Interactions of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: a computational model of human skeletal muscle
|
|
|
- Marshall Oliver
- 5 years ago
- Views:
Transcription
1 Interactions of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: a computational model of human skeletal muscle Feilim Mac Gabhann and Aleksander S. Popel Am J Physiol Heart Circ Physiol 292: , First published Sep 15, 2006; doi: /ajpheart You might find this additional information useful... This article cites 44 articles, 24 of which you can access free at: Updated information and services including high-resolution figures, can be found at: Additional material and information about AJP - Heart and Circulatory Physiology can be found at: This information is current as of January 15, AJP - Heart and Circulatory Physiology publishes original investigations on the physiology of the heart, blood vessels, and lymphatics, including experimental and theoretical studies of cardiovascular function at all levels of organization ranging from the intact animal to the cellular, subcellular, and molecular levels. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD Copyright 2005 by the American Physiological Society. ISSN: , ESSN: Visit our website at
2 Am J Physiol Heart Circ Physiol 292: H459 H474, First published September 15, 2006; doi: /ajpheart Interactions of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: a computational model of human skeletal muscle Feilim Mac Gabhann and Aleksander S. Popel Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, Maryland Submitted 14 June 2006; accepted in final form 7 September 2006 Mac Gabhann F, Popel AS. Interactions of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: a computational model of human skeletal muscle. Am J Physiol Heart Circ Physiol 292: H459 H474, First published September 15, 2006; doi: /ajpheart The vascular endothelial growth factor (VEGF) family of cytokines is involved in the maintenance of existing adult blood vessels as well as in angiogenesis, the sprouting of new vessels. To study the proangiogenic activation of VEGF receptors (VEGFRs) by VEGF family members in skeletal muscle, we develop a computational model of VEGF isoforms (VEGF 121, VEGF 165), their cell surface receptors, and the extracellular matrix in in vivo tissue. We build upon our validated model of the biochemical interactions between VEGF isoforms and receptor tyrosine kinases (VEGFR-1 and VEGFR-2) and nonsignaling neuropilin-1 coreceptors in vitro. The model is general and could be applied to any tissue; here we apply the model to simulate the transport of VEGF isoforms in human vastus lateralis muscle, which is extensively studied in physiological experiments. The simulations predict the distribution of VEGF isoforms in resting (nonexercising) muscle and the activation of VEGFR signaling. Little of the VEGF protein in muscle is present as free, unbound extracellular cytokine; the majority is bound to the cell surface receptors or to the extracellular matrix. However, interstitial sequestration of VEGF 165 does not affect steadystate receptor binding. In the absence of neuropilin, VEGF 121 and VEGF 165 behave similarly, but neuropilin enhances the binding of VEGF 165 to VEGFR-2. This model is the first to study VEGF tissue distribution and receptor activation in human muscle, and it provides a platform for the design and evaluation of therapeutic approaches. angiogenesis; cytokine; growth factor; mathematical model VASCULAR ENDOTHELIAL GROWTH factor (VEGF) is a critical family of cytokines involved in the process of angiogenesis (or neovascularization), the outgrowth of new microvessels from existing vasculature (30, 36). VEGF family members are diffusible secreted dimers that bind to and activate receptor tyrosine kinases on endothelial cells and stimulate proliferation and migration. They are also involved in the chemotactic guidance of the nascent sprouts. The involvement of VEGF in the angiogenic process identifies it as a target for proangiogenic therapy in skeletal muscle. The increase of muscle vascularization is a possible route for the amelioration of peripheral vascular diseases, in which the delivery of oxygen is reduced because of the loss or partial loss of functional vasculature (15, 32). To study the effects of VEGF on muscle vascularization, we first investigate the behavior of VEGF and VEGF receptors (VEGFRs) in healthy, resting muscle. Although the action of VEGF on cells in vitro has been extensively studied (30, 36), it is difficult to measure the same parameters (receptor activation, for example) in vivo. Here, we build a model of VEGF transport in skeletal muscle, to understand the distribution and availability of VEGF in human tissue, as well as the activation of VEGFR-1 and VEGFR-2, the primary receptor tyrosine kinases for VEGF on blood microvascular endothelial cells. The VEGF family consists of four genes in humans, and of these VEGF-A is the best studied. Each of the genes encodes multiple splice isoforms that can generate proteins of varying lengths. The two most abundant isoforms of VEGF-A are VEGF 121 and VEGF 165. The additional 44 amino acids of the longer isoform encode a domain that binds neuropilin-1 as well as a heparin-binding domain that allows the extracellular matrix (ECM) to sequester that isoform (while VEGF 121 remains freely diffusible). The complexity of the interactions between the various members of the VEGF family and their receptors (including ligandindependent receptor-receptor coupling interactions) is difficult, if not currently impossible, to analyze without the benefit of a computational model. This is especially true in vivo, where it is difficult to make many of the measurements (in regard to receptor binding, for example) that can be performed in vitro. The model takes the measurements that can be made, and a detailed kinetic network, and predicts the concentrations of molecular species that cannot currently be experimentally measured. In addition to better understanding physiological behavior, establishing a detailed model of the skeletal muscle VEGF system would allow us to test therapeutic interventions in silico. We have previously published models of the interactions between VEGF family members and VEGFR-1 and VEGFR-2 in vitro (19, 20), as well as a validated model of the biochemical interaction network of VEGF 121, VEGF 165, VEGFR-2, and neuropilin-1 (18). That study demonstrated that simulations of the interactions between VEGF 165, VEGFR-2, and neuropilin agreed with all published results of VEGF 165 and VEGF 121 binding to and activation of VEGFR-2 on endothelial cells. Here we extend that interaction network to in vivo tissue, by including the ECM as well as multiple cell types (myocytes and endothelial cells) and the geometry of the tissue. The model is general and could be applied to any tissue. In this study we focus on the human vastus lateralis muscle, widely studied in physiological investigations, particularly in relation to the effects of exercise on muscle vascularization (4, 8, 9, 33). Despite uncertainty in the values of some of the parameters, we can make several conclusions about the in vivo functioning of the VEGF system. Address for reprint requests and other correspondence: F. Mac Gabhann, Dept. of Biomedical Engineering, Johns Hopkins Univ. School of Medicine, 720 Rutland Ave., 613 Traylor Bldg., Baltimore, MD ( feilim@jhu.edu). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact /07 $8.00 Copyright 2007 the American Physiological Society H459
3 H460 METHODS Glossary [V 121], [V 165] Concentration of unbound VEGF 121 or VEGF 165 in the interstitial space [M ECM], [M EBM], [M MBM] Concentration of unbound glycosaminoglycan (GAG) chains in ECM, endothelial basement membrane (EBM), or myocyte basement membrane (MBM) [V 165M ECM], [V 165M EBM], [V 165M MBM] Concentration of VEGF bound to GAG chains [R 1], [R 2], [N 1] Concentration of unbound VEGFR-1, VEGFR-2, or neuropilin-1 on endothelial cell surface [V 121R 1], [V 165R 1], [V 121R 2], [V 165R 2], [V 165N 1] Concentration of VEGF bound to receptors [R 2V 165N 1] Concentration of cell surface VEGF 165 bridging VEGFR-2 and neuropilin [R 1N 1], [V 121R 1N 1] Concentration of VEGFR-1-neuropilin complex with and without VEGF 121 q V,m Secretion rate of VEGF from myocytes k on, k off Kinetic rates of binding and unbinding k c Coupling rate of neuropilin-bound or VEGFRbound VEGF to a second receptor k dissoc Dissociation rate of two receptors s R1, s R2, s N1 Insertion rate of new receptors into the membrane Internalization rate of receptors k int Development of Computational Model VEGF transport and binding. We constructed a model of VEGF transport and interactions with its receptors in skeletal muscle in vivo (Fig. 1). The skeletal muscle is composed of three primary elements: myocytes (muscle fibers), blood vessels, and the interstitial space between them. This interstitial space contains a proteoglycan-based matrix and is divided into three regions: the ECM and two denser basement membranes, one surrounding the myocytes (MBM) and the other on the abluminal surface of the endothelial cells of blood vessels (EBM). VEGF is secreted by myocytes and binds to cell surface receptors on endothelial cells; in the case of VEGF 165, it may also be sequestered by VEGF binding sites in the ECM and basement membranes. The signaling ligand-receptor complexes formed by VEGF 121, VEGF 165, and their receptors are shown in Fig. 1, B and C, along with the interactions between the ligands and receptors. Both VEGF 121 and VEGF 165 bind to VEGFR-1 and VEGFR-2: V 121 R 1 7V 121 R 1 V 121 R 2 7V 121 R 2 V 165 R 1 7V 165 R 1 V 165 R 2 7V 165 R 2 In addition, VEGF 165 binds to neuropilin-1: V 165 N 1 7V 165 N 1 VEGF 165-VEGFR-2 and VEGF 165-neuropilin-1 can diffuse laterally on the cell membrane, and VEGF 165 binds to the complementary receptor to couple VEGFR-2 and neuropilin-1 together: V 165 R 2 N 1 7R 2 V 165 N 1 V 165 N 1 R 2 7R 2 V 165 N 1 While VEGFR-2 and neuropilin-1 do not bind each other but are coupled by VEGF 165, VEGFR-1 binds directly to neuropilin-1, and this complex is permissive for binding VEGF 121 but not VEGF 165: R 1 N 1 7R 1 N 1 V 121 R 1 N 1 7V 121 R 1 N 1 V 121 R 1 N 1 7V 121 R 1 N 1 The interactions between VEGF 165 and the interstitial binding sites are: V 165 M ECM 7V 165 M ECM V 165 M EBM 7V 165 M EBM V 165 M MBM 7V 165 M MBM Computational model. We assume that the concentration of free (unbound) VEGF is uniform across the interstitial space. The Damkohler number (ratio of diffusion time to reaction time) is significantly less than 1, indicating that diffusion is significantly faster than the kinetics of binding to and unbinding from ECM glycosaminoglycan (GAG) chains or cell surface receptors. The possible formation of diffusion-limited VEGF gradients will thus not be studied here; instead our model represents average VEGF in the interstitium and average receptor binding on the cell surface. Spatial variability in binding due to gradient formation may be important for angiogenic signaling in vivo (17) but is not dealt with here. Thus the system is described by a set of coupled nonlinear ordinary differential equations. For interstitial proteins these equations are as follows: d V 121 /dt q V121,m k on,vr1 V 121 R 1 k off,vr1 V 121 R 1 k on,vr2 V 121 R 2 k off,vr2 V 121 R 2 k on,vr1 V 121 R 1 N 1 k off,vr1 V 121 R 1 N 1 d V 165 /dt q V165,m k on,vm V 165 M ECM k off,vm V 165 M ECM k on,vm V 165 M EBM k off,vm V 165 M EBM k on,vm V 165 M MBM k off,vm V 165 M MBM k on,vr1 V 165 R 1 k off,vr1 V 165 R 1 k on,vr2 V 165 R 2 k off,vr2 V 165 R 2 k on,vn1 V 165 N 1 k off,vn1 V 165 N 1 d M ECM /dt k on,vm V 165 M ECM k off,vm V 165 M ECM d M EBM /dt k on,vm V 165 M EBM k off,vm V 165 M EBM d M MBM /dt k on,vm V 165 M MBM k off,vm V 165 M MBM d V 165 M ECM /dt k on,vm V 165 M ECM k off,vm V 165 M ECM d V 165 M EBM /dt k on,vm V 165 M EBM k off,vm V 165 M EBM d V 165 M MBM /dt k on,vm V 165 M MBM k off,vm V 165 M MBM
