New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
|
|
|
- Kerry Manning
- 5 years ago
- Views:
Transcription
1 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal Status POM Indication Mild to moderate atopic dermatitis (eczema) in patients aged 2 yrs and over for 1) short term treatment of signs and symptoms and 2) intermittent longterm treatment for prevention of progression to flares Dosage Apply to the affected area twice daily Cost Pimecrolimus 1% cream 30g g tubes of pimecrolimus are also available and 100g tubes will be available later in the year Possible Number of Suffolk 30,000 to 40,000 patients in Suffolk Patients have atopic dermatitis Number Needed to Treat Treatment Alternatives Future Alternatives Possible Future Indications Not calculated Emollients, bath oils & soap substitutes to combat dry skin, topical corticosteroids, oral antihistamines, systemic corticosteroids, ciclosporin tacrolimus Hydrocortisone 1% cream 0.72 Clobetasone 0.05% cream 1.94 Betamethasone 0.1% cream 1.54 Clobetasol 0.05% cream 2.82 Tacrolimus 0.03% Tacrolimus 0.1% (All 30g size taken from BNF 44 th Ed & MIMS Jan 2003) Not known Not known This is an NHS Suffolk document that has been adopted by the WSCCG
2 Reviewer s Comments The prospect of a non-steroidal treatment for atopic dermatitis is welcome. However little evidence has been provided to ensure that this product is both safe and efficacious in the longer term. Whilst there is probably a place for its use in Suffolk it is felt that it should not be seen as first line treatment until further evidence is available. Thus the first instance it may be wise to limit its use to those with severe disease until the safety profile is more established. The product is more costly than current treatments, however its use may be justified in the longer term if the safety profile is found to be good as it is well known that the use of corticosteroids is not without problem. The D&TC should be aware that in January 2002 it classified tacrolimus ointment as RED consultant prescription only. Evidence Reviewed Paper, Review, Abstract etc. Smith K Cambridgeshire Joint Prescribing Group Submission Pimecrolimus Molecule of the month January 2002 Pimecrolimus Pimecrolimus Cream MTRAC Review and Summary Sheet Published MTRAC Nov 2002 Medlineplus Pimecrolimus Elidel Information for USA patients Published Novartis Level of evidence Level of evidence adapted from Quick and Clean : authorative health technology assessment for local health care contracting Andrew Stevens, Duncan Collin-Jones & John Gabbay Health Trends Vol 27 No IV Page 2 of 5 May be freely copied by NHS agencies
3 Review Atopic dermatitis is a chronic inflammatory disease with a lifetime prevalence of 15-20%. It affects all age groups, however prevalence is highest amongst children with 15-20% affected. 80% of patients will present by the age of 5. Tacrolimus ointment was the first topical macrolide immunomodulator for the treatment atopic dermatitis. It is indicated for moderate to severe atopic dermatitis unresponsive to conventional therapy in children over the age of 2 years and adults. Pimecrolimus is the second topical macrolide immunomodulator to be licensed for the treatment of atopic dermatitis. It is indicated for a use in a different patient population to tacrolimus ointment. Pimecrolimus 1% cream can be used on all skin areas including the head, face, neck and intertriginous areas. The therapeutic efficacy of pimecrolimus 1% cream in the management of atopic dermatitis has been evaluated in 2,260 patients aged 2 years and above, in 7 randomised, double blind, vehicle controlled trials - 4 in adults and 3 in children (aged 2-17). Four trials evaluated short-term acute treatment - 2 studies in children over 6 weeks and 2 studies in adults over 3 weeks. In these studies, pimecrolimus 1% cream applied twice daily for up to 6 weeks, significantly reduced the signs and symptoms of atopic dermatitis compared to vehicle. In one dose ranging trial in adults with moderate to severe disease, pimecrolimus 1% cream was less effective than betamethasone valerate 0.1% cream. In the two adults trials, emollients were not allowed to be used during the study period. Three trials have evaluated long-term intermittent use - 1 in children and 2 in adults. Treatment with pimecrolimus 1% cream applied twice daily at the first signs or symptoms of atopic dermatitis, for up to one year, to prevent progression to flare reduced the incidence of disease flare. Conventional therapy was used in the event of a flare. In the adult trial this was emollients and a high potency corticosteroid % prednicarbate. In the children trial it was emollients and a moderately potent or potent corticosteroid - clobetasone 0.05% or hydrocortisone butyrate 0.1% respectively. Those treated with pimecrolimus had reduced topical corticosteroid use compared to vehicle and had a longer mean time to first flare. In the trial conducted in the children, 51.5% using the vehicle alone discontinued therapy compared to 31.6% of those using pimecrolimus. In the adult trial the respective figures were 37.5% and 22.9%. Page 3 of 5 May be freely copied by NHS agencies
