DIAGNOSTIC ACCREDITATION PROGRAM. Spirometry Quality Control Plan
|
|
|
- Cora Terry
- 6 years ago
- Views:
Transcription
1 DIAGNOSTIC ACCREDITATION PROGRAM Spirometry Quality Control Plan
2 Table of Contents Introduction...1 Spirometry Definitions and Requirements...2 Spirometry Requirements Daily Quality Control (see p.7 to 9)... 4 Weekly Quality Control (see p.10 to 13)... 5 Monthly Quality Control (see p.14 to 15)... 5 Semi-annual Reporting... 5 Log Book... 5 Spirometer Calibration...7 Calibration Syringe Requirements... 7 Performing Calibration or a Calibration Check... 8 Enter Ambient Conditions... 8 Perform Calibration or Calibration Check... 9 Spirometer Linearity Testing Linearity Testing Biological Control (BioQC) Testing Establishing the BioQC Normal Range Monthly BioQC Testing Predicted Sets Submission of Data to the DAP References Table of Contents i
3 Introduction Spirometry is a useful diagnostic test commonly performed in a variety of settings; however, accurate results are dependent on careful technique, and proper equipment calibration and maintenance. The American Thoracic Society (ATS) and European Respiratory Society (ERS) have recommended a number of procedures to reduce variability including the weekly testing of flow volume measurements. Under the College of Physicians and Surgeons of British Columbia, Diagnostic Accreditation Program (DAP) Spirometry Quality Control Program, facility personnel at each site will perform quality control procedures according to the DAP protocol. Only one set of data is required from each spirometer. If the spirometer is used in more than one site, the patient data submitted should be representative of this. Results are submitted to the DAP twice a year and will give an indication if any areas of concern exist with the spirometer or performance of the tests. An individual report with recommendations will be sent to the facility at the end of each reporting cycle. Level 1 and level 2 (formerly known as category IIA and IIB) facilities and private clinics performing spirometry require participation in the DAP Spirometry Quality Control Program as part of their accreditation. Continuing accreditation is based upon the satisfactory review of quality assurance and control data submitted to the DAP. Exemption: If the spirometer used exclusively is the COPD-6 spirometer, as approved by the Medical Services Commission for case finding, DAP accreditation is not required. The DAP Spirometry QC Program follows the recommendations of the American Thoracic Society and European Respiratory Society Task Force: Standardisation of Lung Function Testing: Standardisation of spirometry (2005). 1 Introduction 1
4 Spirometry Definitions and Requirements Calibration/calibration check: Calibration is the procedure for establishing the relationship between sensor-determined values of flow or volume, and the actual flow or volume, using a validated 3-L calibration syringe. Current ambient conditions are entered prior to calibration and the injection rates are varied according to equipment manufacturer s recommendations to adjust the output with a known input volume. Calibration check is different in that it does not adjust the spirometer; rather it verifies that the spirometer is within calibration limits. As the validated QC syringe may lose accuracy over time, re-validation is performed according to manufacturer s recommendations. Proof of revalidation is submitted to the DAP annually. Linearity testing: A spirometer is considered linear if its output is directly proportional to its input, regardless of the flow rate. Loss of linearity is a primary problem with flow sensing spirometers, therefore syringe flow volume loops are performed on a weekly basis to ensure system linearity. The therapist injects the full volume of the validated 3-L syringe into the spirometer at various rates and records the reported volumes. The peak flow rates include one injection at a rate of less than 2 liters per second, one injection at a rate of four to six liters per second, and one injection at a rate of greater than eight liters per second. The FVC results must be within the acceptable ranges and the maximum difference between the reported volumes should be less than.105 liters (105 ml) to document that the equipment is within control limits. Enter one set of linearity data per month on the DAP worksheets provided, and include the graphical printout. Data is submitted twice per year, on or before January 15 and July 1. Biologic controls (BioQC): A healthy non-smoking individual will perform spirometry testing monthly to assess the overall operational status of the spirometry system. Results are monitored to assess changes in equipment performance that may be undetected in routine calibration. A second BioQC subject should be identified and able to fill in if the primary BioQC subject cannot perform this function due to absence or illness. Enter one set of BioQC data per month on the DAP worksheets provided. Data is submitted twice per year, on or before January 15 and July 15. Spirometry Requirements The spirometer must be able to accumulate volume for greater than 15 seconds and measure a volume of greater than eight liters (BTPS) with an accuracy of ± 3% of reading or ± 0.05 liters, whichever is greater. The facility must have a room thermometer with an accuracy of ± 1 C. If room temperature changes by more than 2 C, then another calibration must be performed prior to patient testing. Measure height of patients and BioQC subjects accurately. Calibration or calibration checks are performed daily or prior to patient testing. Linearity checks (flow volume loops) should be performed weekly and after equipment repairs, maintenance or software changes. A biologic control (BioQC) should be performed monthly and after equipment repairs, maintenance or software changes. Spirometry Definitions and Requirements 2
5 A log of all relevant testing data, service, repairs or software changes, should be maintained. Spirometry Definitions and Requirements 3
6 The term quality control can be defined as the process of monitoring the accuracy and reproducibility of a test procedure but it also takes into account a number of aspects, any one of which may impact patient results. These include the components that make up the pre-test, test, and post-test: patient education and preparation procedure equipment reporting of results The following chart outlines the steps necessary to ensure proper functioning of the spirometry equipment, and the frequency with which they need to be performed by the therapist, in order to produce accurate patient results. Detailed instructions follow in each section. Equipment Procedure Frequency ATS/ERS Limits of Acceptability / Recommended Limits Spirometer Calibration or Calibration Check with 3-L calibration syringe Spirometer Linearity tests with 3-L calibration syringe Daily or prior to patient testing + 3.5% of 3 L ( L) Weekly Loops at these flow ranges: < 2 L/s, 4 L/s to 6 L/s, > 8 L/s *see p. 12 for acceptable results Spirometer Biologic control (BioQC) Monthly FVC and FEV1 < 3% PEFR < 10% Syringe Leak test Weekly No leaks Daily Quality Control (see p.7 to 9) 1. Perform calibration or calibration check at least daily or prior to patient testing, as per manufacturer recommendations. 2. Print out a copy of the calibration results. The measured values must be between liters. 3. Verify that the calibration has passed (see #2 above) prior to patient testing. 4
7 Weekly Quality Control (see p.10 to 13) 1. Perform 3-L calibration syringe leak test. 2. Perform linearity test with a validated 3-L calibration syringe run in a patient file, named Syringe, QC, and record results on the DAP Monthly Spirometry Linearity Testing worksheets. 3. Verify that the linearity tests fall within the acceptable ranges for accuracy and reproducibility. Monthly Quality Control (see p.14 to 15) 1. Perform BioQC testing: The biologic normal control is performed in the patient file designated for that individual (e.g. BioQC1 or BioQC2). Only one (1) BioQC is required to be tested each month. When this data is entered into the DAP Spirometry QC worksheets, a number of calculations are performed automatically. Unexpected changes in the BioQC values may alert the user to equipment problems that were not present, or undetected, when calibration procedures were performed. 2. Verify that the BioQC values are acceptable based on their established ranges. Semi-annual Reporting 1. Submit to the DAP on or before January 15 and July 15: Log Book a. Worksheets for linearity report (including printout of graphical data). b. Worksheets for BioQC results (including printouts of graphical data). c. Calibration print-outs for day of each monthly BioQC result submitted. d. Printouts of 10 random and anonymized patient spirograms with interpretation, performed during the six-month reporting period. A log book should be kept to record the following: ambient temperature barometric pressure relative humidity calibration FVC values with the 3-L syringe calibration pass or fail leak test pass or fail equipment service, troubleshooting performed, software upgrades, etc. 5
8 Example of log book: Date Initials Temp C 24/08/ 12 BP mm/hg RH% Calibration: FVC ( ) Cal P/F Leak test Comments SW Pass Pass ATS 2005 software upgrade 6
