BUILD-3: A Randomized, Controlled Trial of Bosentan in
|
|
|
- Ethelbert Norton
- 5 years ago
- Views:
Transcription
1 BUILD-3: A Randomized, Controlled Trial of Bosentan in Idiopathic Pulmonary Fibrosis Talmadge E. King, Jr., MD; Kevin K. Brown, MD; Ganesh Raghu, MD; Roland M. du Bois, MD; David Lynch, MD; Fernando Martinez, MD; Dominique Valeyre, MD; Isabelle Leconte, PhD; Adele Morganti, MSc; Sébastien Roux, MD; Juergen Behr, MD Online Data Supplement Supplementary Methods, Section 1 Central reading of surgical lung biopsy slides Surgical lung biopsy slides were assessed at each investigational site or, upon investigator request, by a central reader at a core (central) laboratory before randomization. After randomization of the first two patients at each investigational site, the surgical lung biopsy slides from these patients were reviewed by a central reader at a core (central) laboratory to ensure that each site was enrolling patients with a proven diagnosis of IPF (i.e., by the identification of the pattern of usual interstitial pneumonia [UIP]). Supplementary Methods, Section 2 Quantification of honeycombing on high resolution computed tomography Quantification of honeycombing on high resolution computed tomography (HRCT) was assessed by: (i) a radiologist and a pulmonologist at each site, (ii) two radiologists at each site, or (iii) by a central reader at a core (central) laboratory Page 1
2 upon investigator request and before randomization. The radiologists reviewed the HRCT scan images and quantitated the amount of honeycombing at three levels by comparison with a set of standards provided for this study. Extensive honeycombing was defined as that involving >5% of the parenchyma in three or more out of six thoracic zones: Left lung viewed at the levels of tracheal carina, inferior pulmonary veins, and 1 cm above the dome of the diaphragm Right lung viewed at the levels of tracheal carina, inferior pulmonary veins, and 1 cm above the dome of the diaphragm After randomization of the first two patients at each investigational site, the HRCT images from these patients were reviewed by a central reader at a core (central) laboratory to ensure that each site was enrolling patients within the defined extent of honeycombing. Supplementary Methods, Section 3 Exclusion criteria Patients were not enrolled in the BUILD-3 trial if they exhibited severe concomitant illness limiting life expectancy (<); severe restrictive lung disease (forced vital capacity [FVC] <50% of predicted or <1.2 L [formula reported in {E1}], diffusing capacity for carbon monoxide [DL CO ] <30% of predicted [formula reported in {E2}], or residual volume [RV] 120% of predicted [formula reported in {E3}]); obstructive lung disease (forced expiratory volume in 1 second [FEV 1 ] FVC <0.65); a documented, sustained improvement in IPF up to 12 months prior to randomization; Page 2
3 recent pulmonary or upper respiratory tract infection ( 4 weeks prior to randomization); acute or chronic impairment (other than dyspnea) limiting ability to comply with study requirements; chronic heart failure; serum levels of alanine aminotransferase or aspartate aminotransferase >1.5 upper limit of normal; moderate-to-severe hepatic impairment; and, serum creatinine 2.5 mg dl 1. In addition, patients were not enrolled if, within 4 weeks preceding randomization, they received chronic treatment for IPF with: oral corticosteroids (>20 mg per day prednisone or equivalent), immunosuppressive or cytotoxic drugs, antifibrotic drugs, or N-acetylcysteine. Patients treated using glibenclamide (glyburide) and calcineurin inhibitors within 1 week preceding randomization were also not enrolled. Supplementary Methods, Section 4 Study management The BUILD-3 trial was conducted in accordance with the Declaration of Helsinki and its amendments, and applicable regulations and laws. Approval was obtained from all relevant ethics committees and institutional review boards prior to study start. All patients provided written, informed consent. All authors had full access to data and vouch for the accuracy and completeness of data and analyses reported here. A Steering Committee comprising eight academic researchers oversaw trial design, study conduct, approval of statistical analyses, review and interpretation of data, the writing and the decision to publish this manuscript. The BUILD-3 trial had a group sequential design with two interim analyses for superiority and futility, Page 3
4 conducted when 50% and 75% of events were observed. Monitoring of safety data and conduct of planned interim analyses were performed by an independent Data Safety and Monitoring Board. At all safety interim analyses and at both efficacy interim analyses, continuation of the trial was recommended by the Data Safety Monitoring Board. Supplementary Methods, Section 5 Randomization and concealment of treatment allocation Patients were assigned a unique randomization number via a centralized Interactive Randomization System (Cenduit/Fisher Clinical Services AG, Allschwil, Switzerland), which designated which study treatment was to be dispensed at randomization, at each patient visit to the site, and each time a patient s dose was adjusted. The randomization code was generated using Visual Basic 6.0 (Microsoft, Redmond, WA, United States). The investigators, study staff, patients, monitors, and study sponsor remained blinded to treatment assignment until study database closure. Supplementary Methods, Section 6 Detailed schedule of assessments At baseline, assessments included medical history, physical examination, electrocardiogram, vital signs, height, body weight, complete laboratory tests, PFT results and measurement of resting arterial blood gas. At randomization, assessments included medical history, physical examination, concomitant Page 4
