APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe
|
|
|
- Ethelbert Smith
- 6 years ago
- Views:
Transcription
1 APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size)
2 Medication in Green Zone Change in Yellow Zone (all recommendations are for 7-14 days) ICS MONOTHERAPY Flixotide Evohaler (Fluticasone propionate) 125 mcg 1 puff bid Increase to 4 puffs bid 2 Flixotide Accuhaler (Fluticasone propionate) mcg 1 puff bid Increase to 2 puffs bid Pulmicort Turbuhaler (Budesonide) 200 mcg 1 puff bid Increase to 4 puffs bid 400 mcg 1 puff bid Increase to 3 puffs bid Easyhaler Budesonide DPI 200 mcg 1 puff bid Increase to 4 puffs bid 400 mcg 1 puff bid Increase to 3 puffs bid QVAR Metered Dose Inhaler (Beclomethasone) 100 mcg 2 puffs bid Prednisolone mg daily ii Asmabec Clickhaler DPI (Beclomethasone) 250 mcg 1 puff bid Option 1: Increase to 4 puffs bid i Alvesco Inhaler (Ciclesonide) 80 mcg 1 puff od Increase to 4 puffs od 160 mcg 1 puff od Increase to 4 puffs od 160 mcg 2 puffs od Increase to 4 puffs bid Asmanex Twisthaler (Mometasone) 400 mcg 1 puff od ICS/LABA COMBINATION THERAPY Seretide Accuhaler (Fluticasone propionate/salmeterol) 100/50 mcg 1 puff bid Add Flixotide Accuhaler 100 mcg 3 puffs bid 250/50 mcg 1 puff bid Add Flixotide Accuhaler 250 mcg 3 puffs bid 500/50 mcg 1 puff bid Add Flixotide Accuhaler 500 mcg 1 puff bid Seretide Evohaler (Fluticasone propionate/salmeterol) 125/25 mcg 1 puff bid Add Flixotide Evohaler 125 mcg 3 puffs bid 250/25 mcg 1 puff bid Add Flixotide Evohaler 250 mcg 3 puffs bid 250/25 mcg 2 puffs bid Add Flixotide Evohaler 250 mcg 2 puffs bid Symbicort Turbuhaler (Budesonide/Formoterol) 200/6 mcg 1 puff bid Increase to 4 puffs bid 200/6 mcg 2 puffs bid Add Pulmicort Turbohaler 400 mcg 2 puffs bid Douresp Spiromax DPI (Budesonide/Formoterol) 160/4.5 mcg 1 puff bid Increase to 4 puffs bid 320/9 mcg 1 puff bid Add Budesonide DPI 400 mcg 2 puffs bid Relvar Ellipta (Fluticasone furoate/vilanterol) 100/25 mcg 1 puff od 200/25 mcg 1 puff od Fostair MDI (Beclomethasone/Formoterol) 200/6 mcg 1 puff bid Option 1: Increase to 4 puffs bid i Fostair Nexthaler DPI (Beclomethasone/Formoterol) 200/6 mcg 1 puff bid Option 1: Increase to 4 puffs bid i Flutiform MDI (Fluticasone propionate/formoterol) 50/5 mcg 1 puff bid Increase to 4 puffs bid 125/5 mcg 1 puff bid Increase to 4 puffs bid 250/5 mcg 1 puff bid Increase to 4 puffs bid Special considerations In patients with a history of sudden and severe exacerbations, and/or presenting with PEF Prednisolone 30-50mg daily for or FEV1 60% of personal best/predicted 5-7 days ii If patient fails to improve clinically within 2-3 days of increase in inhaled medication, and/or Prednisolone 30-50mg daily for has a rapid clinical deterioration, and/or has a peak expiratory flow or FEV1 that falls to 5-7 days as rescue therapy ii 60% of their personal best value From: Kouri A, Boulet L-P, Kaplan A, Gupta S. An Evidence-based, point-of-care tool to guide completion of asthma action plans in practice. Eur Respir J i: this dose exceeds product monograph total daily dose limits intended for chronic daily use. A short term dose increase beyond these limits is unlikely to carry any significant safety risks, however formal safety testing data are not available and the decision to pursue this approach should be based on patient and clinician comfort ii: Ask patients to contact the health care provider to consider a prednisolone prescription and/or provide a standing prescription for prednisolone 30-50mg daily for 5-7 days. Ensure that patients are appropriately counseled about the risks of short-term prednisolone use