4 H461 where q is secretion rate and k on and k off are kinetic rates of binding and unbinding, respectively. Although we note VEGF as secreted from myocytes, VEGF secreted from other sources, e.g., endothelial cells, if significant, could be included in the same term by adding all the sources of VEGF together. For cell surface receptors we have the following equations: the measured capillary-to-fiber ratio. From this, we calculate the capillary density and size based on the vascular volume of 1.4% (cm 3 /cm 3 ). For example, for a fiber cross-sectional area of 3,500 m 2, the microvascular density would be 360 capillaries/mm 2,witha7- m characteristic diameter; for 3,000- m 2 fiber area, the microvascular d R 1 /dt s R1 k int,r1 R 1 k on,vr1 V 121 R 1 k off,vr1 V 121 R 1 k on,vr1 V 165 R 1 k off,vr1 V 165 R 1 k c,r1n1 R 1 N 1 k dissoc R 1 N 1 d R 2 /dt s R2 k int,r2 R 2 k on,vr2 V 121 R 2 k off,vr2 V 121 R 2 k on,vr2 V 165 R 2 k off,vr2 V 165 R 2 k c,r2,vn1 V 165 N 1 R 2 k off,vr2 R 2 V 165 N 1 d N 1 /dt s N1 k int,n1 N 1 k on,vn1 V 165 N 1 k off,vn1 V 165 N 1 k c,vr2,n1 V 165 R 2 N 1 k off,vn1 R 2 V 165 N 1 d V 121 R 1 /dt k int,vr1 V 121 R 1 k on,vr1 V 121 R 1 k off,vr1 V 121 R 1 k c,r1n1 V 121 R 1 N 1 k dissoc V 121 R 1 N 1 ] d V 121 R 2 /dt k int,vr2 V 121 R 2 k on,vr2 V 121 R 2 k off,vr2 V 121 R 2 d V 165 R 1 /dt k int,vr1 V 165 R 1 k on,vr1 V 165 R 1 k off,vr1 V 165 R 1 d V 165 R 2 /dt k int,vr2 V 165 R 2 k on,vr2 V 165 R 2 k off,vr2 V 165 R 2 k c,vr2,n1 V 165 R 2 N 1 k off,vn1 R 2 V 165 N 1 d V 165 N 1 /dt k int,vn1 V 165 N 1 k on,vn1 V 165 N 1 k off,vn1 V 165 N 1 k c,r2,vn1 V 165 N 1 R 2 k off,vr2 R 2 V 165 N 1 d R 2 V 165 N 1 /dt k int,vr2 R 2 V 165 N 1 k c,vr2,n1 V 165 R 2 N 1 k off,vn1 R 2 V 165 N 1 k c,r2,vn1 V 165 N 1 R 2 k off,vr2 R 2 V 165 N 1 d V 121 R 1 N 1 /dt k int,vr1 V 121 R 1 N 1 k c,r1n1 [V 121 R 1 ] N 1 k dissoc V 121 R 1 N 1 k on,vr1 V 121 R 1 N 1 k off,vr1 V 121 R 1 N 1 d R 1 N 1 /dt k int,r1 R 1 N 1 k c,r1n1 R 1 N 1 k dissoc R 1 N 1 k on,vr1 V 121 R 1 N 1 k off,vr1 [V 121 R 1 N 1 ] Each of the VEGF binding sites cell surface receptors as well as interstitial GAG chains comes into equilibrium with the VEGF concentration in the interstitium when the system has reached steady state. The coupled set of ordinary differential equations is solved for appropriate values of the parameters (below) to find the steady-state concentrations of all molecular species. Steady state here represents resting muscle; the effects of the exercising muscle on the dynamics of VEGF secretion and transport will not be considered here. Model Parameters The parameters required for simulation of this model fall into three categories: geometry, kinetic rates, and initial concentrations; they are given in Tables 1 3. Geometry. The model is general and applicable to any tissue. In this study, we use the geometric parameters typical for skeletal muscle, in particular human vastus lateralis muscle, a tissue extensively studied in human physiological experiments, particularly those involving exercise, as summarized in Table 1. The extracellular water fraction of this tissue is taken to be 8.4% (cm 3 /cm 3 ), based on 7.9 ml/100 g wet muscle (16, 38) and a muscle density of 1.06 g/ml (23, 35). Of this, the capillary blood volume is taken to be 1.4% (cm 3 /cm 3 ) (34). The capillary-to-fiber ratio in human vastus lateralis has been measured as 1.38 and the cross-sectional area of fibers as 5,232 m 2 for sedentary men (8), although muscle fiber size and capillary density in the vastus lateralis vary with age, sex, and exercise training (4, 8, 9, 33, 42). However, to match the measured vascular space, this gives a characteristic capillary diameter of 8.6 m and a microvascular density of 240 capillaries/mm 2 (when adjusted for extracellular space). The capillary size is larger than measured capillary diameters (5 7.5 m) in human skeletal muscle (11, 41), while the density is too low to deliver required oxygen to the tissue according to the analysis of McGuire and Secomb (22). Thus we assume that the fiber crosssectional area was overestimated because of muscle contraction and other artifacts of tissue handling (as suggested in Ref. 22) but maintain density would be 420 capillaries/mm 2, with a 6.5- m characteristic diameter. We take the latter value. This diameter is consistent with measurements of capillary diameter (11, 41), and the density is consistent with the oxygen consumption analysis (22). The nonextracellular volume is composed of the skeletal muscle fibers and the endothelial cells themselves. The endothelial cells are assumed to have a thickness of 0.5 m (consistent with measurements; Ref. 11) surrounding the vessels and thus occupy a volume of 0.46% (cm 3 /cm 3 ). The remaining volume 91.14% (cm 3 /cm 3 ) is composed of fibers. The muscle fibers are not cylindrical. The measured perimeter of the fibers is 14% greater than that predicted for a cylinder of equivalent cross-sectional area (8). Thus, in calculating the surface area of the fibers, a correction of 1.14 is applied to the cylindrical surface area determined from the volume fraction of fibers. The fibers have a perimeter of 222 m, and the surface area of fibers per unit volume is 674 cm 2 fiber surface/cm 3 tissue. Similarly, the blood vessels are not cylindrical. The abluminal surface area of capillaries is affected by the elliptical shape of the vessel and bulging caused by the nucleus. As an upper limit, the perimeter to cross-sectional area correction has been measured for tumor microvessels (which have been noted to be more irregular than normal muscle vessels) as 1.23 (28, 29). For the muscle microvessels, we assume a surface area correction of 10% (1.10), and the surface area of microvessels per unit volume is 109 cm 2 endothelial cell surface/cm 3 tissue. The results of our simulations are not significantly affected by a change in the surface area correction (data not shown). Both the vessels and the muscle fibers have associated basement membranes. The thickness of these was measured in resting human vastus lateralis as 42 and 24 nm, respectively (21). These thicknesses are used to calculate the volumes of each type of basement membrane in the tissue (Table 1). The remaining interstitial space is designated as ECM.
5 H462 Fig. 1. Vascular endothelial growth factor (VEGF) transport in skeletal muscle. A: the tissue is characterized by long muscle fibers of (approximately) constant cross section and a capillary network in the interstitial space between the fibers. VEGF is secreted by the muscle fibers into the interstitial space, which is composed of 3 sections: the basement membranes (BM) surrounding the muscle fibers and blood vessels and the extracellular matrix (ECM). VEGF 165 binds to (and is sequestered by) glycosaminoglycan (GAG) chains in the interstitial space; VEGF 121 does not. Both isoforms bind to the cell surface signaling receptors, although the pattern of binding is different for the 2 isoforms. B and C: VEGF isoform interactions with VEGF receptor (VEGFR)-1 (C), VEGFR-2 (B), and neuropilin-1. VEGF 121 and VEGF 165 are the 2 most abundant isoforms of VEGF in human tissue. Both isoforms bind VEGFR-1 and VEGFR-2, receptor tyrosine kinases. Only VEGF 165 binds to neuropilin-1 and to the GAG chains on the proteoglycans of the ECM and BM. VEGF 165 can bind to both VEGFR-2 and neuropilin-1 simultaneously and thus can couple these receptors together on the endothelial cell surface (with rate k c). VEGFR-1 and neuropilin-1 interact directly, and this complex is permissive for VEGF 121 binding to VEGFR-1 but not VEGF 165. Muscle fibers are long, multinucleate cells. We divide these into smaller structures (similar to mononuclear cell size) by applying the concept of the myonuclear domain (MD), the volume of the fiber under the control of a single nucleus (1). More specifically for this study of cell surface receptors, we use the term to refer to the surface area of the fiber cell membrane under the control of each nucleus. Secretion from the fibers is thus given in units of molecules per MD per second, rather than molecules per fiber per second. One study of nuclear density in human vastus lateralis gave a MD of 2,600 m 2, with 94 nuclei per millimeter of length of a fiber of cross section 3,600 m 2 and a perimeter correction of 15% (elderly male subjects) (12). Soleus muscles of healthy human males, with 150 nuclei/mm and cross-sectional areas of 2,000 m 2,haveaMDof 1,200 m 2 (27). We assume that young, healthy male vastus lateralis would have higher density of nuclei (120/mm) and calculate a MD of 1,850 m 2. These geometric parameters are incorporated into the kinetic parameters and concentrations of the model (Tables 1 3), and the results are therefore tissue specific. Tissue geometry changes result in changes to the kinetic parameters and concentrations in the model and affect predictions of both the VEGF distribution and VEGFR occupancy. Kinetics. The kinetic rates for VEGF isoform binding to and unbinding from VEGFRs and neuropilins are based on experimental measurements and similar to those used in previous models (18) and are summarized in Table 2. The ECM binding sites for VEGF are a diverse group of proteins and proteoglycans with different affinities.