5 medications, FVC, DL CO, health-related quality of life using 36-item Short-Form questionnaire (SF-36v2 ) [E4] and the EuroQol Group 5 Dimension Self-Report Questionnaire (EQ-5D) [E5], and baseline dyspnea index [E6]. At monthly intervals, liver function tests were performed as well as 2 weeks after each up-titration of study treatment. Every 4 months, assessments of concomitant medications, vital signs, body weight, FVC, and DL CO were performed until End of Study. At Month 12, assessments of SF 36, EQ-5D, and transition dyspnea index (TDI) [E6] were performed. At each patient s individual End of Study visit (or End of Study Treatment visit in cases of premature discontinuation) assessments included vital signs, body weight, FVC, DL CO, SF 36, EQ-5D, and TDI. Clinical status at BUILD-3 End of Study was obtained for all patients who did not complete the study due to withdrawal of consent. Supplementary Methods, Section 7 Statistical methods for analyses of secondary, exploratory, and safety measures Secondary and exploratory endpoints were evaluated in the All-Randomized Set and summarized descriptively. Treatment comparisons were carried out at the nominal two-sided 0.05 type I error. Dichotomous variables were analyzed using the Fisher exact test. Continuous variables were assessed using the Wilcoxon rank-sum test with asymptotic approximation. Time-to-event variables were analyzed using logrank testing with asymptotic approximation. No correction for multiple testing was performed. Page 5
6 No imputation methods were applied to the proportion of patients who experienced IPF worsening or death at, at End of Study, or to the time to IPF worsening up to End of Study excluding death, and the time to death up to End of Study. Censoring for time to death up to End of Study in patients without an event was performed using the latest available measurements (i.e., from the date of last contact or End of Study visit). For other endpoints, the last post-baseline observation carried forward was used for all patients except in cases of IPF worsening or death. In cases of IPF worsening or death, the worst post-baseline value derived or observed at was used for changes from baseline to in health-related quality of life and forced vital capacity. For TDI and changes in DL CO at the fixed values ( 9 for TDI, 1 mmol kpa 1 min 1 for DL CO ) were used. Safety analyses were performed on the Safety Set, which comprised all randomized patients who received study drug at least once and had at least one safety assessment post baseline. Data were summarized descriptively. Page 6
7 Table E1 Events contributing to IPF worsening or death up to End of Study Events contributing to primary endpoint, n (%) Death (Primary endpoint, all causes) Acute exacerbation of IPF PFT/IPF worsening Confirmed Substituted* *PFT/IPF worsening events with missing confirmation Bosentan (N=407) 158 (38.8) 11 (2.7) 19 (4.7) 128 (31.4) 120 (29.5) 8 (2.0) Placebo (N=209) 94 (45.0) 6 (2.9) 6 (2.9) 82 (39.2) 71 (34.0) 11 (5.3) Page 7
8 Table E2 Changes from baseline to in health-related quality of life measured using the individual domains and health transition score of the SF-36 questionnaire Bosentan (N=407) Mean ± SD Placebo (N=209) Mean ± SD Treatment effect (95% CI) Physical functioning 61.1 ± 25.4* 58.2 ± ± 28.9* 52.8 ± 27.6 ( 3.9, 3.9) Role-physical 63.1 ± ± ± ± 30.9 ( 7.7, 2.2) Pain index 69.9 ± ± ± ± 30.0 ( 4.2, 5.7) General health perceptions 52.1 ± 21.5 ll 48.7 ± ± 24.1 ll 46.9 ± 22.9 ( 6.5, 0.6) Vitality 55.5 ± ± ± ± 24.1 ( 5.4, 2.1) Social functioning 77.6 ± 24.3** 72.5 ± ± 30.5** 69.3 ± 29.7 ( 6.4, 3.4) Page 8
9 Role-emotional 79.3 ± ± ± ± 31.2 ( 8.6, 2.2) Mental health index 73.6 ± ± ± ± 23.5 ( 5.3, 2.2) Health transition score 3.3 ± 0.8* 3.4 ± ± 1.0* 3.2 ± 1.0 ( 0.2, 0.2) *n=379; n=195; n=377; n=196; ll n=376; **n=378; CI, confidence interval Page 9
10 Table E3 Changes from baseline to in health-related quality of life measured using the health state score and visual analog score of the EuroQoL EQ-5D questionnaire Bosentan (N=407) Mean ± SD Placebo (N=209) Mean ± SD Treatment effect (95% CI) Health state score ± 0.185* ± ± 0.386* ± ( 0.098, 0.027) Visual analog score 70.4 ± ± ± ± 23.2 ( 5.4, 2.4) *n=381; n=200; n=374; n=199; CI, confidence interval Page 10
11 Table E4 dyspnea index and transition dyspnea index at Bosentan (N=407) Mean ± SD Placebo (N=209) Mean ± SD Treatment effect (95% CI) dyspnea index Functional impairment 2.8 ± 1.0* 2.7 ± 0.9 Magnitude of task Magnitude of effort 2.6 ± ± 0.9* 2.5 ± ± Mean total score 7.9 ± 2.5 ll 7.6 ± 2.5** Transition dyspnea index 1.7 ± ± ( 0.5, 0.7) *n=402; n=205; n=403; n=206; ll n=400; **n=202; n=383; n=199; CI, confidence interval Page 11
