Pulmonary and Nasal Anti-Inflammatory and Anti-Allergy Inhalation Aerosol Delivery Systems
|
|
|
- Lee Lamb
- 6 years ago
- Views:
Transcription
1 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, 10, Pulmonary and Nasal Anti-Inflammatory and Anti-Allergy Inhalation Aerosol Delivery Systems Xiao Wu, reoluwa. Adedoyin and eidi M. Mansour * Department of Pharmaceutical Sciences-Drug Development Division, University of Kentucky College of Pharmacy, 789 South Limestone Street, Lexington, KY , USA Abstract: Most respiratory infections, diseases and allergic reactions have varying degrees of inflammation. Inflammation is a natural immunodefensive response to the presence of allergens or foreign particles that come into contact or affect the cells and tissues within the respiratory tract. The three main types of therapeutic drug classes available for anti-inflammatory and anti-allergy effects are corticosteroids, antihistamines and decongestants. Corticosteroid drugs for pulmonary inhalation and/or nasal delivery include beclomethasone dipropionate, budesonide, ciclesonide, fluticasone furoate, fluticasone propionate, mometasone furoate, and triamcinolone acetonide. Antihistamine drugs for nasal delivery include azelastine and olopatadine. Two common decongestants available are oxymetazoline and phenylephrine. Another therapeutic class, the anticholinergic agents, such as ipratropium bromide and tiotropium bromide, are used in pulmonary delivery in the treatment of inflammatory diseases such as asthma and chronic obstructive pulmonary disease. The mast cell stabilizer therapeutic class, cromolyn sodium, can be used to prevent and relieve nasal allergic symptoms. Additionally cromolyn sodium was the first dry powder inhaler product for pulmonary drug delivery several decades ago and currently is on the market as a pressurized metered dose inhaler for pulmonary inhalation delivery. Based on the devices used in pulmonary drug delivery, this route can be subdivided into three categories; namely nebulizers, pressurized metered dose inhalers, and dry powder inhalers. Nasal delivery of anti-inflammatory and antiallergy drugs is most commonly available commercially in an aqueous spray form. This article comprehensively reviews and discusses different kinds of drugs used for anti-inflammatory and anti-allergic effects via the pulmonary and nasal delivery route, as well as their mechanisms of action, marketed products, disease state indications while highlighting drug delivery and therapeutic aspects. Keywords: Pulmonary drug delivery, aerosol, nasal spray, anti-inflammatory, anti-allergy, corticosteroids, antihistamines, decongestants, rhinitis, anti-cholinergics, mast cell stabilizers, beta agonists, short-acting, long-acting. 1. INTRDUCTIN T PULMNARY DELIVERY SYSTEMS Pulmonary drug delivery to or via the lungs, is one of the most ancient yet still advancing fields in the world of drug development and delivery. Traditionally, therapy via inhalation is targeted at treatment of localized lung diseases [1]. It has numerous advantages including rapid onset of action, drug confinement to target organ, circumvention of first pass effect, avoidance of drug degradation by the gastrointestinal tract, large lung surface area of low metabolic activity, and lower dosage thus minimizing adverse reactions [2, 3]. There are three major categories of aerosol devices used in pulmonary drug delivery and the device type, together with the nature of the aerosol droplet emitted, will determine the deposition characteristics and location within the respiratory tract [4]. The three main classes are the nebulizers, pressurized metered dose inhalers (pmdis), and dry powder inhalers (DPI). These three main categories differ in their mode of operation, degree of dependency on the patient's inspiratory flow to create the aerosol, particle characteristics, compatible drug formulations and portability. Certain features are expected of aerosol devices for performance and *Address correspondence to this author at the University of Kentucky, College of Pharmacy, Department of Pharmaceutical Sciences-Drug Development Division, 789 South Limestone Street, Lexington, KY , USA; Tel: (859) ; heidi.mansour@uky.edu therapeutic efficacy which include the ability to generate aerosol particles of suitable aerodynamic size for pulmonary inhalation deposition, provide reproducible drug dosing, ease of use, convenient, inexpensive, and portable [4, 5]. Choosing an appropriate pulmonary inhalation device and patient education are important in order to achieve the desired clinical outcomes [6]. Nebulizers are the first commercially marketed pulmonary inhalation devices and the aerosol generated does not depend on the patient's inspiratory flow. They are used most often in in-patient settings and for patients (e.g. neonates, young children, the acutely ill, the elderly, and those with restricted dexterity in coordinating inhalation and device actuation) who may not be able to achieve adequate therapy using the pmdi or DPI device [7, 8]. Nebulizers (which can be jet or ultrasonic) are able to deliver pulmonary drugs in solution while also allowing for dosing adjustments [1]. The jet nebulizers are particularly well suited for administration of aqueous and mixed aqueous/alcohol (i.e. water and solvent) solutions. Nebulization can exhibit variability in lung deposition patterns between patients, a wide range in the aerosol particle size distribution in a given aerosol plume, sterility problems, requires an external power source and a relatively long administration time, and can be relatively high cost [1, 5, 9]. owever, advancement in technology has yielded more portable and smaller hand-held battery-operated nebulizers, can provide the ability to time aerosol delivery and patients inspiration with improved lung
2 216 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No. 3 Wu et al. deposition and decreased aerosol waste to the environment [1]. Nebulizer delivery is generally reserved clinically for acute care, inpatient settings, and select patient populations (e.g. some pediatric and geriatric patients). To circumvent the problems posed by nebulizers, pmdis were developed. pmdis have been the mainstay of asthma inhalation therapy for the past several decades and are more portable than nebulizers [9]. The key components of the formulation are a propellant, the drug (or two drugs), cosolvent mixture, and surfactant [4]. owever, the requirement by the Montreal Protocol to significantly reduce and gradually phase out the use of chlorofluorocarbon propellants due to its depleting effect on the zone layer necessitated the use of alternative propellants/propellant systems such as the hydrofluroalkanes (FA) in pmdis [4, 8]. pmdis have numerous advantages such as their portability, affordability, and ease of use. owever, the presence of the propellant/ cosolvent mixture limits the range of drug substances that can be delivered (due to solubility limits and/or drug degradation problems in solution) and also can have an aftertaste that patients may dislike. Furthermore, there is a need for patients to coordinate actuation and inspiration for enhanced lung deposition [9]. In addition, due to the introduction of new alternative propellants and propellant systems, new issues associated with solubility of active drug entities, stability, aerosol behavior and excipient compatibility have arisen; and as such, extensive research is being devoted to re-formulation of conventional CFC-based pmdis [1]. DPIs are the fastest growing sector of approved pulmonary inhalation products, due in large part to their many advantages. The drug is formulated as an inhalable dry powder [8], and thus, is in the solid state which affords greater drug stability and higher dose delivery than nebulizers and pmdis. DPI devices can be classified as either passive (i.e. aerosol dispersion depends on the patient's inspiratory flow) or active (i.e. aerosol dispersion does not depend on the patient s inspiratory effort) [8]. Exubera, approved by the FDA in 2006, was the first marketed pulmonary product with an active dispersion DPI device; all other currently approved marketed products utilize passive devices [4]. In contrast to pmdis, DPIs are more environmentally friendly and do not cause patient aftertaste due to the absence of propellant. ther advantages include the versatility to deliver a wide range of drugs, portability, and a reduced need of synchronization of actuation and patient inspiration [1, 8, 9]. n the other hand, dry powders can have high oro-pharyngeal deposition if the powder is highly cohesive (i.e. aggregated) and there is drug-inhaler device specificity for each DPI commercial product [1, 5, 6]. It is important to note that the physicochemical properties (e.g. particle size, particle size distribution, particle morphology, surface roughness, crystallinity, ternary/fine component, surface impurities, hygroscopicity, and electrostatic charge) of drugs formulated as respirable dry powders significantly affect the aerosolization performance of DPIs as they directly influence the powder interparticulate forces and particle aggregation/deaggregation [10]. ptimization of these properties is essential in the formulation, stabilization and performance enhancement of dry powders for inhalation delivery Pathogenesis of Inflammation Most respiratory infections and diseases have within their pathogenesis the existence of varying degrees of inflammation. This inflammation is a defensive response to the presence of allergens or foreign particles that come in contact with or affect the cells and tissues within the respiratory tract. Several cell types (including mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines, prostaglandins, and the complement system) are involved in allergic and non-allergic-mediated inflammation [11, 12]. When the pulmonary system senses the presence of allergens, there is a profuse release of these inflammatory mediators which results in smooth muscle contraction, production/secretion of mucus and a cascade of other inflammatory responses, in addition to wheezing, chest tightness, coughing and shortness of breath that is often experienced by patients with pre-existing pulmonary disease (e.g. asthma, chronic obstructive pulmonary disease, etc). The inflammatory response can be prevented or controlled by corticosteroids, which can be natural or synthetic. Corticosteroids act by supressing the formation, release and activity of these endogenous inflammatory cells and mediators thus modifying the body s immune system [11]. Cortisol (also known as hydrocortisone) is the naturally occurring glucocorticoid hormone that is a potent anti-inflammatory Pulmonary Diseases Treated with Anti-Inflammatory Drugs Anti-inflammatory agents such as corticosteroids, leukotriene antagonists, and mast cell stabilizers are used in treatment of many pulmonary diseases having varying degrees of inflammation, including Chronic bstructive Pulmonary Disease (CPD), asthma, bronchitis, mesothelioma, and lung cancer. Asthma is a chronic inflammatory airway disease characterized by airway obstruction that is usually episodic and often reversible with symptoms such as cough, shortness of breath, wheezing, and chest tightness [12, 13]. The goal of asthma treatment is to improve lung function, symptoms, and the ability to perform daily activities, and reduce the risk of exacerbations [14]. Successful treatment of asthma entails the use of inhaled corticosteroids and -2 adrenoceptor agonists as the cornerstone of successful therapy and this combination therapy has been found to be more effective at controlling symptoms, improving lung function and preventing worsening of the symptoms [13]. Corticosteroids however have a wide range of inhibitory activities against different cell types and inflammatory mediators thus, are efficacious in treatment of asthma [15]. Chronic obstructive pulmonary disease (CPD) is a complex chronic inflammatory disease of the lungs (usually characterized by emphysema and bronchitis) that involves different inflammatory cells and mediators. Corticosteroids are a very important drug class used in CPD and asthma therapy to reduce inflammation in the airways thus preventing airway narrowing, reduce edema, reduce secretion of mucus into the airway, and reduce lung damage caused by inflammation [16]. Corticosteroids for respiratory diseases can be taken orally or via inhalation. Common examples of
