MEDICAL ASSISTANCE BULLETIN
|
|
|
- Lewis Phelps
- 5 years ago
- Views:
Transcription
1 ISSUE DATE January 6, 2016 SUBJECT EFFECTIVE DATE January 20, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of COPD Agents Pharmacy Service Leesa M. Allen, Deputy Secretary Office of Medical Assistance Programs IMPORTANT REMINDER: All providers (including all associated service locations - 13 digits) who enrolled on or before March 25, 2011 must revalidate their enrollment information no later than March 24, New enrollment application including all revalidation requirements may be found at Please send in your application(s) as soon as possible. PURPOSE: The purpose of this bulletin is to: SCOPE: 1. Inform providers that the Department of Human Services (Department) will require prior authorization of tiotropium when prescribed for a diagnosis of asthma. 2. Issue updated handbook pages that include instructions on how to request prior authorization of prescriptions for COPD Agents, including the type of medical information needed to evaluate requests for medical necessity. This bulletin applies to all licensed pharmacies and prescribers enrolled in the Medical Assistance (MA) Program and providing services in the fee-for-service (FFS) delivery system, including pharmacy services to residents of long term care facilities. BACKGROUND: The Department s Pharmacy and Therapeutics (P&T) Committee meets semi-annually to review published peer-reviewed clinical literature and make recommendations relating to new drugs in therapeutic classes already included in the Preferred Drug List (PDL), changes in the status of drugs on the PDL from preferred to non-preferred and non-preferred to preferred, * COMMENTS QUESTIONS REGARDING THIS BULLETIN SHOULD BE DIRECTED TO: The appropriate toll free number for your provider type Visit the Office of Medical Assistance Programs Web site at
2 2 new quantity limits, and classes of drugs to be added to or deleted from the PDL. The P&T Committee also recommends new guidelines or modifications to existing guidelines to evaluate requests for prior authorization of prescriptions for medical necessity. DISCUSSION: During the November 3, 2015 meeting, the P&T Committee recommended that the Department require a clinical prior authorization of tiotropium when prescribed for a diagnosis of asthma. At the recommendation of the Committee, the guidelines to determine medical necessity of tiotropium when prescribed for a diagnosis of asthma were reviewed and agreed to by an independent medical specialist. The guidelines were then subject to public review and comment, and subsequently approved for implementation by the Department. The revised clinical review guidelines to determine the medical necessity of COPD Agents are included in the attached updated provider handbook pages. PROCEDURE: The procedures for prescribers to request prior authorization of COPD Agents are located in SECTION I of the Prior Authorization of Pharmaceutical Services Handbook. The Department will take into account the elements specified in the clinical review guidelines (which are included in the provider handbook pages in the SECTION II chapters related to COPD Agents) when reviewing the prior authorization request to determine medical necessity. As set forth in 55 Pa. Code (a), the procedures described in the handbook pages must be followed to ensure appropriate and timely processing of prior authorization requests for drugs that require prior authorization. ATTACHMENTS: Prior Authorization of Pharmaceutical Services Handbook - Updated pages SECTION II COPD Agents
3 I. Requirements for Prior Authorization of Chronic Obstructive Pulmonary Disease (COPD) Agents A. Prescriptions That Require Prior Authorization Prescriptions for COPD Agents that meet any of the following conditions must be prior authorized. 1. A prescription for a non-preferred COPD Agent. See the most recent version of the PDL which includes the list of preferred COPD Agents at: pdf 2. A prescription for either a preferred or non-preferred COPD Agent when there is a record of a recent paid claim for another drug in the same therapeutic class of drugs in PROMISe, the Department s Point-of-Sale On-Line Claims Adjudication System (therapeutic duplication). 3. A prescription for tiotropium for a diagnosis of asthma 4. A prescription for a preferred or non-preferred COPD Agent with a prescribed quantity that exceeds the quantity limit. See Quantity Limits for the list of drugs with quantity limits at: ument/s_ pdf B. Review of Documentation for Medical Necessity In evaluating a request for prior authorization of a prescription for a nonpreferred COPD Agent, the determination of whether the requested prescription is medically necessary will take into account the following: 1. For Daliresp (roflumilast), whether the recipient: a. Has a diagnosis of severe COPD as documented by medical history, physical exam findings, and lung function testing (Forced Expiratory Volume (FEV1) < 50% of predicted) that are consistent with severe COPD according to the most current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines on the diagnosis and management of COPD 1 January 20, 2016
4 b. Has a diagnosis of chronic bronchitis as documented by cough and sputum production for at least 3 months in each of 2 consecutive years c. Had other causes of their chronic airflow limitations excluded d. Continues to experience more than 2 exacerbations of COPD per year requiring Emergency Department visits, hospitalization, or oral steroid use despite maximum therapeutic doses of, intolerance, or contraindication to regular scheduled use of a: i. Long-acting inhaled beta 2 agonist ii. Preferred long-acting inhaled anticholinergic iii. Inhaled corticosteroid e. Does not have moderate to severe liver impairment (Child-Pugh B or more severe) f. Will not be taking strong cytochrome P450 enzyme inducers such as but not limited to rifampin, phenobarbital, carbamazepine, and phenytoin 2 January 20, 2016