9 Spirometer Calibration Calibration Syringe Requirements In order to achieve accurate and reproducible spirometry results, measurements from your spirometer should be regularly checked against a precision calibration syringe. The 2005 ATS/ERS statement Standardisation of Spirometry 1 requires daily calibration or calibration checks of flow and volume spirometers using a validated 3 liter calibration syringe with an accuracy of ± 15 ml or ± 0.5%. Calibration is the procedure for establishing the relationship between sensor-determined values of flow or volume, and the actual flow or volume, using a validated 3-L calibration syringe. Calibration should be performed daily or prior to use. Follow manufacturer s instructions. Spirometer should be set to calibration mode. A calibration check is different from calibration and is the procedure used to validate that the spirometer is within calibration limits, that is to say ± 3.5% of true. A calibration check is required daily or prior to patient testing. Spirometer should be set to calibration check mode. There are no spirometers on the market currently that may be used without at least a calibration check. Syringe re-validation: As wear and tear can affect the accuracy of the 3-L calibration syringe over time, it should be re-validated on a yearly basis or as specified by the manufacturer. There are a number of companies that perform re-validation services of calibration syringes. Proof of syringe validation must be submitted annually to the DAP. Syringe leak test: The 3-L calibration syringe should be tested for leaks and smoothness of operation minimally on a weekly basis. The syringe should be tested from a full (drawn back) position by placing a hand over the outlet and depressing the syringe handle gently. No air should escape. Secondly the syringe should be emptied, and in an empty position should be checked by again placing a hand over the outlet, then pulling gently on the syringe handle. No air should enter the syringe. Syringes that leak may not measure proper volume and should be sent for service. Syringe smoothness test: Move the syringe handle back and forth to check that the action is smooth, without catching or stuttering. Syringes that do not move smoothly may not deliver proper volume and should be sent for service. Spirometer Calibration 7
10 Performing Calibration or a Calibration Check Calibration or calibration checks must be performed every day prior to patient testing or when quality control is performed. Check your manufacturer s manual for calibration or calibration check directions. The directions will be labeled as a calibration or a calibration check. Some systems are calibrated at the manufacturer s site but still require a calibration check daily or prior to patient or quality control testing. The following instructions may not be specific to your spirometer, but the examples are meant to represent how the data may look. The displays will vary slightly based on the spirometer you are using. Enter Ambient Conditions The facility must have a room thermometer with an accuracy of ± 10C. If barometer and/or hygrometer are not available, barometric pressure and relative humidity may be recorded from local weather station sources. From your thermometer, barometer and hygrometer enter: 1. Temperature 2. Barometric pressure 3. Humidity Spirometer Calibration 8
11 Perform Calibration or Calibration Check 1. Set spirometer to calibration mode or calibration check mode. 2. If filters designed specifically for spirometry testing are used, calibration or calibration checks should be done through the filter. 3. Perform a calibration or calibration check using the validated 3-L calibration syringe according to the 2005 ATS/ERS statement Standardisation of Spirometry. 1 Pull the syringe handle out completely and push the 3-liter volume into the spirometer at the correct flow. 4. Repeat the calibration or calibration check at least three separate times at three different flow rates, as per manufacturer instructions. 5. Ensure the calibration results are within the required limits ± 3.5% (or liters to liters). 6. Maintain a copy of the calibration or calibration check in the log book. 9
12 Spirometer Linearity Testing A spirometer is considered linear if its output is directly proportional to its input regardless of flow rate. The resulting volumes should be accurate and reproducible. The 2005 ATS/ERS statement Standardisation of Spirometry 1 states that linearity testing must be performed weekly. The DAP Spirometry QC Program requires that only one of those tests be recorded for each month on the Monthly Spirometry Linearity Testing worksheet. The linearity test is completed by performing syringe flow volume loops to simulate patient testing with a known volume at varying flows. Set the spirometer to patient mode and inject air from a 3-L syringe at a slow, moderate, and fast flow rates. The FVC volumes achieved at each of these flow rates should meet both the accuracy requirement of ± 3.5%, and should show reproducibility to within 105 ml from highest to lowest flow rate (see sections A and B on pages 12 to 13). Linearity Testing 1. Enter ambient conditions as described on page Perform a calibration or calibration check using the 3-L syringe, as described on page Set up a mock patient study. Enter the patient data as follows: First Name: QC Last Name: Syringe Weight: 150 lbs. (or 68 kg) Height: 64 in. (or 163 cm) Age: 40 Spirometer Linearity Testing 10
13 4. Perform linearity tests (flow volume loops) as shown below. a. Set spirometer to patient mode. Attach a 3-L syringe to the spirometer with a tight seal (no leaks). b. Withdraw syringe until fully inflated to a full three liters. c. Perform a minimum of three linearity tests (flow volume loops): Example: one maneuver will be performed with a peak flow of less than two liters/sec one maneuver will be performed with a peak flow between four and six liters/sec one maneuver will be performed with a peak flow greater than eight liters/sec 5. Print out the following: All linearity tests displaying each loop at each flow and the actual numeric results. Ambient temperature is an integral piece of information that must be recorded somewhere on the flow volume loops printout. 6. Verify linearity meets acceptability criteria as defined below 7. Complete DAP Monthly Spirometry Quality Control Program worksheet (tab labeled Linearity): Fill in the values generated for FVC and FEV1 Calculate the highest reading minus the lowest FVC Confirm that FVC and FEV1 values are within acceptable limits. Attach the corresponding graphical print-out. Note: While Linearity testing is required weekly, only one week per month should be recorded for submission to DAP. 8. Submit completed six-month data set to the DAP on or before January 15 and July 15. Spirometer Linearity Testing 11
14 Linearity Acceptance Criteria A. Acceptable results at ambient temperature (accuracy verification): Spirometers may measure volume at body temperature (BTPS) or at ambient/room temperature (ATPS). Most spirometers in physicians offices will report at BTPS. Spirometers reporting at BTPS automatically employ correction factors, at specific temperatures, that convert ATPS to BTPS. Here is an example for an ambient temperature of 21 C: Volume of 3-L syringe = 3.00 L Correction factor at 21 C = Therefore: 3.00 L x = 3.28 L Acceptable accuracy is ± 3.5%. Therefore: o o 3.28 L minus 3.5% = 3.17 L 3.28 plus 3.5% = 3.40 L The acceptable range at 21 C is: 3.17 L 3.40 L The chart below has already calculated the acceptable ranges at each temperature for spirometers reporting at BTPS. In order to verify the accuracy of your measurement, use the chart to determine the FVC acceptable range for each trial, at your ambient temperature: BTPS Chart for Acceptable Ranges (± 3.5%) Factor o C Acceptable Range (L) Factor o C Acceptable Range (L) Factor o C Acceptable Range (L) Spirometer Linearity Testing 12
15 B. Acceptable difference in FVC at three flow rates (reproducibility verification): In order to determine if your spirometer meets reproducibility requirements across various flow rates, the volumes should fall within L (105 ml) from largest FVC to smallest FVC. And Peak Expiratory Flow must fall within target range for each trial. Example 1: The largest FVC trial (3.27) minus the smallest FVC trial (3.18) is less than L (105 ml) and is therefore acceptable. The peak flows are also within the required ranges (less than 2 L/s, 4 6 L/s and greater than 8 L/s). Linearity Test Flow Volume Loops Trial 1 Low flow (less than 2 L/sec) Trial 2 Mid flow (4 to 6 L/sec) Trial 3 High flow (greater than 8 L/sec) Difference in FVC (highest minus lowest: should be < or = 105 ml) FVC (liters) L (90 ml) Peak Expiratory Flow (liters/sec) Example 2: The largest FVC trial (3.28) minus the smallest FVC trial (3.08) is greater than L (105 ml) and therefore is not acceptable. Also, the Peak Expiratory Flow for Trial 1 is 2.56, which is higher than the requirement of less than 2 L/sec and therefore would not be considered an acceptable trial. Linearity Test Flow Volume Loops Trial 1 Low flow (less than 2 L/sec) Trial 2 Mid flow (4 to 6 L/sec) Trial 3 High flow (greater than 8 L/sec) Difference in FVC (highest minus lowest: should be < or = 105 ml) FVC (liters) L (200 ml) Peak Expiratory Flow (liters/sec) Spirometer Linearity Testing 13