12 Table E5 Treatment-emergent adverse events observed in 5% of bosentan-treated patients Bosentan (N=406) n (%) Placebo (N=209) n (%) Total patients with at least one adverse event 396 (97.5%) 203 (97.1%) Total number of adverse events Worsening idiopathic pulmonary fibrosis 133 (32.8%) 76 (36.4%) Upper respiratory tract infection 114 (28.1%) 61 (29.2%) Cough 79 (19.5%) 52 (24.9%) Dyspnea 63 (15.5%) 24 (11.5%) Bronchitis 46 (11.3%) 31 (14.8%) Fatigue 46 (11.3%) 15 (7.2%) Headache 44 (10.8%) 22 (10.5%) Nasopharyngitis 40 (9.9%) 22 (10.5%) Sinusitis 38 (9.4%) 18 (8.6%) Edema peripheral 37 (9.1%) 23 (11.0%) Lower respiratory tract infection 33 (8.1%) 21 (10.0%) Arthralgia 31 (7.6%) 17 (8.1%) Liver function test abnormal 30 (7.4%) 0 (0.0%) Back pain 27 (6.7%) 19 (9.1%) Nausea 27 (6.7%) 15 (7.2%) Alanine aminotransferase increased 27 (6.7%) 7 (3.3%) Dizziness 25 (6.2%) 17 (8.1%) Chest pain 25 (6.2%) 14 (6.7%) Pneumonia 23 (5.7%) 12 (5.7%) Page 12
13 Aspartate aminotransferase increased 23 (5.7%) 6 (2.9%) Page 13
14 Table E6 Treatment-emergent adverse events leading to discontinuation of study treatment Bosentan (N=406) n (%) Placebo (N=209) n (%) Total patients with at least one adverse event* 190 (46.8%) 92 (44.0%) Total number of adverse events* Worsening idiopathic pulmonary fibrosis 121 (29.8%) 76 (36.4%) Liver function test abnormal 18 (4.4%) 0 (0.0%) Alanine aminotransferase increased 15 (3.7%) 1 (0.5%) Aspartate aminotransferase increased 15 (3.7%) 1 (0.5%) Pulmonary function test decreased 6 (1.5%) 4 (1.9%) Hepatic enzyme increased 5 (1.2%) 0 (0.0%) Dyspnea 4 (1.0%) 0 (0.0%) Pneumonia 3 (0.7%) 2 (1.0%) Respiratory failure 3 (0.7%) 0 (0.0%) Transaminases increased 2 (0.5%) 0 (0.0%) Myocardial infarction 1 (0.2%) 1 (0.5%) Acute respiratory distress syndrome 1 (0.2%) 0 (0.0%) Allergic edema 1 (0.2%) 0 (0.0%) Anemia 1 (0.2%) 0 (0.0%) Angina unstable 1 (0.2%) 0 (0.0%) Bilirubin conjugated increased 1 (0.2%) 0 (0.0%) Blood alkaline phosphatase increased 1 (0.2%) 0 (0.0%) Blood bilirubin increased 1 (0.2%) 0 (0.0%) Cardiac arrest 1 (0.2%) 0 (0.0%) Page 14
15 Cerebral hemorrhage 1 (0.2%) 0 (0.0%) Drug hypersensitivity 1 (0.2%) 0 (0.0%) Fluid retention 1 (0.2%) 0 (0.0%) General physical health deterioration 1 (0.2%) 0 (0.0%) Hepatic cirrhosis 1 (0.2%) 0 (0.0%) Hepatic function abnormal 1 (0.2%) 0 (0.0%) Hepatitis acute 1 (0.2%) 0 (0.0%) Lower respiratory tract infection 1 (0.2%) 0 (0.0%) Lung adenocarcinoma 1 (0.2%) 0 (0.0%) Lymphadenopathy 1 (0.2%) 0 (0.0%) Pancytopenia 1 (0.2%) 0 (0.0%) Pruritus allergic 1 (0.2%) 0 (0.0%) Sjögren s syndrome 1 (0.2%) 0 (0.0%) Urosepsis 1 (0.2%) 0 (0.0%) Clostridium difficile colitis 0 (0.0%) 1 (0.5%) Computerized tomogram abnormal 0 (0.0%) 1 (0.5%) Cough 0 (0.0%) 1 (0.5%) Dementia 0 (0.0%) 1 (0.5%) Hypoxia 0 (0.0%) 1 (0.5%) Lobar pneumonia 0 (0.0%) 1 (0.5%) Lung transplant 0 (0.0%) 1 (0.5%) Memory impairment 0 (0.0%) 1 (0.5%) Mesenteric arteriosclerosis 0 (0.0%) 1 (0.5%) Upper respiratory tract infection 0 (0.0%) 1 (0.5%) Vasculitis 0 (0.0%) 1 (0.5%) *Leading to discontinuation of study treatment. Note: The discontinuation of study Page 15
16 treatment could have been reported at BUILD-3 End of Study, and would not therefore be considered a premature discontinuation of study treatment. Page 16
17 Table E7 Treatment-emergent serious adverse events with frequency >1.5% in bosentantreated patients Bosentan (N=406) n (%) Placebo (N=209) n (%) Total patients with at least one serious adverse 129 (31.8%) 74 (35.4%) event Total number of severe adverse events Worsening idiopathic pulmonary fibrosis 41 (10.1%) 17 (8.1%) Pneumonia 16 (3.9%) 10 (4.8%) Lower respiratory tract infection 7 (1.7%) 5 (2.4%) Coronary artery disease 6 (1.5%) 1 (0.5%) Dyspnea 6 (1.5%) 0 (0.0%) Page 17
18 References E1. Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971;103: E2. Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis 1981;123: E3. Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 1982;18: E4. SF-36v2 Health Survey 2000, 2002 by Medical Outcomes Trust and Quality Metric Incorporated. E5. EuroQoL Group: EuroQoL a new facility for the measurement of health-related quality of life. Health Policy 1990;16: E6. Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85: Page 18
Summary of eligibility criteria for the Phase 3 multinational studies
 SUPPLEMENTAL MATERIAL Table 1 Summary of eligibility criteria for the Phase 3 multinational studies CAPACITY (Studies 004 and 006) ASCEND (Study 016) Age 40 to 80 years Confident IPF diagnosis within the
SUPPLEMENTAL MATERIAL Table 1 Summary of eligibility criteria for the Phase 3 multinational studies CAPACITY (Studies 004 and 006) ASCEND (Study 016) Age 40 to 80 years Confident IPF diagnosis within the
DIAGNOSTIC NOTE TEMPLATE
 DIAGNOSTIC NOTE TEMPLATE SOAP NOTE TEMPLATE WHEN CONSIDERING A DIAGNOSIS OF IDIOPATHIC PULMONARY FIBROSIS (IPF) CHIEF COMPLAINT HISTORY OF PRESENT ILLNESS Consider IPF as possible diagnosis if any of the
DIAGNOSTIC NOTE TEMPLATE SOAP NOTE TEMPLATE WHEN CONSIDERING A DIAGNOSIS OF IDIOPATHIC PULMONARY FIBROSIS (IPF) CHIEF COMPLAINT HISTORY OF PRESENT ILLNESS Consider IPF as possible diagnosis if any of the
Randomized Trial of Acetylcysteine in Idiopathic Pulmonary Fibrosis
 original article Randomized Trial of in Idiopathic Pulmonary Fibrosis The Idiopathic Pulmonary Fibrosis Clinical Research Network* ABSTRACT Background has been suggested as a beneficial treatment for idiopathic
original article Randomized Trial of in Idiopathic Pulmonary Fibrosis The Idiopathic Pulmonary Fibrosis Clinical Research Network* ABSTRACT Background has been suggested as a beneficial treatment for idiopathic