5 g. Does not have suicidal ideations h. Was evaluated, treated, and determined to be a candidate for treatment with Daliresp by a psychiatrist if the recipient has a history of prior suicide attempt, bipolar disorder, major depressive disorder, schizophrenia, substance use disorders, anxiety disorders, borderline personality disorder and antisocial personality disorder OR i. For all others, had a mental health evaluation performed by the prescriber and determined to be a candidate for treatment with Daliresp In evaluating a request for a renewal of prior authorization of a prescription for Daliresp, the determination of whether the requested prescription is medically necessary will take into account whether the recipient: a. Has documented improvement in the forced expiratory volume (FEV 1 ) and FEV 1 /forced vital capacity (FVC) ratio, and a decrease in the frequency of COPD exacerbations b. Will not be taking strong cytochrome P450 enzyme inducers such as but not limited to rifampin, phenobarbital, carbamazepine, and phenytoin c. Does not have suicidal ideations 3 January 20, 2016
6 d. Was reevaluated and treated for new onset or worsening symptoms of anxiety and depression and determined to continue to be a candidate for treatment with Daliresp 2. For all other non-preferred COPD Agents, whether the recipient has a documented history of therapeutic failure, intolerance or contraindication of the preferred COPD Agents 3. For therapeutic duplication, whether: a. The recipient is being titrated to, or tapered from, a drug in the same class OR b. Supporting peer-reviewed literature or national treatment guidelines corroborate concomitant use of the medications being requested 4. For tiotropium when prescribed for a diagnosis of asthma, whether the recipient: a. Is 12 years of age or older b. Is currently receiving optimally tolerated doses of all of the following: i. Inhaled glucocorticoids ii. Long acting beta agonists OR c. Has a contraindication or intolerance to optimally titrated doses of all of the following: i. Inhaled glucocorticoids ii. Long acting beta agonists 4 January 20, 2016
7 5. The recipient does not meet the clinical review guidelines listed above, but in the professional judgment of the physician reviewer, the services are medically necessary to meet the medical needs of the recipient. In addition, if a prescription for either a preferred or non-preferred COPD Agent is in a quantity that exceeds the quantity limit, the determination of whether the prescription is medically necessary will also take into account the guidelines set forth in the Quantity Limits Chapter. C. Clinical Review Process Prior authorization personnel will review the request for prior authorization and apply the clinical guidelines in Section B. above, to assess the medical necessity of the request for a prescription for a COPD Agent. If the guidelines in Section B. are met, the reviewer will prior authorize the prescription. If the guidelines are not met, the prior authorization request will be referred to a physician reviewer for a medical necessity determination. Such a request for prior authorization will be approved when, in the professional judgment of the physician reviewer, the services are medically necessary to meet the medical needs of the recipient. D. Dose and Duration of Therapy Requests for prior authorization of Daliresp will be approved for up to 12 months. E. References: 1. Daliresp package insert. Forest Pharmaceuticals, Inc. St. Louis, MO, Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease. 3. American Psychiatric Association Practice Guideline for the Assessment and Treatment of Patients with Suicidal Behaviors, November Busse WW et.al. National Heart Lung and Blood Institute Guidelines for the Diagnosis and Management of Asthma (EPR-3). 5. Peters, S. et.al. Treatment of moderate persistent asthma in adolescents and adults. Up To Date. Accessed October 9, Wenzel, S. Treatment of severe asthma in adolescents and adults. Accessed October 9, January 20, 2016
8 7. Martin, R.J. Alternative and experimental agents for the treatment of asthma. Up To Date. Accessed October 9, January 20, 2016
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE July 5, 2016 SUBJECT EFFECTIVE DATE July 11, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants - Pharmacy Services Leesa M. Allen, Deputy Secretary
ISSUE DATE July 5, 2016 SUBJECT EFFECTIVE DATE July 11, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Anticoagulants - Pharmacy Services Leesa M. Allen, Deputy Secretary
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Analgesics, Narcotic Long Acting and Analgesics, Narcotic Short
ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Analgesics, Narcotic Long Acting and Analgesics, Narcotic Short
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Anticoagulants A. Prescriptions That Require Prior Authorization Prescriptions for Anticoagulants
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Analgesics, Narcotic Long Acting A. Prescriptions That Require Prior Authorization Prescriptions for Analgesics, Narcotic Long Acting
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Analgesics, Narcotic Long Acting A. Prescriptions That Require Prior Authorization Prescriptions for Analgesics, Narcotic Long Acting