4 The unpublished data from a 1-year study in adult patients with moderate to severe disease shows that pimecrolimus 1% cream was less effective in controlling symtoms than potent topical corticosteroid treatment (triamcinolone acetonide 0.1% and hydrocortisone acetate 1%). The long-term effect of pimecrolimus 1% cream on the local skin immune response and on the incidence of skin malignancies is not known. If no improvement occurs after 6 weeks of treatment, or if disease exacerbation occurs, pimecrolimus 1% cream should be discontinued. The Summary of Product Characteristics (SPC) states that it is recommended that pimecrolimus cream should be prescribed by physicians with experience in the topical treatment of atopic dermatitis. Adverse Effects etc. For full information it is important to read the SoPC. The most common adverse events reported in clinical trials were application site reactions - burning, irritation, pruritus & erythema. These generally occurred early in treatment, were mild/moderate and were of short duration. It should not be used on areas affected by acute cutaneous viral infections (e.g. chicken pox, herpes simplex) or clinically infected atopic dermatitis. There may be an increased risk of skin bacterial infections (e.g. impetigo) during treatment. It is suggested that vaccination should take place during a treatment free period. Avoid contact with the eyes & mucous membranes. It should not be used under occlusion. Patients should avoid excessive exposure of the skin to UV light. It should not be used concomitantly with other topical corticosteroids or topical anti-inflammatory products. It should not be used in pregnancy. Breast-feeding mothers may use it, although they must not apply it directly to the breast. Whilst the SoPC in the UK warns against excessive exposure to UV light including the use of a solarium the patient information leaflet in the USA is much more restrictive stating: Avoid sunlight and sun lamps, tanning beds, and treatment with UVA or UVB light. If you need to be outdoors after applying ELIDEL, wear loose fitting clothing that protects the treated area from the sun. Ask your doctor what other type of protection from the sun you should use. Page 4 of 5 May be freely copied by NHS agencies
5 The Medlineplus patient information suggests that patients should stay out of direct sunlight if possible between and They suggest that sunblock and lipsick of at least SPF15 should be worn. Health Economics The prevalence of atopic dermatitis has increased over the past 30 years and now affects 5% of the UK population. Estimates suggest that 15-20% of children aged 7 to 18 years are affected with 2-10% of adults. Most affected individuals develop atopic dermatitis before the age of 5 years. Unfortunately the age breakdown available does not match this but the following table can be constructed: Table 1 to show the various estimates of the prevalence of atopic dermatitis PCT 18% of 0 to 14 years of age 5% of aged 15 years and over Total of Columns 2 & 3 5% of total population Central Ipswich Suffolk Coastal Suffolk West Waveney Total for Suffolk Total for East Commissioning There is a certain discrepancy between the two methods of calculating the prevalence but it can be seen that between 30,000 and 40,000 patients are affected by atopic dermatitis. Not all of these will need treatment with pimecrolimus. Page 5 of 5 May be freely copied by NHS agencies
GREEN. Ropinirole Other PD treatments Benzodiazepines Opioids low potency Anticonvulsants Clonidine
 New Medicine Report (Adopted by the CCG until review and futher notice) Document Status Traffic Light Decision PRAMIPEXOLE For restless legs syndrome Post Suffolk D&TC GREEN Prescribers Rating Possibly
New Medicine Report (Adopted by the CCG until review and futher notice) Document Status Traffic Light Decision PRAMIPEXOLE For restless legs syndrome Post Suffolk D&TC GREEN Prescribers Rating Possibly
0BCore Safety Profile. Pharmaceutical form(s)/strength: Cream 1% DK/H/PSUR/0009/005 Date of FAR:
