic competition by means of topical immunotherapy. Such an immunomodulation has in fact been visualized immunohistologically. Biopsies taken on both treated and untreated sides of the head after unilateral regrowth had started revealed that the peri bulbar round cell infiltrates were denser on the treated side, especially in early stages of treatment (Happle et al. 1978). The seemingly paradoxical finding that an augmentation of the infiltrate by means of topical immunotherapy has the same clinical effect as a pronounced reduction of the infiltrate induced by corticosteroids can be explained when the composition of this augmented infiltrate is studied. Topical immunotherapy modulates the infiltrate resulting in a change of the CD 4/CDs ratio from 4: 1 to 1 : 1. Presumably, this is due to an increased number ofcdt cells (Happle et al. 1986) , a 0 <> b c Fig.3a-c. Lymphocytic infiltrates in alopecia areata. Modulation by different modes of treatment. a No treatment; predominance of CD: cells. b Corticoid therapy; overall reduction of the infiltrate. c Topical immunotherapy; dense infiltrate with equilibrium of CD: and CDt cells
6 576 C. Perret and R. Happle The modulation of the lymphocytic infiltrate in alopecia areata by different modes of therapy is illustrated in Fig Expression of Class I and Class II Antigens In contrast to the case in normal skin, expression of class I antigens (HLA-ABC) and class II antigens (HLA-DR and HLA-DQ) by matrix cells of the hair bulbs in untreated alopecia areata could be demonstrated (Brocker et al. 1987; Hamm et al. 1988; Messenger and Bleehan 1985). HLA-ABC antigens are not only expressed on hair matrix but also in the subinfundibular epithelium. An abnormal expression of HLA-DR antigens could be shown in hair matrix epithelium and in subinfundibular epithelium. Moreover, in long-standing alopecia areata, the expression of HLA-DQ antigens is a frequent finding (Hamm et al. 1988). The abnormal expression of class I antigens seems to be a prerequisite for a specific interaction of cytotoxic T-Iymphocytes with epithelium of the hair matrix. The pathogenetic implications of the abnormal expression of class II antigens are not clear, although it is known that HLA-DR expression is also necessary for antigen presentation. Presumably, this expression is a secondary phenomenon. It is postulated that the mononuclear infiltrate is followed by class II expression, probably induced by interferon that is produced within the T-cell infiltrate (Brocker et al. 1987; Khoury et al. 1986). In treated alopecia areata the expression of these markers is down-modulated. The abnormal expression of class I and class II major histocompatibility antigens in the epithelium of the hair bulb tends to disappear in part or even completely (Brocker et al. 1987) Langerhans Cells In healthy controls, Langerhans cells could not be demonstrated in the hair bulb. In alopecia areata, however, CDt dendritic cells could be demonstrated in a peribulbar and intrabulbar localization even in an early phase of the disease (Brocker et a1.1987; Kohchiyama et al. 1985; Wiesner-Menzel and Happle 1984). This finding, too, indicates that a T-cell mediated immune mechanism plays a role in the Table 1. Comparison of immunohistological findings in alopecia areata before and during topical immunotherapy Peri bulbar lymphocytic infiltrate Composition CDt/CDt Expression of class I and class II antigens on hair matrix cells and subinfundibular epithelium Intrabulbar CDt cells Prior to topical immunotherapy ++ 90% CDt cells 3:1-4: During topical immunotherapy % CDt cells 1 : >0 +--->0
7 Treatment of Alopecia Areata 577 pathogenesis of alopecia areata. In treated alopecia areata, peri- and intrabulbar Langerhans cells (CDt cells) tend to disappear in part or even completely (Brocker et al. 1987). The described immunohistological findings are summarized in Table Antigenic Competition as an Explanation for the Efficacy of Topical Immunotherapy The mechanism of the effect of topical immunotherapy is not fully understood. In order to explain the efficacy, the concept of antigenic competition has been proposed. It is known that application of two contact allergens within a few days influences the effectiveness of sensitization. Painting of 5% dinitrofluorobenzene 7 days prior to sensitization with picryl chloride results in a significant decrease of delayed-type hypersensitivity to picryl chloride (Nakano 1977). In alopecia areata, the characteristic peri bulbar lymphocytic infiltrate might reflect a cell-mediated immune reaction to some hair-associated antigens. With the eliciting of contact allergy, a second antigen is introduced at the same site. The generated suppressor T cells would nonspecifically inhibit the immune response against the hair follicles. Regrowth of hair could then be attributed to a change in the local balance between different T-cell subsets (Happle 1980). The immunohistological findings demonstrated may then be interpreted as the result of an immune modulation, which results in a suppression of the T-cell mediated immune reaction against the hair bulbs and thus leads to hair regrowth. In accordance with this hypothesis are the T-cell nature of the characteristic infiltrate with the predominance of CDt cells, the expression of class I and class II antigens, the presence of Langerhans cells in untreated alopecia areata and - even more importantly - the characteristic modulation of these parameters during topical immunotherapy, as demonstrated in the described immunohistological studies. 2.2 PUV A Therapy Since the first report on treatment of alopecia areata with 8-methoxypsoralen and sunlight by Rollier and Warcewski (1974), the effectiveness ofpuva therapy has been discussed controversially. Weissmann et al. (1978) reported that in all of their patients treated with topical PUV A, hair regrowth was observed. Lassus et al. (1980) found good to excellent results in 73% of patients treated with either topical or oral PUV A, with no significant difference between the modalities. The number of irradiations required for a good result was generally below 40. Recent reports have been less promising. Amer and Garf (1983) could achieve some regrowth in only 40% of patients treated with oral PUV A therapy. Claudy and Gagnaire (1983) treated 23 patients with photochemotherapy, combining oral or topical methoxypsoralen and UVA radiation of the scalp or the whole body. According to their findings, only total body irradiation combined with orally administered methoxypsoralen induced sufficient hair regrowth in 56% of the patients.
8 578 C. Perret and R. HappJe The main disadvantage of this therapy is the high amount of UV A and the large number of sessions needed. Claudy and Gagnaire (1983) used nearly 100 sessions, resulting in an application of up to 785 Jjcm 2. The precise mechanism ofpuva therapy in alopecia areata is not known. An effect on peripheral blood T -lymphocytes may play an important role. It is known that in patients with psoriasis under PUV A therapy a significant decrease of CDt positive cells can be observed (Caranzan et al. 1987). Furthermore, PUVA reduces both allogenic and antigen-specific T-cell responses (Mark et al. 1987). This effect may be explained by the disappearance of epidermal Langerhans cells during PUVA therapy, as observed by Friedmann (1981). 3 Other Therapeutic Modalities In this section we discuss various other therapeutic approaches which, however, we do not recommend because they are either associated with serious side effects or, at least in our judgement, ineffective. 3.1 Corticosteroids In the era prior to topical immunotherapy and PUV A therapy, corticosteroids were the only approach offering some chance for success Systemic Application In 1952 Dillaha and Rothman successfully treated four patients with long-standing alopecia totalis or universalis with high doses of cortisone given systemically. For obvious reasons, this treatment cannot be continued for a longer period of time. This report led to an increasing number of studies investigating several corticosteroids and dose-response relationships (Darvill1963; Kern et al. 1973; Michalowski and Kuczynska 1978; Rony and Cohen 1955; Shelley et al. 1959; Unger and Schemmer 1978; Winter et al. 1976). The main problems of systemic corticosteroid treatment are the high doses needed to induce hair regrowth and the necessity to give these high doses for long-term maintenance, thus giving rise to systemic side effects such as acne, obesity, lenticular opacities, hypertension, and suppression of the adrenals. Furthermore, after discontinuation a relapse is frequently seen. Long-standing alopecia areata and alopecia totalis or universalis show a poor response Intradermal Application In an attempt to prevent the side-effects of a long-term oral corticosteroid therapy, Rony and Cohen (1955) first tried intradermal injection of hydrocortisone.
