Trust Guideline for the Prevention of Tuberculosis and Management of Tuberculosis Exposure in Health Care Workers
|
|
|
- Jasmine Banks
- 5 years ago
- Views:
Transcription
1 A Clinical Guideline For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the: Date of approval: 25/03/2016 Ratified by or reported as approved to (if applicable): To be reviewed before: This document remains current after this date but will be under review To be reviewed by: Workplace Health & Wellbeing Occupational Health Nursing and Medical Staff All Health Care staff, contracted workers, agency workers and students on placement Corporate Tuberculosis (TB), screening staff for TB, occupational health Dr Mark Ferris, Specialty Doctor, Workplace Health & Wellbeing Hilary Winch, Occupational Health Nurse Manager Dr Phillips, Consultant Respiratory Medicine Dr N Elumogo, Consultant Microbiologist Clinical Guidelines Assessment Panel (CGAP) If approved by committee or Governance Lead Chair s Action; tick here Clinical Standards Group and Effectiveness Sub-Board 25/03/2019 Occupational Health Team Reference and / or Trust Docs ID No: CA4010 id 1265 Version No: 4 Description of changes: Compliance links: (is there any NICE related to guidance) If Yes - does the strategy/policy deviate from the recommendations of NICE? If so why? Updated following publication of new NICE guideline January 2016 NICE guidelines [NG33] No This guideline has been approved by the Trust's Clinical Guidelines Assessment Panel as an aid to the diagnosis and management of relevant patients and clinical circumstances. Not every patient or situation fits neatly into a standard guideline scenario and the guideline must be interpreted and applied in practice in the light of prevailing clinical circumstances, the diagnostic and treatment options available and the professional judgement, knowledge and expertise of relevant clinicians. It is advised that the rationale for any departure from relevant guidance should be documented in the patient's case notes. The Trust's guidelines are made publicly available as part of the collective endeavour to continuously improve the quality of healthcare through sharing medical experience and knowledge. The Trust accepts no responsibility for any misunderstanding or misapplication of this document. Review date: 18/11/2018 Available via Trust Docs Page 1 of 12
2 1. Quick reference guideline/s 1.1 New Starter screening for all Health Care Workers (HCW) who are new to the NHS / transferring to the Norfolk and Norwich University Hospital from another NHS organisation. This screening process will be undertaken by Workplace Health & Wellbeing, (See Quick Reference Guide 1.1) 1.2 Contact trace guidance to follow should a HCW be exposed to a case of pulmonary or laryngeal tuberculosis (TB). This will be initiated and managed by Workplace Health & Wellbeing with the assistance of line managers (See Quick Reference Guide 1.2) Quick Reference Guide 1.1 Review date: 18/11/2018 Available via Trust Docs Page 2 of 12
3 TB Screening New Starter Review date: 18/11/2018 Available via Trust Docs Page 3 of 12
4 Quick Reference Guide 1.2 Testing and treating Health Care workers who have had close contact with an individual diagnosed with pulmonary or laryngeal TB Review date: 18/11/2018 Available via Trust Docs Page 4 of 12
5 2.0 Objective of Guideline 2.1 To prevent and manage the risk of a Health Care Worker (HCW) developing tuberculosis (TB) from patient contact. 2.2 To prevent and manage the exposure of TB in HCWs 2.3 To outline the health surveillance and reporting mechanisms for those staff who are regularly exposed to patients with TB or who work in high risk clinical environments for exposure to TB. 3.0 Rationale 3.1 Around 8000 cases of TB are currently reported each year in the United Kingdom. Most cases occur in major cities, particularly in London (HPA 2014) 3.2 The basis for the prevention and control of tuberculosis amongst health care workers (HCWs) is taken from the NICE (2016) Guidance Tuberculosis, which stresses the importance of new starter screening in HCWs, new entrant screening, administration of Bacillus Calmette-Guerin (BCG) vaccination and infection control measures associated with cases of tuberculosis. 4.0 Broad recommendations 4.1 All staff who have contact with patients or clinical specimens must adhere to the Trust Guidelines on the Prevention and Management of Tuberculosis (TB) in the following circumstances: Prior to commencement of employment with the Trust During employment, with safe working practices. If they have had contact with a patient diagnosed with tuberculosis. 4.2 Locum agencies, training organisations and sub-contractors are responsible for ensuring suitable screening and appropriate immunisation have been carried out and that the staff and students concerned can provide evidence of this. 5.0 Responsibility of the Trust 5.1 To ensure the health & safety of patients is not compromised by their exposure to HCWs who are infected with tuberculosis 5.2 To ensure the health & safety of staff members are not compromised by their exposure to patients who are infected with tuberculosis 6.0 Responsibility of Employee 6.1 To attend Workplace Health & Wellbeing (WHWB) for new starter screening / new entrant screening as requested 6.2 To adopt safe practices for patient care in accordance with the Trust Infection Prevention and Control Policy (C10). Review date: 18/11/2018 Available via Trust Docs Page 5 of 12
6 6.3 To promptly report any symptoms suspicious of tuberculosis to Workplace Health & Wellbeing 6.4 To complete an annual health surveillance questionnaire identifying any symptoms associated with tuberculosis, if working in areas identified with an increased risk of exposure to tuberculosis (see12.2). This will also include information on reporting any symptoms promptly to Workplace Health & Wellbeing. 6.5 To inform Workplace Health & Wellbeing if contact with known cases of tuberculosis. 6.6 To attend appropriate screening if requested by Workplace Health & Wellbeing in the event of being in contact with a tuberculosis case. 7.0 Responsibility of HIV infected health care workers 7.1 All HCWs who have been diagnosed as being infected with HIV must seek and follow advice from Workplace Health & Wellbeing regarding tuberculosis. HCWs must not rely on their own assessment of the risk they pose to patients or staff. 8.0 Responsibility of Managers 8.1 To ensure all staff within their departments have had the appropriate new starter TB screening undertaken prior to the commencement of work in their area 8.2 To ensure that all staff have attended immunisation updates / blood tests as indicated on the New Starter Fitness certificate provided by Workplace Health & Wellbeing. 8.3 To undertake an individual risk assessment if a member of staff is identified to them by Workplace Health & Wellbeing as not having evidence of immunity from or vaccination for tuberculosis. 8.4 To inform Infection Prevention and Control and Workplace Health & Wellbeing if a member of staff or patient is diagnosed with or exposed to tuberculosis. 8.5 To provide information to Workplace Health & Wellbeing and Infection Prevention and Control when contact tracing programmes are required. 8.6 To ensure that staff have received training and fit testing for personal protective equipment (PPE) that may be required when there is potential contact with patients known to have tuberculosis Review date: 18/11/2018 Available via Trust Docs Page 6 of 12
