KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
|
|
|
- Felix Cobb
- 5 years ago
- Views:
Transcription
1
2 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated erythematous plaques with silvery scales on elbows, knees, lumbosacral region, and scalp, and nail changes. Erythrodermic psoriasis affects more than 80% body surface area. Generalised pustular psoriasis is widespread erythema studded with superficial pustules which may coalesce to form lakes of pus. Psoriasis can be as mentally and physically disabling as cancer, heart disease, diabetes, hypertension, arthritis and depression. Psoriatic arthritis affects about 16% of Malaysians with psoriasis. Early recognition and treatment prevent deformities. Assessment should be performed at least annually by looking for relevant signs and symptoms:- a. Joint swelling b. Dactylitis c. Significant early morning stiffness >1/2 hour Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality. Psoriasis patients should be screened for metabolic syndrome and risk factors of atherosclerosis-related diseases. Assess physical severity of psoriasis with Psoriasis Area and Severity Index (PASI) or Body Surface Area (BSA). Assess the impact of psoriasis on the quality of life (QoL) of patients with Dermatology Life Quality Index (DLQI) hoice of treatment for pregnant and lactating women should benefit the mother and pose minimal risk to the foetus/baby. This Quick Reference provides key messages and a summary of the main recommendations in the linical Practice Guidelines (PG) Management of Psoriasis Vulgaris. Details of the evidence supporting these recommendations can be found in the above PG, available on the following websites: Ministry of Health Malaysia : Academy of Medicine Malaysia : Malaysian Dermatology Society : 1
3 PRINIPLES OF TREATMENT Management should start with patient education. Treatment of psoriasis should be a combined decision between patients & their healthcare providers. Treatment goal and minimal target set should be based on disease severity and patient s preferences. Treatment goal achieved should be monitored regularly to detect loss of response which may necessitate modification of therapy. ASSESSMENT OF SEVERITY Grading of Psoriasis Severity Grade of severity Mild Moderate Severe Measurement tools BSA 10% PGA mild PASI 10 DLQI 10 BSA >10% to 30% PGA moderate PASI >10 to 20 DLQI >10 to 20 BSA >30% PGA severe or very severe PASI >20 DLQI >20 Interpretation Disease with a minimal impact on the patient s QoL and patient can achieve acceptable symptom control by standard topical therapy Disease that cannot be, or would not be expected to be controlled to an acceptable degree by standard topical therapy, and/or disease that moderately affects the patient s QoL Disease that cannot be, or would not be expected to be controlled by topical therapy and that adversely affect patient s QoL (this include erythrodermic psoriasis, pustular psoriasis and psoriatic arthritis) Treatment Treatment Goals of Various Modalities Minimal targets Time for Evaluation (weeks) Subsequent Evaluation (months) Topical therapy BSA 50 or PASI 50 or DLQI Phototherapy Methotrexate yclosporine Acitretin BSA 75 or PASI 75 or DLQI Infliximab Adalimumab Ustekinumab Etanercept PASI 75 OR PASI 50 to <75 plus DLQI
4 TREATMENT MODALITIES Patients with mild or moderate psoriasis with minimal impairment in QoL (DLQI 5) should be treated with topical agents. Emollient should be used regularly. Tar-based preparations may be used as a first-line topical therapy. Short-term use of potent and very potent topical corticosteroid may be used to clear limited plaques. Mild potency corticosteroid may be used for face, genitalia and body folds. Fixed dose combination of vitamin D analogue and corticosteroid may be used for short-term treatment. Topical vitamin D analogue may be used but dose should not exceed 100g / week. Phototherapy should be offered to patients who have failed topical therapy before starting them on systemic agents. Life time exposure to psoralen plus ultraviolet A (PUVA) and ultraviolet B (UVB) should not exceed 200 and 350 sessions respectively. Systemic / biologic therapy for moderate to severe psoriasis should be initiated by a dermatologist. Pre-treatment assessment and regular monitoring for toxicity should be done during systemic / biologic therapy. Methotrexate or acitretin should be used as first-line systemic therapy. yclosporine may be used as second-line systemic therapy. yclosporine should NOT be used for more than 2 years and avoided in patients with previous PUVA exposure. Biologics should be offered to patients who fail, have intolerance or contraindication to conventional systemic treatment and phototherapy. 1. Dermatology Referral RITERIA OF REFERRAL Indications for referral Diagnostic uncertainty Erythrodermic or pustular psoriasis should be referred urgently for specialist assessment and treatment Patients who have failed adequate trial of topical therapy for 6-12 weeks Severe psoriasis that requires phototherapy or systemic therapy 2. Rheumatology Referral Indications for referral Diagnostic evaluation of patients with suspected Psoriatic Arthritis (PsA) Formulate management plan for PsA 3