3 Region: Canada Instructions: Print on 8.5 x14 paper (Legal size)
4 Medication in Green Zone Change in Yellow Zone (all recommendations are for 7-14 days) ICS MONOTHERAPY Flovent HFA (Fluticasone propionate) 125 mcg 1 puff bid Increase to 4 puffs bid 2 Flovent Diskus (Fluticasone propionate) mcg 1 puff bid Increase to 2 puffs bid Arnuity Ellipta (Fluticasone furoate) 100 mcg 1 puff od 200 mcg 1 puff od Pulmicort Turbuhaler (Budesonide) 200 mcg 1 puff bid Increase to 4 puffs bid 400 mcg 1 puff bid Increase to 3 puffs bid QVAR MDI (Beclomethasone) 100 mcg 2 puffs bid Prednisone mg daily ii Alvesco MDI (Ciclesonide) 100 mcg 1 puff od Increase to 4 puffs od 200 mcg 2 puffs od Increase to 4 puffs bid Asmanex Twisthaler (Mometasone) 400 mcg 1 puff od ICS/LABA COMBINATION THERAPY Advair Diskus (Fluticasone propionate/salmeterol) 100/50 mcg 1 puff bid Add Flovent Diskus 100 mcg 3 puffs bid 250/50 mcg 1 puff bid Add Flovent Diskus 250 mcg 3 puffs bid 500/50 mcg 1 puff bid Add Flovent Diskus 500 mcg 1 puff bid Advair HFA (Fluticasone propionate/salmeterol) 125/25 mcg 1 puff bid Add Flovent HFA 125 mcg 3 puffs bid 250/25 mcg 1 puff bid Add Flovent HFA 250 mcg 3 puffs bid 250/25 mcg 2 puffs bid Add Flovent HFA 250 mcg 2 puffs bid Symbicort Turbuhaler (Budesonide/Formoterol) 200/6 mcg 1 puff bid Increase to 4 puffs bid 200/6 mcg 2 puffs bid Add Pulmicort Turbuhaler 400 mcg 2 puffs bid Zenhale MDI (Mometasone/Formoterol) 50/5 mcg 2 puffs bid Change to Zenhale MDI 200/5mcg 2 puffs bid 100/5 mcg 2 puffs bid Option 1: Change to Zenhale MDI 200/5 mcg 4 puffs bid i 200/5 mcg 2 puffs bid Prednisone mg daily ii Breo Ellipta (Fluticasone furoate/vilanterol) 100/25 mcg 1 puff od 200/25 mcg 1 puff od Special considerations In patients with a history of sudden and severe exacerbations, and/or presenting with PEF or FEV1 60% of personal best/predicted If patient fails to improve clinically within 2-3 days of increase in inhaled medication, and/or has a rapid clinical deterioration, and/or has a peak expiratory flow or FEV1 that falls to 60% of their personal best value daily for 5-7 days as first line therapy ii daily for 5-7 days as rescue (second line) therapy ii i: This dose exceeds product monograph total daily dose limits intended for chronic daily use. A short term dose increase beyond these limits is unlikely to carry any significant safety risks, however formal safety testing data are not available and the decision to pursue this approach should be based on patient and clinician comfort ii: Ask patients to contact the health care provider to consider a prednisone prescription and/or provide a standing prescription for prednisone 30-50mg daily for 5-7 days. Ensure that patients are appropriately counseled about the risks of short-term prednisone use From: Kouri A, Boulet L-P, Kaplan A, Gupta S. An Evidence-based, point-of-care tool to guide completion of asthma action plans in practice. Eur Respir J 2017.