6 Table 1. Geometric parameters of human vastus lateralis muscle H463 Skeletal Muscle Characteristic Value Unit Reference Muscle fibers Cross-sectional area of 1 fiber 3,000 m 2 See text Perimeter of 1 fiber 222 m See text Capillary-fiber ratio Capillary density 420 Capillaries/mm 2 tissue Calculated Muscle fiber density 304 Fibers/mm 2 tissue Calculated Volume fractions Interstitial space 7% cm 3 /cm 3 tissue 16, 38 Fibers 91.14% cm 3 /cm 3 tissue Calculated Microvessels 1.86% cm 3 /cm 3 tissue Calculated Of which vascular space 1.4% cm 3 /cm 3 tissue 34 Microvessels Internal diameter of microvessel 6.52 m Calculated Thickness of endothelial cell 0.5 m 11 External diameter of microvessel 7.52 m Calculated Cross-sectional area of 1 microvessel 44 m 2 Calculated Perimeter of 1 microvessel 26 m Calculated Surface areas Muscle fibers 674 cm 2 /cm 3 tissue Calculated Microvessels 109 cm 2 /cm 3 tissue Calculated Basement Membranes (BMs) Thickness of muscle fiber BM 24 nm 21 Basement membrane volume (muscle fiber) 0.162% cm 3 /cm 3 tissue Calculated 2.3% cm 3 /cm 3 interstitium Calculated Thickness of microvessel BM 43 nm 21 BM volume (microvessel) 0.046% cm 3 /cm 3 tissue Calculated 0.7% cm 3 /cm 3 interstitium Calculated Extracellular matrix volume 6.79% cm 3 /cm 3 tissue Calculated 97% cm 3 /cm 3 interstitium Calculated Myonuclear domain surface area 1,850 m 2 See text Table 2. Kinetic parameters of VEGF system Parameter Measured Parameter Tissue Model Value Unit Value Unit VEGF binding to VEGFR-1 k on M 1 s (pmol/cm 3 tissue) 1 s 1 k off 10 3 s 1 K d 33 pm pmol/cm 3 tissue VEGF binding to VEGFR-2 k on 10 7 M 1 s (pmol/cm 3 tissue) 1 s 1 k off 10 3 s 1 K d 100 pm pmol/cm 3 tissue VEGF 165 binding to NRP-1 k on M 1 s (pmol/cm 3 tissue) 1 s 1 k off 10 3 s 1 K d 312 pm pmol/cm 3 tissue VEGF 165 binding to GAGs k on M 1 s (pmol/cm 3 tissue) 1 s 1 k off 10 2 s 1 K d 24 nm 1.7 pmol/cm 3 tissue Coupling of NRP-1 and VEGFR-2 k cvr2,n (mol/cm 2 ) 1 s (pmol/cm 3 tissue) 1 s 1 k offvr2,n 10 3 s 1 k cvn,r (mol/cm 2 ) 1 s (pmol/cm 3 tissue) 1 s 1 k offvn,r s 1 VEGFR-1 coupling to NRP-1 k cr1,n (mol/cm 2 ) 1 s (pmol/cm 3 tissue) 1 s 1 k dissoc,r1,n 10 2 s 1 VEGFR internalization k int,r (free receptors) s 1 k int,c (bound receptors) s 1 Conversion from in vitro parameters to tissue parameters is based on the geometry (Table 1): (pmol/cm 3 tissue)/(mol/l of interstitial space) and (pmol/cm 3 tissue)/(mol/cm 2 EC), where EC is endothelial cells. The effect of tissue geometry is thus included in the model through the kinetic parameters (this table) and concentrations (Table 3). VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; GAG, glycosaminoglycan; NRP-1, neuropilin-1.
7 H464 Table 3. VEGF concentration and receptor densities for skeletal muscle Measured Parameter Tissue Model Parameter Value Unit Value Unit Free VEGF concentration Human vastus lateralis, rest (13) 1 pm pmol/cm 3 tissue Total VEGF tissue concentration Human vastus lateralis, rest (4, 8, 9, 33) 1 2 pg/ g protein pmol/cm 3 tissue VEGFR1 tissue concentration Human vastus lateralis, rest (4, 9, 33) pg/ g protein pmol/cm 3 tissue 60,000 68,000 No./EC VEGFR2 tissue concentration Human vastus lateralis, rest (4, 9, 33) pg/ g protein pmol/cm 3 tissue 13,000 19,000 No./EC NRP1 tissue concentration Not available: test a range pmol/cm 3 tissue 1, ,000 No./EC ECM binding site density ECM 0.75 M 51 pmol/cm 3 tissue Vessel BM 13 M 6 pmol/cm 3 tissue Myocyte BM 13 M 21 pmol/cm 3 tissue Conversion of receptor densities to tissue concentrations is based on relationship described in Table 2 and the surface area of an endothelial cell (EC; 1,000 m 2 ). VEGF concentration is normalized based on the entire interstitial space, because it diffuses throughout: (pmol/cm 3 tissue)/(mol/l interstitial space). VEGF binding sites in extracellular matrix (ECM) and BMs are based on those volumes: (pmol/cm 3 tissue)/(mol/l ECM), (pmol/cm 3 tissue)/(mol/l EBM), and (mol/cm 3 tissue)/(mol/l MBM), where EBM is endothelial BM and MBM is myocyte BM. The effect of tissue geometry is thus included in the model through both the concentrations (this table) and the kinetic parameters (Table 2). Conversions from pg/mg protein are based on 155 mg protein/g tissue (10) and 45-kDa VEGF, 210-kDa VEGFR, and 240-kDa VEGFR2. Numbers in parentheses are reference numbers. An effective binding affinity is used for the ensemble of sites. VEGF binding affinity to the GAG chains on the proteoglycans of the ECM chains is of a similar order to that of bfgf, and therefore we use similar binding site density and affinity (7). Concentrations. The mean concentration of unbound VEGF has been determined by microdialysis of resting human vastus lateralis to be pm (13). This is consistent with microdialysis measurements of VEGF in normal breast tissue ( pm; Ref. 5); other human tissues have yet to be investigated with this method. It is also consistent with VEGF measurements of extracted nonmalignant cirrhotic or tuberculous ascites: 2 3 pm (ascitic fluid can be assumed to be of similar composition to interstitial fluid; in cancer, the VEGF concentration in this fluid can be up to a hundredfold higher; Refs. 6, 45). We assume that a steady-state concentration of 1 pm VEGF should be attained in our simulations. Two different isoforms of VEGF are studied here, freely diffusible VEGF 121 and matrix-binding VEGF 165, and we run each simulation twice, each assuming one isoform being secreted by itself. Muscle secretes both isoforms, and so the net behavior of VEGF in the muscle lies between the two extremes. We examine the effect of simultaneous secretion of the two isoforms in Fig. 11, described in Simultaneous Secretion of VEGF Isoforms. The 1 pm VEGF interstitial concentration is thus VEGF 121, VEGF 165, or the sum of the two, depending on the simulation. Because there is uncertainty in some of the parameters, e.g., receptor densities, and because the secretion rate of VEGF from myocytes in vivo has not been measured, we use a different secretion rate for each simulation to maintain the 1 pm free VEGF concentration at steady state. The secretion rate is shown in the relevant figure for each simulation. Although VEGFR-1, VEGFR-2, and neuropilin have all been identified as being expressed on the endothelial cells of the vasculature, quantitative estimates of the receptor density in human vastus lateralis have been published only for VEGFR-1 and VEGFR-2 (4, 9, 33). For those receptors, the measured range is equivalent to 10,000 20,000 and 50, ,000 receptors per endothelial cell (based on 155 mg protein/g tissue; Ref. 10). In addition, these measured values do not discriminate between intracellular and cell surface receptors and, in the case of VEGFR-1, secreted receptors (we do not include soluble VEGFR-1 in this model; no information was available on the concentration in tissue). We assume that the number of receptors on each endothelial cell is 10,000 for VEGFR-1 and VEGFR-2, but we also examine several expression levels of all three receptors starting with these estimates to estimate the effect of receptor density on VEGF distribution. Model Solution and Simulations Simulations of VEGF transport in skeletal muscle were developed based on the model described above. The secretion rate for each simulation was adjusted to achieve a steady-state free (unbound) VEGF concentration of 1 pm. We consider the secretion of VEGF 121 and VEGF 165 separately for most simulations. The coupled ordinary differential equations were solved with an adaptive step-size Runge- Kutta integration scheme (5th-order accuracy) implemented in Fortran and run on a desktop PC. RESULTS VEGF Internalization Capacity At steady state, secretion of VEGF must be balanced by the internalization by the receptors. The maximum internalization capacity, I max, is determined by I max k int,c [VR] max but since and then [VR] max [R] total k int R /k int C [R] total s R /k int R I max s R i.e., the total internalization capacity is equal to the insertion rate of receptors. The same conclusion would be arrived at from a mass balance on VEGFRs. The maximum secretion rate q max must not exceed the maximum internalization rate, I max.if
8 both q max and I max (or s R ) are expressed in tissue volume units (mol cm 3 tissue s 1 ), the numerical values are equal. Expressing q max and s R in local secretion or internalization units (molecules/cell/s) for this tissue, myocyte surface area per cm 3 of tissue q max myonuclear domain surface area microvessel surface area per cm 3 of tissue s R endothelial cell surface area 674 cm 2 /cm 3 q max cm s 109 cm 2 /cm 3 2 R cm 2 we obtain q max 0.3s R q max [R] total where s R and q max are measured in molecules per endothelial cell per second and molecules per MD per second, respectively, and R total is measured in molecules per endothelial cell. If the secretion rate exceeds this upper bound, then no steady state can exist in the system and the concentration of VEGF will continue to build up over time. It is possible that this occurs in pathological situations, or for short times (e.g., during exercise), but in resting muscle we will assume that a balance will be maintained and that the secretion rate does not exceed the internalization capacity of the tissue. The effect of high VEGF secretion rate approaching the internalization capacity of the tissue is illustrated in Fig. 2, as the steady-state concentration of VEGF becomes asymptotically high. Fig. 2. Interstitial VEGF concentration depends on secretion rate and receptor density. Increasing secretion rate results in increased VEGF concentration in the interstitial space; increasing receptor density (while keeping secretion rate constant) decreases the concentration as more VEGF binds to the vessels. The solid gray line represents the 1 pm concentration of unbound VEGF 121 or VEGF 165 measured in resting human vastus lateralis (13). Only VEGFR-2 is expressed by the vessels in this simulation. Note the 3 scales for VEGF secretion rate; they are shown here for comparison but are not repeated in subsequent figures. The myonuclear domain (MD) is the surface area of the myocyte per nucleus. EC, endothelial cell. Secretion Rate of VEGF and Receptor Density Determine VEGF Concentration H465 The free (unbound) VEGF concentration is determined by the balance between VEGF secretion and VEGF internalization (Fig. 2). For these simulations, only VEGFR-2 is expressed on the endothelial cells, and either VEGF 121 or VEGF 165 is secreted by the myocytes. Not all receptors are expressed on the vessels of each tissue, and thus the single-receptor case is relevant to certain tissues in addition to being informative in advance of the consideration of multiple receptor expression. A higher secretion rate from the myocytes results in higher VEGF concentration; higher receptor density increases the binding of VEGF to the cell surface and subsequent internalization into the cell, and thus decreases interstitial VEGF. It is of particular note that for the same secretion rate the unbound interstitial VEGF is the same for both VEGF 121 and VEGF 165. Even though VEGF 165 binds to the proteoglycans of the ECM, this does not affect the unbound