Importance of Recognizing and Preventing Blindness From Juvenile Idiopathic Arthritis Associated Uveitis
|
|
|
- Karen Watson
- 5 years ago
- Views:
Transcription
1 Arthritis Care & Research Vol. 64, No. 5, May 2012, pp DOI /acr , American College of Rheumatology REVIEW ARTICLE Importance of Recognizing and Preventing Blindness From Juvenile Idiopathic Arthritis Associated Uveitis STEPHEN D. ANESI AND C. STEPHEN FOSTER The association between rheumatologic and ocular inflammatory disease (OID) is well recognized. Uveitis is one of the leading causes of preventable blindness worldwide (1,2), accounting for 10 15% of causes in the US. One of the most ocularly pernicious forms of uveitis, first described in 1910 (3), is associated with juvenile idiopathic arthritis (JIA), which was previously known as juvenile rheumatoid arthritis or juvenile chronic arthritis. Children younger than 16 years of age represent 5 10% of the population with uveitis (4), and JIA is associated with many of these cases (5,6). Complications from JIA-associated uveitis are well known, especially considering the disease is often occult and not realized by the physician or even the patient until chronic changes and permanent vision loss have occurred. The most recent recommendations for uveitis monitoring in patients with JIA were made in 2006 (7) by the American Academy of Pediatrics in conjunction with a panel of rheumatologists and ophthalmologists. In 2007, the German Uveitis in Childhood Study Group published suggested modifications to these guidelines (8), including a reference to the modified JIA classification by the International League of Associations for Rheumatology (ILAR) in 1995 (9). Screening for uveitis is an underappreciated necessity, as shown by the high percentage of children with complications at initial presentation to a uveitis specialist. One or more complications have been present in 34 67% of children with JIA-associated uveitis (10,11) and have been as prevalent as 86.3% only 3 years after initial presentation. Shockingly, 47% of these patients upon initial presentation were already legally blind (20/ 200 or worse) in at least one eye (10) as a consequence of complications that were likely preventable had the patient been under proper ophthalmologic care. Stephen D. Anesi, MD, C. Stephen Foster, MD, FACS, FACR: Massachusetts Eye Research and Surgery Institution, Cambridge. Address correspondence to C. Stephen Foster, MD, FACS, FACR, 5 Cambridge Center, 8th Floor, Cambridge, MA fosters@uveitis.org. Submitted for publication June 22, 2011; accepted in revised form January 3, Furthermore, complications during long-term followup are profoundly underappreciated, especially by the rheumatologic community. Studies in rheumatologic peerreviewed publications support this, based on observations of patients still under the care of rheumatologists (12,13) but devoid of data from patients who no longer need ongoing rheumatologic care that have progression of eye damage from inadequately treated chronic uveitis. Longterm followup studies of 10 years or less have claimed good outcomes of corrected visual acuity of 20/40 or better ( %) despite high rates of complications ( %) (13,14). A review in the ophthalmologic literature of 43 patients with JIA-associated uveitis with a mean overall duration of uveitis of 12.2 years showed that visual improvement occurred in 70% of patients with therapy, although prognosis was guarded due in part to the large number of patients needing surgery to achieve this (5). Much poorer outcomes are found in studies following patients up to 25 years (15,16). A review of 18 adult patients with JIA-associated uveitis for a mean duration of 20.5 years showed that 100% of eyes had at least one ocular complication (17). There is an obvious need for greater recognition and proper management of JIAassociated uveitis since it too often presents a life-long burden of blindness for children moving into adulthood. JIA has multiple well-documented subsets, including the systemic, polyarticular, and oligoarticular (pauciarticular) forms, with the latter recognized as the most common associated with uveitis (18,19). Established screening guidelines categorize high-risk patients as having oligoarticular, seronegative polyarticular, psoriatic, or other arthritis onset prior to age 7 years with positive antinuclear antibody (ANA) testing and a disease duration under 5 years; it is recommended that these patients be screened at 3-month intervals in the absence of previously diagnosed uveitis (10). A meta-analysis of case series of patients with JIA between 1980 and 2004 (20) found ANAnegative oligoarticular patients have the same risk of developing uveitis as ANA-positive polyarticular patients, and the authors recommend consistency between screening times for these groups (currently 6 months and 3 months, respectively). Rheumatoid factor negativity is typ- 653
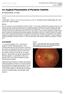
2 654 Anesi and Foster Significance & Innovations Juvenile idiopathic arthritis (JIA) associated uveitis is an ocularly pernicious inflammatory disease with underappreciated vision outcomes in longterm studies. Complications result from chronic inflammation and long-term corticosteroid therapy. A stepladder approach using steroid-sparing therapy should be employed early to induce remission from inflammation. Increased efforts in screening, research, and education are necessary to help find and treat children with JIA-associated uveitis. Figure 1. Band keratopathy, posterior synechiae, and severe cataract in a quiet eye of a patient with juvenile idiopathic arthritis associated uveitis (photo courtesy of C. Stephen Foster). ical (21) and antihistone antibodies are receiving more attention as possible biomarkers for disease chronicity and severity risk (22). Screening for psoriatic arthritis, recognized as a subset of JIA by ILAR in 1995 (23), must also be considered. The annual incidence of all childhood uveitis is estimated to be per 100,000 in North America and 30 per 100,000 in Europe (24 26). JIA reportedly has been associated with anywhere from % of cases, with most reports ranging between 10% and 35% (6). Arthritis is often diagnosed prior to the development of eye findings, but a population of children exists in whom ocular inflammation is present prior to joint disease. Studies have shown a marked disparity between joint inflammation and ocular inflammation. One study of 35 children with JIA-associated uveitis in 1986 showed that uveitis followed the onset of arthritis in 60% of the cases by 1 6 years (mean SD years); however, 31.4% of the cases had uveitis noted at the onset of arthritis and 8.6% showed evidence of chronic ocular inflammation (i.e., band keratopathy, synechiae, cataracts) at initial arthritis onset (19). Additional support of the disparity between joint inflammation and ocular inflammation is shown by the differential response to various treatments, some of which have been shown to reduce inflammation in only one of the two sites. Etanercept, an anti tumor necrosis factor antibody biologic response-modifying agent (BRM), has been shown to lack therapeutic efficacy for uveitis despite its ability to control arthritis (27,28). Conversely, daclizumab, an anti interleukin-2 receptor (anti-cd25) antibody BRM, was shown to be effective in treating ocular inflammation; however, one patient was removed from the study due to uncontrolled joint