agonist in patients 6 years and older with reversible airway disease and acute attacks of bronchospasm
|
|
|
- Barbra Singleton
- 5 years ago
- Views:
Transcription
1 Pharmaceutical Listing 2009 Buyers Guide This listing, organized by indication, is a quick way to find commonly used respiratory and sleep drugs. Consult the manufacturer s full prescribing information before administering any drug to a patient. ALPHA 1 ANTITRYPSIN DEFICIENCY Aralast Generic: Alpha 1 alpha 1 Manufacturer: Baxter Healthcare Indication: Chronic augmentation therapy in patients having congenital deficiency of alpha1 with clinically evident AralastNP Generic: Alpha 1 alpha 1 Manufacturer: Baxter Healthcare Indication: Chronic augmentation therapy in patients having congenital deficiency of alpha 1 with clinically evident Prolastin Generic: Alpha 1 alpha 1 Manufacturer: Talecris Biotherapeutics Inc. Indication: Chronic replacement therapy for patients with congenital deficiency of alpha 1 with clinically demonstrable panacinar Zemaira Generic: Alpha 1 alpha 1 Manufacturer: CSL Behring LLC. Indication: Chronic augmentation and maintenance therapy in individuals with alpha 1 deficiency and clinical evidence of ASTHMA Accolate Tablets Generic: Zafirlukast Description: Leukotriene receptor ant Pharmaceuticals LP Indication: Prophylaxis and continuous treatment of asthma in patients 5 years and older Recommended dose: Adults and children 12 years and older: 20 mg bid (oral); children 5 to 11 years: 10 mg bid (oral) AccuNeb Solution for Inhalation 0.021% and 0.042% Generic: Albuterol Sulfate Inhalation Solution 0.021% and 0.042% Description: Shortacting beta 2 in patients 2 to 12 years with Recommended dose: 0.63mg or 1.25 mg (one 3 ml unitdose vial) tid or qid by Advair Diskus 100/50, 250/50, 500/50 Generic: Fluticasone propionate and salmeterol xinafoate Description: Combination product Indication: Longterm, twicedaily maintenance treatment of asthma in patients 4 years and older. ADVAIR DISKUS 250/50 is indicated for the twicedaily maintenance treatment in patients with COPD associated with chronic bronchitis. This product shouldn t be used for acute exacerbations. Recommended dose: One inhalation bid (a.m. and p.m., approx. 12 hours apart) Advair Diskus HFA 45/21, 115/21, 230/21 Generic: Fluticasone propionate and salmeterol xinafoate Description: Combination product Indication: Longterm, twicedaily maintenance treatment of asthma in patients 12 years and older. This product should not be used for acute exacerbations. inhalations bid (a.m. and p.m., approx. 12 hours apart) Albuterol Inhalation Aerosol Note: A limited number of CFC inhalers remain available; pharmacies can sell these products after the Dec. 31, 2008 phaseout deadline if they still have remaining stock. Description: Shortacting beta 2 Indication: Prevention and relief of bronchospasm in patients 4 years and older with reversible obstructive airway, and prevention of exerciseinduced bronchospasm inhalations q 4 to 6 hours Solution 0.042% Description: Shortacting beta 2 in patients 2 to 12 years with Recommended dose: 1.25 mg (one 3 ml unitdose vial) tid or qid by Solution 0.5% Description: Shortacting beta 2 in patients 2 years and older with and acute attacks of bronchospasm Recommended dose: 2.5 mg (as 0.5 ml diluted in normal saline or other suitable agent) tid or qid by Solution 0.083% Description: Shortacting beta 2 in patients 2 years and older with reversible airway and acute attacks of bronchospasm Recommended dose: 1 vial tid or qid by Albuterol Sulfate Tablets Description: Shortacting beta 2 in patients 6 years and older with reversible airway and acute attacks of bronchospasm (2 mg or 4 mg) orally 3 to 4 times daily Albuterol Sulfate Extended Release Tablets Manufacturer: Mylan Description: Shortacting beta 2 in patients 6 years and older with reversible airway and acute attacks of bronchospasm (4 mg or 8 mg) every 12 hours Alvesco Generic: Ciclesonide Manufacturer: Nycomed Description: Nonhalogenated glucocorticoid prodrug in patients 12 years and older Recommended dose: One puff (80 mcg or 160 mcg) once daily Asmanex Twisthaler Inhalation Powder Generic: Mometasone furoate Manufacturer: Schering Corp. for patients 4 years and older Recommended dose: Initial treatment, 1 inhalation (220 mcg per inhalation) QD (once daily) in the evening. Dosage may be increased to 2 inhalations QD (once daily). For patients maintained on oral corticosteroids: 2 inhalations (440 mcg) bid. Azmacort Inhalation Aerosol Generic: Triamcinolone acetonide Manufacturer: Abbott Laboratories Recommended dose: Adults: 2 inhalations (200 mcg) tid or qid, or 4 inhalations (400 mcg) bid; 6 to 12 years: 1 or 2 inhalations (100 to 200 mcg) tid or qid, or 4 inhalations (200 to 400 mcg) bid Cromolyn Sodium Inhalation Solution USP Description: Mast cell stabilizer Indication: Management of bronchial asthma Recommended dose: One vial qid by Flovent HFA Inhalation Aerosol 44 mcg, 110 mcg, 220 mcg Generic: Fluticasone propionate HFA in patients 4 years and older Recommended dose: 2 puffs bid
2 Foradil Aerolizer Generic: Formoterol fumarate inhalation powder Description: Longacting beta 2 Manufacturer: Schering Corp. Indication: Longterm, twicedaily maintenance treatment of asthma and prevention of bronchospasm in patients 5 years and older with s, and acute prevention of exerciseinduced bronchospasm in patients 12 years and older. This product should not be used for acute exacerbations. Recommended dose: The contents of one capsule (12 mcg) by inhalation every 12 hours Intal Inhaler Generic: Cromolyn sodium for oral inhalation Description: Inhaled mast cell stabilizer Manufacturer: King Indication: Prophylactic agent in the management of patients with bronchial asthma; also may be effective in some patients with exerciseinduced bronchospasm Recommended dose: Patients 5 years and older: 2 puffs qid Maxair Autohaler Generic: Pirbuterol acetate inhalation aerosol Description: Shortacting beta 2 Manufacturer: Graceway Pharmaceuticals LLC Indication: Prevention and reversal of bronchospasm in patients 12 years and older with reversible bronchospasm inhalations (400 mcg) q 4 to 6 hours; one inhalation (200 mcg) q 4 to 6 hours may suffice