VEGF concentration at steady state. The total (free and GAG bound) interstitial VEGF 165 concentration is 47-fold higher (in the simulation of this tissue) than that of VEGF 121. Increasing proteoglycan (or GAG) density or affinity for VEGF (or increased basement membrane thickness) would increase the amount of VEGF 165 held in the matrix. It takes longer to establish a steady state for VEGF 165, as the matrix acts as a capacitor or reservoir for VEGF and must be filled before the steady state can be reached. The similarity between the isoforms in this simulation can be understood by considering the transport of VEGF at steady state. The secretion of VEGF from the muscle fibers must be balanced by the internalization into the endothelial cells. In effect, we have a continuous flux through the system as VEGF is moved across the interstitial space. The ECM, being in equilibrium with the free VEGF, offers no significant impediment to the net transport across the gap. Because the binding affinities of VEGF 121 and VEGF 165 for VEGFR-2 are the same, the same concentration of each VEGF isoform is required to bind the same number of receptors; the receptorbound VEGF population is then internalized. The measured steady-state free VEGF concentration in resting human vastus lateralis is pm (13). We assume that a steady-state concentration of 1 pm of VEGF 121 or VEGF 165 should be attained in our simulations. The secretion rate required to achieve 1 pm at steady state depends on the receptor density (Fig. 2, gray line). Because there is uncertainty in some of the parameters, e.g., receptor densities, and because the secretion rate of VEGF from myocytes in vivo has not been measured, we use a different secretion rate for each simulation to maintain the 1 pm free VEGF concentration at steady state. An alternative way to study this system would be to maintain a constant secretion rate throughout the analysis; however, using the VEGF concentration to determine the secretion rate for each case gives us a better picture of the resting muscle. Starting from the predicted secretion rate, it would be possible to determine the impact of therapeutic interventions (e.g., increasing VEGF secretion or receptor density) or exercise on VEGF distribution, but that is not studied here. The secretion rates predicted for myocytes by the simulation are consistent with measured VEGF secretion rates from cells in vitro: 0.01 molecule cell 1 s 1 for cells derived from rat tibialis anterior (37), 0.48 molecule cell 1 s 1 for C 2 C 12 myo-
9 H466 blasts (43), and pmol cm 3 tissue s 1 for cells from various other tissues (47) (compare to Fig. 2). Distribution of VEGF in Tissue Although for a VEGFR-2-expressing vasculature the same secretion rate of VEGF 165 and VEGF 121 will lead to the same steady-state free VEGF concentration (Fig. 2), the total amount of VEGF in the tissue will be different because of the binding of VEGF 165 to the GAGs in the interstitial space. Thus the distribution of VEGF (free, bound to cell surface receptors, and bound to the matrix) is different for the two isoforms (Fig. 3). VEGF 121 is primarily endothelial cell surface receptor bound, and the proportion that is free decreases as the receptor density increases (Fig. 3A). VEGF 165, on the other hand, has a lower total proportion free (although the ratio of receptor bound to free is the same as for VEGF 121 ) and a high proportion, 20 90% depending on the receptor density, bound to the ECM (Fig. 3B). Of this matrix-bound VEGF, 8% is bound to the basement membrane surrounding the microvessels and 27% to the basement membrane surrounding the muscle fibers. Fig. 3. VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-2. The majority of VEGF of either isoform is bound to cell surface receptors (light gray) or matrix (dark gray, VEGF 165 only). Unbound VEGF decreases as the receptor density increases. The total quantity of extracellular (free, matrix bound, and receptor bound) VEGF for each case is shown in Fig. 4C. Note the 3 scales for VEGF receptor density; they are shown here for comparison but are not repeated in subsequent figures. Coexpression of VEGFR-1 and VEGFR-2 For muscle tissue such as human vastus lateralis that has microvasculature expressing both VEGFR-1 and VEGFR-2, there is again no difference between the VEGF isoforms in the secretion rate from the myocytes required to attain 1 pm steady state interstitial VEGF (Fig. 4A) or the fractional occupancy of either VEGFR (Fig. 4B). The increase of VEGF secretion as the receptor density increases (Fig. 4A) does not alter the fractional occupancy of the receptors (Fig. 4B); this results from the fact that the fractional occupancy is determined by the VEGF concentration available for binding and the receptor binding affinity, and not the secretion rate. The addition of VEGFR-1, a higher-affinity receptor for VEGF, results in competition between these two receptors for VEGF binding, but the fractional occupancy is unchanged (the secretion rate at steady state must be higher to balance the increased binding). The predicted fractional occupancies of both receptors is 2.5%. This is discussed and examined further in Fractional Occupancy of Receptor Tyrosine Kinases. There are differences, however, in the total VEGF content of the tissue and the distribution of VEGF isoforms in the tissue. As the receptor density increases, the percentage of VEGF in the tissue that is surface bound increases significantly (Fig. 4D). This is accompanied by a convergence between the total amounts of VEGF 165 or VEGF 121 in the tissue (Fig. 4C). As more VEGF is bound to the cell surface, the matrix becomes a less important determinant of the VEGF content of the tissue. The free and matrix-bound VEGF content of the tissue for each simulation can be calculated from Fig. 4D: the free VEGF 121 concentration is all of the non-receptor-bound VEGF, whereas free VEGF 165 is 2% of the non-receptor-bound VEGF. This effect of VEGFR-1 expression on the distribution of VEGF in muscle that expresses 10,000 VEGFR-2/endothelial cell is shown in more detail in Fig. 5. The presence of this receptor at densities within the measured range for this muscle decreases the reservoir of VEGF bound to the matrix. Coexpression of VEGFR-2 and Neuropilin-1 The presence of neuropilin on endothelial cells has been demonstrated to increase VEGF 165 binding and activation of VEGFR-2 in vitro (18, 39, 40, 44). In our in vivo simulations, increasing neuropilin density increases the fractional occupancy of VEGFR-2 (Fig. 6B), whereas the binding of VEGF 121 is unaffected. Even a low density of neuropilin (1,000/endothelial cell) results in a significant increase in the binding of VEGF 165 to VEGFR-2. The lower the density of VEGFR-2, the more significant the effect of neuropilin. A higher secretion rate is required to balance this increased VEGFR-2 binding and internalization (Fig. 6A), and the increased binding of VEGF to VEGFR-2 further shifts the VEGF distribution away from interstitial space and toward the microvessel cell surface (Fig. 6, D and E). Coexpression of VEGFR-1, VEGFR-2, and Neuropilin-1 The interaction between VEGFR-1 and neuropilin which form a complex that prevents the binding of VEGF 165 to either receptor results in additional differences in signaling between the isoforms. The presence of neuropilin on a cell that expresses both VEGFR-1 and VEGFR-2 (at a VEGFR-2 density
10 H467 Fig. 4. VEGFR-1-VEGFR-2 coexpression increases tissue VEGF but not fractional occupancy. A: VEGF secretion rate (for 1 pm unbound extracellular VEGF 121 or VEGF 165 concentration). B: fractional occupancy of VEGFR-1 and VEGFR-2. Variation in the density of either receptor does not change the occupancy. C: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. D: % of cell surface-bound VEGF. Notation as in C. Fig. 5. VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-1 and VEGFR-2. VEGFR-2 density is 10,000/EC. High receptor concentrations decrease the proportion of VEGF bound to matrix (dark gray, VEGF 165 only). Unbound VEGF also decreases as the receptor density increases. The total quantity of extracellular (free, matrix bound, and receptor bound) VEGF for each case is shown in Fig. 4C. of 10,000/endothelial cell) does not change VEGF 121 signaling through either VEGFR-1 or VEGFR-2, but does result in an increase in VEGF 165 binding to VEGFR-2 and a decrease in VEGF 165 binding to VEGFR-1 (Fig. 7, A and B). These simultaneous shifts in the receptor-binding profile of VEGF may be synergistic in terms of downstream signaling, as there is recent evidence suggesting that VEGF-VEGFR-1 signaling may modulate or inhibit VEGFR-2 signaling (2, 31, 46). The change in the binding to receptors affects the total VEGF content of the tissue (Fig. 7D) and VEGF distribution (Fig. 7, E and F). It also alters the total internalization of VEGF, and therefore the secretion rate to balance it (Fig. 7C). However, the shape of these curves is not as intuitive as for the previous figures. For low VEGFR-1 density on the endothelial cells of the tissue, a low concentration of neuropilin prevents VEGF 165 binding to VEGFR-1 and increasing neuropilin increases the binding to VEGFR-2. For higher VEGFR-1 density, more of the neuropilin is involved in forming complexes with VEGFR-1 and thus a higher neuropilin density is required to enhance VEGF 165 binding to VEGFR-2. There is a characteristic dip in the curves: this is the point at which neuropilin is effectively inhibiting binding to VEGFR-1 (decreasing secretion rate required and VEGF concentration) but is not present in sufficient quantities to enhance binding to VEGFR-2 (which would increase the required secretion rate and VEGF concentration). The effect of variations in both VEGFR-1 and neuropilin density can be seen more clearly in Fig. 8; VEGFR-2 density is 10,000/endothelial cell for these simulations. The contour graphs show the result of simultaneous variation of both receptors for VEGF 165 secretion; the line graphs represent tissues that express no VEGFR-1 or no neuropilin (Fig. 8, A E, left and bottom, respectively). The results for VEGF 121 are equivalent to the results for VEGF 165 in the absence of neuropilin. The secretion rate is a function primarily of receptor
11 H468 Fig. 6. Neuropilin coexpression increases VEGF 165 bound to VEGFR-2. VEGFR-1 is not expressed. A: VEGF secretion rate (for 1 pm extracellular unbound VEGF 121 or VEGF 165 concentration). B: fractional occupancy of VEGFR-2. C: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. D: % of cell surface-bound VEGF. Notation as in C. E: VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-2 and neuropilin-1. VEGFR-2 density is 10,000/EC. Fig. 7. Neuropilin coexpression decreases VEGF 165 bound to VEGFR-1 (10,000 VEGFR-2/EC). A: fractional occupancy of VEGFR-1. B: fractional occupancy of VEGFR-2. C: VEGF secretion rate (for 1 pm extracellular unbound VEGF 165 or VEGF 121 concentration). D: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. E: % of cell surface-bound VEGF. Notation as in C. F: VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-1, VEGFR-2 and neuropilin-1. VEGFR-1 density is 10,000/EC.
