WARMING THERAPY AND ULTRASOUND THERAPY FOR WOUNDS
|
|
|
- Clyde Summers
- 5 years ago
- Views:
Transcription
1 UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare Benefits of Texas, Inc. UnitedHealthcare of Washington, Inc. UnitedHealthcare SignatureValue & UnitedHealthcare Benefits Plan of California Medical Management Guideline WARMING THERAPY AND ULTRASOUND THERAPY FOR WOUNDS Guideline Number: MMG138.F Effective Date: October 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1 COVERAGE RATIONALE...2 APPLICABLE CODES...2 DESCRIPTION OF SERVICES...2 CLINICAL EVIDENCE...3 U.S. FOOD AND DRUG ADMINISTRATION...7 REFERENCES...7 GUIDELINE HISTORY/REVISION INFORMATION...9 Related Policies None INSTRUCTIONS FOR USE This Medical Management Guideline provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the member specific benefit plan document must be referenced. The terms of the member specific benefit plan document [e.g., Evidence of Coverage (EOC) and Schedule of Benefits (SOB)] may differ greatly from the standard benefit plan upon which this Medical Management Guideline is based. In the event of a conflict, the member specific benefit plan document supersedes this Medical Management Guideline. All reviewers must first identify member eligibility, any federal or state regulatory requirements, and the member specific benefit plan coverage prior to use of this Medical Management Guideline. Other Policies and G uidelines may apply. UnitedHealthcare reserves the right, in its sole discretion, to modify its Policies and Guidelines as necessary. This Medical Management Guideline is provided for informational purposes. It does not constitute medical advice. UnitedHealthcare may also use tools developed by third parties, such as the MCG Care Guidelines, to assist us in administering health benefits. The MCG Care Guidelines are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice. Member benefit coverage and limitations may vary based on the member s benefit plan Health Plan coverage provided by or through UnitedHealthcare of California, UnitedHealthcare Benefits Plan of California, UnitedHealthcare of Oklahoma, Inc., UnitedHealthcare of Oregon, Inc., UnitedHealthcare Benefits of Texas, Inc., or UnitedHealthcare of Washington, Inc. BENEFIT CONSIDERATIONS Before using this policy, please check the member specific benefit plan document and any federal or state mandates, if applicable. Essential Health Benefits for Individual and Small Group For plan years beginning on or after January 1, 2014, the Affordable Care Act of 2010 (ACA) requir es fully insured non-grandfathered individual and small group plans (inside and outside of Exchanges) to provide coverage for ten categories of Essential Health Benefits ( EHBs ). Large group plans (both self-funded and fully insured), and small group ASO plans, are not subject to the requirement to offer coverage for EHBs. However, if such plans choose to provide coverage for benefits which are deemed EHBs, the ACA requires all dollar limits on those benefits to be removed on all Grandfathered and Non-Grandfathered plans. The determination of which benefits constitute EHBs is made on a state by state basis. As such, when using this guideline, it is important to refer to the member specific benefit plan document to determine benefit coverage. Warm ing Therapy and Ultrasound Therapy for Wounds Page 1 of 9
2 COVERAGE RATIONALE Warming therapy or noncontact normothermic wound therapy is unproven and not medically necessary for treating wounds. The safety and efficacy of warming therapy or noncontact normothermic wound therapy for the treatment of chronic wounds has not been established in the published medical literature. Limitations of the existing studies include small samples, a lack of controls and/or randomization, short follow-up times, differences in standard therapy received by the treatment and control groups, and a paucity of statistical analyses of the results. These limitations create difficulties in drawing definitive conclusions about the relative efficacy and/or safety of this therapy for healing chronic wounds. Well-designed trials evaluating the efficacy and safety of warming therapy are needed to demonstrate that warming therapy is beneficial for health outcomes in patients with chronic wounds. Low frequency ultrasound is unproven and not medically necessary for treating wounds. Evidence for the use of low frequency ultrasound to treat wounds is limited and consists of studies that lack adequate sample sizes and proper control groups. Additional research with larger trials is needed to demonstrate that low frequency ultrasound is beneficial for health outcomes in patients with wounds. APPLICABLE CODES The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this guideline does not imply that the service described by the code is a covered or noncovered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. CPT Code HCPCS Code A4639 A6000 E0221 E0231 E0232 Description Low frequency, non-contact, non-thermal ultrasound, including topical application(s), when performed, wound assessment, and instruction(s) for ongoing care, per day CPT is a registered trademark of the American Medical Association Description Replacement pad for infrared heating pad system, each Noncontact wound-warming wound cover for use with the noncontact woundwarming device and warming card Infrared heating pad system Noncontact wound-warming device (temperature control unit, ac adapter and power cord) for use with warming card and wound cover Warming card for use with the noncontact wound-warming device and noncontact wound warming-wound cover DESCRIPTION OF SERVICES Warming therapy or noncontact normothermic wound therapy uses a noncontact wound cover and a warming unit to apply radiant heat and maintain 100% relative humidity in a wound. The intent is to raise the wound temperature to increase blood flow and oxygen to the wound. Low frequency or low energy ultrasound using the Mist Therapy System has been developed to provide simultaneous cleansing and debridement of wounds. Treatment with this device involves holding an ultrasonic handset 1 cm away from the wound and applying a saline solution to the handset, generating a saline mist that is designed to carry low levels of ultrasonic energy into the wound. According to the device manufacturer, this treatment promotes healing of acute, traumatic, and chronic wounds by stimulating cellular activities that contribute to healing and by cleaning the wound surface. The MIST Therapy System is a noncontact, low-frequency ultrasound debridement device that has been developed to promote healing of chronic wounds by removing yellow slough, tissue exudates, fibrin, and bacteria from the wound surface. The main components of the MIST Therapy System are an ultrasound generator; a handheld ultrasound transducer; and a single-use, disposable applicator with a bottle reservoir for sterile saline. The transducer tip vibrates 40,000 times per second to generate ultrasound waves at 40 kilohertz (khz) that are carried to the wound via a saline mist. In addition to wound cleaning, ultrasonic energy has been proposed as a means of stimulating angiogenesis, growth factor production, and other cellular activities that contribute to wound healing (Hayes, 201 7). Warm ing Therapy and Ultrasound Therapy for Wounds Page 2 of 9
