Atopic dermatitis Tacrolimus vs topical corticosteroid use
|
|
|
- Bathsheba Wilson
- 5 years ago
- Views:
Transcription
1 Atopic dermatitis Tacrolimus vs topical corticosteroid use Yolande Langa, BPharm Elsabé van der Merwe, BPharm; MSc (Pharmacology) Abstract Atopic dermatitis (AD), the dermatologic manifestation of the atopic diathesis, has a variety of clinical presentations. It is a chronic and relapsing inflammatory disorder, requiring a multifaceted treatment approach. Topical corticosteroids are the backbone of therapy. However, concerns over adverse drug reactions associated with their long-term application, limit their use. Tacrolimus, on the other hand, has been shown to be effective in stabilising the symptoms of AD in the long-term setting, without the side effects that hamper the use of topical corticosteroids. Long-term safety data up to ten years is available in the literature. Despite this, the US Food and Drug Administration (FDA) s black box warning of possible malignancies has resulted in much debate amongst experts. The main focus of this article is to compare the safety and efficacy of topical corticosteroids to calcineurin inhibitors, particularly tacrolimus. Furthermore, the aim is to evaluate the place of tacrolimus in the therapy of AD. A brief overview of the condition and other treatment modalities will also be discussed. Atopic dermatitis Atopic dermatitis (AD) is an inflammatory skin disease that is characterised by extreme pruritis and frantic scratching, which induces papulation, excoriation, bleeding, oozing and crusting, secondary infection and ultimately thickening or lichenification. 1,2,3,4 It is a chronic disease, with periods of remission and flare-ups. 3,5 Although dermatitis can occur on any part of the body, there are typical locations of involvement that vary with age. 4,5,6 In infants, extensor surfaces, cheeks and the scalp are mainly affected, but the diaper area is often spared. The typical localisation in childhood stages has a flexural distribution, seen mainly around the front of the elbow area, back of the knees, insides of the wrists and ankles, as well as around the neck. In adults, a similar pattern to that observed in childhood is seen, but it is increasingly localised and lichenified. The buttocks and hands are frequently involved. The prevalence of AD is high, particularly in the paediatric population, and if not treated adequately AD has both physical and psychological ramifications. 3,5,6 The incidence of AD in adults in South Africa is not recorded in the literature, but data is available regarding children and adolescents. The South African Family Practice Journal (SAFPJ) reports on a study conducted in Cape Town amongst schoolchildren years of age. A oneyear prevalence rate of 8.3%, which increased to 13.3% on followup, was observed in this group. In 3 11-year-old Xhosa children, a one-year prevalence rate of 1 2.5% was documented. 4 While AD is a disease of childhood, it can progress into adulthood. It is estimated that about 60% of childhood cases resolve by early adolescence, but dry and irritable skin often persists. Recurrences in adults are not uncommon. 2,3 Concomitant atopic diseases such as asthma and allergic rhinitis, as well as family history of AD are predictive of a more persistent course. 2,5 Diagnosis Clinical features for the diagnosis of AD are listed below. Traditionally the Hanifin and Rajika diagnostic criteria, which consist of four major and 23 minor criteria have been used as a basis of diagnosis. 6 There is, however, lack of standardisation around clinical assessment and criteria used. 3 Variations of the above criteria are applied by different authorities. A prerequisite to diagnosis is evidence of itchy skin or pruritis, as well as three or more of the following major criteria 3,4,5,6 : 50 SAPJNovDec10pp50-56.indd 50 12/3/ :34:45 AM
2 CLINICAL REVIEW Typical morphology and distribution Visible flexural lichenification or linearity in adults Visible facial and extensor involvement in infants and children History of generally dry skin History of involvement of skin creases Personal or family history of atopy (asthma, allergic rhinitis, atopic dermatitis) Chronic or chronically-relapsing dermatitis Onset before the age of two years, in patients older than four years Listed below are some minor features of AD 2,4,5,6 : Xerosis (dry skin), dry hair and lips Raised serum IgE Wool intolerance Tendency toward cutaneous infections (especially S. aureus and Herpes simplex) or impaired cell-mediated immunity Palmar hyperlinearity Lateral thinning of the eyebrows Infant cradle cap Facial pallor Recurrent conjunctivitis Food allergies or intolerance Disease flares with emotional changes Increased pruritis when sweating Double fold of lower eyelid A number of other dermatological manifestations may have a similar presentation to AD. It is therefore imperative that a differential diagnosis is done to exclude these dermatological conditions. 5 Table I gives a list of possible differential diagnoses. Table I: Selected differential diagnoses of atopic dermatitis 2,4,7,8 Diagnosis Seborrhoeic dermatitis Psoriasis Irritant contact dermatitis Insect bites Allergic contact dermatitis Scabies Characteristic features Red, shiny, scaly lesions involving the diaper area in infants; absence of family history Lesions distributed over the extensor surfaces, scalp, buttocks; pitted nails History of exposure to irritants; rash in area of exposure; damage to skin barrier; absence of family history Symmetric distribution around the scalp, neck, face and/or extremities; erythemous crops with hypo- or hyperpigmentation; absence of family history Hypersensitivity reaction following sensitisation to a substance; may be superimposed on AD Severe pruritis; J- or S-shaped burrows 5 10 mm in length on palms, soles, genitalia and between fingers Treatment The complex nature of AD as a disease which involves genetic, environmental and immunological factors necessitates individualised therapy. 9 The treatment of AD is directed at symptomatic relief, skin hydration and reduction of inflammation. 10 Figure 1 depicts a summary of available treatment modalities for AD. Topical corticosteroids Topical corticosteroids are the mainstay of therapy for acute exacerbations. The anti-inflammatory effect of topical corticosteroids in the skin is induced through various mechanisms. 3,12 It has been purported that they have an antimitotic activity. This effect is attributed to their efficacy in the treatment of scaling dermatoses, as well as their ability to cause dermal thinning due to fibroblast inhibition. 12 The extent to which they induce cutaneous vasoconstriction and inhibit inflammation corresponds with their potency. 3,12 Topical corticosteroids can be subdivided into four groups. The classification that is used in South Africa is listed below 13 : Very potent Group IV Potent Group III Moderately potent Group II Weak Group I Table II outlines the different preparations available in SA, as well as their respective potencies. Better absorption of topical corticosteroids is observed through areas of inflammation and desquamation compared to normal skin and absorption occurs more readily through the outer dermis in infants than the skin of adults. 