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Antipsychotics
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Antipsychotics A. Prescriptions That Require Prior Authorization Prescriptions for Antipsychotics
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Antipsychotics A. Prescriptions That Require Prior Authorization Prescriptions for Antipsychotics
Clinical Policy: Roflumilast (Daliresp) Reference Number: CP.PMN.46. Line of Business: Medicaid
 Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Multiple Sclerosis Agents
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Multiple Sclerosis Agents a. Prescriptions That Require Prior Authorization Prescriptions for Multiple Sclerosis Agents which meet any
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Multiple Sclerosis Agents a. Prescriptions That Require Prior Authorization Prescriptions for Multiple Sclerosis Agents which meet any
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE February 18, 2015 SUBJECT EFFECTIVE DATE January 21, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Drug List (PDL) Update January 21, 2015 Pharmacy Services Vincent D. Gordon, Deputy
ISSUE DATE February 18, 2015 SUBJECT EFFECTIVE DATE January 21, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Drug List (PDL) Update January 21, 2015 Pharmacy Services Vincent D. Gordon, Deputy
Brand Name: Daliresp. Generic Name: roflumilast. Manufacturer: Forest Pharmaceuticals, Inc.
 Brand Name: Daliresp Generic Name: roflumilast 3, 4, 5 Manufacturer: Forest Pharmaceuticals, Inc. 1, 2,4,5,7 Drug Class: Second Generation Phosphodiesterase 4 (PDE 4) inhibitor Labeled Uses: prophylaxis
Brand Name: Daliresp Generic Name: roflumilast 3, 4, 5 Manufacturer: Forest Pharmaceuticals, Inc. 1, 2,4,5,7 Drug Class: Second Generation Phosphodiesterase 4 (PDE 4) inhibitor Labeled Uses: prophylaxis
Clinical Policy: Roflumilast (Daliresp) Reference Number: CP.PMN.46 Effective Date: Last Review Date: 08.18
 Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11.01.11 Last Review Date: 08.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11.01.11 Last Review Date: 08.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Fluticasone/Salmeterol (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 08/16 Last Review Date: 08/17
 Clinical Policy: (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this
Chronic Obstructive Pulmonary Disease (COPD) Clinical Guideline
 Chronic Obstructive Pulmonary Disease (COPD) Clinical These clinical guidelines are designed to assist clinicians by providing an analytical framework for the evaluation and treatment of patients. They
Chronic Obstructive Pulmonary Disease (COPD) Clinical These clinical guidelines are designed to assist clinicians by providing an analytical framework for the evaluation and treatment of patients. They
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. A. Prescriptions That Require Prior Authorization
 MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Xyrem A. Prescriptions That Require Prior Authorization All prescriptions for Xyrem must be prior authorized. B. Review of Documentation
MEDICAL ASSISTANCE HBOOK I. Requirements for Prior Authorization of Xyrem A. Prescriptions That Require Prior Authorization All prescriptions for Xyrem must be prior authorized. B. Review of Documentation
Rexulti (brexpiprazole)
 Market DC Rexulti (brexpiprazole) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Indiana see State Specific Mandates below *Maryland see State Specific Mandates below *Virginia
Market DC Rexulti (brexpiprazole) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Indiana see State Specific Mandates below *Maryland see State Specific Mandates below *Virginia
CARE OF THE ADULT COPD PATIENT
 CARE OF THE ADULT COPD PATIENT Target Audience: The target audience for this clinical guideline is all MultiCare providers and staff including those associated with our Clinically Integrated Network. The
CARE OF THE ADULT COPD PATIENT Target Audience: The target audience for this clinical guideline is all MultiCare providers and staff including those associated with our Clinically Integrated Network. The
Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD)
 Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD) A Pulmonary Diagnosis Form is filled out by the reviewer for all medical records that are sent to them for review by the CSCC.
Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD) A Pulmonary Diagnosis Form is filled out by the reviewer for all medical records that are sent to them for review by the CSCC.
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Fluticasone/Salmeterol (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan See Important Reminder
Clinical Policy: Fluticasone/Salmeterol (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan See Important Reminder
Guideline for the Diagnosis and Management of COPD
 Guideline for the Diagnosis and Management of COPD Introduction Chronic obstructive pulmonary disease (COPD) is a respiratory disorder largely caused by smoking. It is characterized by progressive, partially
Guideline for the Diagnosis and Management of COPD Introduction Chronic obstructive pulmonary disease (COPD) is a respiratory disorder largely caused by smoking. It is characterized by progressive, partially
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.PMN.69 Effective Date: 11/15 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.69 Effective Date: 11/15 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis.