OFEV MEDIA BACKGROUNDER
 OFEV MEDIA BACKGROUNDER 1 What is OFEV (nintedanib*)? 2 How does OFEV (nintedanib*) work? 3 Data overview 4 OFEV (nintedanib*) approval status 1 What is OFEV (nintedanib*)? OFEV (nintedanib*) is a small
OFEV MEDIA BACKGROUNDER 1 What is OFEV (nintedanib*)? 2 How does OFEV (nintedanib*) work? 3 Data overview 4 OFEV (nintedanib*) approval status 1 What is OFEV (nintedanib*)? OFEV (nintedanib*) is a small
Controversies in Clinical Trials. Pirfenidone for Idiopathic Pulmonary Fibrosis (IPF)
 Controversies in Clinical Trials Pirfenidone for Idiopathic Pulmonary Fibrosis (IPF) Controversies to be highlighted by IPF Post-hoc analyses Story Primary end point selection Changing prespecified endpoints
Controversies in Clinical Trials Pirfenidone for Idiopathic Pulmonary Fibrosis (IPF) Controversies to be highlighted by IPF Post-hoc analyses Story Primary end point selection Changing prespecified endpoints
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
ERS 2016 Congress Highlights Interstitial Lung Disease (ILD)
 ERS 216 Congress Highlights Interstitial Lung Disease (ILD) London, UK September 3 rd 7 th 216 The 26 th European Respiratory Society International Congress, (ERS) the largest respiratory meeting in the
ERS 216 Congress Highlights Interstitial Lung Disease (ILD) London, UK September 3 rd 7 th 216 The 26 th European Respiratory Society International Congress, (ERS) the largest respiratory meeting in the
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Experience with the Compassionate Use Program of nintedanib for the treatment of Idiopathic Pulmonary Fibrosis in Argentina
 ORIGINAL 131 RAMR 2017;2:131-135 ISSN 1852-236X Correspondence Gabriela Tabaj gabrielatabaj@gmail.com Received: 11.15.2016 Accepted: 02.03.2017 Experience with the Compassionate Use Program of nintedanib
ORIGINAL 131 RAMR 2017;2:131-135 ISSN 1852-236X Correspondence Gabriela Tabaj gabrielatabaj@gmail.com Received: 11.15.2016 Accepted: 02.03.2017 Experience with the Compassionate Use Program of nintedanib
Connective Tissue Disorder- Associated Interstitial Lung Disease (CTD-ILD) and Updates
 Connective Tissue Disorder- Associated Interstitial Lung Disease (CTD-ILD) and Updates Maria Elena Vega, M.D Assistant Professor of Medicine Lewis Katz School of Medicine at Temple University Nothing to
Connective Tissue Disorder- Associated Interstitial Lung Disease (CTD-ILD) and Updates Maria Elena Vega, M.D Assistant Professor of Medicine Lewis Katz School of Medicine at Temple University Nothing to
Nintedanib and Pirfenidone: New Medications in the Management of Idiopathic Pulmonary Fibrosis
 Nintedanib and Pirfenidone: New Medications in the Management of Idiopathic Pulmonary Fibrosis Brad Zimmermann, PharmD, MBA Pharmacy Grand Rounds May 02, 2017 Rochester, Minnesota 2017 MFMER slide-1 Objectives
Nintedanib and Pirfenidone: New Medications in the Management of Idiopathic Pulmonary Fibrosis Brad Zimmermann, PharmD, MBA Pharmacy Grand Rounds May 02, 2017 Rochester, Minnesota 2017 MFMER slide-1 Objectives
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
#8 - Respiratory System
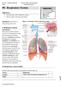 Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
KD : A Phase 2 Trial of KD025 to Assess Safety, Efficacy and Tolerability in Patients with Idiopathic Pulmonary Fibrosis (IPF)
 -207: A Phase 2 Trial of to Assess Safety, Efficacy and Tolerability in Patients with Idiopathic Pulmonary Fibrosis (IPF) K. F. Gibson 1, F. Averill 2, T.E. Albertson 3, D. M. Baratz 4, S. Chaudhary 5,
-207: A Phase 2 Trial of to Assess Safety, Efficacy and Tolerability in Patients with Idiopathic Pulmonary Fibrosis (IPF) K. F. Gibson 1, F. Averill 2, T.E. Albertson 3, D. M. Baratz 4, S. Chaudhary 5,
Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article,, and N-Acetylcysteine for Pulmonary Fibrosis The Idiopathic Pulmonary Fibrosis Clinical Research Network* A bs tr ac t The members
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article,, and N-Acetylcysteine for Pulmonary Fibrosis The Idiopathic Pulmonary Fibrosis Clinical Research Network* A bs tr ac t The members
Clinical Trial Synopsis TL-OPI-516, NCT#
 Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
A case of a patient with IPF treated with nintedanib. Prof. Kreuter and Prof. Heussel
 A case of a patient with IPF treated with nintedanib Prof. Kreuter and Prof. Heussel Case Overview This case describes the history of a patient with IPF who, at the time of diagnosis, had symptoms typical
A case of a patient with IPF treated with nintedanib Prof. Kreuter and Prof. Heussel Case Overview This case describes the history of a patient with IPF who, at the time of diagnosis, had symptoms typical
New Drug Evaluation: Pirfenidone capsules, oral
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Supplementary Online Content
 Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable: Secondary Outcome/Efficacy Variable(s): Statistical Methods:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial. Online Data Supplement
 Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
4/17/2010 C ini n ca c l a Ev E a v l a ua u t a ion o n of o ILD U dat a e t e i n I LDs
 Update in ILDs Diagnosis 101: Clinical Evaluation April 17, 2010 Jay H. Ryu, MD Mayo Clinic, Rochester MN Clinical Evaluation of ILD Outline General aspects of ILDs Classification of ILDs Clinical evaluation