3 CLINICAL EVIDENCE Warming Therapy Results of the studies of noncontact normothermic wound therapy (NNWT) for treatment of stage III and IV pressure ulcers and neuropathic ulcers suggest that NNWT, either alone, or in conjunction with standard care and/or offloading, can increase healing rates and the incidence of complete healing (Whitney et al., 2001; Kloth et al., 2002; McCulloch and Knight, 2002; Alvarez et al., 2003). In some cases, these increases were nonsignificant trends, which may reflect the relatively modest treatment effect combined with the small sample sizes. These studies should be interpreted with caution since all of the studies were supported by the manufacturer of the NNWT system, which introduces the possibility of investigator bias. Small study populations, ranging from 17 to 56 wounds per study, lack of blinding, lack of or insufficient statistical analysis and substantial dropout rates further compromised the quality of the evidence. Patient selection criteria were not defined in some studies. Furthermore, long-term follow-up data assessing wound stability and recurrence rates are not available. In a very small, randomized study evaluating the efficacy of NNWT combined with offloading for treatment of chronic skin wounds associated with osteomyelitis, mean healing time was increased in patients who received NNWT compared with those who received standard care and offloading. However, this difference did not reach statistical significance, and median wound healing times did not differ between groups (Karr, 2003). Ellis et al. (2003) evaluated 33 patients with full-thickness pressure sores who were randomized to receive standard care or radiant heat therapy with a Warm-Up device. The Warm-U p device eradicated bacteria in 6 patients within 2 weeks of starting treatment compared to none in the standard care group. The significance of this study is limited by small sample size. Forty-one patients with pressure ulcers were randomized to receive radiant heat dressing or hydrocolloid dressing. Fifty-seven percent of the patients using the radiant heat dressing device had complete ulcer healing compared to 44% of the control group. However, the difference was not statistically significant (Thomas et al., 2005). Low Frequency Ultrasound Cullum and Liu (2017) performed a systematic review to investigate whether ultrasound helps to heal or improve the symptoms of venous leg ulcers. Eleven randomized controlled trials (RCTs) involving a total of 969 participants were found. Eight studies compared ultrasound with use of no ultrasound for venous leg ulcers and the other three compared ultrasound with sham ultrasound. Seven out of the eleven studies were at high risk of bias, one study was at low risk of bias and bias could not be determine in three studies due to poor reporting. The authors found that the results of one study (337 participants) suggested that high-frequency ultrasound may be associated with more adverse events such as pain and skin redness (moderate quality evidence). The two studies that evaluated low - frequency ultrasound did not report whether participants experienced side effects. It is also uncertain whether either high- or low frequency ultrasound affects participants quality of life. The outcomes of adverse effects, quality of life and cost were not reported for low-frequency ultrasound treatment. The authors concluded that it is uncertain whether therapeutic ultrasound (either high or low frequency) improves the healing of venous leg ulcers. They rated most of the evidence as low or very low quality due to risk of bias and imprecision. Most of the studies did not have many participants, had short follow-up times and had poor study design. A 2016 ECRI Health Technology Assessment identified and reviewed 13 studies relevant to the Mist Therapy System: Diabetic foot ulcers: 2 randomized controlled trials (RCTs) (n = 77) Venous leg ulcers: 4 RCTS (n = 277). Pressure ulcers: 1 nonrandomized comparison study (n = 85); 1 case series (n =11). Chronic wounds: 1 case series (n = 48); 3 systematic reviews; 1 technology asse ssment. The report concluded that the Mist Therapy System shows potential for healing chronic venous leg ulcers and diabetic foot ulcers faster than standard of care. However, available studies are small and do not compare Mist Therapy to other wound care technologies, other than standard care. Evidence is insufficient for drawing conclusions of Mist s effect on pressure ulcers. Additional RCTs are needed that make appropriate comparisons and report results separately for each chronic wound type. White et al. (2015) compared non-contact low-frequency ultrasound (NLFU) in addition to standard of care (SOC) 3 times a week, with SOC alone at least once-weekly in a single-site, blinded randomized control trial. Thirty-six randomized patients with chronic venous ulcers completed treatment (17 NLFU + SOC, 19 SOC). NLFU plus SOC patients showed a -47 % change in wound area; SOC, -39 % change; with a difference of -7.4 %. The median number of infections per patient was two in both groups and the change in quality of life (QoL) scores were not significant. Non-contact low-frequency ultrasound plus SOC patients reported a substantial mean reduction in pain score of points, SOC patients' pain scores reduced by -5.3; with a difference of The authors concluded that Warm ing Therapy and Ultrasound Therapy for Wounds Page 3 of 9
4 the results demonstrated the importance of high-quality wound care and that outcome measures favored NLFU + SOC over SOC, but the differences were not statistically significant. The significance of this study is limited by small sample size and a short follow-up period. Tricco et al. (2015) examined systematic reviews that focused on interventions to treat complex wounds. Ninety -nine systematic reviews of wound care interventions were included in this overview of systematic reviews; 54 were systematic reviews with meta-analysis results and 45 were systematic reviews without a meta-analysis. Six categories of complex wounds were examined: venous leg ulcers, diabetic foot/leg ulcers, pressure ulcers, mixed arterial/venous leg wounds, unspecified mixed complex wounds and infected surgical wounds. The duration of treatment ranged from 2 days to 160 months and the duration of follow-up ranged from 2 days to 195 months. Based on data from systematic reviews including a meta-analysis with an AMSTAR score 8, various interventions for complex wounds were identified. These included bandages or stockings (multi-layer, high compression) and wound cleansing for venous leg ulcers; four-layer bandages for mixed arterial/venous leg ulcers; biologics, low frequency, low intensity noncontact ultrasound, and hydrogel dressings for diabetic leg/foot ulcers; hydrocolloid dressings, electrotherapy, air - fluidized beds, and alternate foam mattresses for pressure ulcers; and silver dressings and ultrasound for mixed complex wounds. Moderate to low quality review evidence found topical negative pressure and vacuum -assisted closure could be used for surgical wound infections. The authors concluded that various interventions can be utilized for treatment of varying types of complex wounds, but that few treatments were consistently effective across all outcomes. A prospective, randomized, controlled, multi-center trial was conducted by Gibbons et al. (2015) to compare percent wound size reduction, proportions healed, pain, and quality-of-life (QOL) outcomes in patients randomized to standard care (SC) alone (n=40) or SC and 40 khz noncontact, low-frequency ultrasound (NLFU) treatments (n=41) 3 times per week for 4 weeks. One hundred and twelve individuals with documented venous s tasis, a venous insufficiency and ulceration (VLU) greater than 30 days duration, measuring 4 cm2 to 50 cm2, and demonstrated arterial flow were enrolled. Index ulcers were 56 % recurrent, with a median duration of 10.3 months and median ulcer area of 11.0 cm2. All participants received protocol-defined SC compression (30 to 40 mm Hg), dressings to promote a moist wound environment, and sharp debridement for a minimum of 1 time per week. Ulcer measurements were obtained weekly using digital planimetry. Pain and QOL scores were assessed at baseline and after 4 weeks of treatment using the visual analog