and systemic symptoms requiring alternate therapy (29). Clinically, JIA is usually associated with anterior uveitis, or inflammation (cells and flare) mainly localized to the anterior chamber. It is typically a nongranulomatous chronic inflammation, often bilateral, that remains asymptomatic in many patients. JIA may manifest before or after the onset of articular involvement, sometimes leading to chronic inflammatory changes in the eye (30). An important concept to consider in ocular inflammation is the fragility of the eye relative to much of the rest of the human anatomy. There are several structures within the eye that do not retain function after, or do not have the ability to recover from, chronic inflammation, edema, or fibrosis; therefore, any amount of inflammation in the eye is a potential permanent threat to vision. One study at a uveitis tertiary care center over 21 years examined complications at initial presentation to the ophthalmologist, which included band keratopathy (31.5%), posterior synechiae (27.5%), cataracts (22.5%), glaucoma (15.3%), ocular hypotony (9.3%), and macular edema (3.0%) (31) (Figure 1). In the same study, 23.7% of these patients had 20/200 or worse vision in the better-seeing eye. These data are stunning, and it is our strong impression that they are unknown to most rheumatologists. Also problematic is that these data are at times refuted by other studies, as in the aforementioned meta-analysis reporting that adverse visual outcomes (acuity worse than 20/40) were less than previously reported (20). These claims are misleading, however, since this study did not account for visual acuity between the patients individual eyes, which is important considering the tremendous advantage of proper stereoscopic vision, which requires good acuity in both eyes; several of the reviewed series were reported by nonophthalmologists. These studies also underrepresent the population with uveitis, again considering patients with JIA-associated uveitis who have little or no rheumatologic followup due to quiescence of joint inflammation but have active ocular disease. Complications are often found in patients with JIAassociated uveitis, especially in those not receiving timely and effective treatment. Proper management of all ocular inflammation is critical to good long-term prognosis, and should include swift referral to a specialist who is comfortable dealing with OID. Another important concept is avoidance of long-term corticosteroid therapy, whether topical, systemic, or locally injected, which invariably leads to complications. Many patients require long-term systemic therapy with nonsteroidal antiinflammatory drugs (NSAIDs) or immunomodulatory therapy (IMT) to control stubborn vision-threatening inflammation refrac-
3 Preventing Blindness From Uveitis in JIA 655 tory to conservative measures. One study from a tertiary care center examining 89 children with JIA-associated uveitis over 18 years showed that 73% of these children were receiving IMT and 40% were treated with IMT and NSAIDs (6). A stepladder approach can be used when deciding upon systemic medication for ocular inflammation, requiring regular examinations and monitoring for drug toxicity (32). Many obstacles are encountered by ophthalmologists managing JIA-associated uveitis in children. They are often forced to manage the large amount of complications these patients have prior to initial presentation. As stated earlier, ocular inflammation occasionally presents with or prior to joint involvement, typically without any warning signs or symptoms noticeable to the child. Many children are too young or naive to notice or mention problems with their vision, or they fear the social stigma of possibly needing glasses. These children are poor advocates for themselves in an incredibly serious matter that they, depending on their age or maturity, often cannot be expected to comprehend. Examining these children may also be difficult, and subtle inflammation may be missed by an untrained or unsuspecting examiner. Sadly, specialists must also shoulder the burden of complications arising because of all the providers who were both inexperienced in properly managing OID and too stubborn to reach out to and comanage with those more knowledgeable; after all, the degree of inflammation may not seem that bad and topical corticosteroid therapy should certainly be able to take care of that. While corticosteroids are sometimes necessary for acute inflammation, long-term treatment must absolutely not be relied on since corticosteroids often are not enough to control the disease and inevitably lead to cataract formation and possibly glaucoma. Parents often advocate for referral themselves after their child has been subjected to repeated problems. Childhood uveitis is an aggressive disease that must be treated aggressively. Proper management includes knowledge of what therapy to use and when to use it, while moving along to something else if one therapy proves to be ineffective. When NSAIDs are ineffective in preventing relapse of uveitis after the child is completely off all corticosteroid therapy, or when inflammation is overtly severe, antimetabolites (i.e., methotrexate, azathioprine, mycophenolate mofetil), calcineurin inhibitors (modified cyclosporin) in conjunction with antimetabolites, and BRMs (i.e., infliximab, adalimumab, rituximab, abatacept) have been used off label to great effect in treating chronic inflammation from JIA-associated uveitis (33 40). Over the past 2 decades, BRMs have garnered greater attention in both the rheumatologic and ophthalmologic communities, given how successful these medications have become in treating some inflammation refractive to other known therapies. Alkylating agents are generally avoided but may be appropriately used in extreme cases. Similar to recent rheumatologic standards, the mission must be remission, treating to a target of no active inflammation with complete stoppage of corticosteroid treatment. Different patients respond to different therapies differently, and we have no way to predict which children will be among the 70% who respond to methotrexate monotherapy and which may require intravenous immunoglobulin in combination with rituximab. When administered under close supervision, medications should provide an escape from the life-long rescue with topical and systemic corticosteroid therapy, and many times in the experience of these authors, the medications can be stopped indefinitely after approximately 2 years of immunomodulation without recurrence of further disease activity. The notion that these medications are too risky is profoundly inaccurate, shown by the abundance of reports and retrospective series in peer-reviewed publications supporting the use of IMT in treating JIA-associated uveitis; prospective trials are, few as they may be, difficult to justify and carry out in this population. What else can be done to lessen the amount of poor outcomes seen so frequently in this disease? Since relying on presentation of arthritis is not always effective in catching the early development of ocular inflammation, we advocate that more assertive action be taken. This can only be possible through wider and earlier screening in preschools and day care programs, and may help lower both the percentage of complications seen at initial presentation to specialists and the number of poor visual outcomes of these children in general. Additionally, children