CHAPTER 5 HW: STEREOISOMERS
|
|
|
- Ashlyn Walker
- 6 years ago
- Views:
Transcription
1 CAPTER 5 W: STEREISMERS CIRALITY AND ENANTIMERS 1. Define the following terms: a. Chiral A chiral substance has the possibility of having right and left handed versions (or it can have an enantiomer). b. Enantiomers (and give an example) Stereoisomers that are non-superimposable mirror images: they mirror but are different spatially, in 3D.. Put an asterisk (*) on all chiral centers in these compounds. Some may have no chiral centers and some may have more than one. F * * no stereocenters * * * * no stereocenters 3. Find the chiral center in each compounds, then draw both enantiomers. * a. b. * *. Draw the enantiomer of each compound. Compound Enantiomer Compound Enantiomer F F C 3 C 3 Page 1
2 DIASTEREMERS AND MES CMPUNDS 5. Define the following terms and give an example of each: a. Diastereomers Stereoisomers that are not enantiomers. The atoms are connected in the same order, but they do not mirror, and they are still different in 3D. b. Meso compound A meso compound has two or more chiral centers, but also has an internal plane of symmetry and is therefore achiral (is superimposable with its mirror image). 6. Draw a diastereomer of each compound. (several answers: keep at least one center, switch at least one) Compound A diastereomer Compound A diastereomer C 3 C 3 C 3 C 3 7. Draw two stereoisomers of the structure below that are diastereomers of each other. 8. Draw a compound with a molecular formula of C 5 10 that is (there are several answers) A chiral compound. A meso compound. An achiral compound that is not a meso compound. A constitutional isomer of your previous structure. Page
3 9. Identify the relationship between each pair of compounds. Are they identical, constitutional isomers, enantiomers, diastereomers, or not isomers? Relationship Enantiomers Constitutional isomers C C 3 C C 3 C C 3 C C 3 Relationship Diastereomers Identical Relationship Identical Not isomers (C 5 vs. C 7 ) 10. A meso compound has no enantiomer, but can have a diastereomer. Explain and give examples. 1 3 A meso compound is achiral, meaning it can t have an enantiomer. It is superimposable with its mirror image (e.g. structure 1 is the mirror image of structure - and they are identical, not different as they would be if they were enantiomers). But compound (meso) is a diastereomer of compound 3- they are stereoisomers that don t mirror. 11. Explain why compound M has no enantiomer and why compound N has no diastereomer. C M C C N C 3 Compound M has an internal mirror plane, so is achiral, meaning it cannot have an enantiomer. Compound N has only 1 chiral center, so can only have an enantiomer. You need at least chiral centers to have diasteromers. 1. For n chiral centers in a compound, there may be n possible stereoisomers.,-dichloropentane has two chiral centers, but only has three stereoisomers. Explain why this is, using structures. ne of the three stereoisomers (3) in,-dichloromethane is meso, since it has an internal plane of symmetry. When a meso compound exists, there will be less than n stereoisomers. 1 3 Page 3
4 R,S DESIGNATINS 13. Circle the four different groups around each chiral center, then rank each group from 1- using priorities for R,S nomenclature Determine the R,S configuration of each chiral center. 3 1 a. 3 C b. c. 1 R C C C S ( is ) 1 looks R, so is S d. e. N R R 1 3 S 1 looks R, so is S 15. Ketamine is an anesthetic used in human and veterinary medicine. It is also used by some recreationally as a club drug. It is often administered as a racemic mixture, although the more active form is the S enantiomer. Which of the structures below is (S)-ketamine? 3 NC NC Why is cis-1-chloro--methylcyclopentane an incomplete name for this structure C 3 C 3 Cis does differentiate this structure from trans, but there are two different structures that have the cis orientation ( enantiomers). R,S naming is necessary to distinguish between the two different enantiomers. Page
5 17. Give the IUPAC name for each compound, including R,S designations. Structure Name (R)-,,5-trichlorohexane (3R,5S)-3,5-dicyclopropyloctane Structure C C 3 C 3 F Name (1R,3R)-1-ethyl-3-fluorocyclohexane (3S,S,5S)-1,3-dibromo-5-chloro- -methylhexane 18. Draw the structure from the following names, including dashed and wedged bonds where appropriate. Structure C 3 C 3 Name (R)-3-methylhexane (3S,6R)-6-isopropyl-3-methyldecane 19. Multiple choice: choose the best answer(s). a. The enantiomer of (S,3S)-3-bromo--chloropentane is: (S,3R)-3-bromo--chloropentane (R,3R)-3-bromo--chloropentane (R,3S)-3-bromo--chloropentane b. The following are diastereomers of (1R,3S)-1-ethyl-3-methylcyclohexane: (1R,3R)-1-ethyl-3-methylcyclohexane (1S,3R)-1-ethyl-3-methylcyclohexane (1S,3S)-1-ethyl-3-methylcyclohexane Page 5
6 FISCER PRJECTINS 0. Draw the following: (several answers for the diastereomer and constitutional isomer) Draw the enantiomer Draw a diastereomer Draw a constitutional isomer C 3 C 3 C 3 C 3 C C C 3 C C 3 C 3 C C 3 1. Draw each structure in a Fischer projection, then decide whether the compound is chiral or achiral. Structure C C 3 C 3 Fischer or or or C C 3 C 3 Chiral or achiral? Chiral Achiral Chiral (not quite symmetric: different ends). Draw Fischer projections of the following compounds to help determine their relationship. Are they identical, enantiomers, diastereomers, constitutional isomers, or not related? 3 C C C C C 3 C 3 C C 3 3 C C C C C 3 (C ) 3 C 3 C C 3 C 3 (C ) 3 C 3 They are enantiomers (non-superimposable mirror images) Page 6