 1 Definition of COPD: COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis. Airflow obstruction may be accompanied by airway hyper-responsiveness
1 Definition of COPD: COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis. Airflow obstruction may be accompanied by airway hyper-responsiveness
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
CME/CE POSTTEST CME/CE QUESTIONS
 CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Indacaterol/Glycopyrrolate (Utibron Neohaler) Reference Number: CP.PMN.147 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important
Clinical Policy: Indacaterol/Glycopyrrolate (Utibron Neohaler) Reference Number: CP.PMN.147 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important
EXACERBATION ASSESSMENT FORM
 EXACERBATION ASSESSMENT FORM ID NUMBER: 0a) Form Completion Date... 0b) Staff Code... Administrative Information 1) Date of clinic visit: 2) What type of Event is this?... Participant/HCU-triggered...
EXACERBATION ASSESSMENT FORM ID NUMBER: 0a) Form Completion Date... 0b) Staff Code... Administrative Information 1) Date of clinic visit: 2) What type of Event is this?... Participant/HCU-triggered...
Drug Use Evaluation: Low Dose Quetiapine
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
EXACERBATION ASSESSMENT FORM
 EXACERBATION ASSESSMENT FORM ID NUMBER: VERSION: 1.0 05/27/14 0a) Form Completion Date... 0b) Staff Code... Instructions: This form should be completed when a participant comes to the clinical center for
EXACERBATION ASSESSMENT FORM ID NUMBER: VERSION: 1.0 05/27/14 0a) Form Completion Date... 0b) Staff Code... Instructions: This form should be completed when a participant comes to the clinical center for
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide
 VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES
 JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
Roflumilast for the management of severe chronic obstructive pulmonary disease
 Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 www.nice.org.uk/ta244 NHS Evidence has accredited the process used by the Centre for Health Technology
Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 www.nice.org.uk/ta244 NHS Evidence has accredited the process used by the Centre for Health Technology
Roflumilast for the management of severe chronic obstructive pulmonary disease
 Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 guidance.nice.org.uk/ta244 NICE has accredited the process used by the Centre for Health Technology Evaluation
Roflumilast for the management of severe chronic obstructive pulmonary disease Issued: January 2012 guidance.nice.org.uk/ta244 NICE has accredited the process used by the Centre for Health Technology Evaluation
Nucala (mepolizumab) Prior Authorization Protocol
 Nucala (mepolizumab) Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
Nucala (mepolizumab) Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
2/4/2019. GOLD Objectives. GOLD 2019 Report: Chapters
 GOLD Objectives To provide a non biased review of the current evidence for the assessment, diagnosis and treatment of patients with COPD. To highlight short term and long term treatment objectives organized
GOLD Objectives To provide a non biased review of the current evidence for the assessment, diagnosis and treatment of patients with COPD. To highlight short term and long term treatment objectives organized
VIDA SIGNIFICANT ASTHMA EXACERBATION (Visits 2-10, 88 and 90-92)
 VIDA SIGNIFICANT ASTHMA EXACERBATION (Visits 2-10, 88 and 90-92) Part. ID: - - Part. Initials: Visit: Visit Date: / / 20 Coordinator ID: (Coordinator Completed) For participants who meet treatment failure
VIDA SIGNIFICANT ASTHMA EXACERBATION (Visits 2-10, 88 and 90-92) Part. ID: - - Part. Initials: Visit: Visit Date: / / 20 Coordinator ID: (Coordinator Completed) For participants who meet treatment failure
Pulmonary Rehabilitation. Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education
 Pulmonary Rehabilitation Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education Pulmonary Rehabilitation Pulmonary Rehabilitation is a multi-disciplinary program of care for patients with chronic
Pulmonary Rehabilitation Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education Pulmonary Rehabilitation Pulmonary Rehabilitation is a multi-disciplinary program of care for patients with chronic
OFFICE OF MENTAL HEALTH AND SUBSTANCE ABUSE SERVICES BULLETIN
 OFFICE OF MENTAL HEALTH AND SUBSTANCE ABUSE SERVICES BULLETIN ISSUE DATE January 13, 2017 EFFECTIVE DATE: Immediately NUMBER: OMHSAS-17-02 SUBJECT: BY: Applied Behavioral Analysis Using Behavioral Specialist
OFFICE OF MENTAL HEALTH AND SUBSTANCE ABUSE SERVICES BULLETIN ISSUE DATE January 13, 2017 EFFECTIVE DATE: Immediately NUMBER: OMHSAS-17-02 SUBJECT: BY: Applied Behavioral Analysis Using Behavioral Specialist
