Vesicular Trafficking of Semaphorin 3A is Activity- Dependent and Differs Between Axons and Dendrites
|
|
|
- Frank Boyd
- 6 years ago
- Views:
Transcription
1 Traffic 6; 7: 6 77 Blackwell Munksgaard Copyright # Blackwell Munksgaard 6 doi:./j x Vesicular Trafficking of Semaphorin A is Activity- Dependent and Differs Between Axons and Dendrites Joris de Wit, Ruud F. Toonen, Joost Verhaagen and Matthijs Verhage, * Department of Functional Genomics, Center for Neurogenomics and Cognitive Research (CNCR), Vrije Universiteit (VU) and VU Medical Center (VUmc), De Boelelaan 87, 8 HV Amsterdam, the Netherlands Neuroregeneration Laboratory, Netherlands Institute for Brain Research, Meibergdreef, 5 AZ Amsterdam, Amsterdam, the Netherlands *Corresponding author: Matthijs Verhage, matthijs@cncr.vu.nl Secreted semaphorins act as guidance cues in the developing nervous system and may have additional functions in mature neurons. How semaphorins are transported and secreted by neurons is poorly understood. We find that endogenous semaphorin A (SemaA) displays a punctate distribution in axons and dendrites of cultured cortical neurons. GFP SemaA shows a similar distribution and co-localizes with secretory vesicle cargo proteins. Live-cell imaging reveals highly dynamic trafficking of GFP SemaA vesicles with distinct properties in axons and dendrites regarding directionality, velocity, mobility and pausing time. In axons, most GFP SemaA vesicles move fast without interruption, almost exclusively in the anterograde direction, while in dendrites many GFP SemaA vesicles are stationary and move equally frequent in both directions. Disruption of microtubules, but not of actin filaments, significantly impairs GFP SemaA transport. Interestingly, depolarization induces a reversible arrest of axonal transport of GFP SemaA vesicles but has little effect on dendritic transport. Conversely, action potential blockade using tetrodotoxin (TTX) accelerates axonal transport, but not dendritic transport. These data indicate that axons and dendrites regulate trafficking of SemaA and probably other secretory vesicles in distinct ways, with axons specializing in fast, uninterrupted, anterograde transport. Furthermore, neuronal activity regulates secretory vesicle trafficking in axons by a depolarizationevoked trafficking arrest. Key words: axon guidance, axonal transport, large dense core vesicle, live-cell imaging, plasticity, secretory granule, semaphorin Received September 5, revised and accepted for publication 9 April 6, published on-line 5 May 6 Secreted semaphorins are essential for the proper wiring of the developing nervous system (). Semaphorin A (SemaA) is a secreted guidance molecule that acts as a chemorepellent for axonal growth cones (). The effects of secreted semaphorins in the nervous system are mediated by a receptor complex consisting of ligandbinding neuropilin- (Npn-) or Npn- and signal-transducing plexins (). Activation of the receptor complex initiates an intricate signaling cascade leading to reorganization of the cytoskeleton (4). SemaA and SemaF null mutant mice show extensive defasciculation of peripheral projections and guidance defects in the central nervous system (CNS) (5 7), which are largely phenocopied in null mutants of their respective receptor components Npn- and Npn- (8 ). Both SemaA and SemaF continue to play a role in the wiring of the postnatal brain by promoting pruning of specific hippocampal projections (). Recent evidence indicates that the role of secreted semaphorins may extend beyond defining the connectivity pattern of the developing brain (). SemaA stimulates growth and branching of cortical dendrites in slice cultures (), and secreted semaphorin-npn- signaling has been shown to regulate the complexity of cortical basal dendrites in vivo (4). Expression of secreted semaphorins persists in adult neurons (5,6) and can be modulated by electrical activity (7,8). Moreover, the secreted semaphorin SemaF can alter synaptic transmission in adult hippocampal slices (9). Together, these studies suggest a role for secreted semaphorins in structural and synaptic plasticity. However, how secreted semaphorins exert their effects on dendritic complexity and synaptic transmission is unclear, as it is unknown how semaphorins are transported and released by neurons. Furthermore, whether transport of secreted semaphorins depends on neuronal activity is unresolved. Here, we investigated the trafficking of the secreted semaphorin SemaA in cortical neurons. SemaA is highly expressed in the developing neocortex (,) and continues to be expressed in subpopulations of cortical neurons in adulthood (5). We show that a previously characterized GFP SemaA fusion protein () closely reproduces the punctate distribution of the endogenous protein in cortical neurons and is transported in secretory vesicles along microtubules. Live-cell imaging of transfected neurons revealed striking differences in the dynamics of GFP SemaA vesicular trafficking in axons and dendrites. Compared to axonal vesicles, dendritic GFP SemaA vesicles moved slower, paused more frequently and were often stationary. Furthermore, axonal trafficking of GFP SemaA was strongly influenced by neuronal activity, whereas dendritic trafficking was not. Thus, both the dynamics and the activity dependence of GFP SemaA trafficking in neurons differ between axons 6
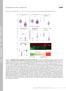
2 Activity-Dependent Vesicular Trafficking of SemaA and dendrites. These data indicate that axons and dendrites regulate trafficking of SemaA and probably other secretory vesicles in distinct ways. Results Punctate distribution of endogenous SemaA in axons and dendrites of cortical neurons We first analyzed the localization of endogenous SemaA protein in neurons. Cultured neurons derived from E8 entorhinal and piriform cortex, cortical regions with high SemaA expression during embryonic development (,), were used. The SemaA C-7 antibody (Ab) used is directed against epitopes in the C-terminus of SemaA. At 6 days in vitro (DIV), prominent SemaA labeling was observed in axons and growth cones (Figure A). SemaA puncta displayed a similar distribution as synapsin-positive synaptic vesicles (Figure B), which were present in the axon shaft and accumulated in the growth cones of immature neurons (). Despite their similar distribution, SemaA puncta showed little colocalization with synaptic vesicles in growth cones and neurites (Figure C), suggesting that SemaA is sorted to a distinct vesicular compartment. In more mature neurons (7 DIV), SemaA showed a punctate distribution in cell body and neurites (Figure D). Microtubule-associated protein- (MAP) labeling was used to identify the somatodendritic compartment (Figure E). Discrete SemaApositive puncta localized to both MAP-positive dendrites and MAP-negative axons (Figure F,H). A similar punctate distribution in neurons was found using two different Abs against SemaA (data not shown). In addition to the labeling in neurites, a punctate SemaA labeling outlined the membrane of neuronal cell bodies (Figure G), suggesting association with the cell surface in this compartment. To confirm the specificity of the Ab used, we stained neurons expressing recombinant GFP SemaA () with the SemaA C-7 Ab. Extensive overlap between SemaA Ab staining and GFP SemaA A B C SemaA D Synapsin E F * * SemaA MAP G H I GFP-SemaA J SemaA K Figure : Endogenous SemaA shows a punctate distribution in neurites of cortical neurons. A C, axonal growth cone (6 DIV) labeled with SemaA C-7 (A) and synapsin (B) antibodies. A, SemaA-positive puncta in axon and growth cone (arrowheads). B, Synapsin labeling. C, d image. D, SemaA immunocytochemistry on mature neuron (7 DIV). E, MAP staining identifies dendrites (arrowheads). F, d image shows punctate SemaA distribution in both dendrites and axons (arrow). Asterisks indicate another axon extending along neuron in (F). G, SemaA-positive puncta outline the cell body and are present in dendrite (arrowheads). MAP signal not shown. H, Detail of axon-showing punctate SemaA labeling. I, GFP SemaA expressed in cortical neuron ( DIV). Image shows segment of axon. J, SemaA immunocytochemistry of the axon shown in (I). K, d image shows extensive overlap of GFP SemaA fluorescence and SemaA antibody staining in yellow (indicated with arrowheads). Scale bar in (A C) is 5 mm and in (D K) is mm. Traffic 6; 7:
3 de Wit et al. fluorescence was observed, showing that the Ab recognizes SemaA (Figure I K). GFP SemaA mimics the punctate distribution of SemaA in cortical axons and dendrites Expression of GFP SemaA in cortical neurons resulted in a characteristic punctate distribution of GFP SemaA fluorescence, similar to endogenous SemaA (Figure ). Discrete GFP SemaA puncta localized to axons and growth cones of DIV 6 neurons (Figure A) and showed little co-localization with synaptic vesicles (Figure C), consistent with the distribution of endogenous SemaA (Figure A C). A punctate GFP SemaA distribution was also observed in cell bodies and dendrites of DIV 6 neurons (Figure D G). In more mature neurons (DIV 4), GFP SemaA puncta were observed in both axons and dendrites and could be detected at large distances from the cell body (Figure H O). GFP SemaA puncta were occasionally found in the vicinity of presynaptic terminals (Figure L O). Quantification showed that 8.9.% (mean SEM) of synapsin-labeled terminals partially overlapped with GFP SemaA puncta (n ¼ 586 synapses measured in three cells). Thus, the expression of GFP SemaA in cortical neurons results in a punctate distribution that closely mimics that of the endogenous protein. SemaA is transported in secretory vesicles Because SemaA is a secreted protein, we co-expressed fluorescent versions of SemaA with fluorescently labeled vesicle cargo proteins to determine whether the SemaA puncta in neurons represent a vesicular compartment. Monomeric red fluorescent protein (mrfp) SemaA showed a punctate distribution in neurons (Figure A), identical to that of GFP SemaA (data not shown). mrfp SemaA was co-expressed with tissue plasminogen activator GFP (tpa GFP), which is transported in secretory vesicles of the regulated secretory pathway in neuroendocrine cells and hippocampal neurons (4,5). In A B C GFP-SemaA D Synapsin E F G GFP-SemaA Synapsin MAP H K L GFP-SemaA I M Synapsin J N O MAP Figure : Punctate localization of GFP SemaA in neurites of cortical neurons. A C, Axonal growth cone (6 DIV). A, GFP SemaA puncta (arrowheads) in axon and growth cone. B, Synapsin labeling. C, d image. Open arrowhead indicates co-localization. D G, Cortical neuron (6 DIV), transfected at DIV. D, GFP SemaA. GFP SemaA puncta are present in axon (indicated with arrow), dendrites and dendritic growth cones (arrowheads). E, Synapsin labeling. F, MAP labeling. G, d image. GFP SemaA puncta occasionally co-localize with synapses (open arrowhead). H O, Cortical neuron (DIV 4). H, GFP SemaA. I, Synapsin labeling. J, MAP labeling. K, d image. Arrow indicates axon. L O, Higher magnification of boxed image in (K). L, GFP SemaA. M, Synapsin labeling. N, MAP labeling. O, d image. Some dendritic GFP SemaA puncta are localized in the vicinity of synapses (open arrowheads). Scale bar in (A C) is 5 mm and in (D O) is mm. 6 Traffic 6; 7: 6 77
4 Activity-Dependent Vesicular Trafficking of SemaA A B C * * * mrfp-semaa D tpa-gfp E F G H I SemaA Secretogranin II Figure : SemaA is transported in secretory vesicles. A F, Cortical neuron (DIV 6) co-transfected with mrfp SemaA (A,D) and secretory granule marker tpa GFP (B,E) at DIV. Note the characteristic labeling of the extracellular substrate by mrfp SemaA (indicated with asterisk), but not by tpa GFP. d image (C,F) shows extensive co-localization (yellow). D F, Higher magnification of boxed area in (C). Arrowheads indicate co-localizing puncta of mrfp SemaA and tpa GFP. G I, DIV 6 cortical neuron double-labeled with SemaA antibody (G) and SgII antibody (H). d image (I) shows co-localization of SemaA and SgII-positive puncta (indicated with arrowheads). Scale bar in (A C) is mm and in (D I) is 5 mm. cortical neurons, tpa GFP displayed a punctate distribution in cell bodies and neurites (Figure B). We observed an extensive co-localization of mrfp SemaA with tpa GFP (Figure A F). Quantification showed that % of mrfp SemaA puncta overlapped with tpa GFP (n ¼ puncta in five cells). Similar co-localization values were obtained for both axons and dendrites. Co-expression of mrfp SemaA with neuropeptide Y Venus, another secretory vesicle cargo protein (5 7), also resulted in extensive co-localization ( % overlap; n ¼ puncta in five cells). In contrast, GFP SemaA showed a modest co-localization with the presynaptic marker VAMP mrfp in axons (. 7.6% overlap; n ¼ 54 puncta in four cells), and little co-localization was observed between GFP SemaA and transferrin-labeled endosomes in dendrites (..7% overlap; n ¼ 46 puncta in four cells). Thus, mrfp SemaA is transported in the same type of vesicle as the exogenously expressed secretory vesicle cargo proteins tpa GFP and NPY Venus. To analyze whether endogenous SemaA localizes to secretory vesicles in neurons, we performed double immunocytochemistry for SemaA and secretogranin II (SgII), a secretory granule marker. This revealed a partial co-localization in cortical neurons (Figure G I). Quantification showed that 4..% (n ¼ puncta in five cells) of SemaA-positive puncta co-localized with SgII-labeled puncta. Similar values were obtained for both axons and dendrites. We attempted to confirm the localization of endogenous SemaA to secretory vesicles at the ultrastructural level, but localization studies at the electron microscopic level using immunogold proved not feasible with the currently available Ab (data not shown). SemaA was originally isolated from brain membrane fractions (). To determine whether some GFP SemaA puncta may reside on the neuronal cell surface, we labeled living, non-permeabilized neurons with an anti-gfp Ab. Live labeling revealed bright clusters of GFP fluorescence on the surface of dendrites of GFP SemaA-expressing neurons (Figure 4A D). Surface expression of GFP SemaA was also observed on axons (Figure 4E), as well as on cell bodies (Figure 4F H), where the punctate staining outlining the cell body was reminiscent of the distribution of endogenously expressed SemaA (Figure G). Attempts to label endogenous SemaA on the cell surface with three different Abs were unsuccessful (data not shown). The punctate cell surface distribution of GFP SemaA was specific, because surface labeling was absent on neurons expressing GFP alone (Figure 4I K). The surface expression of GFP SemaA suggests that GFP SemaA can be secreted from both axons and dendrites and subsequently bind to negative charges on the cell surface (). To test whether secreted GFP SemaA might be released and subsequently endocytosed by neighboring non-transfected neurons, cultured neurons were incubated with GFP SemaA-conditioned medium (CM), obtained from GFP SemaA-expressing HEK9 cells (). GFP SemaA CM did not produce a detectable punctate staining in neurons and did not co-localize with Traffic 6; 7:
5 de Wit et al. A B C D GFP-SemaA Anti-GFP E F G H GFP-SemaA Anti-GFP I J K GFP * * * Anti-GFP Figure 4: SemaA puncta localize to the neuronal cell surface and cytoplasm. A D, Cortical neuron (6 DIV) live-labeled with an anti- GFP antibody. A, GFP SemaA. B, Anti-GFP labeling. C, d image shows dendritic surface GFP SemaA clusters in yellow (indicated with arrowheads). Arrow indicates axon. D, Higher magnification of boxed area in (C) showing surface GFP SemaA clusters on dendrite (arrowheads). E, Detail of axon showing GFP SemaA surface clusters in yellow (arrowheads). F H, Surface GFP SemaA clusters outlining the cell body (arrowheads). Some clusters appear red but not green due to the sensitivity of anti-gfp staining that yields a stronger signal than that of GFP SemaA fluorescence. I K, GFP SemaA surface staining is specific because live-cell labeling of GFPexpressing neurons with GFP antibody shows no surface expression. Asterisk in (I K) indicates the position of the cell body. Scale bar in (A C; E K) is mm and in (D) is 5 mm. simultaneously added transferrin (data not shown). The only labeling observed was on the surface of glial cells on which the neurons were grown, similar to the fluorescent halo often observed around XFP SemaA-expressing neurons (Figure A). This suggests that secreted GFP SemaA adheres mainly to the surface and