with JIA-associated uveitis need proper management of their disease, which includes avoiding long-term corticosteroid monotherapy and moving to IMT when necessary. Providers who feel uncomfortable managing these patients should advocate for the help of others, preferably fellowship-trained uveitis specialists. This would also include providers who are unable to bear the burden of costly off-label medications and the strenuous process of obtaining prior authorization coverage. Herein yet another problem arises, that too few eye specialists exist who are comfortable treating these patients either medically or surgically. The number of such trained ophthalmologists is quite insufficient for the needs of the population affected. Therefore, many ophthalmologists need to partner with a rheumatologist or hematologist in a comanagement strategy that will allow the child to receive such needed care, rather than simply reverting to the expedient of continuing therapy with corticosteroids. An additional problem is that we repeatedly see instances in which such collaboration is not collegial, but rather antagonistic, with the rheumatologist or hematologist uncomfortable taking the responsibility of prescribing medications that possess risk while being dependent upon the ophthalmologist to determine and report that inflammation is sufficient enough to warrant such therapy or more therapy. Some doctors seem comfortable taking the ophthalmologist s word, while others clearly do not seem comfortable, and hence the antagonism, with the patient ultimately experiencing the consequences. Also, active communication between all providers involved is essential in the proper management of IMT, including when it is appropriate to increase or taper therapy. There is a need for increased education for everyone involved in the care of children with JIA-associated uveitis. An emphasis on the importance of recognizing and properly treating JIA-associated uveitis must be made dur-
4 656 Anesi and Foster ing the early phases of physician education. Dedicated lectures and training during medical school, residency, and fellowship programs by faculty who are either uveitis specialists or familiar with the current standard of care regarding management of JIA-associated uveitis are sorely needed; there is a gross deficiency of uveitis faculty throughout the ophthalmology training programs in the US. Rheumatology and pediatric programs alike must educate their residents and fellows in these matters to maximize the support available to these children and their families. An increase in the amount of discussion and research in professional forums is also needed to help heighten the awareness of the debilitating nature and proper management of this disease. In August 2010, an expert panel of ophthalmologists and rheumatologists in the management of JIA-associated uveitis met in Boston to help form a consensus of opinions on the treatment of all forms of uveitis in children, with special emphasis on JIAassociated uveitis. The results of this roundtable meeting and a consensus of the opinions of these experts were published as a childhood uveitis monograph (41). Efforts by organizations such as the Ocular Immunology and Uveitis Foundation (OIUF) and the Childhood Arthritis & Rheumatology Research Alliance (CARRA) to advance research in pediatric uveitis and rheumatologic disease and to provide training for individuals to apply the knowledge gained from such research have made great strides toward increasing awareness and improving outcomes of JIA and JIA-associated uveitis. Last, the families of children with JIA need to be made aware of the dangers of chronic uveitis if left untreated and the need for frequent monitoring. These families must realize that they are their child s best advocate in the fight to prevent blindness from this disease, and that they must be actively involved in their child s disease management and future. Further education of the general public to increase awareness of this disease is the ultimate goal so that children who unknowingly have ocular inflammation can possibly avoid potentially preventable life-long visual impairment. AUTHOR CONTRIBUTIONS Both authors were involved in drafting the article or revising it critically for important intellectual content, and both authors approved the final version to be published. REFERENCES 1. Vadot E, Barth E, Billet P. Epidemiology of uveitis: preliminary results of a prospective study in Savoy. In: Sarri KM, editor. Uveitis update. Amsterdam: Elsevier; p Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80: Ohm J. Bandförmige Hornhauttrübung bei einem neunjährigen Mädchen und ihre Behandlung mit subconjunctivalen Jodaliumeinspirtzungen. Klin Monatsbl Augenheilk 1910;48: Tugal-Tutkun I, Havrlikova K, Power WJ, Foster CS. Changing patterns in uveitis of childhood. Ophthalmology 1996;103: Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritisassociated uveitis. Ophthalmology 1997;104: Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology 2005;112: Cassidy J, Kivlin J, Lindsley C, Nocton J, Section on Rheumatology, Section on Ophthalmology. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 2006;117: Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 2007;46: Fink CW. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J Rheumatol 1995;22: Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology 2004;111: Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis (JIA). Am J Ophthalmol 2007;143: Marvillet I, Terrada C, Quartier P, Quoc EB, Bodaghi B, Prieur AM. Ocular threat in juvenile idiopathic arthritis. Joint Bone Spine 2009;76: Cabral DA, Petty ER, Malleson PN, Ensworth S, McCormick AQ, Shroeder ML. Visual prognosis in children with chronic anterior uveitis and arthritis. J Rheumatol 1994;21: Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, et al. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup study. Arthritis Rheum 2007;56: Skarin A, Elborgh R, Edlund E, Bengtsson-Stigmar E. Longterm follow-up of patients with uveitis associated with juvenile idiopathic arthritis: a cohort study. Ocul Immunol Inflamm 2009;17: Zak M, Fledelius H, Pedersen FK. Ocular complications and visual outcome in juvenile chronic arthritis: a 25-year follow-up study. Acta Ophthalmol Scand 2003;81: Ozdal PC, Vianna RN, Deschenes J. Visual outcome of juvenile rheumatoid arthritis-associated uveitis in adults. Ocul Immunol Inflamm 2005;13: Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology (Oxford) 2002;41: Rosenberg AM, Oen KG. The relationship between ocular and articular disease activity in children with juvenile rheumatoid arthritis and associated uveitis. Arthritis Rheum 1986;29: Carvounis PE, Herman DC, Cha S, Burke JP. Incidence and outcomes of uveitis in juvenile rheumatoid arthritis, a synthesis of the literature. Graefes Arch Clin Exp Ophthalmol 2006;244: Petty RE, Smith JR, Rosenbaum JT. Arthritis and uveitis in children: a pediatric rheumatology perspective. Am J Ophthalmol 2003;135: Nordal EB, Songstad NT, Berntson L, Moen T, Straume B, Rygg M. Biomarkers of chronic uveitis in juvenile idiopathic arthritis: predictive value of antihistone antibodies and antinuclear antibodies. J Rheumatol 2009;36: Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004;111: Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol 2003;135: Paivonsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand 2000;78:84 8.