12 In addition, increased permeability to topical corticosteroids is noted on anatomic sites with a thin epidermis, like the face, compared to thickskinned areas, such as the palms and soles. 3,12 Absorption also depends on the vehicle in the preparation, e.g. ointments result in enhanced absorption and are semi-occlusive. Creams, on the other hand, are usually less potent. 12 The choice of topical corticosteroid is determined by the nature of the condition that is being treated. Generally, the best practice is to start with the lowest potency agent appropriate for the severity and to use it for as short a period of time as possible. In children, short durations of therapy with a low potency corticosteroid are recommended. The adverse effect profile of higher potency topical corticosteroids has limited their use in children and for facial areas. Limiting high potency corticosteroids to areas other than the face and the inner aspects of thighs and axillae, as well as once daily applications reduce the incidence of side effects. 3,12 The use of topical corticosteroids should be discontinued once the skin condition has resolved. 5,12 Clinical trials have shown that these agents are safe and effective for AD when used for up to four weeks. 5 Although topical corticosteroids are safer compared to systemic 51 SAPJNovDec10pp50-56.indd 51 12/3/ :34:45 AM
3 Figure 1: Available treatment modalities for atopic dermatitis 2,3,4,5,7,10,11 Active treatment Atopic dermatitis diagnosis Supportive treatment Topical corticosteroids Mainstay or first line for acute flare-ups Emollient creams To treat xerosis and reduce the need for corticosteroids Topical calcineurin inhibitors Second line agents Sedating antihistamines Dubious role in pruritis, but indicated for sleep disturbance and co-morbid allergies Coal tar # Alternative for patients with mild to moderate AD; has anti-inflammatory and antipruritic effects; may be useful in lichenified lesions Antibiotics For Staphylococcus infections Systemic agents: Ciclosporin Azathioprine # Systemic corticosteroids, short-term Mycophenolate mofetil # Interferon gamma # IV immunoglobulins # For severe, resistant cases Phototherapy: UVA For acute flares UVB For chronic disease PUVA For chronic, severe disease Nonpharmacologic: Dietary restrictions # Allergen reduction # Psychological approaches # There are mixed results or limited evidence regarding the efficacy due to lack of proper randomised trials UVA ultraviolet type A UVB ultraviolet type B PUVA Psoralen potentiated ultraviolet A 52 SAPJNovDec10pp50-56.indd 52 12/3/ :34:46 AM
4 CLINICAL REVIEW Table II: Topical corticosteroids available in South Africa 12,13,14 Potency Examples Dosage form Adult/paediatric appropriateness Body location Very potent Group IV Potent Group III Clobetasol propionate 0.05% Cream, ointment, scalp lotion Adults Resistant, thick lesions; palms, soles and scalp Diflucortolone valerate 0.3% Ointment Beclometasone dipropionate 0.025% Cream Adults Thick lesions; palms, soles and scalp Betamethasone valerate 0.1% Cream, ointment Betamethasone dipropionate 0.05% Diflucortolone valerate 0.1% Fluocinolone acetonide 0.025% Fluticasone propionate 0.05% Fluticasone propionate 0.005% Hydrocortisone butyrate 0.1% Methylprednisolone aceponate 0.1% Mometasone furoate 0.1% Cream, ointment Fatty ointment Cream, ointment, gel Cream Ointment Cream, ointment, lotion, topical emulsion Cream, milk, ointment, fatty ointment Cream, ointment, lotion Moderately potent Group II Weak Group I Betamethasone valerate 0.05% Cream Adults and children Clobetasone butyrate 0.05% Ointment Hydrocortisone acetate 0.5% and 1% Cream, ointment Adults, children and infants Extensive body involvement, excluding face and thin-skinned areas Face, folds, genitals and for extensive use glucocorticoids, systemic side effects can occur, particularly with super potent and potent drugs, or extensive use of lower potency agents. 12 Adverse effects of topical corticosteroids are discussed in more detail later in this article. Calcineurin inhibitors Tacrolimus and pimecrolimus are classified as calcineurin inhibitors. 5,15 This article focuses on tacrolimus, which is a macrolide lactone immunosuppressant. It exerts an inhibitory effect on T-lymphocyte activation and also modulates the release of inflammatory mediators from skin mast cells and basophils. 10,15,16 Primarily, it inhibits the phosphatase activity of calcineurin. Calcineurin is an essential component in the series of events that are necessary in the early stages of T-cell activation. In summary, tacrolimus modulates the key cellular mediators that are imperative in AD pathogenesis. 9,15,16 Pharmacokinetically, tacrolimus is a relatively large and highly lipophilic molecule with a strong affinity for the skin. It exhibits rate-limiting absorption. As the skin heals, there is a proportionate reduction in the absorption of tacrolimus. 9 Although tacrolimus penetrates the skin, systemic absorption is minimal. 9,10 In clinical trials, systemic blood levels were found to be below the limit of quantification. 1,10 Serum concentrations were shown to be minimal in a 3-week, phase II, randomised, double-blind, multicentre study, which was designed to investigate absorption from 0.03%, 0.3% and 0.1% tacrolimus preparations. 1 Based on this, systemic side effects are expected to be minimal, if not absent. 9,15 More detail on adverse effects is supplied later in this article. Comparative data on topical corticosteroids vs tacrolimus A number of studies have been conducted in an effort to establish the safety and efficacy of tacrolimus compared to either vehicle base or different potencies of topical corticosteroids. Outlined below are some of these studies, as well as their results. Kapp et al reported on two short-term, corticosteroid-controlled trials. These were double-blind, randomised, multicentre, comparative studies. 9 Refer to Table III for a summary of the study design and results in the adult arm. In adults, there was rapid improvement of symptoms across all study arms. Also noted was an improvement in disease status and this was progressive in all three groups as assessed by the modified Eczema Area and Severity Index (measi). There was no statistically significant difference in measi score between tacrolimus 0.1% and hydrocortisone butyrate 0.1%. Improvement was also noted in the tacrolimus 0.03% group, although significantly less than in the other two groups, p < SAPJNovDec10pp50-56.indd 53 12/3/ :34:46 AM