substrate of the transfected neuron and does not produce a punctate distribution in neighboring non-transfected cells. Live-cell imaging reveals distinct characteristics of GFP SemaA trafficking in axons and dendrites To analyze the dynamics of GFP SemaA vesicle transport in cortical neurons, we imaged live GFP SemaA-expressing neurons. Figure 5A shows frames of a time-lapse movie of GFP SemaA trafficking in an axonal segment (Supplementary Movie ). The identity of axons and dendrites of imaged neurons was confirmed by retrospective labeling for MAP (data not shown). Vesicles displayed rapid movements in both the anterograde and the retrograde direction, sometimes interrupted by stationary periods, and could be observed to merge, separate or suddenly change direction. GFP SemaA carriers were invariably spherical in shape. The positions of three representative vesicles are indicated in Figure 5A to illustrate different types of movement. Some axonal vesicles remained stationary during imaging (vesicle ), but most moved in the anterograde direction (vesicle ). In addition, some vesicles changed their direction several times during imaging (vesicle ) or moved in the retrograde direction. The corresponding kymograph of this axonal segment is shown in Figure 5C, and the trajectories of the numbered vesicles indicated in Figure 5A have been highlighted. The majority of axonal GFP SemaA vesicles displayed continuous movement in the anterograde direction, with few stationary puncta (Figure 5C). Dendritic transport of GFP SemaA was far less dynamic (Figure 5B, Supplementary Movie ). Most vesicles in this time-lapse sequence remained stationary during the course of imaging (vesicle ). The vesicles that did move often displayed only small movements (vesicle ) or changed their direction several times (vesicle ). The corresponding kymograph shows that vertical lines of stationary vesicles are dominant in dendrites and that continuous movements in the anterograde direction are largely absent (Figure 5D). The difference in dynamics of GFP SemaA trafficking in axons and dendrites is readily observed when both kymographs are compared (Figure 5C,D). To quantify the dynamics of GFP SemaA trafficking, individual GFP SemaA vesicles in axons and dendrites were manually tracked. We discerned four categories of movement: anterograde and retrograde vesicles, bi-directional (vesicles that changed direction during the course of imaging) and stationary vesicles. Velocities were corrected for stationary periods and defined as the total distance 64 Traffic 6; 7: 6 77
6 Activity-Dependent Vesicular Trafficking of SemaA A :44 B :58 :48 : :5 :6 :56 : : :4 C D :6 :7 µm µm E Frequency (%) 5 5 F G H * 6.4 ** Axon Dendrite Axon Dendrite 5. to.. to.8.8 to.6.6 to.4.4 to.. to to.. to.4 Moving puncta (%).4 to.6.6 to.8.8 to.. to.. to.4.4 to.6.6 to.8.8 to. Pausing time (%) Axon Axon Dendrite. to.. to.4.4 to.6 ** Dendrite Figure 5: Legend continued on next page Traffic 6; 7:
7 de Wit et al. Table : Velocities and contribution to total transport of GFP SemaA vesicles in axons and dendrites Velocity (mm/s) Axon Anterograde Retrograde Bi-directional... Stationary 4.9 Dendrite Anterograde..5.8 Retrograde Bi-directional Stationary 47. % of total Data based on n ¼ 6 cells, 4 vesicles tracked. Values represent mean SEM. traveled divided by the time spent moving. A frequency histogram plotting the velocities of GFP SemaA vesicles moving in the anterograde and retrograde direction shows that the velocity distributions for axonal and dendritic vesicles were distinct [Figure 5E; p <., Kolmogorov Smirnov (KS) test]. Anterograde transport in axons was faster than in dendrites, and a population of fast anterograde moving axonal vesicles reached maximum velocities of.6 mm/s (Figure 5E). The average velocity of anterograde vesicles was.78. mm/s (mean SEM) in axons compared with..5 mm/ s in dendrites (Table ). The value of.78 mm/s is consistent with previously reported velocities for anterograde axonal transport vesicles carrying membrane proteins in sensory neurons (8). Similar values were obtained for vesicles carrying NPY Venus (data not shown). The majority of axonal vesicles moved in the anterograde direction (Table ), resulting in net anterograde transport of GFP SemaA vesicles in axons. Because trafficking in the initial dendritic segment was often too dense to distinguish individual vesicles, we measured the trafficking of vesicles in proximal dendrites at least mm away from the cell body. In contrast to axonal vesicles, dendritic vesicles did not display a clear preference for the anterograde or retrograde direction and a large percentage moved bi-directionally (Table ). A comparison of GFP SemaA transport characteristics in axons and dendrites showed significant differences for all three parameters examined. First, the average velocity of all moving vesicles was significantly higher in axons than in dendrites (.65.8 mm/s in axons versus.9.5 mm/s in dendrites; p ¼.; Figure 5F). Second, axons contained more mobile vesicles than dendrites ( % moving vesicles in axons versus % in dendrites; p ¼.5; Figure 5G). Third, moving vesicles in dendrites had longer pausing times (defined as the time vesicles were stationary divided by the total time a moving vesicle could be followed) than axonal vesicles (..5% paused time in axons versus % in dendrites; p ¼.5; Figure 5H). Thus, dendritic GFP SemaA-containing vesicles move slower, are less mobile and pause more frequently than axonal vesicles. Microtubule-dependent axonal transport of GFP SemaA To determine whether SemaA vesicular transport depends on an intact cytoskeleton, GFP SemaA-expressing neurons were treated for h with cytoskeleton-disrupting drugs and subsequently imaged in the presence of the drug. The microtubule network (visualized with an antitubulin Ab) and actin cytoskeleton (visualized with phalloidin) of a representative vehicle-treated neuron are shown in Figure 6A and D, respectively. Treatment with the microtubule-depolymerizing agent nocodazole ( mm) reduced the tubulin labeling in axons and dendrites (Figure 6B) but did not affect the actin cytoskeleton in the same neuron (Figure 6E). Nocodazole treatment did not completely disrupt the microtubule network. Higher doses of nocodazole ( mm) were more effective but also severely disturbed neuronal morphology (data not shown). In addition, a higher dose of nocodazole has Figure 5: Distinct characteristics of GFP SemaA trafficking in axons versus dendrites. A,B, Frames from time-lapse movies showing axonal (A) and dendritic (B) trafficking of GFP SemaA in cortical neurons (DIV 4 7). Cell bodies are located to the left outside of the picture frame. Images have been inverted for clarity. A, Numbers indicate the positions of a stationary vesicle (, red), an anterograde vesicle (, green) and a vesicle moving back and forth (, blue). B, Numbers indicate the positions of a stationary vesicle (, red), a vesicle moving back and forth (, green) and a vesicle displaying a short retrograde displacement (, blue). The identity of axons and dendrites was verified by retrospective immunocytochemistry for MAP (data not shown). C, Kymograph plotting GFP SemaA fluorescence on a line drawn along the axonal segment shown in (A) for each movie frame. The trajectories of the numbered vesicles indicated in (A) are highlighted in the corresponding colors. Stationary vesicles appear as vertical lines, and anterograde vesicles appear as downward sloping lines. Most vesicles display continuous anterograde movement. D, Kymograph depicting GFP SemaA transport in the dendritic segment is shown in (B). The trajectories of the numbered vesicles indicated in (B) are highlighted in the corresponding colors. Most vesicles are stationary, and uninterrupted movement is largely absent. E, Frequency histogram showing velocities of anterograde and retrograde (indicated as negative values) moving vesicles in axons (grey bars) versus dendrites (black bars). Velocities are defined as the total distance traveled divided by the time spent moving. F, The average velocity of all moving vesicles (anterograde, retrograde and bi-directional) is significantly lower in dendrites compared with axons. G, The percentage moving puncta is significantly lower in dendrites compared with axons. H, The pausing time of moving vesicles (expressed as the time vesicles were stationary divided by the total time a moving vesicle could be followed) is significantly higher in dendrites than in axons. All vesicles visible in the first frame of time-lapse movies were manually tracked through the entire movie or as long as they could be followed. N ¼ 6 cells, 4 vesicles tracked. Bar graphs show mean SEM. *p <.5; **p <., Student s t-test. Scale bar in (A,B) is 5 mm. 66 Traffic 6; 7: 6 77