Update in ILDs Diagnosis 101: Clinical Evaluation April 17, 2010 Jay H. Ryu, MD Mayo Clinic, Rochester MN Clinical Evaluation of ILD Outline General aspects of ILDs Classification of ILDs Clinical evaluation
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Case Presentations in ILD. Harold R. Collard, MD Department of Medicine University of California San Francisco
 Case Presentations in ILD Harold R. Collard, MD Department of Medicine University of California San Francisco Outline Overview of diagnosis in ILD Definition/Classification High-resolution CT scan Multidisciplinary
Case Presentations in ILD Harold R. Collard, MD Department of Medicine University of California San Francisco Outline Overview of diagnosis in ILD Definition/Classification High-resolution CT scan Multidisciplinary
Product: Denosumab (AMG 162) Clinical Study Report: month Primary Analysis Date: 21 November 2016 Page 1
 Date: 21 November 2016 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Prolia Name of Active Ingredient: denosumab Title of Study: Randomized, Double-blind,
Date: 21 November 2016 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Prolia Name of Active Ingredient: denosumab Title of Study: Randomized, Double-blind,
2.0 Synopsis. Choline fenofibrate capsules (ABT-335) M Clinical Study Report R&D/06/772. (For National Authority Use Only) Name of Study Drug:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
Regulatory Status FDA-approved indication: Ofev is a kinase inhibitor indicated for the treatment of idiopathic pulmonary fibrosis (IPF) (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.05 Subject: Ofev Page: 1 of 5 Last Review Date: March 17, 2017 Ofev Description Ofev (nintedanib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.45.05 Subject: Ofev Page: 1 of 5 Last Review Date: March 17, 2017 Ofev Description Ofev (nintedanib)
Patient with FVC>90% predicted. Demosthenes Bouros, Vasilios Tzilas University of Athens
 Patient with FVC>90% predicted Demosthenes Bouros, Vasilios Tzilas University of Athens CASE OVERVIEW A 63-year-old, male patient with progressive exertional dyspnoea lasting for 2 years and dry cough
Patient with FVC>90% predicted Demosthenes Bouros, Vasilios Tzilas University of Athens CASE OVERVIEW A 63-year-old, male patient with progressive exertional dyspnoea lasting for 2 years and dry cough
Summary ID# Clinical Study Summary: Study F1J-MC-HMDV
 CT Registry ID# 7108 Page 1 Summary ID# 7108 Clinical Study Summary: Study F1J-MC-HMDV Duloxetine 60 to 120 mg Once Daily Compared with Placebo in the Prevention of Relapse in Generalized Anxiety Disorder
CT Registry ID# 7108 Page 1 Summary ID# 7108 Clinical Study Summary: Study F1J-MC-HMDV Duloxetine 60 to 120 mg Once Daily Compared with Placebo in the Prevention of Relapse in Generalized Anxiety Disorder
Diffuse Interstitial Lung Diseases: Is There Really Anything New?
 : Is There Really Anything New? Sujal R. Desai, MBBS, MD ESTI SPEAKER SUNDAY Society of Thoracic Radiology San Antonio, Texas March 2014 Diffuse Interstitial Lung Disease The State of Play DILDs Is There
: Is There Really Anything New? Sujal R. Desai, MBBS, MD ESTI SPEAKER SUNDAY Society of Thoracic Radiology San Antonio, Texas March 2014 Diffuse Interstitial Lung Disease The State of Play DILDs Is There
Triple kinase inhibitor with phosphodiesterase-5 inhibitor for idiopathic pulmonary fibrosis
 Editorial Triple kinase inhibitor with phosphodiesterase-5 inhibitor for idiopathic pulmonary fibrosis Tomoo Kishaba Department of Respiratory Medicine, Okinawa Chubu Hospital, Uruma City, Okinawa, Japan
Editorial Triple kinase inhibitor with phosphodiesterase-5 inhibitor for idiopathic pulmonary fibrosis Tomoo Kishaba Department of Respiratory Medicine, Okinawa Chubu Hospital, Uruma City, Okinawa, Japan
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials
 ORIGINAL ARTICLE INTERSTITIAL LUNG DISEASE Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials Paul W. Noble 1, Carlo Albera 2, Williamson Z.
ORIGINAL ARTICLE INTERSTITIAL LUNG DISEASE Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials Paul W. Noble 1, Carlo Albera 2, Williamson Z.
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Study No.: LOV Title: Rationale: Phase: IIB Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary-
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Diagnosing Idiopathic Pulmonary Fibrosis on Evidence-Based Guidelines
 Diagnosing Idiopathic Pulmonary Fibrosis on Evidence-Based Guidelines Rebecca Keith, MD Assistant Professor, Division of Pulmonary and Critical Care Medicine National Jewish Health, Denver, CO Objectives
Diagnosing Idiopathic Pulmonary Fibrosis on Evidence-Based Guidelines Rebecca Keith, MD Assistant Professor, Division of Pulmonary and Critical Care Medicine National Jewish Health, Denver, CO Objectives
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs.
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs.
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Hunninghake GM, Hatabu H, Okajima Y, et al. MUC5B promoter
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. GENERIC DRUG NAME / COMPOUND NUMBER: Tofacitinib / CP-690,550
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. GENERIC DRUG NAME / COMPOUND NUMBER: Tofacitinib / CP-690,550
Pulmonary Function Testing: Concepts and Clinical Applications. Potential Conflict Of Interest. Objectives. Rationale: Why Test?