scale and the Short Form-36 Health Survey. After 4 weeks of treatment, the average wound size reduction was 61.6 % ± 28.9 in the NLFU+SC compared to 45 % ± 32.5 in the SC group. Reductions in median and absolute wound area as well as pain scores were also significant. The authors concluded that NLFU therapy with guideline-defined standard VLU care should be considered for healing VLUs not responding to SC alone. They stated that the results of this study warrant further research on barriers to healing and the changes occurring in the tissue of the wound. This was not a blinded study. A randomized, controlled clinical study was conducted by Olyais et al. (2013) to compare the effectiveness of standard treatment and standard treatment plus either high frequency or noncontact low frequency ultrasound when treating chronic wounds, including venous leg ulcers. It was noted that a total of 90 people participated in the study, including 47 men and 43 women. It was also noted that the average age for the participants was 38.3 year old. Out of the 90 participants it was noted that 30 participants received standard care. Standard care was defined as follows: multilayered compression bandaging (40 mm Hg of pressure at the ankle graduated to 17 mm Hg to 20 mm Hg below the knee), nonadherent dressing, and regular debridement. Standard care dressing changes and ultrasound therapy were provided three times per week for 3 months or until healed. It was noted that 30 participants received standard treatment plus high-frequency ultrasound, which was delivered at 1-3 MHz for 5 to 10 minutes. The remaining 30 participants received standard treatment plus low-frequency ultrasound, which was delivered at 40 khz for 4-10 minutes. The final results of this study were reported to show that the outcome of both methods of the ultrasounds therapy was better than standard care alone. The result of this one study does not provide sufficient clin ical data, to change the current unproven status of the use of low frequency ultrasound for the treatment of wounds. Additional studies and randomized trials are required to support and confirm the long and short term findings of this lone study. Yoa et al. (2012) conducted a randomized clinical study designed to determine the effects of non -contact low frequency ultrasound (NCLF-US) devices when used for the treatment of chronic non healing wounds. Subjects were randomly assigned to one of three groups: application of NCLF-US thrice per week (Group 1), NCLF-US once per week (Group 2) and the control (Group 3) that received no NCLF-US. All subjects received standard wound care plus offloading for a total of 4 weeks. Percent area reduction (PAR) of each wound compared to baseline was evaluated weekly. Twelve DFU patients, 2 (16 7%) type 1 and 10 (83 3%) type 2 diabetics, with an average age of 58 ± 10 years and a total of 12 foot ulcers were enrolled. Group 1 showed significant wound area reduction at weeks 3, 4 and 5 compared to baseline, with the greatest PAR, 86% (P < 0 05); Groups 2 and 3 showed 25% PAR and 39% PAR, respectively, but there were no statistically significant differences between Group 2 and Group 3 over time. Based on the information provided by this small randomized study demonstrated that NCLF-US is an effective in treating neuropathic diabetic foot ulcers through at least in part, inhibiting pro -inflammatory cytokines in chronic wound and Warm ing Therapy and Ultrasound Therapy for Wounds Page 4 of 9
5 improving tissue regeneration. Therapeutic application of NFLU three times per week allows for the best wound area reduction. The results of this study must be confirmed in a larger trial. Ennis et al. (2005) conducted a multicenter, double-blinded randomized controlled trial to evaluate the Mist Therapy System for treatment of chronic diabetic foot ulcers. As an adjunct to standard wound care with saline moistened gauze dressings and debridement as needed, patients underwent true or sham Mist treatment in 3 sessions per week for 12 weeks. Although 133 patients were enrolled and considered in the intent-to-treat analysis, 78 patients (59%) were subsequently excluded leaving 27 patients in the treatment group and 28 in the sham group, which comprised the per protocol population. In the per protocol population, at week 11, the treatment group had a 56% decrease in wound exudation compared with the sham group. In the per protocol population, complete closure was achieved in 41% of patients in the treatment group versus 14% in the sham group at 12 weeks. The me an time to healing was 9.1 ± 0.6 weeks for the treatment group versus 11.7 ± 0.2 weeks for the sham group. Despite the improvements in wound exudation and wound closure for the treatment group versus the sham group, Mist Therapy was not associated with any statistically significant improvements in granulation tissue. According to the intent-to-treat analysis, the Mist Therapy System wound healing did not provide any statistically significant improvements in wound healing. For patients who had wounds due to insufficient lower limb blood flow, another RCT found that Mist Therapy was associated with a statistically significant improvement in partial wound healing. After 12 weeks of treatment, greater than 50% wound healing occurred in 63% of patients who underwent Mist Therapy versus 29% of patients who underwent standard wound care alone (Kavros et al, 2007). The small study population limits the validity of this study. Furthermore, this study used 50% rather than 100% wound healing as the measure of succe ss and did not report whether any patients achieved complete wound healing. Watson et al. (2011) assessed the clinical effectiveness of weekly delivery of low dose, high frequency therapeutic ultrasound in conjunction with standard care for hard to heal v enous leg ulcers in a multicenter, two arm randomized controlled trial. The study included 337 patients with a t least one venous leg ulcer of >6 months' duration or >5 cm (2) area and an ankle brachial pressure index of 0.8. The study evaluated weekly administration of low dose, high frequency ultrasound therapy for up to 12 weeks plus standard care compared with standard care alone. The two groups showed no significant difference in the time to healing of the reference leg ulcer. After adjustment for baseline ulcer area, baseline ulcer duration, use of compression bandaging, and study center, there was still no evidence of a difference in time to healing. There was no difference in time to complete healing of all ulcers, with median time to healing of 328 days with standard care and 365 days with ultrasound. There was no evidence of a difference in rates of recurrence of healed ulcers (17/31 with ultrasound vs 14/31 with standard care). There was also no difference between the two groups in health related quality of life; both for the physical component score and the mental component score, but there were significantly more adverse events in the ultrasound group. The authors concluded that low dose, high frequency ultrasound administered weekly for 12 weeks during dressing changes in addition to standard care did not increase ulcer healing rates, affect quality of life, or reduce ulcer recurrence. In a randomized controlled trial, Taradaj et al. (2008) estimated the usefulness of therapeutic ultrasound for healing of venous leg ulcers in 81 patients. Patients in groups 1 and 2 were treated surgically. Patients in groups 3 and 4 were treated conservatively. Patients in groups 1 and 3 were additionally treated with the ultrasound (1 MHz, 0.5 W/ cm (2)) once daily, six times a week for seven weeks. Comparison of the number of complete healed wounds indicated statistically significant differences between groups 1 and 4, 2 and 4, 3 and 4 in favor of groups 1, 2 and 3. Comparison of the other parameters