5 Preventing Blindness From Uveitis in JIA Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003; 121: Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, doublemasked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53: Sen HN, Levy-Clarke G, Faia LJ, Li Z, Yeh S, Barron KS, et al. High-dose daclizumab for the treatment of juvenile idiopathic arthritis-associated active anterior uveitis. Am J Ophthalmol 2009;148: Sainz de la Maza M. Seronegative spondyloarthropathies. In: Foster CS, Vitale AT, editors. Diagnosis and treatment of uveitis. Philadelphia: WB Saunders; p Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol 2007; 143: Lee FF, Foster CS. Pharmacotherapy of uveitis. Expert Opin Pharmacother 2010;11: Weiss AH, Wallace CA, Sherry DD. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr 1998;133: Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001;108: Goebel JC, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol 2011;95: Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology 2008;115: Tappeiner C, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye (Lond) 2009;23: Fledelius HC, Nielsen SM, Nissen KR, Pedersen FK, Zak MS. Anti-TNF treatment of juvenile uveitis. Ugeskr Laeger 2007; 169: In Danish. 38. Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. J Pediatr 2006;149: Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-cd20 monoclonal antibody (rituximab). Rheumatology (Oxford) 2011;50: Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, et al. Abatacept for severe anti tumor necrosis factor refractory juvenile idiopathic arthritis related uveitis. Arthritis Care Res (Hoboken) 2010;62: Foster CS, Gonzalez-Gonzalez LA, Anesi SD, Palafox SK, editors. Childhood uveitis. Manchester Center (VT): Shires Press; 2011.
Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
 European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
Course, complications, and outcome of juvenile arthritis related uveitis
 Major Articles Course, complications, and outcome of juvenile arthritis related uveitis Kourosh Sabri, MB, ChB, FRCOphth, a Rotraud K. Saurenmann, MD, b,a Earl D. Silverman, MD, FRCPC, b,c and Alex V.
Major Articles Course, complications, and outcome of juvenile arthritis related uveitis Kourosh Sabri, MB, ChB, FRCOphth, a Rotraud K. Saurenmann, MD, b,a Earl D. Silverman, MD, FRCPC, b,c and Alex V.
Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis
 Ophthalmology, Article ID 461078, 7 pages http://dx.doi.org/10.1155/2014/461078 Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis Ahmadreza Moradi, 1 Rowayda M. Amin,
Ophthalmology, Article ID 461078, 7 pages http://dx.doi.org/10.1155/2014/461078 Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis Ahmadreza Moradi, 1 Rowayda M. Amin,
Prognostic value of antinuclear antibodies in juvenile idiopathic arthritis and anterior uveitis. Results from a systematic literature review
 ARTIGO DE REvIsÃO Prognostic value of antinuclear antibodies in juvenile idiopathic arthritis and anterior uveitis. Results from a systematic literature review Campanilho-Marques R 1, Bogas M 2, Ramos
ARTIGO DE REvIsÃO Prognostic value of antinuclear antibodies in juvenile idiopathic arthritis and anterior uveitis. Results from a systematic literature review Campanilho-Marques R 1, Bogas M 2, Ramos
Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
 Rheumatology 08;47:10 14 Advance Access publication 1 August 08 doi:10.1093/rheumatology/ken298 Concise Report Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
Rheumatology 08;47:10 14 Advance Access publication 1 August 08 doi:10.1093/rheumatology/ken298 Concise Report Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
Paediatric rheumatology. Epidemiology of uveitis in children over a 10-year period
 Paediatric rheumatology Epidemiology of uveitis in children over a 10-year period L.A.L. Clarke 1, Y. Guex-Crosier 2, M. Hofer 1 1 Immunoallergology and Rheumatology Unit, Department of Paediatrics (DMCP),
Paediatric rheumatology Epidemiology of uveitis in children over a 10-year period L.A.L. Clarke 1, Y. Guex-Crosier 2, M. Hofer 1 1 Immunoallergology and Rheumatology Unit, Department of Paediatrics (DMCP),
Tumour necrosis factor a inhibitors in the treatment of childhood uveitis
 Rheumatology 2006;45:982 989 Advance Access publication 3 February 2006 Tumour necrosis factor a inhibitors in the treatment of childhood uveitis R. K. Saurenmann, A. V. Levin 1, J. B. Rose, S. Parker,
Rheumatology 2006;45:982 989 Advance Access publication 3 February 2006 Tumour necrosis factor a inhibitors in the treatment of childhood uveitis R. K. Saurenmann, A. V. Levin 1, J. B. Rose, S. Parker,
Nausheen Khuddus, MD Melissa Elder, MD, PhD
 Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
iologic Agents in the Treatment of Non-Infectious Uveitis
 What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
Blindsided by Arthritis
 Blindsided by Arthritis Melissa A. Lerman, MD, PhD, MSCE Division of Rheumatology The Children s Hospital of Philadelphia The Perelman SOM at the University of PA No financial disclosures. 1 Case 1 2.5
Blindsided by Arthritis Melissa A. Lerman, MD, PhD, MSCE Division of Rheumatology The Children s Hospital of Philadelphia The Perelman SOM at the University of PA No financial disclosures. 1 Case 1 2.5
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy
 Cann et al. Pediatric Rheumatology (2018) 16:51 https://doi.org/10.1186/s12969-018-0266-5 RESEARCH ARTICLE Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy Open Access Megan
Cann et al. Pediatric Rheumatology (2018) 16:51 https://doi.org/10.1186/s12969-018-0266-5 RESEARCH ARTICLE Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy Open Access Megan
Approach to Pediatric Uveitis. Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service
 Approach to Pediatric Uveitis Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service Epidemiology Uveitis is the 3 rd leading cause of blindness in USA 5-10% of uveitis cases involve children
Approach to Pediatric Uveitis Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service Epidemiology Uveitis is the 3 rd leading cause of blindness in USA 5-10% of uveitis cases involve children
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl
 Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
Uveitis in childhood is a potentially blinding condition with
 Immunology and Microbiology Infiltration of Plasma Cells in the Iris of Children With ANA-Positive Anterior Uveitis Viera Kalinina Ayuso, 1 Marijke R. van Dijk, 2 and Joke H. de Boer 1 1 Department of
Immunology and Microbiology Infiltration of Plasma Cells in the Iris of Children With ANA-Positive Anterior Uveitis Viera Kalinina Ayuso, 1 Marijke R. van Dijk, 2 and Joke H. de Boer 1 1 Department of
The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis
 Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Effective Health Care Program
 Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of an inception cohort of Australian children
 Tiller et al. Pediatric Rheumatology (2018) 16:69 https://doi.org/10.1186/s12969-018-0288-z RESEARCH ARTICLE Open Access Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of
Tiller et al. Pediatric Rheumatology (2018) 16:69 https://doi.org/10.1186/s12969-018-0288-z RESEARCH ARTICLE Open Access Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of
Outcome in Juvenile Rheumatoid Arthritis in India
 Outcome in Juvenile Rheumatoid Arthritis in India Amita Aggarwal, Vikas Agarwal, Debasish Danda and Ramnath Misra From the Department of Immunology, Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Outcome in Juvenile Rheumatoid Arthritis in India Amita Aggarwal, Vikas Agarwal, Debasish Danda and Ramnath Misra From the Department of Immunology, Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Uveitis unplugged: systemic therapy
 Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
University of Bristol - Explore Bristol Research
 Hawkins, M. J., Dick, A. D., Lee, R. W. J., Ramanan, A. V., Carreño, E., Guly, C., & Ross, A. H. (2016). Managing juvenile idiopathic arthritisassociated uveitis. Survey of Ophthalmology, 61(2), 197-210.