16 Biological Control (BioQC) Testing A biological normal quality control (BioQC) refers to a healthy non-smoking individual with normal and stable lung function, who is tested on a regular basis as a control. Frequently office personnel are asked to perform this function. BioQC should be performed monthly and reported on the DAP Monthly Spirometry Quality Control Program worksheets. Each site should identify two (2) individuals with normal lung function who are available to perform BioQC spirometry. However, only one (1) individual is required to perform monthly BioQC spirometry. The second person should be available to fill in if the primary BioQC subject cannot perform this function due to absence or illness. Establishing the BioQC Normal Range 1. In order to establish the normal ranges for the primary and backup BioQC subjects, a minimum of 10 replicates are required. Twenty replicates is the recommendation for statistical accuracy, however 10 replicates will give an adequate baseline with which to compare the monthly QC results. 2. Perform 10 to 20 replicates on each BioQC subject over a period of several days. Ideally this should entail a single test performed each day; however a maximum of 2 tests spread out within any single day (e.g. morning and afternoon) may be used. 3. Use the Normal Range Calculator to determine the acceptable ranges for each person. This worksheet takes the average of the replicates and calculates two standard deviations (SD) which constitutes the normal range for this subject. Go to the Community Spirometry page on the College website and save the Spirometry BioQC Normal Range Calculator to your computer. The calculator is also the last tab on the Monthly Spirometry Quality Control Program worksheets. Fill in the values generated by the BioQC subjects. The average, standard deviation (SD) and coefficient of variation (CV) will automatically be calculated. There should be a maximum of 10% between the highest and lowest FVC and FEV1 values. The calculated coefficient of variation (CV) should be 3% or less. If your site does not have access to Excel, submit the data to the DAP for normal range calculations. 4. Subsequent spirometry testing on each BioQC subject should fall within the ± 2 SD ranges for that subject. The facility should perform troubleshooting if BioQC values fall outside of their acceptable ranges. Biological Control (BioQC) Testing 14
17 Monthly BioQC Testing 1. The BioQC subject should perform spirometry procedures in the same way as a patient. 2. For consistency, the BioQC subject should ideally be tested: a. on the same spirometer b. on the same day of month c. at the same time of day 3. An adequate test requires a minimum of three acceptable FVC maneuvers and adherence to repeatability criteria. Repeatability is achieved when the difference between the largest and the next largest acceptable FVC and FEV1, is less than or equal to 150 ml. The results for FVC and FEV1 do not need to come from the same maneuver. The best trial is chosen based on the largest sum of FVC plus FEV1 from acceptable maneuvers. 4. The BioQC result should be recorded for each month on the Monthly Spirometry Quality Control Program worksheets. Go to the Pulmonary Function page of the College website and save these forms to your computer. Fill in the values generated by the BioQC subject(s). The average, standard deviation (SD) and coefficient of variation (CV) will automatically be calculated. The CVs for FVC and FEV1 should be less than or equal to 3%. Confirm that the results fall within the acceptable ranges for this BioQC subject. If your site does not have access to Excel, submit the data to the DAP for BioQC calculations. 5. Data from different BioQC subjects must be recorded on separate worksheets (see tabs at the bottom of the spreadsheet). Do not mix BioQC #1 data with BioQC #2 data. 6. BioQC results are submitted along with that day s calibration report, to the DAP twice each year on or before January 15 and July
18 Predicted Sets The National Health and Nutrition Survey (NHANES) III (1999) is the ATS-ERS recommended source of normal values for ages four to 80. Submission of Data to the DAP QC must be reviewed and signed off by the physician in charge. Completed data may be submitted to DAP PT/QC Specialist by any of the following: Scan / to: Fax to , Attn: DAP PT/QC Mail to: References Attn: DAP PT/QC Diagnostic Accreditation Program College of Physicians and Surgeons of British Columbia Howe Street Vancouver BC V6C 0B4 1 Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: Predicted Sets 16
Quality Assurance Mapping Your QC Program Equipment and Test Quality. Susan Blonshine RRT, RPFT, FAARC, AE-C
 Quality Assurance Mapping Your QC Program Equipment and Test Quality Susan Blonshine RRT, RPFT, FAARC, AE-C How to Begin Gather resources Define PF scope of service Procedures performed Equipment Describe
Quality Assurance Mapping Your QC Program Equipment and Test Quality Susan Blonshine RRT, RPFT, FAARC, AE-C How to Begin Gather resources Define PF scope of service Procedures performed Equipment Describe
Spirometry and Flow Volume Measurements
 Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Facility Information for Initial Assessment
 DIAGNOSTIC ACCREDITATION PROGRAM 300 669 Howe Street Telephone: 604-733-7758 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca Fax: 604-733-3503 Facility Information for Initial Assessment
DIAGNOSTIC ACCREDITATION PROGRAM 300 669 Howe Street Telephone: 604-733-7758 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca Fax: 604-733-3503 Facility Information for Initial Assessment
Pulmonary Function Laboratory ATS Accreditation Are you prepared? Susan Blonshine RRT, RPFT, FAARC, AE-C
 Pulmonary Function Laboratory ATS Accreditation Are you prepared? Susan Blonshine RRT, RPFT, FAARC, AE-C Why Quality Consistent, accurate, reliable results Cost-effective Diagnosis Misclassification Reduce
Pulmonary Function Laboratory ATS Accreditation Are you prepared? Susan Blonshine RRT, RPFT, FAARC, AE-C Why Quality Consistent, accurate, reliable results Cost-effective Diagnosis Misclassification Reduce
Spirometry Training Courses. Spirometry for. Thoracic Society of Australia and New Zealand. June Developed in partnership with
 Standards for Spirometry Training Courses Companion Document to Standards for the Delivery of Spirometry for Coal Mine Workers Thoracic Society of Australia and New Zealand June 2017 Developed in partnership
Standards for Spirometry Training Courses Companion Document to Standards for the Delivery of Spirometry for Coal Mine Workers Thoracic Society of Australia and New Zealand June 2017 Developed in partnership
Getting Spirometry Right It Matters! Performance, Quality Assessment, and Interpretation. Susan Blonshine RRT, RPFT, AE-C, FAARC
 Getting Spirometry Right It Matters! Performance, Quality Assessment, and Interpretation Susan Blonshine RRT, RPFT, AE-C, FAARC Objectives Sample Title Recognize acceptable spirometry that meets the start
Getting Spirometry Right It Matters! Performance, Quality Assessment, and Interpretation Susan Blonshine RRT, RPFT, AE-C, FAARC Objectives Sample Title Recognize acceptable spirometry that meets the start
Angela Lorenzo MS, RRT, RPFT Adjunct Faculty, Respiratory Care Long Island University, Brooklyn
 Angela Lorenzo MS, RRT, RPFT Adjunct Faculty, Respiratory Care Long Island University, Brooklyn Disclaimer The views in this lecture are those of the presenter and not those of the Department of Veterans
Angela Lorenzo MS, RRT, RPFT Adjunct Faculty, Respiratory Care Long Island University, Brooklyn Disclaimer The views in this lecture are those of the presenter and not those of the Department of Veterans
UNIT TWO: OVERVIEW OF SPIROMETRY. A. Definition of Spirometry
 UNIT TWO: OVERVIEW OF SPIROMETRY A. Definition of Spirometry Spirometry is a medical screening test that measures various aspects of breathing and lung function. It is performed by using a spirometer,
UNIT TWO: OVERVIEW OF SPIROMETRY A. Definition of Spirometry Spirometry is a medical screening test that measures various aspects of breathing and lung function. It is performed by using a spirometer,
SPIROMETRY METHOD. COR-MAN IN / EN Issue A, Rev INNOVISION ApS Skovvænget 2 DK-5620 Glamsbjerg Denmark
 SPIROMETRY METHOD COR-MAN-0000-006-IN / EN Issue A, Rev. 2 2013-07 INNOVISION ApS Skovvænget 2 DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
SPIROMETRY METHOD COR-MAN-0000-006-IN / EN Issue A, Rev. 2 2013-07 INNOVISION ApS Skovvænget 2 DK-5620 Glamsbjerg Denmark Tel.: +45 65 95 91 00 Fax: +45 65 95 78 00 info@innovision.dk www.innovision.dk
Purpose Of This Guide
 AOHC Session 5 April 9, Speaker Disclosures Spirometry Testing in Occupational Health Programs Best Practices for Healthcare Professionals Mary C. Townsend, Dr.P.H M.C. Townsend Associates, LLC Adjunct
AOHC Session 5 April 9, Speaker Disclosures Spirometry Testing in Occupational Health Programs Best Practices for Healthcare Professionals Mary C. Townsend, Dr.P.H M.C. Townsend Associates, LLC Adjunct
Spirometric protocol
 Spirometric protocol Spirometry is the most common of the Pulmonary Function Test, that measures lung function, specifically the amount (volume) and/or speed (flow) of air that can be inhaled and exhaled.