7 3. Identify the relationship between each pair of compounds. Are they identical, constitutional isomers, enantiomers, diastereomers, or not isomers? C 3 C 3 C C 3 C C 3 1 C 3 C 3 1 C 3 C C C 3 3 looks S, so R (C 3 # is in front) C C 3 3 S (C 3 # is in back) C C 3 Relationship Enantiomers (do R,S) Identical (rotate 180 ) C 3 C 3 C 3 C C 3 C 3 C 3 C 3 C C 3 C 3 C 3 C 3 C 3 Relationship Diastereomers Enantiomers PTICAL ACTIVITY AND PYSICAL PRPERTIES F STEREISMERS. Define the following terms. a. Plane-polarized light Light which has been passed through a polarizing filter so the waves are oscillating in one direction. b. ptical rotation A measurement of how a sample (typically of a chiral compound) rotates plane-polarized light. c. Racemic mixture A mixture that has equal amounts of enantiomers (50-50 mix). d. Enantiomeric excess (ee) In a sample which includes both enantiomers, the ee is the % difference between the amount of the major enantiomer and the amount of the minor enantiomer. For example, an 80:0 mixture of enantiomers has an ee of 60 (80 0 = 60%). Page 7
8 5. The product mixture from a chemical reaction is reported as 98 ee. What does this mean? Give the percentages of the components present in the mixture. 98 ee means there is 98% excess of one enantiomer. The mixture is 99% one enantiomer and 1% the other (99% 1% = 98%) 6. Would the following pairs of compounds have identical or different physical properties (boiling points, densities, etc.)? int: determine if they re identical, enantiomers, or diastereomers. iefly explain. ief explanation Identical properties: they are enantiomers. The only phys. prop. that enantiomers don t share is optical rotation. Different properties: they are diastereomers. ief explanation Identical properties: they are identical molecules. Identical properties: they are enantiomers. Page 8
9 7. Multiple choice. iefly explain each choice. a. Compound A has an optical rotation of 13., or [a ]= Compound B should have an optical rotation of (circle one): [a ]= +13. [a ]= -13. [a ]= 0 [a ]= some other number A B These are enantiomers, and their optical rotation has the same magnitude but opposite sign. b. Compound C has a boiling point of 130 C. Compound D should have a boiling point of (circle one): b.p. = 130 b.p. = 0 C b.p. = some other number C 3 C 3 C C 3 D C 3 These are identical (both are cis-1,-dimethylcyclohexane). c. Compound E has an optical rotation of -8.3, or [a ]= Compound F should have an optical rotation of (circle one): [a ]= -8.3 [a ]= +8.3 [a ]= 0 [a ]= some other number Molecule F is achiral it is a meso compound, so it has an optical rotation of 0. E F E and F are diastereomers, so they have different physical properties, and -8.3 and 0 are different. d. Compound G has a density of 0.91 g/ml. Compound should have a density of (circle one): d = 0.91 g/ml d = some other number G These are diastereomers, so they don t have the same physical properties. You can t predict the density of one diastereomer based on the properties of another. Page 9
CHAPTER 5 HW: STEREOISOMERS
 CAPTER 5 W: STEREISMERS CIRALITY AND ENANTIMERS 1. Define the following terms: a. Chiral b. Enantiomers (and give an example) 2. Put an asterisk (*) on all chiral centers in these compounds. Some may have
CAPTER 5 W: STEREISMERS CIRALITY AND ENANTIMERS 1. Define the following terms: a. Chiral b. Enantiomers (and give an example) 2. Put an asterisk (*) on all chiral centers in these compounds. Some may have
Practice Problems for Video CH 5-1:
 CEM 243 Organic Chemistry C-5: Stereochemistry - Chiral Molecules Study Guide: Key Concepts and Practice Problems Key concepts you need to understand: Chirality and chiral centers; stereoisomers; (R,S)
CEM 243 Organic Chemistry C-5: Stereochemistry - Chiral Molecules Study Guide: Key Concepts and Practice Problems Key concepts you need to understand: Chirality and chiral centers; stereoisomers; (R,S)
Stereochemistry - Chirality. Chapter 5 Organic Chemistry, 8th Edition John E. McMurry
 Stereochemistry - Chirality Chapter 5 Organic Chemistry, 8th Edition John E. McMurry Isomerism The two major classes of isomers are constitutional isomers and stereoisomers. Constitutional/structural isomers
Stereochemistry - Chirality Chapter 5 Organic Chemistry, 8th Edition John E. McMurry Isomerism The two major classes of isomers are constitutional isomers and stereoisomers. Constitutional/structural isomers
CHEM 109A Organic Chemistry
 CEM 09A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter Isomers The arrangement of atoms in space MIDTERM Wednesday Feb. Chapter Sections.8.6 melting, boiling points, conformations
CEM 09A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter Isomers The arrangement of atoms in space MIDTERM Wednesday Feb. Chapter Sections.8.6 melting, boiling points, conformations
CHM 220 Stereochemistry
 CM 220 Stereochemistry Stereocenters 1. ow many stereocenters are there in 2,2,4-tribromo-3-methyl-1-pentanol? (A) 2 (B) 3 (C) 4 (D) 5 2. ow many stereocenters are there in 2,3-dibromo-4-methylpentane?
CM 220 Stereochemistry Stereocenters 1. ow many stereocenters are there in 2,2,4-tribromo-3-methyl-1-pentanol? (A) 2 (B) 3 (C) 4 (D) 5 2. ow many stereocenters are there in 2,3-dibromo-4-methylpentane?