9 Treatment of Alopecia Areata 579 With injections given twice per week the authors were able to induce hair regrowth exclusively in the treated area. This regrowth could be maintained by injection at monthly intervals. The effect of intradermal injection has been studied extensively (Abell and Munro 1973; Gombiner and Malkinson 1961; Orentreich et al. 1960; Porter and Burton 1971; Rony and Cohen 1955; Unger and Schemmer 1978). Even corticosteroid injections in the eyebrow area were tried (Berger 1961). The value of this mode of treatment has been reduced by the possible development of dermal or subcutaneous atrophy at the site of intradermal injections. Furthermore, paralymphatic atrophy of the skin has been observed (Kikuchi and Horikawa 1975). The risk of an acute unilocular amaurosis following intradermal corticosteroid injections (von Bahr 1963; Baran 1964; Selmanowitz and Orentreich 1974) has been eliminated, because preparations with crystals of the size which produced such side effects, are no longer in use Topical Application Some authors reported rather promising results (Kern et al. 1973; Pascher et al. 1970; Unger and Schemmer 1978), but most other reports were less enthusiastic (Burton and Schuster 1975; Frentz 1977; Leyden and Kligman 1972). Besides the questionable therapeutic effect, the value of topically applied corticosteroids is further reduced by the almost unavoidable side effects such as acneiform eruptions Evaluation of the Effect of Corticosteroid Treatment Due to these disadvantages neither systemic nor local corticosteroid therapy of alopecia areata is any longer considered to be of much benefit. Systemic application is not feasible because serious side effects occur when sufficient doses are applied. In alopecia areata of the eye brows or when only a few bald patches are present, an attempt with intradermal injections seems justified. It should, however, be borne in mind that in active disease, new lesions may occur at untreated sites. The side effects must be considered. The topical application of corticosteroid solutions or ointments in alopecia areata should be considered a placebo treatment. In our opinion, hair regrowth seen under this mode of therapy should be ascribed to spontaneous remission. 3.2 Minoxidil Minoxidil is a potent peripheral vasodilatator used as a antihypertensive drug. A side effect which was soon observed is a reversible hypertrichosis occurring in most patients receiving minoxidil for a period of some months (Dargie et al. 1977; Devine et al. 1977). Although there is literature supporting the view that the substance can stimulate hair growth in alopecia areata, there are so far no studies providing unequivocal evidence that minoxidil has any effect on this disease.
10 580 c. Perret and R. Happle In 1981, Weiss et al. treated alopecia areata in three patients with topical application of a 1 % minoxidil solution. Two patients showed some regrowth. In an open study of a larger group treated with 1 % topical minoxidil, 11 out of 48 patients showed a cosmetically acceptable regrowth. Hair growth was considered cosmetically acceptable if it was sufficient to cover the scalp and to conceal any areas of residual hair loss (Weiss et al. 1984). Some of these patients at least showed a spontaneous regrowth, because pronounced hair growth is also seen on the untreated side (!) in the published photographs of a "responder." Moreover, after a 6-month period of follow-up, two-thirds of "responders" showed a relapse. In their study comparing 1 % and 5% minoxidil, they report cosmetically acceptable results in 8% (!) after treatment with 1 % minoxidil and 6% (!) success with 5% minoxidil in patients with at least 75% hair loss (Fiedler-Weiss 1987). A double-blind placebo-controlled study with 3% minoxidil gave cosmetically acceptable hair growth in 3 out of 11 patients in the minoxidil group as compared with 1 out of 14 in the placebo group. This result is not statistically significant (Fisher's exact test: p=0.209) (Price 1987a). In order to obtain higher tissue levels and greater efficacy, Fiedler-Weiss et al. (1987) combined topical therapy (5% minoxidil) with 2 x 5 mg minoxidil per day given orally, resulting in cosmetically acceptable response in 12/65 patients (18%). The best results are reported by Yuan-Pei Shi (1986) who obtained cosmetically acceptable hair regrowth in 14/28 patients treated with an 1 % topical minoxidil preparation. Other groups were not able to induce substantial regrowth. After 64 weeks of treatment with 3% topical minoxidil in 30 patients, Price (1987b) observed no or only slight hair growth in all nine patients with alopecia totalis while finding response rates ("cosmetically acceptable") in 45% of the patients with patchy alopecia areata. As an unpleasant side effect, five patients showed growth of abnormal, pigmented facial hair mainly above and lateral to the eyes and over the malar prominences. Frentz (1985) performed a double-blind cross-over study in 23 patients and reported some increase in terminal hair growth in 13 patients. However, a cosmetically satisfying result was seen in only one patient. Vestey and Savin (1986) did not find any difference between patients treated with placebo or with the active compound. Maitland et al. (1984) did not find any cosmetically acceptable hair growth in a study comprising 28 patients. White and Friedmann (1985) could not induce a therapeutically useful regrowth in a double-blind crossover trial in 15 patients. De Prost et al. (1984) found one complete regrowth in their series of 25 patients with a relapse after discontinuation of the therapy. In sum, topical minoxidil is apparently of no benefit in alopecia areata, regardless of the clinical type of the disease. Systemic therapy, too, has no proven efficacy and may have serious side effects. 3.3 Cyclosporin A Cyclosporin A is a potent cytostatic drug with apparent specificity for the immune system. Therefore, it is now widely used as a potent immunosuppressive
11 Treatment of Alopecia Areata 581 agent in organ transplantation and in disorders predominantly affecting the immune system, such as pemphigus vulgaris, bullous pemphigoid, and systemic lupus erythematosus (Biren and Barr 1986). It enhances the T suppressor cell activity and depresses both the amount and the function oft helper cells. In cyclosporin A treated patients, the ratio of peripheral CDt /CD; cells was found to be 0.9 as compared with 1.9 in a control group (Kahan 1982). Therefore, from a theoretical point of view, cyclosporin A should be effective in alopecia areata. Gebhart and coworkers (1986) reported on a patient with alopecia areata universalis of 40 years duration. He had received a renal allograft and was therefore treated with cyclosporin A. The patient experienced considerable regrowth of eyebrows and scalp hair during this treatment. Gupta and coworkers (1988) treated four patients with severe alopecia areata with oral cyclosporin, 6 mg/kg per day for 12 weeks. Excellent response with cosmetically acceptable hair growth over the scalp was seen in three of four patients; the fourth patient showed only vellus hair growth. The clinical side effects were mild and transient. Liver or renal function tests remained within normal limits. In a placebo-controlled trial using topical cyclosporin (2%) in 43 patients, De Prost et al. (1986) found "terminal hair growth in small tufts" in 7/22 patients of the cyclosporin group. Complete hair regrowth was not observed. In the control group no hair regrowth was seen. As the only side effect, a transient folliculitis, possibly due to the oily excipient, was observed in some patients. The authors could demonstrate a shift in the ratio of CDt /CD; cells from 90% CDt /5% CD; cells prior to therapy to 10% CDt /75% CD; during cyclosporin therapy in one patient. These preliminary studies await confirmation. The long-term side effects of treatment with cyclosporin must be considered carefully. At this point in time, we do not have the impression that this therapeutic approach can be further developed to a safe method available for routine treatment of alopecia areata. 3.4 Inosiplex Inosiplex is a synthetic drug which is said to change functional lymphocyte parameters, such as T -lymphocyte rosette formation and augmentation of mitogendependent and antigen-dependent lymphocyte DNA synthesis. It thus should act as an immunomodulator. The few studies reported comprise small groups of patients. Galbraith et al. (1984) reported some hair regrowth in seven out of their nine patients. All of these showed a prompt relapse after discontinuation. In a randomized double-blind study of inosiplex in alopecia totalis the same group reported some regrowth in 11/20 patients (Galbraith et al. 1987). The authors found a "reduced inflammation" in the perifollicular infiltrates in scalp biopsies obtained during therapy. The exact composition and its changes were not examined. Lowy et al. (1985) reported total or partial regrowth in 9 out of 14 patients treated with inosiplex. Prior to treatment seven of these nine patients had autoantibodies that were reported to decrease or even to disappear under treatment with inosiplex.