Spirometric protocol Spirometry is the most common of the Pulmonary Function Test, that measures lung function, specifically the amount (volume) and/or speed (flow) of air that can be inhaled and exhaled.
Katrina M Hynes, MHA RRT RPFT Supervisor - Mayo Clinic Pulmonary Function Laboratory AARC Diagnostic Chair
 Katrina M Hynes, MHA RRT RPFT Supervisor - Mayo Clinic Pulmonary Function Laboratory AARC Diagnostic Chair Conflict of Interest I have no real or perceived conflict of interest that relates to this presentation.
Katrina M Hynes, MHA RRT RPFT Supervisor - Mayo Clinic Pulmonary Function Laboratory AARC Diagnostic Chair Conflict of Interest I have no real or perceived conflict of interest that relates to this presentation.
You Take My Breath Away. Student Information Page 5C Part 1
 You Take My Breath Away Student Information Page 5C Part 1 Students with asthma or other respiratory problems should not participate in this activity because it involves repeated maximal inhalations and
You Take My Breath Away Student Information Page 5C Part 1 Students with asthma or other respiratory problems should not participate in this activity because it involves repeated maximal inhalations and
Independent Health Facilities
 Independent Health Facilities Assessment Protocol for Pulmonary Function Studies INSTRUCTIONS: Please complete ( ) the attached protocol during the assessment. Ensure that all the questions have been answered,
Independent Health Facilities Assessment Protocol for Pulmonary Function Studies INSTRUCTIONS: Please complete ( ) the attached protocol during the assessment. Ensure that all the questions have been answered,
2.0 Scope: This document is to be used by the DCS staff when collecting participants spirometry measurements using the TruFlow Easy-On Spirometer.
 Title: Spirometry Version Date: 2017-MAR-21 Document Effective Date: 2017-MAY-15 Number: Data Collection Site (DCS) Version: 2.3 Number of Pages: SOP_DCS_0012 6 1.0 Purpose: The purpose of this document
Title: Spirometry Version Date: 2017-MAR-21 Document Effective Date: 2017-MAY-15 Number: Data Collection Site (DCS) Version: 2.3 Number of Pages: SOP_DCS_0012 6 1.0 Purpose: The purpose of this document
#7 - Respiratory System
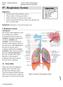 #7 - Respiratory System Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Perform spirometry to measure lung volumes Define and understand the lung volumes and
#7 - Respiratory System Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Perform spirometry to measure lung volumes Define and understand the lung volumes and
MALAYSIAN THORACIC SOCIETY LUNG FUNCTION TESTS EDUCATION PROGRAMME
 INTRODUCTION TO THE MTS LFT EDUCATION PROGRAMME Lung Function Tests (LFT) are vital tests used by the Respiratory Speciality for diagnostic and monitoring purposes. They include Peak Expiratory Flowmetry,
INTRODUCTION TO THE MTS LFT EDUCATION PROGRAMME Lung Function Tests (LFT) are vital tests used by the Respiratory Speciality for diagnostic and monitoring purposes. They include Peak Expiratory Flowmetry,
SPIROMETRY. Marijke Currie (CRFS) Care Medical Ltd Phone: Copyright CARE Medical ltd
 SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
3.0 METHODS. 3.1 Participants
 3.0 METHODS 3.1 Participants There were initially twenty participants studied in this experiment. The age range was between 18-50 years of age. The nine workers came from a private landscape company with
3.0 METHODS 3.1 Participants There were initially twenty participants studied in this experiment. The age range was between 18-50 years of age. The nine workers came from a private landscape company with
Understanding the Basics of Spirometry It s not just about yelling blow
 Understanding the Basics of Spirometry It s not just about yelling blow Carl D. Mottram, RRT RPFT FAARC Technical Director - Pulmonary Function Labs and Rehabilitation Associate Professor of Medicine -
Understanding the Basics of Spirometry It s not just about yelling blow Carl D. Mottram, RRT RPFT FAARC Technical Director - Pulmonary Function Labs and Rehabilitation Associate Professor of Medicine -
Breathing and pulmonary function
 EXPERIMENTAL PHYSIOLOGY EXPERIMENT 5 Breathing and pulmonary function Ying-ying Chen, PhD Dept. of Physiology, Zhejiang University School of Medicine bchenyy@zju.edu.cn Breathing Exercise 1: Tests of pulmonary
EXPERIMENTAL PHYSIOLOGY EXPERIMENT 5 Breathing and pulmonary function Ying-ying Chen, PhD Dept. of Physiology, Zhejiang University School of Medicine bchenyy@zju.edu.cn Breathing Exercise 1: Tests of pulmonary
PREDICTION EQUATIONS FOR LUNG FUNCTION IN HEALTHY, LIFE TIME NEVER-SMOKING MALAYSIAN POPULATION
 Prediction Equations for Lung Function in Healthy, Non-smoking Malaysian Population PREDICTION EQUATIONS FOR LUNG FUNCTION IN HEALTHY, LIFE TIME NEVER-SMOKING MALAYSIAN POPULATION Justin Gnanou, Brinnell
Prediction Equations for Lung Function in Healthy, Non-smoking Malaysian Population PREDICTION EQUATIONS FOR LUNG FUNCTION IN HEALTHY, LIFE TIME NEVER-SMOKING MALAYSIAN POPULATION Justin Gnanou, Brinnell
Technical Assessment of Spirometers Connected in Series
 Technical Assessment of Spirometers Connected in Series Quentin Lefebvre, Thomas Vandergoten, Eric Derom MD PhD, Emilie Marchandise PhD, and Giuseppe Liistro MD PhD BACKGROUND: Office spirometers are now
Technical Assessment of Spirometers Connected in Series Quentin Lefebvre, Thomas Vandergoten, Eric Derom MD PhD, Emilie Marchandise PhD, and Giuseppe Liistro MD PhD BACKGROUND: Office spirometers are now
BETTER SPIROMETRY. Marijke Currie (CRFS) Care Medical Ltd Phone: Copyright CARE Medical ltd
 BETTER SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual
BETTER SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual
Pulmonary Function Testing
 In the Clinic Pulmonary Function Testing Hawa Edriss MD, Gilbert Berdine MD The term PFT encompasses three different measures of lung function: spirometry, lung volumes, and diffusion capacity. In this
In the Clinic Pulmonary Function Testing Hawa Edriss MD, Gilbert Berdine MD The term PFT encompasses three different measures of lung function: spirometry, lung volumes, and diffusion capacity. In this
Spirometry: Introduction
 Spirometry: Introduction Dr. Badri Paudel 1 2 GMC Spirometry Spirometry is a method of assessing lung function by measuring the volume of air the patient can expel from the lungs after a maximal expiration.