3 Pulmonary and Nasal Inhalation Aerosol Delivery Systems Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No Table 1. Marketed Anti-Inflammatory Inhalation Prescription (Rx) Products and Common Brand Names in the United States Drug Name Drug Class Common Brand Name Beclomethasone Dipropionate Budesonide Fluticasone Propionate Corticosteroid Corticosteroid Corticosteroid Beclovent pmdi (GlaxoSmithKline); Qvar pmdi (3M Pharmaceuticals); Vanceril pmdi (Schering Plough) Pulmicort (AstraZeneca); Pulmicort Turbuhaler DPI (AstraZeneca); Pulmicort Flexhaler DPI (AstraZeneca); Pulmicort Respules (AstraZeneca); Symbicort Budesonide/formoterol (AstraZeneca) Flovent FA Inhalation Aerosol (GlaxoSmithKline); Flovent Diskus Inhalation Powder (GlaxoSmithKline); Flovent (GlaxoSmithKline); Flovent Nebules /inhalation suspension (GlaxoSmithKline); Flovent Rotadisk DPI (GlaxoSmithKline); Advair Diskus DPI (+salmeterol xinafoate) (GlaxoSmithKline); Advair pmdi (GlaxoSmithKline); Advair FA pmdi (GlaxoSmithKline) Ciclesonide Corticosteroid Alvesco inhalation aerosol pmdi (Sepracor) Flunisolide Corticosteroid Aerobid Inhalation Solution pmdi (Forest Pharmaceuticals); Aerobid-M pmdi (Forest Pharmaceuticals) Mometasone Furoate Corticosteroid Asmanex Twisthaler inhalation powder (Schering-Plough) Triamcinolone Acetonide Corticosteroid Azmacort inhalation aerosol pmdi (Abbott Laboratories, USA; Discontinued in 2009) Table 2. Marketed Inhalation Prescription (Rx) Products and Common Brand Names Indicated for Use in Inflammatory Pulmonary Diseases such as Asthma and/or Chronic bstructive Pulmonary Disease (CPD) Drug Name Drug Class Common Brand Name Cromolyn Sodium Mast Cell Inhibitor Intal Inhaler pmdi (Aventis); DPI (Fisons) Ipratropium Bromide Anti-cholinergic Atrovent inhalation aerosol (Boehringer Ingelheim); Atrovent FA pmdi; Combivent pmdi (Boehringer Ingelheim) Tiotropium Bromide Anti-cholinergic Spiriva andihaler DPI (Boehringer Ingelheim) inhaled corticosteroids used in reducing inflammation in the airways include fluticasone propionate, budesonide, mometasone and beclomethasone dipropionate. Several studies [17-23] have shown the benefits of combination treatment in the successful management and treatment of many lung diseases (asthma, bronchitis, lung cancer, CPD) due to the fact that multiple etiologies and cell receptors are involved in producing these symptoms. Thus, treatment is often initiated using multiple drug classes (anti inflammatory, -2 adrenoceptor agonists, anticholinergics, anti-infectives) having different modes of action and different targets. Common examples of United States and European marketed anti-inflammatory and antiallergy agents are listed in Tables 1 and ANTI-INFLAMMATRY DRUGS DELIVERED BY PULMNARY INALATIN 2.1. Beclomethasone Dipropionate Beclomethasone dipropionate is a prodrug that undergoes hydrolysis to its active form Beclomethasone -17 monopropionate which has high affinity for the human glucocorticoid receptor. Beclomethasone dipropionate is synthetically related to dexamethasone with the difference being the presence of a chlorine instead of a fluorine at the 9-alpha carbon and having a 16 beta-methyl group instead of a 16 alpha-methyl group. It is a creamy white odorless powder having a molecular formula of C Cl 7 and molecular weight of g/mol [24]. For many decades, nebulized beclomethasone dipropionate has been in use therapeutically showing great clinical efficacy and tolerability in patients with chronic and persistent asthma [7]. It is also delivered as a pmdi. QVAR (beclomethasone dipropionate) can deliver smaller-particle-sized medication to the large, intermediate and small airways [24]. Interestingly, the replacement of CFC with hydrofluoroalkanes (FA) can provide the opportunity to specifically target certain corticosteroids directly to all inflammatory sites that exist in both the upper and lower airways [25]. This commercial product is indicated for use in maintenance treatment of asthma as prophylactic therapy in patients 5 years of age and older. Lipworth et al. (1999) compared the pharmacokinetic profile of Beclazone TM (beclomethasone dipropionate) in its CFC-based vs. FA-based formulations [26]. They found the T max occurred significantly (P<0.05) earlier and the plasma concentrations were significantly higher with formulations containing FA rather than CFC. In addition, the geometric mean values for the AUC were significantly greater and the C max values were significantly greater for the FA-containing formulation compared to the CFC formulation after drug inhalation in healthy volunteers Budesonide Budesonide is a corticosteroid for inhalation use that marketed both as liquid and solid-state formulations. Budesonide is a corticosteroid with potent anti-inflammatory properties. Studies have shown that addition of a long-acting
4 218 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No. 3 Wu et al. -2 agonist (formoterol) to budesonide was more beneficial in controlling asthma, and improving lung function and symptoms [27-29]. Symbicort is a combination drug aerosol pmdi marketed pharmaceutical product indicated for treatment of asthma, inflammation, bronchoconstriction and for maintenance treatment of CPD [15, 30]. It contains the antiinflammatory drug budesonide and formoterol fumarate dihydrate, a long-acting bronchodilator that exerts agonistic effects on -2 adrenergic receptors of the bronchial smooth muscles Fluticasone Propionate Fluticasone propionate is a highly hydrophobic synthetic trifluorinated fatty acid-conjugated corticosteroid used as a long-acting anti inflammatory agent in treatment and management of allergic rhinitis, asthma, and other respiratory diseases. It is delivered as a nasal spray, pmdi, and DPI. It is a white powder with an empirical formula of C F 3 5 S and molecular weight of 500.6g/mol [31]. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol. Comparative effectiveness of certain corticosteroids using the McKenzie vasoconstrictor assay demonstrated that fluticasone propionate is a potent agonist of the human glucocorticoid receptor with activity 18 times greater than dexamethasone and about two times greater than of beclomethasone-17-pripionate and about 3 times greater than that of budesonide. owever, even though the McKenzie assay is the standard in vivo method for comparing topical potency and bioequivalence of steroids based on the degree of vasoconstriction, certain studies have shown that the degree of topical potency of corticosteroids does not necessarily correlate to their efficacy when used as an inhaled anti inflammatory agent [31-33]. Nevertheless, Frois et al. (2009) conducted a review comparing the clinical effectiveness and tolerability of certain corticosteroids in use in asthma therapy, and concluded that fluticasone was reported to have significantly greater improvement in lung function and better asthma symptom control than budesonide [34]. Fluticasone propionate pmdi consists of an FA propellant (1,1,1,2-tetrafluoroethane) and a micronized microcrystalline suspension of the drug. No other excipients are included in the formulation [31]. n the other hand, Flovent Diskus is a specially designed for DPI delivery having a specific multi-unit dose delivery device (i.e. the diskus) and contains individual double-foil blister strips consisting of microfine fluticasone propionate and large lactose carrier particles [35]. In clinical trials comparing the efficacy of inhaled powder and orally administered tablets of fluticasone propionate, the inhaled powder demonstrated far greater clinical efficacy in maintaining asthma stability and improving lung function compared to oral tablets and placebo; thus demonstrating that the anti inflammatory effect of fluticasone propionate is due to direct local effect on the lung cells rather than systemic [35]. In DPIs containing physical blends of micronized flutihydrate carrier particles, it was experimentally demonstrated that the hydrophobicity of FP (fluorinated corticosteroid having a conjugated propionate moiety) may strongly influence the interfacial properties favoring aggregation, thereby resulting in a subsequent decrease in aerosolization of the drug [36]. Advair Diskus is a marketed combination drug multiunit-dose DPI containing the long-acting corticosteroid fluticasone propionate and salmeterol xinofoate (a long-acting beta-adrenergic receptor agonist) [37, 38]. It is used in treatment of asthma and CPD. This drug combination is supplied both as a DPI and pmdi. Rojas et al. (2007) compared the effect of a salmeterol xinofoate/fluticasone propionate combination treatment vs. fluticasone propionate alone on the maintenance treatment of moderate persistent asthma being treated only with inhaled short-acting -2 agonists [39]. They concluded that significantly more patients attained better control of their asthma during treatment with the combination drug therapy (46%) compared with fluticasone propionate alone (32%) Ciclesonide Ciclesonide is a non-halogenated glucocorticoid with a molecular formula of C and a molecular weight of 540.7g/mol [40]. It is a white to yellow-white powder and is formulated and delivered as a nasal spray (e.g. mnaris ) for the treatment of allergic rhinitis or as an inhalation aerosol in maintenance therapy of asthma as prophylactic therapy (Alvesco ) [41]. Alvesco inhalation aerosol contains ciclesonide in a solution mixture of propellant FA-134a (1,1,1,2 tetrafluoroethane) and ethanol. It is a novel, safe and highly efficacious aerosol metered dose inhaler formulation due to the fact that it is lung targeted, lung activated and lung limited [41, 42]. Ciclesonide demonstrates about 99% protein binding in serum thus implying the presence of very little amounts of des-ciclesonide systemically. This suggests a very low potential for occurrence of systemic adverse effects after inhalation of ciclesonide [43]. In addition, due to the very high affinity desciclesonide has for glucocorticoid receptors in the lungs, Alvesco demonstrates improved efficacy, low systemic bioavailablity, fewer occurrence of adverse effects, and enhanced safety profile [41]. When compared with other corticosteroids like budesonide and fluticasone, several studies have indicated the similarity of all three drugs in improving lung function and asthma symptoms however, ciclesonide was shown to demonstrate a better safety profile with less cortisol suppression [41, 44, 45]. Ciclesonide has been shown to be as effective as other inhaled corticosteroids at initiating, maintaining, and controlling asthma symptoms with a positive safety profile and is well tolerated [46] Flunisolide Flunisolide is a corticosteroid indicated in maintenance treatment of asthma as prophylactic therapy and for asthma patients requiring systemic corticosteroid administration. It is a white to creamy white crystalline powder with a molecular weight of g/mol [47].