Hawkins, M. J., Dick, A. D., Lee, R. W. J., Ramanan, A. V., Carreño, E., Guly, C., & Ross, A. H. (2016). Managing juvenile idiopathic arthritisassociated uveitis. Survey of Ophthalmology, 61(2), 197-210.
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES HUMIRA PEDIATRIC
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Positions. Training. Education & Research
 Stephen Damien Anesi, MD 42 Pope Hill Road Milton Massachusetts 02186 home (617) 322-1835 cell (310) 709-6600 office (781) 891-6377 fax (781) 647-1430 sanesi@mersi.com stephenanesi@yahoo.com Positions
Stephen Damien Anesi, MD 42 Pope Hill Road Milton Massachusetts 02186 home (617) 322-1835 cell (310) 709-6600 office (781) 891-6377 fax (781) 647-1430 sanesi@mersi.com stephenanesi@yahoo.com Positions
E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G.
 E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G. Chasnyk St. Petersburg State Pediatric Medical University, St.
E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G. Chasnyk St. Petersburg State Pediatric Medical University, St.
Clinical Course of Uveitis in Children in a Tertiary Ophthalmology Center in Northwest Iran
 http://www.cjmb.org Open Access Original Article Crescent Journal of Medical and Biological Sciences Vol. 4, No. 4, October 2017, 200 204 eissn 2148-9696 Clinical Course of Uveitis in Children in a Tertiary
http://www.cjmb.org Open Access Original Article Crescent Journal of Medical and Biological Sciences Vol. 4, No. 4, October 2017, 200 204 eissn 2148-9696 Clinical Course of Uveitis in Children in a Tertiary
Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis
 Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis Reference: D12X02 NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients
Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis Reference: D12X02 NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
CLINICAL SCIENCES. Antineutrophil Cytoplasmic Antibody Associated Active Scleritis
 CLINICAL SCIENCES Antineutrophil Cytoplasmic Antibody Associated Active Scleritis Lani T. Hoang, MD; Lyndell L. Lim, MBBS, FRANZCO; Brian Vaillant, MD; Dongseok Choi, PhD; James T. Rosenbaum, MD Objective:
CLINICAL SCIENCES Antineutrophil Cytoplasmic Antibody Associated Active Scleritis Lani T. Hoang, MD; Lyndell L. Lim, MBBS, FRANZCO; Brian Vaillant, MD; Dongseok Choi, PhD; James T. Rosenbaum, MD Objective:
The Hospital for Sick Children Technology Assessment at SickKids (TASK)
 The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
Stephen Damien Anesi, MD office (781) fax (781)
 Stephen Damien Anesi, MD office (781) 891-6377 fax (781) 647-1430 sanesi@mersi.com Positions 2016-present Partner 2011-present Sub-Investigator Ocular Immunology and Uveitis Foundation, Waltham, MA 2011-2016
Stephen Damien Anesi, MD office (781) 891-6377 fax (781) 647-1430 sanesi@mersi.com Positions 2016-present Partner 2011-present Sub-Investigator Ocular Immunology and Uveitis Foundation, Waltham, MA 2011-2016
Clinical features of children with juvenile idiopathic arthritis using the ILAR classification criteria: A community-based cohort study in Taiwan
 Journal of Microbiology, Immunology and Infection (2013) 46, 288e294 Available online at www.sciencedirect.com journal homepage: www.e-jmii.com ORIGINAL ARTICLE Clinical features of children with juvenile
Journal of Microbiology, Immunology and Infection (2013) 46, 288e294 Available online at www.sciencedirect.com journal homepage: www.e-jmii.com ORIGINAL ARTICLE Clinical features of children with juvenile
Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior Uveitis in a Korean Population
 J Korean Med Sci 2009; 24: 722-8 ISSN 1011-8934 DOI: 10.3346/jkms.2009.24.4.722 Copyright The Korean Academy of Medical Sciences Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior
J Korean Med Sci 2009; 24: 722-8 ISSN 1011-8934 DOI: 10.3346/jkms.2009.24.4.722 Copyright The Korean Academy of Medical Sciences Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior
Clinical and Biochemical Characteristics of Children with Juvenile Idiopathic Arthritis
 ORIGINAL ARTICLE Clinical and Biochemical Characteristics of Children with Juvenile Idiopathic Arthritis Shakeel Ahmed 1, Syed Rehan Ali 1, Sidra Ishaque 1 and Nabil Sami 2 ABSTRACT Objective: To determine
ORIGINAL ARTICLE Clinical and Biochemical Characteristics of Children with Juvenile Idiopathic Arthritis Shakeel Ahmed 1, Syed Rehan Ali 1, Sidra Ishaque 1 and Nabil Sami 2 ABSTRACT Objective: To determine
Juvenile idiopathic arthritis-associated uveitis
 Clarke et al. Pediatric Rheumatology (2016) 14:27 DOI 10.1186/s12969-016-0088-2 REVIEW Open Access Juvenile idiopathic arthritis-associated uveitis Sarah L. N. Clarke 1,2, Ethan S. Sen 1,2* and Athimalaipet
Clarke et al. Pediatric Rheumatology (2016) 14:27 DOI 10.1186/s12969-016-0088-2 REVIEW Open Access Juvenile idiopathic arthritis-associated uveitis Sarah L. N. Clarke 1,2, Ethan S. Sen 1,2* and Athimalaipet
Infliximab for the treatment of refractory scleritis
 1 Massachussets Eye Research and Surgery Institution, Cambridge, Massachusetts, USA 2 BayView Clinic, Mumbai, India 3 Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, USA 4 Brigham
1 Massachussets Eye Research and Surgery Institution, Cambridge, Massachusetts, USA 2 BayView Clinic, Mumbai, India 3 Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, USA 4 Brigham
Clinical Study Usefulness of Adalimumab in the Treatment of Refractory Uveitis Associated with Juvenile Idiopathic Arthritis
 Mediators of Inflammation Volume 2013, Article ID 560632, 6 pages http://dx.doi.org/10.1155/2013/560632 Clinical Study Usefulness of Adalimumab in the Treatment of Refractory Uveitis Associated with Juvenile
Mediators of Inflammation Volume 2013, Article ID 560632, 6 pages http://dx.doi.org/10.1155/2013/560632 Clinical Study Usefulness of Adalimumab in the Treatment of Refractory Uveitis Associated with Juvenile
Amjevita (adalimumab-atto)
 *- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
*- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
Horizon Scanning Technology Summary. Abatacept (Orencia) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Review Golimumab in refractory uveitis associated to juvenile idiopathic arthritis: multicentre study of 7 cases and literature review
 Review Golimumab in refractory uveitis associated to juvenile idiopathic arthritis: multicentre study of 7 cases and literature review N. Palmou-Fontana 1, V. Calvo-Río 1, J.L. Martín-Varillas 1, C. Fernández-Díaz
Review Golimumab in refractory uveitis associated to juvenile idiopathic arthritis: multicentre study of 7 cases and literature review N. Palmou-Fontana 1, V. Calvo-Río 1, J.L. Martín-Varillas 1, C. Fernández-Díaz
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
New Drugs for Uveitis. Medical Eye Unit St Thomas Hospital
 New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
TRANSPARENCY COMMITTEE. Opinion. 29 November 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 November 2006 HEXATRIONE 2% suspension for injection (intra-articular) Box containing one 2-ml vial - CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 November 2006 HEXATRIONE 2% suspension for injection (intra-articular) Box containing one 2-ml vial - CIP code:
and eyes and have also looked at histocompatibility and 1980 were identified as having had either rate (ESR) and all ANA results were noted. ANA.