Spirometry: Introduction Dr. Badri Paudel 1 2 GMC Spirometry Spirometry is a method of assessing lung function by measuring the volume of air the patient can expel from the lungs after a maximal expiration.
Top 5 Pitfalls of Spirometry And How to Avoid Them
 Don t Blow It Make Every Breath Count Top 5 Pitfalls of Spirometry And How to Avoid Them Is your company moving into the respiratory clinical trials market? Maybe you ve ventured into spirometry, learned
Don t Blow It Make Every Breath Count Top 5 Pitfalls of Spirometry And How to Avoid Them Is your company moving into the respiratory clinical trials market? Maybe you ve ventured into spirometry, learned
2.0 Scope: This document is to be used by the DCS staff when collecting participants spirometry measurements using the TruFlow Easy-On Spirometer.
 Title: Spirometry Version Date: 2014-AUG-20 Effective Date: 2014-OCT-15 Data Collection Site (DCS) Version: 2.2 Document Number: Number of Pages: SOP_DCS_0012 7 1.0 Purpose: The purpose of this document
Title: Spirometry Version Date: 2014-AUG-20 Effective Date: 2014-OCT-15 Data Collection Site (DCS) Version: 2.2 Document Number: Number of Pages: SOP_DCS_0012 7 1.0 Purpose: The purpose of this document
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C
 MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
S P I R O M E T R Y. Objectives. Objectives 2/5/2019
 S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
PULMONARY FUNCTION TEST(PFT)
 PULMONARY FUNCTION TEST(PFT) Objectives: By the end of the present lab, students should be able to: 1. Record lung volumes and capacities and compare them with those of a typical person of the same gender,
PULMONARY FUNCTION TEST(PFT) Objectives: By the end of the present lab, students should be able to: 1. Record lung volumes and capacities and compare them with those of a typical person of the same gender,
Guide to Fulfillment of Measurement Uncertainty
 DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
behaviour are out of scope of the present review.
 explained about the test, a trial may be done before recording the results. The maneuver consists initially of normal tidal breathing. The subject then inhales to maximally fill the lungs. This is followed
explained about the test, a trial may be done before recording the results. The maneuver consists initially of normal tidal breathing. The subject then inhales to maximally fill the lungs. This is followed
WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System
 WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System To obtain information about warranty for this product contact
WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System To obtain information about warranty for this product contact
S P I R O M E T R Y. Objectives. Objectives 3/12/2018
 S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
Care Bundle. Adult patients with COPD
 Care Bundle Adult patients with COPD Version 2 July 2014 What is a care bundle? A care bundle is a set of interventions that, when used together, significantly improve patient outcomes. The measures chosen
Care Bundle Adult patients with COPD Version 2 July 2014 What is a care bundle? A care bundle is a set of interventions that, when used together, significantly improve patient outcomes. The measures chosen
Testing Spirometers: Are the Standard Curves of the American Thoracic Society Sufficient?
 Testing Spirometers: Are the Standard Curves of the American Thoracic Society Sufficient? Quentin Lefebvre Eng, Thomas Vandergoten Eng, Eric Derom MD PhD, Emilie Marchandise PhD, and Giuseppe Liistro MD
Testing Spirometers: Are the Standard Curves of the American Thoracic Society Sufficient? Quentin Lefebvre Eng, Thomas Vandergoten Eng, Eric Derom MD PhD, Emilie Marchandise PhD, and Giuseppe Liistro MD
SPIROMETRY. Performance and Interpretation for Healthcare Professionals. Faculty of Respiratory Physiology IICMS. Version 1 April 2015
 SPIROMETRY Performance and Interpretation for Healthcare Professionals Faculty of Respiratory Physiology IICMS Version 1 April 2015 Authors: Maria Mc Neill & Geraldine Nolan www.iars.ie Contents Page Background
SPIROMETRY Performance and Interpretation for Healthcare Professionals Faculty of Respiratory Physiology IICMS Version 1 April 2015 Authors: Maria Mc Neill & Geraldine Nolan www.iars.ie Contents Page Background
AviagenBrief. Evaluating Comparative Broiler Performance through Trials INTRODUCTION KEY CONSIDERATIONS. November 2018
 AviagenBrief Evaluating Comparative Broiler Performance through Trials INTRODUCTION November 2018 Carefully planned broiler trials will allow the customer to clearly evaluate the effect of the treatment,
AviagenBrief Evaluating Comparative Broiler Performance through Trials INTRODUCTION November 2018 Carefully planned broiler trials will allow the customer to clearly evaluate the effect of the treatment,
SPIROMETRY TECHNIQUE. Jim Reid New Zealand
 Jim Reid New Zealand The Basics Jim Reid Spirometry measures airflow and lung volumes, and is the preferred lung function test in COPD. By measuring reversibility of obstruction, it is also diagnostic
Jim Reid New Zealand The Basics Jim Reid Spirometry measures airflow and lung volumes, and is the preferred lung function test in COPD. By measuring reversibility of obstruction, it is also diagnostic
Assessment of accuracy and applicability of a new electronic peak flow meter and asthma monitor
 Eur Respir J 18; 12: 45 42 DOI: 18/1.8.5 Printed in UK - all rights reserved Copyright ERS Journals Ltd 18 European Respiratory Journal ISSN - 1 Assessment of accuracy and applicability of a new electronic
Eur Respir J 18; 12: 45 42 DOI: 18/1.8.5 Printed in UK - all rights reserved Copyright ERS Journals Ltd 18 European Respiratory Journal ISSN - 1 Assessment of accuracy and applicability of a new electronic
Mammography Quality Control: A Refresher
 Mammography Quality Control: A Refresher MATT WAIT, MS, DABR Objectives Attendees will re-familiarize themselves with the purpose of quality assurance and quality control Attendees will re-familiarize
Mammography Quality Control: A Refresher MATT WAIT, MS, DABR Objectives Attendees will re-familiarize themselves with the purpose of quality assurance and quality control Attendees will re-familiarize
PULMONARY FUNCTION. VOLUMES AND CAPACITIES
 PULMONARY FUNCTION. VOLUMES AND CAPACITIES The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer (spiro = breath, meter = to measure). A bell spirometer
PULMONARY FUNCTION. VOLUMES AND CAPACITIES The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer (spiro = breath, meter = to measure). A bell spirometer
Niche News August 2017 August 2017
 Niche News August 2017 August 2017 In This Issue Greetings! Introducing the new EasyOne Air Spirometer LiteAire Spacer - Get Better Pricing with Bulk Buy FeNO "Value Proposition" White Paper EasyOne Pro
Niche News August 2017 August 2017 In This Issue Greetings! Introducing the new EasyOne Air Spirometer LiteAire Spacer - Get Better Pricing with Bulk Buy FeNO "Value Proposition" White Paper EasyOne Pro
Peak Expiratory Flow Is Not a Quality Indicator for Spirometry*
 Original Research PULMONARY FUNCTION TESTING Peak Expiratory Flow Is Not a Quality Indicator for Spirometry* Peak Expiratory Flow Variability and FEV 1 Are Poorly Correlated in an Elderly Population Matthew
Original Research PULMONARY FUNCTION TESTING Peak Expiratory Flow Is Not a Quality Indicator for Spirometry* Peak Expiratory Flow Variability and FEV 1 Are Poorly Correlated in an Elderly Population Matthew
Center for Family Health Policy
 Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
Spirometry. Obstruction. By Helen Grim M.S. RRT. loop will have concave appearance. Flows decreased consistent with degree of obstruction.