7 9.0 Role of Workplace Health & Wellbeing 9.1 New Starter Screening To undertake the appropriate new starter screening for TB when employees commence employment with the Trust, as per quick reference guide HCWs who are new to the NHS / transferring to the Norfolk and Norwich University Hospital from another NHS organisation who will be working with patients or clinical specimens should not start work in a high risk area (see 12.2) until they have completed a TB health check (or they can provide evidence that the TB health check has been completed in the preceding 12 months). The TB health check is defined as an assessment of personal and family history, symptom questionnaire and either reliable evidence of TB skin testing in the last 5 years (or IGRA) or a BCG scar check by Occupational Health). Staff can start work in other clinical areas if they have completed a symptom check questionnaire and on condition that the TB health check is undertaken after commencement of employment Staff who are new to the NHS / transferring from another NHS organisation who will not have patient contact or contact with clinical specimens should not start work if they have signs or symptoms of TB HCWs and other NHS employees who have contact with patients or clinical specimens who are previously unvaccinated (that is, without adequate documentation or a BCG scar) and have a Mantoux (tuberculin) test result of less than 5mm or negative interferon-gamma release assay (IGRA), who are not immuno-compromised, should be offered the BCG vaccination irrespective of their age. If the BCG vaccination is contra-indicated or refused, the risks should be explained to the individual and supplemented with written advice, the outcome recorded in their occupational health record and their line manager informed that they are not protected against infection with tuberculosis. The importance of reporting possible symptoms of tuberculosis promptly will be re-emphasised. If the person still declines BCG vaccination, he or she should not work where there is a risk of exposure to TB. The employer will need to consider each case individually, taking account of employment and health and safety obligations Routine screening tests for HIV infection before BCG vaccination of HCWs are not appropriate but enquiries should include questions to determine whether the individual is at risk of HIV infection. If they are at significant risk, HIV testing (with counselling) will be offered; this will be undertaken when the tuberculin test / interferon gamma test is undertaken A Mantoux test of 5 mm or larger (regardless of BCG history) should prompt an IGRA, as NICE guidance, to confirm latent TB regardless of previous exposure or symptoms. If the IGRA is positive, a chest x-ray will be arranged. The HCW will subsequently be seen by a doctor at Workplace Health and Wellbeing to consider appropriate management. Review date: 18/11/2018 Available via Trust Docs Page 7 of 12
8 9.1.7 New entrants arriving from a country where the annual incidence of tuberculosis is greater than 40/100,000, or new NHS employees who have had contact with patients in settings where tuberculosis is highly prevalent, should be screened with an IGRA. If the test is positive, then the individual will have a chest x-ray and be seen by an occupational health physician to discuss referral to a TB clinic. If a new employee has lived or worked in a country with a high annual incidence of tuberculosis (or travelled there for a period of 3 months or more within the last 12 months) then they will be considered and treated as a New Entrant. A list of such countries is published by the World Health Organization (WHO). Estimates of tuberculosis incidence by country, 2014 (sorted by country) is found via the following link: If the IGRA is negative and there is not reliable evidence of previous BCG, then the individual should be offered BCG vaccination, in line with the Green Book, after undertaking a HIV risk assessment. 9.2 HIV infected health care workers Provide guidance on restrictions for HIV infected HCWs. Guidance from the Expert Advisory Group on AIDS (EAGA) is that HIV infected HCWs who are well on antiretroviral treatment (viral load undetectable and CD4>500 cells/mµ), who have been screened for tuberculosis by occupational health and have taken chemoprophylaxis if indicated, no longer need to be restricted from working with patients who have tuberculosis. Workplace Health & Wellbeing will liaise with their treating consultant to receive regular updates regarding viral load and CD4 count, in order to review the requirement for restrictions If an HIV infected HCW does not meet this criteria, then their exposure to patients with tuberculosis may need to be restricted. Workplace Health & Wellbeing will inform their line manager of the requirement for the restriction, but not the reason for it To stress to this group of staff the importance of, and the responsibility to, report symptoms which may be suggestive of tuberculosis To ensure that HIV infected HCWs do not receive the BCG vaccination. Workplace Health & Wellbeing will undertake an HIV risk assessment and, if appropriate, offer HIV counselling and testing at the time of undertaking a Mantoux test / IGRA. 9.3 HCWs who have contact with Tuberculosis Contact tracing will be implemented by Workplace Health & Wellbeing if the index (source) case has pulmonary or laryngeal tuberculosis. Workplace Health & Wellbeing will be alerted about the commencement of a HCW contact trace by the following staff: For exposure to inpatients with suspected tuberculosis - the Infection Prevention and Control Team will inform Workplace Health & Wellbeing once confirmed diagnosis. Review date: 18/11/2018 Available via Trust Docs Page 8 of 12
9 For exposure to outpatients with suspected tuberculosis - the TB Liaison Nurse Specialist will inform Workplace Health & Wellbeing of the confirmed diagnosis. Mortuary staff wear appropriate respiratory protection for known tuberculosis post mortems. If they are exposed to an unexpected tuberculosis case, the Senior Mortuary Technician (in charge) will advise Workplace Health & Wellbeing both at the time of the exposure and when verification of the pathology has been received The HCW contact will be categorised as either a 'casual contact' or 'close contact'. Casual contact includes staff members attending tuberculosis patients in a routine manner. Close contact is defined as staff who have undertaken any of the following procedures: mouth to mouth resuscitation without appropriate protection, prolonged care of a patient who requires a high level of dependant nursing care, repeated chest physiotherapy, involved in a tuberculosis infected case at post mortem or bronchoalveolar lavage without use of correct protective procedures. Prolonged care will not generally be considered to have occurred until a cumulative total exceeding 8 hours of high dependency nursing has occurred. If the source patient has had two weeks or more of anti-tuberculosis treatment the risk is significantly reduced Close contacts of tuberculosis cases will be screened as advised in the NICE (2016) guidance (see Quick reference guide 1.2). Contact tracing should be undertaken for the period that the case had respiratory symptoms, including cough. If this is unknown, contacts should be traced from 3 months preceding the first positive sputum smear. Tracing is extended backwards, a month at a time, if clinical indications in contacts suggest that transmission has occurred Possible close contacts will be identified by the ward / department and Workplace Health & Wellbeing will make contact with them to determine whether contact screening is required HCWs with HIV who are close contacts require active disease excluded and to then be given treatment for latent TB infection. They will be seen in Workplace Health and Wellbeing to assess whether they have symptoms of tuberculosis, arrange an IGRA and CXR and onward referral to a respiratory physician (in consultation with their treating HIV physician) HCWs in casual contact with a case of tuberculosis will (unless immunocompromised) only need to be reassured and reminded of the possible symptoms (over the next twelve month period) Those staff who work in areas of increased risk of tuberculosis (see 12.2) will receive annual health surveillance questionnaires to complete and return to Workplace Health & Wellbeing. Review date: 18/11/2018 Available via Trust Docs Page 9 of 12