 Archives of Disease in Childhood, 1986, 61, 168-172 Antinuclear antibody studies in juvenile chronic arthritis A M LEAK, B M ANSELL, AND S J BURMAN Division of Rheumatology, Canadian Red Cross Memorial
Archives of Disease in Childhood, 1986, 61, 168-172 Antinuclear antibody studies in juvenile chronic arthritis A M LEAK, B M ANSELL, AND S J BURMAN Division of Rheumatology, Canadian Red Cross Memorial
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Regional vs. Systemic Therapy. Corticosteroids. Regional vs. Systemic Therapy for Uveitis. Considerations
 Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis
 Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis Reference: NHS England D12/P/b NHS England INFORMATION READER
Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis Reference: NHS England D12/P/b NHS England INFORMATION READER
Title: Predictive factors of relapse, in patients with JIA in remission, after discontinuation of synthetic disease-modifying antirheumatic drugs.
 Title: Predictive factors of relapse, in patients with JIA in remission, after discontinuation of synthetic disease-modifying antirheumatic drugs. Background Juvenile idiopathic arthritis (JIA) is not
Title: Predictive factors of relapse, in patients with JIA in remission, after discontinuation of synthetic disease-modifying antirheumatic drugs. Background Juvenile idiopathic arthritis (JIA) is not
BIOLOGIC THERAPY : A NEW OPTION FOR TREATMENT JUVENILE IDIOPATHIC ARTHRITIS DR TON THAT HOANG
 BIOLOGIC THERAPY : A NEW OPTION FOR TREATMENT JUVENILE IDIOPATHIC ARTHRITIS DR TON THAT HOANG INTRODUCTION JIA is the most common chronic rheumatic inflammatory disease of childhood. If not successfully
BIOLOGIC THERAPY : A NEW OPTION FOR TREATMENT JUVENILE IDIOPATHIC ARTHRITIS DR TON THAT HOANG INTRODUCTION JIA is the most common chronic rheumatic inflammatory disease of childhood. If not successfully
Combined Infliximab and Rituximab in Necrotising Scleritis
 This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology
 Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology Paediatric Rheumatology Expert Meeting, London 4 th December 29 Dr. Richard Veselý, Dr. Emma Sala Soriano Paediatric Investigation
Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology Paediatric Rheumatology Expert Meeting, London 4 th December 29 Dr. Richard Veselý, Dr. Emma Sala Soriano Paediatric Investigation
Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry
 Henderson et al. Pediatric Rheumatology (2016) 14:9 DOI 10.1186/s12969-016-0069-5 RESEARCH ARTICLE Open Access Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology
Henderson et al. Pediatric Rheumatology (2016) 14:9 DOI 10.1186/s12969-016-0069-5 RESEARCH ARTICLE Open Access Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis. Reference: NHS England: /PS
 Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]
![Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]](/thumbs/82/86218480.jpg) Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Update on Enthesitis-Related Arthritis, a Subtype of Juvenile Idiopathic Arthritis
 Hong Kong Bull Rheum Dis 2010;10:15-19 Review Article Update on Enthesitis-Related Arthritis, a Subtype of Juvenile Idiopathic Arthritis Tsz-Leung Lee Abstract: Keywords: Enthesitis related arthritis (ERA)
Hong Kong Bull Rheum Dis 2010;10:15-19 Review Article Update on Enthesitis-Related Arthritis, a Subtype of Juvenile Idiopathic Arthritis Tsz-Leung Lee Abstract: Keywords: Enthesitis related arthritis (ERA)
Juvenile Psoriatic Arthritis (JPsA): juvenile arthritis with psoriasis?