 1 2 Spirometry By Helen Grim M.S. RRT 3 4 Obstruction loop will have concave appearance. Flows decreased consistent with degree of obstruction. Volumes may be normal, but can decrease with severity of
1 2 Spirometry By Helen Grim M.S. RRT 3 4 Obstruction loop will have concave appearance. Flows decreased consistent with degree of obstruction. Volumes may be normal, but can decrease with severity of
Stability of the EasyOne ultrasonic spirometer for use in general practice
 Blackwell Publishing AsiaMelbourne, AustraliaRESRespirology1323-77992006 Blackwell Publishing Asia Pty Ltd2006113306310MiscellaneousCalibration of an ultrasonic spirometerjae Walters et al. Respirology
Blackwell Publishing AsiaMelbourne, AustraliaRESRespirology1323-77992006 Blackwell Publishing Asia Pty Ltd2006113306310MiscellaneousCalibration of an ultrasonic spirometerjae Walters et al. Respirology
Vitalograph Alpha Spirometer
 Product Catalogue Vitalograph Alpha Spirometer Dedicated all-in-one desktop unit Simple to use Rapid and low cost testing Clear and bright color screen Accurate Fleisch pneumotachograph Measures VC, FVC,
Product Catalogue Vitalograph Alpha Spirometer Dedicated all-in-one desktop unit Simple to use Rapid and low cost testing Clear and bright color screen Accurate Fleisch pneumotachograph Measures VC, FVC,
Standardisation of spirometry
 Eur Respir J 2005; 26: 319 338 DOI: 10.1183/09031936.05.00034805 CopyrightßERS Journals Ltd 2005 SERIES ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING Edited by V. Brusasco, R. Crapo and
Eur Respir J 2005; 26: 319 338 DOI: 10.1183/09031936.05.00034805 CopyrightßERS Journals Ltd 2005 SERIES ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING Edited by V. Brusasco, R. Crapo and
Method Comparison Report Semi-Annual 1/5/2018
 Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
TECHNICAL BULLETIN, MEDICAL OCCUPATIONAL AND ENVIRONMENTAL HEALTH SPIROMETRY IN OCCUPATIONAL HEALTH PROGRAMS
 TECHNICAL BULLETIN, MEDICAL OCCUPATIONAL AND ENVIRONMENTAL HEALTH SPIROMETRY IN OCCUPATIONAL HEALTH PROGRAMS APPROVED FOR PUBLIC RELEASE; DISTRIBUTION IS UNLIMITED. HEADQUARTERS, DEPARTMENT OF THE ARMY
TECHNICAL BULLETIN, MEDICAL OCCUPATIONAL AND ENVIRONMENTAL HEALTH SPIROMETRY IN OCCUPATIONAL HEALTH PROGRAMS APPROVED FOR PUBLIC RELEASE; DISTRIBUTION IS UNLIMITED. HEADQUARTERS, DEPARTMENT OF THE ARMY
How to Perform Spirometry
 Purpose How to Perform Spirometry This guideline provides recommendations regarding best practice to support high quality spirometry practice for KINNECT Pre-Employment Medicals. Scope This guideline provides
Purpose How to Perform Spirometry This guideline provides recommendations regarding best practice to support high quality spirometry practice for KINNECT Pre-Employment Medicals. Scope This guideline provides
PBSI-EHR Off the Charts!
 PBSI-EHR Off the Charts! Enhancement Release 3.2.1 TABLE OF CONTENTS Description of enhancement change Page Encounter 2 Patient Chart 3 Meds/Allergies/Problems 4 Faxing 4 ICD 10 Posting Overview 5 Master
PBSI-EHR Off the Charts! Enhancement Release 3.2.1 TABLE OF CONTENTS Description of enhancement change Page Encounter 2 Patient Chart 3 Meds/Allergies/Problems 4 Faxing 4 ICD 10 Posting Overview 5 Master
Spirometry Training Courses
 Spirometry Training Courses A Position Paper of The Australian and New Zealand Society of Respiratory Science & The Thoracic Society of Australia and New Zealand At the time of the preparation of this
Spirometry Training Courses A Position Paper of The Australian and New Zealand Society of Respiratory Science & The Thoracic Society of Australia and New Zealand At the time of the preparation of this
Sterile U Network. Quality Control for Table-top Steam Sterilizers. Introduction: T U T O R I A L S
 3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
3M Attest Products TM TM Sterile U Network T U T O R I A L S Quality Control for Table-top Steam Sterilizers Introduction: Many health care professionals in office-based settings are not aware that they
In order to diagnose lung diseases doctors
 You Take My Breath Away Activity 5C NOTE: This activity is designed to follow You Really Are Full of Hot Air! Activity Objectives: After completing You Really Are Full of Hot Air! Activity 5B, students
You Take My Breath Away Activity 5C NOTE: This activity is designed to follow You Really Are Full of Hot Air! Activity Objectives: After completing You Really Are Full of Hot Air! Activity 5B, students
Spirometry: FEVER DISEASE DIABETES HOW RELIABLE IS THIS? 9/2/2010 BUT WHAT WE PRACTICE: Spirometers are objective tools
 SPIROMETRY PRINCIPLES, PROCEDURE AND QA Spirometry: Dr. Rahul Kodgule CHEST RESEARCH FOUNDATION, PUNE FEVER ISCHAEMIC HEART DISEASE DIABETES BUT WHAT WE PRACTICE: Spirometers are objective tools to diagnose
SPIROMETRY PRINCIPLES, PROCEDURE AND QA Spirometry: Dr. Rahul Kodgule CHEST RESEARCH FOUNDATION, PUNE FEVER ISCHAEMIC HEART DISEASE DIABETES BUT WHAT WE PRACTICE: Spirometers are objective tools to diagnose
DOELAP On-Site Assessment Requirements Checklist Page 1 of 24 Participant:
 DOELAP On-Site Assessment Requirements Checklist Page 1 of 24 General Requirements Y, N, N/A Demonstration of Conformance G.1 Latest version of protocols or procedures G.2 Latest version of dosimeter specifications
DOELAP On-Site Assessment Requirements Checklist Page 1 of 24 General Requirements Y, N, N/A Demonstration of Conformance G.1 Latest version of protocols or procedures G.2 Latest version of dosimeter specifications
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN
 Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
Individual Study Table Referring to Part of the Dossier. Volume:
 Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Original Contributions
 Original Contributions Comparison of a New Desktop Spirometer (Spirospec) with a Laboratory Spirometer in a Respiratory Out-Patient Clinic François Swart, Macé M Schuurmans MD, Johannes C Heydenreich,
Original Contributions Comparison of a New Desktop Spirometer (Spirospec) with a Laboratory Spirometer in a Respiratory Out-Patient Clinic François Swart, Macé M Schuurmans MD, Johannes C Heydenreich,
Content Indica c tion Lung v olumes e & Lung Indica c tions i n c paci c ties
 Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
Office Spirometry Guide
 Office Spirometry Guide MD Spiro 803 Webster Street, Lewiston ME 04240 Telephone 207-786-7808 1-800-588-3381 Fax 207-786-7280 www.mdspiro.com e-mail: sales@mdspiro.com Why should you perform spirometry
Office Spirometry Guide MD Spiro 803 Webster Street, Lewiston ME 04240 Telephone 207-786-7808 1-800-588-3381 Fax 207-786-7280 www.mdspiro.com e-mail: sales@mdspiro.com Why should you perform spirometry
PULMONARY FUNCTION TESTING. By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS)
 PULMONARY FUNCTION TESTING By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS) PULMONARY FUNCTION TESTS CATEGORIES Spirometry Lung volumes