10 9.4 HCWs who have a confirmed diagnosis of Tuberculosis If a HCW is diagnosed with tuberculosis, whether occupationally acquired or not, liaison will occur between Workplace Health & Wellbeing, the treating Respiratory Physician, the TB Community Nurse and the Infection Prevention and Control Team. Tuberculosis contact tracing procedures will be initiated by the Infection Prevention and Control Team/TB Community Nurse for patient contacts and by Workplace Health & Wellbeing for staff contacts. If identified as a work acquired disease, then a RIDDOR report will be submitted anonymously to the Health & Safety Executive by Workplace Health & Wellbeing. The Consultant in Communicable Disease Control will be informed by the respiratory team and will initiate any wider screening (family members etc.) that may be required Role of Infection Prevention and Control 10.1 To inform Workplace Health & Wellbeing of any suspected or confirmed cases of pulmonary or laryngeal tuberculosis To initiate contact tracing of any patient contacts from an inpatient case Education and training 11.1 Staff education will occur both during the pre-employment screening process and on the Trust Risk Management Induction and Mandatory training sessions Individual training / updates will be given on an opportunistic basis, when undertaking immunisation updates. Staff will be reminded that BCG does not offer complete protection and so the requirement to be aware of and to report relevant symptoms remains after immunisation Clinical Audit Standards derived from guideline (audit will be undertaken as part of Workplace Health & Wellbeing s rolling audit programme) 12.1 Workplace Health & Wellbeing Audit statements: All HCWs working in the higher risk areas for tuberculosis will have had their immunity to tuberculosis assessed prior to commencement of work in the Trust. All HCWs who do not have a BCG scar or reliable history of having had BCG vaccination will have a Mantoux test and a BCG vaccination administered, if nonimmune and not contraindicated. All New Entrants will have an IGRA prior to commencing work within the Trust. An annual surveillance questionnaire will be completed by staff working in higher risk areas. Review date: 18/11/2018 Available via Trust Docs Page 10 of 12
11 12.2 Staff at higher risk of exposure to tuberculosis are those who have: Regular contact with TB patients or clinical specimens. Worked in a high risk clinical setting for 4 weeks or longer. Higher risk areas for TB are as follows: Mortuary Histopathology Respiratory Medicine Thoracic Surgery GUM Clinic EAU (Medical) Oncology/Haematology Renal Dialysis Unit 13.0 Summary of development and consultation process undertaken before registration and dissemination This guideline has been developed by Workplace Health & Wellbeing in consultation with Respiratory Medicine, Microbiology and the TB Group within the Norfolk and Norwich University Hospital NHS Trust References and related documents: 1. Norfolk & Norwich University Hospital NHS Trust, Trust Guideline for the management of patients with confirmed or suspected tuberculosis C10 Trust Doc ID 627 v3, NNUH Trust Intranet. 2. Norfolk & Norwich University Hospital NHS Trust, Personal Protective Equipment Procedure. Document 040 V1.5, NNUH Trust Intranet. 3. National Institute for Health and Clinical Excellence, Tuberculosis (NG33). January Available at (accessed 16/02/16) 4. Department of Health 2007, Health Clearance for tuberculosis, hepatitis B, hepatitis C and HIV: New healthcare workers. Available at ublicationsandstatistics/publications/publicationspolicyandguidance/dh_ Accessed 26/09/15) 5. Public Health England Tuberculosis: the green book, chapter 32https:// Joint Tuberculosis Committee of the British Thoracic Society. Control and prevention of tuberculosis in the United Kingdom: Code of Practice, Thorax (2000): Review date: 18/11/2018 Available via Trust Docs Page 11 of 12
12 7. Meredith S, Watson J, Citron K, Cockcroft A, Darbyshire J. Are healthcare workers in England and Wales at increased risk of tuberculosis? BMJ 1996; 313: Public Health England. (2014) Tuberculosis in the UK: 2014 report. Public Health England: London. Available at (accessed 13/09/15) 9. Public Health England. (2013)Tuberculosis (TB) by country: rates per 100,000 people. Available at (accessed 13/09/15) 10. NHS Plus. Policy for Occupational Health Management of Tuberculosis Review date: 18/11/2018 Available via Trust Docs Page 12 of 12
Trust Guideline for the inclusion of women at High Risk of Breast Cancer in the NHS Breast Screening Programme
 Trust Guideline for the inclusion of women at High Risk of Breast Cancer in the NHS Breast Screening Programme For Use in: By: For: Division responsible for document: Key words: Name and job title of document
Trust Guideline for the inclusion of women at High Risk of Breast Cancer in the NHS Breast Screening Programme For Use in: By: For: Division responsible for document: Key words: Name and job title of document
9. Screening in Special Situations
 9. Screening in Special Situations Screening is the practice of identifying a condition or illness, which could benefit from early diagnosis, preventative or curative intervention. 318 Screening should
9. Screening in Special Situations Screening is the practice of identifying a condition or illness, which could benefit from early diagnosis, preventative or curative intervention. 318 Screening should
INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust)
 A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC20 VACCINATION PROGRAMME FOR STAFF AND
A member of: Association of UK University Hospitals INFECTION PREVENTION AND CONTROL POLICY AND PROCEDURES Sussex Partnership NHS Foundation Trust (The Trust) IPC20 VACCINATION PROGRAMME FOR STAFF AND
Audit of management of TB in HIV co-infected patients: survey of clinic arrangements
 Audit of management of TB in HIV co-infected patients: survey of clinic arrangements Please complete this questionnaire if your clinic/department provides TB treatment and care for adult patients, but
Audit of management of TB in HIV co-infected patients: survey of clinic arrangements Please complete this questionnaire if your clinic/department provides TB treatment and care for adult patients, but
Trust Protocol for the Administration of Intravenous Methylprednisolone for Thyroid Eye Disease A Protocol. The Clinical Investigation Unit (CIU)
 A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
Guideline for the Use of Granulocyte Colony Stimulating Factor (G-CSF) for Adults in Oncology and Haematology
 (G-CSF) for Adults in Oncology and Haematology For Use in: By: Oncology and Haematology Inpatients and Outpatients Oncologists and Haematologists For: Division responsible for document: Key words: Name
(G-CSF) for Adults in Oncology and Haematology For Use in: By: Oncology and Haematology Inpatients and Outpatients Oncologists and Haematologists For: Division responsible for document: Key words: Name
STAFF SCREENING AND IMMUNISATION POLICY
 2GETHER NHS FOUNDATION TRUST GLOUCESTERSHIRE HOSPITALS NHS FOUNDATION TRUST GLOUCESTERSHIRE CARE SERVICES STAFF SCREENING AND IMMUNISATION POLICY Version: 1 Consultation: Ratified by: Date ratified: Name
2GETHER NHS FOUNDATION TRUST GLOUCESTERSHIRE HOSPITALS NHS FOUNDATION TRUST GLOUCESTERSHIRE CARE SERVICES STAFF SCREENING AND IMMUNISATION POLICY Version: 1 Consultation: Ratified by: Date ratified: Name
Division 2, Surgical and Anaesthetics Directorates All surgical and anaesthetics staff Patients with an implanted spinal cord stimulator For:
 Joint Clinical Guideline for Patients with an implanted spinal cord stimulator (SCS) For Use in: Division 2, Surgical and Anaesthetics Directorates By: All surgical and anaesthetics staff Patients with
Joint Clinical Guideline for Patients with an implanted spinal cord stimulator (SCS) For Use in: Division 2, Surgical and Anaesthetics Directorates By: All surgical and anaesthetics staff Patients with
Staff Immunisation Policy
 Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
Tuberculosis Procedure ICPr016. Table of Contents
 Tuberculosis Procedure ICPr016 Table of Contents Tuberculosis Procedure ICPr016... 1 What is Tuberculosis?... 2 Any required definitions/explanations... 2 NHFT... 2 Tuberculosis (TB)... 3 Latent TB...
Tuberculosis Procedure ICPr016 Table of Contents Tuberculosis Procedure ICPr016... 1 What is Tuberculosis?... 2 Any required definitions/explanations... 2 NHFT... 2 Tuberculosis (TB)... 3 Latent TB...