12 582 C. Perret and R. Happle 4 Practical Guidelines As shown above, a consistently effective and safe method in treating each form of alopecia areata is sti111acking. Therefore, the decision about the mode of treatment must be individualized for each patient. Patients with extensive forms of alopecia areata often suffer from severe emotional distress. A sympathetic approach and psychological guidance are of utmost importance. We propose the following practical guidelines. 4.1 Evaluation of the Patient's Need for Therapy The most important step is to evaluate the patient's need for therapy. Patients who are well balanced may be helped by prescription of a well-fitting wig and, even more importantly, by the assurance that the hair loss is not a sign of serious internal disease. It should be stressed that spontaneous regrowth may occur at any time. In these patients we tend to refrain from proposing topical immunotherapy or other therapeutic modalities entailing possible side effects. Obviously, this group of patients is comparatively small. Most of the patients ask for a more active approach. 4.2 Treatment of Alopecia Areata in Children In children under 16 years of age, neither topical immunotherapy nor PUV A is feasible. We do not perform an active treatment in this age group. The patients (and their parents) should be reassured that hair regrowth may occur at any time. They should be informed that if this is not the case, therapeutic modalities are available when the patient has become older. If necessary, a well-fitting wig should be prescribed to reduce psychosocial problems. 4.3 Treatment of Mild Alopecia Areata in Adults In view of the high rate of spontaneous remission, we prefer to reassure the patient. He should be told that the risk-benefit ratio of the available therapeutic modalities is unfavorable in this stage of the disease, and that waiting for spontaneous regrowth is by far the best approach. In patients who cannot be convinced of this point of view, a placebo treatment, e.g., topical application of a mild corticosteroid, may be performed. 4.4 Treatment of Extensive Alopecia Areata in Adults In this group of patients, topical immunotherapy is the treatment of choice. The patient should be thoroughly informed about the experimental character of the treatment, the lack of sufficient toxicological data, the chance for regrowth, the
13 Treatment of Alopecia Areata 583 possible side effects, and the possible failure to respond. He should be told that the induction of an allergic contact dermatitis is a desired effect and one that is necessary for a good result. A local ethical committee should be asked for consent. As an alternative, PUV A therapy may be considered. The lower success rate and the need for frequent visits (three times a week for a period of several months) should be taken into account. References Abell E, Munro DD (1973) Intralesional treatment of alopecia areata with triamcinolone acetonide by jet injector. Br J DermatoI88:55-59 Amer MA, EI Garf A (1983) Photochemotherapy and alopecia areata. lnt J DermatoI22: Baran LR (1964) Le risque d'amaurose au cours du traitement local des alopecies par corticotherapie injectable. Bull Soc Fr Dermatol Syphiligr 71:25-28 Berger RA (1961) Alopecia areata of the eyebrows - corticosteroids. Arch Dermatol 83: Biren CA, Barr RJ (1986) Dermatologic application of cyclosporine. Arch DermatoI122: Brocker EB, Echternacht-Happle K, Hamm H, Happle R (1987) Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest DermatoI88: Burton JL, Shuster S (1975) Large doses of glucocorticoid in the treatment of alopecia areata. Acta Derm Venereol (Stockh) 55: Caranzan FR, Bene MC, Faure GC (1987) Peripheral blood T-lymphocyte subsets in the followup of psoriasis patients under PUVA therapy. Photodermatology 4: Case PC, Mitchell AJ, Swanson NA, Vanderveen EE, Ellis CN, Headington JT (1984) Topical therapy of alopecia areata with squaric acid dibutyl ester. J Am Acad Dermatol 10: Claudy AL, Gagnaire D (1983) PUVA treatment of alopecia areata. Arch DermatoI119: Dargie HJ, Dollery CT, Daniel J (1977) Minoxidil in resistant hypertension. Lancet ii: Darvill FT (1963) Steroid therapy in alopecia universalis. A case report and review of the literature. Arch DermatoI87: De Prost Y, Paquez F, Baspeyraz M, Touraine R (1984) Traitement des pelades severes par applications locales de minoxidil. Ann Dermatol Venereolll1:613--{)14 De Prost Y, Teillac D, Paquez F, Carrugi L, Bachelez H, Touraine R (1986) Placebo-controlled trial of topical cyclosporin in severe alopecia areata. Lancet 11: Devine BL, Fife R, Trust PM (1977) Minoxidil for severe hypertension after failure of other hypotensive drugs. Br Med J 2: Dillaha CJ, Rothman S (1952) Treatment of alopecia areata totalis and universalis with cortisone acetate. J Invest DermatoI18:5-6 Fiedler-Weiss VC (1987) Topical minoxidil solution (1 % and 5%) in the treatment of alopecia areata. J Am Acad DermatoI16: Fiedler-Weiss VC, Rumsfield J, Buys CM, West DP, Wendrow A (1987) Evaluation of oral minoxidil in the treatment of alopecia areata. Arch DermatoI123: Frentz G (1977) Topical treatment of extended alopecia. Dermatologica 155: Frentz G (1985) Topical minoxidil for extended areate alopecia. Acta Derm Venereol (Stockh) 65: Friedmann PS (1981) Disappearance of epidermal Langerhans cells during PUVA therapy. Br J DermatoI105: Galbraith GMP, Thiers BH, Fudenberg HH (1984) An open-label trial ofimmunomodulation therapy with inosiplex (Isoprinosine) in patients with alopecia totalis and cell-mediated immunodeficiency. J Am Acad Dermatolll:
14 584 C. Perret and R. Happle Galbraith GMP, Thiers BH, lensen 1, Hoehler F (1987) A randomized double-blind study of inosiplex (Isoprinosine) therapy in patients with alopecia totalis. 1 Am Acad DermatoI16: Gebhart W, Schmidt JB, Schemper M, Spona 1, Kopsa H, Zazgornik 1 (1986) Cyclosporin-Ainduced hair growth in human renal allograft recipients and alopecia areata. Arch Dermatol Res 278: Gombiner A, Malkinson FD (1961) Triamcinolone suspension in alopecia areata. Arch DermatoI83: Gupta AK, Ellis CM, Tellner DC, Goldfarb MT, Voorhees 11 (1988) Treatment of severe alopecia areata with oral cyclosporin. 1 Invest Dermato190:565 Hamm H, Klemmer S, Kreuzer I, Steijlen PM, Happle R, Brocker EB (1988) HLA-DR and HLA-DQ antigen expression of anagen and telogen hair bulbs in long-standing alopecia areata. Arch Dermatol Res 280: Happle R (1979) DNCB-Therapie der Alopecia areata. Z Hautkr 54: Happle R (1980) Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res 267: Happle R (1985a) The potential hazards of dinitrochlorobenzene. Arch Dermato1121: Happle R (1985b) Immunologische Behandlung der Alopecia areata. Hautarzt 36 (Suppl VII): Happle R (1986) Alopecia areata und Alopecia totalis: Pathogenese und topische Immuntherapie mit Diphencyprone. Therapiewoche 36: Happle R, Echternacht K (1977) Induction of hair growth in alopecia areata with DNCB. Lancet II: Happle R, Cebulla K, Echternacht-Happle K (1978) Dinitrochlorobenzene therapy for alopecia areata. Arch Dermatol114: Happle R, Kalveram Kl, Buchner U, Echternacht-Happle K, Goggelmann W, Summer KH (1980) Contact allergy as a therapeutic tool for alopecia areata: application of squaric acid dibutylester. Dermatologica 161: Happle R, Hausen BM, Wiesner-Menzel L (1983) Diphencyprone in the treatment of alopecia areata. Acta Derm Venereol (Stockh) 63:49-52 Happle R, Klein HM, Macher E (1986) Topical immunotherapy changes the composition of the peribulbar infiltrate in alopecia areata. Arch Dermatol Res 278: Kahan BD (1982) Cyclosporin A: a selective anti-t cell agent. Clin HaematoI11: Kern F, Hoffmann WH, Hambrick GW, Blizzard RM (1973) Alopecia areata. Immunologic studies and treatment with prednisone. Arch Dermatoll07: Khoury EL, Price VH, Wong EL, Greenspan ls (1986) HLA-DR expression by hair follicle epithelial cells in alopecia areata: evidence that it is secondary to the lymphoid infiltration. 1 Invest Dermatol 86:484 Kietzmann H, Hardung H, Christophers E (1985) Therapie der Alopecia areata mit Diphenylcyclopropenon. Hautarzt 36: Kikuchi I, Horikawa S (1975) Perilymphatic atrophy of the skin. Arch Dermatoll11: Kohchiyama A, Hatamochi A, Ueki H (1985) Increased number ofokt6-positive dendritic cells in the hair follicles of patients with alopecia areata. Dermatologica 171 : Lassus A, Kuanto U, 10hansson E, luvakoski T (1980) PUVA treatment for alopecia areata. Dermatologica 161: Leyden 11, Kligman AM (1972) Treatment of alopecia areata with steroid solution. Arch Dermatol 106:924 Lowy M, Ledoux-Corbusier M, Achten G, Wybran 1 (1985) Clinical and immunologic response to Isoprinosine in alopecia areata and alopecia universalis: association with autoantibodies. 1 Am Acad DermatoI12:78-84 MacDonald-Hull S, Norris IF (1988) Diphencyprone in the treatment oflong-standing alopecia areata. Br 1 DermatoI119: Maitland 1M, Aldridge RD, Main RA, White Ml, Ormerod AD (1984) Topical minoxidil in the treatment of alopecia areata. Br Med 1 288: Martin ES (1984) Treatment of alopecia areata profiting from "natural" allergy to nickel. Arch DermatoI120:
15 Treatment of Alopecia Areata 585 Messenger AG, Bleehen SS (1985) Expression of HLA-DR by anagen hair follicles in alopecia areata. J Invest DermatoI85: Michalowski R, Kuczynska L (1978) Long-term intramuscular triamcinolon-acetonide therapy in alopecia areata totalis and universalis. Arch Derm Res 261:73-76 Monk B, Williams HC (1988) Topical diphencyprone therapy in alopecia totalis. Br J Dermatol 119 (SuppI33):16-17 Mork NJ, Gaudernack G, Braathen LR (1987) Effect ofuva and PUVA on allo-activating and antigen-presenting capacity of human epidermal Langerhans cells. Photodermatology 4:66-72 Nakano Y (1977) Antigenic competition in the induction of contact sensitivity in mice. Immunology 33 : Ochsendorf FR, Mitron G, Milbradt R (1988) Treatment of alopecia areata with diphenylcyclopropenone. Z Hautkr 63: Orecchia G, Douville H (1988) A 3-year treatment of alopecia areata with squaric acid dibutylester. Ann 1st Derm Clin Sper 42: Orentreich N, Sturm HM, Weidman AI, Pelzig A (1960) Local injection of steroids and hair regrowth in alopecias. Arch Dermatol 82: Pascher F, Kurtin S, Andrade R (1970) Assay of 0.2% fluocinolone acetonide cream for alopecia areata and totalis. Dermatologica 141: Peereboom-Wynia JDR, van Joost T, Stolz E, Prins MEF (1986) Markers of immunologic injury in progressive alopecia areata. J Cutan PathoI13: Perret C, Brocker EB, Wiesner-Menzel L, Happle R (1982) In situ demonstration of T cells in alopecia areata. Arch Dermatol Res 273: Perret C, Wiesner-Menzel L, Happle R (1984) Immunohistochemical analysis oft-cell subsets in the peri bulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol (Stockh) 64:26-30 Perret CM, Steijlen PM, Happle R (1990, in press) Alopecia areata - pathogenesis and topical immunotherapy. Int J Dermatol (in press) Porter D, Burton JL (1971) A comparison of intra-lesional triamcinolone hexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol 85: Price VH (1987 a) Double-blind, placebo-controlled evaluation of topical minoxidil in extensive alopecia areata. J Am Acad Dermato116: Price VH (1987b) Topical minoxidil (3%) in extensive alopecia areata, including long-term efficacy. J Am Acad DermatoI16: Rhodes EL, Dolman W, Kennedy C, Taylor RR (1981) Alopecia areata regrowth induced by primula obconica. Br J DermatoI104: Rollier R, Warcewski Z (1974) Le traitement de la pelade par la Meladinine. Bull Soc Fr Dermatol Syphiligr 81 :97 Rony JR, Cohen DM (1955) The effect of cortisone in alopecia areata. J Invest DermatoI25: Rosenberg E, Drake L (1976) Discussion of Dunaway DA. Alopecia areata. Arch Dermatol 112:256 Selmanowitz VJ, Orentreich N (1974) Cutaneous corticosteroid injection and amaurosis. Arch DermatoI110: Shelley WB, Hamn JS, Lehman JM (1959) Long-term triamcinolone therapy of alopecia universalis. Arch Dermatol 80:91/433-93/435 Todes-Taylor N, Turner R, Wood GS, Stratte PT, Morhenn VB (1984) T cell subpopulations in alopecia areata. J Am Acad DermatoI11: Unger WP, Schemmer RJ (1978) Corticosteroids in the treatment of alopecia totalis. Arch DermatoI114: Valsecchi R, Cainelli T, Foiadelli L, Rossi A (1986) Topical immunotherapy of alopecia areata. A follow-up study. Acta Derm VenereoI66: Vestey JP, Savin J A (1986) A trial of 1 % minoxidil used topically for severe alopecia areata. Acta Derm Venereol (Stockh) 66: von Bahr G (1963) Multiple embolisms in the fundus of an eye after an injection in the scalp. Acta OpthalmoI41:85-91
16 586 C. Perret and R. Happle: Treatment of Alopecia Areata Weiss VC, West DP, Mueller CE (1981) Topical minoxidil in alopecia areata. JAm Acad Dermatol 5: Weiss VC, West DP, Fu TS Robinson LA, Cook B, Cohen RL, Chambers DA (1984) Alopecia areata treated with topical minoxidil. Arch DermatoI120: Weissmann I, Hofmann C, Wagner G, Plewig G, Braun-Falco 0 (1978) PUVA-therapy for alopecia areata. An investigative study. Arch Dermatol Res 262: White SJ, Friedmann PS (1985) Topical minoxidillacks efficacy in alopecia areata. Arch Dermato1121:591 Wiesner-Menzel L, Happle R (1984) Intrabulbar and peribulbar accumulation of dentritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res 276: Winter RJ, Kern F, Blizzard RM (1976) Prednisone therapy for alopecia areata. A follow-up report. Arch DermatoI112: Yuan-Pei Shi (1986) Topical minoxidil in the treatment of alopecia areata and male-pattern alopecia. Arch Dermato1122:506
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/22370
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/22370
Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata
 Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
OBSERVATION. Predictive Model for Immunotherapy of Alopecia Areata With Diphencyprone
 OBSERVATION Predictive Model for Immunotherapy of Alopecia Areata With Diphencyprone Marni C. Wiseman, MD, FRCPC; Jerry Shapiro, MD, FRCPC; Nina MacDonald, RN, BScN; Harvey Lui, MD, FRCPC Background: Immunotherapy
OBSERVATION Predictive Model for Immunotherapy of Alopecia Areata With Diphencyprone Marni C. Wiseman, MD, FRCPC; Jerry Shapiro, MD, FRCPC; Nina MacDonald, RN, BScN; Harvey Lui, MD, FRCPC Background: Immunotherapy
Successful Treatment of Alopecia Areata-Like Hair Loss with the Contact Sensitizer Squaric Acid Dibutylester (SADBE) in C3H/HeJ Mice
 Successful Treatment of Alopecia Areata-Like Hair Loss with the Contact Sensitizer Squaric Acid Dibutylester (SADBE) in C3H/HeJ Mice Pia Freyschmidt-Paul, John P. Sundberg,* Rudolf Happle, Kevin J. McElwee,*
Successful Treatment of Alopecia Areata-Like Hair Loss with the Contact Sensitizer Squaric Acid Dibutylester (SADBE) in C3H/HeJ Mice Pia Freyschmidt-Paul, John P. Sundberg,* Rudolf Happle, Kevin J. McElwee,*
Ann Dermatol Vol. 25, No. 1,
 Ann Dermatol Vol. 25, No. 1, 2013 http://dx.doi.org/10.5021/ad.2013.25.1.12 ORIGINAL ARTICLE Combination Therapy with Cyclosporine and Psoralen Plus Ultraviolet A in the Patients with Severe Alopecia Areata:
Ann Dermatol Vol. 25, No. 1, 2013 http://dx.doi.org/10.5021/ad.2013.25.1.12 ORIGINAL ARTICLE Combination Therapy with Cyclosporine and Psoralen Plus Ultraviolet A in the Patients with Severe Alopecia Areata:
Diphencyprone (DCP) treatment
 Page 1 of 5 Diphencyprone (DCP) treatment Introduction This leaflet gives you information about Diphencyprone (DCP) treatment for alopecia areata and answers some of the commonly asked questions. What
Page 1 of 5 Diphencyprone (DCP) treatment Introduction This leaflet gives you information about Diphencyprone (DCP) treatment for alopecia areata and answers some of the commonly asked questions. What
REVIEW Alopecia areata: Treatment options
 REVIEW Alopecia areata: Treatment options E. Kasumagić-Halilović, A. Prohić Department of Dermatovenerology, University Clinical Center, Sarajevo, Bosnia anad Herzegovina ABSTRACT Alopecia areata (AA)
REVIEW Alopecia areata: Treatment options E. Kasumagić-Halilović, A. Prohić Department of Dermatovenerology, University Clinical Center, Sarajevo, Bosnia anad Herzegovina ABSTRACT Alopecia areata (AA)
Ann Dermatol Vol. 27, No. 6,
 IK Yeo, et al Ann Dermatol Vol. 27, No. 6, 2015 http://dx.doi.org/10.5021/ad.2015.27.6.676 ORIGINAL ARTICLE Comparison of High-Dose Corticosteroid Pulse Therapy and Combination Therapy Using Oral Cyclosporine
IK Yeo, et al Ann Dermatol Vol. 27, No. 6, 2015 http://dx.doi.org/10.5021/ad.2015.27.6.676 ORIGINAL ARTICLE Comparison of High-Dose Corticosteroid Pulse Therapy and Combination Therapy Using Oral Cyclosporine
Corresponding author: Prof. Katarzyna Borowska, MD, PhD.,
 Our Dermatology Online Efficacy and tolerance of 2,3-diphenylcyclopropenone in propylene glycol versus 2,3-diphenylcyclopropenone in isopropanol - a novel formula designed for the treatment of alopecia
Our Dermatology Online Efficacy and tolerance of 2,3-diphenylcyclopropenone in propylene glycol versus 2,3-diphenylcyclopropenone in isopropanol - a novel formula designed for the treatment of alopecia
CLINICAL REVIEW. Management of alopecia areata. M J Harries, 1 J Sun, 2 3 R Paus, 1 4 L E King 5. For the full versions of these articles see bmj.