Siliq. Siliq (brodalumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.28 Subject: Siliq Page: 1 of 5 Last Review Date: December 8, 2017 Siliq Description Siliq (brodalumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.28 Subject: Siliq Page: 1 of 5 Last Review Date: December 8, 2017 Siliq Description Siliq (brodalumab)
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #51 (NQF 0091): Chronic Obstructive Pulmonary Disease (COPD): Spirometry Evaluation National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #51 (NQF 0091): Chronic Obstructive Pulmonary Disease (COPD): Spirometry Evaluation National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM
 What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM Covering: Basic Definition New assessment criteria Some newer treatments BiPAP Not Covering: Definitions: Chronic Obstructive Pulmonary
What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM Covering: Basic Definition New assessment criteria Some newer treatments BiPAP Not Covering: Definitions: Chronic Obstructive Pulmonary
IL-5 Antagonists (IgG1 kappa) Fasenra (benralizumab) Nucala (mepolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.07 Subject: IL-5 Antagonists (IgG1 kappa) Page: 1 of 6 Last Review Date: June 22, 2018 IL-5 Antagonists
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.07 Subject: IL-5 Antagonists (IgG1 kappa) Page: 1 of 6 Last Review Date: June 22, 2018 IL-5 Antagonists
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date: Last Review Date: 08.17
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Clinical Policy: Opioid Analgesics Reference Number: CP.PMN.97 Effective Date: Last Review Date: 02.19
 Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Alabama Medicaid Pharmacy Override
 Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Long Term Care Formulary RS -29
 RESTRICTED USE Asthma/COPD Management 1 of 6 PROTOCOL: Asthma Glossary of Medication Acronyms: SABA: short-acting beta agonist (e.g. salbutamol) SABD: short-acting bronchodilator (e.g. ipratropium or SABA)
RESTRICTED USE Asthma/COPD Management 1 of 6 PROTOCOL: Asthma Glossary of Medication Acronyms: SABA: short-acting beta agonist (e.g. salbutamol) SABD: short-acting bronchodilator (e.g. ipratropium or SABA)
What is COPD? COPD Pharmacotherapy. COPD Mortality Is Increasing
 COPD Pharmacotherapy Chronic Bronchitis What is COPD? 75% 17.5% Emphysema Laura C. Feemster, MD, MS Assistant Professor University of Washington Division of Pulmonary & Critical Care April 23,2015 COPD
COPD Pharmacotherapy Chronic Bronchitis What is COPD? 75% 17.5% Emphysema Laura C. Feemster, MD, MS Assistant Professor University of Washington Division of Pulmonary & Critical Care April 23,2015 COPD
CEDAC FINAL RECOMMENDATION
 CEDAC FINAL RECOMMENDATION ROFLUMILAST (Daxas Nycomed Canada Inc.) Indication: Chronic Obstructive Pulmonary Disease Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
CEDAC FINAL RECOMMENDATION ROFLUMILAST (Daxas Nycomed Canada Inc.) Indication: Chronic Obstructive Pulmonary Disease Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 Page 1 of 9 PURPOSE To assure that DOP inmates with Pulmonary Diseases are receiving high quality Primary Care for their condition. POLICY All DOP Primary Care Providers and Chronic Disease Nurses are
Page 1 of 9 PURPOSE To assure that DOP inmates with Pulmonary Diseases are receiving high quality Primary Care for their condition. POLICY All DOP Primary Care Providers and Chronic Disease Nurses are
ADASUVE (LOXAPINE) INHALATION POWDER. EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS
 ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
Defining COPD. Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist
 Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
Defining COPD Georgina Grantham Community Respiratory Team Leader/ Respiratory Nurse Specialist Defining COPD Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Treatment. Assessing the outcome of interventions Traditionally, the effects of interventions have been assessed by measuring changes in the FEV 1
 58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
The Louis de la Parte Florida Mental Health Institute
 Data Brief December 2003 Mary Rose Murrin, M.A. Kelley Dhont, M.S. David Thornton, M.A. The Louis de la Parte Florida Mental Health Institute Children s Psychotropic Medication Use by Age and Diagnostic
Data Brief December 2003 Mary Rose Murrin, M.A. Kelley Dhont, M.S. David Thornton, M.A. The Louis de la Parte Florida Mental Health Institute Children s Psychotropic Medication Use by Age and Diagnostic
XOLAIR (omalizumab) Prior Authorization
 MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
PA Update: Oral Cystic Fibrosis Modulators