POLICY FOR THE PREVENTION AND CONTROL OF TUBERCULOSIS
 POLICY FOR THE PREVENTION AND CONTROL OF TUBERCULOSIS Policy No: 7.20 Approval Date: Review Date: Lead Director: Under Review Under Review Under Review Page 1 of 7 Polic y_for_the_prevention_and_control_of_tuberculosis
POLICY FOR THE PREVENTION AND CONTROL OF TUBERCULOSIS Policy No: 7.20 Approval Date: Review Date: Lead Director: Under Review Under Review Under Review Page 1 of 7 Polic y_for_the_prevention_and_control_of_tuberculosis
Trust Guideline for the Use of Parenteral Vancomycin and Teicoplanin in Adults
 A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy
 PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy 1 Policy title Protection from Occupational Acquired /Transmitted Communicable Diseases Policy COR 60 reference Policy category
PROTECTION FROM OCCUPATIONAL ACQUIRED/TRANSMITTED COMMUNICABLE DISEASES Policy 1 Policy title Protection from Occupational Acquired /Transmitted Communicable Diseases Policy COR 60 reference Policy category
Immunisation Requirements for Health Care Workers - Quick Reference Guide
 TARGET AUDIENCE All health care workers (HCW) at Alfred Health and Women s @ Sandringham. This includes employees, agency, contractors, Visiting Medical Officers (VMOs), locums, students, volunteers and
TARGET AUDIENCE All health care workers (HCW) at Alfred Health and Women s @ Sandringham. This includes employees, agency, contractors, Visiting Medical Officers (VMOs), locums, students, volunteers and
A Clinical Guideline for the use of Intravenous Aminophylline in Acute Severe Asthma in Children
 For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
Trust Guideline for the Management of Patient Controlled Analgesia (PCA) in Adults
 Patient Controlled Analgesia (PCA) in Adults A clinical guideline recommended for use For Use in: In all Clinical Areas By: Anaesthetists, Ward Nurses, Recovery Staff Acute Pain Service Staff For: Adult
Patient Controlled Analgesia (PCA) in Adults A clinical guideline recommended for use For Use in: In all Clinical Areas By: Anaesthetists, Ward Nurses, Recovery Staff Acute Pain Service Staff For: Adult
Infections What is new and what is important?
 Infections What is new and what is important? 2 What am I going to talk about? Imported infections Meningitis changes Flu vaccine issues TB NICE guidance changes 3 Imported infections - Zika Transmitted
Infections What is new and what is important? 2 What am I going to talk about? Imported infections Meningitis changes Flu vaccine issues TB NICE guidance changes 3 Imported infections - Zika Transmitted
Interferon gamma release assays and the NICE 2011 guidelines on the diagnosis of latent tuberculosis
 CLINICAL AUDIT Clinical Medicine 2013, Vol 13, No 4: 362 6 Interferon gamma release assays and the NICE 2011 guidelines on the diagnosis of latent tuberculosis Helen R Mujakperuo, Richard D Thompson and
CLINICAL AUDIT Clinical Medicine 2013, Vol 13, No 4: 362 6 Interferon gamma release assays and the NICE 2011 guidelines on the diagnosis of latent tuberculosis Helen R Mujakperuo, Richard D Thompson and
Interventions to improve screening for latent TB: effectiveness and outcomes
 Interventions to improve screening for latent TB: effectiveness and outcomes Fotinie Ntziora, Thushan DeSilva, Natasha Baker, Debbie Talbot, Louise Byrne, Karen Sherry, Sandra Booth, Janice Hobson, Karen
Interventions to improve screening for latent TB: effectiveness and outcomes Fotinie Ntziora, Thushan DeSilva, Natasha Baker, Debbie Talbot, Louise Byrne, Karen Sherry, Sandra Booth, Janice Hobson, Karen
Trust Guideline for the Management of: Abnormal Pre-operative Thyroid Function Tests in Adults. Anaesthetists Abnormal Pre-op Thyroid Function Test
 For Use in: By: For: Pre-operative Thyroid Function Tests in Adults Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager:
For Use in: By: For: Pre-operative Thyroid Function Tests in Adults Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager:
Immunisation Requirements and Mandatory Health Screenings
 Immunisation Requirements and Mandatory Health Screenings The purpose of pre-employment screening is to ensure that you are fit for the position you have applied for and that you don t have any condition
Immunisation Requirements and Mandatory Health Screenings The purpose of pre-employment screening is to ensure that you are fit for the position you have applied for and that you don t have any condition
Louise Teare, Consultant Microbiologist
 Staff Immunisation and Health Screening Policy Policy Register No: 08037 Status: Public Developed in response to: Best Practice Contributes to CQC Outcomes: 12 Consulted With Individual/Body Date Director
Staff Immunisation and Health Screening Policy Policy Register No: 08037 Status: Public Developed in response to: Best Practice Contributes to CQC Outcomes: 12 Consulted With Individual/Body Date Director
Call: Visit:
 Candidate details are logged on Arithon. Ensure all personal information is completed in the tabs. All candidate documents are to be original sight stamp verified and uploaded per document. All conversations
Candidate details are logged on Arithon. Ensure all personal information is completed in the tabs. All candidate documents are to be original sight stamp verified and uploaded per document. All conversations
Glucocorticoid replacement, Steroids, Acute Illness Dr Rupa Ahluwalia, Consultant Physician (NNUH)
 For Use in: By: For: Division responsible for document: Key words: Name and job title of document author s: Name and job tile of document author s Line Manager: Supported by: Assessed and approved by the:
For Use in: By: For: Division responsible for document: Key words: Name and job title of document author s: Name and job tile of document author s Line Manager: Supported by: Assessed and approved by the:
Management of Healthcare Workers Infected With A Blood-borne Virus (HIV, Hepatitis B, Hepatitis C)
 Management of Healthcare Workers Infected With A Blood-borne Virus (HIV, Hepatitis B, Hepatitis C) Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet.
Management of Healthcare Workers Infected With A Blood-borne Virus (HIV, Hepatitis B, Hepatitis C) Solent NHS Trust policies can only be considered to be valid and up-to-date if viewed on the intranet.