 For the full versions of these articles see bmj.com Management of alopecia areata M J Harries, 1 J Sun, 2 3 R Paus, 1 4 L E King 5 1 Epithelial Sciences, School of Translational Medicine, University of
For the full versions of these articles see bmj.com Management of alopecia areata M J Harries, 1 J Sun, 2 3 R Paus, 1 4 L E King 5 1 Epithelial Sciences, School of Translational Medicine, University of
A Comparative Study of Oral Cyclosporine and Betamethasone Minipulse Therapy in the Treatment of Alopecia Areata
 Cyclosporine and Steroid Minipulse Therapy in AA pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 5, 2016 http://dx.doi.org/10.5021/ad.2016.28.5.569 ORIGINAL ARTICLE A Comparative Study of Oral
Cyclosporine and Steroid Minipulse Therapy in AA pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 5, 2016 http://dx.doi.org/10.5021/ad.2016.28.5.569 ORIGINAL ARTICLE A Comparative Study of Oral
STUDY. Histopathologic Features of Alopecia Areata
 Histopathologic Features of Alopecia Areata A New Look David A. Whiting, MD, FRCP(Edin) STUDY Background: A peribulbar lymphocytic infiltrate is the expected histologic feature of alopecia areata, but
Histopathologic Features of Alopecia Areata A New Look David A. Whiting, MD, FRCP(Edin) STUDY Background: A peribulbar lymphocytic infiltrate is the expected histologic feature of alopecia areata, but
Autoimmune Hair Loss (Alopecia Areata) Transferred by T Lymphocytes to Human Scalp Explants on SCID Mice
 Autoimmune Hair Loss (Alopecia Areata) Transferred by T Lymphocytes to Human Scalp Explants on SCID Mice Amos Gilhar, Yehuda Ullmann, Tamara Berkutzki, Bedia Assy, and Richard S. Kalish* Skin Research
Autoimmune Hair Loss (Alopecia Areata) Transferred by T Lymphocytes to Human Scalp Explants on SCID Mice Amos Gilhar, Yehuda Ullmann, Tamara Berkutzki, Bedia Assy, and Richard S. Kalish* Skin Research
INTRALESIONAL PLATELET RICH PLASMA vs INTRALESIONAL TRIAMCINOLONE IN THE TREATMENT OF ALOPECIA AREATA: A COMPARATIVE STUDY
 International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 4 Issue 1 Coden: IJMRHS Copyright @214 ISSN: 2319-5886 Received: 3 rd Oct 214 Revised: 7 th Dec 214 Accepted: 17 th Dec
International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 4 Issue 1 Coden: IJMRHS Copyright @214 ISSN: 2319-5886 Received: 3 rd Oct 214 Revised: 7 th Dec 214 Accepted: 17 th Dec
Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls
 Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls U033 July 30 th 2017 7:30-8:30AM Paradi Mirmirani, MD The Permanente Medical Group, Vallejo, CA Regional Director, Hair Disorders
Diagnosis and Management of the Hair Loss Patient: Pearls and Pitfalls U033 July 30 th 2017 7:30-8:30AM Paradi Mirmirani, MD The Permanente Medical Group, Vallejo, CA Regional Director, Hair Disorders
Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment of Periungual Warts
 Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Snapshot Dx Quiz: September 2018 Detailed Answers
 Snapshot Dx Quiz: September 2018 Detailed Answers Cynthia X. Wang, BA 1 Milan J. Anadkat, MD 1,2 1 Washington University School of Medicine, St. Louis, Missouri 2 Division of Dermatology, St. Louis, Missouri
Snapshot Dx Quiz: September 2018 Detailed Answers Cynthia X. Wang, BA 1 Milan J. Anadkat, MD 1,2 1 Washington University School of Medicine, St. Louis, Missouri 2 Division of Dermatology, St. Louis, Missouri
Treatment Options in Alopecia Areata
 Chapter 09 Treatment Options in Alopecia Areata Nilgun Senturk* Department of Dermatology, Ondokuz Mayis University School of Medicine, Turkey * Corresponding Author: Nilgun Senturk, Department of Dermatology,
Chapter 09 Treatment Options in Alopecia Areata Nilgun Senturk* Department of Dermatology, Ondokuz Mayis University School of Medicine, Turkey * Corresponding Author: Nilgun Senturk, Department of Dermatology,
6. Contact allergic reactions in patients with atopic eczema
 Acta Derm Venereol 2005; Suppl. 215: 28 32 6. Contact allergic reactions in patients with atopic eczema JOHN MCFADDEN Medicament allergy is a not uncommon problem both with antibiotics and topical corticosteroids.
Acta Derm Venereol 2005; Suppl. 215: 28 32 6. Contact allergic reactions in patients with atopic eczema JOHN MCFADDEN Medicament allergy is a not uncommon problem both with antibiotics and topical corticosteroids.
GENERAL OVERVIEW OF TYPES OF HAIR LOSS AND ALOPECIA TELOGEN EFFLUVIUM
 GENERAL OVERVIEW OF TYPES OF HAIR LOSS AND ALOPECIA TELOGEN EFFLUVIUM Telogen effluvium is a form of diffuse hair loss that occurs during the telogen or resting phase of the hair growth cycle. Telogen
GENERAL OVERVIEW OF TYPES OF HAIR LOSS AND ALOPECIA TELOGEN EFFLUVIUM Telogen effluvium is a form of diffuse hair loss that occurs during the telogen or resting phase of the hair growth cycle. Telogen
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Diphencyprone (DPC) treatment for Alopecia Areata
 University Teaching Trust Diphencyprone (DPC) treatment for Alopecia Areata Irving Building Dermatology 0161 206 1846 All Rights Reserved 2017. Document for issue as handout. What is diphencyprone treatment?