5 Pulmonary and Nasal Inhalation Aerosol Delivery Systems Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No Aerobid TM /Aerobid-M Inhaler is a pmdi containing a microcrystalline suspension of flunisolide hemihydrate in a mixture of propellants, a dispersing/suspending agent (sorbitan trioleate), and in the case of Aerobid-M, the addition of a flavoring agent (menthol) [48]. Newman et al. (1996) conducted a comparative evalution of deposition pattern and efficacy of flunisolide by different devices and demonstrated that use of a multidose hand-held nebulizer (Respimat Soft mist inhaler TM ) which delivered an ethanolic solution of flunisolide had much higher whole lung deposition than the pmdi or pmdi-spacer combination [49]. As described earlier due to the Montreal Protocol, other flunisolide formulations without the CFC propellants have been developed. For example, Tzou et al. (1999) invented an aerosol formulation comprised of flunisolide, an FA propellant comprising 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3, 3-heptafluoropropane, and a mixture thereof, and sufficient ethanol to solubilize the drug [48]. This FA formulation eliminates problems of increased particle size, rapid flocculation, irreversible particle aggregation and bulk separation; thus resulting in a higher respirable fraction of drug vs. the CFC based propellants [48] Mometasone Furoate Mometasone furoate is a synthetic corticosteroid with an empirical formula of C C l2 6, a molecular weight of g/mol, and is used as an anti-inflammatory agent [11]. Mometasone furoate shows in vitro binding affinity for the human glucocorticoid receptor about 12 times that of dexamethasone, 7 times that of triamcinolone, 5 times that of budesonide, and 1.5 times greater than that of fluticasone, though the clinical significance of this is unknown [11]. Asmanex Twisthaler is a cap-activated multidose DPI containing micronized respirable mometasone furoate as its active ingredient physically blended with large non-respirable lactose carrier particles. It is indicated for the maintenance treatment of asthma as prophylactic therapy [11]. It uses a stabilized agglomerate formulation to measure and deliver accurate drug doses over a range of inspiratory flow rates [50]. Mometasone furoate delivered via dry powder inhaler for once daily maintenance treatment of asthma was found to be both safe and efficacious in both adults and children [14]. Furthermore, mometasone furoate shows comparative efficacy with budesonide, beclomethasone dipropionate and fluticasone and has been shown to be superior in improving lung function, controlling asthma symptoms and improving quality of life in persistent asthmatics being treated with short-acting -2 agonists or twice daily maintenance therapy with certain inhaled corticosteroids [51] Triamcinolone Acetonide Triamcinolone acetonide is a potent corticosteroid derivative of triamcinolone, with a chemical formula of C F 6 and a molecular weight of 434.5g/mol [52]. It is formulated and delivered as cream, ointment, dental paste, injection, nasal spray, and inhaler. Triamcinolone is about 1 or 2 times more potent than prednisolone but triamcinolone acetonide is about 8 times more potent than prednisolone [53]. Azmacort Inhalation Aerosol is a metered-dose pmdi that contains a microcrystalline suspension of triamcinolone acetonide in the propellant dichlorodifluoromethane and dehydrated alcohol USP 1% w/w [53]. It is indicated in the maintenance treatment of asthma as prophylactic therapy, and for asthma patients that need systemic corticosteroid administration, where adding Azmacort may reduce or eliminate the need for the systemic corticosteroids [53]. owever, it was voluntarily withdrawn from the market in 2009 by the manufacturer and not reformulated with an FA propellant [54]. 3. TER DRUGS DELIVERED BY TE PULMNARY RUTE FR TE TREATMENT F ASTMA AND CPD 3.1. Cromolyn Sodium Cromolyn sodium is the disodium salt of 5,5'-[(2- hydroxytrimethylene) dioxy]bis [4-oxo-4-1-benzopyran-2- carboxylate] with molecular formula C Na 2 11 and molecular weight of g/mol [55]. Cromolyn sodium (disodium cromoglycate) is a mast cell stabilizer which prevents release of inflammatory mediators such as histamine and SRS-A (slow reacting substance of anaphylaxis) from mast cells thus preventing bronchoconstriction that manifests in response to exposure to an antigen [55]. Cromolyn sodium is supplied as nasal spray (allergic rhinitis), inhalation solution (asthma prophylaxis), ophthalmic solution (keratitis, vernal conjunctivitis, vernal keratoconjunctivitis) and oral solution (systemic mast cell disease) [55]. In the management of asthma today, cromolyn sodium is delivered via nebulizer or pmdi. Interestingly, it was the first dry powder inhaler (DPI) ever marketed many decades ago but is not available as a DPI currently. Blends of disodium cromoglycate (DSCG) and lactose monohydrate have been shown to demonstrate superior aerosol performance (increased fine particle fraction) and this phenomenon is attributed to the ability of DSCG to reduce the high interfacial interactions occurring between particles [56]. Intal inhaler is a pressurized metered dose inhaler used to deliver cromolyn sodium via inhalation. It contains two propellants CFC-12 (dichlorodifluoromethane) and CFC-114 (dichlorotetrafluoroethane) but has been discontinued by the manufacturer due to the ozone layer depletion effects of CFCs [57]. owever, despite this, cromolyn sodium nebulizer solution and FA-containing propellants are still available for use by asthma suffers Ipratropium Bromide Ipratropium bromide is an anti-cholinergic drug formulated and supplied as a nasal spray, pmdi, and nebulized inhalation solution. It is a cholinergic antagonist of acetylcholine at the cholinergic receptors, and suppresses the increase of of cyclic guanosine monophosphate (cyclic GMP) levels due to the interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle [58, 59]. Inhalation aerosol delivery to the lungs by pmdi or nebulized solution is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with
6 220 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No. 3 Wu et al. CPD pulmonary disease, including chronic bronchitis and emphysema [58, 59]. In addition, combination drug delivery of albuterol and ipratropium bromide (Combivent ) have been formulated, as a pmdi and nebulized solution, for treatment of severe bronchospasm in patients with CPD. This is a combination of a short-acting beta-adrenergic bronchodilator (albuterol) and an anticholinergic agent (ipratropium bromide) to widen the air passages and help the asthmatic patient breathe more easily [60]. Analyses of the influence of physical blends of different lactose monohydrate carrier batches with ipratropium bromide indicate that there is no significant difference in the carrier influence on the fluidization and entrainment properties of ipratropium bromide formulation aerosols [36] Tiotropium Bromide Tiotropium bromide is a long-acting anti-cholinergic agent which exerts its pharmacological activity by inhibiting the M3-receptors of the smooth muscles leading to bronchodilation [61]. Like ipratropium bromide, it is an anticholinergic drug but tiotropium is long-acting. It is indicated for long-term once a day maintenance treatment of bronchospasm associated with CPD [62]. It has a molecular weight of 490.4g/mol and a molecular formula of C N 4 S 2 Br 2 [63]. Spiriva andialer consists of individual capsules containing a dry powder formulation of tiotropium bromide monohydrate blended with lactose monohydrate that the patient must actively load into the unitdose inhaler device, the andialer [62, 63]. F S Cl F F Beclomethasone dipropionate Budesonide Fluticasone propionate F Cl Cl S F F Mometasone furoate Ciclesonide Fluticasone furoate Cl N N N F N Triamcinolone acetonide Azelastine lopatadine N N N xymetazoline Phenylephrine Fig. (1). Chemical structures of corticosteroids (beclomethasone dipropionate, budesonide, ciclesonide, fluticasone furoate, fluticasone propionate, mometasone furoate, and triamcinolone acetonide), antihistamines (azelastine and olopatadine) and decongestants (oxymetazoline and phenylephrine) available in the nasal sprays or drops for prevention or relief of nasal allergy and inflammation