 0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD)
 Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Atomoxetine (First known as Tomoxetine) (Adopted by the CCG until review and further notice)
 New Medicine Report Document Status Atomoxetine (First known as Tomoxetine) (Adopted by the CCG until review and further notice) Post Suffolk D&TC Traffic Light Decision RED Date of Last Revision 12.07.04
New Medicine Report Document Status Atomoxetine (First known as Tomoxetine) (Adopted by the CCG until review and further notice) Post Suffolk D&TC Traffic Light Decision RED Date of Last Revision 12.07.04
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
Atopic Eczema with detail on how to apply wet wraps
 Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elidel 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of cream contains 10 mg of pimecrolimus. For a full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elidel 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of cream contains 10 mg of pimecrolimus. For a full list of excipients,
New Medicine Report (Adopted by the CCG until review and further
 New Medicine Report (Adopted by the CCG until review and further DEXIBUPROFEN notice) Document Status Decision made at Suffolk D&TC 7 th September 2006 Traffic Light Decision Double Green Prescriber s
New Medicine Report (Adopted by the CCG until review and further DEXIBUPROFEN notice) Document Status Decision made at Suffolk D&TC 7 th September 2006 Traffic Light Decision Double Green Prescriber s
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) Elidel 10mg/g cream DATE: , VERSION 12.0
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) Elidel 10mg/g cream DATE: 23.11.2016, VERSION 12.0 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Eczema mainly affects children
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) Elidel 10mg/g cream DATE: 23.11.2016, VERSION 12.0 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Eczema mainly affects children
Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82
 Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Protopic 0.03% ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of Protopic 0.03% ointment contains 0.3 mg of tacrolimus
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Protopic 0.03% ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of Protopic 0.03% ointment contains 0.3 mg of tacrolimus
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Pharmacologic Treatment of Atopic Dermatitis
 J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
RECLASSIFICATION SUBMISSION. EUMOVATE Eczema and Dermatitis Cream. (Clobetasone butyrate 0.05%) From Prescription Only Medicine
 RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 3Q17 July August
 BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
The safety and effectiveness of Dupixent in pediatric patients have not been established (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal
 TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal INSTRUCTIONS 1. Read the article. 2. Take the test, record your answers in the test answer section (Section B)
TRIGGERS & TREATMENT OF ATOPIC DERMATITIS COA#PCIA0809 CE Activity provided by PCI Journal INSTRUCTIONS 1. Read the article. 2. Take the test, record your answers in the test answer section (Section B)
PACKAGE LEAFLET: INFORMATION FOR THE USER. Tacrolimus 0.1% Ointment Tacrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Tacrolimus 0.1% Ointment Tacrolimus Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
PACKAGE LEAFLET: INFORMATION FOR THE USER Tacrolimus 0.1% Ointment Tacrolimus Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tacrolimus 0.1% Ointment Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g ointment contains tacrolimus monohydrate corresponding to 1.0
1 NAME OF THE MEDICINAL PRODUCT Tacrolimus 0.1% Ointment Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g ointment contains tacrolimus monohydrate corresponding to 1.0
New Medicine Report (Adopted by the CCG until review and further
 New Medicine Report (Adopted by the CCG until review and further GRASS ALLERGEN TREATMENT notice) Document Status Decision following Suffolk D&TC meeting Traffic Light Decision Red for 2007 with review
New Medicine Report (Adopted by the CCG until review and further GRASS ALLERGEN TREATMENT notice) Document Status Decision following Suffolk D&TC meeting Traffic Light Decision Red for 2007 with review
Apply a thin layer of elidel 1% cream to the affected skin twice daily and rub in gently and completely.