5 Region: USA Instructions: Print on 8.5 x14 paper (Legal size)
6 Medication in Green Zone Asthma Action Plan Yellow Zone Formulation Table Change in Yellow Zone (all recommendations are for 7-14 days ICS MONOTHERAPY Flovent HFA (Fluticasone propionate) 44 mcg 1 puff bid Increase to 4 puffs bid 110 mcg 1 puff bid Increase to 4 puffs bid 220 mcg 1 puff bid Increase to 4 puffs bid Flovent Diskus (Fluticasone propionate) mcg 1 puff bid Increase to 2 puffs bid Arnuity Ellipta (Fluticasone furoate) 100 mcg 1 puff od 200 mcg 1 puff od Pulmicort Flexhaler (Budesonide) 90 mcg 1 puff bid Increase to 4 puffs bid 180 mcg 1 puff bid Increase to 4 puffs bid 360 mcg 1 puff bid Increase to 3 puffs bid QVAR MDI (Beclomethasone) 40 mcg 1 puff bid Increase to 4 puffs bid 80 mcg 1 puff bid Increase to 4 puffs bid 80 mcg 2 puffs bid Prednisone mg daily ii Alvesco MDI (Ciclesonide) 80 mcg 1 puff od Increase to 4 puffs od 160 mcg 1 puff od Increase to 4 puffs od 160 mcg 2 puffs od Increase to 4 puffs bid Asmanex HFA (Mometasone) 400 mcg 1 puff od ICS/LABA COMBINATION THERAPY Advair Diskus (Fluticasone propionate/salmeterol) 100/50 mcg 1 puff bid Add Flovent Diskus 100 mcg 3 puffs bid 250/50 mcg 1 puff bid Add Flovent Diskus 250 mcg 3 puffs bid 500/50 mcg 1 puff bid Add Flovent Diskus 500 mcg 1 puffs bid Advair HFA (Fluticasone propionate/salmeterol) 115/21 mcg 1 puff bid Add Flovent HFA 110 mcg 3 puffs bid 230/21 mcg 1 puff bid Add Flovent HFA 220 mcg 3 puffs bid 230/21 mcg 2 puffs bid Add Flovent HFA 220 mcg 2 puffs bid Symbicort (Budesonide/Formoterol) 80/4.5 mcg 2 puffs bid Add Pulmicort Flexhaler 360 mcg 2 puffs bid 160/4.5 mcg 2 puffs bid Add Pulmicort Flexhaler 360 mcg 2 puffs bid Dulera MDI (Mometasone/Formoterol) 100/5 mcg 2 puffs bid Option 1: Change to Dulera MDI 200/5 mcg 4 puffs bid i 200/5 mcg 2 puffs bid daily ii Breo Ellipta (Fluticasone furoate/vilanterol) 100/25 mcg 1 puff od 200/25 mcg 1 puff od Special considerations In patients with a history of sudden and severe exacerbations, and/or presenting with PEF or FEV1 60% of personal best/predicted If patient fails to improve clinically within 2-3 days of increase in inhaled medication, and/or has a rapid clinical deterioration, and/or has a peak expiratory flow or FEV1 that falls to 60% of their personal best value daily for 5-7 days as first line therapy ii daily for 5-7 days as rescue (second line) therapy ii i: This dose exceeds product monograph total daily dose limits intended for chronic daily use. A short term dose increase beyond these limits is unlikely to carry any significant safety risks, however formal safety testing data are not available and the decision to pursue this approach should be based on patient and clinician comfort ii: Ask patients to contact the health care provider to consider a prednisone prescription and/or provide a standing prescription for prednisone 30-50mg daily for 5-7 days. Ensure that patients are appropriately counseled about the risks of short-term prednisone use From: Kouri A, Boulet L-P, Kaplan A, Gupta S. An Evidence-based, point-of-care tool to guide completion of asthma action plans in practice. Eur Respir J 2017.