7 Pulmonary and Nasal Inhalation Aerosol Delivery Systems Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No NASAL DELIVERY F ANTI-INFLAMMATRY AND ANTI-ALLERGY DRUGS 4.1. Introduction Nasal delivery of anti-inflammatory and anti-allergy drugs is available most commonly and commercially as pharmaceutical products in two forms; namely, as a pressurized aerosol and as a spray pump (aqueous) [64]. The pressurized aerosol form delivers a predetermined dose of dry medication when activated. The more commonly used pump delivers a water-based spray, which may provide some moisturizing and soothing effect as well as antiinflammatory action. Patients who feel that the drip in their nose and throat increases when using the aqueous spray pump form may prefer the aerosol. In contrast, the spray is favored if the pressurized aerosol causes irritation or excessive drying of the nasal membranes. The nasal delivery devices include nasal inhalers, nasal sprays, and nebulizers. They can be classified into single and multi-use intranasal delivery devices for both liquid and powder formulations. A nasal spray is usually in an aqueous medium and delivered by instilling a fine mist into the nostril by action of a hand-operated pump mechanism. It contains a drug or drugs for either locally or generally alleviation of inflammation or allergy symptoms, including sinusitis [65, 66], rhinosinusitis [67-70], rhinitis [71-74], rhinopharyngitis [75] and hay fever [76]. Inflammation often occurs as a result of an allergic reaction, and people who suffer from nasal allergies, such as hay fever, dust mite and pet allergies, tend to experience a variety of symptoms due to this inflammation [77]. Inflammation of the nasal passage forces fluid out of the nasal tissues, resulting in a runny and blocked nose. ther symptoms include sneezing, and watery, itchy eyes. The three main types of drugs available for antiinflammatory and anti-allergy effect are corticosteroids [78-92], antihistamines [73, 93-97] and decongestants. Corticosteroid drugs include beclomethasone dipropionate [68, ], budesonide [ ], ciclesonide [74, ], fluticasone furoate [71, 111, 112], fluticasone propionate [ ], mometasone furoate [ ] and triamcinolone acetonide [ ]. Antihistamine drugs include azelastine [94, 126] and olopatadine [127]. Two common decongestants available in the nasal sprays or drops form are oxymetazoline and phenylephrine. Their chemical structures and names are shown in Fig. (1) and Table 3, respectively Nasal Steroid Sprays Corticosteroid medicines are derivatives of corticosteroid hormones that are produced naturally by the adrenal glands. The corticosteroids have potent anti-inflammatory effects and are very effective in treating allergic inflammation in the nose. The best therapeutic efficacy could be obtained when the sprays are used on a regular preventative basis. With seasonal allergies, daily use of these sprays should begin one to two weeks before the allergy season and continue throughout the season. In year round or perennial allergic rhinitis, particularly if unresponsive to treatments, daily use of intranasal steroids has been found very effective in controlling symptoms, particularly nasal congestion. Nasal steroids may also help improving the sense of smell, which is frequently diminished in allergic rhinitis. The medication may work by reducing swelling high up in the nose, where the area for smell is located. Decreasing the swelling allows more air containing the odors to reach the nerves that are responsible for the sense of smell [128]. Table 3. Popular Anti-Inflammatory and Anti-Allergy Drugs Incorporated in the Marketed Nasal Sprays and their Chemical Names Drugs Beclomethasone dipropionate Budesonide Ciclesonide Fluticasone furoate Fluticasone propionate Mometasone furoate monohydrate Triamcinolone acetonide Azelastine hydrochloride lopatadine hydrochloride xymetazoline hydrochloride Phenylephrine Ipratropium bromide monohydrate Cromolyn sodium Chemical Names 9-chloro-11,17,21-trihydroxy-16 -methylpregna-1,4-diene-3,20-dione 17,21-dipropionate (RS)-11-beta, 16-alpha, 17, 21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16, 17-acetal with butyraldehyde pregna-1,4-diene-3,20-dione, 16,17-[[R-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-oxopropoxy)- (11,16 ) (6, 11, 16, 17 )-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4- dien-17-yl 2-furancarboxylate S-(fluoromethyl) 6,9-difluoro dihydroxy-16 -methyl-3-oxoandrosta-1,4-diene-17 -carbothioate, 17- propionate 9,21-Dichloro-11ß,17-dihydroxy-16a-methylpregna-1,4-diene-3,20-dione 17-(2 furoate) monohydrate 9-Fluoro-11ß,16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone (±)-1-(2)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1-azepin-4-yl)-, monohydrochloride (Z)-11-[3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride 3-(4,5-dihydro-1-imidazol-2-ylmethyl)-2,4-dimethyl-6-tert-butyl-phenol hydrochloride (R)-3-[-1-hydroxy-2-(methylamino)ethyl]phenol 8-azoniabicyclo (3.2.1) octane,3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo,syn)-, (±) disodium 5, 5'-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4-1-benzopyran-2-carboxylate]
8 222 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No. 3 Wu et al Antihistamines Excessive histamine function is the primary cause of allergic reaction in people. istamine is a chemical released from allergic cells in the body, such as mast cells and basophils, usually in response to an allergen. When histamine is released by allergic cells in the nose, it can cause sneezing, runny nose, itching nose, nasal congestion and post-nasal drip. These are the symptoms of hay fever, also known allergic rhinitis. istamine works by attaching itself to the histamine receptors on the surface of cells and thereby causing its effects. uman cells have three different types of histamine receptors, including 1, 2 and 3. It is mainly through the 1 receptor that histamine causes symptoms of allergy. Antihistamine drugs compete with histamine for 1 histamine receptor sites. By occupying the histamine receptor sites, they prevent histamine from binding to them and causing allergic symptoms [73, 129, 130]. Antihistamines are most effective when taken continuously during the allergy season. They usually begin working between 30 to 60 minutes after being taken [94]. owever, histamine is only one of the many chemicals involved in the allergic reaction, which explains why relief from antihistamines is usually only partial. Astelin Nasal Spray (azelastine hydrochloride), Astepro Nasal Spray 0.1% (azelastine hydrochloride), Astepro Nasal Spray 0.15% (azelastine hydrochloride) and Patanase Nasal Spray (olopatadine hydrochloride) are the local antihistamines available in the market. They have gained popularity with sufferers of allergic rhinitis Nasal Decongestants Allergies can cause a congested or stuffy nose. This congestion results when membranes lining the nose become swollen. Topical decongestants are medications used to relieve nasal congestion after applied directly to the nasal cavity. These drugs act on alpha-adrenergic receptors in the mucosa of the respiratory tract to produce vasoconstriction of the blood vessels in the nose and paranasal sinuses, which temporarily reduces the swelling associated with inflammation of the mucous membranes lining the nasal passages [129]. Commonly used decongestant nasal sprays often contain oxymetazoline (Afrin and other brands) and phenylephrine (Neosynephrine and other brands), which are direct agonists, acting directly on the adrenergic receptor system. Topical nasal sprays work more quickly than oral decongestants (a few minutes compared to thirty minutes). More, they are safer by reducing the side-effects associated with systemically-acting decongestants, such as high blood pressure, heart palpitations, sleeplessness, nervousness and dizziness. The most common side effects from decongestant nasal sprays and nose drops are sneezing and temporary burning, stinging, or dryness. These effects are usually temporary and do not need medical attention. It is important, however, not to use these topical agents for longer than 3 to 5 days, because rebound congestion may occur after repeated doses over a short period of time. When this happens, the nose remains stuffy or gets worse with every dose. The only way to stop the cycle is to stop using the drug. The stuffiness should then go away within about a week. If the cycle is not membranes, which lead to a condition known as rhinitis medicamentosa [131]. Rhinitis medicamentosa refers to an inflammation in the nose that is caused by the use of medications. Symptoms include severe stuffiness, burning, bleeding, and dryness of the nose ther Nasal Sprays Ipratropium Bromide Nasal Spray Ipratropium bromide is an anticholinergic agent [ ]. Its structural formula is shown in Fig. (2). Ipratropium bromide inhibits vagally-mediated reflexes by antagonizing the action of acetylcholine at the cholinergic receptor [132, 136]. Acetylcholine is a chemical that signals the mucous glands in the nose to produce mucous. Allergic reactions can trigger excessive acetylcholine activity on the mucous glands. In humans, ipratropium bromide has anti-secretory properties and, when applied intranasally, occupies the same receptor on the glands as does acetylcholine and in this way reduces watery hypersecretion from mucosal glands in the nose [ ]. C 3 C(C 3 ) 2 N + C C C C 2 Fig. (2). Chemical structure of ipratropium bromide. Side effects of ipratropium bromide are infrequent, because it minimally crosses the nasal and gastrointestinal membrane and the blood-brain barrier, resulting in a reducetion of the systemic anticholinergic effects (e.g., neurologic, ophthalmic, cardiovascular and gastrointestinal effects) that are seen with tertiary anticholinergic amines. owever, there are still adverse events reported by a small percentage of the patients receiving ipratropium bromide. These adverse effects include dry nose, dry mouth, nasal irritation, nose bleeding, dizziness, ocular irritation, blurred vision, conjunctivitis, hoarseness, cough, and taste perversion [134] Cromolyn Sodium Nasal Spray The chemical structure of cromolyn sodium is shown in Fig. (3). It works to reduce nasal inflammation as the noncorticosteroid treatment of choice. Many of the symptoms and signs of allergic reactions are caused by chemicals such as histamine that are released from mast cells, a type of cell that is found in the lungs, nose and eyelids. Cromolyn sodium acts on mast cells to stabilize them, thereby preventing the release of histamine and other mediators that would normally attract inflammatory cells [137]. Cromolyn sodium was approved by the FDA in In 1997, the FDA approved over-the-counter status for the nasal solution to treat seasonal allergic rhinitis. Since cromolyn sodium is strictly a controller medication, rather than a reliever medication, it must be taken before allergic exposure, usually at least 2 weeks prior, due to its slow onset of effectiveness [138, 139]. The drug tends Br -