Clinical diagnosis and management of tuberculosis, and measures for its prevention and control
 Quick reference guide Issue date: March 2006 Clinical diagnosis and management of tuberculosis, and measures for its prevention and control Clinical Guideline 33 Developed by the National Collaborating
Quick reference guide Issue date: March 2006 Clinical diagnosis and management of tuberculosis, and measures for its prevention and control Clinical Guideline 33 Developed by the National Collaborating
By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author s Line Manager:
 For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
(a) Infection control program. The facility must establish an infection control program under which it--
 420-5-10-.17 Infection Control. (1) The facility must establish and maintain an infection control program designed to provide a safe, sanitary, and comfortable environment and to help prevent the development
420-5-10-.17 Infection Control. (1) The facility must establish and maintain an infection control program designed to provide a safe, sanitary, and comfortable environment and to help prevent the development
New NICE guideline updates recommendations for diagnosing latent tuberculosis
 Tel: 0845 003 7782 www.nice.org.uk Ref: 2011/053 PRESS RELEASE New NICE guideline updates recommendations for diagnosing latent tuberculosis The National Institute for Health and Clinical Excellence (NICE)
Tel: 0845 003 7782 www.nice.org.uk Ref: 2011/053 PRESS RELEASE New NICE guideline updates recommendations for diagnosing latent tuberculosis The National Institute for Health and Clinical Excellence (NICE)
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 TB Partial Update Appendix 1 - Scope NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Tuberculosis: interferon gamma tests for the diagnosis of latent tuberculosis (partial
TB Partial Update Appendix 1 - Scope NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Tuberculosis: interferon gamma tests for the diagnosis of latent tuberculosis (partial
COFM Immunization Policy 2016
 COFM Immunization Policy 2016 Council of Ontario Faculties of Medicine June 2016 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy 2016
COFM Immunization Policy 2016 Council of Ontario Faculties of Medicine June 2016 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy 2016
NICE guideline Published: 13 January 2016 nice.org.uk/guidance/ng33
 Tuberculosis NICE guideline Published: 13 January 2016 nice.org.uk/guidance/ng33 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tuberculosis NICE guideline Published: 13 January 2016 nice.org.uk/guidance/ng33 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Tuberculosis: interferon gamma tests for the diagnosis of latent tuberculosis (partial update) 1.1 Short title Tuberculosis
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Tuberculosis: interferon gamma tests for the diagnosis of latent tuberculosis (partial update) 1.1 Short title Tuberculosis
Management of AIDS/HIV Infected Healthcare Workers Policy
 Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
Peggy Leslie-Smith, RN
 Peggy Leslie-Smith, RN EMPLOYEE HEALTH DIRECTOR - AVERA TRAINING CONTENT 1. South Dakota Regulations 2. Iowa Regulations 3. Minnesota Regulations 4. Interferon Gamma Release Assay (IGRA)Testing 1 SOUTH
Peggy Leslie-Smith, RN EMPLOYEE HEALTH DIRECTOR - AVERA TRAINING CONTENT 1. South Dakota Regulations 2. Iowa Regulations 3. Minnesota Regulations 4. Interferon Gamma Release Assay (IGRA)Testing 1 SOUTH
Respiratory Viruses Policy
 Respiratory Viruses Policy Page 1 of 8 Document Control Sheet Name of document: Version: 3 Status: Owner: File location / Filename: Respiratory viruses policy Date of this version: February 2013 Infection
Respiratory Viruses Policy Page 1 of 8 Document Control Sheet Name of document: Version: 3 Status: Owner: File location / Filename: Respiratory viruses policy Date of this version: February 2013 Infection
This SOP applies to all staff employed by NHS Greater Glasgow & Clyde and locum staff on fixed term contracts and volunteer staff.
 Page 1 of 8 SOP Objective To ensure that Healthcare Workers (HCWs) are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients clinical
Page 1 of 8 SOP Objective To ensure that Healthcare Workers (HCWs) are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients clinical
Joint Trust Clinical Guideline for Monitoring of patients with Guillain Barré Syndrome (GBS)
 A Clinical Guideline For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author
A Clinical Guideline For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author
ATTACHMENT 2. New Jersey Department of Health Tuberculosis Program FREQUENTLY ASKED QUESTIONS
 1. QUESTION Is it required to submit the Annual Report of TB Testing in Schools Form (TB-57) to the New Jersey Department of Health,? NO. The TB-57 form is completed by the school nurse and kept on-site
1. QUESTION Is it required to submit the Annual Report of TB Testing in Schools Form (TB-57) to the New Jersey Department of Health,? NO. The TB-57 form is completed by the school nurse and kept on-site
Policy Objective. This policy applies to all staff employed by NHS Greater Glasgow & Clyde and locum staff on fixed term contracts.
 1 of 9 Policy Objective To ensure that Healthcare Workers are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients clinical conditions
1 of 9 Policy Objective To ensure that Healthcare Workers are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients clinical conditions
Trust Guideline for the management of Parapneumonic Effusion in children
 A clinical guideline recommended for use For Use in: By: For: Division responsible for document: Buxton ward, Children s Assessment Unit (CAU) All staff Children with parapneumonic effusion Women and Children
A clinical guideline recommended for use For Use in: By: For: Division responsible for document: Buxton ward, Children s Assessment Unit (CAU) All staff Children with parapneumonic effusion Women and Children
Guideline for the Management of Continuous IV Vancomycin Infusion in Neonates on NICU A Clinical Guideline recommended for use
 Guideline for the Management of Continuous IV Vancomycin Infusion in A Clinical Guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Name and job title of document
Guideline for the Management of Continuous IV Vancomycin Infusion in A Clinical Guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Name and job title of document
POLICY TITLE: HEALTH CARE PERSONNEL IMMUNIZATION Former Policy Title: DOCUMENT NAME: Health Care Personnel Immunization Policy-LG Health
 Former Policy Title: Policy Author: Norma J. Ferdinand Effective Date: 12/1/12 Policy Owner: Bobbi Jo Hurst Last Review Date: 12/1/12 POLICY PURPOSE: The purpose of this Policy is to protect the health
Former Policy Title: Policy Author: Norma J. Ferdinand Effective Date: 12/1/12 Policy Owner: Bobbi Jo Hurst Last Review Date: 12/1/12 POLICY PURPOSE: The purpose of this Policy is to protect the health
Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines
 Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines Historically, Latent Tuberculosis Infection (LTBI) diagnosis was based on risk assessment, chest x-ray (CXR)
Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines Historically, Latent Tuberculosis Infection (LTBI) diagnosis was based on risk assessment, chest x-ray (CXR)
NHS SHETLAND TUBERCULOSIS ANNUAL REPORT
 NHS SHETLAND TUBERCULOSIS ANNUAL REPORT 2014-2015 Prepared by Wendy Hatrick October 2015 NHS Shetland Tuberculosis Annual Report 2013-14 Acronyms and Abbreviations AIDS Acquired Immuno-Deficiency Syndrome
NHS SHETLAND TUBERCULOSIS ANNUAL REPORT 2014-2015 Prepared by Wendy Hatrick October 2015 NHS Shetland Tuberculosis Annual Report 2013-14 Acronyms and Abbreviations AIDS Acquired Immuno-Deficiency Syndrome
Latent Tuberculosis Infection (LTBI) Questions and Answers for Health Care Providers
 Latent Tuberculosis Infection (LTBI) Questions and Answers for Health Care Providers Who Should Be Screened for Latent Tuberculosis Infection (LTBI)?... 2 What tests are used to screen for LTBI?... 2 How
Latent Tuberculosis Infection (LTBI) Questions and Answers for Health Care Providers Who Should Be Screened for Latent Tuberculosis Infection (LTBI)?... 2 What tests are used to screen for LTBI?... 2 How
(13) "Pulmonary tuberculosis" means tuberculosis that affects the lungs.
 ACTION: Withdraw Final DATE: 12/06/2005 8:17 AM 5122-3-09 Integrated behavioral healthcare system (IBHS) tuberculosis control program. (A) The purpose of this rule shall be to establish a policy that will
ACTION: Withdraw Final DATE: 12/06/2005 8:17 AM 5122-3-09 Integrated behavioral healthcare system (IBHS) tuberculosis control program. (A) The purpose of this rule shall be to establish a policy that will
Joint Trust Guideline for the Management of: CFS/ME (Chronic Fatigue Syndrome / Myalgic Encephalopathy) in Children and Young People
 A Clinical Guideline For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author
A Clinical Guideline For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author
TB Clinical Guidelines: Revision Highlights March 2014
 TB Clinical Guidelines: Revision Highlights March 2014 AIR TRAVEL & TB CONTROL With respect to non-ambulance air travel of patients diagnosed with or suspected as having active Mycobacterium tuberculosis,
TB Clinical Guidelines: Revision Highlights March 2014 AIR TRAVEL & TB CONTROL With respect to non-ambulance air travel of patients diagnosed with or suspected as having active Mycobacterium tuberculosis,
Administration of Tuberculin Purified Protein Derivative (PPD) 2TU per 0.1ml to patients attending BCG clinics with identified TB at risk factors.