Stereochemistry. Dr. Sapna Gupta
 Stereochemistry Dr. Sapna Gupta Introduction Stereo left and right handedness Any carbon that has four different groups will show chirality. hirality: the mirror image of the compound will not superimpose
Stereochemistry Dr. Sapna Gupta Introduction Stereo left and right handedness Any carbon that has four different groups will show chirality. hirality: the mirror image of the compound will not superimpose
A. CONFORMATIONAL ISOMERS
 EMISTRY 104 elp Sheet #3 Organic (Part II): ISOMERS hapters 2.9 (structural isomers), 6.5 (geometric isomers), 6.11 (constitutional isomers), 7.2f (optical isomers) Do the topics appropriate for your lecture
EMISTRY 104 elp Sheet #3 Organic (Part II): ISOMERS hapters 2.9 (structural isomers), 6.5 (geometric isomers), 6.11 (constitutional isomers), 7.2f (optical isomers) Do the topics appropriate for your lecture
Chapter 5 Stereochemistry
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith Modified by Dr. Juliet Hahn Chapter 5 Stereochemistry 1 Stereoisomers of 2,3-dibromobutane Figure 5.9 Pair of enantiomers: A and B Pairs of diastereomers:
Organic Chemistry, Fifth Edition Janice Gorzynski Smith Modified by Dr. Juliet Hahn Chapter 5 Stereochemistry 1 Stereoisomers of 2,3-dibromobutane Figure 5.9 Pair of enantiomers: A and B Pairs of diastereomers:
Chirality. Chapter 5 Stereochemistry. Chiral Carbons. Stereoisomers. Mirror Planes of Symmetry. (R), (S) Nomenclature
 Organic hemistry, 5 th Edition L. G. Wade, Jr. hapter 5 Stereochemistry hirality andedness : right glove doesn t fit the left hand. Mirror-image object is different from the original object. Jo Blackburn
Organic hemistry, 5 th Edition L. G. Wade, Jr. hapter 5 Stereochemistry hirality andedness : right glove doesn t fit the left hand. Mirror-image object is different from the original object. Jo Blackburn
Stereochemistry: 3-D Chemistry. 3-D Chemistry. Enantiomers
 Stereochemistry: 3-D Chemistry 3-D Chemistry Enantiomers If a molecule and its mirror image are not superimposable these molecules are isomers; in particular, these mirror image isomers are called enantiomers
Stereochemistry: 3-D Chemistry 3-D Chemistry Enantiomers If a molecule and its mirror image are not superimposable these molecules are isomers; in particular, these mirror image isomers are called enantiomers
As a result, IV and V are chiral molecules and are enantiomers.
 5.1 lassify each of the following objects as to whether it is chiral or achiral: (a) A screwdriver achiral (d) A tennis shoe chiral (g) A car chiral (b) A baseball bat achiral (e) An ear chiral (h) A hammer
5.1 lassify each of the following objects as to whether it is chiral or achiral: (a) A screwdriver achiral (d) A tennis shoe chiral (g) A car chiral (b) A baseball bat achiral (e) An ear chiral (h) A hammer
CHAPTER 5: STEREOISOMERS
 APTER 5: STEREISMERS SIMPLE STEREISMERS NSTITUTINAL ISMERS VERSUS STEREISMERS A constitutional isomer A stereoisomer Page 1 IRALITY (ANDEDNESS) hiral object: Achiral object: Are these objects chiral or
APTER 5: STEREISMERS SIMPLE STEREISMERS NSTITUTINAL ISMERS VERSUS STEREISMERS A constitutional isomer A stereoisomer Page 1 IRALITY (ANDEDNESS) hiral object: Achiral object: Are these objects chiral or
Stereochemistry 1.1 INTRODUCTION
 1 Stereochemistry 1.1 INTRODUTION The isomers which have same arrangement of atoms but differ only due to different orientation of atoms in space within the molecule are called Stereoisomers and the phenomenon
1 Stereochemistry 1.1 INTRODUTION The isomers which have same arrangement of atoms but differ only due to different orientation of atoms in space within the molecule are called Stereoisomers and the phenomenon
Chemistry 261 Homework 3: Chapters 5, 6 Out: 11/20/17 Due: 11/30/17 Point Value: 9 points (7 EC points possible when combined with part I)
 Chemistry 261 omework 3: Chapters 5, 6 ut: 11/20/17 Due: 11/30/17 Point Value: 9 points (7 EC points possible when combined with part I) The following homework assignment contains 28 questions valued at
Chemistry 261 omework 3: Chapters 5, 6 ut: 11/20/17 Due: 11/30/17 Point Value: 9 points (7 EC points possible when combined with part I) The following homework assignment contains 28 questions valued at
3.7A: Compounds with multiple stereocenters
 Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
CH 3 H C 2 H 5. C Cl C H. H 3 C Cl H H 5 C 2. H Cl. Cl H. C Cl H. H Cl. Problem Set 7: Stereochemistry-2
 Problem Set 7: Stereochemistry-2 hemistry 260 Organic hemistry 1. ow many constitutional isomers of 7 16 have stereogenic centres? (1) 1 (2) 2 (3) 3 (4) 4 (5) none 2. Indicate which of the following compounds
Problem Set 7: Stereochemistry-2 hemistry 260 Organic hemistry 1. ow many constitutional isomers of 7 16 have stereogenic centres? (1) 1 (2) 2 (3) 3 (4) 4 (5) none 2. Indicate which of the following compounds
CHEM 261 Feb. 16, stereogenic center H C C C
 77 EM 261 Feb. 16, 2017 eview of hiral enters 3 quinine - anti-malarial drug from the bark of the tree inchona officinalis malaria is cause by Plasmodium species transmitted by Anopheles mosquito example.
77 EM 261 Feb. 16, 2017 eview of hiral enters 3 quinine - anti-malarial drug from the bark of the tree inchona officinalis malaria is cause by Plasmodium species transmitted by Anopheles mosquito example.
CHAPTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS
 C26 09/17/2013 10:52:31 Page 379 CHAPTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center of asymmetry
C26 09/17/2013 10:52:31 Page 379 CHAPTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center of asymmetry
Chemistry I (Organic) Stereochemistry LECTURE 2 Stereogenic elements & enantiomers
 1 Chemistry I (Organic) Stereochemistry LECTURE 2 Stereogenic elements & enantiomers Alan C. Spivey a.c.spivey@imperial.ac.uk Oct 2011 2 Format & scope of lecture Isomers & stereoisomers some definitions
1 Chemistry I (Organic) Stereochemistry LECTURE 2 Stereogenic elements & enantiomers Alan C. Spivey a.c.spivey@imperial.ac.uk Oct 2011 2 Format & scope of lecture Isomers & stereoisomers some definitions
Two chiral centres (diastereoisomers)
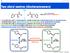 1 Two chiral centres (diastereoisomers) Mirror N S N Two enantiomers differ by absolute configuration N N 2 N 2 N A molecule with 1 stereogenic centre exists as 2 stereoisomers or enantiomers Enantiomers
1 Two chiral centres (diastereoisomers) Mirror N S N Two enantiomers differ by absolute configuration N N 2 N 2 N A molecule with 1 stereogenic centre exists as 2 stereoisomers or enantiomers Enantiomers
Carbohydrates. Chapter 18
 Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
1. Draw a standard line bond structure for compounds of the following molecular formulas:
 TF ame: LS1a Fall 06 Problem Set #1 Due Friday 9/29 at noon in your TF s drop box on the 2 nd floor of Science Center all questions including extra ones should be turned in 1. Draw a standard line bond
TF ame: LS1a Fall 06 Problem Set #1 Due Friday 9/29 at noon in your TF s drop box on the 2 nd floor of Science Center all questions including extra ones should be turned in 1. Draw a standard line bond
Problem Set 2 Out: November 5, 1999 Due Back: November 12, 1999 Chemistry 221, 1999
 Problem Set ut: November 5, 1999 Due Back: November 1, 1999 hemistry 1, 1999 Answers to the following problems should be written, in order and labeled, on 8 1/ x 11 inch paper. Answers written on the problem
Problem Set ut: November 5, 1999 Due Back: November 1, 1999 hemistry 1, 1999 Answers to the following problems should be written, in order and labeled, on 8 1/ x 11 inch paper. Answers written on the problem
Student Handout. This experiment allows you to explore the properties of chiral molecules. You have
 Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
CHEM J-6 June 2014
 CEM1611 2014-J-6 June 2014 rlistat (shown below) is a drug for obesity management which acts by inhibiting the absorption of dietary fats. Indicate all stereogenic centres on the structure below. 6 rlistat
CEM1611 2014-J-6 June 2014 rlistat (shown below) is a drug for obesity management which acts by inhibiting the absorption of dietary fats. Indicate all stereogenic centres on the structure below. 6 rlistat
Stereo-Isomers in Pesticides. Martin Rodler, CropLife International
 Stereo-Isomers in Pesticides Martin Rodler, CropLife International Outline Relevance of Stereo-Isomers for Pesticides Regulatory Evaluation of Pesticides Introduction to Stereo-Isomerism Enantiomers Diastereomers
Stereo-Isomers in Pesticides Martin Rodler, CropLife International Outline Relevance of Stereo-Isomers for Pesticides Regulatory Evaluation of Pesticides Introduction to Stereo-Isomerism Enantiomers Diastereomers
Level 3 Chemistry, 2007
 Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
Level 3 hemistry, 2007 Annotated answers to this organic paper. Q1 QUESTIN NE Give the proper name that gives the structure a unique name (a) Give the systematic IUPA names for the following molecules
Final Exam, Page 1 of 15. Your full signature
 Final Exam, Page 1 of 15 Your full signature Print your full name Your SID If you are making up an I grade Indicate the semester you took the course Name of the instructor for that course: This exam has
Final Exam, Page 1 of 15 Your full signature Print your full name Your SID If you are making up an I grade Indicate the semester you took the course Name of the instructor for that course: This exam has
Organic/Biochem Test #2 Takehome Name: Spring 2012 Page 1 of 6. Multiple choice: Circle the best answer for each of the following questions.
 Spring 2012 Page 1 of 6 Multiple choice: Circle the best answer for each of the following questions. 1. The general formula for a carboxylic acid is: a) RCHO b) RCOR c) R-OH d) R-COOH 2. In which of the
Spring 2012 Page 1 of 6 Multiple choice: Circle the best answer for each of the following questions. 1. The general formula for a carboxylic acid is: a) RCHO b) RCOR c) R-OH d) R-COOH 2. In which of the
CHE 102 Exam 3 CH 3 CHCOOH. CH 3 CH 2 CH 2 O d. e. f. CH3 COO g. h. i. O O CH 3 CCH 2 CCH 3
 CE 102 Exam 3 1. (1 pt each) Classify the following molecules as aldehydes, ketones, carboxylic acids, or esters: a. b. c. C C 3 CC C 2 C 2 CC 3 C 3 C 2 C 2 d. e. f. CCC 3 C 3 C 3 CC 2 C 2 C C3 C g. h.
CE 102 Exam 3 1. (1 pt each) Classify the following molecules as aldehydes, ketones, carboxylic acids, or esters: a. b. c. C C 3 CC C 2 C 2 CC 3 C 3 C 2 C 2 d. e. f. CCC 3 C 3 C 3 CC 2 C 2 C C3 C g. h.
Stereoisomerism There are two types of stereoisomerism, E-Z Isomerism and Optical Isomerism.