 Butbul Aviel et al. Pediatric Rheumatology 2013, 11:11 RESEARCH Open Access Juvenile Psoriatic Arthritis (JPsA): juvenile arthritis with psoriasis? Yonatan Butbul Aviel 1, Pascal Tyrrell 1, Rayfel Schneider
Butbul Aviel et al. Pediatric Rheumatology 2013, 11:11 RESEARCH Open Access Juvenile Psoriatic Arthritis (JPsA): juvenile arthritis with psoriasis? Yonatan Butbul Aviel 1, Pascal Tyrrell 1, Rayfel Schneider
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373
 Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Case Report Psoriatic Juvenile Idiopathic Arthritis Associated with Uveitis: A Case Report
 Case Reports in Rheumatology Volume 2013, Article ID 595890, 4 pages http://dx.doi.org/10.1155/2013/595890 Case Report Psoriatic Juvenile Idiopathic Arthritis Associated with Uveitis: A Case Report Davide
Case Reports in Rheumatology Volume 2013, Article ID 595890, 4 pages http://dx.doi.org/10.1155/2013/595890 Case Report Psoriatic Juvenile Idiopathic Arthritis Associated with Uveitis: A Case Report Davide
Efficacy of adalimumab in young children with juvenile idiopathic arthritis and chronic uveitis: a case series
 La Torre et al. BMC Research Notes 2014, 7:316 CASE REPORT Open Access Efficacy of adalimumab in young children with juvenile idiopathic arthritis and chronic uveitis: a case series Francesco La Torre
La Torre et al. BMC Research Notes 2014, 7:316 CASE REPORT Open Access Efficacy of adalimumab in young children with juvenile idiopathic arthritis and chronic uveitis: a case series Francesco La Torre
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
ISPUB.COM. An Atypical Presentation of Posterior Scleritis. A Ramanathan, A Gaur CASE REPORT
 ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
Review Article Gender and Spondyloarthropathy-Associated Uveitis
 Ophthalmology Volume 2013, Article ID 928264, 6 pages http://dx.doi.org/10.1155/2013/928264 Review Article Gender and Spondyloarthropathy-Associated Uveitis Wendy M. Smith MayoClinic,200FirstStreetSW,Rochester,MN55905,USA
Ophthalmology Volume 2013, Article ID 928264, 6 pages http://dx.doi.org/10.1155/2013/928264 Review Article Gender and Spondyloarthropathy-Associated Uveitis Wendy M. Smith MayoClinic,200FirstStreetSW,Rochester,MN55905,USA
University of Bristol - Explore Bristol Research. Peer reviewed version. Link to published version (if available): / X.
 Sen, E. S., & Ramanan, A. V. (2016). Biologic drugs in pediatric rheumatology. International Journal of Rheumatic Diseases, 19(6), 533-535. https://doi.org/10.1111/1756-185x.12924 Peer reviewed version
Sen, E. S., & Ramanan, A. V. (2016). Biologic drugs in pediatric rheumatology. International Journal of Rheumatic Diseases, 19(6), 533-535. https://doi.org/10.1111/1756-185x.12924 Peer reviewed version
National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD
 xxxxx xxxxxx xxxxxx xxxxxxx - xxxxxxxxx x National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD Dear xxxxx, 1st September 2011 Comments on the August
xxxxx xxxxxx xxxxxx xxxxxxx - xxxxxxxxx x National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD Dear xxxxx, 1st September 2011 Comments on the August
Orencia (abatacept) DRUG.00040
 Market DC Orencia (abatacept) DRUG.00040 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Comments Quantity Limit Orencia (abatacept) - AGP, VA MCD only 4 vials per 28
Market DC Orencia (abatacept) DRUG.00040 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Comments Quantity Limit Orencia (abatacept) - AGP, VA MCD only 4 vials per 28
COVER FOCUS AT A GLANCE. BY LISA J. FAIA, MD, and KIMBERLY A. DRENSER, MD, PhD
 PEDIATRIC UVEITIS: CHALLENGING FOR OPHTHALMOLOGISTS, PATIENTS, AND PARENTS Management of these complicated diseases differs between pediatric and adult patient populations. BY LISA J. FAIA, MD, and KIMBERLY
PEDIATRIC UVEITIS: CHALLENGING FOR OPHTHALMOLOGISTS, PATIENTS, AND PARENTS Management of these complicated diseases differs between pediatric and adult patient populations. BY LISA J. FAIA, MD, and KIMBERLY
HLA Associations in a Cohort of Children With Juvenile Idiopathic Arthritis With and Without Uveitis
 Retina HLA Associations in a Cohort of Children With Juvenile Idiopathic Arthritis With and Without Uveitis Sheila T. Angeles-Han, 1 3 Courtney McCracken, 1 Steven Yeh, 3 Se Ryeong Jang, 1 Kirsten Jenkins,
Retina HLA Associations in a Cohort of Children With Juvenile Idiopathic Arthritis With and Without Uveitis Sheila T. Angeles-Han, 1 3 Courtney McCracken, 1 Steven Yeh, 3 Se Ryeong Jang, 1 Kirsten Jenkins,
UVEITIS AND IDIOPATHIC JUVENILE ARTHRITIS IN SPAIN. EPIDEMIOLOGICAL AND THERAPEUTIC ASPECTS
 ARCH SOC ESP OFTALMOL 2009; 84: 133-138 ORIGINAL ARTICLE UVEITIS AND IDIOPATHIC JUVENILE ARTHRITIS IN SPAIN. EPIDEMIOLOGICAL AND THERAPEUTIC ASPECTS UVEÍTIS Y ARTRITIS IDIOPÁTICA JUVENIL. EPIDEMIOLOGÍA,
ARCH SOC ESP OFTALMOL 2009; 84: 133-138 ORIGINAL ARTICLE UVEITIS AND IDIOPATHIC JUVENILE ARTHRITIS IN SPAIN. EPIDEMIOLOGICAL AND THERAPEUTIC ASPECTS UVEÍTIS Y ARTRITIS IDIOPÁTICA JUVENIL. EPIDEMIOLOGÍA,
Risk Factors and Biomarkers for the Occurrence of Uveitis in Juvenile Idiopathic Arthritis
 ARTHRITIS & RHEUMATOLOGY Vol. 70, No. 10, October 2018, pp 1685 1694 DOI 10.1002/art.40544 2018 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College
ARTHRITIS & RHEUMATOLOGY Vol. 70, No. 10, October 2018, pp 1685 1694 DOI 10.1002/art.40544 2018 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College
PATHOGENIC IMPLICATIONS OF AGE OF ONSET IN JUVENILE RHEUMATOID ARTHRITIS
 25 1 PATHOGENIC IMPLICATIONS OF IN JUVENILE RHEUMATOID ARTHRITIS DONITA B. SULLIVAN, JAMES T. CASSIDY, and ROSS E. PETTY An analysis of age of onset in juvenile rheumatoid arthritis was performed in the
25 1 PATHOGENIC IMPLICATIONS OF IN JUVENILE RHEUMATOID ARTHRITIS DONITA B. SULLIVAN, JAMES T. CASSIDY, and ROSS E. PETTY An analysis of age of onset in juvenile rheumatoid arthritis was performed in the
Emerging therapies for the treatment of uveitis: clinical trial observations
 Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
APPLICATION FOR SPECIAL AUTHORITY. Subsidy for Tocilizumab
 APPLICATION FOR SPECIAL AUTHORITY Fm SA1781 Subsidy f Tocilizumab Application Categy Page Cytokine release syndrome - Initial application... 2 Previous use - Initial application... 2 Rheumatoid Arthritis
APPLICATION FOR SPECIAL AUTHORITY Fm SA1781 Subsidy f Tocilizumab Application Categy Page Cytokine release syndrome - Initial application... 2 Previous use - Initial application... 2 Rheumatoid Arthritis
Optical coherence tomography findings in a child with posterior scleritis
 European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
Primary Results Citation 2
 Table S1. Adalimumab clinical trials 1 ClinicalTrials.gov Rheumatoid Arthritis 3 NCT00195663 Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study. A multicenter, randomized, double-blind clinical
Table S1. Adalimumab clinical trials 1 ClinicalTrials.gov Rheumatoid Arthritis 3 NCT00195663 Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study. A multicenter, randomized, double-blind clinical
ADALIMUMAB Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form Submit request via: Fax
 Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Humira (adalimumab) require a prior
Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Humira (adalimumab) require a prior