5 for both comparisons. 9 Table IV gives a summary of results in the paediatric arm. In children, both tacrolimus treatment groups showed a statistically significant improvement compared to the hydrocortisone acetate group, p for both comparisons. Tacrolimus 0.1% was significantly better compared to the 0.03% formulation, p < The overall results of these two studies show that in the shortterm tacrolimus is as effective as moderately potent to potent topical corticosteroids and more effective compared to mild or weak potency topical corticosteroids. 9 Tacrolimus was also shown to have similar efficacy to a moderately potent steroid in a phase III, randomised, open-label study comparing tacrolimus 1% to betamethasone valerate 0.12% over three weeks. 16 Long-term data is also available. In a 6-month comparative trial, Reitamo et al investigated the difference in efficacy between tacrolimus 1% and hydrocortisone butyrate 1% applied to the trunk and extremities, as well as hydrocortisone acetate 1% applied to head and neck areas. 17 Table V tabulates the results. A greater number of patients responded to tacrolimus compared to the hydrocortisone arm. The difference was statistically significant with p < There was also an improvement noted on the health-related quality of life (HRQOL) with tacrolimus compared to the topical corticosteroids. 16 In other long-term, noncomparative studies in which both adults and children were observed for one year, prolonged tacrolimus use showed progressive and sustained improvement in symptoms of AD. 9 Adverse effects The main adverse drug reaction observed with the use of tacrolimus is transient skin burning. 2,5,9 Generally, this is mild to moderate in intensity and does not necessitate withdrawal of treatment, as it diminishes over time. 9,13,16 Other side effects include mild, transient pruritis and skin erythema. All these side effects are limited to areas of application. 9,16 As a topical immunomodulatory agent, tacrolimus carries the theoretical risk of increasing skin infections, like Herpes simplex infections, eczema herpeticum, fungal dermatitis, furunculosis, or warts. 10,13,15,18 Some studies did report a Table III: Different strengths of tacrolimus ointment vs hydrocortisone butyrate 9,16 Study design Duration 3 weeks Number of participants 570 adult patients AD severity moderate to severe Regimen twice daily applications Treatment Tacrolimus 0.1% Tacrolimus 0.03% Hydrocortisone butyrate 0.1% Sample size (n) Mean measi reduction 77.3% 67.5% 73.4% PGE 49.2% 37.6% 51.4% DS 85.0% 79.9% 79.2% Skin burning 8.7% 6.8% 1.2% PGE physician s global evaluation of clinical response: percentage of patients with 90% improvement or more; measi modified Eczema Area and Severity Index; DS percentage of patients with 50% improvement in disease status Table IV: Different strengths of tacrolimus ointment vs hydrocortisone acetate 9,16 Study design Duration 3 weeks Number of participants 560 children, age 2 15 years AD severity moderate to severe Regimen twice daily applications Treatment Tacrolimus 0.1% Tacrolimus 0.03% Hydrocortisone acetate 1% Sample size (n) Mean measi reduction 75.9% 65.8% 37.3% PGE 48.4% 38.5% 15.7% DS 83.5% 80.2% 51.4% Skin burning 5.6% 1.1% 1.2% PGE physician s global evaluation of clinical response: percentage of patients with 90% improvement or more; measi modified Eczema Area and Severity Index; DS percentage of patients with 50% improvement in disease status 54 SAPJNovDec10pp50-56.indd 54 12/3/ :34:47 AM
6 CLINICAL REVIEW higher incidence of viral skin infections during initial treatment with tacrolimus compared to corticosteroids; other studies not. 9,10,16 Overall, long-term treatment (6 12 months) is not associated with an increased prevalence of cutaneous infections; therefore tacrolimus does not seem to impair cellmediated immunity. 9,10,16,18 However, it is important to note that application of tacrolimus preparations to infected areas is not recommended. 9,13,18 Acne is one problem for which a causal relationship with longterm use of the drug cannot be ruled out. 16 Flushing, especially with alcohol ingestion, has also been reported in a number of studies. 11,13,16 An increased risk of malignancies is another concern with tacrolimus. 13,15,16 As such, there is a black box warning against tacrolimus placed by the FDA in 2005 due to lack of long-term safety data at the time and the potential risk for the development of malignancies, mainly lymphoma and skin cancer. This was based on information derived from animal studies. 2,5,19 However, to date there is no data to suggest that tacrolimus is associated with an increased risk of cancer in either children or adults. 9 No increased risk of nonmelanoma skin cancer was found in posthoc reviews, neither was squamous cell skin cancer reported in any clinical trials with tacrolimus ointment. 9,10,16 A literature search also did not produce any evidence to confirm any malignancy risk, but some authors are still concerned. 4,10,18 Study design Table V: Tacrolimus 0.1% compared to hydrocortisone-based preparations 9,16,17 Duration maximum 6 months Number of participants 972 adults AD severity moderate to severe Regimen twice daily applications Primary end point 60% improvement in measi at 3 months The mean % reduction in measi and median reduction in DQLI were also measured at 3 months Treatment Tacrolimus 0.1% Hydrocortisone butyrate 1% trunk and extremities Hydrocortisone acetate 1% head and neck Sample size (n) combined Primary end point reached Mean measi reduction Nonresponders DQLI median decrease 72.6% 52.3% 73% 62% 52 patients (10.7%) 124 patients (25.6%) 74.3% 69.2% measi - modified Eczema Area and Severity Index; DQLI - Dermatology Quality of Life Index (a higher score represents a lower quality of life) Nevertheless, tacrolimus carries a warning for minimal exposure to sunlight or UV light, as a precautionary measure against local malignancies. 5,18 Alternatively, patients can be counselled to use sun protection creams. 5,9,10,15,18 Adverse drug reactions associated with the use of corticosteroids range from skin atrophy, striae, telangiectasia, acne, glaucoma, adrenocortical insufficiency and, in extreme cases, Cushing s syndrome. 5,12,16,17 However, there is no conclusive evidence that correctly used topical agents cause significant systemic side effects. 5,18 In fact, literature suggests that when used for periods up to four weeks, topical corticosteroids are safe and effective for the treatment of AD flare-ups. 5 Conversely, it is the long-term use or overuse that is associated with adverse effects. 2,5 Regarding skin atrophy, no cases were reported for tacrolimus. On the contrary, in a 12-month study of tacrolimus 0.1% in adults, some patients experienced reversal of symptoms. It was unclear whether this was due to absence of steroids or directly attributable to tacrolimus. 9 The atrophogenic potential of tacrolimus was specifically investigated in a randomised, phase II, double-blind, placebocontrolled trial with 14 AD patients and 12 healthy subjects. Tacrolimus 0.1%, 0.3%, bethamethasone valerate 0.1%, and vehicle were applied twice, on day 1 and again 3 4 days later, to nonaffected abdominal skin and occluded with bandages. Skin thinning was only observed with betamethasone. 9 In contrast, four 16-week randomised trials with topical corticosteroids failed to show any clinically significant skin thinning. Concerns of patients and parents about topical corticosteroid use may be out of proportion to the true risk. 2 In summary, many experts agree that skin thinning with topical corticosteroids is minimal if they are used intermittently and correctly. 2,4,5,18 Interestingly, in the phase III bethamethasone 0.12% comparative study mentioned earlier, the overall safety ratings by patients were 88% for tacrolimus and 91% for the cortisone preparation. 16 Discussion The above results suggest that tacrolimus 0.1% is as effective as potent topical corticosteroids and more effective than mild topical corticosteroids, such as hydrocortisone acetate 1%, for treating atopic dermatitis in the short term. Long-term efficacy and safety are even more promising. Having looked at the different studies, as well as the adverse effect profile of both topical corticosteroids and tacrolimus, the question arises as to the place in therapy for tacrolimus in AD. In South Africa, tacrolimus (Protopic ) is registered for the treatment of moderate to severe atopic dermatitis in adolescents 16 years and adults. The 0.03% strength is to be applied initially as a thin layer twice daily to affected skin 55 SAPJNovDec10pp50-56.indd 55 12/3/ :34:47 AM