9 Pulmonary and Nasal Inhalation Aerosol Delivery Systems Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No to be more effective in younger people with higher levels of lge. It is a particularly well tolerated medication with minimal side effects (e.g., sneezing, nasal irritation, or stinging). Rare cases of nasal bleeding or residual bad taste are reported. These are no systemic or body-wide side effects. NaC 2 C 2 C C Fig. (3). Chemical structure of cromolyn sodium. CNa 5. CURRENT NASAL SPRAYS AVAILABLE N TE MARKET A summary of marketed pharmaceutical products of nasal anti-inflammatory and anti-allergy sprays is shown in Table Beconase AQ Nasal Spray The active component of Beconase AQ Nasal Sprat is beclomethasone dipropionate, Beconase AQ Nasal Spray is a metered-dose, manual pump spray unit containing a microcrystalline suspension of beclomethasone dipropionate, monohydrate equivalent to 42 mcg of beclomethasone dipropionate, calculated on the dried basis, in an aqueous medium containing microcrystalline cellulose, carboxymethylcellulose sodium, dextrose, benzalkonium chloride, polysorbate 80, and 0.25% v/w phenylethyl alcohol. The p through expiry is 5.0 to 6.8. Following topical administration, beclomethasone dipropionate produces anti-inflammatory and vasoconstrictor effects. Beconase AQ Nasal Spray is indicated for the relief of the symptoms of seasonal or perennial allergic and nonallergic (vasomotor) rhinitis [ ] and also for the prevention of recurrence of nasal polyps following surgical removal [140]. The mechanisms responsible for the anti-inflammatory action of beclomethasone dipropionate are unknown. Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic mediated inflammation. The direct relationship of these findings to the effects of beclomethasone dipropionate on allergic rhinitis symptoms is not known Rhinocort Aqua Nasal Spray The active component of Rhinocort Aqua Nasal Spray is budesonide, an anti-inflammatory synthetic corticosteroid. Rhinocort Aqua is an unscented, metered-dose, manualpump spray formulation containing a micronized suspension Table 4. Marketed Pharmaceutical Products of Nasal Anti-Inflammatory and Anti-Allergy Sprays Drug classes Drugs Brand names Manufacturers Disease state indications Rx vs. TC Corticosteroids Antihistamines Decongestants Anticholinergic agent Mast cell stabilizer Beclometasone dipropionate Budesonide Beconase AQ GlaxoSmithKline Relieve the symptoms of hay fever, prevent regrowth of nasal polyps following surgical removal Rhinocort Aqua Astrazeneca Treat seasonal or perennial allergic rhinitis Rx Ciclesonide mnaris Sepracor Inc. Treat seasonal or perennial allergic rhinitis Rx Fluticasone furoate Veramyst GlaxoSmithKline Treat seasonal or year-round allergy symptoms Rx Fluticasone propionate Mometasone furoate monohydrate Triamcinolone acetonide Azelastine hydrochloride lopatadine hydrochloride xymetazoline hydrochloride Flonase Nasonex Nasacort AQ Astepro Patanase Afrin GlaxoSmithKline Schering Corporation Sanofi-aventis U.S. LLC Meda Pharmaceuticals Inc. Alcon laboratories, Inc. Schering-Plough Treat seasonal or perennial allergic rhinitis, nonallergic rhinitis Treat seasonal and perennial nasal allergy symptoms, prevent most seasonal nasal allergy symptoms, treat nasal polyps Treat seasonal or perennial allergic rhinitis Treat seasonal or perennial allergic rhinitis Treat seasonal allergic rhinitis Relieve congestion associated with allergies, hay fever and sinus irritation Phenylephrine Sinex Vicks Relieve sinus congestion TC Ipratropium bromide monohydrate Atrovent Boehringer Ingelheim Pharmaceuticals, Inc. Cromolyn sodium NasalCrom BlackSmith Brands Relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis Prevents and relieves nasal allergy symptoms like runny/itchy nose, sneezing and allergic stuffy nose without drowsiness Rx Rx Rx Rx Rx Rx TC Rx TC
10 224 Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 2011, Vol. 10, No. 3 Wu et al. of budesonide in an aqueous medium. Microcrystalline cellulose and carboxymethyl cellulose sodium, dextrose anhydrous, polysorbate 80, disodium edetate, potassium sorbate, and purified water are contained in this medium; hydrochloric acid is added to adjust the p to a target of 4.5 [141]. Rhinocort Aqua Nasal Spray is indicated for the management of nasal symptoms of seasonal or perennial allergic rhinitis [104, 142]. The precise mechanism of corticosteroid actions in seasonal and perennial allergic rhinitis is not known mnaris Nasal Spray mnaris Nasal Spray delivers the corticosteroid ciclesonide as a hypotonic aqueous spray via a metered-dose manual pump. mnaris Nasal Spray also contains microcrystalline cellulose, carboxymethylcellulose sodium, hypromellose, potassium sorbate and edetate sodium; and hydrochloric acid to adjust the p to 4.5 [143]. Like Rhinocort Aqua Nasal Spray, mnaris Nasal Spray is also indicated for the management of nasal symptoms of seasonal or perennial allergic rhinitis [74, 92, ]. In well designed trials, intranasal ciclesonide 200 μg once daily was more effective than placebo in terms of reducing nasal symptoms in adolescents and adults with moderate to severe seasonal allergic rhinitis or moderately severe perennial allergic rhinitis [108]. Systemic exposure to ciclesonide and its active metabolite desisobutyryl-ciclesonides is low after intranasal administration. igh protein binding (~ 99%) and rapid first-pass clearance further reduce systemic exposure to the drug Veramyst Nasal Spray The active component of Veramyst Nasal Spray is fluticasone furoate, a synthetic corticosteroid derived from fluticasone. It is marketed by GlaxoSmithKline for the treatment of seasonal and year-round allergy symptoms in adults and children 2 years old and older [71, 111, 112]. Inactive ingredients in Veramyst Nasal Spray include 0.015% w/w benzalkonium chloride, dextrose anhydrous, edetate disodium, microcrystalline cellulose, carboxymethylcellulose sodium, polysorbate 80, and purified water [144] Flonase Nasal Spray The active component of Flonase Nasal Spray is fluticasone propionate. Flonase Nasal Spray is an aqueous suspension of microfine fluticasone propionate for topical administration to the nasal mucosa by means of a metering, atomizing spray pump. Flonase Nasal Spray also contains microcrystalline cellulose and carboxymethyl-cellulose sodium, dextrose, 0.02% w/w benzalkonium chloride, polysorbate 80, and 0.25% w/w phenylethyl alcohol, and has a p between 5 and 7 [145]. Flonase Nasal Spray is indicated for the management of the nasal symptoms of seasonal and perennial allergic [114, 146, 147] and nonallergic rhinitis in adults and pediatric patients [148] 4 years of age and older Nasonex Nasal Spray Nasonex Nasal Spray contains the active ingredient a metered-dose, manual pump spray unit containing an aqueous suspension of mometasone furoate monohydrate equivalent to 0.05% w/w mometasone furoate calculated on the anhydrous basis; in an aqueous medium containing glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, sodium citrate, citric acid, benzalkonium chloride, and polysorbate 80 [149]. The p is between 4.3 and 4.9. Mometasone furoate works by stopping the cells in the nasal lining from producing various inflammation-causing chemicals that are normally released when the body reacts to allergens or irritation. These include prostaglandins and various other inflammatory substances. By preventing these inflammatory chemicals from being released in the nose, mometasone furoate reduces the inflammation and relieves its related symptoms. The nasal spray delivers mometasone directly to the area where it is needed, which reduces the chance of side effects occurring elsewhere in the body. Nasonex Nasal Spray treats the nasal symptoms of seasonal allergic [118, 119, 150, 151] and perennial allergic rhinitis [118, 152, 153], in adults and pediatric patients [ ] 2 years of age or older. It can also be used to prevent the nasal symptoms of seasonal allergic rhinitis in adult and adolescent patients 12 years and older. Treatment should be start 2 to 4 weeks prior to the anticipated start of the pollen season for this purpose. Moreover, Nasonex Nasal Spray is indicated for the treatment of nasal polyps in patients 18 years of age and older [158, 159] Nasacort AQ Nasal Spray The active ingredient of Nasacort AQ Nasal Spray is triamcinolone acetonide. Nasacort AQ Nasal Spray is a thixotropic, water-based metered-dose pump spray formulation unit containing a microcrystalline suspension of triamcinolone acetonide in an aqueous medium. Microcrystalline cellulose, carboxymethylcellulose sodium, polysorbate 80, dextrose, benzalkonium chloride, and edentate disodium are contained in this aqueous medium; hydrochloric acid or sodium hydroxide may be added to adjust the p to a target of 5.0 within range of 4.5 and 6.0 [160]. Nasacort AQ Nasal Spray treats nasal symptoms of seasonal and perennial allergic rhinitis in adults and children 2 years of age and older [ ] Astepro Nasal Spray Astepro Nasal Spray is an antihistamine formulated as a metered-spray solution for intranasal administration. The active ingredient is azelastine hydrochloride, a white, almost odorless, crystalline powder with a bitter taste. It has a molecular weight of It is sparingly soluble in water, methanol, and propylene glycol and slightly soluble in ethanol, octanol, and glycerine. It has a melting point of about 225 C and the p of a saturated solution is between 5.0 and 5.4. Astepro Nasal Spray also contains sorbitol, sucralose, hypromellose, sodium citrate, edetate disodium and benzalkonium chloride in an isotonic aqueous solution [3]. It provides relief from seasonal allergic rhinitis and perennial allergic rhinitis symptoms including nasal congestion without added decongestants like pseudoephedrine [73, 126, 161, 162].