 Stereoisomerism There are two types of stereoisomerism, E-Z Isomerism and Optical Isomerism. E Z Isomerism This type tells us about the positions of substituent's either side of the carbon carbon double
Stereoisomerism There are two types of stereoisomerism, E-Z Isomerism and Optical Isomerism. E Z Isomerism This type tells us about the positions of substituent's either side of the carbon carbon double
adducts of cis or trans alkenes with reagents adding cis or trans is troublesome. Even for those who can write the Fischer
 Mnemonic use for aiding students to determine erythro vs threo stereochemistry in additions to internal alkenes John H. MacMillan Ph.D. Department of Chemistry, Temple University, Philadelphia, Pa. 19122
Mnemonic use for aiding students to determine erythro vs threo stereochemistry in additions to internal alkenes John H. MacMillan Ph.D. Department of Chemistry, Temple University, Philadelphia, Pa. 19122
Dr. Nafith Abu Tarboush. Tarek Khrisat
 1 Dr. Nafith Abu Tarboush June 18 th 2013 Tarek Khrisat 1 Lecture Outline: Aldoses and Ketoses Optical isomers -Fischer projection -Enantiomers, Diastereomers, and Epimers Isomers: Compounds with the same
1 Dr. Nafith Abu Tarboush June 18 th 2013 Tarek Khrisat 1 Lecture Outline: Aldoses and Ketoses Optical isomers -Fischer projection -Enantiomers, Diastereomers, and Epimers Isomers: Compounds with the same
DEPARTMENT OF MEDICINAL CHEMISTRY SCHOOL OF PHARMACY
 KEY DEPARTMET F MEDIIAL EMISTRY SL F PARMAY BASI PARMAEUTIAL SIEE FR TE PRATIIG PARMAIST MED 527, Fall 2008 Quiz #4, Dr. Desai, 100 points; ovember 4, 2008 AME (In APITAL Letters LY) I affirm that I have
KEY DEPARTMET F MEDIIAL EMISTRY SL F PARMAY BASI PARMAEUTIAL SIEE FR TE PRATIIG PARMAIST MED 527, Fall 2008 Quiz #4, Dr. Desai, 100 points; ovember 4, 2008 AME (In APITAL Letters LY) I affirm that I have
MEDCHEM 562. Physicochemical Properties of Drugs: Lecture 2; Kent Kunze. Stereochemistry (Double the trouble. or double the fun?)
 1 MEDCHEM 562 Physicochemical Properties of Drugs: Lecture 2; Kent Kunze Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center or site of
1 MEDCHEM 562 Physicochemical Properties of Drugs: Lecture 2; Kent Kunze Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center or site of
ANSWERS: Isomers. 2) A and F
 NSWERS: Isomers 1) For cis and trans isomers to occur a carbon-carbon double bond must be present as this prevents any rotation about this bond, and the atoms or groups of atoms attached to the two carbon
NSWERS: Isomers 1) For cis and trans isomers to occur a carbon-carbon double bond must be present as this prevents any rotation about this bond, and the atoms or groups of atoms attached to the two carbon
Chem 1120 Final 210 points Dr. Luther Giddings
 Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
CHAPTER 27 CARBOHYDRATES SOLUTIONS TO REVIEW QUESTIONS
 27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
Anomeric carbon Erythritol is achiral because of a mirror plane in the molecule and therefore, the product is optically inactive.
 APTER 22 Practice Exercises 22.1 2 2 2 2 2 2 2 2 D-Ribulose L-Ribulose D-Xyulose L-Xyulose (one pair of enantiomers) (a second pair of enantiomers) 22.3 2 Anomeric carbon Glycosidic bond 3 () Methyl -D-mannopyranoside
APTER 22 Practice Exercises 22.1 2 2 2 2 2 2 2 2 D-Ribulose L-Ribulose D-Xyulose L-Xyulose (one pair of enantiomers) (a second pair of enantiomers) 22.3 2 Anomeric carbon Glycosidic bond 3 () Methyl -D-mannopyranoside
6.1 Cis Trans Isomers
 6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
6.1 CIS TRANS ISMERS 179 two dimensions on the pages of a book. The use of models is an invaluable aid in understanding the material presented in this chapter. You are strongly encouraged to build models
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Carbohydrates CHAPTER SUMMARY
 14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
14 2 cellulose 2 2 arbohydrates 2 amylose APTER SUMMARY 14.1 hemical Nature of arbohydrates - Polyhydroxy Aldehydes and Ketones arbohydrates are a class of organic biopolymers which consist of polyhydroxy
Sheet #8 Dr. Nafeth Abu-Tarboush
 1 arbohydrates There are two topic goals in our study of carbohydrates: Monosaccharides: to recognize their structure, properties, & their stereochemistry. The nature of di-, oligo- & polysaccharides.
1 arbohydrates There are two topic goals in our study of carbohydrates: Monosaccharides: to recognize their structure, properties, & their stereochemistry. The nature of di-, oligo- & polysaccharides.
Stereochemistry: biological significance of isomerism
 S Stereochemistry: biological significance of isomerism Craig Wheelock October 17 th, 2008 craig.wheelock@ki.se http://www.metabolomics.se/ (copies of slides can be downloaded from my homepage) Learning
S Stereochemistry: biological significance of isomerism Craig Wheelock October 17 th, 2008 craig.wheelock@ki.se http://www.metabolomics.se/ (copies of slides can be downloaded from my homepage) Learning
CHEM 203 HOMEWORK 4 Chemistry of Alkenes - II. Answer the above questions by writing a detailed mechanism for the conversion of A into lanosterol.