also demonstrated more efficient therapy effects in groups 1, 2 and 3 than in group 4. There were no statistical differences in all examined parameters between groups 1, 2 a nd 3. The investigators concluded that ultrasound is an efficient and useful method only in conservatively treated venous leg ulcers. They state also that there are no special reasons for application of the ultrasound in surgically treated patients. They comment further that a well-conducted surgical operation is much more effective for a healing process than conservative pharmacological procedures. The small study population limits the validity of this study. Driver et al. (2011) performed a meta-analysis and summarized the effects of a noncontact low-frequency ultrasound (NLFU) therapy on healing of chronic wounds. The meta-analysis included 8 published studies that reported effects of NLFU on wound size and healing rate of chronic wounds in 444 patients. Mean time to healing was 8.2 weeks, with 42% of wounds healed by 12 weeks. The wound volume was reduced by 79.7% over a mean of 12 weeks. According to the authors, noncontact low-frequency ultrasound for treatment of chronic wounds was associated with consistent and substantial wound size reductions, as well as favorable rates of healing. However, the quality of the evidence was limited by small patient numbers and lack of appropriate comparison groups. Voight et al. (2011) conducted a systematic review and meta-analysis to examine low-frequency (20-30 khz) ultrasound delivered at either low or high intensity. The objective of the review was to determine whether low - frequency ultrasound used as an adjunctive therapy improves the outcomes of complete healin g and reduction of size of chronic lower limb wounds. Eight randomized controlled trials were identified. Three of these trials were Warm ing Therapy and Ultrasound Therapy for Wounds Page 5 of 9
6 unpublished papers presented at conferences. Results demonstrated that early healing (at 5 months) in patients with venous stasis and diabetic foot ulcers was favorably influenced by both high- and low-intensity ultrasound delivered at a low frequency-either via contact or noncontact techniques. However, according to the authors, the quality of the evidence is in general of lower quality for both types of ultrasound, and especially for low-frequency low-intensity noncontact ultrasound because of significant biases. The authors stated that although it appears from the meta-analysis performed that low-frequency low-intensity noncontact ultrasound is more effective at complete healing than standard of care, the quality of the evidence as it relates to biases was poor. The study by Ennis et al. (2005) had a significant protocol violation that resulted in 42 patients being dropped f rom the study. This protocol violation was a result of an inversion of the treatment distances of the treatment protocol between active and sham devices. Furthermore, in the study by Kavros et al. (2007) it was indicated in the acknowledgments that Cellera tion (manufacturer of the ultrasound device) supported in the assistance of the manuscript, calling into question the potential quality of the evidence. According to the authors, although there appears to be some clinical benefit with the use of low frequency ultrasound, more and larger size randomized controlled trials should be completed on the longer term clinical effects (i.e., >3 months) for this therapy. Cullum et al. (2010) assessed whether ultrasound (US) increases the healing of venous leg ulcers by searching several databases for randomized controlled trials (RCTs) comparing US with no US. Two authors independently assessed the search results and selected eligible studies. Details from included studies were summarized using a data extraction sheet, and double-checked. Eight trials were included; all had unclear, or high, risks of bias, with differences in duration of follow-up, and US regimens. Six trials evaluated high frequency US and five of these reported healing at 7-8 weeks. Significantly more patients healed with US than without it at 7-8 weeks, but later assessments at 12 weeks showed the increased risk of healing with US was no longer statistically significant. One poor-quality study of high-frequency US found no evidence of an effect on healing after three weeks' treatment. Two trials evaluated low frequency US and reported healing at different time points. Both trials reported no evidence of a difference in the proportion of ulcers healed with US compared with no US: both were significantl y underpowered. The authors concluded that the trials evaluating US for venous leg ulcers are small, poor-quality and heterogeneous. They state that there is no reliable evidence that US hastens healing of venous ulcers. There is a small amount of weak evidence of increased healing with US, but this requires confirmation in larger, high-quality RCTs. They conclude that there is no evidence of a benefit associated with low frequency US. Al-Kurdi et al. (2008) reviewed results from 8 small trials (average enrollment 44 patients) with comparator treatments of either sham ultrasound or standard of care. Studies had either a medium or high risk of bias, which indicates a reduction in study validity. Typical study limitations included lack of allocation concealme nt and failure to report baseline characteristics, randomization methods, and blinding of outcome assessors. The authors concluded that the available evidence suggests that there may be a benefit from ultrasound therapy in healing venous leg ulcers, however due to the poor quality of the studies; these results need to be interpreted with caution. In a National Institute for Health and Clinical Excellence (NICE) guidance for MIST Therapy System for the Promotion of Wound Healing in Chronic and Acute Wounds, NICE states that the MIST Therapy System shows potential to enhance the healing of chronic, hard-to-heal, complex wounds, compared with standard methods of wound management. However, the amount and quality of published evidence on the relative effective ness of the MIST Therapy System is not sufficient, at this time, to support the case for routine adoption of the MIST Therapy System (NICE, 2011, Reaffirmed June 2016). A 2016 Hayes Health Technology Brief on the Mist Therapy System reported that a literature search identified 4 studies (n=12 to 210 patients) that evaluated MIST therapy as an adjunct to standard wound care (SWC) for the treatment of diabetic foot ulcers (DFUs) or lower extremity arterial wounds. Overall, a low-quality body of evidence suggests that MIST therapy as an adjunct to SWC may enhance wound healing compared with SWC alone. Three of the studies reported a statistically significant increase in wound reduction and healing when MIST therapy was used. Results from 1 cohort study suggest improvements in wound healing for patients undergoing MIST therapy compared with those undergoing SWC alone; however, no differences were identified between groups when evaluating outcomes by wound categories (ischemic or neuropathic wounds). Limitations of the individual studies include small sample size, inadequate follow-up, lack of blinding of outcome assessor, retrospective design (1 study), substantial attrition, a lack of power analysis, and limited reporting on safety outcomes. An interventional phase 2 clinical study is in progress to evaluate the effect of low frequency, low intensity ultrasound treatment on wound healing and health-related quality of life with a randomized clinical trial of patients (n=120) with venous ulcers or diabetic ulcers. The estimated study completion date is September ClinicalTrials.gov identifier: NCT Warm ing Therapy and Ultrasound Therapy for Wounds Page 6 of 9