PULMONARY FUNCTION TESTING By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS) PULMONARY FUNCTION TESTS CATEGORIES Spirometry Lung volumes
Spirometry in primary care
 Spirometry in primary care Wednesday 13 th July 2016 Dr Rukhsana Hussain What is spirometry? A method of assessing lung function Measures volume of air a patient can expel after a full inspiration Recorded
Spirometry in primary care Wednesday 13 th July 2016 Dr Rukhsana Hussain What is spirometry? A method of assessing lung function Measures volume of air a patient can expel after a full inspiration Recorded
ADVANCED TECHNOLOGY CONSORTIUM (ATC) CREDENTIALING PROCEDURES FOR LUNG BRACHYTHERAPY IMPLANT PROTOCOLS
 ACOSOG-RTOG Lung Brachytherapy QA Page 1 of 8 ADVANCED TECHNOLOGY CONSORTIUM (ATC) CREDENTIALING PROCEDURES FOR LUNG BRACHYTHERAPY IMPLANT PROTOCOLS FACILITY QUESTIONNAIRE Institutions wishing to enter
ACOSOG-RTOG Lung Brachytherapy QA Page 1 of 8 ADVANCED TECHNOLOGY CONSORTIUM (ATC) CREDENTIALING PROCEDURES FOR LUNG BRACHYTHERAPY IMPLANT PROTOCOLS FACILITY QUESTIONNAIRE Institutions wishing to enter
TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS. Otthon. Ultrasonic handheld spirometer
 TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS Otthon Ultrasonic handheld spirometer OTTHON ULTRASONIC HANDHELD SPIROMETER TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS OTTHON
TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS Otthon Ultrasonic handheld spirometer OTTHON ULTRASONIC HANDHELD SPIROMETER TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS OTTHON
MEDIUM-FLOW PNEUMOTACH TRANSDUCER
 MEDIUM-FLOW PNEUMOTACH TRANSDUCER SS11LA for MP3x and MP45 System TSD117 & TSD117-MRI for MP150/MP100 System RX117 Replacement Airflow Head See also: AFT series of accessories for airflow and gas analysis
MEDIUM-FLOW PNEUMOTACH TRANSDUCER SS11LA for MP3x and MP45 System TSD117 & TSD117-MRI for MP150/MP100 System RX117 Replacement Airflow Head See also: AFT series of accessories for airflow and gas analysis
Office Based Spirometry
 Osteopathic Family Physician (2014)1, 14-18 Scott Klosterman, DO; Woodson Crenshaw, OMS4 Spartanburg Regional Family Medicine Residency Program; Edward Via College of Osteopathic Medicine - Virginia Campus
Osteopathic Family Physician (2014)1, 14-18 Scott Klosterman, DO; Woodson Crenshaw, OMS4 Spartanburg Regional Family Medicine Residency Program; Edward Via College of Osteopathic Medicine - Virginia Campus
Peak expiratory flow and the resistance of the mini-wright peak flow meter
 Eur Respir J, 1996, 9, 828 833 DOI: 10.1183/09031936.96.090828 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1996 European Respiratory Journal ISSN 0903-1936 TECHNICAL NOTE Peak expiratory
Eur Respir J, 1996, 9, 828 833 DOI: 10.1183/09031936.96.090828 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1996 European Respiratory Journal ISSN 0903-1936 TECHNICAL NOTE Peak expiratory
Training at the Namibian Uranium Institute Schedule and Course Overview for 2017 Radiation Safety and Occupational Medicine
 Tel: +264-64-402393 Fax: +264-64-402394 E-Mail: info@namibianuranium.org Website: www.namibianuranium.org P.O. Box 2747 Swakopmund, Namibia 17, Cottage Avenue, Tamariskia Swakopmund Training at the Namibian
Tel: +264-64-402393 Fax: +264-64-402394 E-Mail: info@namibianuranium.org Website: www.namibianuranium.org P.O. Box 2747 Swakopmund, Namibia 17, Cottage Avenue, Tamariskia Swakopmund Training at the Namibian
DESIGN, ANALYSIS AND IMPLEMENTATION OF SPIROMETER
 DESIGN, ANALYSIS AND IMPLEMENTATION OF SPIROMETER Asmita Parve Mokal1, Dr. M.J. Sheikh2, Bipin D. Mokal3 1M Tech Student, Department of Mechanical Engg, B.D.C.O.E., Sevagram 442102 2Prof and HOD, Department
DESIGN, ANALYSIS AND IMPLEMENTATION OF SPIROMETER Asmita Parve Mokal1, Dr. M.J. Sheikh2, Bipin D. Mokal3 1M Tech Student, Department of Mechanical Engg, B.D.C.O.E., Sevagram 442102 2Prof and HOD, Department
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 26782 First edition 2009-07-15 Anaesthetic and respiratory equipment Spirometers intended for the measurement of time forced expired volumes in humans Matériel d'anesthésie et
INTERNATIONAL STANDARD ISO 26782 First edition 2009-07-15 Anaesthetic and respiratory equipment Spirometers intended for the measurement of time forced expired volumes in humans Matériel d'anesthésie et
Respiratory Physiology In-Lab Guide
 Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
Synergy Respiratory Care Dr. Lyle Melenka
 1 2 Synergy Respiratory Care Dr. Lyle Melenka Respiratory Care 2 Accredited Pulmonary Function Labs Cardiac Care 2 Accredited Cardiac Exercise Stress Testing labs WoW Wellness of Workers Early Identification
1 2 Synergy Respiratory Care Dr. Lyle Melenka Respiratory Care 2 Accredited Pulmonary Function Labs Cardiac Care 2 Accredited Cardiac Exercise Stress Testing labs WoW Wellness of Workers Early Identification
IQspiro. Version Operation Manual Rev. E
 IQspiro Version 10.0 Operation Manual 3-100-1120 Rev. E Notice The information in this manual is subject to change without notice. Midmark Corporation shall not be liable for technical or editorial omissions
IQspiro Version 10.0 Operation Manual 3-100-1120 Rev. E Notice The information in this manual is subject to change without notice. Midmark Corporation shall not be liable for technical or editorial omissions
Pulmonary Function Testing. Ramez Sunna MD, FCCP
 Pulmonary Function Testing Ramez Sunna MD, FCCP Lecture Overview General Introduction Indications and Uses Technical aspects Interpretation Patterns of Abnormalities When to perform a PFT 1. Evaluation
Pulmonary Function Testing Ramez Sunna MD, FCCP Lecture Overview General Introduction Indications and Uses Technical aspects Interpretation Patterns of Abnormalities When to perform a PFT 1. Evaluation
Pulmonary Rehabilitation. Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education
 Pulmonary Rehabilitation Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education Pulmonary Rehabilitation Pulmonary Rehabilitation is a multi-disciplinary program of care for patients with chronic
Pulmonary Rehabilitation Palmetto GBA, Jurisdiction 11 MAC Provider Outreach and Education Pulmonary Rehabilitation Pulmonary Rehabilitation is a multi-disciplinary program of care for patients with chronic
EVect of breathing circuit resistance on the measurement of ventilatory function
 9 Department of Respiratory Medicine, The Alfred Hospital and Monash University Medical School, Melbourne, Victoria, Australia 311 D P Johns C M Ingram S Khov P D Rochford E H Walters Correspondence to:
9 Department of Respiratory Medicine, The Alfred Hospital and Monash University Medical School, Melbourne, Victoria, Australia 311 D P Johns C M Ingram S Khov P D Rochford E H Walters Correspondence to:
What do pulmonary function tests tell you?