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
Drug Class Monograph
 Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Stepping down asthma treatment guidelines
 Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Stepping-down combination ICS/LABA asthma inhaler therapy: Adults 18yrs
 Step Down guidance Stepping-down combination ICS/LABA asthma inhaler therapy: Adults 18yrs Important Complete asthma control needs to be achieved for at least 12 weeks before attempting to step patients
Step Down guidance Stepping-down combination ICS/LABA asthma inhaler therapy: Adults 18yrs Important Complete asthma control needs to be achieved for at least 12 weeks before attempting to step patients
CHARM ASTHMA TREATMENT GUIDELINE
 NHS City and Hackney Prescribing Guidelines Adults ( 12 years of age) CHARM ASTHMA TREATMENT GUIDELINE Written by: Hetal Dhruve (Specialist Respiratory Pharmacist, City and Hackney CCG) Checked by: Prof
NHS City and Hackney Prescribing Guidelines Adults ( 12 years of age) CHARM ASTHMA TREATMENT GUIDELINE Written by: Hetal Dhruve (Specialist Respiratory Pharmacist, City and Hackney CCG) Checked by: Prof
Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred inhaler choices
 ENHCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred
ENHCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred
Inhaled Corticosteroid Dose Comparison in Asthma
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
Adult Summary flowchart for Asthma Switch and Step Down to preferred inhaler choices
 HVCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to preferred
HVCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to preferred
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
Dose. Route. Units. Given. Dose. Route. Units. Given
 Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12
 North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
Respiratory Inhalers. Identification Guide Version 3
 Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
The Medical Letter. on Drugs and Therapeutics
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
Drug Effectiveness Review Project Summary Report
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Alberta Childhood Asthma Pathway for Primary Care
 Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
COPD Medications Coverage Summary Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes
 COPD Medications Coverage Summary Drug Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes Ventolin MDI + generics Yes Yes Ventolin Diskus NO NO Yukon Pharmacare/Chronic Disease Program
COPD Medications Coverage Summary Drug Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes Ventolin MDI + generics Yes Yes Ventolin Diskus NO NO Yukon Pharmacare/Chronic Disease Program
Aerospan (flunisolide)
 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Select Inhaled Respiratory Agents
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Orally Inhaled Corticosteroids to 2022
 Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
Asthma - An update BTS Asthma Guidelines 2016
 Asthma - An update BTS Asthma Guidelines 2016 Dr Ian Clifton Overview Diagnosis Supported self-management Non-pharmacological management Drugs / inhaled therapy Difficult asthma services Case discussions
Asthma - An update BTS Asthma Guidelines 2016 Dr Ian Clifton Overview Diagnosis Supported self-management Non-pharmacological management Drugs / inhaled therapy Difficult asthma services Case discussions
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless?
 Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
Medicines Optimisation Team Standard Operating Procedure for Audit: High Dose Inhaled Corticosteroids
 Cannock Chase Clinical Commissioning Group South East Staffordshire and Seisdon Peninsula Clinical Commissioning Group Stafford and Surrounds Clinical Commissioning Group East Staffordshire Clinical Commissioning
Cannock Chase Clinical Commissioning Group South East Staffordshire and Seisdon Peninsula Clinical Commissioning Group Stafford and Surrounds Clinical Commissioning Group East Staffordshire Clinical Commissioning
SAMPLE. mg by mouth every day for day(s) Prednisolone. Other Medicine: Medicine Dose How long Directions
 Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
Include patients: with a confirmed diagnosis of asthma who have been free of asthma symptoms for 3 months or more.
 Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK
 fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
Clinical Policy: Dupilumab (Dupixent) Reference Number: ERX.SPA.49 Effective Date:
 Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Question I was one of the first dry power devices available in the US Flovent, Serevent and Advair are all available in this device
 What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date: Last Review Date: 08.17
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Key features and changes to these four components of asthma care include:
 Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
Asthma Treatment Guideline for Adults (aged 17 and over)