5 ALGORITHM 1: MANAGEMENT OF PSORIASIS VULGARIS IN PRIMARY ARE PSORIASIS PATIENT PRESENTING TO PRIMARY ARE Articular symptoms / signs suggestive of PsA Joint swelling Dactylitis Significant early morning stiffness >1/2 hour 1. Assess Severity Arthritis (PsA) o-morbidities 2. Educate patient Presence of co-morbidities such as obesity, hypertension, diabetes, depression etc. YES YES REFER TO RHEUMATOLOGIST SEVERITY MANAGE / REFER TO RELEVANT SPEIALITY Mild (BSA 10% or PASI 10) Moderate (BSA >10% to 30% or PASI >10 to 20) Severe (BSA >30% or PASI >20) Erythrodermic or generalised pustular psoriasis: urgent referral is indicated Topical Therapy DLQI 10 DLQI >10 Assess DLQI REFER TO DERMATOLOGIST Re-assess in 6 weeks Optimise topical therapy DLQI 5 RESPONDER NO DLQI >5 Assess DLQI YES Regular follow-up as indicated Annual assessment:- Document severity Assess co-morbidities and articular symptoms Optimise topical treatment BSA PASI DLQI Responder - Body Surface Area - Psoriasis Area and Severity Index - Dermatology Life Quality Index - BSA 50% reduction or PASI 50 achieved 4
6 ALGORITHM 2: TREATMENT OF PSORIASIS VULGARIS PSORIASIS VULGARIS Mild (BSA 10% or PASI 10) Moderate (BSA >10% to 30% or PASI >10 to 20) Severe BSA >30% or PASI >20 Topical Therapy DLQI 10 Assess DLQI DLQI >10 Phototherapy Failed / contraindicated / not available Tar (First line therapy) Systemic Therapy Dithranol (Large plaque) orticosteroids (Short-term therapy) Methotrexate (First-line) Acitretin yclosporine (Short- term therapy) Vitamin D analogues (<100g/week) Failed / contraindicated / intolerant with BSA >30% or PASI >20 or DLQI >20 alcineurin inhibitors (Face & Flexures only) Biologics Ustekinumab Adalimumab Etanercept Infliximab 5
7 DRUG TOPIAL OSTIOSTEROIDS Mild Hydrocortisone 1% ream / Ointment Moderate Betamethasone 17-Valerate 0.025% ream / Ointment lobetasone Butyrate 0.05% ream / Ointment Ointment Potent Betamethasone 17- Valerate 0.1% ream / Ointment Mometasone Furoate 0.1% ream / Ointment Very Potent lobetasol Propionate 0.05% ream / Ointment Recommended Medication Dosing, Side Effects and ontraindications REOMMENDED DOSAGE 1-2 times daily Worsening of untreated infection, contact dermatitis, perioral dermatitis, acne, depigmentation, dryness, hypertrichosis, secondary infection, skin atrophy, pruritus, tingling/stinging, rosacea, folliculitis, photosensitivity SIDE EFFETS ONTRAINDIATIONS SPEIAL PREAUTION DRUG INTERATION Untreated bacterial, fungal, or viral skin lesions, in rosacea, and in perioral dermatitis Avoid prolonged use on the face Avoid use on face and body folds Limit continuous use to <4 weeks Limit to 60g/week Once daily Avoid prolonged use on face 1-2 times daily TAR-BASED PREPARATION 1-2 times daily TOPIAL VITAMIN D ANALOGUE alcipotriol 50 mcg/g ream / Ointment alcipotriol 50 mcg/ml Scalp Solution alcipotriol Hydrate 50 mcg/g & Betamethasone Dipropionate 0.5 mg/g Ointment / Gel DITHRANOL PREPARATIONS SALIYLI AID 2-10% REAM / OINTMENT Twice daily Once daily % suitable for overnight treatment for skin 1-2% short contact therapy 30 mins -1 hour Twice daily Dermatitis, folliculitis, irritation, photo-sensitivity Itching, erythema, burning, paraesthesia, dermatitis, photosensitivity Worsening of untreated infection, contact dermatitis, perioral dermatitis, acne, depigmentation, dryness, hypertrichosis, secondary infection, skin atrophy, pruritus, tingling/stinging, rosacea, folliculitis, photosensitivity Local burning sensation and irritation, stains skin, hair and fabrics Sensitivity, excessive drying, irritation, salicylism with excessive use Avoid in acutely inflammed lesions, and pustular psoriasis Hypercalcemia or evidence of vitamin D toxicity Acutely inflammed and pustular psoriasis Avoid use on face and body folds Limit continuous use to <2weeks Limit to 30g/week Avoid contact with eyes, genital / rectal areas Avoid use in 1 st trimester of pregnancy Avoid use on face Avoid excessive exposure to sunlight and sunlamps Pregnancy Breast feeding Avoid use near eyes and sensitive areas of skin PREGNANY ATEGORY Avoid broken or inflamed skin 6
8
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
Topical Calcipotriol Algorithm
 Topical Calcipotriol Algorithm Is this patient an adult previously diagnosed with psoriasis by a doctor? Do the skin patches look the same as those diagnosed as psoriasis? Is this psoriasis covering an