7 until the lesion clears. If this fails or results are inadequate, then the 0.1% preparation should be started. Treatment should be stopped if there is no improvement after three weeks. In patients 2 15 years old, the 0.03% strength may be applied twice daily for up to three weeks, then the application should be reduced to once daily until the lesion clears. 13 Topical corticosteroids are effective in most patients 5,18 and given the fact that tacrolimus 0.1% is about 1.4 to 3.6 times more expensive per 30 g than moderately potent and potent original steroid preparations, 20 the cost-effectiveness of topical tacrolimus vs steroids still needs to be established. 4 Several authorities recommend that tacrolimus be used as a second-line therapy, for moderate to severe AD in the following scenarios: For face and neck areas where high potency steroids would be required to control symptoms 9,10,18 Where long-term treatment with steroids is required 10,18 In patients with evident signs of steroid toxicity 18 In patients who are resistant to topical corticosteroids (rarely seen) 2,9,10,18 In patients who are intolerant to conventional therapies 2,9,10 The National Institute for Health and Clinical Excellence (NICE) gives the following recommendation: Topical tacrolimus is recommended, within its licensed indications, as an option for the second-line treatment of moderate to severe atopic eczema in adults and children aged two years and older that has not been controlled by topical corticosteroids, where there is a serious risk of important adverse effects from further topical corticosteroid use, particularly irreversible skin atrophy. NICE also recommends that topical corticosteroids are to be used as first-line treatment for episodic worsening (flare-ups) of atopic eczema. They should be used intermittently, in order to reduce exposure to corticosteroids. 21 Conclusion Tacrolimus offers an alternative to the treatment of moderate to severe atopic dermatitis and steroid-resistant AD. It may be useful for atopic dermatitis at sensitive sites such as the face, where the use of potent topical steroids carries a high risk of thinning of the skin and telangiectasia. 1 Tacrolimus 0.1% may also be useful for patients who depend on the constant use of potent steroids. The cost-effectiveness, as well as long-term risk of infections and cancers remain to be determined. 4,18 r References 1. Ruzicka T, Bieber T, Schöpf E, Rubins A, Dobozy A, Bos JD, et al. A shortterm trial of tacrolimus ointment for atopic dermatitis. N Engl J Med 1997; 337: Williams HC. Atopic dermatitis. N Engl J Med 2005; 352(22): National Institute for Health and Clinical Excellence. Frequency of application of topical costicosteroids for atopic eczema. Technology Appraisal 81. August Available from URL 4. Jordaan HF, Visser WI. The diagnosis and management of atopic dermatitis. SA Fam Pract 2009; 51(5): Buys LM. Treatment options for atopic dermatitis. Am Fam Physician 2007; 75: Available from URL 6. Edelstein JA, Sinert RH. Dermatitis, atopic. emedicine June (Online) (Access August). Available from URL com/article/ print 7. Correale C, Walker C, Murphy L, Craig T. Atopic dermatitis: A review of diagnosis and treatment. Am Fam Physician 1999; 60: (Access August). Available from URL 8. Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: A new approach to papular urticaria. Pediatrics 2006; 118(1): e Available from URL full/peds v1 9. Kapp A, Allen BR, Reitamo S. Atopic dermatitis management with tacrolimus ointment (Protopic ). Journal of Dermatological Treatment 2003; 14(Suppl 1): Cheer SM, Plosker GL. Tacrolimus ointment: A review of its therapeutic potential as a topical therapy in atopic dermatitis. Am J Clin Dermatol 2001; 2(6): Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol 2004; 50: Available from URL ClinicalResearch_atopic%20Dermatitis%20Part%20I.pdf 12. Goldstein BG, Goldstein AO. General principles of dermatologic therapy and topical corticosteroid use. UptoDate December (Online) (Access July). Licenced subscription at URL Rossiter D, editor. South African Medicines Formulary (SAMF). Ninth edition. Cape Town: Health and medical publishing group; p , Eczema guide. Topical corticosteroids. (Online) (Access July). Available from URL topical_corticosteroids.html 15. Pascual JC, Fleischer AB. Tacrolimus ointment (Protopic ) for atopic dermatitis. Skin Therapy Letter 2004; 9. Available from URL skintherapyletter.com/2004/9.9/1.html 16. Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis. Clin Drug Invest 2006; 26(5): Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. British Journal of Dermatology 2005; 152: Conroy S. New products for eczema. Arch Dis Child Educ Pract Ed 2004; 89: ep23-6. Available from URL U.S. Food and Drug Administration. Safety: Elidel (pimecrolimus), Protopic (tacrolimus). (Online) (Access September). Available from URL SafetyAlertsforHumanMedicalProducts/ucm htm 20. Monthly Index of Medical Specialities (MIMS). 2010; 50(7) 21. National Institute for Health and Clinical Excellence. Tacrolimus and pimecrolimus for atopic eczema. Technology Appraisal 82. August Available from URL 56 SAPJNovDec10pp50-56.indd 56 12/3/ :34:47 AM
Atopic dermatitis: tacrolimus vs. topical corticosteroid use
 Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Atopic dermatitis: tacrolimus vs. topical corticosteroid use Langa Y, BPharm Van der Merwe E, BPharm, MSc (Pharmacology) Correspondence to: Elsabe van der Merwe, e-mail: elsabev@medikredit.co.za Keywords:
Medication Policy Manual. Topic: Dupixent, dupilumab Date of Origin: March 10, Committee Approval: March 10, 2017 Next Review Date: May 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru493 Topic: Dupixent, dupilumab Date of Origin: March 10, 2017 Committee Approval: March 10, 2017
New Medicine Report. Pimecrolimus. RED- Hospital only Date of Last Revision 6 th March 2003
 New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
New Medicine Report Document Status Pimecrolimus Reviewed by Suffolk D&T RED- Hospital only Date of Last Revision 6 th March 2003 Approved Name Pimecrolimus Trade Name Elidel Manufacturer Novartis Legal
What is atopic dermatitis?