 Asthma Treatment Guideline for Adults (aged 17 and over) Sharon Andrew MLCSU January 2019 (Review date 0 January 2022) VERSION CONTROL. Please access via the LMMG website to ensure that the correct version
Asthma Treatment Guideline for Adults (aged 17 and over) Sharon Andrew MLCSU January 2019 (Review date 0 January 2022) VERSION CONTROL. Please access via the LMMG website to ensure that the correct version
Not available 100/6mcg 2 BD formoterol (Fostair MDI) 100/6mcg 33
 COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
Guide to Inhaled Treatment Choices
 Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices
 Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
COPD Update: Focus on Intensifying LABA, LAMA and ICS Therapy
 Update: Focus on Intensifying LABA, LAMA and ICS Therapy B.C. Provincial Academic Detailing Service February 2017 Background In Canada, approximately 20 inhaled medications are approved to treat Chronic
Update: Focus on Intensifying LABA, LAMA and ICS Therapy B.C. Provincial Academic Detailing Service February 2017 Background In Canada, approximately 20 inhaled medications are approved to treat Chronic
Clinical Policy: Mepolizumab (Nucala) Reference Number: ERX.SPA.214 Effective Date:
 Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
ASTHMA TREATMENT GUIDE (ADULTS)
 ASTHMA TREATMENT GUIDE (ADULTS) The BTS/SIGN guideline provides a wide range of information and guidance on the treatment of patients with asthma. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/
ASTHMA TREATMENT GUIDE (ADULTS) The BTS/SIGN guideline provides a wide range of information and guidance on the treatment of patients with asthma. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
The Inflammatory Response. Inflammation in the Airway. The Inflammatory Response. Inflammation in the Airway. Inflammation in the Airway
 The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Position within the Organisation
 ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
Why Asthma Still Kills The National Review of Asthma Deaths (NRAD)
 APPROVED FINAL VERSION NHS Protect Why Asthma Still Kills The National Review of Asthma Deaths (NRAD) Summary of Recommendations for GP Practices and Community Pharmacies Author: Anne Henry Contact: anne.henry@nhs.net
APPROVED FINAL VERSION NHS Protect Why Asthma Still Kills The National Review of Asthma Deaths (NRAD) Summary of Recommendations for GP Practices and Community Pharmacies Author: Anne Henry Contact: anne.henry@nhs.net
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES
 CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
Disclosure. Case. Objectives. Case Continued. Inhalers. Asthma: A GINA Update to the NAEPP 2007 Guidelines 1/20/2015
 Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
AWMSG SECRETARIAT ASSESSMENT REPORT
 AWMSG SECRETARIAT ASSESSMENT REPORT Fluticasone furoate/vilanterol (as trifenatate) (Relvar Ellipta ) inhalation powder, 184/22 inhalation powder Reference number: 1216 FULL SUBMISSION This report has
AWMSG SECRETARIAT ASSESSMENT REPORT Fluticasone furoate/vilanterol (as trifenatate) (Relvar Ellipta ) inhalation powder, 184/22 inhalation powder Reference number: 1216 FULL SUBMISSION This report has
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
AIRDUO RESPICLICK (fluticasone-salmeterol) aerosol DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol
 DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Reference Guide for Caring for Pediatric Patients with Asthma
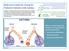 Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS
 Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date:
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 07/05/18 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
Cinqair (reslizumab injection for intravenous use)
 Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
MANAGING ASTHMA. Nancy Davis, RRT, AE-C
 MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
Clinical Policy: Dupilumab (Dupixent) Reference Number: ERX.SPA.49 Effective Date:
 Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
(PLACE PATIENT LABEL HERE) Date: Time: Assessment nurse: Sign: STOP!
 ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
ANTINEOPLASTIC DRUGS CHAPTER 21. Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component
 ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
Greater Manchester Asthma Management Plan 2018 Inhaler therapy options for adult patients (18 and over) with asthma
 Greater Manchester Asthma Management Plan 2018 Inhaler therapy options for adult patients (18 and over) with asthma Non-pharmacological options for ALL patients, consider at ALL stages Make sure diagnosis
Greater Manchester Asthma Management Plan 2018 Inhaler therapy options for adult patients (18 and over) with asthma Non-pharmacological options for ALL patients, consider at ALL stages Make sure diagnosis
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
COPD Medicine. No one ever showed me how to use this. Wendy Happel; RRT, COPD Educator Krystal Fedoris; RRT-NPS, BA, COPD Educator
 Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD)
 Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