 EM 203 MEWK 4 hemistry of Alkenes - II 1. The following questions may have occurred to you: (i) do carbocations occur in living systems? (ii) an an olefin (a Lewis base) react with a carbocation (a Lewis
EM 203 MEWK 4 hemistry of Alkenes - II 1. The following questions may have occurred to you: (i) do carbocations occur in living systems? (ii) an an olefin (a Lewis base) react with a carbocation (a Lewis
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Hydrolytic transformations involving amide-, ester bonds are the easiest to perform
 Hydrolytic reactions Hydrolytic transformations involving amide-, ester bonds are the easiest to perform Proteins used for these reactions are proteases, esterases or lipases. A favourite class of enzymes
Hydrolytic reactions Hydrolytic transformations involving amide-, ester bonds are the easiest to perform Proteins used for these reactions are proteases, esterases or lipases. A favourite class of enzymes
Organic. Carbon Chemistry
 Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
Today Organic Carbon Chemistry Organic You know more than you think already What you will need Lewis dot, VSEPR VB, hybrid orbitals, MO electronegativity intermolecular forces Two hurdles we will deal
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Part I => CARBS and LIPIDS. 1.2 Monosaccharides 1.2a Stereochemistry 1.2b Derivatives
 Part I => CARBS and LIPIDS 1.2 Monosaccharides 1.2a Stereochemistry 1.2b Derivatives Section 1.2a: Stereochemistry Synopsis 1.2a monosaccharide (greek) sugar - Monosaccharides are carbonyl polyols (or
Part I => CARBS and LIPIDS 1.2 Monosaccharides 1.2a Stereochemistry 1.2b Derivatives Section 1.2a: Stereochemistry Synopsis 1.2a monosaccharide (greek) sugar - Monosaccharides are carbonyl polyols (or
Polarization and Circular Dichroism (Notes 17)
 Polarization and Circular Dichroism - 2014 (Notes 17) Since is vector, if fix molec. orient., E-field interact (absorb) with molecule differently when change E-orientation (polarization) Transitions can
Polarization and Circular Dichroism - 2014 (Notes 17) Since is vector, if fix molec. orient., E-field interact (absorb) with molecule differently when change E-orientation (polarization) Transitions can
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
13. ORGANIC CHEMISTRY
 1. ORGANIC EMISTRY III) ALKENES SYNOPSIS Alkenes are unsaturated hydrocarbons. These contain a C =C. They contain two hydrogens less than corresponding alkanes. Double bonded carbon undergoes hybridisation.
1. ORGANIC EMISTRY III) ALKENES SYNOPSIS Alkenes are unsaturated hydrocarbons. These contain a C =C. They contain two hydrogens less than corresponding alkanes. Double bonded carbon undergoes hybridisation.
7.6 BICYCLIC AND POLYCYCLIC COMPOUNDS. A. Classification and Nomenclature 290 CHAPTER 7 CYCLIC COMPOUNDS. STEREOCHEMISTRY OF REACTIONS
 290 CAPTER 7 CYCLIC CMPUNDS. STERECEMISTRY F REACTINS 105 C 60 C C Figure 7.12 The orbitals that overlap to form each C C bond in cyclopropane do not lie along the straight line between the carbon atoms.
290 CAPTER 7 CYCLIC CMPUNDS. STERECEMISTRY F REACTINS 105 C 60 C C Figure 7.12 The orbitals that overlap to form each C C bond in cyclopropane do not lie along the straight line between the carbon atoms.
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS!
 Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
Long time ago, people who sacrifice their sleep, family, food, laughter, and other joys of life were called SAINTS. But now, they are called STUDENTS! Monosaccharaides Q. Can hydrolysis occur at anytime
CHM 2200C April 28, (Please Print)
 M 2200 April 28, 2005 Final Exam Name KEY (Please Print) Points The test consists of six pages. Print your name legibly on each page now. A seventh page contains approximate pk values for some acids. You
M 2200 April 28, 2005 Final Exam Name KEY (Please Print) Points The test consists of six pages. Print your name legibly on each page now. A seventh page contains approximate pk values for some acids. You
N NH 3 NHCNH 2. 3 Lysine (Lys)
 Make sure You have FIVE Different C 110 May, 2007 Pages Including this Cover Page. Cover Page You may pick up your graded exam after 120 minutes Final Exam 5/08/07 until 12/01/07 DETAC TIS SEET AND USE
Make sure You have FIVE Different C 110 May, 2007 Pages Including this Cover Page. Cover Page You may pick up your graded exam after 120 minutes Final Exam 5/08/07 until 12/01/07 DETAC TIS SEET AND USE
1. Butane. 2. trans-2-methylcyclohexanol. 3. 1,2-dimethylcyclohexene. Chem 131 Spring 2018 Exam I Practice
 Chem 131 Spring 2018 Exam I Practice Thursday s examination will contain 31 questions valued at 3.5 points/question, with some bonus possibilities available Name KEY Name the following compounds. 1. Butane
Chem 131 Spring 2018 Exam I Practice Thursday s examination will contain 31 questions valued at 3.5 points/question, with some bonus possibilities available Name KEY Name the following compounds. 1. Butane
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Introduction to Carbohydrates
 Introduction to Carbohydrates 1. A six-carbon aldose has four chiral centers as follows: 2R, 3R, 4S, and 5R. A. Draw the sugar in linear and cyclic form B. Draw the form that would predominate in solution.
Introduction to Carbohydrates 1. A six-carbon aldose has four chiral centers as follows: 2R, 3R, 4S, and 5R. A. Draw the sugar in linear and cyclic form B. Draw the form that would predominate in solution.
Fundamentals of Organic Chemistry. CHAPTER 6: Carbohydrates
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
(A) (B) (C) (D) (E) (A) 5. When (R)-butan-2-ol is treated with TsCl in pyridine, the product formed is.