 What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
What is atopic dermatitis? Complex inflammatory skin disorder intense pruritus cutaneous hyperreactivity immune dysregulation Chronic with exacerbations and remissions Affects all ages, but more common
Children s Hospital Of Wisconsin
 Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Children s Hospital Of Wisconsin Co-Management Guidelines To support collaborative care, we have developed guidelines for our community providers to utilize when referring to, and managing patients with,
Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82
 Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tacrolimus and pimecrolimus for atopic eczema Technology appraisal guidance Published: 25 August 2004 nice.org.uk/guidance/ta82 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Pharmacologic Treatment of Atopic Dermatitis
 J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
J KMA Pharmacotherapeutics Pharmacologic Treatment of Atopic Dermatitis Chun Wook Park, MD Department of Dermatology, Hallym University College of Medicine E mail : dermap@paran.com J Korean Med Assoc
15 minute eczema consultation
 THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
THERAPY WORKSHOP 15 minute eczema consultation History Current treatments Examination Treatment Plan Written action plan Soap substitute/bath oil Antiseptic baths Emollients Topical steroids Other treatments
Dermatitis (inflammatory skin condition) Nonallergic. dermatitis. Non-atopic eczema (non- IgE mediated)
 Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
Atopic Eczema Dermatitis (inflammatory skin condition) Allergic dermatitis -eczema Nonallergic dermatitis Atopic eczema (IgE mediated) Non-atopic eczema (non- IgE mediated) Pathophysiology of Eczema Allergy
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Eucrisa Page: 1 of 7 Last Review Date: June 22, 2018 Eucrisa Description Eucrisa (crisaborole)
Eucrisa. Eucrisa (crisaborole) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.25 Subject: Eucrisa Page: 1 of 6 Last Review Date: September 15, 2017 Eucrisa Description Eucrisa
The Itch That Rashes. Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah
 The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
The Itch That Rashes Sarah D. Cipriano, MD, MPH, MS Resident, Dermatology University of Utah 1 Conflict of Interest No conflict of interest Will discuss off label use of medications 2 3 Most likely diagnosis?
Topical Immunomodulator Step Therapy Program
 Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Atopic Eczema with detail on how to apply wet wraps
 Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
Atopic Eczema with detail on how to apply wet wraps Dr Carol Hlela Consultant Dermatologist Head of Unit, Department of Dermatology, Paediatrics Red Cross Children s Hospital, UCT Red Cross War Memorial
RELEVANT DISCLOSURES ATOPIC DERMATITIS / ECZEMA MANAGING ECZEMA IN INFANTS AND CHILDREN
 RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
RELEVANT DISCLOSURES MANAGING ECZEMA IN INFANTS AND CHILDREN Advisory board member - MEDA (Elidel), Speaking honoraria Bayer (Advantan) Advisory board, consultant, speaker: Pfizer, Abbvie, Janssen, Elli
Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis
 Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
Original Article Comparative efficacy of topical mometasone furoate 0.1% cream vs topical tacrolimus 0.03% ointment in the treatment of atopic dermatitis Md Alauddin Khan *, Lubna Khondker **, Dilshad
過敏病科中心. Allergy Centre. Eczema. Allergy Centre 過敏病科中心. Allergy Centre. For enquiries and appointments, please contact us at:
 Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
Allergy Centre 過敏病科中心 Eczema For enquiries and appointments, please contact us at: Allergy Centre 9/F, Li Shu Pui Block Hong Kong Sanatorium & Hospital 2 Village Road, Happy Valley, Hong Kong Tel: 2835
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
CHAPTER 1. Eczema Basics
 CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
CHAPTER 1 Eczema Basics Definition Eczema is an inflammatory skin condition, characterised by ichtyosis (dry skin), erythema (redness), excoriation (interruption of the skin), scratching lesions, lichenification
The safety and effectiveness of Dupixent in pediatric patients have not been established (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.30 Subject: Dupixent Page: 1 of 6 Last Review Date: September 15, 2017 Dupixent Description Dupixent
Dupixent (dupilumab)
 Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Dupixent (dupilumab) Line(s) of Business: HMO; PPO; QUEST Integration Effective Date: TBD POLICY A. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Protopic 0.03% ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of Protopic 0.03% ointment contains 0.3 mg of tacrolimus
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Protopic 0.03% ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of Protopic 0.03% ointment contains 0.3 mg of tacrolimus
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
UPDATES IN ATOPIC DERMATITIS
 UPDATES IN ATOPIC DERMATITIS Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute, Scottsdale, AZ LEARNING OBJECTIVES Discuss epidemiology, risk factors, and causes of
UPDATES IN ATOPIC DERMATITIS Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute, Scottsdale, AZ LEARNING OBJECTIVES Discuss epidemiology, risk factors, and causes of
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Eczema. By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University
 Eczema By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University Dermatitis= Eczema =Spongiosis Eczema Atopic Seborrheic Contact Allergic Irritant Nummular Asteatotic Stasis Neurodermatitis/Lichen Simplex
Eczema By:- Dr. Naif Al-Shahrani Salman bin Abdazziz University Dermatitis= Eczema =Spongiosis Eczema Atopic Seborrheic Contact Allergic Irritant Nummular Asteatotic Stasis Neurodermatitis/Lichen Simplex
0BCore Safety Profile. Pharmaceutical form(s)/strength: Cream 1% DK/H/PSUR/0009/005 Date of FAR:
 0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
0BCore Safety Profile Active substance: Pimecrolimus Pharmaceutical form(s)/strength: Cream 1% P-RMS: DK/H/PSUR/0009/005 Date of FAR: 06.06.2013 4.3 Contraindications Hypersensitivity to pimecrolimus,
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level
 Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Constitutional eczema
 Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Patient information Constitutional eczema What is constitutional eczema? Constitutional eczema, also called atopic eczema, is a form of eczema that mainly occurs in childhood. Eczema usually starts before
Learning Circle: Jan 26, 2011 Childhood Eczema
 Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
Learning Circle: Jan 26, 2011 Childhood Eczema Wingfield Rehmus, MD MPH BC Children s Hospital Clinical Assistant Professor, UBC Department of Paediatrics Associate Member, UBC Department of Dermatology
ATOPIC ECZEMA. What are the aims of this leaflet?
 ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
ATOPIC ECZEMA What are the aims of this leaflet? This leaflet has been written to help you understand more about atopic eczema. It tells you what it is, what causes it, what can be done about it, and where
Managing and Minimizing Flare-ups in Atopic Dermatitis
 Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
Managing and Minimizing Flare-ups in Atopic Dermatitis Importance of the skin barrier & how commonly used drugs are impacting it Dr. Benjamin Barankin, MD FRCPC Medical Director & Founder of Toronto Dermatology
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2116-3 Program Prior Authorization/Medical Necessity Medications Dupixent (dupilumab) P&T Approval Date 1/2017, 5/2017, 7/2017
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland
 Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
Professor Rohan Ameratunga Clinical Immunologist and Allergist Auckland 16:30-17:25 WS #170: Eczema Management 17:35-18:30 WS #182: Eczema Management (Repeated) Managing ECZEMA A/Prof Rohan Ameratunga
Thursday, 21 October :53 - Last Updated Thursday, 11 November :27
 1 / 15 2 / 15 3 / 15 4 / 15 Pityriasis Alba Background Pityriasis alba is a nonspecific dermatitis of unknown etiology that causes erythematous scaly patches. These resolve and leave areas of hypopigmentation
1 / 15 2 / 15 3 / 15 4 / 15 Pityriasis Alba Background Pityriasis alba is a nonspecific dermatitis of unknown etiology that causes erythematous scaly patches. These resolve and leave areas of hypopigmentation
8-year surveillance 2016 Atopic eczema in under 12s (2007) NICE guideline CG57
 8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
8-year 2016 Atopic eczema in under 12s (2007) NICE guideline CG57 Appendix A: decision matrix year Diagnosis 57 01 What criteria should be used to diagnose atopic eczema in children and how do they vary
KEY MESSAGES. Psoriasis patients are more prone to cardiovascular diseases, stroke, lymphoma and non-melanoma skin cancers, and increased mortality.
 KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
KEY MESSAGES Psoriasis is a genetically determined, systemic immune-mediated chronic inflammatory disease that affects primarily the skin and joints. Psoriasis Vulgaris is characterised by well-demarcated
Skin disorders. Seborrhoeic dermatitis Search date April 2010 Luigi Naldi ...
 Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Seborrhoeic Search date April 21 Luigi Naldi.................................................. ABSTRACT INTRODUCTION: Seborrhoeic affects at least 1% of the population. Malassezia (Pityrosporum) ovale
Psoriasis: Causes, Symptoms, And Treatment
 Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Psoriasis: Causes, Symptoms, And Treatment We all know that a healthy immune system is good. But, do you know that an overactive immune system can cause certain conditions like Psoriasis? Read on to find
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tacrolimus 0.1% Ointment Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g ointment contains tacrolimus monohydrate corresponding to 1.0
1 NAME OF THE MEDICINAL PRODUCT Tacrolimus 0.1% Ointment Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g ointment contains tacrolimus monohydrate corresponding to 1.0
ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS. Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine
 ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine THE PLAN Is it atopic dermatitis? What is atopic dermatitis? Guidelines
ATOPIC DERMATITIS: A BLUEPRINT FOR SUCCESS Sierra Wolter MD, FAAD Pediatric Dermatology University of Arizona, College of Medicine THE PLAN Is it atopic dermatitis? What is atopic dermatitis? Guidelines
Vitiligo is a skin depigmentation disorder
 THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Recommended diagnosis and management of atopic eczema
 n DRUG REVIEW Recommended diagnosis and management of atopic eczema Andrew MacKenzie MRCP(UK) and Olivia Schofield FRCP(Edin) SPL The pathogenesis of atopic eczema is complex and its management requires
n DRUG REVIEW Recommended diagnosis and management of atopic eczema Andrew MacKenzie MRCP(UK) and Olivia Schofield FRCP(Edin) SPL The pathogenesis of atopic eczema is complex and its management requires
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD)
 Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Diagnosis: ATOPIC DERMATITIS (AD) Texas Children's Hospital Dermatology Service PCP Referral Guidelines- Atopic Dermatitis (AD) PATIENT ADVICE: Unfortunately, there is no cure for atopic dermatitis, so
Drug allergy and Skin Disorders. Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey
 Drug allergy and Skin Disorders Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey The best screening test for anaphylaxis is? A. histamine
Drug allergy and Skin Disorders Timothy Craig, DO, FACOI Professor of Medicine and Pediatrics Distinguished Educator Penn State University, Hershey The best screening test for anaphylaxis is? A. histamine
Atopic Dermatitis: Therapeutic Challenges
 Atopic Dermatitis: Therapeutic Challenges PDA August 14, 2009 Jon Hanifin OHSU, Portland Dominant Concepts in Atopic Dermatitis Allergy / Immunology Era: 1915-2006 The Epidermal Era: 2006---- Barrier dysfunction
Atopic Dermatitis: Therapeutic Challenges PDA August 14, 2009 Jon Hanifin OHSU, Portland Dominant Concepts in Atopic Dermatitis Allergy / Immunology Era: 1915-2006 The Epidermal Era: 2006---- Barrier dysfunction
Time to Learn. 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service
 Time to Learn 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service The Red Face Rosacea Acne Seborrhoeic eczema eczema Psoriasis Slapped cheek syndrome Fungal infection Erysipelas...
Time to Learn 6 th March 2018 Dr. Shirin Chakera GPwSI Integrated Dermatology Service The Red Face Rosacea Acne Seborrhoeic eczema eczema Psoriasis Slapped cheek syndrome Fungal infection Erysipelas...
TCIs are only available on prescription and are usually started by a dermatology specialist.
 (TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
(TCIs) What are topical calcineurin inhibitors? Topical calcineurin inhibitors are treatments that alter the immune system and have been developed for controlling eczema. There are two types available:
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
Atopic dermatitis/ eczema (a condition that makes skin red and itchy):
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Atopic dermatitis/ eczema (a condition that makes skin red and itchy): Atopic dermatitis (AD) is a common skin condition with
Biologic Therapies for Atopic Dermatitis and Beyond
 Biologic Therapies for Atopic Dermatitis and Beyond Jonathan Corren, M.D. Departments of Medicine and Pediatrics, David Geffen School of Medicine at UCLA Disclosures Genentech - research Medimmune/AZ -
Biologic Therapies for Atopic Dermatitis and Beyond Jonathan Corren, M.D. Departments of Medicine and Pediatrics, David Geffen School of Medicine at UCLA Disclosures Genentech - research Medimmune/AZ -
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elidel 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of cream contains 10 mg of pimecrolimus. For a full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Elidel 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g of cream contains 10 mg of pimecrolimus. For a full list of excipients,
Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57
 Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
Atopic eczema in under 12s: diagnosis and management Clinical guideline Published: 12 December 2007 nice.org.uk/guidance/cg57 NICE 2007. All rights reserved. Contents Introduction... 4 Child-centred care...