The Medical Letter. on Drugs and Therapeutics. Drug Some Formulations OTC/Rx Usual Dosage Comments Class Comments Cost 1
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
Allergies and Asthma 5/21/2013. Objectives. Allergic Rhinitis (AR): Risk Factor for ASTHMA. Rhinitis and Asthma
 Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Medications Affecting The Respiratory System
 Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Aerospan (flunisolide)
 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
PRODUCT INFORMATION FLIXONASE ALLERGY & HAYFEVER 24 HOUR
 PRODUCT INFORMATION FLIXONASE ALLERGY & HAYFEVER 24 HOUR NAME Fluticasone propionate DESCRIPTION FLIXONASE ALLERGY & HAYFEVER 24 HOUR Fluticasone Aqueous Nasal Spray (0.05 w/w) is an aqueous suspension
PRODUCT INFORMATION FLIXONASE ALLERGY & HAYFEVER 24 HOUR NAME Fluticasone propionate DESCRIPTION FLIXONASE ALLERGY & HAYFEVER 24 HOUR Fluticasone Aqueous Nasal Spray (0.05 w/w) is an aqueous suspension
Foundations of Pharmacology
 Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Asthma Description. Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways.
 Asthma Asthma Description Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways. Symptoms of asthma In susceptible individuals, this inflammation causes recurrent
Asthma Asthma Description Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways. Symptoms of asthma In susceptible individuals, this inflammation causes recurrent
FLOMIST Aqueous Nasal Spray (Fluticasone propionate)
 Published on: 10 Jul 2014 FLOMIST Aqueous Nasal Spray (Fluticasone propionate) Composition FLOMIST Aqueous Nasal Spray Each spray delivers: Fluticasone Propionate BP...50 mcg Fluticasone Propionate BP...
Published on: 10 Jul 2014 FLOMIST Aqueous Nasal Spray (Fluticasone propionate) Composition FLOMIST Aqueous Nasal Spray Each spray delivers: Fluticasone Propionate BP...50 mcg Fluticasone Propionate BP...
Asthma medications: Know your options - MayoClinic.com. Asthma medications: Know your options
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
ANTINEOPLASTIC DRUGS CHAPTER 21. Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component
 ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
Package leaflet: Information for the patient. Dymista Nasal Spray 137 micrograms / 50 micrograms per actuation Nasal Spray, Suspension
 Package leaflet: Information for the patient Dymista Nasal Spray 137 micrograms / 50 micrograms per actuation Nasal Spray, Suspension Azelastine hydrochloride/fluticasone propionate Read all of this leaflet
Package leaflet: Information for the patient Dymista Nasal Spray 137 micrograms / 50 micrograms per actuation Nasal Spray, Suspension Azelastine hydrochloride/fluticasone propionate Read all of this leaflet
10/18/2012. Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C
 Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
PATIENT INFORMATION LEAFLET
 N-CORT 0.055% nasal spray, suspension It is used by spraying into the nose. Active substance: (w/w%) Triamcinolone acetonide 0.055 PATIENT INFORMATION LEAFLET Excipients: Benzalkonium chloride 50%, sodium
N-CORT 0.055% nasal spray, suspension It is used by spraying into the nose. Active substance: (w/w%) Triamcinolone acetonide 0.055 PATIENT INFORMATION LEAFLET Excipients: Benzalkonium chloride 50%, sodium
Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD)
 Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD) COPD COPD is a chronic, irreversible obstruction of airflow that is usually progressive. Symptoms include cough, excess mucus production,
Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD) COPD COPD is a chronic, irreversible obstruction of airflow that is usually progressive. Symptoms include cough, excess mucus production,
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
BRINGING THE PATIENT S VOICE TO NASAL DRUG DELIVERY
 BRINGING THE PATIENT S VOICE TO NASAL DRUG DELIVERY Nasal MDI s were phased out in favour of aqueous sprays when CFC propellants were banned. However, here, Louise Righton, Global Market Development Manager,
BRINGING THE PATIENT S VOICE TO NASAL DRUG DELIVERY Nasal MDI s were phased out in favour of aqueous sprays when CFC propellants were banned. However, here, Louise Righton, Global Market Development Manager,
Fluticasone propionate, 50 mcg per spray. Please read this leaflet carefully before you use FLIXONASE ALLERGY & HAYFEVER 24 HOUR.
 FLIXONASE ALLERGY & HAYFEVER 24 HOUR Fluticasone propionate, 50 mcg per spray Consumer Medicine Information What is in this leaflet? Please read this leaflet carefully before you use FLIXONASE ALLERGY
FLIXONASE ALLERGY & HAYFEVER 24 HOUR Fluticasone propionate, 50 mcg per spray Consumer Medicine Information What is in this leaflet? Please read this leaflet carefully before you use FLIXONASE ALLERGY
31 - Respiratory System
 31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
Glossary of Asthma Terms
 HealthyKidsExpress@bjc.org Asthma Words to Know Developed in partnership with Health Literacy Missouri Airways (Bronchi, Bronchial Tubes): The tubes in the lungs that let air in and out of the body. Airway
HealthyKidsExpress@bjc.org Asthma Words to Know Developed in partnership with Health Literacy Missouri Airways (Bronchi, Bronchial Tubes): The tubes in the lungs that let air in and out of the body. Airway
Asthma. Definition. Symptoms
 Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
FLONASE (fluticasone propionate) Nasal Spray, 50 mcg
 FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. DESCRIPTION Fluticasone propionate, the active component of FLONASE Nasal
FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. DESCRIPTION Fluticasone propionate, the active component of FLONASE Nasal
PRINCIPAL MEDICATION OPTIONS FOR RHINITIS
 SEE INDICATED SUMMARY STATEMENT (SS#) DISCUSSION FOR SUPPORTING DATA ALLERGIC RHINITIS (AR): SEASONAL (SAR) AND PERENNIAL (PAR) MONOTHERAPY ORAL Antihistamines, oral (H1 receptor antagonists) (SS# 61-64)
SEE INDICATED SUMMARY STATEMENT (SS#) DISCUSSION FOR SUPPORTING DATA ALLERGIC RHINITIS (AR): SEASONAL (SAR) AND PERENNIAL (PAR) MONOTHERAPY ORAL Antihistamines, oral (H1 receptor antagonists) (SS# 61-64)
Chapter 7. Anticholinergic (Parasympatholytic) Bronchodilators. Mosby items and derived items 2008, 2002 by Mosby, Inc., an affiliate of Elsevier Inc.
 Chapter 7 Anticholinergic (Parasympatholytic) Bronchodilators Clinical Indications for Use Indication for anticholinergic bronchodilator COPD maintenance Indication for combined anticholinergic and β-agonist
Chapter 7 Anticholinergic (Parasympatholytic) Bronchodilators Clinical Indications for Use Indication for anticholinergic bronchodilator COPD maintenance Indication for combined anticholinergic and β-agonist
NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate
 NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about NASONEX. It does not contain all
NASONEX Aqueous Nasal Spray 0.05% Mometasone Furoate Monohydrate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about NASONEX. It does not contain all
Significance. Asthma Definition. Focus on Asthma
 Focus on Asthma (Relates to Chapter 29, Nursing Management: Obstructive Pulmonary Diseases, in the textbook) Asthma Definition Chronic inflammatory disorder of airways Causes airway hyperresponsiveness
Focus on Asthma (Relates to Chapter 29, Nursing Management: Obstructive Pulmonary Diseases, in the textbook) Asthma Definition Chronic inflammatory disorder of airways Causes airway hyperresponsiveness
History & Development
 RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
Allergies Formulary. Self Care
 How does the guidance apply to this formulary? For full information see: NHSE Guidance to CCGs A quick reference guide for professionals is also available which contains hyperlinks to the conditions for
How does the guidance apply to this formulary? For full information see: NHSE Guidance to CCGs A quick reference guide for professionals is also available which contains hyperlinks to the conditions for
Pharmacotherapy for Allergic Rhinitis
 Pharmacotherapy for Allergic Rhinitis William Reisacher, MD FACS FAAOA Assistant Professor Weill Cornell Medical College The Impact of Allergic Rhinitis Allergic rhinitis affects approximately 50 million
Pharmacotherapy for Allergic Rhinitis William Reisacher, MD FACS FAAOA Assistant Professor Weill Cornell Medical College The Impact of Allergic Rhinitis Allergic rhinitis affects approximately 50 million
Respiratory Pharmacology
 Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
(pedi) Patient Name: date of birth:
 (pedi) Patient Name: date of birth:_ Date: I am being seen on: a) self referral _ b) physician referral from Dr. Please share the main reasons for your office visit today (check all those that apply):
(pedi) Patient Name: date of birth:_ Date: I am being seen on: a) self referral _ b) physician referral from Dr. Please share the main reasons for your office visit today (check all those that apply):
Asthma By Mayo Clinic staff
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
The Inflammatory Response. Inflammation in the Airway. The Inflammatory Response. Inflammation in the Airway. Inflammation in the Airway
 The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
Allergy and inflammation
 and inflammation 1 Allergic population hyper-producers of IgE consistently increasing western societies: ~20% of general population 2 Allergic population 3 Allergic triggers 4 Allergic triggers abnormal
and inflammation 1 Allergic population hyper-producers of IgE consistently increasing western societies: ~20% of general population 2 Allergic population 3 Allergic triggers 4 Allergic triggers abnormal
BECONASE AQ - beclomethasone dipropionate monohydrate spray, suspension Stat Rx USA LLC
 BECONASE AQ - beclomethasone dipropionate monohydrate spray, suspension Stat Rx USA LLC DESCRIPTION Beclomethasone dipropionate, monohydrate, the active component of BECONASE AQ Nasal Spray, is an anti-inflammatory
BECONASE AQ - beclomethasone dipropionate monohydrate spray, suspension Stat Rx USA LLC DESCRIPTION Beclomethasone dipropionate, monohydrate, the active component of BECONASE AQ Nasal Spray, is an anti-inflammatory
Function of the Respiratory System. Exchange CO2 (on expiration) for O2 (on inspiration)
 Function of the Respiratory System Exchange CO2 (on expiration) for O2 (on inspiration) Upper Respiratory Tract Includes: Nose Mouth Pharynx Larynx Function: Warms and humidifies the inspired air Filters
Function of the Respiratory System Exchange CO2 (on expiration) for O2 (on inspiration) Upper Respiratory Tract Includes: Nose Mouth Pharynx Larynx Function: Warms and humidifies the inspired air Filters
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
Sinusitis. What are the sinuses? Who develops sinusitis?