 ELIDEL Atopic Dermatitis Treatment Composition One gram of cream contains 10 mg of pimecrolimus. Indications Atopic dermatitis (eczema) (AD). Elidel 1% cream is indicated for the short-term (acute) treatment
ELIDEL Atopic Dermatitis Treatment Composition One gram of cream contains 10 mg of pimecrolimus. Indications Atopic dermatitis (eczema) (AD). Elidel 1% cream is indicated for the short-term (acute) treatment
Vulval dermatoses. Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough
 Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
Topical Immunomodulator Step Therapy Program
 Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis
 Dermatology The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis The image to the left shows an image of skin cells and the proteins which
Dermatology The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis The image to the left shows an image of skin cells and the proteins which
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
PHOTOTHERAPY. With narrowband UVB, the light tubes produce a narrow part of the UVB spectrum. Two wavelengths
 Phototherapy (light therapy) refers to the use of ultraviolet (UV) light to treat moderate to severe eczema in children and adults. Phototherapy is a second-line treatment option that is available at specialist
Phototherapy (light therapy) refers to the use of ultraviolet (UV) light to treat moderate to severe eczema in children and adults. Phototherapy is a second-line treatment option that is available at specialist
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
Atopic dermatitis/ eczema (a condition that makes skin red and itchy):
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
RELEVANT DISCLOSURES ATOPIC DERMATITIS / ECZEMA MANAGING ECZEMA IN INFANTS AND CHILDREN
 RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
Legal Status Indication Treatment of Type 1 and Type 2 diabetes
 New Medicine Report (Adopted by the CCG until review and further notice) Document Status Recombinant human insulin analogues Following Suffolk D&TC Meeting Traffic Light Decision Green Date of Last Revision
New Medicine Report (Adopted by the CCG until review and further notice) Document Status Recombinant human insulin analogues Following Suffolk D&TC Meeting Traffic Light Decision Green Date of Last Revision
LRI Children s Hospital
 Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Atopic Eczema Care LRI Children s Hospital Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team approval date: May 2017 Version: V 4 Revision due: May 2020 Written by: K.
Vitiligo is a skin depigmentation disorder
 THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland
 Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
Steroid use in managing your child s Atopic Eczema
 Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Steroid use in managing your child s Atopic Eczema Clinical Nurse Specialist for Paediatric Dermatology (01284) 713575 Step up step down approach: Addressograph Severe Call your General Practitioner (GP)
Clinico Pathological Test SCPA605-Essential Pathology
 Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
Clinico Pathological Test SCPA605-Essential Pathology Somphong Narkpinit, M.D. Department of Pathogbiology, Faculty of Science, Mahidol University e-mail : somphong.nar@mahidol.ac.th Pathogenesis of allergic
What s Topical About Topicals?
 What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Oral PUVA Phototherapy
 Information for patients: Oral PUVA Phototherapy Brooke Building Phototherapy Unit 0161 206 1343 All Rights Reserved 2017. Document for issue as handout. This booklet aims to answer the most commonly asked
Information for patients: Oral PUVA Phototherapy Brooke Building Phototherapy Unit 0161 206 1343 All Rights Reserved 2017. Document for issue as handout. This booklet aims to answer the most commonly asked
Paediatric Eczema. Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012
 Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
Pediatric Dermatology. Wingfield Rehmus, MD MPH BC Children s Hospital
 Pediatric Dermatology Wingfield Rehmus, MD MPH BC Children s Hospital Conflict of interest! No financial conflict of interest! Individual products shown are examples only not a product endorsement Pediatric
Pediatric Dermatology Wingfield Rehmus, MD MPH BC Children s Hospital Conflict of interest! No financial conflict of interest! Individual products shown are examples only not a product endorsement Pediatric
NEW ZEALAND DATA SHEET
 EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
Elidel Cream Pimecrolimus 1 % w/w Consumer Medicine Information
 Elidel Cream Pimecrolimus 1 % w/w Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Elidel. It does not contain all the available information. It does
Elidel Cream Pimecrolimus 1 % w/w Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Elidel. It does not contain all the available information. It does
What is atopic dermatitis?