 零 不 不 (A) 1. What is the carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone? (A) an enol (B) a Grignard reagent (C) an acetylide (D) a carbocation (E) an
零 不 不 (A) 1. What is the carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone? (A) an enol (B) a Grignard reagent (C) an acetylide (D) a carbocation (E) an
Sheet #10 Dr. Mamoun Ahram Sec 1,2,3 15/07/2014. Carbohydrates 2
 Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
Chapter 18. Carbohydrates with an Introduction to Biochemistry. Carbohydrates with an Introduction to Biochemistry page 1
 Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
Chapter 18 Carbohydrates with an Introduction to Biochemistry Carbohydrates with an Introduction to Biochemistry page 1 Introduction to Proteins, Carbohydrates, Lipids, and Bioenergetics Metabolism and
Chapter 7 Carbohydrates
 Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
Chapter 7 Carbohydrates Definition of Carbohydrates carbohydrate: hydrate of carbon ; C n ( 2 ) m Examples: glucose (C 6 12 6 or C 6 ( 2 ) 6 ), sucrose (C 12 22 11 or C 12 ( 2 ) 11 ) saccharide: simple
Dr. Nafith Abu Tarboush. Rana N. Talj
 2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
Alkane C-C single bond (propane) Alkene C=C double bond (propene) Alcohol - OH group (1-propanol) major. minor
 Functional group* and name? Alkane - single bond (propane) *alkanes not really regarded as a functional group Alkene = double bond (propene) Addition of an unsymmetrical reagent to unsymmetrical alkene
Functional group* and name? Alkane - single bond (propane) *alkanes not really regarded as a functional group Alkene = double bond (propene) Addition of an unsymmetrical reagent to unsymmetrical alkene
Org/Biochem Final Lec Form, Spring 2012 Page 1 of 6
 Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
Page 1 of 6 Missing Complete Protein and Question #45 Key Terms: Fill in the blank in the following 25 statements with one of the key terms in the table. Each key term may only be used once. Print legibly.
Organic Chemistry. Carey/Giuliano 10th edition. Chapter 10
 Organic Chemistry Carey/Giuliano 10th edition Chapter 10 Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education.
Organic Chemistry Carey/Giuliano 10th edition Chapter 10 Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education.
Please read and sign the Honor Code statement below:
 CHEM 3311 Exam #1 Name Dr. Minger June 6, 2016 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
CHEM 3311 Exam #1 Name Dr. Minger June 6, 2016 Please read and sign the Honor Code statement below: I pledge that on my honor, as a University of Colorado at Boulder student, I have neither given nor received
Oregon State University
 H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
UNSATURATED HYDROCARBONS
 hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
Alkenes and Alkynes: Structure and Nomenclature
 hapter 3 3 : Structure and Nomenclature APTER SUMMARY 3.1 Introduction to Alkenes are hydrocarbons in which there is at least one carbon-carbon double bond; alkynes have at least one carbon-carbon triple
hapter 3 3 : Structure and Nomenclature APTER SUMMARY 3.1 Introduction to Alkenes are hydrocarbons in which there is at least one carbon-carbon double bond; alkynes have at least one carbon-carbon triple
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Chapter 24: Carbohydrates
 Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
Chapter 24: Carbohydrates [Sections: 24.1 24.10] 1. Carbohydrates definition naturally occuring compounds derived from carbon, oxygen and hydrogen the net molecular formula comes from each carbon having
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/11/e1701208/dc1 Supplementary Materials for Competitive chiral induction in a 2D molecular assembly: Intrinsic chirality versus coadsorber-induced chirality This
advances.sciencemag.org/cgi/content/full/3/11/e1701208/dc1 Supplementary Materials for Competitive chiral induction in a 2D molecular assembly: Intrinsic chirality versus coadsorber-induced chirality This
Alkynes. Amides. Aromatics. Hydration of Alkenes. Thiols Salts. Acetal Formation
 ` Learning Commons 3 Alkynes Amides Aromatics ydration of Alkenes Thiols Salts Acetal Formation 5 USER GUIDE 7 UNIT I 9 ACIDS & BASES 11 1.1 ACIDS & BASE CEMISTRY 13 1.2 GRAM EQUIVALENTS- NORMALITY-
` Learning Commons 3 Alkynes Amides Aromatics ydration of Alkenes Thiols Salts Acetal Formation 5 USER GUIDE 7 UNIT I 9 ACIDS & BASES 11 1.1 ACIDS & BASE CEMISTRY 13 1.2 GRAM EQUIVALENTS- NORMALITY-
Carbohydrate Structure and Nomenclature. Essentials of Glycobiology 1 April 2004
 1 Carbohydrate Structure and Nomenclature Essentials of Glycobiology 1 April 2004 Nathaniel Finney Dept. of Chemistry and Biochemistry UCSD nfinney@chem.ucsd.edu 2 Lecture utline 1. Carbohydrates - definition
1 Carbohydrate Structure and Nomenclature Essentials of Glycobiology 1 April 2004 Nathaniel Finney Dept. of Chemistry and Biochemistry UCSD nfinney@chem.ucsd.edu 2 Lecture utline 1. Carbohydrates - definition
Chapter 7- Alkenes: Structure and Reactivity. Ashley Piekarski, Ph.D. Alkene
 Chapter 7- Alkenes: Structure and Reactivity Ashley Piekarski, Ph.D. Alkene What is an alkene func
Chapter 7- Alkenes: Structure and Reactivity Ashley Piekarski, Ph.D. Alkene What is an alkene func
Alkanes. 1. Predict the molecular formula of an alkane with 13 carbon atoms The table below lists the boiling points of some alkanes.
 Alkanes 1. Predict the molecular formula of an alkane with 13 carbon atoms.... [Total 1 mark]. The table below lists the boiling points of some alkanes. alkane number of carbon atoms molecular formula
Alkanes 1. Predict the molecular formula of an alkane with 13 carbon atoms.... [Total 1 mark]. The table below lists the boiling points of some alkanes. alkane number of carbon atoms molecular formula
Chemistry 14C Winter 2017 Final Exam Part B Page 1
 Chemistry 14C Winter 2017 Final Exam Part B Page 1 Please use the backs of the data table pages and other exam pages for scratch space. Please do not use the exam margins for this purpose. Begin this exam
Chemistry 14C Winter 2017 Final Exam Part B Page 1 Please use the backs of the data table pages and other exam pages for scratch space. Please do not use the exam margins for this purpose. Begin this exam
Chapter 24: Carbohydrates
 Chapter 24: Carbohydrates Photosynthesis: : Energy storage Sun, chlorophyll 6 CO 2 + 6 2 O C 6 ( 2 O) 6 + 6 O 2 Metabolism Glucose Land plants Universal Chlorophyll 3% to 6% of the total incident solar
Chapter 24: Carbohydrates Photosynthesis: : Energy storage Sun, chlorophyll 6 CO 2 + 6 2 O C 6 ( 2 O) 6 + 6 O 2 Metabolism Glucose Land plants Universal Chlorophyll 3% to 6% of the total incident solar
Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils
 Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils Distillation relies on the fact that the substance with the greatest vapor pressure will be enriched in the vapor phase
Isolation and Purification of Organic Compounds Steam Distillation of Essential Oils Distillation relies on the fact that the substance with the greatest vapor pressure will be enriched in the vapor phase
Enantiomers: a new issue in pharmacotherapy of depression?