7 Professional Societies Association for the Advancement of Wound Care (AAWC) The AAWC released a guideline on the care of pressure ulcers. While nonconta ct ultrasound therapy was included as a potential second-line treatment if first-line treatments failed to induce wound healing, the strength of the evidence supporting this decision was low (Level C), indicating that there is limited evidence for this tec hnology (AAWC, 2010a). In a 2010 guideline for venous ulcers, the AAWC states that more evidence is needed to evaluate the use of low frequency ultrasound for venous ulcers (AAWC, 2010b). American College of Foot and Ankle Surgeons The American College of Foot and Ankle Surgeons guideline for Diabetic Foot Disorders states that several new ultrasound devices are being used to debride the wound and provide ultrasonic therapy. The guideline also indicates that low-intensity pulsed ultrasound (LIPUS) has also been suggested as a useful adjunct in promoting healing of Charcot fractures. The guideline states that although promising in theory, none of these adjunctive treatments have yet been conclusively proven effective through large prospective multicenter, r andomized trials (Frykberg, 2006). National Institute for Health and Care Excellence (NICE) The NICE guideline for diabetic foot problems prevention and management recommends one or more of the following as standard care for treating diabetic foot ulcers: offloading; control of foot infection; control of ischemia; wound debridement; and wound dressings. NICE recommends that negative pressure wound therapy should be considered after surgical debridement for diabetic foot ulcers, on the advice of the multidisciplinary foot care service. Dermal or skin substitutes as an adjunct to standard care can be considered when treating diabetic foot ulcers, only when healing has not progressed, and on the advice of the multidisciplinary foot care service (NICE 2016). American Podiatric Medical Association The American Podiatric Medical Association in collaboration with the Society for Vascular Surgery and the Society for Vascular Medicine developed a 2016 clinical practice guideline for the management of diabetic foot. Their recommendations do not include warming therapy, noncontact normothermic wound therapy or low frequency ultrasound for the treatment of wounds (Hingorani et al. 2016). U.S. FOOD AND DRUG ADMINISTRATION (FDA) Warm-Up Active Wound Therapy received 510(k) approval from the FDA on March 28, 1997 as a wound and burn occlusive heated dressing. See the following Web site for more information: (Use product code MSA) (Accessed August 11, 2017) The Mist Therapy System is regulated by the FDA as a Class II device and is classified as an ultrasound wound cleaner. This device was approved via the FDA 510(k) process in April In May 2005, FDA granted marketing clearance to Celleration for the MIST Therapy System 5.1 with an expanded indication. The approved indication for use is to produce "a low-energy ultrasound-generated mist to promote wound healing through wound cleansing and maintenance debridement by the removal of yellow slough, fibrin, tissue exudates, and bacteria. In 2014 the FDA approved the UltraMIST System (K140782), a smaller, sleeker and user-friendly design. See the following Web site for more information: (Accessed August 11, 2017) See the following website for additional devices (use product code NRB): (Accessed August 11, 2017) In November 2005, the FDA issued a document that outlines the controls applicable to all low -frequency ultrasound devices: Class II Special Controls Guidance Document: Low Energy Ultrasound Wound Cleaner. This document provides nonbinding recommendations to address risks associated with these devices (i.e., delayed wound healing, inflammation, thermal damage, infection, and electrical shock) and assure the devices' safety and effectiveness. See the following Web site for more information: (Accessed August 11, 2017) REFERENCES Al-Kurdi D, Bell-Syer SE, Flemming K. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev Jan 23;(1):CD Alvarez OM, Rogers RS, Booker JG, et al. Effect of noncontact normothermic wound therapy on the healing of neuropathic (diabetic) foot ulcers: an interim analysis of 20 patients. J Foot Ankle Surg. 2003;42(1): Warm ing Therapy and Ultrasound Therapy for Wounds Page 7 of 9
8 Association for the Advancement of Wound Care (AAWC). Pressure Ulcer Guideline. October 2010a. Association for the Advancement of Wound Care (AAWC). Venous Ulcer Guideline. December 2010b. Cullum NA, Al-Kurdi D, Bell-Syer SE. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev Jun 16;6:CD Cullum N and Liu Z. Therapeutic ultrasound for venous leg ulcers. Cochrane Datab ase of Systematic Reviews Issue 5. Art. No.: CD Driver VR, Yao M, Miller CJ. Noncontact low-frequency ultrasound therapy in the treatment of chronic wounds: a meta-analysis. Wound Repair Regen. 2011;19(4): ECRI Institute. Health Technology Assessment. Mist Therapy System (Alliqua Biomedical, Inc.) Noncontact, Lowfrequency Ultrasound for Healing Chronic Wounds. December Ellis SL, Finn P, Noone M, et al. Eradication of methicillin-resistant Staphylococcus aureus from pressure sores using warming therapy. Surg Infect (Larchmt) Spring;4(1):53-5. Ennis WJ, Foremann P, Mozen N, et al. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8): Erratum in: Ostomy Wound Manage. 2005;51(9):14. Frykberg RG, Zgonis T, Armstrong DG, et al. American College of Foot and Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline. J Foot Ankle Surg 2006 Sep-Oct;45(5):S2-66. Gibbons GW, Orgill DP, Serena TE, et al. A prospective, randomized, controlled trial comparing the effects of noncontact, low-frequency ultrasound to standard care in healing venous leg ulcers. Ostomy Wound Manage. 2015; 61(1): Hayes, Inc. Hayes Medical Technology Directory Report. Noncontact Low-Frequency Ultrasound Using the MIST Therapy System (Celleration Inc.) for Treatment of Lower Extremity Arterial and Diabetic Foot Ulcers. Lansdale, PA: Hayes. June 30, Hayes, Inc. Hayes Medical Technology Directory Report. Noncontact Low-Frequency Ultrasound Using the MIST Therapy System (Celleration Inc.) for Treatment of Venous Leg Ulcers. Lansdale, PA: Hayes. June 30, Amended August 3, Hingorani, Anil, LaMuraglia, Glenn, Henke, Peter, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the Ame rican Podiatric Medical Association and the Society for Vascular Medicine. Journal of Vascular Surgery. February 2016;63(25). Karr JC. External thermoregulation of wounds associated with lower-extremity osteomyelitis: a pilot study. J Am Podiatr Med Assoc. 2003;93(1): Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: The Mayo Clinic Experience, Adv Skin Wound Care. 2007; 20(4): Kloth LC, Berman JE, Nett M, et al. A randomized controlled clinical trial to evaluate the effects of noncontact normothermic wound therapy on chronic full-thickness pressure ulcers. Adv Skin Wound Care. 2002;15(6): McCulloch J, Knight CA. Noncontact normothermic wound therapy and offloading in the treatment of neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage. 2002;48(3): National Institute for Health and Care Excellence (NICE). NG19. Diabetic foot problems: prevention and management. January National Institute for Health and Care Excellence (NICE); The MIST Therapy system for the promotion of wound healing; London, UK: NICE; July Reaffirmed June Olyaie M, Rad FS, Elahifar MA, et al. High-frequency and noncontact low frequency ultrasound therapy for venous leg ulcer treatment: a randomized, controlled study. Ostomy Wound Manage Aug;59(8): Taradaj J, Franek A, Brzezinska-Wcislo L, et al. The use of therapeutic ultrasound in venous leg ulcers: a randomized, controlled clinical trial. Phlebology. 2008;23(4): Thomas DR, Diebold MR, Eggemeyer LM. A controlled, randomized, comparative study of a radiant heat bandage on the healing of stage 3-4 pressure ulcers: a pilot study. J Am Med Dir Assoc Jan-Feb;6(1):46-9. Tricco AC, Antony J, Vafaei A, et al. Seeking effective interventions to treat complex wounds: An overview of systematic reviews. BMC Med. 2015;13:89. Voigt J, Wendelken M, Driver V et al; Low-frequency ultrasound (20-40 khz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials; Int J Low Extrem Wounds; 2011; 10 (4): Warm ing Therapy and Ultrasound Therapy for Wounds Page 8 of 9