8 Activity-Dependent Vesicular Trafficking of SemaA A dmso B nocodazole C latrunculin A Tubulin Tubulin Tubulin D dmso E nocodazole F latrunculin A Phalloidin Phalloidin Phalloidin G Frequency (%).9 I J 9 K * * ** dmso noco lat. A dmso noco lat. A dmso noco lat. A.8 to.6.4 to.. to.8.6 to.4. to. to.4.6 to.8 Moving puncta (%) dmso noco. to..4 to.6.8 to.. to.4 H Frequency (%) to.6.4 to.. to.8.6 to.4. to Pausing time (%). to.4.6 to.8 dmso lat. A. to..4 to.6.8 to.. to.4 Figure 6: Microtubule-based transport of GFP SemaA in cortical neurons. Neurons (DIV 5 6) were treated for h at 7 C with vehicle (dmso), mm nocodazole (noco) or 5 mm latrunculin A (lat. A) and subsequently imaged in the presence of the drug. A C, Anti-tubulin-stained microtubule networks of a vehicle-treated neuron (A), nocodazole-treated neuron (B) and latrunculin A-treated neuron (C). D F, Phalloidin-labeled actin cytoskeleton of the same neurons shown in (A C). Images of different treatment conditions were taken with identical settings. G, Frequency histogram showing velocities of anterograde and retrograde (indicated as negative values) moving vesicles in axons of vehicle- (grey bars) and nocodazole- (black bars) treated neurons. H, Frequency histogram showing velocities of anterograde and retrograde axonal vesicles in vehicle- (grey bars) and latrunculin- (white bars) treated neurons. I, Nocodazole treatment results in a significant decrease in average velocity of all moving vesicles in axons. J, Nocodazole treatment significantly decreases the percentage of moving vesicles. K, Nocodazole treatment significantly increases the pausing time of moving vesicles. Latrunculin A treatment has no significant effect on average velocity, percentage mobile vesicles and pausing time of GFP SemaA vesicles. Values obtained for vehicle-treated neurons do not differ significantly from untreated neurons (data not shown). Bar graphs show mean SEM. N ¼ 9 neurons, 4 vesicles tracked (dmso); n ¼ neurons, 67 vesicles tracked (noco); and n ¼ 9 neurons, vesicles tracked (lat. A). Statistical significance was determined using Student s t-test, and overall group differences were analyzed using one-way analysis of variance (ANOVA). *p <.5; **p <.. Scale bar in (A F) is mm. Traffic 6; 7:
9 de Wit et al. been shown to disrupt neuronal morphology to such an extent that the actin cytoskeleton appears to be affected as well (9). Treatment with the actin filament depolymerizing drug latrunculin A (5 mm) strongly reduced actin-labeling (Figure 6F) but left the microtubule network intact (Figure 6C). Following nocodazole treatment, anterograde and retrograde GFP SemaA transport in axons was strongly reduced (Table ). The frequency histogram in Figure 6G shows a significant shift toward lower velocities for both retrograde and anterograde moving vesicles in nocodazoletreated neurons compared with vehicle-treated cells (Figure 6G; p <.5, KS test). Nocodazole treatment significantly reduced the average velocity of all GFP SemaA axonal vesicles (.7.7 mm/s in vehicle-treated cells versus.45.7 mm/s in nocodazole-treated cells; p ¼.4; Figure 6I). In addition, the percentage of moving vesicles was reduced in nocodazole-treated cells ( % in vehicle-treated cells versus. 7.% in nocodazole-treated cells; p ¼.; Figure 6J). Furthermore, nocodazole treatment resulted in an increase in pausing time (4..% in vehicle-treated cells versus % in nocodazole-treated cells; p ¼.8; Figure 6K). In contrast, none of these parameters was affected by treatment with latrunculin A. The velocities of anterograde and retrograde vesicles in latrunculin A-treated cells showed a similar distribution as in vehicle-treated cells (Figure 6H; p >.5, KS test). Average velocity, percentage moving vesicles and pausing time were not significantly different in latrunculin A-treated cells (Figure 6I K and Table ). Thus, disruption of the microtubule network, but not of actin filaments, reduced the average velocity and mobility and increased the pausing time of GFP SemaA vesicles. GFP SemaA trafficking is activity dependent We next examined whether trafficking of GFP SemaA in cortical neurons depends on neuronal activity. Neurons were transferred to the imaging chamber and subsequently imaged in depolarizing Tyrode s solution containing 6 mm KCl. Depolarization strongly reduced the average velocity of GFP SemaA vesicles in axons (.7.5 mm/s in control neurons versus..4 mm/s in depolarized neurons; p <.; Figure 7A and Table ). The effect of 6 mm KCl-induced depolarization on the velocity of moving vesicles was dependent on the presence of Ca þ in the extracellular medium, because imaging in Tyrode s containing 6 mm KCl, 5 mm EGTA and mm Ca þ did not significantly affect the velocity of moving vesicles in axons (Figure 7A). Conversely, blocking action potentials with mm tetrodotoxin (TTX) caused an increase in the average velocity of axonal moving vesicles (.7.5 mm/s in control neurons versus.. mm/s in TTX-treated neurons; p ¼.8; Figure 7A). Similar effects were found when the percentage mobile vesicles was analyzed in axons of depolarized and TTX-treated neurons. Depolarization induced a strong decrease in the percentage moving vesicles in axons ( % in control neurons versus. 4.9% in depolarized cells; p <.; Figure 7B). Treatment with TTX caused a significant increase in the percentage of mobile vesicles ( % in control neurons versus % in TTX-treated cells; p ¼.96; Figure 7B). Analysis of the velocity profile of anterograde and retrograde moving vesicles in axons showed a significant shift toward lower velocities in 6 mm KCl-treated cells (Figure 7C; p <., KS test) and a significant shift toward higher velocities in TTX-treated cells (Figure 7D; p <., KS test). Both retrograde and anterograde moving vesicles were affected by these treatments (Figure 7C,D and Table ). The velocity profile of axons imaged in 5 mm EGTA and mm Ca þ did not differ significantly from controls (p >.5, KS test). In contrast to the activity-dependent trafficking of GFP SemaA vesicles in axons, dendritic GFP SemaA trafficking was largely unaffected by neuronal activity. Depolarizing neurons with 6 mm KCl or blockade of action potentials with TTX had no significant effect on the velocity of GFP SemaA vesicles in dendrites (Figure 7E and Table 4). In dendrites, depolarization caused a significant decrease in the percentage of moving vesicles (4.8.7% in control neurons versus 5.6.7% in depolarized neurons; p ¼.7; Figure 7F). Treatment with TTX did not affect the percentage moving vesicles in dendrites (Figure 7F). The distribution of velocities of anterograde and retrograde vesicles did not differ significantly in 6 mm KCl-, TTX- or 5 mm EGTA, mm Ca þ -treated dendrites (data not shown). Thus, neuronal activity strongly affects both velocity and mobility of GFP SemaA vesicles in axons but has a relatively minor effect on GFP SemaA trafficking in dendrites. Increased neuronal activity results in reduced axonal transport of GFP SemaA, whereas reduced neuronal activity causes an increase in GFP SemaA transport in axons. Depolarization significantly reduced the percentage mobile vesicles in both axons and dendrites (Figure 7B,F and Tables and 4). To test whether the increased number of stationary vesicles in stimulated neurons correlates with an increased SemaA secretion, we took advantage of GFP SemaA s cell surface binding properties (Figure 4). GFP SemaA-transfected neurons were stimulated for 5 min with control or 6 mm KCl-containing Tyrode s in the presence of anti-gfp Ab. Depolarization resulted in an increase in the number of GFP SemaA surface puncta along neuronal processes (Figure 7G). Quantification of the number of surface puncta per length of MAP-positive neurite showed a significant increase in SemaA surface expression in depolarized neurons (4.9. surface puncta in control neurons versus.9. surface puncta per mm MAP-positive neurite in depolarized neurons; p ¼.9; Figure 7H). Thus, the increased number of stationary vesicles in 68 Traffic 6; 7: 6 77