University Teaching Trust Diphencyprone (DPC) treatment for Alopecia Areata Irving Building Dermatology 0161 206 1846 All Rights Reserved 2017. Document for issue as handout. What is diphencyprone treatment?
Antonella Tosti Fredric Brandt Endowed Professor of Dermatology & Cutaneous Surgery
 Dermoscopy in the evaluation and treatment of hair loss Antonella Tosti Fredric Brandt Endowed Professor of Dermatology & Cutaneous Surgery DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Antonella Tosti, MD
Dermoscopy in the evaluation and treatment of hair loss Antonella Tosti Fredric Brandt Endowed Professor of Dermatology & Cutaneous Surgery DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Antonella Tosti, MD
Clinical Policy Title: Alopecia areata
 Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21,2016 Next Review Date:
Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21,2016 Next Review Date:
Topical immunomodulation. Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University
 Topical immunomodulation Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University Vitiligo Atopic dermatitis Seborrheic dermatitis Oral lichen planus Anogenital warts Bowen s disease actinic
Topical immunomodulation Charoen Choonhakarn,MD Division of Dermatology Khon Kaen University Vitiligo Atopic dermatitis Seborrheic dermatitis Oral lichen planus Anogenital warts Bowen s disease actinic
Clinical Policy Title: Alopecia areata
 Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next Review Date:
Clinical Policy Title: Alopecia areata Clinical Policy Number: 16.02.03 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next Review Date:
Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])
![Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV]) Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])](/thumbs/89/99382632.jpg) Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
RESENT TREATMENT OF ALOPECIA AREATA
 RESENT TREATMENT OF ALOPECIA AREATA Dr. Simant Ankit 1* and Prof. Dr. Tongxiang Zeng 1 1 Department of Dermatology & Venereology, Jingzhou Central Hospital, Yangtze University, Jingzhou, Hubei, China ABSTRACT
RESENT TREATMENT OF ALOPECIA AREATA Dr. Simant Ankit 1* and Prof. Dr. Tongxiang Zeng 1 1 Department of Dermatology & Venereology, Jingzhou Central Hospital, Yangtze University, Jingzhou, Hubei, China ABSTRACT
During the last 20 years, the number of topical
 THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
THERAPEUTICS FOR THE CLINICIAN Cumulative Irritation Potential of Adapalene 0.1% Cream and Gel Compared With Tretinoin Microsphere 0.04% and 0.1% Jonathan S. Dosik, MD; Kenneth Homer, MS; Stéphanie Arsonnaud
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Adhesive tape impression 1 Diff Quik in EPD diagnosis, 583 Allergy(ies). See also Food allergy; specific types, e.g., Culicoides hypersensitivity
Note: Page numbers of article titles are in boldface type. A Adhesive tape impression 1 Diff Quik in EPD diagnosis, 583 Allergy(ies). See also Food allergy; specific types, e.g., Culicoides hypersensitivity
Dermatology GP Referral Guidelines
 Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
in alopeeia areata are not entirely defined. Sabouraud (1) made the fundamental observation
 MORPHOLOGIC CHANGES IN PILOSEBACEOUS UNITS AND ANAGEN HAIRS IN ALOPECIA AREATA* The normal growth of scalp hair is a result of a continuous, rapid proliferation of tissue by the matrix of the hair bulb
MORPHOLOGIC CHANGES IN PILOSEBACEOUS UNITS AND ANAGEN HAIRS IN ALOPECIA AREATA* The normal growth of scalp hair is a result of a continuous, rapid proliferation of tissue by the matrix of the hair bulb
The New England Journal of Medicine. Review Article. Pathophysiology. Treatment in Men
 The New England Journal of Medicine Review Article Drug Therapy A LASTAIR J.J. WOOD, M.D., Editor TREATMENT OF HAIR LOSS VERA H. PRICE, M.D. HAIR loss is a common and distressing symptom. With the approval
The New England Journal of Medicine Review Article Drug Therapy A LASTAIR J.J. WOOD, M.D., Editor TREATMENT OF HAIR LOSS VERA H. PRICE, M.D. HAIR loss is a common and distressing symptom. With the approval
Topical Immunomodulator Step Therapy Program
 Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Clinical Policy Title: Alopecia areata
 Clinical Policy Title: Alopecia areata Clinical Policy Number: CCP.1134 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: August 1, 2018 Next Review Date:
Clinical Policy Title: Alopecia areata Clinical Policy Number: CCP.1134 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: August 1, 2018 Next Review Date:
Review Article. Management of alopecia areata: an update. Imran Majid and Abid Keen. BJMP 2012;5(3):a530. Introduction
 BJMP 2012;5(3):a530 Review Article Management of alopecia areata: an update Imran Majid and Abid Keen Abstract Alopecia areata is a common, non-scarring, autoimmune disorder affecting any hair-bearing
BJMP 2012;5(3):a530 Review Article Management of alopecia areata: an update Imran Majid and Abid Keen Abstract Alopecia areata is a common, non-scarring, autoimmune disorder affecting any hair-bearing
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
CHAPTER 22 Brocq s alopecia (pseudopelade of Brocq) and burnt out scarring alopecia
 CHAPTER 22 Brocq s alopecia (pseudopelade of Brocq) and burnt out scarring alopecia BROCQ S ALOPECIA The term pseudopelade of Brocq is a source of much confusion and fruitless debate, and should be abandoned.
CHAPTER 22 Brocq s alopecia (pseudopelade of Brocq) and burnt out scarring alopecia BROCQ S ALOPECIA The term pseudopelade of Brocq is a source of much confusion and fruitless debate, and should be abandoned.
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Triamcinolone and vitiligo
 P ford residence southampton, ny Triamcinolone and vitiligo Dermatologica. 1970;140(3):195-206. Treatment of localized vitiligo with intradermal injections of triamcinolone acetonide. Kandil E. PMID: 5414362;
P ford residence southampton, ny Triamcinolone and vitiligo Dermatologica. 1970;140(3):195-206. Treatment of localized vitiligo with intradermal injections of triamcinolone acetonide. Kandil E. PMID: 5414362;
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma
 An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
A case of rosacea fulminans in a pregnant woman
 Hong Kong J. Dermatol. Venereol. (2018) 26, 122-126 Views and Practice A case of rosacea fulminans in a pregnant woman JE Seol, SH Park, JU Kim, GJ Cho, SH Moon, H Kim Introduction Rosacea fulminans (RF)
Hong Kong J. Dermatol. Venereol. (2018) 26, 122-126 Views and Practice A case of rosacea fulminans in a pregnant woman JE Seol, SH Park, JU Kim, GJ Cho, SH Moon, H Kim Introduction Rosacea fulminans (RF)
What's New in Oncodermatopathology: Immunotherapy Reactions
 What's New in Oncodermatopathology: Immunotherapy Reactions Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania March
What's New in Oncodermatopathology: Immunotherapy Reactions Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania March
Clinico Pathological Test SCPA605-Essential Pathology
 Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
Research Article Angiotensin Converting Enzyme Activity in Alopecia Areata
 Enzyme Research Volume 2014, Article ID 694148, 4 pages http://dx.doi.org/10.1155/2014/694148 Research Article Angiotensin Converting Enzyme Activity in Alopecia Areata Mohammad Reza Namazi, 1,2,3 Armaghan
Enzyme Research Volume 2014, Article ID 694148, 4 pages http://dx.doi.org/10.1155/2014/694148 Research Article Angiotensin Converting Enzyme Activity in Alopecia Areata Mohammad Reza Namazi, 1,2,3 Armaghan
TrichoScan as a Method to Determine Hair Root Pattern in Patients with Scalp Psoriasis
 2010;18(3):146-1 CLINICAL ARTICLE TrichoScan as a Method to Determine Hair Root Pattern in Patients with Scalp Psoriasis Emina Kasumagić-Halilović 1, Asja Prohić 1, Begler Begović 2 1 University Department
2010;18(3):146-1 CLINICAL ARTICLE TrichoScan as a Method to Determine Hair Root Pattern in Patients with Scalp Psoriasis Emina Kasumagić-Halilović 1, Asja Prohić 1, Begler Begović 2 1 University Department
five series of cases were studied. Many of the patients treated by one
 PHOTOSENSITIZATION IN THE TREATMENT OF PSORIASIS' ERVIN EPSTEIN, M.D. Oakland, Calif. Reviewing the symposium on the "Practical Management of Psoriasis" (1) one is impressed by the popularity gained by
PHOTOSENSITIZATION IN THE TREATMENT OF PSORIASIS' ERVIN EPSTEIN, M.D. Oakland, Calif. Reviewing the symposium on the "Practical Management of Psoriasis" (1) one is impressed by the popularity gained by
A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients
 Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Alopecia areata (AA) is regarded as a nonscarring, in-
 The Pathogenesis of Alopecia Areata in Rodent Models REVIEW ARTICLE Kevin J. McElwee, Pia Freyschmidt-Paul, John P. Sundberg n and Rolf Ho mann Department of Dermatology, Philipp University, Marburg, Germany.