 Pulmonary Function Testing Michael Wert, MD Assistant Professor Clinical Department of Internal Medicine Division of Pulmonary, Critical Care, and Sleep Medicine The Ohio State University Wexner Medical
Pulmonary Function Testing Michael Wert, MD Assistant Professor Clinical Department of Internal Medicine Division of Pulmonary, Critical Care, and Sleep Medicine The Ohio State University Wexner Medical
VANCOUVER POLICE DEPARTMENT
 VANCOUVER POLICE DEPARTMENT REPORT TO THE VANCOUVER POLICE BOARD REPORT DATE: April 10, 2013 BOARD MEETING DATE: April 16, 2013 BOARD REPORT # 1304C01 Regular TO: FROM: Vancouver Police Board Service and
VANCOUVER POLICE DEPARTMENT REPORT TO THE VANCOUVER POLICE BOARD REPORT DATE: April 10, 2013 BOARD MEETING DATE: April 16, 2013 BOARD REPORT # 1304C01 Regular TO: FROM: Vancouver Police Board Service and
ER75 Electro-Acoustic Ear Simulator. Operating Manual
 ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
 MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
Dräger Alcotest Handheld Product Suite
 Dräger Alcotest Handheld Product Suite D-7404-2014 Roadside alcohol testing can be challenging and unpredictable. That s why Dräger designed Alcotest handheld breath testers to be accurate and easy to
Dräger Alcotest Handheld Product Suite D-7404-2014 Roadside alcohol testing can be challenging and unpredictable. That s why Dräger designed Alcotest handheld breath testers to be accurate and easy to
Digital Pathology and CAP Guidelines
 Digital Pathology and CAP Guidelines Frequently asked questions The VENTANA family of digital pathology products empowers you with the convenience of a comprehensive image and workflow solution. When used
Digital Pathology and CAP Guidelines Frequently asked questions The VENTANA family of digital pathology products empowers you with the convenience of a comprehensive image and workflow solution. When used
GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7
 GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7 NOTICE: This document is version controlled and was produced as a part of the GEX Information Program which requires that all Series 100
GEX Recommended Procedure Eff. Date: 09/21/10 Rev.: D Pg. 1 of 7 NOTICE: This document is version controlled and was produced as a part of the GEX Information Program which requires that all Series 100
Cardiovascular and Respiratory Systems
 Cardiovascular and Respiratory Systems Learning Objectives 1. State the parts of the cardiovascular and respiratory systems and give the functions of each part. 2. Identify the parts of the cardiovascular
Cardiovascular and Respiratory Systems Learning Objectives 1. State the parts of the cardiovascular and respiratory systems and give the functions of each part. 2. Identify the parts of the cardiovascular
Policy # MI/SER/02/v03 Page 1 of 9 Policy & Procedure Manual
 Policy # MI/SER/02/v03 Page 1 of 9 Section: Subject Title: AxSYM System Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: October 28, 2003 AXSYM
Policy # MI/SER/02/v03 Page 1 of 9 Section: Subject Title: AxSYM System Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: October 28, 2003 AXSYM
your bioassay is in good hands: transfer from a CRO perspective MIKE MERGES March 4, 2013
 your bioassay is in good hands: transfer from a CRO perspective MIKE MERGES March 4, 2013 Contact Information Michael Merges, M.S. Director Analytical Development Solutions Development & Clinical Services
your bioassay is in good hands: transfer from a CRO perspective MIKE MERGES March 4, 2013 Contact Information Michael Merges, M.S. Director Analytical Development Solutions Development & Clinical Services
#8 - Respiratory System
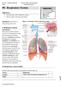 Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
TAMPA ELECTRIC COMPANY ENERGY SUPPLY HEARING CONSERVATION PROGRAM
 TABLE OF CONTENTS TITLE PAGE # PURPOSE / INTRODUCTION / SCOPE 1, 2 ROLES AND RESPONSIBILITIES 2 EMPLOYEE TRAINING 3 ENGINEERING CONTROLS 4 NOISE EXPOSURE EVALUATION 4 SIGNAGE / POSTING 4-5 AUDIOMETRIC
TABLE OF CONTENTS TITLE PAGE # PURPOSE / INTRODUCTION / SCOPE 1, 2 ROLES AND RESPONSIBILITIES 2 EMPLOYEE TRAINING 3 ENGINEERING CONTROLS 4 NOISE EXPOSURE EVALUATION 4 SIGNAGE / POSTING 4-5 AUDIOMETRIC
MES 9000 MUSCULOSKELETAL EVALUATION SYSTEM
 MES 9000 MUSCULOSKELETAL EVALUATION SYSTEM The new MES 9000. Now you can completely and objectively evaluate and document Dynamic Range of Motion, Static and Dynamic EMG and Muscle Testing All with one
MES 9000 MUSCULOSKELETAL EVALUATION SYSTEM The new MES 9000. Now you can completely and objectively evaluate and document Dynamic Range of Motion, Static and Dynamic EMG and Muscle Testing All with one
Small Volume Nebulizer Treatment (Hand-Held)
 Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Performing a Methacholine Challenge Test
 powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
Automated external defibrillators
 MEDICAL DEVICES Automated external defibrillators Our advice www.hpra.ie Automated external defibrillators MEDICAL DEVICES Automated external defibrillators An automated external defibrillator (AED) is
MEDICAL DEVICES Automated external defibrillators Our advice www.hpra.ie Automated external defibrillators MEDICAL DEVICES Automated external defibrillators An automated external defibrillator (AED) is
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Pulmonary Function Tests. Mohammad Babai M.D Occupational Medicine Specialist
 Pulmonary Function Tests Mohammad Babai M.D Occupational Medicine Specialist www.drbabai.com Pulmonary Function Tests Pulmonary Function Tests: Spirometry Peak-Flow metry Bronchoprovocation Tests Body
Pulmonary Function Tests Mohammad Babai M.D Occupational Medicine Specialist www.drbabai.com Pulmonary Function Tests Pulmonary Function Tests: Spirometry Peak-Flow metry Bronchoprovocation Tests Body
Patient assessment - spirometry
 Patient assessment - spirometry STEP 1 Learning objectives This module will provide you with an understanding of spirometry and the role it plays in aiding the diagnosis of lung diseases, particularly
Patient assessment - spirometry STEP 1 Learning objectives This module will provide you with an understanding of spirometry and the role it plays in aiding the diagnosis of lung diseases, particularly
RESPIRATORY PHYSIOLOGY Pre-Lab Guide
 RESPIRATORY PHYSIOLOGY Pre-Lab Guide NOTE: A very useful Study Guide! This Pre-lab guide takes you through the important concepts that where discussed in the lab videos. There will be some conceptual questions
RESPIRATORY PHYSIOLOGY Pre-Lab Guide NOTE: A very useful Study Guide! This Pre-lab guide takes you through the important concepts that where discussed in the lab videos. There will be some conceptual questions