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Disclosure Statement. Epidemiological Data
 EVALUATION OF THE MEDICATION UTILIZATION OF COPD PATIENTS AT THE MIAMI VA HEALTHCARE SYSTEM Simone Edgerton, PharmD. PGY 1 Pharmacy Resident Miami VA Healthcare System Miami, Florida Simone.edgerton2@va.gov
EVALUATION OF THE MEDICATION UTILIZATION OF COPD PATIENTS AT THE MIAMI VA HEALTHCARE SYSTEM Simone Edgerton, PharmD. PGY 1 Pharmacy Resident Miami VA Healthcare System Miami, Florida Simone.edgerton2@va.gov
Turning Science into Real Life Roflumilast in Clinical Practice. Roland Buhl Pulmonary Department Mainz University Hospital
 Turning Science into Real Life Roflumilast in Clinical Practice Roland Buhl Pulmonary Department Mainz University Hospital Therapy at each stage of COPD I: Mild II: Moderate III: Severe IV: Very severe
Turning Science into Real Life Roflumilast in Clinical Practice Roland Buhl Pulmonary Department Mainz University Hospital Therapy at each stage of COPD I: Mild II: Moderate III: Severe IV: Very severe
Provider Respiratory Inservice
 Provider Respiratory Inservice 2 Welcome Opening Remarks We will cover: Definition of Asthma & COPD Evidence based guidelines for diagnosis, evaluation, and management of asthma Evidence based guidelines
Provider Respiratory Inservice 2 Welcome Opening Remarks We will cover: Definition of Asthma & COPD Evidence based guidelines for diagnosis, evaluation, and management of asthma Evidence based guidelines
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
Proposed Revision to Med (i)
 Proposed Revision to Med 501.02 (i) I. Purpose This rule has been adopted to enable the Board to best protect public health and safety while providing a framework for licensees to effectively treat and
Proposed Revision to Med 501.02 (i) I. Purpose This rule has been adopted to enable the Board to best protect public health and safety while providing a framework for licensees to effectively treat and
Bridges to Excellence Chronic Obstructive Pulmonary Disease Care Recognition Program Guide
 Bridges to Excellence Chronic Obstructive Pulmonary Disease Care Recognition Program Guide Altarum Bridges to Excellence 3520 Green Court, Suite 300 Ann Arbor, MI 48105 bte@altarum.org www.bridgestoexcellence.org
Bridges to Excellence Chronic Obstructive Pulmonary Disease Care Recognition Program Guide Altarum Bridges to Excellence 3520 Green Court, Suite 300 Ann Arbor, MI 48105 bte@altarum.org www.bridgestoexcellence.org
Clinical Practice Guideline: Asthma
 Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Pharmacy Prior Authorization Clinical Guideline for Attention Deficit Disorder/Attention Deficit Hyperactivity CNS Stimulants
 AETNA BETTER HEALTH Pharmacy Prior Authorization Clinical Guideline for Attention Deficit Disorder/Attention Deficit Hyperactivity CNS Stimulants Formulary amphetamine/dextroamphetamine IR, ER (generic
AETNA BETTER HEALTH Pharmacy Prior Authorization Clinical Guideline for Attention Deficit Disorder/Attention Deficit Hyperactivity CNS Stimulants Formulary amphetamine/dextroamphetamine IR, ER (generic
Indacaterol. Powder capsule administered by a Breezhaler (Onbrez ) or Neohaler (Acapta ) Mechanism of Action Ultra Long-acting beta-2 agonist (LABA)
 Indacaterol Powder capsule administered by a Breezhaler (Onbrez ) or Neohaler (Acapta ) Mechanism of Action Ultra Long-acting beta-2 agonist (LABA) Clinical Application Indications: The long-term maintenance
Indacaterol Powder capsule administered by a Breezhaler (Onbrez ) or Neohaler (Acapta ) Mechanism of Action Ultra Long-acting beta-2 agonist (LABA) Clinical Application Indications: The long-term maintenance
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
Antidepressants for treatment of depression.
 JR3 340 1 of 9 PSYCHOTROPIC MEDICATIONS PURPOSE The use of psychotropic medication as part of a youth's comprehensive mental health treatment plan may be beneficial. The administration of psychotropic
JR3 340 1 of 9 PSYCHOTROPIC MEDICATIONS PURPOSE The use of psychotropic medication as part of a youth's comprehensive mental health treatment plan may be beneficial. The administration of psychotropic
Drug Prior Authorization Guideline NUCALA (mepolizumab)
 Drug Prior Authorization Guideline MB9914 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below Restricted to Pulmonology, Allergy, and
Drug Prior Authorization Guideline MB9914 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below Restricted to Pulmonology, Allergy, and
Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy. Date Prescription will Start Denying at the
 Maryland Medicaid Pharmacy Program & News ViewsNovember 2013 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy The Antipsychotic Peer Review Program is Expanding!