 What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
Volume 2; Number 2 February 2008
 Volume 2; Number 2 February 2008 CONTENTS Page 2 Page 3 Page 3 Page 4 Page 6 New Drug Assessment: Rufinamide (Inovelon) New Drug Assessment: Zoledronic acid infusion (Aclasta) New Drug Assessment: Mesalazine
Volume 2; Number 2 February 2008 CONTENTS Page 2 Page 3 Page 3 Page 4 Page 6 New Drug Assessment: Rufinamide (Inovelon) New Drug Assessment: Zoledronic acid infusion (Aclasta) New Drug Assessment: Mesalazine
Dupixent (dupilumab)
 Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis
 Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Recommended management of eczema in older patients
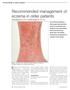 Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Treating dermatomyositis
 Treating dermatomyositis David Fiorentino, MD, PhD Stanford University School of Medicine Department of Dermatology Department of Medicine (Rheumatology) September 25, 2010 DM affects many organs Approaching
Treating dermatomyositis David Fiorentino, MD, PhD Stanford University School of Medicine Department of Dermatology Department of Medicine (Rheumatology) September 25, 2010 DM affects many organs Approaching
Thursday, 21 October :53 - Last Updated Thursday, 11 November :27
 1 / 15 2 / 15 3 / 15 4 / 15 Pityriasis Alba Background Pityriasis alba is a nonspecific dermatitis of unknown etiology that causes erythematous scaly patches. These resolve and leave areas of hypopigmentation
1 / 15 2 / 15 3 / 15 4 / 15 Pityriasis Alba Background Pityriasis alba is a nonspecific dermatitis of unknown etiology that causes erythematous scaly patches. These resolve and leave areas of hypopigmentation
STUDY. 1% Pimecrolimus, 0.005% Calcipotriol, and 0.1% Betamethasone in the Treatment of Intertriginous Psoriasis
 STUDY 1% Pimecrolimus, 0.005% Calcipotriol, and 0.1% Betamethasone in the Treatment of Intertriginous Psoriasis A Double-blind, Randomized Controlled Study Alexander Kreuter, MD; Anna Sommer, MD; Julia
STUDY 1% Pimecrolimus, 0.005% Calcipotriol, and 0.1% Betamethasone in the Treatment of Intertriginous Psoriasis A Double-blind, Randomized Controlled Study Alexander Kreuter, MD; Anna Sommer, MD; Julia
Atopic dermatitis: tacrolimus vs. topical corticosteroid use
 Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Additional analysis on the assessment report: The Effectiveness and Cost Effectiveness of Pimecrolimus and Tacrolimus for the Atopic Eczema.