DIPROSONE OV Cream and Ointment do not contain preservatives, parabens or lanolin.
 PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
PRODUCT INFORMATION DIPROSONE OV (OPTIMISED VEHICLE) CREAM AND OINTMENT NAME OF THE MEDICINE DIPROSONE OV Cream and Ointment (Betamethasone dipropionate) Chemistry Abstracts Service (CAS) registry number:
Vulval dermatoses. Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough
 Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
Vulval dermatoses Dr Fiona Lewis, Consultant Dermatologist St John s Institute of Dermatology, London & Heatherwood & Wexham Park Hospital, Slough Pigmentation Vulvodynia Ulcers Genetic Pruritus VULVAL
The role of the practice nurse in managing psoriasis in primary care
 The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
The role of the practice nurse in managing psoriasis in primary care Item type Authors Publisher Journal Article Buckley, David Nursing in General Practice Nursing in general practice Downloaded 16-Sep-2016
o topical and systemic
 Corticosteroid: o topical and systemic Aznan Lelo, Yunita Sari Pane Dep. Farmakologi dan Terapeutik, Fakultas Kedokteran Universitas Sumatera Utara 8&11, 4 Februari 2009, KBK, FK USU, Medan Dermatitis
Corticosteroid: o topical and systemic Aznan Lelo, Yunita Sari Pane Dep. Farmakologi dan Terapeutik, Fakultas Kedokteran Universitas Sumatera Utara 8&11, 4 Februari 2009, KBK, FK USU, Medan Dermatitis
Atopic dermatitis (AD), which can produce
 Original Article 485 Efficacy and Safety of Topically Applied Tacrolimus Ointment in Patients with Moderate to Severe Atopic Dermatitis Wen-Rou Wong, MD; Hsiang-Ju Tsai, MD; Hong-Shang Hong, MD, PhD Background:
Original Article 485 Efficacy and Safety of Topically Applied Tacrolimus Ointment in Patients with Moderate to Severe Atopic Dermatitis Wen-Rou Wong, MD; Hsiang-Ju Tsai, MD; Hong-Shang Hong, MD, PhD Background:
SUMMARY OF THE PRODUCT CHARACTERISTICS
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Zoviduo 50 mg/g and 10 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram cream contains 50 mg aciclovir and 10 mg hydrocortisone.
NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION
 NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
Core Safety Profile. AT/H/PSUR/0013/002 Date of FAR:
 ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
ore afety Profile Active substance: Methylprednisolon aceponate Pharmaceutical form(s)/strength: ream / 0.1 % Fatty ointment / 0.1 % utaneous emulsion / 0.1 % intment / 0.1 % utaneous solution / 0.1 %
What s Topical About Topicals?
 What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
What s Topical About Topicals? Megha M. Tollefson, MD Associate Professor of Dermatology and Pediatrics July 29, 2017 2015 MFMER 3513105-1 Disclosures None 2015 MFMER 3513105-2 Outline Topical steroids
Research Review PRODUCT REVIEW. About the Reviewer. Dr Jennifer Pilgrim MB ChB (Otago), FRACP. Atopic eczema
 Research Review PRODUCT REVIEW Methylprednisolone aceponate 0.1% ointment/cream [ADVANTAN ] About the Reviewer Dr Jennifer Pilgrim MB ChB (Otago), FRACP A graduate of Otago University, Dr Jennifer Pilgrim
Research Review PRODUCT REVIEW Methylprednisolone aceponate 0.1% ointment/cream [ADVANTAN ] About the Reviewer Dr Jennifer Pilgrim MB ChB (Otago), FRACP A graduate of Otago University, Dr Jennifer Pilgrim
ATOPIC DERMATITIS IN CHILDREN. Simple choice questions
 ATOPIC DERMATITIS IN CHILDREN Simple choice questions 1. Which is the principal cause of atopic dermatitisdevelopment in little age children? A. Psychoemotional factors. B. Habitual antigens. C. Food allergy.
ATOPIC DERMATITIS IN CHILDREN Simple choice questions 1. Which is the principal cause of atopic dermatitisdevelopment in little age children? A. Psychoemotional factors. B. Habitual antigens. C. Food allergy.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
RECLASSIFICATION SUBMISSION. EUMOVATE Eczema and Dermatitis Cream. (Clobetasone butyrate 0.05%) From Prescription Only Medicine
 RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
RECLASSIFICATION SUBMISSION EUMOVATE Eczema and Dermatitis Cream (Clobetasone butyrate 0.05%) From Prescription Only Medicine To Pharmacist Only Medicine NOVEMBER 2000 MEETING PART A 1. International non-proprietary
Dermatology elective for yr. 5. Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015
 Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
Dermatology elective for yr. 5 Natta Rajatanavin, MD. Div. of dermatology Dep. Of Medicine, Ramathibodi Hospital Mahidol University 23 rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives
Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital
 Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
Atopic dermatitis definition Management of eczema in infants and children Assoc Prof David Orchard Director, Department of Dermatology Royal Children s Hospital Atopic dermatitis is long lasting (chronic)
forniture parafarmaceutiche
 User's Manual forniture parafarmaceutiche CONTENTS forniture parafarmaceutiche Dermatitis of the Scalp Seborrheic Dermatitis Treatments Atopshield Lotion The mechanism of action of Atopshield Lotion Indications
User's Manual forniture parafarmaceutiche CONTENTS forniture parafarmaceutiche Dermatitis of the Scalp Seborrheic Dermatitis Treatments Atopshield Lotion The mechanism of action of Atopshield Lotion Indications
BETNOVATE Betamethasone 17-valerate
 BETNOVATE Betamethasone 17-valerate Betamethasone valerate is referred to as Betnovate throughout this information. PRESENTATION BETNOVATE Cream (non-greasy base) 0.1% betamethasone as 17-valerate in a
BETNOVATE Betamethasone 17-valerate Betamethasone valerate is referred to as Betnovate throughout this information. PRESENTATION BETNOVATE Cream (non-greasy base) 0.1% betamethasone as 17-valerate in a
Paediatric Eczema. Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012
 Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
Paediatric Eczema Dr Manjeet Joshi Consultant Dermatologist 16 th May 2012 Classification of the principal forms of eczema EXOGENOUS ENDOGENOUS Irritant Allergic contact Photoallergic contact Eczematous
An Everyday Guide to Eczema
 An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
An Everyday Guide to Eczema By Dr. Kristel Polder, Board-Certified Dermatologist Developed in Partnership with Who is affected by eczema? 32 million people in the US 1 in 5 children 1 in 12 adults *www.eczema.org
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil)
 Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
GROUP 15 TOPICAL PREPARATIONS
 - 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