 Sinusitis Health experts estimate that 37 million Americans are affected by sinusitis every year. Americans spend nearly $6 billion each year on health care costs related to sinusitis. Sinusitis is an
Sinusitis Health experts estimate that 37 million Americans are affected by sinusitis every year. Americans spend nearly $6 billion each year on health care costs related to sinusitis. Sinusitis is an
ASTHMA. Epidemiology. Pathophysiology. Diagnosis. IAP UG Teaching slides
 BRONCHIAL ASTHMA ASTHMA Epidemiology Pathophysiology Diagnosis 2 CHILDHOOD ASTHMA Childhood bronchial asthma is characterized by Airway obstruction which is reversible Airway inflammation Airway hyper
BRONCHIAL ASTHMA ASTHMA Epidemiology Pathophysiology Diagnosis 2 CHILDHOOD ASTHMA Childhood bronchial asthma is characterized by Airway obstruction which is reversible Airway inflammation Airway hyper
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide
 PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
Respiratory Pharmacology. Manuel Otero Lopez Department of Anaesthetics and Intensive Care Hôpital Européen Georges Pompidou, Paris, France
 Respiratory Pharmacology Manuel Otero Lopez Department of Anaesthetics and Intensive Care Hôpital Européen Georges Pompidou, Paris, France Programme Bronchomotor tone Drugs and factors influencing airway
Respiratory Pharmacology Manuel Otero Lopez Department of Anaesthetics and Intensive Care Hôpital Européen Georges Pompidou, Paris, France Programme Bronchomotor tone Drugs and factors influencing airway
Asthma in Pregnancy. Asthma. Chronic Airway Inflammation. Objective Measures of Airflow. Peak exp. flow rate (PEFR)
 Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
your breathing problems worsen quickly. you use your rescue inhaler, but it does not relieve your breathing problems.
 MEDICATION GUIDE ADVAIR DISKUS [ad vair disk us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is the most important information I should know about ADVAIR DISKUS?
MEDICATION GUIDE ADVAIR DISKUS [ad vair disk us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is the most important information I should know about ADVAIR DISKUS?
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET ALANASE Beclometasone dipropionate Aqueous Nasal Spray 50 µg & 100 µg per actuation Presentation ALANASE Aqueous Nasal Spray (50 micrograms per actuation) is an almost white opaque
NEW ZEALAND DATA SHEET ALANASE Beclometasone dipropionate Aqueous Nasal Spray 50 µg & 100 µg per actuation Presentation ALANASE Aqueous Nasal Spray (50 micrograms per actuation) is an almost white opaque
FLONASE (fluticasone propionate) Nasal Spray, 50 mcg
 1 2 3 4 5 6 FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. 7 8 9 10 11 12 DESCRIPTION Fluticasone propionate, the active
1 2 3 4 5 6 FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. 7 8 9 10 11 12 DESCRIPTION Fluticasone propionate, the active
ATROVENT NASAL and ATROVENT NASAL FORTE PRODUCT INFORMATION
 ATROVENT NASAL and ATROVENT NASAL FORTE PRODUCT INFORMATION DESCRIPTION Approved Name Chemical Name Ipratropium bromide (1R, 3r, 5S, 8r)-8-Isopropyl-3-( tropoyloxytropanium bromide. C 20 H 30 NO 3 Br.
ATROVENT NASAL and ATROVENT NASAL FORTE PRODUCT INFORMATION DESCRIPTION Approved Name Chemical Name Ipratropium bromide (1R, 3r, 5S, 8r)-8-Isopropyl-3-( tropoyloxytropanium bromide. C 20 H 30 NO 3 Br.
Staying Healthy. with Asthma. Illustrations by paulsharp.com
 Staying Healthy with Asthma Illustrations by paulsharp.com Lungs & Asthma What is Asthma? Inflammation or swelling of airways that leads to: 1) Mucous production deep inside the airways. 2) Temporary difficulty
Staying Healthy with Asthma Illustrations by paulsharp.com Lungs & Asthma What is Asthma? Inflammation or swelling of airways that leads to: 1) Mucous production deep inside the airways. 2) Temporary difficulty
FLONASE (fluticasone propionate) Nasal Spray, 50 mcg
 FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. DESCRIPTION Fluticasone propionate, the active component of FLONASE Nasal
FLONASE (fluticasone propionate) Nasal Spray, 50 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE GENTLY BEFORE USE. DESCRIPTION Fluticasone propionate, the active component of FLONASE Nasal
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Allergy Relief 50 microgram Nasal Spray (Fluticasone propionate)
 Information for the user Allergy Relief 50 microgram Nasal Spray (Fluticasone propionate) Read all of this leaflet carefully before you start taking this medicine because it contains important information
Information for the user Allergy Relief 50 microgram Nasal Spray (Fluticasone propionate) Read all of this leaflet carefully before you start taking this medicine because it contains important information
ARIA. At-A-Glance Pocket Reference 2007
 ARIA_Glance_2007_8pg:ARIA_Glance_English 9/14/07 3:10 PM Page 1 ARIA At-A-Glance Pocket Reference 2007 1 st Edition NEW ARIA UPDATE BASED ON THE ALLERGIC RHINITIS AND ITS IMPACT ON ASTHMA WORKSHOP REPORT
ARIA_Glance_2007_8pg:ARIA_Glance_English 9/14/07 3:10 PM Page 1 ARIA At-A-Glance Pocket Reference 2007 1 st Edition NEW ARIA UPDATE BASED ON THE ALLERGIC RHINITIS AND ITS IMPACT ON ASTHMA WORKSHOP REPORT
Oral Agents. Fml Limits. Available Strengths NF NF
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Allergy Medications LAST REVIEW: 9/12/2017 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 9/16, 5/15, 9/14
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Allergy Medications LAST REVIEW: 9/12/2017 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 9/16, 5/15, 9/14
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
PREPARATION FOR ALLERGY TESTING *** Please read this information at least one week before your upcoming visit.