10 Activity-Dependent Vesicular Trafficking of SemaA Table : Axonal transport of GFP SemaA following disruption of the cytoskeleton Vehicle Nocodazole Latrunculin A Velocity (mm/s) % of total Velocity (mm/s) % of total Velocity (mm/s) % of total Anterograde Retrograde Bi-directional Stationary Vehicle: n ¼ 9 neurons, 4 vesicles tracked; nocodazole ( mm): n ¼ neurons, 67 vesicles tracked; latrunculin A (5 mm): n ¼ 9 neurons, vesicles tracked. Values represent mean SEM. processes of depolarized cells correlates with an increased secretion of GFP SemaA. Depolarization induces a reversible arrest of anterograde and retrograde axonal trafficking To determine whether the depolarization-induced arrest in axonal trafficking of GFP SemaA was reversible, we applied a brief s pulse of 6 mm KCl Tyrode s delivered from a pipette positioned near the imaged cell. Figure 8A shows frames of a time-lapse movie of GFP SemaA trafficking in an axonal segment (Supplementary Movie ). Depolarization induced a rapid and robust decrease in axonal transport, which increased again after the stimulus had ended (Figure 8A, Supplementary Movie ). Kymograph analysis showed that the trajectories of most moving vesicles were altered upon stimulus (Figure 8B). After the onset of depolarization, anterograde vesicles transiently slowed down (vesicle ) or became stationary (vesicle ). A similar decrease in velocity was observed for retrograde moving vesicles (vesicle ). Vesicles accelerated again with various delays after the stimulus had ended, causing the reappearance of downward sloping lines corresponding to fast, anterograde moving vesicles in the kymograph (Figure 8B). The trajectories of vesicles in axons stimulated with a s pulse of non-depolarizing control Tyrode s were unaltered (data not shown). Comparison of the velocity profiles of vesicles before and during stimulus showed a significant shift toward lower values for both anterograde and retrograde traffic during depolarization (Figure 8C; p <., KS test). Vesicles showed a normal distribution of anterograde and retrograde velocities after the stimulus had ended (Figure 8D; p >.5, KS test). Quantification showed significant reductions in both the average velocity (.8.9 mm/s before versus.. mm/s during stimulus; p <.; Figure 8E) and the percentage moving vesicles ( % before versus % during stimulus; p <.; Figure 8F) during the 6 mm KCl stimulus. Average velocity and percentage moving vesicles returned to control values after the stimulus had ended (Figure 8E,F). Delivery of a train of action potentials (8 AP, Hz) induced a similar transient decrease in the average velocity of axonal vesicles (Figure 8G). The percentage moving vesicles in axons and the velocity and percentage moving vesicles in dendrites were not affected by field stimulation (data not shown). Thus, depolarization causes a reversible arrest of axonal trafficking of GFP SemaA. Discussion In this study, we investigated the localization and trafficking of the secreted semaphorin SemaA in cortical neurons. SemaA displayed a punctate distribution in axons and dendrites, which was closely reproduced by GFP SemaA. Live-cell imaging revealed distinct differences in the dynamics of GFP SemaA trafficking in axons and dendrites. Furthermore, axonal trafficking of GFP SemaA was strongly dependent on neuronal activity, whereas dendritic transport of GFP SemaA was not. Thus, both the dynamics and the activity dependence of GFP SemaA trafficking in cortical neurons differ between axons and dendrites, indicating that axons and dendrites regulate trafficking of SemaA vesicles in distinct ways. Differential dynamics of SemaA vesicular trafficking in axons and dendrites Expression of GFP SemaA or mrfp SemaA in neurons invariably resulted in a punctate distribution in axons and dendrites, which closely reproduced that of endogenous SemaA. These puncta co-localized extensively with exogenously expressed secretory vesicle cargo proteins tpa GFP or NPY Venus. Both tpa GFP and NPY Venus are sorted into the regulated secretory pathway and are transported in large dense core vesicles (LDCVs) in cultured hippocampal neurons and neuroendocrine cells (4 7). Ultrastructural studies confirm that endogenous NPY localizes to LDCVs in rat brain as well (,). The observed co-localization with established secretory vesicle markers suggests that SemaA can be sorted into the regulated pathway of secretion in neurons. In agreement with this, endogenous SemaA displayed a partial co-localization with SgII, a marker for secretory vesicles (). The remaining SemaA puncta that did not co-localize with SgII might represent membrane-associated SemaA, although we were unable to label endogenous surface SemaA. Alternatively, these puncta could represent SemaA in secretory vesicles that lack SgII. The granin composition of secretory vesicles can vary between individual cells (,), and SgII labels only a subpopulation of secretory Traffic 6; 7:
11 de Wit et al. vesicles in non-neuronal cells (4). We were unable to distinguish between immature or mature secretory vesicles, because a SgII Ab that distinguishes between these types of secretory vesicles in PC cells (5) did not work on cortical neurons. Interestingly, the co-localization between SemaA and secretory vesicle cargoes tpa and NPY suggests that proteins as diverse as NPY, a small peptide, and SemaA, a large glycoprotein, may be stored in the same vesicle, co-transported to their destination and co-released upon the same stimulus. Live-cell imaging revealed a highly dynamic behavior of secretory vesicles carrying GFP SemaA. The observed rapid movements, interrupted by stationary periods, and A B.4 9 **. 8 * ***.4 ***. Control High K + High K + TTX Control High K + High K + TTX Ca + Ca + Moving puncta (%) C Frequency (%) E Control High K F Control High K + High K + TTX Ca + D Frequency (%).8 to.6.4 to.. to.8.6 to.4. to. to.4.6 to.8. to..4 to.6.8 to.. to to.4. to..8 to.6.4 to. Moving puncta (%). to.8.6 to.4. to. to.4 ** Control High K + High K + TTX Ca + Control TTX.6 to.8. to..4 to.6.8 to.. to.4.6 to.8. to. G Surface puncta / µm MAP 6 4 Control High K + Control H 4 8 * High K + Figure 7: Legend continued on next page 7 Traffic 6; 7: 6 77
12 Activity-Dependent Vesicular Trafficking of SemaA Table : Axonal transport of GFP SemaA following depolarization or activity blockade Control High K þ High K þ Ca þ TTX Velocity (mm/s) % of total Velocity (mm/s) % of total Velocity (mm/s) % of total Velocity (mm/s) % of total Anterograde Retrograde Bi-directional Stationary Control (Tyrode s): n ¼ 8 neurons, 9 vesicles tracked; high K þ (6 mm KCl): n ¼ neurons, vesicles tracked; high K þ Ca þ (6 mm KCl, mm Ca þ,5mm EGTA): n ¼ neurons, 78 vesicles tracked; TTX ( mm): n ¼ neurons, 5 vesicles tracked. Values represent mean SEM. Table 4: Dendritic transport of GFP SemaA following depolarization or activity blockade Control High K þ High K þ Ca þ TTX Velocity (mm/s) % of total Velocity (mm/s) % of total Velocity (mm/s) % of total Velocity (mm/s) % of total Anterograde Retrograde Bi-directional Stationary Control (Tyrode s): n ¼ 9 neurons, 5 vesicles tracked; high K þ (6 mm KCl): n ¼ neurons, 74 vesicles tracked; high K þ Ca þ (6 mm KCl, mm Ca þ,5mm EGTA): n ¼ 9 neurons, 5 vesicles tracked; TTX ( mm): n ¼ 7 neurons, 74 vesicles tracked. Values represent mean SEM. sudden reversals of direction are consistent with previous observations of microtubule-based transport in non-neuronal cells (6 9). Indeed, axonal transport of GFP SemaA was dependent on the integrity of the microtubule cytoskeleton. In contrast, disruption of actin filaments did not affect GFP SemaA axonal transport. However, our data do not exclude a role for actin-based local trafficking in the F-actin cortex near the cell membrane (4,4). The axonal and dendritic trafficking of secretory vesicles in neurons is poorly characterized, as most studies have relied on the use of neuroendocrine cells to