12 H469 Fig. 8. VEGFR-1 and neuropilin-1 balance and tissue VEGF effects (10,000 VEGFR-2/EC). Contour graphs show results for VEGF 165 only. Line graphs accompanying each contour graph represent simulation results for zero VEGFR-1 expression (left) and zero neuropilin expression (bottom). Units for contour graphs are the same as for line graphs. A: fractional occupancy of VEGFR-1. B: fractional occupancy of VEGFR-2. C: VEGF secretion rate (for 1 pm extracellular unbound concentration of VEGF 121 or VEGF 165). D: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. E: % of cell surface-bound VEGF. density (Fig. 8C), as the receptors increase the total amount of VEGF bound and internalized into the endothelial cells, unless VEGFR-1 and neuropilin-1 are present in close to equal quantities; then they bind to each other and are not significantly involved in VEGF 165 binding to VEGFR-2. The competition between VEGFR-1 and VEGFR-2 for VEGF binding is governed by neuropilin (Fig. 8, A and B); in the absence of neuropilin, the occupancies of VEGFR-1 and VEGFR-2 are not determined by receptor density. High neuropilin results in high VEGFR-2 occupancy and low VEGFR-1 occupancy, but an excess of VEGFR-1 over neuropilin will result in the neuropilin binding VEGFR-1 rather than VEGF 165 and decreasing its effects. The distribution of VEGF in the tissue follows the VEGF secretion and correlates with receptor density (Fig. 8, D and E). Fractional Occupancy of Receptor Tyrosine Kinases The fractional occupancy of the receptor tyrosine kinases VEGFR-1 and VEGFR-2 was predicted by these simulations to be quite low, 2% and 1%, respectively, in the absence of neuropilin (Fig. 4B). The impact of receptor density on the fractional occupancy of each of the receptors is given in more detail in Fig. 9. VEGFR-2 is present at 10,000/endothelial cell; VEGFR-1 and neuropilin densities are shown. The solid lines in Fig. 9, A and B, represent iso-vegfr-1 lines; the dotted lines represent iso-neuropilin lines. The fractional occupancies are plotted against the secretion rate that is required to obtain a steady-state free VEGF concentration of 1 pm. While the presence of neuropilin can lead to increased fractional occupancy of VEGFR-2 (Fig. 9B), the fractional occupancy of VEGFR-1 decreases almost to zero (Fig. 9A). This raises some interesting questions about the function of receptor occupancy and sensitivity to increased VEGF concentrations (see DISCUSSION). It should be noted that at high neuropilin concentrations, the solid (iso-vegfr-1) lines converge, as the effect of VEGFR-1 density is decreased. VEGF Distribution and VEGF Secretion Rate VEGF tissue content and VEGF distribution demonstrate a dependence on VEGF secretion rate (Fig. 9C), falling along a single line irrespective of the receptor densities. This implies that the receptor densities do not independently determine the
13 H470 Fig. 9. Fractional occupancy of VEGFR-1 and VEGFR-2 in resting skeletal muscle. Only VEGF 165 is presented; VEGF 121 behaves as for VEGF 165 with no neuropilin (NRP) present. A: fractional occupancy of VEGFR-1. VEGFR-2 is present at 10,000/EC; VEGFR-1 and neuropilin densities are shown. Solid lines represent iso-vegfr-1 lines; dotted lines represent iso-neuropilin lines. Fractional occupancies are plotted against the secretion rate that is required to obtain a steady-state free VEGF 165 concentration of 1 pm. B: fractional occupancy of VEGFR-2. Notation as in A. C: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF (squares, right y-axis) and VEGF cell surface tissue distribution (circles, left y-axis). VEGF distribution in the tissue; rather, they determine the VEGF secretion rate required to maintain the VEGF interstitial concentration (1 pm in this case). It is the secretion rate that determines the VEGF distribution within the tissue. Matrix VEGF Binding Site Density The GAGs of the ECM and basement membranes do not have an impact on the steady-state binding of either VEGF isoform to VEGFR-1 (Fig. 10A) or VEGFR-2 (Fig. 10B). Decreasing the matrix affinity (increasing the numerical value of the dissociation constant) does not affect the VEGF secretion rate (Fig. 10C) but does decrease the total VEGF content (Fig. 10D) by decreasing the matrix-bound fraction of VEGF (Fig. 10, E and F). Decreasing the matrix density would have the same effect (data not shown). This reservoir of VEGF may have significant impact on the dynamics of VEGF binding to its receptors and represents a potential source (through proteolytic cleavage) of additional receptor-binding VEGF. Simultaneous Secretion of VEGF Isoforms Human muscle and other tissues express multiple isoforms of VEGF. In mouse skeletal muscle, VEGF 164 mrna is 10-fold more abundant than VEGF 120 (26) (mouse VEGF isoforms are 1 amino acid shorter than human). Simulations of VEGF 121 and VEGF 165 simultaneous secretion (Fig. 11) demonstrate that a 10-to-1 ratio of VEGF 165 to VEGF 121 results in similar binding profiles (Fig. 11, A and B) and VEGF distribution (Fig. 11, E and F) as VEGF 165 alone. Only at high neuropilin densities (100,000/endothelial cell) are the fractional occupancies of the receptors (Fig. 11, A and B) and the total secretion rate of VEGF (Fig. 11C) significantly affected by the presence of VEGF 121 at a level of 10% or less of total VEGF. That is, at lower neuropilin densities, VEGF 121 would have to make up a higher proportion of the VEGF present to observe changes in receptor occupancy. DISCUSSION The model described here and the simulations performed are the first to provide an insight into the activation of VEGFRs in human tissue. Building on a validated model of the biochemical interactions between the VEGF isoforms and their receptors (18), we introduce ECM sequestration of VEGF 165 and the impact of the geometry of the tissue on the secretion and internalization of VEGF. The model developed is general and can be applied to other tissues; here it is applied to the resting human vastus lateralis. This tissue was chosen because it is the subject of many physiological studies in humans and is the typical model tissue for the effects of exercise on vasculature changes in skeletal muscle (4, 8, 9, 33). Using geometric parameters, VEGF concentrations, and VEGFR densities typical of this tissue, we investigated the steady-state activation of VEGFRs as well as the distribution of VEGF within the tissue and the effects of receptor density on these results. There are also therapeutic applications for this work, since inducing angiogenesis in peripheral arterial disease (PAD) may be a promising therapeutic strategy (15, 32). The simulations predict that in the absence of neuropilin expression on the vasculature of skeletal muscle, VEGF 165 and VEGF 121 behave similarly at steady state: for a given free VEGF concentration, fractional receptor activation is the same, even as the relative densities of VEGFR-1 and VEGFR-2 are varied. Of course, the absolute value of the receptor activation, e.g., number of active receptors per cell, is increased as the receptor density increases, but the fractional occupancy is the same. There is uncertainty over whether fractional occupancy or absolute receptor activation is the more meaningful determinant of VEGF signaling. It is likely that the absolute number of
14 H471 Fig. 10. VEGF sequestration in the ECM does not affect steady-state cell surface binding (10,000 VEGFR-2, 10,000 VEGFR-1 per EC). GAG, glycosaminoglycan chains of interstitial proteoglycans to which VEGF 165 binds. Increasing affinity is represented by lower values (i.e., affinity decreases to the right). A: fractional occupancy of VEGFR-1. B: fractional occupancy of VEGFR-2. C: VEGF secretion rate (for 1 pm extracellular, unbound VEGF 121, or VEGF 165 concentration). D: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. E: % of cell surface-bound VEGF. Notation as in C. F: VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-1, VEGFR-2, and neuropilin-1. 10,000 Neuropilin-1/EC. Fig. 11. Balance of VEGF isoform secretion (10,000 VEGFR-2, 10,000 VEGFR-1 per EC). A: fractional occupancy of VEGFR-1. B: fractional occupancy of VEGFR-2. C: VEGF secretion rate [for 1 pm extracellular, unbound total VEGF (VEGF 121 VEGF 165) concentration]. D: total extracellular (unbound, receptor bound, and matrix bound) concentration of VEGF. E: % of cell surface-bound VEGF. Notation as in C. F: VEGF distribution in skeletal muscle with microvasculature expressing VEGFR-1, VEGFR-2, and neuropilin-1. 10,000 Neuropilin-1/EC.