for some patients Metaproterenol Sulfate (available as oral syrup, 10 mg and 20 mg tablets) Description: Shortacting beta 2 Indication: For bronchial asthma and reversible bronchospasm associated with bronchitis and Recommended dose: Oral dosing also would be tid or qid ProAir HFA Inhalation Aerosol Description: shortacting beta 2 Manufacturer: Teva Specialty Pharmaceuticals LLC Indication: Treatment and prevention of bronchospasm with s and prevention of exerciseinduced bronchospasm in patients 4 years and older Recommended dose: For patients 12 years and older: two inhalations repeated every 4 to 6 hours. For exerciseinduced bronchospasm prevention, patients 12 years or older: two inhalations 15 to 30 minutes before exercise. Proventil HFA Inhalation Aerosol Description: Shortacting beta 2 Manufacturer: ScheringPlough Indication: Treatment and prevention of bronchospasm with s and prevention of exerciseinduced bronchospasm in patients 4 years and older inhalations q 4 to 6 hours Pulmicort Respules 0.25 mg and 0.5 mg Generic: Budesonide inhalation suspension of asthma and as prophylactic therapy in children 12 months to 8 years Recommended dose: One respule once or twice daily as directed by Pulmicort Flexhaler Generic: Budesonide inhalation powder in patients 6 years and older. This product should not be used for acute exacerbations. Recommended dose: Inhalation of 90 mcg to 360 mcg twice daily QVAR Inhalation Aerosol 40 mcg and 80 mcg per actuation Generic: Beclomethasone dipropionate HFA inhalation aerosol Manufacturer: Teva Specialty Pharmaceuticals LLC in patients 5 years and older Recommended dose: Low dose: 80 to 160 mcg as 40 to 80 mcg BID; medium dose: 160 to 320 mcg; high dose: 320 mcg bid (the highest dose recommended dosage). For children ages 5 to 11 years: starting dose of 40 mcg bid up to 80 mcg bid maximum recommended Serevent Diskus Generic: Salmeterol xinafoate inhalation powder Description: Longacting beta 2 Indication: Longterm, twicedaily maintenance treatment of asthma and in prevention of bronchospasm in patients 4 years and older with reversible obstructive airway ; also for longterm maintenance treatment of bronchospasm associated with chronic obstructive pulmonary. This product should not be used for acute exacerbations. Recommended dose: Patients 4 years and older: one inhalation (50 mcg) bid (a.m. and p.m., approx. q 12 hours) Singulair Tablets, Chewable Tablets, and Granule Packets Generic: Montelukast sodium Description: Leukotriene receptor ant Manufacturer: Merck & Co. Inc. Indication: Prophylaxis and chronic treatment of asthma in patients 12 months and older; Prevention of exercisedinduced bronchoconstriction in patients 15 years and older Recommended dose: Patients 15 years and older: one 10 mg tablet daily at night; 6 to 14 years: one 5 mg chewable tablet daily at night; 2 to 5 years: one 4 mg chewable tablet or granule packet daily taken at night; 12 to 24 months: one 4 mg granule packet. Symbicort Generic: Budesonide/formoterol fumarate inhalation powder Description: Combination product Indication: For the longterm maintenance treatment of asthma in patients 12 years and older. Recommended dose: One to two sprays orally twice daily Theophylline Description: Methylxanthine symptoms and reversible airflow obstruction associated with chronic asthma, chronic bronchitis, or COPD. Recommended dose: Initial dose is 10 mg/kg/day with adjustments made to maintain a steady plasma level of 5 mcg/ml to 15 mcg/ml Tilade Inhaler Note: Tilade is being discontinued. It will remain available until current supplies are depleted. Generic: Nedocromil sodium inhalation aerosol Description: Mast cell stabilizer Manufacturer: King Indication: Maintainance therapy in the management of patients with mildtomoderate asthma Recommended dose: For patients 6 years and up, two inhalations qid Ventolin HFA Inhalation Aerosol Description: Shortacting beta 2 Indication: Treatment and prevention of bronchospasm in with s, and for prevention of exerciseinduced asthma Recommended dose: Patients 4 years and older: two puffs repeated q 4 to 6 hours; for some patients, one puff may be sufficient VoSpire ER ExtendedRelease Tablets Description: Controlled released shortacting beta 2 Manufacturer: Dava in patients 6 years and older with Recommended dose: Patients 12 years and up: 8 mg every 12 hours; 6 to 12 years: 4 mg every 12 hours Xolair Generic: Omalizumab Description: Humanized IgG1 monoclonal antibody Manufacturer: Genentech Inc. Indication: Relief of asthma in patients 12 years and older with moderatetosevere persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are controlled inadequately with inhaled corticosteroids Recommended dose: 150 mg to 375 mg administered subcutaneously every 2 weeks to 4 weeks
3 Pharmaceutical Listing 2009 Buyers Guide Xopenex Inhalation Solution Generic: Levalbuterol HCl Description: Shortacting beta 2 Indication: Treatment or prevention of bronchospasm in patients 6 years and older with s Recommended dose: Patients 6 to 11 years: 0.31 mg tid by ; 12 years and up: 0.63 mg tid by Xopenex HFA Inhaler Generic: Levalbuterol HCl Description: Shortacting beta 2 Indication: For the treatment or prevention of bronchospasm in patients with reversible obstructive airway Recommended dose: 1 to 2 inhalations every 4 to 6 hours Zyflo Generic: Zileuton tablets Description: Leukotriene formation inhibitor Manufacturer: Critical Therapeutics Inc. Indication: Prevention and chronic treatment of asthma in patients 12 years and older Recommended dose: 600 mg qid Zyflo CR Generic: Zileuton extended release tablets Description: Leukotriene formation inhibitor Manufacturer: Critical Therapeutics Inc. Indication: Prevention and chronic treatment of asthma in patients 12 years and older 600 mg tablets (1200 mg) bid CHRONIC OBSTRUCTIVE PULMONARY DISEASE Acetylcysteine Solution 10% and 20% USP Description: Mucolytic agent Indication: Adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions Recommended dose: 3 to 5 ml of the 20% solution or 6 to 10 ml of the 10% solution diluted with an appropriate vehicle 3 to 4 times a day. Advair Diskus 100/50, 250/50, 500/50 Albuterol Inhalation Aerosol Solution 0.5% Solution 0.083% ATROVENT