Maryland Medicaid Pharmacy Program & News ViewsNovember 2013 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy The Antipsychotic Peer Review Program is Expanding!
CLINICAL POLICY Clinical Policy: Extended Release Opioid Analgesics
 Reference Number: AZ.CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid- AHCCCS Revision Log See Important Reminder at the end of this policy for important regulatory and
Reference Number: AZ.CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid- AHCCCS Revision Log See Important Reminder at the end of this policy for important regulatory and
Louisiana Medicaid. Provider Update. Volume 27, Issue 3 May/June UNISYS Health Information Management Acquired. by Molina Healthcare
 Louisiana Medicaid Provider Update Volume 27, Issue 3 May/June 2010 UNISYS Health Information Management Acquired by Molina Healthcare Effective May 1, 2010, Molina Healthcare purchased the Health Information
Louisiana Medicaid Provider Update Volume 27, Issue 3 May/June 2010 UNISYS Health Information Management Acquired by Molina Healthcare Effective May 1, 2010, Molina Healthcare purchased the Health Information
Global Initiative for Asthma (GINA) What s new in GINA 2015?
 Global Initiative for Asthma (GINA) What s new in GINA 2015? GINA Global Strategy for Asthma Management and Prevention What s new in GINA 2015 (1) Add-on tiotropium by soft-mist inhaler is a new other
Global Initiative for Asthma (GINA) What s new in GINA 2015? GINA Global Strategy for Asthma Management and Prevention What s new in GINA 2015 (1) Add-on tiotropium by soft-mist inhaler is a new other
Nucala. Nucala (mepolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.07 Subject: Nucala Page: 1 of 5 Last Review Date: December 2, 2016 Nucala Description Nucala (mepolizumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.07 Subject: Nucala Page: 1 of 5 Last Review Date: December 2, 2016 Nucala Description Nucala (mepolizumab)
See Important Reminder at the end of this policy for important regulatory and legal information.
 Effective Date: 04.18 Last Review Date: 04.18 Line of Business: Medicaid- AHCCCS Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Effective Date: 04.18 Last Review Date: 04.18 Line of Business: Medicaid- AHCCCS Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Antipsychotic Medications Age and Step Therapy
 Market DC *- Florida Healthy Kids Antipsychotic Medications Age and Step Therapy Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Virginia Medicaid See State Specific Mandates *Indiana
Market DC *- Florida Healthy Kids Antipsychotic Medications Age and Step Therapy Override(s) Approval Duration Prior Authorization 1 year Quantity Limit *Virginia Medicaid See State Specific Mandates *Indiana
In 2002, it was reported that 72 of 1000
 REPORTS Aligning Patient Care and Asthma Treatment Guidelines Eric Cannon, PharmD Abstract This article describes how the National Asthma Education and Prevention Program Guidelines for the Diagnosis and
REPORTS Aligning Patient Care and Asthma Treatment Guidelines Eric Cannon, PharmD Abstract This article describes how the National Asthma Education and Prevention Program Guidelines for the Diagnosis and
Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene
 Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene Emily S. Wan, John E. Hokanson, James R. Murphy, Elizabeth A. Regan, Barry J. Make, David A. Lynch, James D. Crapo, Edwin K.
Clinical and radiographic predictors of GOLD-Unclassified smokers in COPDGene Emily S. Wan, John E. Hokanson, James R. Murphy, Elizabeth A. Regan, Barry J. Make, David A. Lynch, James D. Crapo, Edwin K.