 Pulmonary Function Testing: Concepts and Clinical Applications David M Systrom, MD Potential Conflict Of Interest Nothing to disclose pertinent to this presentation BRIGHAM AND WOMEN S HOSPITAL Harvard
Pulmonary Function Testing: Concepts and Clinical Applications David M Systrom, MD Potential Conflict Of Interest Nothing to disclose pertinent to this presentation BRIGHAM AND WOMEN S HOSPITAL Harvard
New Horizons The Future of IPF and ILD
 New Horizons The Future of IPF and ILD Talmadge E. King, Jr., M.D. Julius R. Krevans Distinguished Professorship in Internal Medicine Chair, Department of Medicine University of California San Francisco
New Horizons The Future of IPF and ILD Talmadge E. King, Jr., M.D. Julius R. Krevans Distinguished Professorship in Internal Medicine Chair, Department of Medicine University of California San Francisco
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Synopsis. Adalimumab M Clinical Study Report R&D/09/060. (For National Authority Use Only) to Part of Dossier: Name of Study Drug:
 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Volume: (For National Authority Use Only) Name of Active Ingredient: Page: Title of Study: A Multi-Center,
Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Volume: (For National Authority Use Only) Name of Active Ingredient: Page: Title of Study: A Multi-Center,
Clinical Trial Results Database Page 1
 Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis
 The new england journal of medicine original article A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis Talmadge E. King, Jr., M.D., Williamson Z. Bradford, M.D., Ph.D., Socorro
The new england journal of medicine original article A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis Talmadge E. King, Jr., M.D., Williamson Z. Bradford, M.D., Ph.D., Socorro
PFTs ACOI Board Review 2018
 PFTs ACOI Board Review 2018 Thomas F. Morley, DO, MACOI, FCCP, FAASM Professor of Medicine Chairman Department of Internal Medicine Director of the Division of Pulmonary, Critical Care and Sleep Medicine
PFTs ACOI Board Review 2018 Thomas F. Morley, DO, MACOI, FCCP, FAASM Professor of Medicine Chairman Department of Internal Medicine Director of the Division of Pulmonary, Critical Care and Sleep Medicine
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
Therapies for Idiopathic Pulmonary Fibrosis Pharmacologic, Non-Pharmacologic
 Therapies for Idiopathic Pulmonary Fibrosis Pharmacologic, Non-Pharmacologic Amy Olson, MD, MSPH Associate Professor, Division of Pulmonary and Critical Care Medicine National Jewish Health, Denver, CO
Therapies for Idiopathic Pulmonary Fibrosis Pharmacologic, Non-Pharmacologic Amy Olson, MD, MSPH Associate Professor, Division of Pulmonary and Critical Care Medicine National Jewish Health, Denver, CO
Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis
 Thorax Online First, published on March 22, 2012 as 10.1136/thoraxjnl-2011-201184 Interstitial lung disease < Additional materials are published online only. To view these files please visit the journal
Thorax Online First, published on March 22, 2012 as 10.1136/thoraxjnl-2011-201184 Interstitial lung disease < Additional materials are published online only. To view these files please visit the journal
Idiopathic pulmonary fibrosis (IPF) is a
 Eur Respir J 2011; 38: 176 183 DOI: 10.1183/09031936.00114010 CopyrightßERS 2011 Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema S.L. Schmidt*,
Eur Respir J 2011; 38: 176 183 DOI: 10.1183/09031936.00114010 CopyrightßERS 2011 Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema S.L. Schmidt*,
UMEC/VI vs. UMEC in subjects who responded to UMEC UMEC/VI vs. VI in subjects who responded to VI
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis
 ORIGINAL ARTICLE INTERSTITIAL LUNG DISEASES FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis Ganesh Raghu 1, Mary
ORIGINAL ARTICLE INTERSTITIAL LUNG DISEASES FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis Ganesh Raghu 1, Mary
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW
 SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW Lung disease can be a serious complication of scleroderma. The two most common types of lung disease in patients with scleroderma are interstitial
SCLERODERMA LUNG DISEASE: WHAT THE PATIENT SHOULD KNOW Lung disease can be a serious complication of scleroderma. The two most common types of lung disease in patients with scleroderma are interstitial
Sponsor Novartis. Generic Drug Name. NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia
 Page 1 Sponsor Novartis Generic Drug Name NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia Approved Indication Investigational. Study Number CA2206 Title A
Page 1 Sponsor Novartis Generic Drug Name NA (not existing yet) Therapeutic Area of Trial Parkinson s Disease L-dopa induced dyskinesia Approved Indication Investigational. Study Number CA2206 Title A
This was a randomized, double-blind, placebo-controlled, fixed-dose, parallel-group study.
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: HMR3647A_4019. Study Code: Telithromycin. Generic drug name:
 Sponsor/company: Generic drug name: These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription
Sponsor/company: Generic drug name: These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription
Clinical Trial Synopsis
 Clinical Trial Synopsis Title of Study: A Phase III, Open-Label, Fixed-Dose Study to Determine the Safety of Long-Term Administration of TAK-375 in Subjects With Chronic Insomnia Protocol Number: Name
Clinical Trial Synopsis Title of Study: A Phase III, Open-Label, Fixed-Dose Study to Determine the Safety of Long-Term Administration of TAK-375 in Subjects With Chronic Insomnia Protocol Number: Name
2.0 Synopsis. Adalimumab M Clinical Study Report Final R&D/15/1093. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
Respiratory Disease. Dr Amal Damrah consultant Neonatologist and Paediatrician
 Respiratory Disease Dr Amal Damrah consultant Neonatologist and Paediatrician Signs and Symptoms of Respiratory Diseases Cardinal Symptoms Cough Sputum Hemoptysis Dyspnea Wheezes Chest pain Signs and Symptoms
Respiratory Disease Dr Amal Damrah consultant Neonatologist and Paediatrician Signs and Symptoms of Respiratory Diseases Cardinal Symptoms Cough Sputum Hemoptysis Dyspnea Wheezes Chest pain Signs and Symptoms
NINTEDANIB MEDIA BACKGROUNDER
 NINTEDANIB MEDIA BACKGROUNDER 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. International treatment guidelines for IPF 1. What is nintedanib? Nintedanib (OFEV a ) is a small molecule
NINTEDANIB MEDIA BACKGROUNDER 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. International treatment guidelines for IPF 1. What is nintedanib? Nintedanib (OFEV a ) is a small molecule
Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD)
 Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD) A Pulmonary Diagnosis Form is filled out by the reviewer for all medical records that are sent to them for review by the CSCC.