study vesicle transport (4,4 4). We show here that distinct differences exist between secretory vesicle transport in the axonal and the dendritic domain with regard to directionality, velocity, mobility and pausing time. Axonal secretory vesicles undergo rapid anterograde transport toward distant terminals, whereas dendritic vesicles are largely stationary or move slowly, with no clear preference for the anterograde or retrograde direction. This divergence in axonal and dendritic vesicular trafficking may be due to several reasons. First, microtubules in axons are of uniform polarity, with their plus ends oriented away from the cell body, whereas dendrites contain microtubules of mixed polarity (44,45). The different microtubule organization in these two Figure 7: Trafficking of GFP SemaA in cortical neurons is activity dependent. Neurons (DIV 4 5) were imaged in Tyrode s solution (control); in depolarizing Tyrode s-containing 6 mm KCl (high K þ ); in Tyrode s-containing 6 mm KCl, 5 mm EGTA and mm Ca þ (high K þ Ca þ ) or in Tyrode s-containing mm TTX. Application of 6 mm KCl Tyrode s effectively destained FM 4-64-loaded synapses (data not shown). Prolonged KCl-induced depolarization ( h) in the presence or absence of Ca þ did not affect the viability of neurons, based on propidium iodide staining (data not shown). A D, Axonal trafficking; E,F, Dendritic trafficking. A, 6 mm KCl-induced depolarization results in a strong decrease in the average velocity of moving vesicles in axons compared with control neurons. Treatment with mm TTX significantly increases the average velocity. B, Depolarization induces a significant decrease in the percentage moving vesicles in axons, whereas TTX has the opposite effect. C, Frequency histogram showing velocities of anterograde and retrograde (indicated as negative values) moving vesicles in axons of control (grey bars) and 6 mm KCl- (black bars) treated neurons. D, Frequency histogram showing velocities of anterograde and retrograde axonal vesicles in control (grey bars) and TTX- (dark grey bars) treated neurons. E, The average velocity of moving vesicles in dendrites is not affected by depolarization or TTX. F, Depolarization significantly decreases the percentage moving vesicles in dendrites. Data based on 8 neurons per condition for axonal transport, 7 vesicles tracked; 7 neurons per condition for dendritic transport, 898 vesicles tracked. G,H, Depolarization increases GFP SemaA surface expression. G, Representative dendrites of control (left panel) and 6 mm KCl- (right panel) treated neurons (DIV 4), transfected with GFP SemaA (green), surface stained with anti-gfp (red) and labeled with anti-map (blue) following permeabilization. Dendrites are shown with (top) and without (bottom) GFP SemaA signal. Arrowheads indicate surface puncta. Scale bar, 5 mm. H, Depolarization results in a significant increase in the number of GFP SemaA surface puncta per mm dendrite (n ¼ 8 neurons per condition, 6 puncta). Bar graphs show mean SEM. Statistical significance was determined using Student s t-test, and overall group differences were analyzed using one-way analysis of variance (ANOVA). *p <.5; **p <.; ***p <.. Traffic 6; 7:
13 de Wit et al. A : B : :5 6 mm KCI : : :5 6 µm : C Frequency (%) to.6.4 to.. to.8.6 to.4. to. to.4.6 to.8. to. Before During.4 to.6.8 to.. to.4 D Frequency (%) to.6.4 to.. to.8.6 to.4. to. to.4.6 to.8. to. Before After.4 to.6.8 to.. to.4 E F G ***. Moving puncta (%) 8 6 ** *.4. Before During After Before During After Before During After Figure 8: Depolarization induces a reversible arrest in axonal trafficking of GFP SemaA. A brief -s depolarizing pulse of 6 mm KCl Tyrode s was applied to the cells (DIV 4 5) after s of imaging; neurons were imaged for an additional min. A, Frames from time-lapse movie of axonal GFP SemaA trafficking. Cell body located to the left outside of the picture frame. Images have been inverted. Numbers indicate the positions of a retrograde vesicle (, red) and two anterograde vesicles (, green;, blue). B, Kymograph of the axonal segment shown in (A). The trajectories of the numbered vesicles indicated in (A) are highlighted in the corresponding colors. Anterograde vesicles appear as downward sloping lines. The onset and end of the stimulus are indicated by black arrowheads on either side of the kymograph and the black bar on the right. The trajectories of most moving vesicles are altered upon the onset of stimulus. C, Frequency histogram showing velocities of anterograde and retrograde (indicated as negative values) moving vesicles in the same axonal segment before (grey bars) and during (black bars) stimulus. Before was defined as the last frames before onset of stimulus; during was defined as the last frames of the -s stimulus. D, Frequency histogram showing velocities of anterograde and retrograde vesicles in the same axonal segment before (grey bars) and after (dark grey bars) stimulus. After was defined as the last frames of the timelapse movie. E, The average velocity of GFP SemaA vesicles shows a significant decrease during stimulus. F, The percentage moving puncta is significantly decreased during stimulus. G, The average velocity of GFP SemaA vesicles shows a significant decrease during field stimulus (8 action potentials, Hz). The field stimulus used effectively destained FM 4-64-loaded presynaptic terminals (data not shown). Bar graphs show mean SEM. N ¼ 6 neurons for 6 mm KCl-stimulated cells, 44 vesicles tracked; n ¼ 5 neurons for fieldstimulated cells, 74 vesicles tracked. Statistical significance was determined using Student s t-test, and overall group differences were analyzed using one-way analysis of variance (ANOVA). *p <.5; **p <.; ***p <.. Scale bar in (A) is mm. 7 Traffic 6; 7: 6 77
14 Activity-Dependent Vesicular Trafficking of SemaA domains may account for the net anterograde trafficking of axonal vesicles and the lack of significant net anterograde or retrograde transport of vesicles in proximal dendrites. The high proportion of bi-directionally moving vesicles in dendrites may also result from the mixed polarity of microtubules in dendrites causing vesicles to change track more frequently than in axons, which could also account for the longer pausing time between dendritic vesicle movements. Second, a differential distribution of MAPs, which can interfere with vesicle movement along microtubules (46 49), could contribute to different dynamics of secretory vesicle trafficking in axons and dendrites. Third, a polarized distribution of motor proteins might contribute to the difference in vesicle trafficking in axons and dendrites (5). The frequency histogram in Figure 5 shows a distinct population of fast anterogradely moving vesicles in axons. The average velocity in the anterograde direction in axons is in agreement with fast axonal transport (5), which is carried out by kinesin family member (KIF) proteins (5,5). In Caenorhabditis elegans, the fast anterograde axonal transport of LDCVs was shown to depend in part on the microtubule motor UNC- 4 (KIFA) (54). It is not known which kinesins mediate secretory granule trafficking in vertebrate neurons (5). Activity-dependent trafficking of SemaA in axons Previous studies investigating the effects of secretagogues on vesicle motility in neuroendocrine cells have produced conflicting results. Elevation of intracellular Ca þ or Ba þ levels increases secretory granule movement in PC cells (4,55) but has no effect on secretory granule trafficking in NSY cells (4). Results on secretory granule transport and exocytosis obtained in cell lines may not always translate to neurons (5). We find that in cortical neurons, depolarization caused a strong, reversible decrease of GFP SemaA trafficking in axons. Conversely, blockade of action potentials resulted in an increase in axonal GFP SemaA transport. Interestingly, depolarization altered the velocity and mobility of anterograde, as well as retrograde moving vesicles in axons. The finding that both anterograde and retrograde trafficking are affected suggests that the function of kinesin (mediating plus-end oriented transport along microtubules) and dynein (mediating minus-end oriented transport along microtubules) motor proteins was altered upon depolarization. The