C. Assess clinical response after the first three months of treatment.
 Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Juvenile Idiopathic Arthritis (JIA)
 Juvenile Idiopathic Arthritis (JIA) Kaveh Ardalan, MD, MS Division of Rheumatology Ann & Robert H. Lurie Children s Hospital of Chicago Assistant Professor, Pediatrics and Medical Social Sciences Northwestern
Juvenile Idiopathic Arthritis (JIA) Kaveh Ardalan, MD, MS Division of Rheumatology Ann & Robert H. Lurie Children s Hospital of Chicago Assistant Professor, Pediatrics and Medical Social Sciences Northwestern
Juvenile idiopathic arthritis (JIA) represents the most frequent
 Immunology and Microbiology Elevated S100A8/A9 and S100A12 Serum Levels Reflect Intraocular Inflammation in Juvenile Idiopathic Arthritis- Associated Uveitis: Results From a Pilot Study Karoline Walscheid,
Immunology and Microbiology Elevated S100A8/A9 and S100A12 Serum Levels Reflect Intraocular Inflammation in Juvenile Idiopathic Arthritis- Associated Uveitis: Results From a Pilot Study Karoline Walscheid,
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
TRIALS. Ramanan et al. Trials 2014, 15:14
 Ramanan et al. Trials 2014, 15:14 TRIALS STUDY PROTOCOL Open Access A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate
Ramanan et al. Trials 2014, 15:14 TRIALS STUDY PROTOCOL Open Access A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate
Subpopulations within juvenile psoriatic arthritis: A review of the literature
 Clinical & Developmental Immunology, June December 2006; 13(2 4): 377 380 Subpopulations within juvenile psoriatic arthritis: A review of the literature MATTHEW L STOLL 1 & PETER A NIGROVIC 1,2, 1 Division
Clinical & Developmental Immunology, June December 2006; 13(2 4): 377 380 Subpopulations within juvenile psoriatic arthritis: A review of the literature MATTHEW L STOLL 1 & PETER A NIGROVIC 1,2, 1 Division
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION
 UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR
 THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR SEGMENT: A REVIEW New pharmacologic treatment options are urgently needed. BY STEVEN YEH, MD, and JESSICA G. SHANTHA, MD Noninfectious uveitis (NIU)
THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR SEGMENT: A REVIEW New pharmacologic treatment options are urgently needed. BY STEVEN YEH, MD, and JESSICA G. SHANTHA, MD Noninfectious uveitis (NIU)
Blau's Disease / Juvenile Sarcoidosis
 https://www.printo.it/pediatric-rheumatology/gb/intro Blau's Disease / Juvenile Sarcoidosis Version of 2016 1. WHAT IS BLAU S DISEASE/JUVENILE SARCOIDOSIS 1.1 What is it? Blau syndrome is a genetic disease.
https://www.printo.it/pediatric-rheumatology/gb/intro Blau's Disease / Juvenile Sarcoidosis Version of 2016 1. WHAT IS BLAU S DISEASE/JUVENILE SARCOIDOSIS 1.1 What is it? Blau syndrome is a genetic disease.
Learning Objectives and Assessment Methodologies Combined Medicine-Pediatrics Rheumatology Elective
 Learning Objectives and Assessment Methodologies Combined Medicine-Pediatrics Rheumatology Elective Overview: Med-Peds PGY2 s, PGY3 s and PGY4 s can elect to spend one four-week rotation with the adult
Learning Objectives and Assessment Methodologies Combined Medicine-Pediatrics Rheumatology Elective Overview: Med-Peds PGY2 s, PGY3 s and PGY4 s can elect to spend one four-week rotation with the adult
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
Pharmacy Prior Authorization
 Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Literature Review. Biological therapy in treatment of uveitis. Contemporary trends. A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med)
 Literature Review Biological therapy in treatment of uveitis. Contemporary trends A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy of NAMS
Literature Review Biological therapy in treatment of uveitis. Contemporary trends A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy of NAMS
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
Ophthalmology Subcommittee of PTAC Meeting held 30 October (minutes for web publishing)
 Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
The Use of Methotrexate in Juvenile Idiopathic Arthritis: A Single Center Experience
 HK J Paediatr (new series) 2006;11:191-198 The Use of Methotrexate in Juvenile Idiopathic Arthritis: A Single Center Experience PPW LEE, TL LEE, WHS WONG, YL LAU Abstract Key words In the recent decade,
HK J Paediatr (new series) 2006;11:191-198 The Use of Methotrexate in Juvenile Idiopathic Arthritis: A Single Center Experience PPW LEE, TL LEE, WHS WONG, YL LAU Abstract Key words In the recent decade,
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Surgery in patients with uveitis. Lyndell Lim and Anthony Hall
 Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Department of Paediatrics Clinical Guideline. Guideline for the child with possible arthritis (joint swelling/pain, loss of function)
 Department of Paediatrics Clinical Guideline Guideline for the child with possible arthritis (joint swelling/pain, loss of function) Definition: Juvenile Idiopathic Arthritis (JIA) is defined as arthritis
Department of Paediatrics Clinical Guideline Guideline for the child with possible arthritis (joint swelling/pain, loss of function) Definition: Juvenile Idiopathic Arthritis (JIA) is defined as arthritis