9 Watson JM, Kang'ombe AR, Soares MO, et al; VenUS III Team; Use of weekly, low dose, high frequency ultrasound for hard to heal venous leg ulcers: the VenUS III randomized controlled trial; BMJ; 2011 Mar 8; 342:d1092 White J, Ivins N, Wilkes A, et al. Non-contact low-frequency ultrasound therapy compared with UK standard of care for venous leg ulcers: A single-centre, assessor-blinded, randomised controlled trial. Int Wound J Jan 25. Whitney JD, Salvadalena G, Higa L, et al. Treatment of pressure ulcers with noncontact normothermic wound therapy: healing and warming effects. J WOCN. 2001;28(5): Yao M, Hasturk H, Kantarci A, Gu G, et al. A pilot study evaluating noncontact low frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers. Int Wound J Nov 19. GUIDELINE HISTORY/REVISION INFORMATION Date 10/01/2017 Action/Description Updated supporting information to reflect the most current clinical evidence, FDA information and references; no change to coverage rationale or lists of applicable codes Archived previous policy version MG138.E Warm ing Therapy and Ultrasound Therapy for Wounds Page 9 of 9
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Description. Section: Medicine Effective Date: April 15, 2015 Subsection: Medicine Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Non-Contact Ultrasound Treatment for Wounds
 Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. MTG Review Decision Document
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Centre for Health Technology Evaluation MTG Review Decision Document Review of MTG5: The MIST Therapy system for the promotion of wound healing This guidance
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Centre for Health Technology Evaluation MTG Review Decision Document Review of MTG5: The MIST Therapy system for the promotion of wound healing This guidance
Non-Contact Ultrasound Treatments for Wounds
 Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
Non-Contact Ultrasound Treatment for Wounds
 Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 7/2018 Origination: 1/2008 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 7/2018 Origination: 1/2008 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
HYSTERECTOMY FOR BENIGN CONDITIONS
 UnitedHealthcare Commercial Medical Policy HYSTERECTOMY FOR BENIGN CONDITIONS Policy Number: 2018T0572G Effective Date: September 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
UnitedHealthcare Commercial Medical Policy HYSTERECTOMY FOR BENIGN CONDITIONS Policy Number: 2018T0572G Effective Date: September 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare
BRONCHIAL THERMOPLASTY UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare
HYSTERECTOMY FOR BENIGN CONDITIONS
 HYSTERECTOMY FOR BENIGN CONDITIONS UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 104.7 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
HYSTERECTOMY FOR BENIGN CONDITIONS UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 104.7 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Use of Non-Contact Low Frequency Ultrasound in Wound Care
 Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
NON-SURGICAL ENDODONTICS
 NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.02 Effective Date: February 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.02 Effective Date: February 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
NON-SURGICAL ENDODONTICS
 NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.03 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.03 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare Oxford Clinical Policy Policy Number: RESPIRATORY 009.11 T2 Effective Date: August 1, 2017 Table of Contents Page BRONCHIAL THERMOPLASTY... 1 INSTRUCTIONS FOR USE...
BRONCHIAL THERMOPLASTY UnitedHealthcare Oxford Clinical Policy Policy Number: RESPIRATORY 009.11 T2 Effective Date: August 1, 2017 Table of Contents Page BRONCHIAL THERMOPLASTY... 1 INSTRUCTIONS FOR USE...
Coverage Summary. Wound Treatments
 Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE
 UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS
 UnitedHealthcare Oxford Clinical Policy ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS Policy Number: DME 029.15 T2 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR
UnitedHealthcare Oxford Clinical Policy ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS Policy Number: DME 029.15 T2 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR
Clinical Policy Title: Air fluidized beds
 Clinical Policy Title: Air fluidized beds Clinical Policy Number: 16.02.10 Effective Date: May 1, 2018 Initial Review Date: March 6, 2018 Most Recent Review Date: April 10, 2018 Next Review Date: April
Clinical Policy Title: Air fluidized beds Clinical Policy Number: 16.02.10 Effective Date: May 1, 2018 Initial Review Date: March 6, 2018 Most Recent Review Date: April 10, 2018 Next Review Date: April
ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS
 UnitedHealthcare Commercial Medical Policy ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS Policy Number: WOU001 Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Medical Policy ELECTRICAL STIMULATION AND ELECTROMAGNETIC THERAPY FOR WOUNDS Policy Number: WOU001 Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
doi: /ptj The online version of this article, along with updated information and services, can be
 Therapy for Adjunctive Treatment of Nonhealing Wounds: Retrospective Analysis Autumn L Bell and Joseph Cavorsi PHYS THER. Published online October 10, 2008 doi: 10.2522/ptj.20080009 The online version
Therapy for Adjunctive Treatment of Nonhealing Wounds: Retrospective Analysis Autumn L Bell and Joseph Cavorsi PHYS THER. Published online October 10, 2008 doi: 10.2522/ptj.20080009 The online version
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
LITHOTRIPSY FOR SALIVARY STONES
 UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare Benefits of Texas,
UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare Benefits of Texas,
My Diabetic Patient Has No Pulses; What Should I Do?
 Emily Malgor, MD Assistant Professor of Surgery University of Oklahoma, Oklahoma City My Diabetic Patient Has No Pulses; What Should I Do? There are no disclosures. Background Diabetes affects 387 million
Emily Malgor, MD Assistant Professor of Surgery University of Oklahoma, Oklahoma City My Diabetic Patient Has No Pulses; What Should I Do? There are no disclosures. Background Diabetes affects 387 million
CASE 1: TYPE-II DIABETIC FOOT ULCER
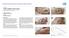 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
HIGH- VOLTAGE PULSED CURRENT (HVPC) ELECTRICAL STIMULATION FOR TREATMENT OF DIABETIC FOOT ULCER (DFU) - A REVIEW
 Review Article Allied Science International Journal of Pharma and Bio Sciences ISSN 0975-6299 HIGH- VOLTAGE PULSED CURRENT (HVPC) ELECTRICAL STIMULATION FOR TREATMENT OF DIABETIC FOOT ULCER (DFU) - A REVIEW
Review Article Allied Science International Journal of Pharma and Bio Sciences ISSN 0975-6299 HIGH- VOLTAGE PULSED CURRENT (HVPC) ELECTRICAL STIMULATION FOR TREATMENT OF DIABETIC FOOT ULCER (DFU) - A REVIEW
Ophthalmologic Policy. Vascular Endothelial Growth Factor (VEGF) Inhibitors
 Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Mean percent reduction in ulcer area from baseline at six weeks 62 % SANTYL Ointment + supportive care* + sharp debridement 1 (P<0.
 Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Coverage Guideline. BioniCare System (formerly the BIO-1000 System) DEFINITION COVERAGE CRITERIA MEDICAL BACKGROUND
 Coverage Guideline System (formerly the BIO-1000 System) Disclaimer: Please note that Baptist Health Plan updates Coverage Guidelines throughout the year. A printed version may not be most up to date version
Coverage Guideline System (formerly the BIO-1000 System) Disclaimer: Please note that Baptist Health Plan updates Coverage Guidelines throughout the year. A printed version may not be most up to date version
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
Review report of MTG5: The MIST Therapy system for the promotion of wound healing
 Review report of MTG5: The MIST Therapy system for the promotion of wound healing Produced by: The Birmingham and Brunel Consortium External Assessment Centre Authors: Zulian Liu, Research Fellow, Institute
Review report of MTG5: The MIST Therapy system for the promotion of wound healing Produced by: The Birmingham and Brunel Consortium External Assessment Centre Authors: Zulian Liu, Research Fellow, Institute
Wound Healing Community Outreach Service
 Wound Healing Community Outreach Service Wound Management Education Plan January 2011 December 2011 Author: Michelle Gibb Nurse Practitioner Wound Management Wound Healing Community Outreach Service Institute
Wound Healing Community Outreach Service Wound Management Education Plan January 2011 December 2011 Author: Michelle Gibb Nurse Practitioner Wound Management Wound Healing Community Outreach Service Institute
Cahaba Medicare Policy Primer 1,2 for Apligraf
 Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Ray Norris, Rachel Henchy
 Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
BUPRENORPHINE (PROBUPHINE & SUBLOCADE )
 UnitedHealthcare Oxford Clinical Policy BUPRENORPHINE (PROBUPHINE & SUBLOCADE ) Policy Number: PHARMACY 291.6 T2 Effective Date: July 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
UnitedHealthcare Oxford Clinical Policy BUPRENORPHINE (PROBUPHINE & SUBLOCADE ) Policy Number: PHARMACY 291.6 T2 Effective Date: July 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
Medical Affairs Policy
 Service: Acupuncture Therapy PUM 250-0002-1803 Medical Affairs Policy Medical Policy Committee Approval 03/16/18 Effective Date 07/01/18 Prior Authorization Needed Yes-if not an exclusion of the health
Service: Acupuncture Therapy PUM 250-0002-1803 Medical Affairs Policy Medical Policy Committee Approval 03/16/18 Effective Date 07/01/18 Prior Authorization Needed Yes-if not an exclusion of the health
Advancing the science of wound bed preparation
 Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
DIABETIC ULCERS V PRESSURE ULCERS SO, WHAT DO YOU CALL IT?
 DIABETIC ULCERS V PRESSURE ULCERS SO, WHAT DO YOU CALL IT? Arthur Stone, DPM Mary Sieggreen, MSN,CNS,NP,CVN Faculty Disclosure Dr. Stone has listed an affiliation with: Advisory Board Member..FXI, Inc.
DIABETIC ULCERS V PRESSURE ULCERS SO, WHAT DO YOU CALL IT? Arthur Stone, DPM Mary Sieggreen, MSN,CNS,NP,CVN Faculty Disclosure Dr. Stone has listed an affiliation with: Advisory Board Member..FXI, Inc.
Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration
 Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration Harding K G, Price P, Robinson B, Thomas S, Hofman D Record Status
Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration Harding K G, Price P, Robinson B, Thomas S, Hofman D Record Status
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
Sarah Medrano, RN, BSN, WOCN; and Mary Jo Beneke, RN, BS, CWOCN Yuma Regional Medical Center, Yuma, Arizona
 Sound Evidence coustic Pressure Wound Therapy to ebride Unstageable Pressure Ulcers in the cute Care Setting: Case Series Sarah Medrano, RN, BSN, WCN; and Mary Jo Beneke, RN, BS, CWCN Yuma Regional Medical
Sound Evidence coustic Pressure Wound Therapy to ebride Unstageable Pressure Ulcers in the cute Care Setting: Case Series Sarah Medrano, RN, BSN, WCN; and Mary Jo Beneke, RN, BS, CWCN Yuma Regional Medical
ORIGINAL ARTICLE. Judith White 1, Nicola Ivins 2, Antony Wilkes 1, Grace Carolan-Rees 1 &KeithGHarding 2
 International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE Non-contact low-frequency ultrasound therapy compared with UK standard of care for venous leg ulcers: a single-centre, assessor-blinded, randomised
International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE Non-contact low-frequency ultrasound therapy compared with UK standard of care for venous leg ulcers: a single-centre, assessor-blinded, randomised
Disclosures. Outpatient NPWT Options Free up Hospital Beds, but Do They Work? Objectives. Clinically Effective: Does it Work?
 4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145
 Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145 Effective Date: 05/17 Last Review Date: 06/17 See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145 Effective Date: 05/17 Last Review Date: 06/17 See Important Reminder at the end of this policy for important regulatory
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
NEGATIVE PRESSURE WOUND THERAPY
 NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BUPRENORPHINE (PROBUPHINE & SUBLOCADE )
 UnitedHealthcare Oxford Clinical Policy BUPRENORPHINE (PROBUPHINE & SUBLOCADE ) Policy Number: PHARMACY 291.5 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
UnitedHealthcare Oxford Clinical Policy BUPRENORPHINE (PROBUPHINE & SUBLOCADE ) Policy Number: PHARMACY 291.5 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
Topical antimicrobials (antiseptics) Iodine, Silver, Honey
 Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
MEDICALLY NECESSARY ORTHODONTIC TREATMENT
 UnitedHealthcare Dental Coverage Guideline MEDICALLY NECESSARY ORTHODONTIC TREATMENT Guideline Number: DCG003.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT
UnitedHealthcare Dental Coverage Guideline MEDICALLY NECESSARY ORTHODONTIC TREATMENT Guideline Number: DCG003.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT
Granulox Update on evidence and science
 Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude
 Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
EMBOLIZATION OF THE OVARIAN AND ILIAC VEINS FOR PELVIC CONGESTION SYNDROME
 UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare Benefits of Texas,
UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare Benefits of Texas,
Diabetic/Neuropathic Foot Ulcer Assessment Guide South West Regional Wound Care Program Last Updated April 7,
 Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
DIABETES AND THE AT-RISK LOWER LIMB:
 DIABETES AND THE AT-RISK LOWER LIMB: Shawn M. Cazzell Disclosure of Commercial Support: Dr. Shawn Cazzell reports the following financial relationships: Speakers Bureau: Organogenesis Grants/Research Support:
DIABETES AND THE AT-RISK LOWER LIMB: Shawn M. Cazzell Disclosure of Commercial Support: Dr. Shawn Cazzell reports the following financial relationships: Speakers Bureau: Organogenesis Grants/Research Support:
V.A.C. Therapy Patient Guide. Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you.