Targeting Neuropilin-1 to Inhibit VEGF Signaling in Cancer: Comparison of Therapeutic Approaches
 Targeting Neuropilin-1 to Inhibit VEGF Signaling in Cancer: Comparison of Therapeutic Approaches Feilim Mac Gabhann *, Aleksander S. Popel Department of Biomedical Engineering, Johns Hopkins University
Targeting Neuropilin-1 to Inhibit VEGF Signaling in Cancer: Comparison of Therapeutic Approaches Feilim Mac Gabhann *, Aleksander S. Popel Department of Biomedical Engineering, Johns Hopkins University
A Two-Compartment Model of VEGF Distribution in the Mouse
 A Two-Compartment Model of VEGF Distribution in the Mouse Phillip Yen., Stacey D. Finley*., Marianne O. Engel-Stefanini, Aleksander S. Popel Department of Biomedical Engineering, Johns Hopkins University
A Two-Compartment Model of VEGF Distribution in the Mouse Phillip Yen., Stacey D. Finley*., Marianne O. Engel-Stefanini, Aleksander S. Popel Department of Biomedical Engineering, Johns Hopkins University
Differential binding of VEGF isoforms to VEGF receptor 2 in the presence of neuropilin-1: a computational model
 Am J Physiol Heart Circ Physiol 288: H2851 H2860, 2005. First published February 11, 2005; doi:10.1152/ajpheart.01218.2004. Differential binding of VEGF isoforms to VEGF receptor 2 in the presence of neuropilin-1:
Am J Physiol Heart Circ Physiol 288: H2851 H2860, 2005. First published February 11, 2005; doi:10.1152/ajpheart.01218.2004. Differential binding of VEGF isoforms to VEGF receptor 2 in the presence of neuropilin-1:
Simulation of Chemotractant Gradients in Microfluidic Channels to Study Cell Migration Mechanism in silico
 Simulation of Chemotractant Gradients in Microfluidic Channels to Study Cell Migration Mechanism in silico P. Wallin 1*, E. Bernson 1, and J. Gold 1 1 Chalmers University of Technology, Applied Physics,
Simulation of Chemotractant Gradients in Microfluidic Channels to Study Cell Migration Mechanism in silico P. Wallin 1*, E. Bernson 1, and J. Gold 1 1 Chalmers University of Technology, Applied Physics,
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-12-1-0137 TITLE: Computational models of anti-vegf therapies in prostate cancer PRINCIPAL INVESTIGATOR: Mac Gabhann, Dr. Feilim CONTRACTING ORGANIZATION: Johns Hopkins University,
AD Award Number: W81XWH-12-1-0137 TITLE: Computational models of anti-vegf therapies in prostate cancer PRINCIPAL INVESTIGATOR: Mac Gabhann, Dr. Feilim CONTRACTING ORGANIZATION: Johns Hopkins University,
Determination of bioavailability
 Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Pharmaceutics 2 Bioavailability Bioavailability is the rate and extent to which an administered drug reaches the systemic circulation. For example, if 100 mg of a drug is administered orally and 70 mg
Identification of influential proteins in the classical retinoic acid signaling pathway
 Ghaffari and Petzold Theoretical Biology and Medical Modelling (2018) 15:16 https://doi.org/10.1186/s12976-018-0088-7 RESEARCH Open Access Identification of influential proteins in the classical retinoic
Ghaffari and Petzold Theoretical Biology and Medical Modelling (2018) 15:16 https://doi.org/10.1186/s12976-018-0088-7 RESEARCH Open Access Identification of influential proteins in the classical retinoic
BMBF Forsys Partner Project: A Systems Biology Approach towards Predictive Cancer Therapy
 ling and ling and BMBF Forsys Partner Project: A Systems Biology Approach towards Predictive Cancer Therapy H. Perfahl, A. Lapin, M. Reuss Germany holger.perfahl@ibvt.uni-stuttgart.de 1 ling and Cooperation
ling and ling and BMBF Forsys Partner Project: A Systems Biology Approach towards Predictive Cancer Therapy H. Perfahl, A. Lapin, M. Reuss Germany holger.perfahl@ibvt.uni-stuttgart.de 1 ling and Cooperation
Pharmacokinetics of Anti-VEGF Agent Aflibercept in Cancer Predicted by Data-Driven, Molecular-Detailed Model
 Citation: CPT: Pharmacometrics & Systems Pharmacology (2015) 4, 641 649; VC 2015 ASCPT All rights reserved doi:10.1002/psp4.12040 ORIGINAL ARTICLE Pharmacokinetics of Anti-VEGF Agent Aflibercept in Cancer
Citation: CPT: Pharmacometrics & Systems Pharmacology (2015) 4, 641 649; VC 2015 ASCPT All rights reserved doi:10.1002/psp4.12040 ORIGINAL ARTICLE Pharmacokinetics of Anti-VEGF Agent Aflibercept in Cancer
Citation: Chen, Wei (2015) Modelling of Tumour-induced Angiogenesis. Doctoral thesis, Northumbria University.
 Citation: Chen, Wei (2015) Modelling of Tumour-induced Angiogenesis. Doctoral thesis, Northumbria University. This version was downloaded from Northumbria Research Link: http://nrl.northumbria.ac.uk/30235/
Citation: Chen, Wei (2015) Modelling of Tumour-induced Angiogenesis. Doctoral thesis, Northumbria University. This version was downloaded from Northumbria Research Link: http://nrl.northumbria.ac.uk/30235/
Balance point characterization of interstitial fluid volume regulation
 Am J Physiol Regul Integr Comp Physiol 297: R6 R16, 2009. First published May 6, 2009; doi:10.1152/ajpregu.00097.2009. Balance point characterization of interstitial fluid volume regulation R. M. Dongaonkar,
Am J Physiol Regul Integr Comp Physiol 297: R6 R16, 2009. First published May 6, 2009; doi:10.1152/ajpregu.00097.2009. Balance point characterization of interstitial fluid volume regulation R. M. Dongaonkar,
Gas Exchange in the Tissues
 Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease
 Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
Roles of Flow Mechanics in Vascular Cell Biology in Health and Disease Shu Chien Dept. of Bioengineering & Medicine UC, San Diego Presented by Ming-Shaung Ju Dept. of Mech. Eng., NCKU, Tainan Background
NANO 243/CENG 207 Course Use Only
 L9. Drug Permeation Through Biological Barriers May 3, 2018 Lipids Lipid Self-Assemblies 1. Lipid and Lipid Membrane Phospholipid: an amphiphilic molecule with a hydrophilic head and 1~2 hydrophobic tails.
L9. Drug Permeation Through Biological Barriers May 3, 2018 Lipids Lipid Self-Assemblies 1. Lipid and Lipid Membrane Phospholipid: an amphiphilic molecule with a hydrophilic head and 1~2 hydrophobic tails.
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL. PhD THESIS
 UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
Modeling Tumor-Induced Angiogenesis in the Cornea. An Honors Thesis. Presented by Heather Harrington. Group members Marc Maier Lé Santha Naidoo
 Modeling Tumor-Induced Angiogenesis in the Cornea An Honors Thesis Presented by Heather Harrington Group members Marc Maier Lé Santha Naidoo Submitted May 2005 Guidance Committee Approval: Professor Nathaniel
Modeling Tumor-Induced Angiogenesis in the Cornea An Honors Thesis Presented by Heather Harrington Group members Marc Maier Lé Santha Naidoo Submitted May 2005 Guidance Committee Approval: Professor Nathaniel
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
A Cell-Based Model Exhibiting Branching and Anastomosis during Tumor-Induced Angiogenesis
 Biophysical Journal Volume 92 May 2007 3105 3121 3105 A Cell-Based Model Exhibiting Branching and Anastomosis during Tumor-Induced Angiogenesis Amy L. Bauer,* Trachette L. Jackson,* and Yi Jiang y *Department
Biophysical Journal Volume 92 May 2007 3105 3121 3105 A Cell-Based Model Exhibiting Branching and Anastomosis during Tumor-Induced Angiogenesis Amy L. Bauer,* Trachette L. Jackson,* and Yi Jiang y *Department
HORMONES (Biomedical Importance)
 hormones HORMONES (Biomedical Importance) Hormones are the chemical messengers of the body. They are defined as organic substances secreted into blood stream to control the metabolic and biological activities.
hormones HORMONES (Biomedical Importance) Hormones are the chemical messengers of the body. They are defined as organic substances secreted into blood stream to control the metabolic and biological activities.
Transport and Concentration of Charged Molecules in a Lipid Membrane
 Transport and Concentration of Charged Molecules in a Lipid Membrane Johannes S. Roth 1, Matthew R. Cheetham 1,2, and Stephen D. Evans* 1 1 University of Leeds, 2 Current Address: University of Oxford
Transport and Concentration of Charged Molecules in a Lipid Membrane Johannes S. Roth 1, Matthew R. Cheetham 1,2, and Stephen D. Evans* 1 1 University of Leeds, 2 Current Address: University of Oxford
Microcirculation. Lecture Block 11 (contributions from Brett Burton)
 Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
The recruitment of leukocytes and plasma proteins from the blood to sites of infection and tissue injury is called inflammation
 The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte
arxiv: v1 [q-bio.cb] 10 Feb 2016
![arxiv: v1 [q-bio.cb] 10 Feb 2016 arxiv: v1 [q-bio.cb] 10 Feb 2016](/thumbs/80/80689297.jpg) Development of a Computationally Optimized Model of Cancer-induced Angiogenesis through Specialized Cellular Mechanics arxiv:1602.03244v1 [q-bio.cb] 10 Feb 2016 Abstract Dibya Jyoti Ghosh California, United
Development of a Computationally Optimized Model of Cancer-induced Angiogenesis through Specialized Cellular Mechanics arxiv:1602.03244v1 [q-bio.cb] 10 Feb 2016 Abstract Dibya Jyoti Ghosh California, United
Drug Delivery via GLIADEL Wafer for Treatment of Glioblastoma Multiforme (GBM)
 Drug Delivery via GLIADEL Wafer for Treatment of Glioblastoma Multiforme (GBM) William Leif Ericksen, Lyndsey Fortin, Cheryl Hou, Katrina Shum BEE 453 May 1, 2008 Table of Contents I. Executive Summary.
Drug Delivery via GLIADEL Wafer for Treatment of Glioblastoma Multiforme (GBM) William Leif Ericksen, Lyndsey Fortin, Cheryl Hou, Katrina Shum BEE 453 May 1, 2008 Table of Contents I. Executive Summary.