 Additional analysis on the assessment report: The Effectiveness and Cost Effectiveness of Pimecrolimus and Tacrolimus for the Atopic Eczema. Conducted by PenTAG. Page 1 of 6 Further Analysis of the Eczema
Additional analysis on the assessment report: The Effectiveness and Cost Effectiveness of Pimecrolimus and Tacrolimus for the Atopic Eczema. Conducted by PenTAG. Page 1 of 6 Further Analysis of the Eczema
Teledermatology Paediatric eczema. Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital
 Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
Teledermatology Paediatric eczema Dr Carolyn Charman Consultant Dermatologist Royal Devon and Exeter Hospital NHS e-referral teledermatology Rapid access to diagnosis / management advice from local integrated
Atopic dermatitis Tacrolimus vs topical corticosteroid use
 Atopic dermatitis Tacrolimus vs topical corticosteroid use Yolande Langa, BPharm Elsabé van der Merwe, BPharm; MSc (Pharmacology) Abstract Atopic dermatitis (AD), the dermatologic manifestation of the
Atopic dermatitis Tacrolimus vs topical corticosteroid use Yolande Langa, BPharm Elsabé van der Merwe, BPharm; MSc (Pharmacology) Abstract Atopic dermatitis (AD), the dermatologic manifestation of the
Core Safety Profile. AT/H/PSUR/0013/002 Date of FAR:
 ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
New Medicine Report. Anakinra Classification RED (Adopted by the CCG until review and further notice) Date of Last Revision 5 th July 2002
 New Medicine Report Document Status Anakinra Classification RED (Adopted by the CCG until review and further notice) Post Suffolk D&TC Date of Last Revision 5 th July 2002 Approved Name Trade Name Manufacturer
New Medicine Report Document Status Anakinra Classification RED (Adopted by the CCG until review and further notice) Post Suffolk D&TC Date of Last Revision 5 th July 2002 Approved Name Trade Name Manufacturer
Dermatology Literary Review
 Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
Emollients and ageing skin: optimising effectiveness and safety of Nursing, Vol. 25, No.11, pages 596-598. 6 Article outlines the causes of dryness in ageing skin and concludes that emollients are the
PRESCRIBING INFORMATION. Cream 0.025% Topical Corticosteroid
 PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) report Title and TA publication number of static topic: Final decision:
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) report Title and TA publication number of static topic: Final decision:
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
METHYLPREDNISOLONE ACEPONATE (MPA)
 RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
RE AFETY PRFILE ETHYLPREDNILNE AEPNATE (PA) 4.2 Posology and method of administration PA is to be applied thinly once daily to the affected areas and rubbed in lightly. PA cream/ointment/fatty ointment:
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
ATOPIC ECZEMA. What are the aims of this leaflet?
 ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
GROUP 15 TOPICAL PREPARATIONS
 - 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
- 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
Atopic Dermatitis and Topical Antipsoriatics
 Atopic Dermatitis and Topical Antipsoriatics Goal(s): Restrict dermatological drugs only for funded OHP diagnoses. Moderate/severe psoriasis and moderate/severe atopic dermatitis treatments are funded
Atopic Dermatitis and Topical Antipsoriatics Goal(s): Restrict dermatological drugs only for funded OHP diagnoses. Moderate/severe psoriasis and moderate/severe atopic dermatitis treatments are funded
PUVA Phototherapy. Information for patients and visitors. Dermatology Department Medicine Group
 PUVA Phototherapy Dermatology Department Medicine Group This leaflet has been designed to give you important information about your condition / procedure, and to answer some common queries that you may
PUVA Phototherapy Dermatology Department Medicine Group This leaflet has been designed to give you important information about your condition / procedure, and to answer some common queries that you may
Learning Circle: Jan 26, 2011 Childhood Eczema
 Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
FASTUM 2.5% gel PACKAGE LEAFLET: INFORMATION FOR THE USER. What is in this leaflet? 1. WHAT FASTUM GEL IS AND WHAT IT IS USED FOR
 PACKAGE LEAFLET: INFORMATION FOR THE USER FASTUM 2.5% gel KETOPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines the
PACKAGE LEAFLET: INFORMATION FOR THE USER FASTUM 2.5% gel KETOPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines the
Pharmacy Benefit Determination Policy
 Policy Subject: Atopic Dermatitis Agents Policy Number: SHS PBD18 Category: Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS PPO ASO s:
Policy Subject: Atopic Dermatitis Agents Policy Number: SHS PBD18 Category: Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS PPO ASO s:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
Allergy Medications. Antihistamines. are very safe. Although usually taken as tablets, they may be prescribed as a liquid or syrup for young children
 The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
The Itch That Rashes. Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah
 The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
CONSUMER MEDICINE INFORMATION CLOTRIZONE CREAM
 CONSUMER MEDICINE INFORMATION CLOTRIZONE CREAM Clotrimazole 1% w/w and hydrocortisone acetate 1% w/w What is in this leaflet This leaflet answers some common questions about It does not contain all the
CONSUMER MEDICINE INFORMATION CLOTRIZONE CREAM Clotrimazole 1% w/w and hydrocortisone acetate 1% w/w What is in this leaflet This leaflet answers some common questions about It does not contain all the
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
Skin disorders. Seborrhoeic dermatitis Search date April 2010 Luigi Naldi ...
 Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Hydrocortisone Cream 1% 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Cream containing 1% micronised hydrocortisone For excipients, see 6.1
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Hydrocortisone Cream 1% 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Cream containing 1% micronised hydrocortisone For excipients, see 6.1
Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate
 Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate Read all of this leaflet carefully before you start using this medicine because it contains important information
Patient Leaflet : Information for the user Locoid Cream 0.1% w/w Hydrocortisone butyrate Read all of this leaflet carefully before you start using this medicine because it contains important information
Managing and Minimizing Flare-ups in Atopic Dermatitis
 Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
過敏病科中心. Allergy Centre. Eczema. Allergy Centre 過敏病科中心. Allergy Centre. For enquiries and appointments, please contact us at:
 Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Patient Group Directions for the supply of medication by Community Pharmacists under the Common Ailments Service
 Patient Group Directions for the supply of medication by Community Pharmacists under the Common Ailments Service PGD No. Drug Indication Page 130 Chloramphenicol 0.5% eye drops Conjunctivitis (Bacterial)
Patient Group Directions for the supply of medication by Community Pharmacists under the Common Ailments Service PGD No. Drug Indication Page 130 Chloramphenicol 0.5% eye drops Conjunctivitis (Bacterial)
Drug allergy and Skin Disorders. Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey
 Drug allergy and Skin Disorders Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey The best screening test for anaphylaxis is? A. histamine
Drug allergy and Skin Disorders Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey The best screening test for anaphylaxis is? A. histamine
13 August Fucidin-H vs. Hydrocortisone in Atopic Dermatitis.
 FUH9401DK 13 August 2002 Page 11 of108 2 SYNOPSIS Study code number FUH9401 DKStudy Study title Fucidin-H vs. Hydrocortisone in Atopic Dermatitis. Study Subtitle A comparative study of Fucidin-H cream
FUH9401DK 13 August 2002 Page 11 of108 2 SYNOPSIS Study code number FUH9401 DKStudy Study title Fucidin-H vs. Hydrocortisone in Atopic Dermatitis. Study Subtitle A comparative study of Fucidin-H cream
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP)
 CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
Constitutional eczema
 Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Making decisions about available treatments
 TREATMENTS Making decisions about available treatments The aim of this ebooklet is to help you think through the choices that are available to you. It includes: the factors which might influence your decisions.
TREATMENTS Making decisions about available treatments The aim of this ebooklet is to help you think through the choices that are available to you. It includes: the factors which might influence your decisions.
(minutes for web publishing)
 Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
DATA SHEET. Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
 DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
MALE GENITAL (PENIS) LICHEN SCLEROSUS
 MALE GENITAL (PENIS) LICHEN SCLEROSUS What are the aims of this leaflet? This leaflet has been written to help you understand more about male genital lichen sclerosus (also known as balanitis xerotica
MALE GENITAL (PENIS) LICHEN SCLEROSUS What are the aims of this leaflet? This leaflet has been written to help you understand more about male genital lichen sclerosus (also known as balanitis xerotica
NEW ZEALAND DATA SHEET 1 LOCOID 2 QUALITATIVE AND QUANTITATIVE COMPOSTION 3 PHARMACEUTICAL FORM 4 CLINICAL PARTICULARS
 NEW ZEALAND DATA SHEET 1 LOCOID Lipocream Ointment Topical Emulsion (Locoid Crelo ) Scalp Lotion hydrocortisone butyrate 2 QUALITATIVE AND QUANTITATIVE COMPOSTION Each formulation contains active ingredient
NEW ZEALAND DATA SHEET 1 LOCOID Lipocream Ointment Topical Emulsion (Locoid Crelo ) Scalp Lotion hydrocortisone butyrate 2 QUALITATIVE AND QUANTITATIVE COMPOSTION Each formulation contains active ingredient