 V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
Jonathan I. Rosenblum, DPM 1 ; Michael I. Gazes, DPM 2 ; Nachum Greenberg, MD 1
 ORIGINAL RESEARCH Surface Acoustic Wave Patch Therapy Affects Tissue Oxygenation In Ischemic Feet Jonathan I. Rosenblum, DPM 1 ; Michael I. Gazes, DPM 2 ; Nachum Greenberg, MD 1 WOUNDS 2014;26(10):301-305
ORIGINAL RESEARCH Surface Acoustic Wave Patch Therapy Affects Tissue Oxygenation In Ischemic Feet Jonathan I. Rosenblum, DPM 1 ; Michael I. Gazes, DPM 2 ; Nachum Greenberg, MD 1 WOUNDS 2014;26(10):301-305
FIXED PROSTHODONTICS
 FIXED PROSTHODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG017.02 Effective Date: May 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
FIXED PROSTHODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG017.02 Effective Date: May 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
ROUTINE FOOT CARE. Policy Number: OUTPATIENT T1 Effective Date: October 1, 2017
 ?; ] ROUTINE FOOT CARE UnitedHealthcare Oxford Clinical Policy Policy Number: OUTPATIENT 023.22 T1 Effective Date: October 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
?; ] ROUTINE FOOT CARE UnitedHealthcare Oxford Clinical Policy Policy Number: OUTPATIENT 023.22 T1 Effective Date: October 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
PANNICULECTOMY AND BODY CONTOURING PROCEDURES
 Oxford UnitedHealthcare Oxford Clinical Policy PANNICULECTOMY AND BODY CONTOURING PROCEDURES Policy Number: SURGERY 038.24 T2 Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
Oxford UnitedHealthcare Oxford Clinical Policy PANNICULECTOMY AND BODY CONTOURING PROCEDURES Policy Number: SURGERY 038.24 T2 Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device
 VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Forward-looking Statement Disclaimer
 Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
NEGATIVE PRESSURE WOUND THERAPY
 Oxford NEGATIVE PRESSURE WOUND THERAPY UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 110.1 T2 Effective Date: January 1, 2019 Instructions for Use Table of Contents Page CONDITIONS OF
Oxford NEGATIVE PRESSURE WOUND THERAPY UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 110.1 T2 Effective Date: January 1, 2019 Instructions for Use Table of Contents Page CONDITIONS OF
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers
 A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
Corporate Medical Policy
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Ultrasound Transient Elastography for Liver Fibrosis
 Ultrasound Transient Elastography for Liver Fibrosis Protocol: RAD042 Effective Date: June 1, 2018 Table of Contents Page COMMERCIAL, MEDICARE & MEDICAID COVERAGE RATIONALE... 1 DESCRIPTION OF SERVICES...
Ultrasound Transient Elastography for Liver Fibrosis Protocol: RAD042 Effective Date: June 1, 2018 Table of Contents Page COMMERCIAL, MEDICARE & MEDICAID COVERAGE RATIONALE... 1 DESCRIPTION OF SERVICES...
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema
 End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
SPINRAZA (NUSINERSEN)
 SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
ORIGINAL PAPERS. Comparison of High-Frequency and MIST Ultrasound Therapy for the Healing of Venous Leg Ulcers
 ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 6, 969 975 ISSN 1899 5276 Copyright by Wroclaw Medical University Akram Beheshti 1, A, C, F, Younes Shafigh 2, A, B, Hossien Parsa 2, E, F, Amir A. Zangivand
ORIGINAL PAPERS Adv Clin Exp Med 2014, 23, 6, 969 975 ISSN 1899 5276 Copyright by Wroclaw Medical University Akram Beheshti 1, A, C, F, Younes Shafigh 2, A, B, Hossien Parsa 2, E, F, Amir A. Zangivand
MOTORIZED SPINAL TRACTION
 MOTORIZED SPINAL TRACTION UnitedHealthcare Commercial Medical Policy Policy Number: PAI010 Effective Date: January 1, 2019 Table of Contents Page COVERAGE RATIONALE... 1 APPLICABLE CODES... 1 DESCRIPTION
MOTORIZED SPINAL TRACTION UnitedHealthcare Commercial Medical Policy Policy Number: PAI010 Effective Date: January 1, 2019 Table of Contents Page COVERAGE RATIONALE... 1 APPLICABLE CODES... 1 DESCRIPTION
Peripheral Subcutaneous Field Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy Title: Debridement of diabetic foot ulcers
 Clinical Policy Title: Debridement of diabetic foot ulcers Clinical Policy Number: CCP.1200 Effective Date: January 1, 2016 Initial Review Date: October 19, 2015 Most Recent Review Date: October 2, 2018
Clinical Policy Title: Debridement of diabetic foot ulcers Clinical Policy Number: CCP.1200 Effective Date: January 1, 2016 Initial Review Date: October 19, 2015 Most Recent Review Date: October 2, 2018
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity. Original Effective Date: 7/8/2015
 Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
Subject: Gastrointestinal Electrical Stimulation (GES) and Vagus Nerve Blocking Therapy (VBLOC) for Obesity Original Effective Date: 7/8/2015 Policy Number: MCP-243 Revision Date(s): Review Date: 12/16/15,
Emil Schmidt Wound Care specialist SDHB - Otago
 Prospective randomised trial of low frequency ultrasound debridement (LFUD) in management of lower limb wounds Krysa J, Schmidt E, Thomson I, van Rij A Emil Schmidt Wound Care specialist SDHB - Otago Background
Prospective randomised trial of low frequency ultrasound debridement (LFUD) in management of lower limb wounds Krysa J, Schmidt E, Thomson I, van Rij A Emil Schmidt Wound Care specialist SDHB - Otago Background
Venous. Arterial. Neuropathic (e.g. diabetic foot ulcer) Describe Wound Types & Stages of. Pressure Ulcers. Identify Phases of Healing & Wound Care
 A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F
 Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F Record Status This is a critical abstract of an economic evaluation that
Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F Record Status This is a critical abstract of an economic evaluation that