 Administration of Tuberculin Purified Protein Derivative (PPD) 2TU per 0.1ml to patients attending BCG clinics with identified TB at risk factors. Special notes PPD is an unlicensed medicine and therefore
Administration of Tuberculin Purified Protein Derivative (PPD) 2TU per 0.1ml to patients attending BCG clinics with identified TB at risk factors. Special notes PPD is an unlicensed medicine and therefore
Safe Use of Latex Policy
 Safe Use of Latex Policy Lead Manager: Andy Crawford- Head of Clinical Governance Responsible Director: Jointly by: Director of Human Resources Medical Director Approved by: Jointly by: Health & Safety
Safe Use of Latex Policy Lead Manager: Andy Crawford- Head of Clinical Governance Responsible Director: Jointly by: Director of Human Resources Medical Director Approved by: Jointly by: Health & Safety
Examples COMPLETED. Immunization Forms
 Important Notes: Examples of COMPLETED Immunization Forms - The form MUST be completed, signed and dated by the physician. - The form MUST also be signed and dated by the student. - Chest X-rays should
Important Notes: Examples of COMPLETED Immunization Forms - The form MUST be completed, signed and dated by the physician. - The form MUST also be signed and dated by the student. - Chest X-rays should
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS. All clinical areas of the Trust. Prevent spread of Hepatitis B virus
 Trust Policy and Procedure PP(15)027 Document Ref No: HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS For use in: For use by: For use for: Document owner: Status: All clinical
Trust Policy and Procedure PP(15)027 Document Ref No: HEPATITIS B GUIDANCE FOR THE PROTECTION OF HEALTH CARE WORKERS AND PATIENTS For use in: For use by: For use for: Document owner: Status: All clinical
Preventing Tuberculosis (TB) Transmission in Ambulatory Surgery Centers. Heidi Behm, RN, MPH TB Controller HIV/STD/TB Program
 Preventing Tuberculosis (TB) Transmission in Ambulatory Surgery Centers Heidi Behm, RN, MPH TB Controller HIV/STD/TB Program Topics of Discussion TB Overview Epidemiology of TB in Oregon Annual Facility
Preventing Tuberculosis (TB) Transmission in Ambulatory Surgery Centers Heidi Behm, RN, MPH TB Controller HIV/STD/TB Program Topics of Discussion TB Overview Epidemiology of TB in Oregon Annual Facility
Trust Guideline for the Management and Administration of Intravenous Iron in Adults under the Gastroenterology Directorate
 A clinical guideline recommended for use For Use in: The Gastroenterology Directorate By: Registered nurses competent in the administration of intravenous therapy and medical staff For: Adult patients
A clinical guideline recommended for use For Use in: The Gastroenterology Directorate By: Registered nurses competent in the administration of intravenous therapy and medical staff For: Adult patients
Immunization Policy. "UIC/COD-sponsored graduate education program" is one for which UIC/COD maintains academic responsibility.
 I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
I. PURPOSE Immunization Policy TITLE: CLINICAL HEALTHCARE PROVIDERS - IMMUNIZATIONS AND HEALTH REQUIREMENTS To prevent or reduce the risk of transmission of vaccine-preventable and other communicable diseases
Primary Care and TB Control Dr Helen Booth Consultant Thoracic Physician, UCLH Clinical Lead, Integrated TB NCL-Service
 Primary Care and TB Control Dr Helen Booth Consultant Thoracic Physician, UCLH Clinical Lead, Integrated TB NCL-Service North Central London TB Service TBService@nhs.net After Action Review Could we have
Primary Care and TB Control Dr Helen Booth Consultant Thoracic Physician, UCLH Clinical Lead, Integrated TB NCL-Service North Central London TB Service TBService@nhs.net After Action Review Could we have
A clinical guideline recommended for use
 A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Reported as approved to the: The Nephrology Directorate, NNUH. All Medical & Nursing Staff who have
A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Reported as approved to the: The Nephrology Directorate, NNUH. All Medical & Nursing Staff who have
COFM Immunization Policy
 COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy This policy applies to all undergraduate medical students attending an Ontario medical
COUNCIL OF ONTARIO FACULTIES OF MEDICINE An affiliate of the Council of Ontario Universities COFM Immunization Policy This policy applies to all undergraduate medical students attending an Ontario medical
Trust Guideline for the Management of: Condition or Procedure in Adults and / or Children
 Trust Guideline for the Management of: Condition or Procedure in Adults and / or Children A clinical guideline recommended For use in: By: For: Division responsible for document: Key words: Name of document
Trust Guideline for the Management of: Condition or Procedure in Adults and / or Children A clinical guideline recommended For use in: By: For: Division responsible for document: Key words: Name of document
HEALTHWEST PROCEDURE. No Revised by: Effective: December 1, 1995 Revised: April 19, 2017 Environment of Care Committee
 HEALTHWEST PROCEDURE Revised by: Effective: December 1, 1995 Revised: April 19, 2017 Environment of Care Committee Approved by: Subject: Tuberculosis Infection Control Julia Rupp, Executive Director I.
HEALTHWEST PROCEDURE Revised by: Effective: December 1, 1995 Revised: April 19, 2017 Environment of Care Committee Approved by: Subject: Tuberculosis Infection Control Julia Rupp, Executive Director I.
Trust Guideline on Routine Oxygen Saturation Measurement on the New-born (Pulse Oximetry)
 Trust Guideline on Routine Oxygen Saturation Measurement on the New-born For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of
Trust Guideline on Routine Oxygen Saturation Measurement on the New-born For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of
Equality of Opportunity Committee
 Equality of Opportunity Committee EOC(3)-12-09 (p8) Inquiry into Discrimination against People Living with HIV by Healthcare Professionals and Providers Written evidence from Abertawe Bro Morgannwg University
Equality of Opportunity Committee EOC(3)-12-09 (p8) Inquiry into Discrimination against People Living with HIV by Healthcare Professionals and Providers Written evidence from Abertawe Bro Morgannwg University
This SOP applies to all staff employed by NHS Greater Glasgow & Clyde and locum staff on fixed term contracts and volunteer staff.