HFA Inhalation Aerosol Generic: Ipratropium bromide HFA Description: Anticholinergic of bronchospasm associated with COPD, including chronic bronchitis and Recommended dose: 2 puffs qid Brovana Inhalation Solution Generic: Arformoterol tartrate Description: Longacting beta 2 Indication: Twice daily, longterm maintenance treatment of bronchoconstriction in patients with COPD, which includes chronic bronchitis and. Recommended dose: 15 mcg administered twice a day (a.m. and p.m.) by Combivent Inhalation Aerosol Generic: Ipratropium bromide and albuterol sulfate Description: Combination drug Indication: For patients with COPD on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator inhalations qid DuoNeb Inhalation Solution Generic: Ipratropium bromide 0.5 mg and albuterol sulfate 3.0 mg Description: Combination drug bronchospasm associated with COPD in patients requiring more than one bronchodilator Recommended dose: 3 ml vial qid by Foradil Aerolizer Ipratropium Bromide Inhalation Solution 0.02% Description: Anticholinergic of bronchospasm associated with COPD, including chronic bronchitis and Recommended dose: 500 mcg (one vial) tid or qid by oral Ipratropium bromide 0.5 mg and albuterol sulfate 3.0 mg /3mL Solution Description: Combination drug bronchospasm associated with COPD in patients requiring more than one bronchodilator Recommended dose: 3 ml vial qid by Metaproterenol Sulfate (oral syrup and tablets) Perforomist Inhalation Solution Generic: Formoterol fumarate inhalation solution Description: Solution for inhalation Manufacurer: Dey Indication: For the long term treatment of bronchoconstriction in COPD Recommended dose: Twicedaily Serevent Diskus Spiriva HandiHaler Generic: Tiotropium bromide inhalation powder Description: Anticholinergic Indication: Longterm, oncedaily maintenance treatment of bronchospasm associated with COPD, including chronic bronchitis and Recommended dose: The inhalation of the contents of one capsule once daily using the HandiHaler inhalation device Theophylline CYSTIC FIBROSIS Acetylcysteine Solution 10% and 20% USP Description: Mucolytic agent Indication: Adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions Recommended dose: 1 ml to 10 ml of 20% solution (or 2 ml to 20 ml 10% solution) nebulized q 6 to 8 hours Pulmozyme Inhalation Solution Generic: Dornase alfa Description: Recombinant human deoxyribonuclease Manufacturer: Genentech Inc. Indication: Management of cystic fibrosis patients to improve pulmonary function Recommended dose: One singleuse ampule inhaled once daily by TOBI Solution for Inhalation Generic: Tobramycin Description: Aminoglycoside antibiotic Manufacturer: Novartis Pharmaceutical Corp. Indication: Management of cystic fibrosis patients 6 years and older with P. aeruginosa Recommended dose: 300 mg by bid for 28 days, 28 days off, repeat INSOMNIA Ambien Generic: Zolpidem tartrate Manufacturer: sanofiaventis U.S. Indication: Shortterm treatment difficulties with sleep initiation Recommended dose: 5 mg or 10 mg immediately before bedtime AmbienCR Generic: Zolpidem tartrate, controlled release Manufacturer: sanofiaventis U.S. Indication: Shortterm treatment difficulties with sleep onset and/or maintenance Recommended dose: 6.25 mg or 12.5 mg before bedtime Butisol Sodium, Tablets and Elixer Generic: Butabarbital sodium Description: Sedative, Manufacturer: Medpointe Pharmaceuticals Indication: Insomnia Recommended dose: Adults: as a daytime sedative, 15 to 30 mg tid or qid; as a, 50 to 100 mg daily. Children: as a daytime sedative, 7.5 to 30 mg depending on age, weight, and degree of sedation required Dalmane Generic: Flurazepam HCl
4 Manufacturer: Valeant Pharmaceuticals International Indication: For the treatment of insomnia characterized by difficulty in falling asleep, frequent noctural awakenings, and/or early morning awakening Recommended dose: 15 mg or 30 mg before bedtime Doral 15 mg Tablets Generic: Quazepam tablets Manufacturer: Questcor Indication: Shortterm treatment difficult in falling asleep, frequent nocturnal awakenigns, and/or early morning awakenings before bedtime Edluar 5mg, 10mg Sublingual Tablet Generic: Zolpidem sublingual tablets Manufacturer: Meda AB Indication: Shortterm treatment difficulties in sleep initiation before bedtime Estazolam Indication: Shortterm management characterized by difficulty in falling asleep, frequent noctural awakenings, and/or early morning awakening Recommended dose: 1 mg or 2 mg at bedtime Flurazepam HCl Indication: For the treatment of insomnia characterized by difficulty in falling asleep, frequent noctural awakenings, and/or early morning awakening Recommended dose: 15 mg or 30 mg before bedtime Halcion Generic: Triazolam Description: Triazolobenzodiazepine agent Manufacturer: Pfizer Inc. Indication: Shortterm treatment Recommended dose: mg or 0.25 mg before bedtime LUNESTA Generic: Eszopiclone Indication: Insomnia Recommended dose: 1 mg, 2 mg, or 3 mg before bedtime Restoril Generic: Temazepam capsules Manufacturer: Mallinckrodt Inc. Indication: Shortterm treatment Recommended dose: 7.5 mg, 15 mg, 22.5 mg, or 30 mg before bedtime Rozerem Generic: Ramelteon tablets Manufacturer: Takeda Pharmaceuticals America Inc. Indication: For treatment of insomnia characterized by difficulty with sleep onset Recommended dose: One 8 mg tablet at bedtime Somnote Generic: Chloral hydrate capsules USP 500 mg Description: Hypnotic agent Manufacturer: Breckenridge Pharmaceutical Inc. Indication: Shortterm treatment of simple insomnia Recommended dose: Adults: 500 mg before retiring; Children: 50 mg/kg body weight Sonata Capsules Generic: Zaleplon Manufacturer: King Indication: Shortterm treatment Recommended dose: 5 or 10 mg Temazepam Capsules Indication: Shortterm treatment Recommended dose: 15 mg or 30 mg before bedtime Triazolam Description: Triazolobenzodiazepine agent Indication: Shortterm treatment Recommended dose: mg or 0.25 mg before bedtime ZolpiMist Oral Spray Generic: Zolpidem oral spray Manufacturer: Novadel Indication: Shortterm treatment difficult in falling asleep Recommended dose: One spray (5 mg) or two sprays (10 mg) before bedtime Zolpidem tartrate Indication: Shortterm treatment difficulties with sleep initiation