Topical Calcipotriol Algorithm Is this patient an adult previously diagnosed with psoriasis by a doctor? Do the skin patches look the same as those diagnosed as psoriasis? Is this psoriasis covering an
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Dimethyl fumarate for treating moderate to severe Draft scope (pre-referral) Draft remit/appraisal objective To appraise
Horizon Scanning Centre March Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798
 Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
Psoriasis. What is Psoriasis? What causes psoriasis? Medical Topics Psoriasis
 1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Psoriasis management. A/Prof Amanda Oakley Dermatologist, Waikato
 Psoriasis management A/Prof Amanda Oakley Dermatologist, Waikato AbbVie Breakfast Session, 14 June 2014 Disclosure This breakfast session is sponsored by Abbvie Autoimmune skin disorders Psoriasis Eczema
Psoriasis management A/Prof Amanda Oakley Dermatologist, Waikato AbbVie Breakfast Session, 14 June 2014 Disclosure This breakfast session is sponsored by Abbvie Autoimmune skin disorders Psoriasis Eczema
TREATMENT OPTIONS FOR PSORIASIS. Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17
 TREATMENT OPTIONS FOR PSORIASIS Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17 PSORIASIS A chronic, non-infectious inflammatory skin condition that has no cure Characterised
TREATMENT OPTIONS FOR PSORIASIS Sandra Hanlon Dermatology Senior Charge Nurse NHS Ayrshire and Arran 07/03/17 PSORIASIS A chronic, non-infectious inflammatory skin condition that has no cure Characterised
Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])
![Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV]) Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])](/thumbs/89/99382632.jpg) Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
The role of the practice nurse in managing psoriasis in primary care
 The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis. Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement
 Psoriasis Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea Courses Inc. All rights reserved. No part
Psoriasis Andrei Metelitsa, MD, FRCPC, FAAD Clinical Associate Professor, Dermatology, U of C Co-Director, Institute for Skin Advancement Copyright 2017 by Sea Courses Inc. All rights reserved. No part
USTEKINUMAB Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA GUIDELINES FOR USE
 Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Psoriasis. Overview. Epidemiology. Epidemiology 08/08/2015. Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital
 Overview Psoriasis 1. Epidemiology of psoriasis 2. Histology Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital Aug 2015 3. Types of psoriasis 4. Assessing severity 5. Treatments Topical
Overview Psoriasis 1. Epidemiology of psoriasis 2. Histology Dr Nigel Burrows Consultant Dermatologist Addenbrooke s Hospital Aug 2015 3. Types of psoriasis 4. Assessing severity 5. Treatments Topical
COMPARATIVE STUDY OF THE EFFICACY OF VARIOUS TOPICAL TREATMENT MODALITIES AND PHOTOTHERAPY FOR PSORIASIS VULGARIS: A REVIEW
 COMPARATIVE STUDY OF THE EFFICACY OF VARIOUS TOPICAL TREATMENT MODALITIES AND PHOTOTHERAPY FOR PSORIASIS VULGARIS: A REVIEW Dr. Shweta Aryal *, Prof. Dr. Liu Jin Xian, Dr. Gong Shao Zhi and Dr. Jyoti Karki
COMPARATIVE STUDY OF THE EFFICACY OF VARIOUS TOPICAL TREATMENT MODALITIES AND PHOTOTHERAPY FOR PSORIASIS VULGARIS: A REVIEW Dr. Shweta Aryal *, Prof. Dr. Liu Jin Xian, Dr. Gong Shao Zhi and Dr. Jyoti Karki
PSORIASIS BEST PRACTICE IN MANAGEMENT
 PSORIASIS BEST PRACTICE IN MANAGEMENT Objectives Discuss pathology of psoriasis Review types of psoriasis Review triggers and factors affecting disease severity Common comorbidity review Review first and
PSORIASIS BEST PRACTICE IN MANAGEMENT Objectives Discuss pathology of psoriasis Review types of psoriasis Review triggers and factors affecting disease severity Common comorbidity review Review first and
Psoriasis. Causes of Psoriasis
 Psoriasis Psoriasis is a common, chronic, relapsing/remitting, immune-mediated systemic disease characterized by skin lesions including red, scaly patches, papules, and plaques, which usually itch. The
Psoriasis Psoriasis is a common, chronic, relapsing/remitting, immune-mediated systemic disease characterized by skin lesions including red, scaly patches, papules, and plaques, which usually itch. The
Horizon Scanning Centre May Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524
 Horizon Scanning Centre May 2014 Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre May 2014 Brodalumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 5524 This briefing is based on information available at the time of research and a limited literature