Question by Question (QXQ) Instructions for the Pulmonary Diagnosis Form (PLD) A Pulmonary Diagnosis Form is filled out by the reviewer for all medical records that are sent to them for review by the CSCC.
A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS
 A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS A STRATEGY FOR DEVELOPING AN IPF MANAGEMENT PLAN BASED ON REALISTIC PATIENT GOALS Indication Esbriet (pirfenidone) is indicated for the treatment of
A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS A STRATEGY FOR DEVELOPING AN IPF MANAGEMENT PLAN BASED ON REALISTIC PATIENT GOALS Indication Esbriet (pirfenidone) is indicated for the treatment of
Lung-Volume Reduction Surgery ARCHIVED
 Lung-Volume Reduction Surgery ARCHIVED Policy Number: Original Effective Date: MM.06.008 04/15/2005 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST 03/22/2013 Section: Surgery Place(s) of
Lung-Volume Reduction Surgery ARCHIVED Policy Number: Original Effective Date: MM.06.008 04/15/2005 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST 03/22/2013 Section: Surgery Place(s) of
Progress in Idiopathic Pulmonary Fibrosis
 Progress in Idiopathic Pulmonary Fibrosis David A. Lynch, MB Disclosures Progress in Idiopathic Pulmonary Fibrosis David A Lynch, MB Consultant: t Research support: Perceptive Imaging Boehringer Ingelheim
Progress in Idiopathic Pulmonary Fibrosis David A. Lynch, MB Disclosures Progress in Idiopathic Pulmonary Fibrosis David A Lynch, MB Consultant: t Research support: Perceptive Imaging Boehringer Ingelheim
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Update on Therapies for Idiopathic Pulmonary Fibrosis. Outline
 Update on Therapies for Idiopathic Pulmonary Fibrosis Paul Wolters Associate Professor University of California, San Francisco Outline Classification of Interstitial lung disease Clinical classification
Update on Therapies for Idiopathic Pulmonary Fibrosis Paul Wolters Associate Professor University of California, San Francisco Outline Classification of Interstitial lung disease Clinical classification
IPF AND OTHER FIBROSING LUNG DISEASE: WHAT DRUGS MIGHT WORK AND ON WHOM DO THEY W ORK?
 IPF AND OTHER FIBROSING LUNG DISEASE: WHAT DRUGS MIGHT WORK AND ON WHOM DO THEY W ORK? KEVIN K. BROWN, MD PROFESSOR AND VICE CHAIRMAN, DEPARTMENT OF MEDICINE NATIONAL JEWISH HEALTH DENVER, CO Kevin K.
IPF AND OTHER FIBROSING LUNG DISEASE: WHAT DRUGS MIGHT WORK AND ON WHOM DO THEY W ORK? KEVIN K. BROWN, MD PROFESSOR AND VICE CHAIRMAN, DEPARTMENT OF MEDICINE NATIONAL JEWISH HEALTH DENVER, CO Kevin K.
Indian Journal of Basic & Applied Medical Research; September 2013: Issue-8, Vol.-2, P
 Original article: Study of pulmonary function in different age groups Dr.Geeta J Jagia*,Dr.Lalita Chandan Department of Physiology, Seth GS Medical College, Mumbai, India *Author for correspondence: drgrhegde@gmail.com
Original article: Study of pulmonary function in different age groups Dr.Geeta J Jagia*,Dr.Lalita Chandan Department of Physiology, Seth GS Medical College, Mumbai, India *Author for correspondence: drgrhegde@gmail.com
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Pulmonary manifestations of CTDs Diagnosis, differential diagnosis and treatment
 Prague, June 2014 Pulmonary manifestations of CTDs Diagnosis, differential diagnosis and treatment Katerina M. Antoniou, MD, PhD As. Professor in Thoracic Medicine ERS ILD Group Secretary Medical School,
Prague, June 2014 Pulmonary manifestations of CTDs Diagnosis, differential diagnosis and treatment Katerina M. Antoniou, MD, PhD As. Professor in Thoracic Medicine ERS ILD Group Secretary Medical School,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/573. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
Idiopathic pulmonary fibrosis (IPF) is a progressive. Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes
 Eur Respir Rev 2012; 21: 124, 161 167 DOI: 10.1183/09059180.00001112 CopyrightßERS 2012 REVIEW: IPF Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes Vincent
Eur Respir Rev 2012; 21: 124, 161 167 DOI: 10.1183/09059180.00001112 CopyrightßERS 2012 REVIEW: IPF Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes Vincent
Official ATS/ERS/JRS/ALAT Clinical Practice Guidelines: Treatment of Idiopathic Pulmonary Fibrosis
 Official ATS/ERS/JRS/ALAT Clinical Practice Guidelines: Treatment of Idiopathic Pulmonary Fibrosis An Update of the 2011 Clinical Practice Guideline Online Supplement Ganesh Raghu, Bram Rochwerg, Yuan
Official ATS/ERS/JRS/ALAT Clinical Practice Guidelines: Treatment of Idiopathic Pulmonary Fibrosis An Update of the 2011 Clinical Practice Guideline Online Supplement Ganesh Raghu, Bram Rochwerg, Yuan
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Niaspan Name of Active Ingredient: Page: Niacin extended-release
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
TBLB is not recommended as the initial biopsy option in cases of suspected IPF and is unreliable in the diagnosis of rare lung disease (other than
 TBLB is not recommended as the initial biopsy option in cases of suspected IPF and is unreliable in the diagnosis of rare lung disease (other than PAP) BAL is not required as a diagnostic tool in patients
TBLB is not recommended as the initial biopsy option in cases of suspected IPF and is unreliable in the diagnosis of rare lung disease (other than PAP) BAL is not required as a diagnostic tool in patients
Title 1 Descriptive title identifying the study design, population, interventions, and, if applicable, trial acronym
 SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
Interstitial Lung Disease ILD: Definition
 Interstitial Lung Disease 2007 Paul F. Simonelli,, MD, PhD, FCCP Clinical Director Center for Interstitial Lung Disease Columbia University Medical Center 1. ILD is not one disorder ILD: Definition 2.