 Page 1 of 9 Review SOP Objective To ensure that Healthcare Workers (HCWs) are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients
Page 1 of 9 Review SOP Objective To ensure that Healthcare Workers (HCWs) are aware of the actions and precautions necessary to minimise the risk of outbreaks and the importance of diagnosing patients
Occupational Health Hepatitis B and C Clinical Employment Policy
 Occupational Health Hepatitis B and C Clinical Employment Policy Including Exposure Prone Procedures and Haemodialysis Please be advised that the Trust discourages the retention of hard copies of policies
Occupational Health Hepatitis B and C Clinical Employment Policy Including Exposure Prone Procedures and Haemodialysis Please be advised that the Trust discourages the retention of hard copies of policies
CUSOM Student Health Immunization Requirements
 CUSOM Student Health Immunization Requirements Regulatory and legislative authorities require that students demonstrate immunization, immunity and/or protection from multiple contagious diseases before
CUSOM Student Health Immunization Requirements Regulatory and legislative authorities require that students demonstrate immunization, immunity and/or protection from multiple contagious diseases before
Ionising Radiation Policy
 Ionising Radiation Policy CONTENTS 1. University Policy. 2. Procedures / Guidance. 2.1 Responsibilities of the Deans of Schools and/or Heads of Departments 2.2 Radiation Protection Advisor / Radiation
Ionising Radiation Policy CONTENTS 1. University Policy. 2. Procedures / Guidance. 2.1 Responsibilities of the Deans of Schools and/or Heads of Departments 2.2 Radiation Protection Advisor / Radiation
Northwestern Polytechnic University
 Clinical Tuberculosis Assessment by Health Care Provider Clinicians should review and verify the information in the Tuberculosis (TB) Screening Questionnaire (attached). Persons answering YES to any questions
Clinical Tuberculosis Assessment by Health Care Provider Clinicians should review and verify the information in the Tuberculosis (TB) Screening Questionnaire (attached). Persons answering YES to any questions
TRUST WIDE DOCUMENT DOCUMENT NUMBER: ELHT Version 1
 i TRUST WIDE DOCUMENT DOCUMENT TITLE: SEASONAL INFLUENZA PLAN DOCUMENT NUMBER: ELHT Version 1 DOCUMENT PURPOSE: Seasonal Influenza (Flu) Plan sets out a coordinated and evidence-based approach to planning
i TRUST WIDE DOCUMENT DOCUMENT TITLE: SEASONAL INFLUENZA PLAN DOCUMENT NUMBER: ELHT Version 1 DOCUMENT PURPOSE: Seasonal Influenza (Flu) Plan sets out a coordinated and evidence-based approach to planning
New Entrant Screening and Latent TB Get screened and find out if you have TB infection before you develop TB disease!
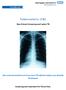 New Entrant Screening and Latent TB Get screened and find out if you have TB infection before you develop TB disease! Screening and treatment for TB are free. What does this leaflet cover? What is Tuberculosis
New Entrant Screening and Latent TB Get screened and find out if you have TB infection before you develop TB disease! Screening and treatment for TB are free. What does this leaflet cover? What is Tuberculosis
8. Contact Tracing. Sputum bacteriology A case of TB whose sputum is smear positive, defined as a patient with minimum of one sputum specimen HSE/HPSC
 8. Contact Tracing Contact tracing is a systematic process with three main objectives: To identify and initiate treatment of secondary cases To identify TB infected contacts in order to offer treatment
8. Contact Tracing Contact tracing is a systematic process with three main objectives: To identify and initiate treatment of secondary cases To identify TB infected contacts in order to offer treatment
Table 9. Policy for Tuberculosis Surveillance and Screening
 Policy for Tuberculosis Surveillance and Screening Purpose: to identify active cases of tuberculosis or latent TB among residents and staff of the nursing home in order to prevent transmission in this
Policy for Tuberculosis Surveillance and Screening Purpose: to identify active cases of tuberculosis or latent TB among residents and staff of the nursing home in order to prevent transmission in this
Joint Trust Guideline for the Management of Methylprednisolone Sodium Succinate Infusion for Child or Young Person A Clinical Guideline
 A Clinical Guideline For Use in: Children s Department By: Registered Paediatric Nurses and Medical Staff Children with active inflammatory bowel disease, For: active rheumatological conditions or other
A Clinical Guideline For Use in: Children s Department By: Registered Paediatric Nurses and Medical Staff Children with active inflammatory bowel disease, For: active rheumatological conditions or other
APPENDIX 1 State Summary Tables
 APPENDIX 1 State Summary Tables X SUMMARY TABLE Element 1: Affected HCW Health Care Worker means all persons, whether paid or unpaid, including but not limited to employees, staff, contractors, clinicians,
APPENDIX 1 State Summary Tables X SUMMARY TABLE Element 1: Affected HCW Health Care Worker means all persons, whether paid or unpaid, including but not limited to employees, staff, contractors, clinicians,
Trust Policy 218 Ionising Radiation Safety Policy
 Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
Trust Policy 218 Ionising Radiation Safety Policy Purpose Date Version August 2016 7 To ensure that Plymouth Hospitals NHS Trust complies with all relevant legislation with regard to the use of ionising
VARICELLA ZOSTER (CHICKENPOX/SHINGLES) INFECTION CONTROL PROCEDURE
 Reference Number: UHB 076 Version Number: 2 Date of Next Review: 23 June 2018 Previous Trust/LHB Reference Number: IPCD Policy No 8 T/45 VARICELLA ZOSTER (CHICKENPOX/SHINGLES) INFECTION CONTROL PROCEDURE
Reference Number: UHB 076 Version Number: 2 Date of Next Review: 23 June 2018 Previous Trust/LHB Reference Number: IPCD Policy No 8 T/45 VARICELLA ZOSTER (CHICKENPOX/SHINGLES) INFECTION CONTROL PROCEDURE
RUTGERS POLICY. Errors or changes? Contact: Rutgers University Occupational Health Department
 RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
RUTGERS POLICY Section: 40.3.2 Section Title: Legacy UMDNJ policies associated with Risk Management Policy Name: Housestaff Immunizations and Health Requirements Formerly Book: 00-01-40-45:00 Approval
Smoking cessation interventions and services
 National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
A rash case Infection control management of measles
 A rash case Infection control management of measles 23 th June 2013 PanCeltic Meeting Dr Jo Hargreaves Microbiology SpR University Hospital of Wales, Cardiff Acknowledgements Dr Harriet Hughes Nicola Bevan,
A rash case Infection control management of measles 23 th June 2013 PanCeltic Meeting Dr Jo Hargreaves Microbiology SpR University Hospital of Wales, Cardiff Acknowledgements Dr Harriet Hughes Nicola Bevan,
PREVENTION OF TUBERCULOSIS. Dr Amitesh Aggarwal
 PREVENTION OF TUBERCULOSIS Dr Amitesh Aggarwal 25 to 50 % of persons exposed to intimate contact with active PTB - latent infection with TB. Exposure to index case for 12 hours - high risk of infection.
PREVENTION OF TUBERCULOSIS Dr Amitesh Aggarwal 25 to 50 % of persons exposed to intimate contact with active PTB - latent infection with TB. Exposure to index case for 12 hours - high risk of infection.