Clinical Policy: Opioid Analgesics Reference Number: CP.PMN.97 Effective Date: Last Review Date: 02.18
 Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Lecture Notes. Chapter 4: Chronic Obstructive Pulmonary Disease (COPD)
 Lecture Notes Chapter 4: Chronic Obstructive Pulmonary Disease (COPD) Objectives Define COPD Estimate incidence of COPD in the US Define factors associated with onset of COPD Describe the clinical features
Lecture Notes Chapter 4: Chronic Obstructive Pulmonary Disease (COPD) Objectives Define COPD Estimate incidence of COPD in the US Define factors associated with onset of COPD Describe the clinical features
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Re-Submission. roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd. Published 11 September
 Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
COPD. Helen Suen & Lexi Smith
 COPD Helen Suen & Lexi Smith What is COPD? Chronic obstructive pulmonary disease: a non reversible, long term lung disease Characterized by progressively limited airflow and an inability to perform full
COPD Helen Suen & Lexi Smith What is COPD? Chronic obstructive pulmonary disease: a non reversible, long term lung disease Characterized by progressively limited airflow and an inability to perform full
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Policy Reference Number: CP.PMN.53 Effective Date: 09.12.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of this
Clinical Policy: Policy Reference Number: CP.PMN.53 Effective Date: 09.12.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of this
UNDERSTANDING COPD MEDIA BACKGROUNDER
 UNDERSTANDING COPD MEDIA BACKGROUNDER What is COPD? Chronic Obstructive Pulmonary Disease (COPD) also called emphysema and/or chronic obstructive bronchitis* is a preventable lung disease caused by the
UNDERSTANDING COPD MEDIA BACKGROUNDER What is COPD? Chronic Obstructive Pulmonary Disease (COPD) also called emphysema and/or chronic obstructive bronchitis* is a preventable lung disease caused by the
CLINICAL PATHWAY. Acute Medicine. Chronic Obstructive Pulmonary Disease
 CLINICAL PATHWAY Acute Medicine Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease Table of Contents (tap to jump to page) INTRODUCTION 1 Scope of this Pathway 1 Pathway Contacts
CLINICAL PATHWAY Acute Medicine Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease Table of Contents (tap to jump to page) INTRODUCTION 1 Scope of this Pathway 1 Pathway Contacts
ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS
 R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
«Impact of a pharmaceutical intervention to improve adherence of inhaled medication in asthma and COPD patients»
 «Impact of a pharmaceutical intervention to improve adherence of inhaled medication in asthma and COPD patients» Baseline data from a randomized controlled trial 5 th Swiss Health Services Research Symposium,
«Impact of a pharmaceutical intervention to improve adherence of inhaled medication in asthma and COPD patients» Baseline data from a randomized controlled trial 5 th Swiss Health Services Research Symposium,
Table of Contents. 1.0 Policy Statement...1
 Division of Medical Assistance General Clinical Policy No. A-6 Table of Contents 1.0 Policy Statement...1 2.0 Policy Guidelines...1 2.1 Eligible Recipients...1 2.1.1 General Provisions...1 2.1.2 EPSDT
Division of Medical Assistance General Clinical Policy No. A-6 Table of Contents 1.0 Policy Statement...1 2.0 Policy Guidelines...1 2.1 Eligible Recipients...1 2.1.1 General Provisions...1 2.1.2 EPSDT
Omalizumab (Xolair) NHLBI normal ranges by age for FEV 1/FVC 8-19 years of age 85%; years of age 80%;
 DRAFT Medical Coverage Policy Injectable Agents for Asthma and Chronic Idiopathic Urticaria Fasenra, Nucala, Xolair, Cinqair EFFECTIVE DATE: 03 01 2018 POLICY LAST UPDATED: 03 20 2018 OVERVIEW This policy
DRAFT Medical Coverage Policy Injectable Agents for Asthma and Chronic Idiopathic Urticaria Fasenra, Nucala, Xolair, Cinqair EFFECTIVE DATE: 03 01 2018 POLICY LAST UPDATED: 03 20 2018 OVERVIEW This policy
Course Handouts & Disclosure
 COPD: Disease Trajectory and Hospice Eligibility Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Hospice Education Network Course Handouts & Disclosure To download presentation
COPD: Disease Trajectory and Hospice Eligibility Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Hospice Education Network Course Handouts & Disclosure To download presentation
Drug Use Research & Management Program Phone Fax Month/Year of Review: End date of literature search Generic Name:
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Month/Year of Review: February 2012 End date of literature
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Month/Year of Review: February 2012 End date of literature
ASTHMA CARE FOR CHILDREN BASKET OF CARE SUBCOMMITTEE Report to: Minnesota Department of Health. June 22, 2009
 This document is made available electronically by the Minnesota Legislative Reference Library as part of an ongoing digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp ASTHMA CARE FOR CHILDREN
This document is made available electronically by the Minnesota Legislative Reference Library as part of an ongoing digital archiving project. http://www.leg.state.mn.us/lrl/lrl.asp ASTHMA CARE FOR CHILDREN
Abbreviated Class Review: Chronic Obstructive Pulmonary Disease (COPD)
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Executive summary: Month/Year of Review: February
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Executive summary: Month/Year of Review: February
Drug prescriptions (Pharm) Exposure (36/48 months)
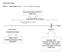 ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
ANNEX SECTION PART A - Study design: Figure 1 overview of the study design Drug prescriptions (Pharm) 2006-2010 Exposure (36/48 months) flexible time windows (e.g. 90 days) time index date (hospital discharge)
Chronic Obstructive Pulmonary Disease
 Chronic Obstructive Pulmonary Disease CareOregon Pharmacy Abridged sample of presentation content Home Equipment Pathophysiology Exacerbations Guidelines Lifestyle Modification Medication Management Sample
Chronic Obstructive Pulmonary Disease CareOregon Pharmacy Abridged sample of presentation content Home Equipment Pathophysiology Exacerbations Guidelines Lifestyle Modification Medication Management Sample