- 105 - GROUP 15 15.1 DERMATOLOGICAL PREPARATIONS 15.1.1 TOPICAL ANTIFUNGALS CLOTRIMAZOLE Indication: Treatment of susceptible fungal infections, dermatophytoses, superficial mycoses, and cutaneous candidiasis
Contact Dermatitis In Atopic Patients
 Contact Dermatitis In Atopic Patients Jenny Murase, MD Palo Alto Foundation Medical Group Director of Patch Testing University of California, San Francisco Associate Clinical Professor Disclosures Consultant
Contact Dermatitis In Atopic Patients Jenny Murase, MD Palo Alto Foundation Medical Group Director of Patch Testing University of California, San Francisco Associate Clinical Professor Disclosures Consultant
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Technology Appraisals and Guidance Information Services Static List Review (SLR) Title and TA publication number of static topic: Final decision: TA82;
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 3Q17 July August
 BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
BRAND NAME Dupixent GENERIC NAME dupilumab MANUFACTURER Regeneron DATE OF APPROVAL March 28, 2017 PRODUCT LAUNCH DATE First week of April 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory EUMOSONE. Clobetasone Cream IP
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory EUMOSONE Clobetasone Cream IP QUALITATIVE AND QUANTITATIVE COMPOSITION EUMOSONE contains : Clobetasone Butyrate IP 0.05
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory EUMOSONE Clobetasone Cream IP QUALITATIVE AND QUANTITATIVE COMPOSITION EUMOSONE contains : Clobetasone Butyrate IP 0.05
Recommended management of eczema in older patients
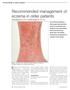 Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Recommended management of eczema in older patients Victoria Sherman MA, MRCP and Daniel Creamer BSc, MD, FRCP Our series Prescribing in older people gives practical advice for successful management of
Skin Manifestations of Allergy
 Dermatologic Manifestations of Allergy William Reisacher MD FACS FAAOA Assistant Professor Weill Cornell Medical College Skin Manifestations of Allergy Atopic dermatitis (atopic eczema, eczema) Contact
Dermatologic Manifestations of Allergy William Reisacher MD FACS FAAOA Assistant Professor Weill Cornell Medical College Skin Manifestations of Allergy Atopic dermatitis (atopic eczema, eczema) Contact
PNW EPC Drug Effectiveness Review Project Summary Report Atopic Dermatitis New Drug Evaluation: Dupilumab
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Skin Deep: Or is It? Practical Pearls from a Pediatric Dermatologist
 Skin Deep: Or is It? Practical Pearls from a Pediatric Dermatologist I have no conflicts of interest 6 yo boy referred for AD. On topical HC and food elimination diet s/p topical triamcinolone to body
Skin Deep: Or is It? Practical Pearls from a Pediatric Dermatologist I have no conflicts of interest 6 yo boy referred for AD. On topical HC and food elimination diet s/p topical triamcinolone to body
COMMON SKIN CONDITIONS IN PRIMARY CARE. Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio
 COMMON SKIN CONDITIONS IN PRIMARY CARE Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio DISCLOSURE The Speaker and members of the planning committee do not have a conflict of interest
COMMON SKIN CONDITIONS IN PRIMARY CARE Ibrahim M. Zayneh, MD Dermatology Private Practice, Portsmouth, Ohio DISCLOSURE The Speaker and members of the planning committee do not have a conflict of interest
ATOPIC DERMATITIS: PATHOPHYSIOLOGY AND PHARMACOLOGY OF MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP
 ATOPIC DERMATITIS: PATHOPHYSIOLOGY AND PHARMACOLOGY OF MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP Disclosures Promotional Speaker for Pfizer, Inc. Any unlabeled/unapproved uses of drugs or products
ATOPIC DERMATITIS: PATHOPHYSIOLOGY AND PHARMACOLOGY OF MANAGEMENT PEGGY VERNON, RN, MA, C-PNP, DCNP, FAANP Disclosures Promotional Speaker for Pfizer, Inc. Any unlabeled/unapproved uses of drugs or products
Cite this article as: BMJ, doi: /bmj d3 (published 24 February 2005)
 Cite this article as: BMJ, doi:10.1136/bmj.38376.439653.d3 (published 24 February 2005) Primary care Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis:
Cite this article as: BMJ, doi:10.1136/bmj.38376.439653.d3 (published 24 February 2005) Primary care Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis:
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA
 What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
What you need to know about ECZEMA The Irish Skin Foundation is a national charity with a mission to improve quality of life for people with skin conditions, promote skin health and the prevention of skin
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNOVATE - S
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNOVATE - S Betamethasone Valerate and Salicylic Acid Skin Ointment QUALITATIVE AND QUANTITATIVE COMPOSITION BETNOVATE
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNOVATE - S Betamethasone Valerate and Salicylic Acid Skin Ointment QUALITATIVE AND QUANTITATIVE COMPOSITION BETNOVATE
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION. Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.
 PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
Integumentary System
 Integumentary System Integumentary System Skin, hair, and nails. Skin: Epidermis: outer layer. Dermis: also called corium, or true skin. Subcutaneous fascia: innermost layer. Integumentary Glands Sudoriferous:
Integumentary System Integumentary System Skin, hair, and nails. Skin: Epidermis: outer layer. Dermis: also called corium, or true skin. Subcutaneous fascia: innermost layer. Integumentary Glands Sudoriferous:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
Psoriasis. What is Psoriasis? What causes psoriasis? Medical Topics Psoriasis
 1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
1 Psoriasis What is Psoriasis? Psoriasis is a long standing inflammatory non-contagious skin disease which waxes and wanes with triggering factors. There is a genetic predisposition in psoriasis. Internationally,
NEW ZEALAND DATA SHEET
 EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
What is Psoriasis? Common Areas Affected. Type Who Does it Affect Characteristics
 What is? is a term derived from the Greek word psōra which means itch and is a common, long lasting, inflammatory skin condition which affects 1-3% of the UK population and about 80 million people worldwide.
What is? is a term derived from the Greek word psōra which means itch and is a common, long lasting, inflammatory skin condition which affects 1-3% of the UK population and about 80 million people worldwide.