cellular mechanisms that regulate motor function are still incompletely understood, but phosphorylation appears to be one important mechanism to control kinesin and dynein motor proteins (56,57). Phosphorylation of kinesin light chains, through a signaling cascade involving cyclin-dependent kinase 5 (cdk5) and glycogen synthase kinase (GSK), inhibits kinesin-based motility and leads to the dissociation of kinesin from transport vesicles (58,59). Thus, kinesin function can be regulated by cellsignaling pathways. Other regulators of kinesin-based motility are camp (6) and calmodulin (6). Calciumdependent binding of calmodulin negatively regulates kinesin-based motility, either by decreasing its motor function (6) or by inhibiting its binding to microtubules (6,6). This mechanism could provide a link between depolarizationinduced Ca þ influx and reduction of anterograde axonal trafficking mediated by kinesins. Dyneins are complex proteins consisting of multiple subunits, and the regulation of dynein motility can occur at multiple levels (57). Mechanisms regulating dynein-based retrograde trafficking include phosphorylation (64 66) and interacting proteins such as dynactin (67,68). Alternatively, neuronal activity could modify substrate properties such as the phosphorylation state of MAPs, thereby altering the regulation of vesicle trafficking along microtubules by MAPs. Inhibition of GSK-mediated phosphorylation of tau, the major MAP in axons, results in an inhibition of anterograde organelle transport in PC cells (69). Regulation of secretory vesicle trafficking by neuronal activity could serve to control the delivery of vesicle cargo to specific locations. The reversible arrest of trafficking during depolarization could indicate a transient fusion of vesicles with the plasma membrane. In line with this, we found that the increased number of stationary vesicles in processes of depolarized neurons correlated with an increased number of SemaA surface puncta. This suggests that during stimulus, axonal vesicles might slow down and release their cargo along the axon, re-endocytose again (6,7) and move on when the stimulus has ended. Thus, a localized stimulus could induce a local release from SemaA carrying vesicles. A similar cycling with the plasma membrane during transport has been suggested for vesicles transporting N-methyl-D-aspartate receptor subunits in young neurons (7). Apparently, the mechanisms that regulate axonal secretory vesicle trafficking do not operate to the same extent in dendrites. The dendritic transport of GFP SemaA may be carried out by motor proteins that lack regulation by signaling pathways or neuronal activity. The difference in both the dynamics and the activity dependence of GFP SemaA trafficking in axons and dendrites raises the question whether these characteristics also affect release of SemaA from these two compartments. Surface-bound GFP SemaA puncta were found both on axons and on dendrites, suggesting that SemaA can be secreted from both compartments. Work in neuroendocrine cells has shown that LDCV secretion is limited by vesicle mobility (55,7,7). In hippocampal neurons, secretory vesicles near the plasma membrane display substantial movement before fusion (5). The rapid activity-dependent anterograde transport of secretory vesicles in axons suggests that the axonal compartment is the predominant site for SemaA secretion, in contrast to dendrites where vesicles move slowly or remain stationary. Future work should determine whether the dynamics of SemaA release differ between the axonal and the dendritic domain and whether the activity-dependent release of secreted semaphorins can occur at synaptic sites. Traffic 6; 7:
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Neuronal plasma membrane
 ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
Neuronal plasma membrane
 ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
ORGANELLES ORGANELLES Neuronal plasma membrane The neuronal plasma membrane contains several local domains with unique properties Presynaptic terminal Endoplasmic Reticulum In neurons the Nissl bodies
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons
 Bury and Sabo Neural Development 2014, 9:13 RESEARCH ARTICLE Open Access Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons Luke AD Bury 1 and Shasta L
Bury and Sabo Neural Development 2014, 9:13 RESEARCH ARTICLE Open Access Dynamic mechanisms of neuroligin-dependent presynaptic terminal assembly in living cortical neurons Luke AD Bury 1 and Shasta L
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
Supplementary Figure 1 Bidirectional optogenetic modulation of the tonic activity of CEA PKCδ + neurons in vitro. a, Top, Cell-attached voltage recording illustrating the blue light-induced increase in
Supplementary information. The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic. Spindle Orientation
 Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb.201510064 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Method for aster 3D tracking, extended characterization of
Supplemental material JCB Tanimoto et al., http ://www.jcb.org /cgi /content /full /jcb.201510064 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Method for aster 3D tracking, extended characterization of
Supplementary Figure S1 (a) (b)
 Supplementary Figure S1: IC87114 does not affect basal Ca 2+ level nor nicotineinduced Ca 2+ influx. (a) Bovine chromaffin cells were loaded with Fluo-4AM (1 μm) in buffer A containing 0.02% of pluronic
Supplementary Figure S1: IC87114 does not affect basal Ca 2+ level nor nicotineinduced Ca 2+ influx. (a) Bovine chromaffin cells were loaded with Fluo-4AM (1 μm) in buffer A containing 0.02% of pluronic
Nature Methods: doi: /nmeth.4257
 Supplementary Figure 1 Screen for polypeptides that affect cellular actin filaments. (a) Table summarizing results from all polypeptides tested. Source shows organism, gene, and amino acid numbers used.
Supplementary Figure 1 Screen for polypeptides that affect cellular actin filaments. (a) Table summarizing results from all polypeptides tested. Source shows organism, gene, and amino acid numbers used.
SUPPLEMENTARY INFORMATION
 Figure S1. Loss of Ena/VASP proteins inhibits filopodia and neuritogenesis. (a) Bar graph of filopodia number per stage 1 control and mmvvee (Mena/ VASP/EVL-null) neurons at 40hrs in culture. Loss of all
Figure S1. Loss of Ena/VASP proteins inhibits filopodia and neuritogenesis. (a) Bar graph of filopodia number per stage 1 control and mmvvee (Mena/ VASP/EVL-null) neurons at 40hrs in culture. Loss of all
Cd k5 Ph o s p h o r y l at i o n o f Mu n c18-1 En h a n c e s Sy n a p t i c
 Cd k5 Ph o s p h o r y l at i o n o f Mu n c18-1 En h a n c e s Sy n a p t i c Tr a n s m i s s i o n Sabine K Schmitz 1 Matthijs Verhage 1 Ruud F Toonen 1 1 Functional Genomics, Center for Neurogenomics
Cd k5 Ph o s p h o r y l at i o n o f Mu n c18-1 En h a n c e s Sy n a p t i c Tr a n s m i s s i o n Sabine K Schmitz 1 Matthijs Verhage 1 Ruud F Toonen 1 1 Functional Genomics, Center for Neurogenomics
Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C. N. Petrok, J.
 SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Brooks and Wallingford, http://www.jcb.org/cgi/content/full/jcb.201204072/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Quantification of ciliary compartments in control
Supplemental material Brooks and Wallingford, http://www.jcb.org/cgi/content/full/jcb.201204072/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Quantification of ciliary compartments in control
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1 SNARE Probes for FRET/2pFLIM Analysis Used in the Present Study. mturquoise (mtq) and Venus (Ven) are in blue and yellow, respectively. The soluble N-ethylmaleimide-sensitive
Supplementary Figure 1 Supplementary Figure 1 SNARE Probes for FRET/2pFLIM Analysis Used in the Present Study. mturquoise (mtq) and Venus (Ven) are in blue and yellow, respectively. The soluble N-ethylmaleimide-sensitive
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Drd1a-Cre driven ChR2 expression in the SCN. (a) Low-magnification image of a representative Drd1a-ChR2 coronal brain section (n = 2) showing endogenous tdtomato fluorescence (magenta).