Nursing and Midwifery students only. Section 1: Information
 Nursing and Midwifery students only. Section 1: Information Students enrolled in programs offered by our School are REQUIRED to provide evidence of their immunisation status for the diseases listed in
Nursing and Midwifery students only. Section 1: Information Students enrolled in programs offered by our School are REQUIRED to provide evidence of their immunisation status for the diseases listed in
Trust Guideline for the Management of: Adult patients requiring anticoagulation with Warfarin (including reversal)
 (including reversal) A Clinical Guideline recommended for use: For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author
(including reversal) A Clinical Guideline recommended for use: For Use in: By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
TB in Corrections Phoenix, Arizona
 TB in Corrections Phoenix, Arizona March 24, 2011 Contact Investigation in the Correctional Setting Jessica Quintero, BAAS March 24, 2011 Jessica Quintero, BAAS has the following disclosures to make: No
TB in Corrections Phoenix, Arizona March 24, 2011 Contact Investigation in the Correctional Setting Jessica Quintero, BAAS March 24, 2011 Jessica Quintero, BAAS has the following disclosures to make: No
2017/2018 Annual Volunteer Tuberculosis Notice
 Lewis Center for Educational Research Academy for Academic Excellence Norton Science and Language Academy Business Offices 17500 Mana Road Apple Valley, CA 92307 E-mail: hr@lcer.org 760-946-5414 Fax 760-946-9193
Lewis Center for Educational Research Academy for Academic Excellence Norton Science and Language Academy Business Offices 17500 Mana Road Apple Valley, CA 92307 E-mail: hr@lcer.org 760-946-5414 Fax 760-946-9193
in pregnancy Document Review History Version Review Date Reviewed By Approved By
 GYNAECOLOGY/ ANTENATAL CARE WIRRAL WOMEN & CHILDREN S HOSPITAL Guideline No: Hepatitis B management in pregnancy VERSION 1 AMENDMENTS MADE: N/A DATE OF ISSUE: May 2012 DATE OF REVIEW: May 2015 REVIEW INTERVAL:
GYNAECOLOGY/ ANTENATAL CARE WIRRAL WOMEN & CHILDREN S HOSPITAL Guideline No: Hepatitis B management in pregnancy VERSION 1 AMENDMENTS MADE: N/A DATE OF ISSUE: May 2012 DATE OF REVIEW: May 2015 REVIEW INTERVAL:
Chapter 7 Tuberculosis (TB)
 Chapter 7 Tuberculosis (TB) TB infection vs. TB disease Information about TB TB skin testing Active TB disease TB risk factors Role of Peel Public Health in TB prevention and control Environmental and
Chapter 7 Tuberculosis (TB) TB infection vs. TB disease Information about TB TB skin testing Active TB disease TB risk factors Role of Peel Public Health in TB prevention and control Environmental and
SPECIALTY TRAINEE IN ORTHODONTICS GLASGOW DENTAL HOSPITAL AND SCHOOL AND INVERCLYDE ROYAL HOSPITAL, GREENOCK
 SPECIALTY TRAINEE IN ORTHODONTICS GLASGOW DENTAL HOSPITAL AND SCHOOL AND INVERCLYDE ROYAL HOSPITAL, GREENOCK Job Profile SPECIALTY TRAINING POST IN ORTHODONTICS JOB PROFILE One full time specialty training
SPECIALTY TRAINEE IN ORTHODONTICS GLASGOW DENTAL HOSPITAL AND SCHOOL AND INVERCLYDE ROYAL HOSPITAL, GREENOCK Job Profile SPECIALTY TRAINING POST IN ORTHODONTICS JOB PROFILE One full time specialty training
Trust Guideline for Management of Faltering Growth (Failure to Thrive) in Babies and Young Children.
 Trust Guideline for Management of Faltering Growth (Failure to Thrive) in. A Clinical Guideline For use in: By: For: Division responsible for document: Women and Children Key words: Buxton Ward, Children
Trust Guideline for Management of Faltering Growth (Failure to Thrive) in. A Clinical Guideline For use in: By: For: Division responsible for document: Women and Children Key words: Buxton Ward, Children
Sharps injuries and exposure to blood and high risk body fluids SOP:
 Clinical Sharps injuries and exposure to blood and high risk body fluids SOP: Document Control Summary Status: Replacement. Replaces: Management of clinical sharps injuries and exposure to blood and high
Clinical Sharps injuries and exposure to blood and high risk body fluids SOP: Document Control Summary Status: Replacement. Replaces: Management of clinical sharps injuries and exposure to blood and high
Shingles Procedure. (IPC Policy Manual)
 Shingles Procedure (IPC Policy Manual) DOCUMENT CONTROL: Version: 1.1 Ratified by: Clinical Policy Approval Group Date ratified: 3 July 2018 Name of originator/author: Senior Clinical Nurse Specialist
Shingles Procedure (IPC Policy Manual) DOCUMENT CONTROL: Version: 1.1 Ratified by: Clinical Policy Approval Group Date ratified: 3 July 2018 Name of originator/author: Senior Clinical Nurse Specialist
Trust Guideline for the Management of Sedation in Painless Imaging Procedures in Children
 A clinical guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Children s Day Ward (CDW), Children s Assessment Unit (CAU), Buxton Ward, Radiology. Paediatric
A clinical guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Children s Day Ward (CDW), Children s Assessment Unit (CAU), Buxton Ward, Radiology. Paediatric
AUTISM ACTION PLAN FOR THE ROYAL BOROUGH OF GREENWICH
 AUTISM ACTION PLAN FOR THE ROYAL BOROUGH OF GREENWICH NATIONAL CONTEXT Fulfilling and Rewarding Lives (2010) is the Government s strategy for adults with Autistic Spectrum Disorders. It sets out the Government
AUTISM ACTION PLAN FOR THE ROYAL BOROUGH OF GREENWICH NATIONAL CONTEXT Fulfilling and Rewarding Lives (2010) is the Government s strategy for adults with Autistic Spectrum Disorders. It sets out the Government
London Strategic Clinical Networks. Quality Standard. Version 1.0 (2015)
 London Strategic Clinical Networks Quality Standard Version 1.0 (2015) Supporting the delivery of equitable, high quality AKI care through collaboration www.londonaki.net @LondonAKI Overview The management
London Strategic Clinical Networks Quality Standard Version 1.0 (2015) Supporting the delivery of equitable, high quality AKI care through collaboration www.londonaki.net @LondonAKI Overview The management
Student Health Requirements Master of Arts, Biomedical Sciences Program
 Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
Student Health Requirements Master of Arts, Biomedical Sciences Program All students in medically related programs, just as physicians in practice, are required to be current with required immunizations
Health care workers with serious communicable diseases who is being protected?
 Health care workers with serious communicable diseases who is being protected? Dr Alison Rimmer Consultant Occupational Physician and Clinical Director Sheffield Occupational Health Service Serious communicable
Health care workers with serious communicable diseases who is being protected? Dr Alison Rimmer Consultant Occupational Physician and Clinical Director Sheffield Occupational Health Service Serious communicable
TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks
 1 TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks 2 Page 1 4 NHS Lothian Infection Prevention and Control Study Day On
1 TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks 2 Page 1 4 NHS Lothian Infection Prevention and Control Study Day On
Thorax Online First, published on December 8, 2009 as /thx
 Thorax Online First, published on December 8, 2009 as 10.1136/thx.2009.119677 Title Page Cost effectiveness of the NICE guidelines for screening for latent tuberculosis infection: the Quantiferon-TB gold
Thorax Online First, published on December 8, 2009 as 10.1136/thx.2009.119677 Title Page Cost effectiveness of the NICE guidelines for screening for latent tuberculosis infection: the Quantiferon-TB gold
Chickenpox Procedure. (IPC Policy Manual)
 Chickenpox Procedure (IPC Policy Manual) DOCUMENT CONTROL: Version: 1 Ratified by: Clinical Policy Approval Group Date ratified: 3 July 2018 Name of originator/author: Senior Clinical Nurse Specialist
Chickenpox Procedure (IPC Policy Manual) DOCUMENT CONTROL: Version: 1 Ratified by: Clinical Policy Approval Group Date ratified: 3 July 2018 Name of originator/author: Senior Clinical Nurse Specialist