Recommended dose: 5 mg or 10 mg immediately before bedtime NARCOLEPSY/CATAPLEXY Dexedrine Generic: Dextroamphetamine sulfate Description: Amphetamine Indication: Narcolepsy Recommended dose: Adults: 5 mg to 60 mg daily in divided doses; 6 to 12 years: 5 mg daily DextroStat Generic: Dextroamphetamine sulfate Description: Amphetamine Manufacturer: Shire US Inc. Indication: Narcolepsy Recommended dose: Patients 12 years and up 5 mg to 60 mg daily in divided doses. Give first dose on awakening; additional doses at intervals of 4 to 6 hours. Start with 10 mg daily, raise in increments of 10 mg at weekly intervals until optimal level is achieved; 6 to 12 years: 5 mg daily. NUVIGIL Generic: Armodafinil tablets system stimulant, analeptic Manufacturer: Cephalon Inc. Indication: Improve wakefulness in patients with exessive sleepiness associated with narcolepsy, obstructive sleep apnea/hypopnea syndrome, and shift work sleep disorder Recommended dose: For patients with OSAHS or narcolepsy, 150 mg or 250 mg in a single dose in the morning. For patients with shift work sleep disorder, 150 mg given daily approximately 1 hour prior to the start of their work shift. Note: In patients with OSAHS, doses up to 250 mg/day, given as a single dose, have been welltolerated, but there is no consistent evidence that this dose confers additional benefit beyond that of the 150 mg/day dose. Provigil Generic: Modafinil tablets system stimulant, analeptic Manufacturer: Cephalon Inc. Indication: Improve wakefulness in patients with exessive sleepiness associated with narcolepsy, obstructive sleep apnea/hypopnea syndrome, and shift work sleep disorder Recommended dose: 100 mg to 200 mg once daily Ritalin Generic: Methylphenidate hydrochloride tablets USP Description: Mild central nervous system stimulant Manufacturer: Novartis Pharmaceuticals Corp. Indication: Narcolepsy Recommended dose: Adults: 20 to 30 mg daily; 6 years and older: 5 mg bid Xyrem Oral Solution Generic: Sodium oxybate system depressant. (This drug only is available via Xyrem Success Program, a restricted distribution risk management program and registry to approved practitioners.) Manufacturer: Jazz excessive daytime sleepiness and cataplexy in patients with narcolepsy Recommended dose: 4.5 g/day up to 9 g/day NEONATAL RESPIRATORY DISTRESS SYNDROME CAFCIT Injection 20mg/mL and Oral Solution 20mg/mL Generic: Caffeine citrate system stimulant Manufacturer: Bedford Laboratories Indication: Shortterm treatment of apnea of prematurity in infants 28 to 33 weeks old Recommended dose: Initial dose:
5 Pharmaceutical Listing 2009 Buyers Guide 1 ml/kg intravenously over 30 minutes; Maintenance dose: 0.25 ml/kg every 24 hours CUROSURF Intratracheal Suspension Generic: Poractant alfa Description: Pulmonary surfactant Indication: Treatment (rescue) of RDS in premature infants Recommended dose: 2.5 ml/kg birth weight with subsequent doses of 1.25 ml/kg birth weight every 12 hours if needed Infasurf Intratracheal Suspension Generic: Calfactant Description: Pulmonary surfactant Manufacturer: Forest Indication: Prevention of RDS in premature infants at high risk for RDS and for the treatment (rescue) of premature infants who develop RDS Recommended dose: 3 ml/kg body weight at birth Survanta Intratracheal Suspension Generic: Beractant Description: Pulmonary surfactant Manufacturer: Abbott Laboratories Inc. Indication: Prevention and treatment (rescue) of RDS in premature infants Recommended dose: 100 mg of phospholipids/kg birth weight (4 ml/kg) PULMONARY HYPERTENSION Epoprostenol sodium Description: Prostacyclin analog Manufacturer: Teva Parenteral Medicines Inc. Indication: Longterm intravenous treatment of primary pulmonary hyptertension and pulmonary hypertension associated with the scleroderma spectrum of in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy. Recommended dose: Chronic infusion should be initiated at 2 ng/ kg/min and increased in increments of 2 ng/kg/min every 15 minutes or longer until doselimiting pharmacologic effects are elicited or until a tolerance limit to the drug is established and further increases in infusion rate are not clinically warranted. Flolan Generic: Epoprostenol sodium Description: Prostacyclin analog Indication: Longterm intravenous treatment of primary pulmonary hyptertension and pulmonary hypertension associated with the scleroderma spectrum of in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy. Recommended dose: Chronic infusion should be initiated at 2 ng/ kg/min and increased in increments of 2 ng/kg/min every 15 minutes or longer until doselimiting pharmacologic effects are elicited or until a tolerance limit to the drug is established and further increases in infusion rate are not clinically warranted. Letairis Generic: Ambrisentan Description: TypeA selective endothelin receptor ant Manufacturer: Gilead Sciences Inc. pulmonary arterial hypertension in subjects with WHO class II or III symptoms to improve exercise capacity and delay clinical worsening. Recommended dose: Initial dose: 5 mg once daily. The dose may be increased to 10 mg once daily if the 5 mg dose is tolerated. Remodulin Generic: Treprostinil sodium Description: Prostacyclin analog Manufacturer: United Therapeutics Corp. Indication: Continuous subcutaneous infusion for the treatment of pulmonary arterial hypertension in patients with NYHA Class IIIV symptoms to diminish symptoms associated with exercise Recommended dose: Supplied in 20 ml vials in concentrations of 1.0 mg/ml, 2.5 mg/ml, 5.0 mg/ ml, and 10.0 mg/ml. Infusion rate is initiated at 1.25 ng/kg/min. If this initial dose cannot be tolerated, the infusion rate should be reduced to ng/kg/min. Revatio Tablets Generic: Sildenafil citrate Description: Phosphodiesterase type5 (PDE5) inhibitor Manufacturer: Pfizer Inc. Indication: For the treatment of pulmonary arterial hypertension (WHO Group I) to improve exercise ability Recommended dose: One 20 mg tablet tid Tracleer Tablets Generic: Bosentan Description: Endothelin receptor Manufacturer: Actelion Pharmaceuticals US Inc. pulmonary arterial hypertension in patients with WHO class III or IV symptoms, to improve exercise ability and decrease the rate of clinical worsening Recommended dose: 62.5 