Supplementary Figure 1 Drd1a-Cre driven ChR2 expression in the SCN. (a) Low-magnification image of a representative Drd1a-ChR2 coronal brain section (n = 2) showing endogenous tdtomato fluorescence (magenta).
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) (B) (C) (D) (E)
 Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
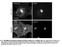 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a)
 1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
syx M18 M13 M18 M13 E *** M18 M13
 syx D syx M13 F % with cluster 8 6 4 G 3 rnd Syx rnd M13 ** rnd M13 H E % with anule 8 6 4 bg syx rnd F cell S a c Supplementary Fig 1. Quantification of membrane protein clusters beneath anules. TIRF-M
syx D syx M13 F % with cluster 8 6 4 G 3 rnd Syx rnd M13 ** rnd M13 H E % with anule 8 6 4 bg syx rnd F cell S a c Supplementary Fig 1. Quantification of membrane protein clusters beneath anules. TIRF-M
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Chapter 4 Neuronal Physiology
 Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Nature Medicine doi: /nm.2860
 Supplemental Figure Legends Supplemental Figure 1: Hypomorphic expression of IFT88 results in olfactory signaling proteins no longer localizing to the ciliary layer. (a) ACIII localizes to the cilia and
Supplemental Figure Legends Supplemental Figure 1: Hypomorphic expression of IFT88 results in olfactory signaling proteins no longer localizing to the ciliary layer. (a) ACIII localizes to the cilia and
Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu
 Distinct contributions of Na v 1.6 and Na v 1.2 in action potential initiation and backpropagation Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu Supplementary figure and legend Supplementary
Distinct contributions of Na v 1.6 and Na v 1.2 in action potential initiation and backpropagation Wenqin Hu, Cuiping Tian, Tun Li, Mingpo Yang, Han Hou & Yousheng Shu Supplementary figure and legend Supplementary
H. An electrical signal travel down the dendrite.
 Nervous System Group Activity Objectives: To be able to describe the nervous system structure and function To understand how neurons communicate using both electrical and chemical signals To know how the
Nervous System Group Activity Objectives: To be able to describe the nervous system structure and function To understand how neurons communicate using both electrical and chemical signals To know how the
cell movement and neuronal migration
 cell movement and neuronal migration Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 Cell migration Cell migration in 3 steps; protrusion,
cell movement and neuronal migration Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 Cell migration Cell migration in 3 steps; protrusion,
d e f Spatiotemporal quantification of subcellular ATP levels in a single HeLa cell during changes in morphology Supplementary Information
 Ca 2+ level (a. u.) Area (a. u.) Normalized distance Normalized distance Center Edge Center Edge Relative ATP level Relative ATP level Supplementary Information Spatiotemporal quantification of subcellular
Ca 2+ level (a. u.) Area (a. u.) Normalized distance Normalized distance Center Edge Center Edge Relative ATP level Relative ATP level Supplementary Information Spatiotemporal quantification of subcellular
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Influenza virus exploits tunneling nanotubes for cell-to-cell spread
 Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the
 Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
CD3 coated cover slips indicating stimulatory contact site, F-actin polymerization and
 SUPPLEMENTAL FIGURES FIGURE S1. Detection of MCs. A, Schematic representation of T cells stimulated on anti- CD3 coated cover slips indicating stimulatory contact site, F-actin polymerization and microclusters.
SUPPLEMENTAL FIGURES FIGURE S1. Detection of MCs. A, Schematic representation of T cells stimulated on anti- CD3 coated cover slips indicating stimulatory contact site, F-actin polymerization and microclusters.
7.06 Spring of PROBLEM SET #6
 7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Supplementary Figure 1: GFAP positive nerves in patients with adenocarcinoma of
 SUPPLEMENTARY FIGURES AND MOVIE LEGENDS Supplementary Figure 1: GFAP positive nerves in patients with adenocarcinoma of the pancreas. (A) Images of nerves stained for GFAP (green), S100 (red) and DAPI
SUPPLEMENTARY FIGURES AND MOVIE LEGENDS Supplementary Figure 1: GFAP positive nerves in patients with adenocarcinoma of the pancreas. (A) Images of nerves stained for GFAP (green), S100 (red) and DAPI
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Large-scale calcium imaging in vivo.
 Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Astrocyte signaling controls spike timing-dependent depression at neocortical synapses
 Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
1. Microfluidic device characteristics and cell compartmentalization
 Supplementary figures: 1. Microfluidic device characteristics and cell compartmentalization Microfluidic channels were molded in PDMS (Fig. S1 A and B) over silicon masters, which were structured with
Supplementary figures: 1. Microfluidic device characteristics and cell compartmentalization Microfluidic channels were molded in PDMS (Fig. S1 A and B) over silicon masters, which were structured with
Structure and Function of Neurons
 CHPTER 1 Structure and Function of Neurons Varieties of neurons General structure Structure of unique neurons Internal operations and the functioning of a neuron Subcellular organelles Protein synthesis
CHPTER 1 Structure and Function of Neurons Varieties of neurons General structure Structure of unique neurons Internal operations and the functioning of a neuron Subcellular organelles Protein synthesis
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic
 Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Supplementary material Legends to Supplementary Figures Figure S1. Figure S2. Figure S3.
 Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex
 Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Muscle and Neuromuscular Junction. Peter Takizawa Department of Cell Biology
 Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
Tyrodes solution in a custom-built imaging chamber as described previously. Images were acquired
 Supplemental Material Supplemental Methods Electrical stimulation of CX-G3-labeled hippocampal neurons Following 5 min incubation in 0.5 µm CX-G3 and washes, 18-20 DIV neurons were imaged in normal Tyrodes
Supplemental Material Supplemental Methods Electrical stimulation of CX-G3-labeled hippocampal neurons Following 5 min incubation in 0.5 µm CX-G3 and washes, 18-20 DIV neurons were imaged in normal Tyrodes
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3443 In the format provided by the authors and unedited. Supplementary Figure 1 TC and SC behaviour during ISV sprouting. (a) Predicted outcome of TC division and competitive Dll4-Notch-mediated
DOI: 10.1038/ncb3443 In the format provided by the authors and unedited. Supplementary Figure 1 TC and SC behaviour during ISV sprouting. (a) Predicted outcome of TC division and competitive Dll4-Notch-mediated
EXAMINING DYNAMIC ASPECTS OF PRESYNAPTIC TERMINAL FORMATION VIA LIVE CONFOCAL MICROSCOPY LUKE ANDREW DASCENZO BURY
 EXAMINING DYNAMIC ASPECTS OF PRESYNAPTIC TERMINAL FORMATION VIA LIVE CONFOCAL MICROSCOPY By LUKE ANDREW DASCENZO BURY Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy
EXAMINING DYNAMIC ASPECTS OF PRESYNAPTIC TERMINAL FORMATION VIA LIVE CONFOCAL MICROSCOPY By LUKE ANDREW DASCENZO BURY Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Diagram of BBB and brain chips.
 Supplementary Figure 1 Diagram of BBB and brain chips. (a) Schematic of the BBB Chip demonstrates the 3 parts of the chip, Top PDMS channel, membrane and Bottom PDMS channel; (b) Image of 2 BBB Chips,
Supplementary Figure 1 Diagram of BBB and brain chips. (a) Schematic of the BBB Chip demonstrates the 3 parts of the chip, Top PDMS channel, membrane and Bottom PDMS channel; (b) Image of 2 BBB Chips,
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
October 26, Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
J. Cell Sci. 129: doi: /jcs : Supplementary information
 Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
April 29, Neurophysiology. Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University,
 April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