Appendix 1: Frequently Asked Questions
 Appendix 1: Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added to the Ontario Drug Benefit (ODB) Formulary
Appendix 1: Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added to the Ontario Drug Benefit (ODB) Formulary
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Core Safety Profile. AT/H/PSUR/0013/002 Date of FAR:
 ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Clinical Trial Report Synopsis A phase 2a, proof of concept trial, testing twice daily application of LEO 124249 ointment 30 mg/g in the treatment of mild to moderate inverse psoriasis Design of trial:
Horizon Scanning Centre March Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209
 Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Predicting the Response to Phototherapy for Psoriasis Patients
 A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
A*STAR-NHG-NTU Skin Research Grant Joint Workshop 17 October 2015 Predicting the Response to Phototherapy for Psoriasis Patients Is it possible? Dr Eugene Tan Consultant Dermatologist National Skin Centre
Follow this and additional works at: Part of the Skin and Connective Tissue Diseases Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 13 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 May 2009 STELARA 45 mg, solution for injection B/1 x 0.5 ml vial (CIP code: 392 586-2) JANSSEN-CILAG Ustekinumab
Psoriasis is a chronic, inflammatory, Prescribing in children
 Psoriasis in children: current approaches to management Laura Proudfoot BSc, MRCP, Elisabeth Higgins MA, FRCP and Judy Davids RGN Our series Prescribing in children gives practical advice for successful
Psoriasis in children: current approaches to management Laura Proudfoot BSc, MRCP, Elisabeth Higgins MA, FRCP and Judy Davids RGN Our series Prescribing in children gives practical advice for successful
A Patient s Guide to. Treatments for Psoriatic Arthritis
 A Patient s Guide to Treatments for Psoriatic Arthritis Who should read this guide? This guide is aimed at people with psoriatic arthritis (abbreviated as PsA), or those who care for a person with this
A Patient s Guide to Treatments for Psoriatic Arthritis Who should read this guide? This guide is aimed at people with psoriatic arthritis (abbreviated as PsA), or those who care for a person with this
METHYLPREDNISOLONE ACEPONATE (MPA)
 RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
Psoriasis: Therapeutic goals
 Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Psoriasis: Therapeutic goals I want to die 50 45 impetiginization infliximab 600 40 35 30 400 25 20 15 200 10 5 0 22-ene 21-feb 23-mar 22-abr 22- may Efalizumab 6 doses: flare + REBOUND CSA 3 21-jun 21-jul
Dermatology GP Referral Guidelines
 Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
Austin Health Dermatology Department holds 5 Clinic sessions to discuss and plan the treatment of with Dermatology conditions. Department of Health clinical urgency categories for specialist clinics Urgent:
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS. NAME OF THE MEDICINAL PRODUCT Daivobet 50 micrograms/g + 0.5 mg/g gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of gel contains 50 micrograms of calcipotriol
SUMMARY OF PRODUCT CHARACTERISTICS. NAME OF THE MEDICINAL PRODUCT Daivobet 50 micrograms/g + 0.5 mg/g gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of gel contains 50 micrograms of calcipotriol
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Package leaflet: Information for the user. Fluticrem 0.05% cream Fluticasone propionate
 Package leaflet: Information for the user Fluticrem 0.05% cream Fluticasone propionate Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Fluticrem 0.05% cream Fluticasone propionate Read all of this leaflet carefully before you start using this medicine because it contains important information
The Natural History of Psoriasis and Treatment Goals
 The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
SUMMARY OF PRODUCT CHARACTERISTICS. Each gram of ointment contains 1 mg of mometasone furoate and 50 mg of salicylic acid
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Elosalic 1 mg/g + 50 mg/g ointment. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of ointment contains 1 mg of mometasone furoate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Elosalic 1 mg/g + 50 mg/g ointment. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of ointment contains 1 mg of mometasone furoate
What is Psoriasis? Common Areas Affected. Type Who Does it Affect Characteristics
 What is? is a term derived from the Greek word psōra which means itch and is a common, long lasting, inflammatory skin condition which affects 1-3% of the UK population and about 80 million people worldwide.