The Pathogenesis of Alopecia Areata in Rodent Models REVIEW ARTICLE Kevin J. McElwee, Pia Freyschmidt-Paul, John P. Sundberg n and Rolf Ho mann Department of Dermatology, Philipp University, Marburg, Germany.
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,350 108,000 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,350 108,000 1.7 M Open access books available International authors and editors Downloads Our
Ciclosporin Microemulsion for Severe Atopic Dermatitis: Experience on Adolescents and Adults in Hong Kong
 ORIGINAL ARTICLES Ciclosporin Microemulsion for Severe Atopic Dermatitis: Experience on Adolescents and Adults in Hong Kong Drs. H. F. Ho, L.Y. Chong, K. M. Ho and W. K. Fung Social Hygiene Service (Dermatology),
ORIGINAL ARTICLES Ciclosporin Microemulsion for Severe Atopic Dermatitis: Experience on Adolescents and Adults in Hong Kong Drs. H. F. Ho, L.Y. Chong, K. M. Ho and W. K. Fung Social Hygiene Service (Dermatology),
Complete remission of alopecia universalis after allogeneic. hematopoetic stem cell transplantation.
 Blood First Edition From www.bloodjournal.org Paper, prepublished by guest online April April 29, 2018. 8, 2004; For personal DOI 10.1182/blood-2004-01-0136 use only. Complete remission of alopecia universalis
Blood First Edition From www.bloodjournal.org Paper, prepublished by guest online April April 29, 2018. 8, 2004; For personal DOI 10.1182/blood-2004-01-0136 use only. Complete remission of alopecia universalis
Tips on getting the most from your alopecia pathology reports. D irector, H a ir C linic, Boston Medical C e n ter
 Tips on getting the most from your alopecia pathology reports Lynne J. Goldberg, MD J a g Bhawan Professor o f Dermatology a n d Pathology & Laboratory Medicine Boston U n iversity School of Medicine D
Tips on getting the most from your alopecia pathology reports Lynne J. Goldberg, MD J a g Bhawan Professor o f Dermatology a n d Pathology & Laboratory Medicine Boston U n iversity School of Medicine D
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
How to decipher a pathology report for alopecia
 How to decipher a pathology report for alopecia DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Lynne J. Goldberg, MD S063-Hair Disorders Made Easier DISCLOSURES I do not have any relationships with industry
How to decipher a pathology report for alopecia DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Lynne J. Goldberg, MD S063-Hair Disorders Made Easier DISCLOSURES I do not have any relationships with industry
Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study
 Report Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study Laita Bokhari, MPhil Med, and Rodney Sinclair, MBBS, MD, FACD Sinclair
Report Treatment of alopecia universalis with topical Janus kinase inhibitors a double blind, placebo, and active controlled pilot study Laita Bokhari, MPhil Med, and Rodney Sinclair, MBBS, MD, FACD Sinclair
Clinical profile of skin diseases in accident and emergency department attenders
 Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Tips on Evaluation and Diagnosis of Scarring Alopecias. Melissa Peck Piliang, MD Dermatology and Anatomic Pathology Cleveland Clinic
 Tips on Evaluation and Diagnosis of Scarring Alopecias Melissa Peck Piliang, MD Dermatology and Anatomic Pathology Cleveland Clinic Disclosures I do not have any relevant relationships with industry Investigator:
Tips on Evaluation and Diagnosis of Scarring Alopecias Melissa Peck Piliang, MD Dermatology and Anatomic Pathology Cleveland Clinic Disclosures I do not have any relevant relationships with industry Investigator:
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP)
 CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISONE AND ITS ANALOGUES
 CORTISONE AND ITS ANALOGUES IN DERMATOLOGY By G. C. WELLS, M.B., M.R.C.P. Senior Lecturer in Dermatology, St. John's Hospital for Diseases of the Skin, London Dermatology is one of the branches of medicine
CORTISONE AND ITS ANALOGUES IN DERMATOLOGY By G. C. WELLS, M.B., M.R.C.P. Senior Lecturer in Dermatology, St. John's Hospital for Diseases of the Skin, London Dermatology is one of the branches of medicine
DERMATOP Ointment (prednicarbate ointment) 0.1% FOR DERMATOLOGIC USE ONLY. NOT FOR USE IN EYES.
 DERMATP intment (prednicarbate ointment) 0.1% FR DERMATLGIC USE NLY. NT FR USE IN EYES. DESCRIPTIN DERMATP intment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate.
DERMATP intment (prednicarbate ointment) 0.1% FR DERMATLGIC USE NLY. NT FR USE IN EYES. DESCRIPTIN DERMATP intment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate.
Dr. Yi-chi M. Kong August 8, 2001 Benjamini. Ch. 19, Pgs Page 1 of 10 TRANSPLANTATION
 Benjamini. Ch. 19, Pgs 379-399 Page 1 of 10 TRANSPLANTATION I. KINDS OF GRAFTS II. RELATIONSHIPS BETWEEN DONOR AND RECIPIENT Benjamini. Ch. 19, Pgs 379-399 Page 2 of 10 II.GRAFT REJECTION IS IMMUNOLOGIC
Benjamini. Ch. 19, Pgs 379-399 Page 1 of 10 TRANSPLANTATION I. KINDS OF GRAFTS II. RELATIONSHIPS BETWEEN DONOR AND RECIPIENT Benjamini. Ch. 19, Pgs 379-399 Page 2 of 10 II.GRAFT REJECTION IS IMMUNOLOGIC
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Vitiligo is a skin depigmentation disorder
 THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and. hydrocortisone ointment, USP)
 CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
What Are the Different Forms?
 SCARRING/CICATRICIAL ALOPECIA Scarring alopecia also known as cicatricial alopecia, refers to a collection of hair loss disorders that may be diagnosed in up to 3% of hair loss patients. It occurs worldwide
SCARRING/CICATRICIAL ALOPECIA Scarring alopecia also known as cicatricial alopecia, refers to a collection of hair loss disorders that may be diagnosed in up to 3% of hair loss patients. It occurs worldwide
Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia
 doi:./j.46-88.2.78.x Journal of Dermatology 22; 9: 27 2 ORIGINAL ARTICLE Evaluation of efficacy and safety of finasteride mg in 77 Japanese men with androgenetic alopecia Akio SATO, Akira TAKEDA 2 Tokyo
doi:./j.46-88.2.78.x Journal of Dermatology 22; 9: 27 2 ORIGINAL ARTICLE Evaluation of efficacy and safety of finasteride mg in 77 Japanese men with androgenetic alopecia Akio SATO, Akira TAKEDA 2 Tokyo
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
What is atopic dermatitis?
 What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
A Vitiligo Update for Pharmacists: Current Practices and Future Advances
 A Vitiligo Update for Pharmacists: Current Practices and Future Advances Dalal Hammoudi Halat, RPh, MSc, PhD Assistant Professor School of Pharmacy, Lebanese International University Disclosure Dalal Hammoudi
A Vitiligo Update for Pharmacists: Current Practices and Future Advances Dalal Hammoudi Halat, RPh, MSc, PhD Assistant Professor School of Pharmacy, Lebanese International University Disclosure Dalal Hammoudi
Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis
 Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Each gram of the ointment contains 0.25 mg Fluocinolone Acetonide in a base containing White Petrolatum.
 FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
AMERICAN JOURNAL OF BIOLOGICAL AND PHARMACEUTICAL RESEARCH
 AMERICAN JOURNAL OF BIOLOGICAL AND PHARMACEUTICAL RESEARCH e-issn - 2348-2184 Print ISSN - 2348-2176 Journal homepage: www.mcmed.us/journal/ajbpr A CURRENT VIEW ON ALOPECIA AREATA J. Dastagiri, M. Shankar*,
AMERICAN JOURNAL OF BIOLOGICAL AND PHARMACEUTICAL RESEARCH e-issn - 2348-2184 Print ISSN - 2348-2176 Journal homepage: www.mcmed.us/journal/ajbpr A CURRENT VIEW ON ALOPECIA AREATA J. Dastagiri, M. Shankar*,
Additional file 2: Details of cohort studies and randomised trials
 Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
DESCRIPTIONS FOR MED 3 ROTATIONS Dermatology A3S
 Regardless of your future field of practice, you will be exposed to a considerable amount of dermatology and this rotation provides you the chance to see a range of skin diseases. You will have the opportunity
Regardless of your future field of practice, you will be exposed to a considerable amount of dermatology and this rotation provides you the chance to see a range of skin diseases. You will have the opportunity
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
IN THE TREATMENT OF SYSTEMIC LUPUS ERYTHEMATOSUS
 THE ROLE OF ACTH AND CORTISONE IN THE TREATMENT OF SYSTEMIC LUPUS ERYTHEMATOSUS The steroids ACTH and cortisone have now been used in the treatment of systemic lupus erythematosus for over five years.
THE ROLE OF ACTH AND CORTISONE IN THE TREATMENT OF SYSTEMIC LUPUS ERYTHEMATOSUS The steroids ACTH and cortisone have now been used in the treatment of systemic lupus erythematosus for over five years.
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil)
 Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
NIH Public Access Author Manuscript J Invest Dermatol. Author manuscript; available in PMC 2015 February 01.
 NIH Public Access Author Manuscript Published in final edited form as: J Invest Dermatol. 2014 August ; 134(8): 2071 2074. doi:10.1038/jid.2014.141. A possible role for IL-17A in establishing Th2 inflammation
NIH Public Access Author Manuscript Published in final edited form as: J Invest Dermatol. 2014 August ; 134(8): 2071 2074. doi:10.1038/jid.2014.141. A possible role for IL-17A in establishing Th2 inflammation
Dutasteride female pattern hair loss management 49, 50 male baldness management 41, 42 Dyeing, see Hair care
 Subject Index Acne keloidalis nuchae, ethnic patients 146, 147 Actinic keratosis, ultraviolet radiation exposure 108 Aging hair effects 4, 5 melanocyte aging 130 132 molecular mechanisms 5 7 pigment loss,
Subject Index Acne keloidalis nuchae, ethnic patients 146, 147 Actinic keratosis, ultraviolet radiation exposure 108 Aging hair effects 4, 5 melanocyte aging 130 132 molecular mechanisms 5 7 pigment loss,
Dermatopathology Workshop Summary, Berlin 2004
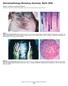 Dermatopathology Workshop Summary, Berlin 2004 David A. Whiting and Rolf Hoffmannw Baylor Hair Research and Treatment Center, Dallas, Texas, USA; wdermatology Practice, Freiburg, Germany Figure 1 Case
Dermatopathology Workshop Summary, Berlin 2004 David A. Whiting and Rolf Hoffmannw Baylor Hair Research and Treatment Center, Dallas, Texas, USA; wdermatology Practice, Freiburg, Germany Figure 1 Case
DATA SHEET. Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
 DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
The Prevention and Management of Acute Skin Reactions Related to Radiation Therapy
 Evidence-Based Series 13-7 IN REVIEW A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Prevention and Management of Acute Skin Reactions Related to Radiation
Evidence-Based Series 13-7 IN REVIEW A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Prevention and Management of Acute Skin Reactions Related to Radiation
This PDF is available for free download from a site hosted by Medknow Publications
 Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
FAST FACTS FOR BOARD REVIEW
 FAST FACTS FOR BOARD REVIEW Series Editor: William W. Huang, MD, MPH Common Hair Disorders Rita Pichardo-Geisinger, MD Dr. Pichardo-Geisinger is Associate Professor of Dermatology, Wake Forest Baptist
FAST FACTS FOR BOARD REVIEW Series Editor: William W. Huang, MD, MPH Common Hair Disorders Rita Pichardo-Geisinger, MD Dr. Pichardo-Geisinger is Associate Professor of Dermatology, Wake Forest Baptist
A Novel Approach for Acne Treatment
 A Novel Approach for Acne Treatment E.V. Ross, M.D.; M.A. Blair, M.D.; B.S. Graham, M.D.; Naval Medical Center, San Diego, CA D.Y. Paithankar, Ph.D.; B.A. Saleh, M.Eng.; Candela Corporation, Wayland, MA
A Novel Approach for Acne Treatment E.V. Ross, M.D.; M.A. Blair, M.D.; B.S. Graham, M.D.; Naval Medical Center, San Diego, CA D.Y. Paithankar, Ph.D.; B.A. Saleh, M.Eng.; Candela Corporation, Wayland, MA
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology is intended for the treatment of: Steroid responsive dermatoses such as psoriasis and seborrheic dermatitis of the hairy regions,
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology is intended for the treatment of: Steroid responsive dermatoses such as psoriasis and seborrheic dermatitis of the hairy regions,
PRESCRIBING INFORMATION. ratio-topisone. Betamethasone dipropionate USP. 0.5 mg Cream, Ointment and Lotion. Topical corticosteroid
 PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
Transplantation. Immunology Unit College of Medicine King Saud University
 Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Skin Deep: Cutaneous Lupus. Dr Sarah Sasson Immunology Registrar, Liverpool Hospital 2016
 Skin Deep: Cutaneous Lupus Dr Sarah Sasson Immunology Registrar, Liverpool Hospital 2016 Introduction: Cutaneous lupus erythematosus LE is an autoimmune disease with a range of clinical manifestations
Skin Deep: Cutaneous Lupus Dr Sarah Sasson Immunology Registrar, Liverpool Hospital 2016 Introduction: Cutaneous lupus erythematosus LE is an autoimmune disease with a range of clinical manifestations
นพ.วาสนภ วช รมน หน วยโรคผ วหน ง คณะแพทยศาสตร โรงพยาบาลรามาธ บด
 Vitiligo Vitiligo Update Acquired pigmentary disorder Depigmented macules and patches นพ.วาสนภ วช รมน หน วยโรคผ วหน ง คณะแพทยศาสตร โรงพยาบาลรามาธ บด Prevalence The prevalence of vitiligo is often said
Vitiligo Vitiligo Update Acquired pigmentary disorder Depigmented macules and patches นพ.วาสนภ วช รมน หน วยโรคผ วหน ง คณะแพทยศาสตร โรงพยาบาลรามาธ บด Prevalence The prevalence of vitiligo is often said
CHAPTER 2. Is severity eruption assessment possible? in polymorphous light
 CHAPTER 2 Is severity assessment in polymorphous light Is severity eruption assessment possible? in polymorphous light Ines Schornagel, eruption Kees Guikers, possible? Huib van Weelden, Carla Bruijnzeel-Koomen
CHAPTER 2 Is severity assessment in polymorphous light Is severity eruption assessment possible? in polymorphous light Ines Schornagel, eruption Kees Guikers, possible? Huib van Weelden, Carla Bruijnzeel-Koomen
Disclosure Statement: Life Cycle of a Hair. Objectives. Life Cycle of a Hair. Hair Classification. Hair Disorders A Case Based Approach
 Hair Disorders A Case Based Approach Daniel Stulberg, M.D. Prof. of Family and Community Medicine University of New Mexico Some slides courtesy EJ Mayeaux, M.D. Disclosure Statement: Co-Author, Dermatologic
Hair Disorders A Case Based Approach Daniel Stulberg, M.D. Prof. of Family and Community Medicine University of New Mexico Some slides courtesy EJ Mayeaux, M.D. Disclosure Statement: Co-Author, Dermatologic
DIPROSONE OV Cream and Ointment do not contain preservatives, parabens or lanolin.
 PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