mg bid for 4 weeks, then increase to maintenance dose of 125 mg bid Ventavis Inhalation Solution Generic: Iloprost Description: Synthetic prostacylin Manufacturer: Actelion Pharmaceuticals US Inc. pulmonary arterial hypertension in patients with NYHA Class III or IV symptoms Recommended dose: Inhalation of 2.5 mcg 6 to 9 times daily using either the Prodose AAD System or the Ineb AAD System RESTLESS LEGS SYNDROME Mirapex mg, 0.25 mg, 0.5 mg Tablets Generic: Pramipexole dihydrochloride Description: Nonergot dopamine Indication: Treatment for those patients with moderatetosevere primary RLS Recommended dose: The recommended starting dose for RLS is mg taken once daily, 2 to 3 hours before bedtime. For patients requiring additional symptomatic relief, the dose may be increased every 4 to 7 days. Requip Tablets Generic: Ropinirole HCl Description: Secondgeneration dopamine Indication: Treatment for those patients with moderatetosevere primary RLS Recommended dose: Adult starting dosage for RLS: 0.25 mg once daily, 1 to 3 hours before bedtime. After two days, the dosage can be increased to 0.5 mg once daily and to 1 mg once daily at the end of the first week of dosing, then titrated as needed to achieve efficacy. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. Ropinirole Description: Secondgeneration dopamine Indication: Treatment for those patients with moderatetosevere primary RLS Recommended dose: Adult starting dosage for RLS is 0.25 mg once daily, 1 to 3 hours before bedtime. After two days, the dosage can be increased to 0.5 mg once daily and to 1 mg once daily at the end of the first week of dosing, then titrated as needed to achieve efficacy. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. SEVERE SEPSIS Xigris Generic: Drotrecogin alfa (activated) Description: Recombinant of human Activated Protein C Manufacturer: Eli Lilly and Co. Indication: Reduction of mortality in adult patients with severe sepsis who have a high risk of death Recommended dose: Administered intravenously at an infusion rate of 24 mcg/kg/hr for a total of 96 hours SMOKING CESSATION Chantix 1 mg Tablets Generic: Varenicline Description: Nicotine receptor binding agent Manufacturer: Pfizer Inc. cessation treatment bid Commit Lozenge, 2 mg and 4 mg Generic: Nicotine cessation for relief of nicotine Recommended dose: One lozenge every 1 to 2 hours to start, reducing use by increasing duration between lozenges. Varies with time to first cigarette.
6 Nicorette Gum, 4 mg and 8 mg Generic: Nicotine cessation for relief of nicotine Recommended dose: Slowly chew one piece of gum when urge to smoke occurs. Maximum dose is 60 mg/day. Nicotine Transdermal Patch cessation for relief of nicotine Recommended dose: Apply one patch to skin daily and leave on for 16 to 24 hours for six to eight weeks. Nicotrol Inhaler Generic: Nicotine inhalation system Description: Nicotine replacement therapy Manufacturer: Pfizer Consumer Healthcare cessation for the relief of nicotine Recommended dose: Initial treatment: 6 to 16 cartridges/day for up to 12 weeks with gradual reduction after 12 weeks as needed Nicotrol NS Generic: Nicotine nasal spray Description: Nicotine replacement therapy Manufacturer: Pfizer Consumer Healthcare cessation for the relief of nicotine Recommended dose: One inhalation per nostril once or twice an hour (one dose = 1 mg nicotine), which can be increased to 80 sprays (40 mg) daily ZYBAN Sustained Release Tablets Generic: Bupropion hydrochloride Description: Nonnicotine aid cessation treatment Recommended dose: 300 mg/day given as 150 mg bid n ADVANCE thanks Michael J. McClusky, RPh, and Health Net Pharmaceutical Services, Rancho Cordova, Calif., for reviewing this guide.
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Foundations of Pharmacology
 Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Medications Affecting The Respiratory System
 Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
Asthma medications: Know your options - MayoClinic.com. Asthma medications: Know your options
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
Medications for Managing COPD in Hospice Patients. Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources
 Medications for Managing COPD in Hospice Patients Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources Goal of medications in COPD Decrease symptoms and/or complications Reduce frequency
Medications for Managing COPD in Hospice Patients Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources Goal of medications in COPD Decrease symptoms and/or complications Reduce frequency
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Select Inhaled Respiratory Agents
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Pain Oral-Intranasal Fentanyl (Abstral, Actiq, Fentora, Lazanda, Onsolis, Subsys)
 Pennsylvania Employees Benefit Trust Fund (PEBTF) and n- Medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
Pennsylvania Employees Benefit Trust Fund (PEBTF) and n- Medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Seebri Neohaler) Reference Number: CP.CPA.150 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Seebri Neohaler) Reference Number: CP.CPA.150 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy
10/18/2012. Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C
 Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Common Inhaled Asthma Medications Dose Comparison and Tips for Use
 Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
Commissioner for the Department for Medicaid Services Selections for Preferred Products
 Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner for the Department for
Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner for the Department for
Women Beware-The Threat of COPD
 Page 1 Speaker: Catherine Cooke attained her Bachelor in Pharmacy from the University of Iowa and then went on to receive her Pharm.D. from the Medical University of South Carolina. Subsequently, she completed
Page 1 Speaker: Catherine Cooke attained her Bachelor in Pharmacy from the University of Iowa and then went on to receive her Pharm.D. from the Medical University of South Carolina. Subsequently, she completed
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless?
 Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
FASENRA (benralizumab)
 FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Key features and changes to these four components of asthma care include:
 Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE
 REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE ID NUMBER: 0a) Date of Collection / / 0b) Staff Code Instructions: This form should be completed during the participant s clinic visit. 1) Are you regularly
REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE ID NUMBER: 0a) Date of Collection / / 0b) Staff Code Instructions: This form should be completed during the participant s clinic visit. 1) Are you regularly
End Stage COPD Guidance Document
 End Stage COPD Guidance Document Suggested Guidelines for the Determination of Hospice Eligibility A patient with severe chronic pulmonary disease that meets the following criteria may be eligible for
End Stage COPD Guidance Document Suggested Guidelines for the Determination of Hospice Eligibility A patient with severe chronic pulmonary disease that meets the following criteria may be eligible for
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
QUANTITY LIMIT CRITERIA. BROVANA (arformoterol tartrate) SEREVENT DISKUS (salmeterol) STRIVERDI RESPIMAT (olodaterol)
 Carelirst. +.V Family of health care plans DRUG CLASS COMBINATIONS QUANTITY LIMIT CRITERIA LONG ACTING BETA2-ADRENERGIC AGONIST, ORAL INHALATION BRAND NAME (generic) LONG-ACTING BETA2-ADRENERGIC AGONISTS:
Carelirst. +.V Family of health care plans DRUG CLASS COMBINATIONS QUANTITY LIMIT CRITERIA LONG ACTING BETA2-ADRENERGIC AGONIST, ORAL INHALATION BRAND NAME (generic) LONG-ACTING BETA2-ADRENERGIC AGONISTS:
Respiratory Medications and Devices Update 2/15
 Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 07/05/18 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
COPD Medicine. No one ever showed me how to use this. Wendy Happel; RRT, COPD Educator Krystal Fedoris; RRT-NPS, BA, COPD Educator
 Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Women Beware The Threat of COPD
 Page 1 Women Beware The Threat of COPD Catherine E. Cooke, PharmD, BCPS, PAHM President, PosiHealth, Inc. & Clinical Associate Professor, University of Maryland School of Pharmacy Supported by an education
Page 1 Women Beware The Threat of COPD Catherine E. Cooke, PharmD, BCPS, PAHM President, PosiHealth, Inc. & Clinical Associate Professor, University of Maryland School of Pharmacy Supported by an education
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Drugs that Affect the Respiratory System BROOKE BENTLEY, PHD, APRN
 Drugs that Affect the Respiratory System BROOKE BENTLEY, PHD, APRN Goals of Therapy Asthma Prevent symptoms COPD Reduce COPD symptoms Decrease use of SABAs Maintain normal pulm function & prevent loss
Drugs that Affect the Respiratory System BROOKE BENTLEY, PHD, APRN Goals of Therapy Asthma Prevent symptoms COPD Reduce COPD symptoms Decrease use of SABAs Maintain normal pulm function & prevent loss
ANTINEOPLASTIC DRUGS CHAPTER 21. Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component
 ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Asthma. Definition. Symptoms
 Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
STRIVERDI RESPIMAT (olodaterol hcl) aerosol
 STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS
 Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
AIRDUO RESPICLICK (fluticasone-salmeterol) aerosol DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol
 DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Assessing Severity. Management of Stable COPD. General Approach. Short Acting Bronchodilators. Staging System (GOLD)
 William P. Saliski Jr. DO Montgomery Pulmonary Consultants Management of Stable COPD Pharmacotherapy Oxygen Smoking Cessation Vaccinations Rehabilitation Surgery Future Discussions Assessing Severity Staging
William P. Saliski Jr. DO Montgomery Pulmonary Consultants Management of Stable COPD Pharmacotherapy Oxygen Smoking Cessation Vaccinations Rehabilitation Surgery Future Discussions Assessing Severity Staging
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Oregon Health Plan prescription benefit updates
 Oregon Health Plan prescription benefit updates EOCCO s prescription program is a pharmacy benefit that offers members a choice of safe and effective medication treatments. The program also helps you save
Oregon Health Plan prescription benefit updates EOCCO s prescription program is a pharmacy benefit that offers members a choice of safe and effective medication treatments. The program also helps you save
Cinqair (reslizumab injection for intravenous use)
 Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Staying Healthy. with Asthma. Illustrations by paulsharp.com
 Staying Healthy with Asthma Illustrations by paulsharp.com Lungs & Asthma What is Asthma? Inflammation or swelling of airways that leads to: 1) Mucous production deep inside the airways. 2) Temporary difficulty
Staying Healthy with Asthma Illustrations by paulsharp.com Lungs & Asthma What is Asthma? Inflammation or swelling of airways that leads to: 1) Mucous production deep inside the airways. 2) Temporary difficulty
Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline. MedStar Health
 Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
500 micrograms/dose Turbohaler dry powder device 500 micrograms/ml injection. 12 micrograms/dose Turbohaler dry powder device
 3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
Proposed Preferred Drug List. Clinical Criteria
 Proposed Preferred Drug List with Clinical Criteria Proposal for TennCare February 7, 2008 Page 1 of 87 PDL Decision Process The primary clinical decision that needs to be made is determining if the drugs
Proposed Preferred Drug List with Clinical Criteria Proposal for TennCare February 7, 2008 Page 1 of 87 PDL Decision Process The primary clinical decision that needs to be made is determining if the drugs
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
The Medical Letter. on Drugs and Therapeutics. Usual Adult Hypnotic Dose 1,2 Some Adverse Effects Comments Cost 3
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date: Last Review Date: 08.17
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Relative Cost/Month. Less than $10. Loratadine Liquid* $10-$15 Cetirizine liquid 1mg/mL*
 Allergy Chlorpheniramine Tablet* Diphenhydramine Tablet* Diphenhydramine Liquid* Loratadine Tablet* Cetirizine Tablet* Loratadine 10mg ODT* Less than $10 Loratadine Liquid* $10-$15 Cetirizine liquid 1mg/mL*
Allergy Chlorpheniramine Tablet* Diphenhydramine Tablet* Diphenhydramine Liquid* Loratadine Tablet* Cetirizine Tablet* Loratadine 10mg ODT* Less than $10 Loratadine Liquid* $10-$15 Cetirizine liquid 1mg/mL*
Asthma/COPD Update with Inhaler Workshop
 Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Medication Acronyms SABA: Short acting
Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Medication Acronyms SABA: Short acting
BNF CHAPTER 3: RESPIRATORY
 3.1 BRONCHODILATORS BNF CHAPTER 3: RESPIRATORY 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated (e.g., Easi-Breathe
3.1 BRONCHODILATORS BNF CHAPTER 3: RESPIRATORY 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated (e.g., Easi-Breathe
Aerospan (flunisolide)
 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
Clinical Guideline for the Diagnosis, Evaluation, and Management of Adults and Children with Asthma
 Clinical Guideline for the Diagnosis, Evaluation, and Management of Adults and Children with Asthma - 2005 Criteria that suggest the diagnosis of Asthma: The symptoms of dyspnea, cough and/or wheezing,
Clinical Guideline for the Diagnosis, Evaluation, and Management of Adults and Children with Asthma - 2005 Criteria that suggest the diagnosis of Asthma: The symptoms of dyspnea, cough and/or wheezing,
Provider Respiratory Inservice
 Provider Respiratory Inservice 2 Welcome Opening Remarks We will cover: Definition of Asthma & COPD Evidence based guidelines for diagnosis, evaluation, and management of asthma Evidence based guidelines
Provider Respiratory Inservice 2 Welcome Opening Remarks We will cover: Definition of Asthma & COPD Evidence based guidelines for diagnosis, evaluation, and management of asthma Evidence based guidelines
FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS*
 FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS* 0 4 Years Age and Adults Potential Adverse Effects Inhaled Corticosteroids (See Figure 18, Estimated Comparative Daily Dosages for ICSs. ) Oral
FIGURE 17. USUAL DOSAGES FOR LONG-TERM CONTROL MEDICATIONS* 0 4 Years Age and Adults Potential Adverse Effects Inhaled Corticosteroids (See Figure 18, Estimated Comparative Daily Dosages for ICSs. ) Oral
Sedative Hypnotics. Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.11 Subject: Sedative Hypnotics Page: 1 of 7 Last Review Date: December 8, 2017 Sedative Hypnotics
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.11 Subject: Sedative Hypnotics Page: 1 of 7 Last Review Date: December 8, 2017 Sedative Hypnotics
Drug Effectiveness Review Project Summary Report
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Asthma COPD Update 2018
 Asthma COPD Update 2018 Roger Hefflinger, Pharm.D. Clinical Associate Professor ISU COP Clinical Teaching Pharmacist Family Medicine Residency of Idaho In support of improving patient care, Idaho State
Asthma COPD Update 2018 Roger Hefflinger, Pharm.D. Clinical Associate Professor ISU COP Clinical Teaching Pharmacist Family Medicine Residency of Idaho In support of improving patient care, Idaho State
Drug Class Monograph
 Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Asthma in Pregnancy. Asthma. Chronic Airway Inflammation. Objective Measures of Airflow. Peak exp. flow rate (PEFR)
 Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Question I was one of the first dry power devices available in the US Flovent, Serevent and Advair are all available in this device
 What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
Learning Objectives 4/26/2012. Review normal lung function and COPD pathophysiology Discuss pharmacological management
 Marliese Gibson PharmD HospiScript, a Catalyst Rx Company May 16, 2012 Chronic Obstructive Pulmonary Disease Learning Objectives Review normal lung function and COPD pathophysiology Discuss pharmacological
Marliese Gibson PharmD HospiScript, a Catalyst Rx Company May 16, 2012 Chronic Obstructive Pulmonary Disease Learning Objectives Review normal lung function and COPD pathophysiology Discuss pharmacological
Sedative Hypnotics. Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.11 Subject: Sedative Hypnotics Page: 1 of 8 Last Review Date: September 15, 2016 Sedative Hypnotics
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.11 Subject: Sedative Hypnotics Page: 1 of 8 Last Review Date: September 15, 2016 Sedative Hypnotics
31 - Respiratory System
 31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
Reference Guide for Caring for Pediatric Patients with Asthma
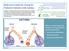 Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Step Therapy Medications
 Step Therapy Medications Step Therapy (ST PA ) is an automated form of prior authorization. It encourages the use of therapies that should be tried first, before other treatments are covered, based on
Step Therapy Medications Step Therapy (ST PA ) is an automated form of prior authorization. It encourages the use of therapies that should be tried first, before other treatments are covered, based on
3/24/2017. Learning Objectives. Current Issues In Airway Pharmacology. Beta-Agonists. Bronchodilating Agents. Adrenergic Autonomic Control
 Learning Objectives Current Issues In Airway Pharmacology Arthur Jones, EdD, RRT This Presentation is Approved for 1.5 CRCE Credit Hours Explain the actions, effects, indications, & contraindications for
Learning Objectives Current Issues In Airway Pharmacology Arthur Jones, EdD, RRT This Presentation is Approved for 1.5 CRCE Credit Hours Explain the actions, effects, indications, & contraindications for
Methacholine Challenge Test
 PATIENT & CAREGIVER EDUCATION Methacholine Challenge Test This information will help you prepare for your methacholine challeng e test at Memorial Sloan Kettering (MSK). A methacholine (meth-a-kole-leen)
PATIENT & CAREGIVER EDUCATION Methacholine Challenge Test This information will help you prepare for your methacholine challeng e test at Memorial Sloan Kettering (MSK). A methacholine (meth-a-kole-leen)
MANAGING ASTHMA. Nancy Davis, RRT, AE-C
 MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Q: Should patients with mild asthma
 1-MINUTE CONSULT CME CREDIT EDUCATIONAL OBJECTIVE: Readers will consider prescribing inhaled corticosteroids to their patients who have mild persistent asthma brief answers to specific clinical questions
1-MINUTE CONSULT CME CREDIT EDUCATIONAL OBJECTIVE: Readers will consider prescribing inhaled corticosteroids to their patients who have mild persistent asthma brief answers to specific clinical questions
Improving Outcomes in COPD
 Neil MacIntyre MD Duke University Durham NC Current treatment guidelines f COPD focus Barriers to providing optimal treatment Diagnosis of COPD EXPOSURE TO RISK FACTORS AND/ OR SYMPTOMS sputum cough dyspnea
Neil MacIntyre MD Duke University Durham NC Current treatment guidelines f COPD focus Barriers to providing optimal treatment Diagnosis of COPD EXPOSURE TO RISK FACTORS AND/ OR SYMPTOMS sputum cough dyspnea
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed Formulary Status. TA Number. Section.
 Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES. SEDATIVE HYPNOTIC AGENTS Generic Brand HICL GCN Exception/Other ZOLPIDEM
 Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
ASTHMA IN THE PEDIATRIC POPULATION
 ASTHMA IN THE PEDIATRIC POPULATION SEARCH Rotation 2 August 23, 2010 Objectives Define asthma as a chronic disease Discuss the morbidity of asthma in pediatrics Discuss a few things that a health center
ASTHMA IN THE PEDIATRIC POPULATION SEARCH Rotation 2 August 23, 2010 Objectives Define asthma as a chronic disease Discuss the morbidity of asthma in pediatrics Discuss a few things that a health center
Allergies and Asthma 5/21/2013. Objectives. Allergic Rhinitis (AR): Risk Factor for ASTHMA. Rhinitis and Asthma
 Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Inhaled Corticosteroid Dose Comparison in Asthma
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
4/3/2018 BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018
 BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018 Approach to treatment List of medication Abbreviations When to use medications Pharmokinetics Pharmacodynamics Step wise therapy both for acute and chronic
BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018 Approach to treatment List of medication Abbreviations When to use medications Pharmokinetics Pharmacodynamics Step wise therapy both for acute and chronic
Nancy Davis, RRT, AE-C
 Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
Breath of Fresh Air. nniversary 20th. New National Asthma Survey: How Does Your Asthma Compare? Long-Acting Bronchodilators: Why All the Fuss?
 Information, news and advice for improving asthma well-being Volume 12, No. 1 Summer 2010 New National Asthma Survey: How Does Your Asthma Compare? nniversary 20th Sometimes if one has to live with a chronic
Information, news and advice for improving asthma well-being Volume 12, No. 1 Summer 2010 New National Asthma Survey: How Does Your Asthma Compare? nniversary 20th Sometimes if one has to live with a chronic
Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management. Colleen Sakon, PharmD BCPS September 27, 2018
 Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management Colleen Sakon, PharmD BCPS September 27, 2018 Disclosures I have no actual or potential conflicts of interest 2 Objectives Summarize
Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management Colleen Sakon, PharmD BCPS September 27, 2018 Disclosures I have no actual or potential conflicts of interest 2 Objectives Summarize
9/14/12. Nicole Paterson, PharmD BCPS Medication Therapy Management Provider Fairview Pharmacy Services, LLC
 Nicole Paterson, PharmD BCPS Medication Therapy Management Provider Fairview Pharmacy Services, LLC } Describe the pathophysiology of asthma and Chronic Obstructive Pulmonary Disease (COPD). } Review the
Nicole Paterson, PharmD BCPS Medication Therapy Management Provider Fairview Pharmacy Services, LLC } Describe the pathophysiology of asthma and Chronic Obstructive Pulmonary Disease (COPD). } Review the
Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists. Learning Objectives.
 Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists Learning Objectives Identify the risk factors for COPD and the clinical features that differentiate
Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists Learning Objectives Identify the risk factors for COPD and the clinical features that differentiate
Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017
 Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
History & Development
 RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
PULMONARY ARTERIAL HYPERTENSION AGENTS
 Approvable Criteria: PULMONARY ARTERIAL HYPERTENSION AGENTS Brand Name Generic Name Length of Authorization Adcirca tadalafil Calendar Year Adempas riociguat Calendar Year Flolan epoprostenol sodium Calendar
Approvable Criteria: PULMONARY ARTERIAL HYPERTENSION AGENTS Brand Name Generic Name Length of Authorization Adcirca tadalafil Calendar Year Adempas riociguat Calendar Year Flolan epoprostenol sodium Calendar
Secretary for Health and Family Services Selections for Preferred Products
 Secretary for Health and Family Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Secretary for Health and Family Services based
Secretary for Health and Family Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Secretary for Health and Family Services based
South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina
 South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina 29202-8206 Pharmacy and Therapeutics (P&T) Committee Meeting MINUTES 1. Call To Order A meeting of the
South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina 29202-8206 Pharmacy and Therapeutics (P&T) Committee Meeting MINUTES 1. Call To Order A meeting of the
Clinical Policy: Dupilumab (Dupixent) Reference Number: ERX.SPA.49 Effective Date:
 Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS:
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS: LOB: Respiratory Disorders Medi-Cal REVIEW HISTORY (MONTH/YEAR) 12/17,12/16, 5/15,
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS: LOB: Respiratory Disorders Medi-Cal REVIEW HISTORY (MONTH/YEAR) 12/17,12/16, 5/15,
Asthma By Mayo Clinic staff
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
Taking Control of Asthma Through Proper Medication Selection and the Use of Asthma Action Plans Julie M. Koehler, Pharm.D., FCCP
 Taking Control of Asthma Through Proper Medication Selection and the Use of Asthma Action Plans Julie M. Koehler, Pharm.D., FCCP Associate Dean for Clinical Education and External Affiliations & Professor
Taking Control of Asthma Through Proper Medication Selection and the Use of Asthma Action Plans Julie M. Koehler, Pharm.D., FCCP Associate Dean for Clinical Education and External Affiliations & Professor
Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients
 Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients Maren A. McGurran, PharmD, BCPS; Lisa M. Richter, PharmD, BCPS, BCCCP; Nathan D. Leedahl,
Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients Maren A. McGurran, PharmD, BCPS; Lisa M. Richter, PharmD, BCPS, BCCCP; Nathan D. Leedahl,
The Latest Medications A Pharmacological Update for RTs
 The Latest Medications A Pharmacological Update for RTs Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Georgia State University Objectives
The Latest Medications A Pharmacological Update for RTs Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Georgia State University Objectives