What is? is a term derived from the Greek word psōra which means itch and is a common, long lasting, inflammatory skin condition which affects 1-3% of the UK population and about 80 million people worldwide.
SUMMARY OF PRODUCT CHARACTERISTICS. Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of ointment contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of ointment contains
Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis
 NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
Etanercept: a new option in paediatric plaque psoriasis
 : a new option in paediatric plaque psoriasis Steve Chaplin MSc, MRPharmS, Medical Writer, Dr David Atherton MA, MB, BChir, FRCP, Honorary Consultant in Paediatric Dermatology, Great Ormond Street Hospital
: a new option in paediatric plaque psoriasis Steve Chaplin MSc, MRPharmS, Medical Writer, Dr David Atherton MA, MB, BChir, FRCP, Honorary Consultant in Paediatric Dermatology, Great Ormond Street Hospital
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
(minutes for web publishing)
 Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
It is estimated that about 26,000 new cases of
 Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
Ontario Public Drug Programs. Inflectra (infliximab) Frequently Asked Questions
 Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Betamethasone dipropionate
 NEW ZEALAND DATA SHEET Daivobet 50/500 ointment Calcipotriol 50 microgram/g Betamethasone 500 microgram/g present as dipropionate NAME OF THE MEDICINE Daivobet 50/500 ointment OH H H H 2 O HO OH Calcipotriol
NEW ZEALAND DATA SHEET Daivobet 50/500 ointment Calcipotriol 50 microgram/g Betamethasone 500 microgram/g present as dipropionate NAME OF THE MEDICINE Daivobet 50/500 ointment OH H H H 2 O HO OH Calcipotriol
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
Psoriasis. Jessica Kaffenberger, M.D. Assistant Professor of Dermatology Division of Dermatology The Ohio State University Wexner Medical Center
 Psoriasis Jessica Kaffenberger, M.D. Assistant Professor of Dermatology Division of Dermatology The Ohio State University Wexner Medical Center Learning objectives Recognize the different types of psoriasis
Psoriasis Jessica Kaffenberger, M.D. Assistant Professor of Dermatology Division of Dermatology The Ohio State University Wexner Medical Center Learning objectives Recognize the different types of psoriasis
Dermatology elective for yr. 5. Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015
 Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
COMMON SKIN CONDITIONS IN PRIMARY CARE. Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio
 COMMON SKIN CONDITIONS IN PRIMARY CARE Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio DISCLOSURE The Speaker and members of the planning committee do not have a conflict of interest
COMMON SKIN CONDITIONS IN PRIMARY CARE Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio DISCLOSURE The Speaker and members of the planning committee do not have a conflict of interest
An otherwise healthy 12-year-old
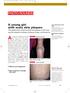 1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
Time to Learn. 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service
 Time to Learn 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service The Red Face Rosacea Acne Seborrhoeic eczema eczema Psoriasis Slapped cheek syndrome Fungal infection Erysipelas...
Time to Learn 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service The Red Face Rosacea Acne Seborrhoeic eczema eczema Psoriasis Slapped cheek syndrome Fungal infection Erysipelas...