Interstitial Lung Disease 2007 Paul F. Simonelli,, MD, PhD, FCCP Clinical Director Center for Interstitial Lung Disease Columbia University Medical Center 1. ILD is not one disorder ILD: Definition 2.
Title A Phase II study of oral LBH589 in adult patients with refractory cutaneous T-Cell lymphoma
 Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Development and validity testing of an IPF-specific version of the St George s Respiratory Questionnaire
 < Additional data are published online only. To view these files please visit the journal online (http://thorax.bmj.com). 1 School of Nursing, Faculty of Health and Social Care, University of Salford,
< Additional data are published online only. To view these files please visit the journal online (http://thorax.bmj.com). 1 School of Nursing, Faculty of Health and Social Care, University of Salford,
Efficacy Study of Zoledronic Acid and Teriparatide Combination Therapy in Women With Osteoporosis
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CZOL446H2409 Previous Study Return to List Next Study Efficacy Study of Zoledronic Acid and Combination Therapy in Women With
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CZOL446H2409 Previous Study Return to List Next Study Efficacy Study of Zoledronic Acid and Combination Therapy in Women With
INTERSTITIAL LUNG DISEASES: FOCUS ON IDIOPATHIC PULMONARY FIBROSIS (IPF)
 INTERSTITIAL LUNG DISEASES: FOCUS ON IDIOPATHIC PULMONARY FIBROSIS (IPF) Marilyn K. Glassberg Csete, M.D. Professor of Medicine, Surgery, and Pediatrics Director, Interstitial and Rare Lung Disease Program
INTERSTITIAL LUNG DISEASES: FOCUS ON IDIOPATHIC PULMONARY FIBROSIS (IPF) Marilyn K. Glassberg Csete, M.D. Professor of Medicine, Surgery, and Pediatrics Director, Interstitial and Rare Lung Disease Program
Differential diagnosis
 Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Current approaches to the diagnosis and treatment of idiopathic pulmonary fibrosis in Europe: the AIR survey
 REVIEW IDIOPATHIC PULMONARY FIBROSIS Current approaches to the diagnosis and treatment of idiopathic pulmonary fibrosis in Europe: the AIR survey Vincent Cottin 1,2 Affiliations: 1 Hospices Civils de Lyon,
REVIEW IDIOPATHIC PULMONARY FIBROSIS Current approaches to the diagnosis and treatment of idiopathic pulmonary fibrosis in Europe: the AIR survey Vincent Cottin 1,2 Affiliations: 1 Hospices Civils de Lyon,
PNEUMOLOGIA 2018 Milano, giugno 2018 INTERSTIZIOPATIE E MALATTIE RARE. Il futuro dell IPF: dove stiamo andando. Carlo Albera
 PNEUMOLOGIA 2018 Milano, 14 16 giugno 2018 INTERSTIZIOPATIE E MALATTIE RARE Il futuro dell : dove stiamo andando Carlo Albera Università di Torino, Scuola di Medicina Dipartimento di Scienze Cliniche e
PNEUMOLOGIA 2018 Milano, 14 16 giugno 2018 INTERSTIZIOPATIE E MALATTIE RARE Il futuro dell : dove stiamo andando Carlo Albera Università di Torino, Scuola di Medicina Dipartimento di Scienze Cliniche e
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective:
 GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
INTERSTITIAL LUNG DISEASE. Radhika Reddy MD Pulmonary/Critical Care Long Beach VA Medical Center January 5, 2018
 INTERSTITIAL LUNG DISEASE Radhika Reddy MD Pulmonary/Critical Care Long Beach VA Medical Center January 5, 2018 Interstitial Lung Disease Interstitial Lung Disease Prevalence by Diagnosis: Idiopathic Interstitial
INTERSTITIAL LUNG DISEASE Radhika Reddy MD Pulmonary/Critical Care Long Beach VA Medical Center January 5, 2018 Interstitial Lung Disease Interstitial Lung Disease Prevalence by Diagnosis: Idiopathic Interstitial
Imaging: how to recognise idiopathic pulmonary fibrosis
 REVIEW IDIOPATHIC PULMONARY FIBROSIS Imaging: how to recognise idiopathic pulmonary fibrosis Anand Devaraj Affiliations: Dept of Radiology, St George s Hospital, London, UK. Correspondence: Anand Devaraj,
REVIEW IDIOPATHIC PULMONARY FIBROSIS Imaging: how to recognise idiopathic pulmonary fibrosis Anand Devaraj Affiliations: Dept of Radiology, St George s Hospital, London, UK. Correspondence: Anand Devaraj,
C.S. HAWORTH 1, A. WANNER 2, J. FROEHLICH 3, T. O'NEAL 3, A. DAVIS 4, I. GONDA 3, A. O'DONNELL 5
 Inhaled Liposomal Ciprofloxacin in Patients With Non-Cystic Fibrosis Bronchiectasis and Chronic Pseudomonas aeruginosa: Results From Two Parallel Phase III Trials (ORBIT-3 and -4) C.S. HAWORTH 1, A. WANNER
Inhaled Liposomal Ciprofloxacin in Patients With Non-Cystic Fibrosis Bronchiectasis and Chronic Pseudomonas aeruginosa: Results From Two Parallel Phase III Trials (ORBIT-3 and -4) C.S. HAWORTH 1, A. WANNER
Differential diagnosis
 Differential diagnosis Idiopathic pulmonary fibrosis (IPF) is part of a large family of idiopathic interstitial pneumonias (IIP), one of four subgroups of interstitial lung disease (ILD). Differential
Differential diagnosis Idiopathic pulmonary fibrosis (IPF) is part of a large family of idiopathic interstitial pneumonias (IIP), one of four subgroups of interstitial lung disease (ILD). Differential