 PREPARATION FOR ALLERGY TESTING *** Please read this information at least one week before your upcoming visit. In order to obtain valid and useful skin testing results, you will need to stop the use of
PREPARATION FOR ALLERGY TESTING *** Please read this information at least one week before your upcoming visit. In order to obtain valid and useful skin testing results, you will need to stop the use of
Monocast Description Indications
 Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
Nancy Davis, RRT, AE-C
 Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr AVAMYS fluticasone furoate nasal spray FOR NASAL USE ONLY This leaflet is part III of a three-part "Product Monograph" published when AVAMYS was approved for sale in Canada
PART III: CONSUMER INFORMATION Pr AVAMYS fluticasone furoate nasal spray FOR NASAL USE ONLY This leaflet is part III of a three-part "Product Monograph" published when AVAMYS was approved for sale in Canada
Triage Information: 1. Duration of HPSJ Membership 2. Age 3. Fill history of Seasonal Allergy Medications
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 5/28/2015 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/15, 9/14
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 5/28/2015 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/15, 9/14
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
3 RESPIRATORY SYSTEM
 3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
PRESCRIBING INFORMATION. IPRATROPIUM BROMIDE NASAL SOLUTION, 0.03% Nasal Spray. Only
 PRESCRIBING INFORMATION IPRATROPIUM BROMIDE NASAL SOLUTION, 0.03% Nasal Spray Only DESCRIPTION The active ingredient in ipratropium bromide nasal solution is ipratropium bromide monohydrate. It is an anticholinergic
PRESCRIBING INFORMATION IPRATROPIUM BROMIDE NASAL SOLUTION, 0.03% Nasal Spray Only DESCRIPTION The active ingredient in ipratropium bromide nasal solution is ipratropium bromide monohydrate. It is an anticholinergic
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION APO-TRIAMCINOLONE AQ Children 4 to 12 years Triamcinolone Acetonide Aqueous Nasal Spray 55 mcg/metered Spray Apotex Standard This leaflet is part III of a three-part Product
PART III: CONSUMER INFORMATION APO-TRIAMCINOLONE AQ Children 4 to 12 years Triamcinolone Acetonide Aqueous Nasal Spray 55 mcg/metered Spray Apotex Standard This leaflet is part III of a three-part Product
MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol
![MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol](/thumbs/78/76835983.jpg) MEDICATION GUIDE ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol ADVAIR HFA 115/21 (fluticasone propionate 115 mcg and salmeterol 21 mcg) Inhalation
MEDICATION GUIDE ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol ADVAIR HFA 115/21 (fluticasone propionate 115 mcg and salmeterol 21 mcg) Inhalation
Case Study. Allergic Rhinitis 5/18/2015
 John A. Fling, M.D. Professor Allergy/Immunology University of North Texas Health Science Center, Fort Worth, Texas Case Study 38 year old male with a history of nasal congestion, clear nasal discharge
John A. Fling, M.D. Professor Allergy/Immunology University of North Texas Health Science Center, Fort Worth, Texas Case Study 38 year old male with a history of nasal congestion, clear nasal discharge
PATIENT INFORMATION LEAFLET
 N-CORT 0.055% Nasal Spray For nasal use. PATIENT INFORMATION LEAFLET Active substance(s): Triamcinolone acetonide (w/w%) 0.055 Excipient(s): Benzalkonium chloride 50%, sodium hydroxide, hydrochloric acid,
N-CORT 0.055% Nasal Spray For nasal use. PATIENT INFORMATION LEAFLET Active substance(s): Triamcinolone acetonide (w/w%) 0.055 Excipient(s): Benzalkonium chloride 50%, sodium hydroxide, hydrochloric acid,
Annex II. Scientific conclusions
 Annex II Scientific conclusions 5 Scientific conclusions Beclometasone dipropionate (BDP) is a glucocorticoid and a prodrug of the active metabolite, beclometasone-17-monopropionate. Beclometasone dipropionate
Annex II Scientific conclusions 5 Scientific conclusions Beclometasone dipropionate (BDP) is a glucocorticoid and a prodrug of the active metabolite, beclometasone-17-monopropionate. Beclometasone dipropionate
APOHEALTH BUDESONIDE HAYFEVER NASAL INHALATION. 16α, 17α - 22 R, S-propylmethylenedioxypregna - 1, 4 - diene - 11ß, 21-diol-3, 20-dione
 APOHEALTH BUDESONIDE HAYFEVER NASAL INHALATION NAME OF THE MEDICINE Budesonide Chemical Name: 16α, 17α - 22 R, S-propylmethylenedioxypregna - 1, 4 - diene - 11ß, 21-diol-3, 20-dione Structural Formula:
APOHEALTH BUDESONIDE HAYFEVER NASAL INHALATION NAME OF THE MEDICINE Budesonide Chemical Name: 16α, 17α - 22 R, S-propylmethylenedioxypregna - 1, 4 - diene - 11ß, 21-diol-3, 20-dione Structural Formula:
Drug/Device Combination Products: Bioequivalence
 Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
SAN DIEGO ALLERGY ASTHMA & IMMUNOLOGY CONSULTANTS, INC
 SAN DIEGO ALLERGY ASTHMA & IMMUNOLOGY CONSULTANTS, INC BERNARD A. FEIGENBAUM, M.D. FACP, FAAAAI 9850 GENESEE AVE, SUITE 355 CLINICAL ASSISTANT PROFESSOR OF MEDICINE & OTOLARYNGOLOGY, NYU LA JOLLA, CA 92037
SAN DIEGO ALLERGY ASTHMA & IMMUNOLOGY CONSULTANTS, INC BERNARD A. FEIGENBAUM, M.D. FACP, FAAAAI 9850 GENESEE AVE, SUITE 355 CLINICAL ASSISTANT PROFESSOR OF MEDICINE & OTOLARYNGOLOGY, NYU LA JOLLA, CA 92037
MANAGING ASTHMA. Nancy Davis, RRT, AE-C
 MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
Allergic Rhinitis: When to Refer to an Allergist
 Allergic Rhinitis: When to Refer to an Allergist Kirsten Kloepfer, MD, MS Assistant Professor of Pediatrics Section of Pulmonary, Allergy and Sleep Medicine Disclosures NIH K23 American Academy of Allergy,
Allergic Rhinitis: When to Refer to an Allergist Kirsten Kloepfer, MD, MS Assistant Professor of Pediatrics Section of Pulmonary, Allergy and Sleep Medicine Disclosures NIH K23 American Academy of Allergy,
Respiratory Medications and Devices Update 2/15
 Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
Allergy Medications. Antihistamines. are very safe. Although usually taken as tablets, they may be prescribed as a liquid or syrup for young children
 The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
The treatments prescribed for allergy control the symptoms and reactions; they do not cure the condition. However, using treatments as prescribed can show a huge change in a patient s health, mood and
RHINOCORT AQUA (budesonide) NASAL SPRAY 32 mcg and 64 mcg. Unannotated Proposed Package Insert NDA
 DRAFT PRESCRIBING INFORMATION 1(11) RINOCORT AQUA (budesonide) NASAL SPRAY 32 mcg and 64 mcg Unannotated Proposed Package Insert NDA 20-746 DRAFT PRESCRIBING INFORMATION 2(11) RINOCORT AQUA (budesonide)
DRAFT PRESCRIBING INFORMATION 1(11) RINOCORT AQUA (budesonide) NASAL SPRAY 32 mcg and 64 mcg Unannotated Proposed Package Insert NDA 20-746 DRAFT PRESCRIBING INFORMATION 2(11) RINOCORT AQUA (budesonide)
PATIENT INFORMATION. ADVAIR DISKUS [AD vair DISK us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation
![PATIENT INFORMATION. ADVAIR DISKUS [AD vair DISK us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation PATIENT INFORMATION. ADVAIR DISKUS [AD vair DISK us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation](/thumbs/74/71128680.jpg) PATIENT INFORMATION ADVAIR DISKUS [AD vair DISK us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is ADVAIR DISKUS? ADVAIR DISKUS combines the inhaled corticosteroid
PATIENT INFORMATION ADVAIR DISKUS [AD vair DISK us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is ADVAIR DISKUS? ADVAIR DISKUS combines the inhaled corticosteroid
Hayfever. Allergic reaction. Prognosis
 Hayfever Hay fever is a type of allergic rhinitis caused by pollen or spores. Allergic rhinitis is a condition where an allergen (something that causes an allergic reaction) makes the inside of your nose
Hayfever Hay fever is a type of allergic rhinitis caused by pollen or spores. Allergic rhinitis is a condition where an allergen (something that causes an allergic reaction) makes the inside of your nose
The Inflammatory Response
 RSPT 2317 The Inflammatory Response A general definition inflammation is the response of vascularized tissue to injury. First described in the first century A.D. and revised in the 20 th century as follows
RSPT 2317 The Inflammatory Response A general definition inflammation is the response of vascularized tissue to injury. First described in the first century A.D. and revised in the 20 th century as follows
NASONEX Aqueous Nasal Spray Mometasone Furoate. Schering-Plough
 NASONEX Aqueous Nasal Spray Mometasone Furoate Schering-Plough COMPOSITION NASONEX Aqueous Nasal Spray is a metered-dose, manual pump spray unit containing a suspension of mometasone furoate. Each metered-dose
NASONEX Aqueous Nasal Spray Mometasone Furoate Schering-Plough COMPOSITION NASONEX Aqueous Nasal Spray is a metered-dose, manual pump spray unit containing a suspension of mometasone furoate. Each metered-dose
Air Flow Limitation. In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation.
 Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
New Therapies for Asthma
 New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
Common Inhaled Asthma Medications Dose Comparison and Tips for Use
 Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
2 What you need to know before you use Beconase. Package Leaflet: Information for the user
 Package Leaflet: Information for the user Beconase Aqueous Nasal Spray beclometasone dipropionate 50 micrograms Read all of this leaflet carefully before you start using this medicine because it contains
Package Leaflet: Information for the user Beconase Aqueous Nasal Spray beclometasone dipropionate 50 micrograms Read all of this leaflet carefully before you start using this medicine because it contains
Asthma. doh.sd.gov/statistics/2009brfss/asthma.pdf
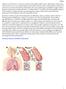 is an obstructive or restrictive condition which inhibits airflow due to inflammation of the airway. This obstruction occurs via increased edema in the small bronchioles walls as well as greater production
is an obstructive or restrictive condition which inhibits airflow due to inflammation of the airway. This obstruction occurs via increased edema in the small bronchioles walls as well as greater production
INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT
 - 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
- 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
Lungs SLO Practice (online) Page 1 of 5
 Lungs SLO Practice (online) Page 1 of 5 1. A 15 year- old teen has asthma. A nebulizer treatment has been ordered. The type of medication most likely to be used in this treatment for asthma management
Lungs SLO Practice (online) Page 1 of 5 1. A 15 year- old teen has asthma. A nebulizer treatment has been ordered. The type of medication most likely to be used in this treatment for asthma management
Azelastine Nasal Spray 1 mg / 1 ml
 Package Leaflet: Information for the User Allergodil 0,1% nasal spray, solution Active substance: Azelastine hydrochloride Read all of this leaflet carefully because it contains important information for
Package Leaflet: Information for the User Allergodil 0,1% nasal spray, solution Active substance: Azelastine hydrochloride Read all of this leaflet carefully because it contains important information for