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Apremilast Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 1/1/2018 Next
Psoriasis and Psoriatic Arthritis Combined Clinic Electronic Medical Records Form
 Psoriasis and Psoriatic Arthritis Combined Clinic Electronic Medical Records Form NAME: AGE: SEX: CHIEF COMPLAINT: HISTORY OF PRESENT ILLNESS: The patient presents for the initial evaluation of [psoriasis
Psoriasis and Psoriatic Arthritis Combined Clinic Electronic Medical Records Form NAME: AGE: SEX: CHIEF COMPLAINT: HISTORY OF PRESENT ILLNESS: The patient presents for the initial evaluation of [psoriasis
NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION
 NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
Inflectra Frequently Asked Questions
 Inflectra Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Earlier in 2016, Inflectra (infliximab) was added to the Ontario Drug Benefit (ODB) Formulary as a Limited
Inflectra Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Earlier in 2016, Inflectra (infliximab) was added to the Ontario Drug Benefit (ODB) Formulary as a Limited
Horizon Scanning Centre January Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652
 Horizon Scanning Centre January 2013 Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652 This briefing is based on information available at the time of research and a limited literature search. It is not
Horizon Scanning Centre January 2013 Apremilast for psoriasis SUMMARY NIHR HSC ID: 2652 This briefing is based on information available at the time of research and a limited literature search. It is not
TRANSPARENCY COMMITTEE OPINION. 26 April 2006
 TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
TRANSPARENCY COMMITTEE OPINION 26 April 2006 REMICADE 100 mg powder for concentrate for solution for infusion Box of 1 (CIP code: 562 070.1) Applicant : laboratoires Schering Plough List I Drug for hospital
Supplementary Tables. Psoriasis a
 Supplementary Tables Supplementary Table S1 Age-sex distribution of patients with psoriasis and PPP subdivided by the department specified in a claim with a diagnosis code of psoriasis and PPP in Japanese
Supplementary Tables Supplementary Table S1 Age-sex distribution of patients with psoriasis and PPP subdivided by the department specified in a claim with a diagnosis code of psoriasis and PPP in Japanese
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Chemical structure of calcipotriol
 PRODUCT INFORMATION DAIVONEX CREAM AUST R 57354 Calcipotriol 50 microgram/g NAME OF THE MEDICINE: CALCIPOTRIOL DESCRIPTION Calcipotriol is a white or almost white crystalline substance. It is a vitamin
PRODUCT INFORMATION DAIVONEX CREAM AUST R 57354 Calcipotriol 50 microgram/g NAME OF THE MEDICINE: CALCIPOTRIOL DESCRIPTION Calcipotriol is a white or almost white crystalline substance. It is a vitamin
0BCore Safety Profile. Pharmaceutical form(s)/strength: Cream 1% DK/H/PSUR/0009/005 Date of FAR:
 0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
Ixekizumab for treating moderate to severe plaque psoriasis [ID904]
![Ixekizumab for treating moderate to severe plaque psoriasis [ID904] Ixekizumab for treating moderate to severe plaque psoriasis [ID904]](/thumbs/86/94899473.jpg) Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Patients who achieved the primary criterion for response i.e.: complete clearance or a reduction
 MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
MC 9101 F Study Page3 ABSTRACT Background: Cyclosporin A has been shown to be an effective systemic treatment in severe psoriasis but with the disadvantage of dose-dependent toxic effects particularly
Ustekinumab for the treatment of moderate to severe psoriasis
 DOI: 10.3310/hta13suppl3/10 Health Technology Assessment 2009; Vol. 13: Suppl. 3 Ustekinumab for the treatment of moderate to severe psoriasis E Gospodarevskaya, J Picot, K Cooper, E Loveman* and A Takeda
DOI: 10.3310/hta13suppl3/10 Health Technology Assessment 2009; Vol. 13: Suppl. 3 Ustekinumab for the treatment of moderate to severe psoriasis E Gospodarevskaya, J Picot, K Cooper, E Loveman* and A Takeda
PACKAGE LEAFLET 1 April 2017
 PACKAGE LEAFLET 1 April 2017 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Monovo 1 mg/g, crème Mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains
PACKAGE LEAFLET 1 April 2017 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Monovo 1 mg/g, crème Mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains
Final Appraisal Report. Etanercept (Enbrel. Pfizer Limited. Advice No: 0210 March Recommendation of AWMSG
 Final Appraisal Report Etanercept (Enbrel ) Pfizer Limited Advice No: 0210 March 2010 Recommendation of AWMSG Etanercept (Enbrel ) is not recommended for use within NHS Wales for the treatment of chronic
Final Appraisal Report Etanercept (Enbrel ) Pfizer Limited Advice No: 0210 March 2010 Recommendation of AWMSG Etanercept (Enbrel ) is not recommended for use within NHS Wales for the treatment of chronic
Psoriasis. Dermatology. History. Examination. Background. Objective. Discussion. Keywords: psoriasis; skin diseases. Philip Clarke
 Dermatology Psoriasis Philip Clarke Background Psoriasis is one of the more common rashes presenting to general practice. Objective This article outlines the assessment and management of psoriasis in the
Dermatology Psoriasis Philip Clarke Background Psoriasis is one of the more common rashes presenting to general practice. Objective This article outlines the assessment and management of psoriasis in the
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
calcipotriol and betamethasone dipropionate gel Topical Antipsoriatic Agent Vitamin D Analogue / Corticosteroid
 PRODUCT MONOGRAPH Pr DOVOBET Gel calcipotriol and betamethasone dipropionate gel 50 mcg/g calcipotriol (as monohydrate) and 0.5 mg/g betamethasone (as dipropionate) gel Topical Antipsoriatic Agent Vitamin
PRODUCT MONOGRAPH Pr DOVOBET Gel calcipotriol and betamethasone dipropionate gel 50 mcg/g calcipotriol (as monohydrate) and 0.5 mg/g betamethasone (as dipropionate) gel Topical Antipsoriatic Agent Vitamin
Psoriasis the latest recommendations for management: where can primary care make a real difference?