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Skeletal muscle VEGF gradients in peripheral arterial disease: simulations of rest and exercise
 Am J Physiol Heart Circ Physiol 293: H3740 H3749, 2007. First published September 21, 2007; doi:10.1152/ajpheart.00009.2007. Skeletal muscle VEGF gradients in peripheral arterial disease: simulations of
Am J Physiol Heart Circ Physiol 293: H3740 H3749, 2007. First published September 21, 2007; doi:10.1152/ajpheart.00009.2007. Skeletal muscle VEGF gradients in peripheral arterial disease: simulations of
Production of Exosomes in a Hollow Fiber Bioreactor
 Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
Lecture 6: Allosteric regulation of enzymes
 Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis
 The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis Nam Deuk Kim, Ph.D. Pusan National University Contents Part 1. The process of angiogenesis Part 2. The role of angiopoietins
The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis Nam Deuk Kim, Ph.D. Pusan National University Contents Part 1. The process of angiogenesis Part 2. The role of angiopoietins
Dynamic Role of the Cardiac Jelly
 61 Chapter 6 Dynamic Role of the Cardiac Jelly Before looping, when the embryonic heart is still a straight tube, the cardiac jelly occupies the bulk of the heart tube walls. Despite its preeminence in
61 Chapter 6 Dynamic Role of the Cardiac Jelly Before looping, when the embryonic heart is still a straight tube, the cardiac jelly occupies the bulk of the heart tube walls. Despite its preeminence in
Processing of VEGF-C and -D by the Proprotein Convertases: Importance in Angiogenesis, Lymphangiogenesis, and Tumorigenesis
 Processing of VEGF-C and -D by the Proprotein Convertases: Importance in Angiogenesis, Lymphangiogenesis, and Tumorigenesis ii Colloquium Digital Library of Life Sciences This e-book is an original work
Processing of VEGF-C and -D by the Proprotein Convertases: Importance in Angiogenesis, Lymphangiogenesis, and Tumorigenesis ii Colloquium Digital Library of Life Sciences This e-book is an original work
Conditional spectrum-based ground motion selection. Part II: Intensity-based assessments and evaluation of alternative target spectra
 EARTHQUAKE ENGINEERING & STRUCTURAL DYNAMICS Published online 9 May 203 in Wiley Online Library (wileyonlinelibrary.com)..2303 Conditional spectrum-based ground motion selection. Part II: Intensity-based
EARTHQUAKE ENGINEERING & STRUCTURAL DYNAMICS Published online 9 May 203 in Wiley Online Library (wileyonlinelibrary.com)..2303 Conditional spectrum-based ground motion selection. Part II: Intensity-based
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 6. Villous Growth
 Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Microcirculation and Edema. Faisal I. Mohammed MD, PhD.
 Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema- L1 L2
 Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Close to site of release (at synapse); binds to receptors in
 Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
The Angiopoietin Axis in Cancer
 Ang2 Ang1 The Angiopoietin Axis in Cancer Tie2 An Overview: The Angiopoietin Axis Plays an Essential Role in the Regulation of Tumor Angiogenesis Growth of a tumor beyond a limiting size is dependent upon
Ang2 Ang1 The Angiopoietin Axis in Cancer Tie2 An Overview: The Angiopoietin Axis Plays an Essential Role in the Regulation of Tumor Angiogenesis Growth of a tumor beyond a limiting size is dependent upon
Containing the cell: Cell membrane
 48 The organisms called bacteria (singular, bacterium) are made up of prokaryotic cells. Prokaryotic cells are very different from and much simpler than eukaryotic cells in their basic structure and organization.
48 The organisms called bacteria (singular, bacterium) are made up of prokaryotic cells. Prokaryotic cells are very different from and much simpler than eukaryotic cells in their basic structure and organization.
Lippincott Questions Pharmacology
 Lippincott Questions Pharmacology Edition Two: Chapter One: 1.Which one of the following statements is CORRECT? A. Weak bases are absorbed efficiently across the epithelial cells of the stomach. B. Coadministration
Lippincott Questions Pharmacology Edition Two: Chapter One: 1.Which one of the following statements is CORRECT? A. Weak bases are absorbed efficiently across the epithelial cells of the stomach. B. Coadministration
Mathematical Model of Cartilage Regeneration via Hydrogel Honors Thesis, Wittenberg University Department of Mathematics
 Daniel Marous Mathematical Model of Cartilage Regeneration via Hydrogel Honors Thesis, Wittenberg University Department of Mathematics Abstract Because of the large number of individuals with cartilage
Daniel Marous Mathematical Model of Cartilage Regeneration via Hydrogel Honors Thesis, Wittenberg University Department of Mathematics Abstract Because of the large number of individuals with cartilage
Chemistry 106: Drugs in Society Lecture 19: How do Drugs Elicit an Effect? Interactions between Drugs and Macromolecular Targets 11/02/17
 Chemistry 106: Drugs in Society Lecture 19: How do Drugs Elicit an Effect? Interactions between Drugs and Macromolecular Targets 11/02/17 Targets for Therapeutic Intervention: A Comparison of Enzymes to
Chemistry 106: Drugs in Society Lecture 19: How do Drugs Elicit an Effect? Interactions between Drugs and Macromolecular Targets 11/02/17 Targets for Therapeutic Intervention: A Comparison of Enzymes to
Endocrine System. Chapter 7
 Endocrine System Chapter 7 15 Endocrine Endocrine System: System Cont. collection of structures (glands,cells) which secrete hormones directly into the Chapter 7 circulation to affect metabolism, reproduction,
Endocrine System Chapter 7 15 Endocrine Endocrine System: System Cont. collection of structures (glands,cells) which secrete hormones directly into the Chapter 7 circulation to affect metabolism, reproduction,
SUPPLEMENTAL MATERIAL
 1 SUPPLEMENTAL MATERIAL Response time and signal detection time distributions SM Fig. 1. Correct response time (thick solid green curve) and error response time densities (dashed red curve), averaged across
1 SUPPLEMENTAL MATERIAL Response time and signal detection time distributions SM Fig. 1. Correct response time (thick solid green curve) and error response time densities (dashed red curve), averaged across
The MOLECULES of LIFE
 The MOLECULES of LIFE Physical and Chemical Principles Solutions Manual Prepared by James Fraser and Samuel Leachman Chapter 16 Principles of Enzyme Catalysis Problems True/False and Multiple Choice 1.
The MOLECULES of LIFE Physical and Chemical Principles Solutions Manual Prepared by James Fraser and Samuel Leachman Chapter 16 Principles of Enzyme Catalysis Problems True/False and Multiple Choice 1.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice
 Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Angiogenesis in Human Development. Vascular Development
 Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 16, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 16, 2017 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
Part I. Boolean modelling exercises
 Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
Part I. Boolean modelling exercises. Glucose repression of Icl in yeast In yeast Saccharomyces cerevisiae, expression of enzyme Icl (isocitrate lyase-, involved in the gluconeogenesis pathway) is important
Collin County Community College BIOL Muscle Physiology. Muscle Length-Tension Relationship
 Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Drug Receptor Interactions and Pharmacodynamics
 Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
The dynamic regulation of blood vessel caliber
 INVITED BASIC SCIENCE REVIEW The dynamic regulation of blood vessel caliber Colleen M. Brophy, MD, Augusta, Ga BACKGROUND The flow of blood to organs is regulated by changes in the diameter of the blood
INVITED BASIC SCIENCE REVIEW The dynamic regulation of blood vessel caliber Colleen M. Brophy, MD, Augusta, Ga BACKGROUND The flow of blood to organs is regulated by changes in the diameter of the blood
PHARMACODYNAMICS II. The total number of receptors, [R T ] = [R] + [AR] + [BR] (A = agonist, B = antagonist, R = receptors) = T. Antagonist present
![PHARMACODYNAMICS II. The total number of receptors, [R T ] = [R] + [AR] + [BR] (A = agonist, B = antagonist, R = receptors) = T. Antagonist present PHARMACODYNAMICS II. The total number of receptors, [R T ] = [R] + [AR] + [BR] (A = agonist, B = antagonist, R = receptors) = T. Antagonist present](/thumbs/73/69197936.jpg) Pharmacology Semester 1 page 1 of 5 PHARMACODYNAMICS II Antagonists Are structurally similar to the binding site of a receptor and thus show affinity towards the receptor. However, they have zero intrinsic
Pharmacology Semester 1 page 1 of 5 PHARMACODYNAMICS II Antagonists Are structurally similar to the binding site of a receptor and thus show affinity towards the receptor. However, they have zero intrinsic
A METAPOPULATION MODEL OF GRANULOMA FORMATION IN THE LUNG DURING INFECTION WITH MYCOBACTERIUM TUBERCULOSIS. Suman Ganguli.
 MATHEMATICAL BIOSCIENCES http://www.mbejournal.org/ AND ENGINEERING Volume, Number 3, August 5 pp. 535 56 A METAPOPULATION MODEL OF GRANULOMA FORMATION IN THE LUNG DURING INFECTION WITH MYCOBACTERIUM TUBERCULOSIS
MATHEMATICAL BIOSCIENCES http://www.mbejournal.org/ AND ENGINEERING Volume, Number 3, August 5 pp. 535 56 A METAPOPULATION MODEL OF GRANULOMA FORMATION IN THE LUNG DURING INFECTION WITH MYCOBACTERIUM TUBERCULOSIS
Rq : Serum = plasma w/ fibrinogen and other other proteins involved in clotting removed.
 Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Mechanisms Regulating Interstitial Fluid Volume
 165 Lymphology 11 (1978) 165-169 Mechanisms Regulating Interstitial Fluid Volume H.O. Fadnes 1, R.K. Reed 1, K. Aukland Institute of Physiology, University of Bergen, Bergen, Norway Summary The present
165 Lymphology 11 (1978) 165-169 Mechanisms Regulating Interstitial Fluid Volume H.O. Fadnes 1, R.K. Reed 1, K. Aukland Institute of Physiology, University of Bergen, Bergen, Norway Summary The present
UNIT 4: BLOOD VESSELS
 UNIT 4: BLOOD VESSELS Dr. Moattar Raza Rizvi NRS237, Physiology Generalized Structure of Blood Vessels 1 Tunica interna (tunica intima) Endothelial layer that lines the lumen of all vessels In vessels
UNIT 4: BLOOD VESSELS Dr. Moattar Raza Rizvi NRS237, Physiology Generalized Structure of Blood Vessels 1 Tunica interna (tunica intima) Endothelial layer that lines the lumen of all vessels In vessels
Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins
 Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away
Supplementary Information
 Supplementary Information Lymphatic endothelial cells support tumor growth in breast cancer Esak Lee a, Niranjan B. Pandey a, and Aleksander S. Popel a,b a Department of Biomedical Engineering, Johns Hopkins
Supplementary Information Lymphatic endothelial cells support tumor growth in breast cancer Esak Lee a, Niranjan B. Pandey a, and Aleksander S. Popel a,b a Department of Biomedical Engineering, Johns Hopkins
BENG 221 Report - Simulation of Glucose Diffusion in a Cylindrical Cell Juyuong Baek, Jason Dang, Ali Ebrahim, Wei Ren
 BENG 221 Report - Simulation of Glucose Diffusion in a Cylindrical Cell Juyuong Baek, Jason Dang, Ali Ebrahim, Wei Ren Introduction Metabolism is the set of chemical reactions that happen in living organisms
BENG 221 Report - Simulation of Glucose Diffusion in a Cylindrical Cell Juyuong Baek, Jason Dang, Ali Ebrahim, Wei Ren Introduction Metabolism is the set of chemical reactions that happen in living organisms
Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416)
 Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416) Muscle Fiber Geometry Muscle fibers are linked together by collagenous connective tissue. Endomysium surrounds individual fibers, perimysium collects bundles
Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416) Muscle Fiber Geometry Muscle fibers are linked together by collagenous connective tissue. Endomysium surrounds individual fibers, perimysium collects bundles
MATHEMATICAL MODELING OF PERIFUSION CELL CULTURE EXPERIMENTS ON GNRH SIGNALING
 MATHEMATICAL MODELING OF PERIFUSION CELL CULTURE EXPERIMENTS ON GNRH SIGNALING N Ezgi Temamogullari a,, H Frederik Nijhout b, Michael C Reed a arxiv:1606.00463v1 [q-bio.cb] 9 Dec 2015 Abstract a Department
MATHEMATICAL MODELING OF PERIFUSION CELL CULTURE EXPERIMENTS ON GNRH SIGNALING N Ezgi Temamogullari a,, H Frederik Nijhout b, Michael C Reed a arxiv:1606.00463v1 [q-bio.cb] 9 Dec 2015 Abstract a Department
Blood Vessels. Chapter 20
 Blood Vessels Chapter 20 Summary of the Characteristics of Arteries and Veins Characteristic Artery Vein Wall thickness thick thin Shape in cross section round flattened Thickest tunic media externa Collagen
Blood Vessels Chapter 20 Summary of the Characteristics of Arteries and Veins Characteristic Artery Vein Wall thickness thick thin Shape in cross section round flattened Thickest tunic media externa Collagen
Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue.
 GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
Transport through membranes
 Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 9700 BIOLOGY
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 9700 BIOLOGY 9700/34
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2012 question paper for the guidance of teachers 9700 BIOLOGY 9700/34
The Effect of Vascularization and Tissue Type on Cryosurgical Procedures. Reed Magleby Amanda Schallop Eric Shulman Joshua Sterling
 The Effect of Vascularization and Tissue Type on Cryosurgical Procedures Reed Magleby Amanda Schallop Eric Shulman Joshua Sterling BEE 453: Computer-Aided Engineering: Applications to Biomedical Processes
The Effect of Vascularization and Tissue Type on Cryosurgical Procedures Reed Magleby Amanda Schallop Eric Shulman Joshua Sterling BEE 453: Computer-Aided Engineering: Applications to Biomedical Processes
Life History of A Drug
 DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
A Dynamic model of Pulmonary Vein Electrophysiology. Harry Green 2 nd year Ph.D. student University of Exeter
 A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
CAPILLARY FLUID EXCHANGE
 CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
6. Unusual and Influential Data
 Sociology 740 John ox Lecture Notes 6. Unusual and Influential Data Copyright 2014 by John ox Unusual and Influential Data 1 1. Introduction I Linear statistical models make strong assumptions about the
Sociology 740 John ox Lecture Notes 6. Unusual and Influential Data Copyright 2014 by John ox Unusual and Influential Data 1 1. Introduction I Linear statistical models make strong assumptions about the
COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010
 COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010 Office of Clinical Research Training and Medical Education National
COMPARTMENTAL ANALYSIS OF DRUG DISTRIBUTION Juan J.L. Lertora, M.D., Ph.D. Director Clinical Pharmacology Program September 23, 2010 Office of Clinical Research Training and Medical Education National
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Mathematical Model Solid Tumor at the Stage of Angiogenesis with Immune Response
 Mathematical Model Solid Tumor at the Stage of Angiogenesis with Immune esponse Deep Shikha Dixit, Deepak Kumar, Sanjeev Kr,ajesh Johri. Department of Mathematics, CET IILM, Gr. Noida.. Department of Mathematics,
Mathematical Model Solid Tumor at the Stage of Angiogenesis with Immune esponse Deep Shikha Dixit, Deepak Kumar, Sanjeev Kr,ajesh Johri. Department of Mathematics, CET IILM, Gr. Noida.. Department of Mathematics,
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1
 BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
μ i = chemical potential of species i C i = concentration of species I
 BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
Model-based quantification of the relationship between age and anti-migraine therapy
 6 Model-based quantification of the relationship between age and anti-migraine therapy HJ Maas, M Danhof, OE Della Pasqua Submitted to BMC. Clin. Pharmacol. Migraine is a neurological disease that affects
6 Model-based quantification of the relationship between age and anti-migraine therapy HJ Maas, M Danhof, OE Della Pasqua Submitted to BMC. Clin. Pharmacol. Migraine is a neurological disease that affects
BEE 453. May 4, Ravi Sood, David Nahlik, Weston Nichols, and Evan Graham
 Laser Irradiation of Tumors for the Treatment of Cancer: An Analysis of Blood Flow, Temperature and Oxygen Transport BEE 453 May 4, 2007 Ravi Sood, David Nahlik, Weston Nichols, and Evan Graham 2 Executive
Laser Irradiation of Tumors for the Treatment of Cancer: An Analysis of Blood Flow, Temperature and Oxygen Transport BEE 453 May 4, 2007 Ravi Sood, David Nahlik, Weston Nichols, and Evan Graham 2 Executive
Chapter 21. Blood Vessels and Circulation
 Chapter 21 Openstax: Chapter 20 Blood Vessels and Circulation Chapter 21 Learning Outcomes After completing Chapter 21, you will be able to: 1. Distinguish among the types of blood vessels based on their
Chapter 21 Openstax: Chapter 20 Blood Vessels and Circulation Chapter 21 Learning Outcomes After completing Chapter 21, you will be able to: 1. Distinguish among the types of blood vessels based on their
Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue
 Megan McCain ES240 FEM Final Project December 19, 2006 Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue Cardiac myocytes are the cells that constitute the working muscle of the heart.
Megan McCain ES240 FEM Final Project December 19, 2006 Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue Cardiac myocytes are the cells that constitute the working muscle of the heart.
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Modeling of human factor Va inactivation by activated protein C
 Bravo et al. BMC Systems Biology 2012, 6:45 RESEARCH ARTICLE Modeling of human factor Va inactivation by activated protein C Maria Cristina Bravo 1, Thomas Orfeo 2, Kenneth G Mann 2 and Stephen J Everse
Bravo et al. BMC Systems Biology 2012, 6:45 RESEARCH ARTICLE Modeling of human factor Va inactivation by activated protein C Maria Cristina Bravo 1, Thomas Orfeo 2, Kenneth G Mann 2 and Stephen J Everse
EXTRACELLULAR MATRIX (pp 9-17)
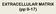 EXTRACELLULAR MATRIX (pp 9-17) Extracellular Matrix (ECM) Apart from specific cells, tissues contain matrix of macromolecules in the extracellular space- Extracellular Matrix. ECM is secreted by cells
EXTRACELLULAR MATRIX (pp 9-17) Extracellular Matrix (ECM) Apart from specific cells, tissues contain matrix of macromolecules in the extracellular space- Extracellular Matrix. ECM is secreted by cells
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
Globular proteins Proteins globular fibrous
 Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM
 Quarterly Journal of Experimental Physiology (1981) 66, 91-98 91 Printed in Great Britain ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM R. B. FISHER University Laboratory of Physiology, Oxford (RECEIVED
Quarterly Journal of Experimental Physiology (1981) 66, 91-98 91 Printed in Great Britain ACTIVE TRANSPORT OF SALICYLATE BY RAT JEJUNUM R. B. FISHER University Laboratory of Physiology, Oxford (RECEIVED
Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM. Real-time automated measurements of cell motility inside your incubator
 Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM Real-time automated measurements of cell motility inside your incubator See the whole story Real-time cell motility visualization and
Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM Real-time automated measurements of cell motility inside your incubator See the whole story Real-time cell motility visualization and
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH- TITLE: PRINCIPAL INVESTIGATOR: CONTRACTING ORGANIZATION: REPORT DATE: TYPE OF REPORT: Annual PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
AD Award Number: W81XWH- TITLE: PRINCIPAL INVESTIGATOR: CONTRACTING ORGANIZATION: REPORT DATE: TYPE OF REPORT: Annual PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
Concentration of drug [A]
![Concentration of drug [A] Concentration of drug [A]](/thumbs/74/70683906.jpg) Pharmacology Semester 1 page 1 of 5 PHARMACODYNAMICS 1 Receptor occupancy - mass action The interaction of a drug with a receptor is reversible due to interactions via weak bonds (not covalent). [A] +
Pharmacology Semester 1 page 1 of 5 PHARMACODYNAMICS 1 Receptor occupancy - mass action The interaction of a drug with a receptor is reversible due to interactions via weak bonds (not covalent). [A] +
EXERCISE Transport Mechanisms in the Body
 EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
DRUG DISTRIBUTION. Distribution Blood Brain Barrier Protein Binding
 DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment
 Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
Comprehensive and Easy Course Notes for BIOL1040 Exams and Assessment MODULE 1: PRINCIPLES OF CELL FUNCTION Membrane Structure & Function Cellular membranes are fluid mosaics of lipids and proteins Phospholipids