1.* Dosage. A. Adults
 Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Drug Class Review. Controller Medications for Asthma
 Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report
Asthma Guidelines and Pharmacological Treatment. Dr James Wilkinson
 Asthma Guidelines and Pharmacological Treatment Dr James Wilkinson Asthma is a common disease in the UK 5.4 million people in the UK are currently receiving treatment for asthma: 4.3 million adults (1
Asthma Guidelines and Pharmacological Treatment Dr James Wilkinson Asthma is a common disease in the UK 5.4 million people in the UK are currently receiving treatment for asthma: 4.3 million adults (1
CAMPER APPLICATION PACKET
 CAMPER APPLICATION PACKET Monday- Friday June 12-16, 2017 Rockport, Texas DEADLINE FOR SUBMITTING ALL FORMS: THE IMPORTANCE OF COMPLETING ALL CAMP FORMS Although it may seem like a lot of paperwork, the
CAMPER APPLICATION PACKET Monday- Friday June 12-16, 2017 Rockport, Texas DEADLINE FOR SUBMITTING ALL FORMS: THE IMPORTANCE OF COMPLETING ALL CAMP FORMS Although it may seem like a lot of paperwork, the
MDI Bonanza. Dwayne Griffin, DO
 MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
Test Your Inhaler Knowledge
 A Breath of Fresh Air: Updates in COPD Management Jennifer Austin Szwak, PharmD, BCPS, DPLA University of Chicago Medicine The speaker has nothing to disclose Abbreviations COPD: Chronic obstructive pulmonary
A Breath of Fresh Air: Updates in COPD Management Jennifer Austin Szwak, PharmD, BCPS, DPLA University of Chicago Medicine The speaker has nothing to disclose Abbreviations COPD: Chronic obstructive pulmonary
Correct Use of Inhaler Devices
 PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
FASENRA (benralizumab)
 FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Pediatric Asthma: Pharmacotherapy. Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado
 Pediatric Asthma: Pharmacotherapy Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado Pediatric Asthma: Pharmacotherapy Disclosures/Conflicts of Interest:
Pediatric Asthma: Pharmacotherapy Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado Pediatric Asthma: Pharmacotherapy Disclosures/Conflicts of Interest:
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Dupixent) Reference Number: CP.PHAR.336 Effective Date: 05.01.17 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Dupixent) Reference Number: CP.PHAR.336 Effective Date: 05.01.17 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017
 Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
Protocol Title: Adult Asthma Protocol Effective Date: 4/27/2016 Version: 1.0 Approval By: CCC Clinical Delivery Steering Planned Review Date: 4/27/2017 1 Purpose & Objective This protocol provides evidence-based
Your Inhaler Devices & You
 1 Your Inhaler Devices & You COUNSEL ON THE APPROPRIATE USE OF A: METERED DOSE INHALER (MDI) DRY POWDER INHALER (DPI) DISCUSS THE APPROPRIATE USAGE OF A PEAK FLOW METER AND SPACER/HOLDING CHAMBER DEVICE
1 Your Inhaler Devices & You COUNSEL ON THE APPROPRIATE USE OF A: METERED DOSE INHALER (MDI) DRY POWDER INHALER (DPI) DISCUSS THE APPROPRIATE USAGE OF A PEAK FLOW METER AND SPACER/HOLDING CHAMBER DEVICE
Foundations of Pharmacology
 Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Asthma. Definition. Symptoms
 Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
Preschool Asthma What you need to know in 10 minutes
 Preschool Asthma What you need to know in 10 minutes Alan Kaplan MD CCFP(EM) FCFP Family Physician Airways Group of Canada Respiratory Medicine section CFPC Faculty/Presenter Disclosure Faculty: Alan Kaplan
Preschool Asthma What you need to know in 10 minutes Alan Kaplan MD CCFP(EM) FCFP Family Physician Airways Group of Canada Respiratory Medicine section CFPC Faculty/Presenter Disclosure Faculty: Alan Kaplan
GMMMG Asthma Formulary Inhaler Options August 2017
 Regular Preventer Beclometasone Beclometasone Beclometasone Brand name Qvar Easi-Breathe Clenil Modulite Easyhaler Device MDI MDI DPI Strengths 50 microgram 100 microgram 200 microgram Adult asthma 2 inhalations
Regular Preventer Beclometasone Beclometasone Beclometasone Brand name Qvar Easi-Breathe Clenil Modulite Easyhaler Device MDI MDI DPI Strengths 50 microgram 100 microgram 200 microgram Adult asthma 2 inhalations
Clinical Policy: Mepolizumab (Nucala) Reference Number: ERX.SPA.214 Effective Date:
 Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
US max daily dose i Food and Drug Administration [2]
![US max daily dose i Food and Drug Administration [2] US max daily dose i Food and Drug Administration [2]](/thumbs/88/115964542.jpg) APPENDIX 2 etable 1. Regulatory limits on total daily dose for asthma medications Drugs Canadian max daily dose i Health Canada [1] US max daily dose i Food and Drug Administration [2] European max daily
APPENDIX 2 etable 1. Regulatory limits on total daily dose for asthma medications Drugs Canadian max daily dose i Health Canada [1] US max daily dose i Food and Drug Administration [2] European max daily
New Drug Evaluation: mepolizumab for injection
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