 Dermatology Psoriasis the latest recommendations for management: where can primary care make a real difference? Dr Stephen Kownacki Executive chair, Primary Care Dermatology Society (PCDS) This session
Dermatology Psoriasis the latest recommendations for management: where can primary care make a real difference? Dr Stephen Kownacki Executive chair, Primary Care Dermatology Society (PCDS) This session
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Monovo 1 mg/g ointment. Mometasone furoate
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Monovo 1 mg/g ointment Mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains important information
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Monovo 1 mg/g ointment Mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains important information
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis
 Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Single Technology Appraisal (STA) Tildrakizumab for treating moderate to severe plaque psoriasis Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
STELARA DATA SHEET NAME OF THE MEDICINE DESCRIPTION V L C L V H C H 1 C H 2 C H 3. Fab. F(ab)' 2. hinge
 DATA SHEET NAME OF THE MEDICINE Ustekinumab (rmc). CAS Registry Number: 815610-63-0. DESCRIPTION (ustekinumab) is a human IgG1kappa monoclonal antibody with an approximate molecular weight of 148,600 daltons.
DATA SHEET NAME OF THE MEDICINE Ustekinumab (rmc). CAS Registry Number: 815610-63-0. DESCRIPTION (ustekinumab) is a human IgG1kappa monoclonal antibody with an approximate molecular weight of 148,600 daltons.
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice)
 Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
Using Your ESP* in Pharmacy: How to Improve Treatment Adherence and Patient Outcomes in Psoriasis (*Expanded Scope of Practice) Patient Case Study in Psoriasis Patient Case Study in Psoriasis William Smith,
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
PSORIASIS PSORIASIS. Anatomic sites FACTS ABOUT PROSIASIS PSORIASIS 11/16/2017 STIGMATA OF PSORIASIS
 PSORIASIS Dr. Sami Alsuwaidan President, Saudi Society of Dermatology & Dermatologic Surgery Associate Professor and Consultant Department of Dermatology, College of Medicine King Saud University PSORIASIS
PSORIASIS Dr. Sami Alsuwaidan President, Saudi Society of Dermatology & Dermatologic Surgery Associate Professor and Consultant Department of Dermatology, College of Medicine King Saud University PSORIASIS
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Monovo 1 mg/g cutaneous emulsion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of cutaneous emulsion contains 1 mg mometasone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Monovo 1 mg/g cutaneous emulsion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram of cutaneous emulsion contains 1 mg mometasone
Psoriasis in Jordan: a single center experience
 Psoriasis in Jordan: a single center experience Shefaa Almashagbeh MD *, Deifallah Alsharari MD *, Hayat Khasawneh MD *, Diana Aljammal * MD, Hamzeh Al-housamieh MD* ABSTRACT Objectives: To evaluate the
Psoriasis in Jordan: a single center experience Shefaa Almashagbeh MD *, Deifallah Alsharari MD *, Hayat Khasawneh MD *, Diana Aljammal * MD, Hamzeh Al-housamieh MD* ABSTRACT Objectives: To evaluate the
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
Psoriasis is an inflammatory condition of the skin that
 Mayo Clin Proc, September 2001, Vol 76 Topical Therapies for Localized Psoriasis 943 Concise Review for Clinicians Concise Review for Clinicians Topical Therapies for Localized Psoriasis PATRICIA M. WITMAN,
Mayo Clin Proc, September 2001, Vol 76 Topical Therapies for Localized Psoriasis 943 Concise Review for Clinicians Concise Review for Clinicians Topical Therapies for Localized Psoriasis PATRICIA M. WITMAN,
Package leaflet: Information for the user. Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf calcipotriol/betamethasone
 Package leaflet: Information for the user Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf calcipotriol/betamethasone Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user Calcipotriol/Betamethason Sandoz 50 microgram/g + 0,5 mg/g, zalf calcipotriol/betamethasone Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user. Monovo 1 mg/g cutaneous emulsion. mometasone furoate
 Package leaflet: Information for the user Monovo 1 mg/g cutaneous emulsion mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Monovo 1 mg/g cutaneous emulsion mometasone furoate Read all of this leaflet carefully before you start using this medicine because it contains important information
This questionnaire was used both during the face-to-face interviews with the
 Additional file 1: Primary research questionnaire This questionnaire was used both during the face-to-face interviews with the dermatologists and during the expert panel 1. During the last month, how many
Additional file 1: Primary research questionnaire This questionnaire was used both during the face-to-face interviews with the dermatologists and during the expert panel 1. During the last month, how many
Psoriasis. and Biologic Treatments. Choose the best treatment to regain your quality of life.