 Should be cited as: Psychiatria Polska 2013, tom XLVII, numer 3 strony 511 516 Enantiomers: a new issue in pharmacotherapy of depression? Department of Biochemistry and Clinical Chemistry, Medical University
Should be cited as: Psychiatria Polska 2013, tom XLVII, numer 3 strony 511 516 Enantiomers: a new issue in pharmacotherapy of depression? Department of Biochemistry and Clinical Chemistry, Medical University
You must answer FOUR of the SIX questions. Use a SEPARATE answer book for EACH question.
 UNIVERSITY OF EAST ANGLIA School of Pharmacy Main Series UG Examination 2016-17 DRUG DESIGN AND MECHANISMS OF DRUG ACTION PHA-5001Y Time allowed: 2 hours You must answer FOUR of the SIX questions. Use
UNIVERSITY OF EAST ANGLIA School of Pharmacy Main Series UG Examination 2016-17 DRUG DESIGN AND MECHANISMS OF DRUG ACTION PHA-5001Y Time allowed: 2 hours You must answer FOUR of the SIX questions. Use
CHAPTER 23. Carbohydrates
 CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
CAPTER 23 Carbohydrates 1 Introduction Carbohydrates are naturally occurring compounds of carbon, hydrogen, and oxygen. Carbohydrates have the empirical formula C 2. Carbohydrates have the general formula
High School Name/ Team ID: ID Numbers:
 Solutions for Chemistry of Life Exam 1. Carbohydrates are one of the four classes of primary metabolites in humans (components that are required for life). This question will deal with glucose, a product
Solutions for Chemistry of Life Exam 1. Carbohydrates are one of the four classes of primary metabolites in humans (components that are required for life). This question will deal with glucose, a product
FOUNDATION IN SCIENCE (CFSI) CHM1204: CHEMISTRY 2 FINAL EXAMINATION: JANUARY 2014 SESSION
 CHM1204 (F)/Page 1 of 11 INTI INTERNATIONAL UNIVERSITY FOUNDATION IN SCIENCE (CFSI) CHM1204: CHEMISTRY 2 FINAL EXAMINATION: JANUARY 2014 SESSION Instructions: This paper consists of FIVE (5) questions.
CHM1204 (F)/Page 1 of 11 INTI INTERNATIONAL UNIVERSITY FOUNDATION IN SCIENCE (CFSI) CHM1204: CHEMISTRY 2 FINAL EXAMINATION: JANUARY 2014 SESSION Instructions: This paper consists of FIVE (5) questions.
For more info visit
 Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
The Foundations of Biochemistry
 c01thefoundationsofbiochemistry.qxd 12/6/12 4:09 PM Page S-1 chapter The Foundations of Biochemistry 1 1. The Size of ells and Their omponents (a) If you were to magnify a cell 10,000-fold (typical of
c01thefoundationsofbiochemistry.qxd 12/6/12 4:09 PM Page S-1 chapter The Foundations of Biochemistry 1 1. The Size of ells and Their omponents (a) If you were to magnify a cell 10,000-fold (typical of
Developing a Biocascade Process: Concurrent Ketone Reduction-Nitrile Hydrolysis of 2-Oxocycloalkanecarbonitriles
 Developing a Biocascade Process: Concurrent Ketone Reduction-Nitrile Hydrolysis of 2-Oxocycloalkanecarbonitriles Elisa Liardo, a Nicolás Ríos-Lombardía, b Francisco Morís, b Javier González-Sabín,*,b and
Developing a Biocascade Process: Concurrent Ketone Reduction-Nitrile Hydrolysis of 2-Oxocycloalkanecarbonitriles Elisa Liardo, a Nicolás Ríos-Lombardía, b Francisco Morís, b Javier González-Sabín,*,b and
Chapter 7 Structure and Synthesis of Alkenes. Introduction
 Chapter 7 Structure and Synthesis of Alkenes Introduction ydrocarbon with carbon-carbon double bonds Sometimes called olefins, oil-forming gas Planar Pi bond is the functional group. More reactive than
Chapter 7 Structure and Synthesis of Alkenes Introduction ydrocarbon with carbon-carbon double bonds Sometimes called olefins, oil-forming gas Planar Pi bond is the functional group. More reactive than
I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy
 Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides
 Educational Goals Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides 1. Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine molecule, be able to give the systematic
Educational Goals Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides 1. Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine molecule, be able to give the systematic
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling
 Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling In the above mentioned scheme sertraline have four different isomers possible.
Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling In the above mentioned scheme sertraline have four different isomers possible.
CHEM 261 Mar 9, same
 93 EM 261 Mar 9, 2017 Review: ydrogenation The addition of 2 to an alkene Example: cyclohexene 2 Pd same or stereochemistry: 3 4 2 1 S R Aside: = deuterium = 2 (1 p + and 1 e - ) Product is a meso compound
93 EM 261 Mar 9, 2017 Review: ydrogenation The addition of 2 to an alkene Example: cyclohexene 2 Pd same or stereochemistry: 3 4 2 1 S R Aside: = deuterium = 2 (1 p + and 1 e - ) Product is a meso compound