 Psoriasis and Biologic Treatments Choose the best treatment to regain your quality of life. PSORIASIS, What is this disease? Psoriasis is a common disease, that affects up to 4% of the population. It usually
Psoriasis and Biologic Treatments Choose the best treatment to regain your quality of life. PSORIASIS, What is this disease? Psoriasis is a common disease, that affects up to 4% of the population. It usually
Psoriasis. and Biologic Treatments. Choose the best treatment to regain your quality of life. Edition 4
 Psoriasis and Biologic Treatments Choose the best treatment to regain your quality of life. Edition 4 PSORIASIS, What is this disease? Psoriasis is a common disease, that affects up to 4% of the population.
Psoriasis and Biologic Treatments Choose the best treatment to regain your quality of life. Edition 4 PSORIASIS, What is this disease? Psoriasis is a common disease, that affects up to 4% of the population.
EXAMINING THE CRUCIAL COALITION BETWEEN DERMATOLOGY AND RHEUMATOLOGY IN PSORIATIC ARTHRITIS
 EXAMINING THE CRUCIAL COALITION BETWEEN DERMATOLOGY AND RHEUMATOLOGY IN PSORIATIC ARTHRITIS ACTIVITY 1: EARLY COLLABORATION IN THE TREATMENT OF PSA Key Slides COMMON COMORBIDITIES OF PSORIATIC DISEASE
EXAMINING THE CRUCIAL COALITION BETWEEN DERMATOLOGY AND RHEUMATOLOGY IN PSORIATIC ARTHRITIS ACTIVITY 1: EARLY COLLABORATION IN THE TREATMENT OF PSA Key Slides COMMON COMORBIDITIES OF PSORIATIC DISEASE
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ustekinumab_stelara 02/2010 10/2018 10/2019 10/2018 Description of Procedure or Service Plaque psoriasis
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ustekinumab_stelara 02/2010 10/2018 10/2019 10/2018 Description of Procedure or Service Plaque psoriasis
GROUP 15 TOPICAL PREPARATIONS
 - 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
- 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
What you need to know about your child s PSORIASIS. Psoriasis
 What you need to know about your child s PSORIASIS Ps Psoriasis The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health
What you need to know about your child s PSORIASIS Ps Psoriasis The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Scalp Topical Suspension safely and effectively. See full prescribing information for Taclonex
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Scalp Topical Suspension safely and effectively. See full prescribing information for Taclonex
EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE SHAMPOO IN THERAPY OF PSORIASIS OF THE SCALP
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Platonova et al. SJIF Impact Factor 2.786 Volume 4, Issue 04, 238-246. Research Article ISSN 2278 4357 EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Platonova et al. SJIF Impact Factor 2.786 Volume 4, Issue 04, 238-246. Research Article ISSN 2278 4357 EFFICACY AND SAFETY OF CLOBETASOL PROPIONATE
Psoriasis, Incidence, Quality of Life, Psoriatic Arthritis, Prevalence
 1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
Elements of Successful PBS Applications. Barbara Radulski RN. Copyright
 Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Elements of Successful PBS Applications Barbara Radulski RN PBS Requirements April 1 2006 THE RULES PBS Requirements 18 years and over Psoriasis x 6 months Failed to achieve an adequate response to 3 systemic
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis
 Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
DATA SHEET. Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
 DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
Package leaflet: Information for the patient. Mometasone furoate 0.1%w/w Ointment (mometasone furoate)
 Package leaflet: Information for the patient Mometasone furoate 0.1%w/w Ointment (mometasone furoate) Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Mometasone furoate 0.1%w/w Ointment (mometasone furoate) Read all of this leaflet carefully before you start using this medicine because it contains important
