Select Inhaled Respiratory Agents
|
|
|
- Merilyn Waters
- 5 years ago
- Views:
Transcription
1 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided in the applicable contract. Medical technology is constantly evolving, and we reserve the right to review and update Medical Policy periodically. Inhaled Corticosteroid Products Based on review of available data, the Company may consider the inhaled corticosteroid products Aerospan (flunisolide), Alvesco (ciclesonide), Asmanex Twisthaler (mometasone furoate), Asmanex HFA (mometasone furoate), and Pulmicort Flexhaler (budesonide) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Aerospan (flunisolide), Alvesco (ciclesonide), Asmanex Twisthaler (mometasone furoate), Asmanex HFA (mometasone furoate), or Pulmicort Flexhaler (budesonide) when the following criterion is met: There is clinical evidence or patient history that suggests the use of Arnuity Ellipta (fluticasone furoate), Flovent Diskus (fluticasone propionate), Flovent HFA (fluticasone propionate), or QVAR (beclomethasone dipropionate) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Aerospan (flunisolide), Alvesco (ciclesonide), Asmanex Twisthaler (mometasone furoate), Asmanex HFA (mometasone furoate), or Pulmicort Flexhaler (budesonide) WITHOUT clinical evidence or patient history that suggests the use of Arnuity Ellipta (fluticasone furoate), Flovent Diskus (fluticasone propionate), Flovent HFA (fluticasone propionate), or QVAR (beclomethasone propionate) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Inhaled Long Acting Beta Agonists (LABAs) Based on review of available data, the Company may consider the inhaled long acting beta agonists Arcapta Neohaler (indacaterol) and Foradil Aerolizer (formoterol fumarate) to be eligible for coverage when the below patient selection criterion is met: Page 1 of 7
2 Coverage eligibility will be considered for Arcapta Neohaler (indacaterol) or Foradil Aerolizer (formoterol fumarate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of Striverdi Respimat (olodaterol) or Serevent Diskus (salmeterol xinafoate) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Arcapta Neohaler (indacaterol) or Foradil Aerolizer (formoterol fumarate) WITHOUT clinical evidence or patient history that suggests the use of Striverdi Respimat (olodaterol) or Serevent Diskus (salmeterol xinafoate) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Nebulized Long Acting Beta Agonists (LABAs) Based on review of available data, the Company may consider the nebulized long acting beta agonists Brovana (arformoterol tartrate) and Perforomist (formoterol fumarate) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Brovana (arformoterol tartrate) or Perforomist (formoterol fumarate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of TWO of the following preferred bronchodilating agents for COPD (chronic obstructive pulmonary disease): Serevent Diskus (salmeterol xinafoate), Spiriva Respimat (tiotropium bromide), Spiriva HandiHaler (tiotropium bromide), Anoro Ellipta (umeclidinium/vilanterol), Striverdi Respimat (olodaterol), or Incruse Ellipta (umeclidinium) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Brovana (arformoterol tartrate) or Perforomist (formoterol fumarate) WITHOUT clinical evidence or patient history that suggests the use of TWO of the following preferred bronchodilating agents for COPD (chronic obstructive pulmonary disease): Serevent Diskus (salmeterol xinafoate), Spiriva Respimat (tiotropium bromide), Spiriva HandiHaler (tiotropium bromide), Anoro Ellipta (umeclidinium/vilanterol), Striverdi Respimat (olodaterol), or Incruse Ellipta (umeclidinium) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Page 2 of 7
3 Inhaled Corticosteroid/Long Acting Beta Agonist Combination Products (ICS/LABAs) Based on review of available data, the Company may consider the inhaled corticosteroid/long acting beta agonist combination product Dulera (mometasone furoate/formoterol furoate) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Dulera (mometasone furoate/formoterol furoate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of Advair Diskus (fluticasone propionate/salmeterol), Advair HFA (fluticasone propionate/salmeterol), Breo Ellipta (fluticasone furoate/vilanterol), or Symbicort (budesonide/formoterol fumarate dihydrate) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Dulera (mometasone furoate/formoterol furoate) WITHOUT clinical evidence or patient history that suggests the use of Advair Diskus (fluticasone propionate/salmeterol), Advair HFA (fluticasone proprionate/salmeterol), Breo Ellipta (fluticasone furoate/vilanterol), or Symbicort (budesonide/formoterol fumarate dihydrate) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Inhaled Long Acting Antimuscarinic Agents (LAMAs) Based on review of available data, the Company may consider the inhaled long acting antimuscarinic agents Tudorza Pressair (aclidinium bromide) and Seebri Neohaler (glycopyrrolate) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Tudorza Pressair (aclidinium bromide) or Seebri Neohaler (glycopyrrolate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of Spiriva Respimat (tiotropium bromide), Spiriva HandiHaler (tiotropium bromide), or Incruse Ellipta (umeclidinium) will be/was ineffective or will/did cause an adverse reaction to the patient. Page 3 of 7
4 Based on review of available data, the Company considers the use of Tudorza Pressair (aclidinium bromide) or Seebri Neohaler (glycopyrrolate) WITHOUT clinical evidence or patient history that suggests the use of Spiriva Respimat (tiotropium bromide), Spiriva HandiHaler (tiotropium bromide), or Incruse Ellipta (umeclidinium) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Inhaled Long Acting Antimuscarinic Agent/Long Acting Beta Agonist Combination Products (LAMA/LABAs) Based on review of available data, the Company may consider the inhaled long acting antimuscarinic agent/long acting beta agonist combination products Stiolto Respimat (tiotropium bromide/olodaterol), Bevespi Aerosphere (glycopyrrolate/formoterol fumarate), and Utibron Neohaler (indacaterol/glycopyrrolate) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Stiolto Respimat (tiotropium bromide/olodaterol), Bevespi Aerosphere (glycopyrrolate/formoterol fumarate), or Utibron Neohaler (indacaterol/glycopyrrolate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of Anoro Ellipta (umeclidinium/vilanterol) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Stiolto Respimat (tiotropium bromide/olodaterol), Bevespi Aerosphere (glycopyrrolate/formoterol fumarate), or Utibron Neohaler (indacaterol/glycopyrrolate) WITHOUT clinical evidence or patient history that suggests the use of Anoro Ellipta (umeclidinium/vilanterol) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Page 4 of 7
5 Inhaled Short Acting Beta Agonists (SABAs) Based on review of available data, the Company may consider the short acting beta agonists Proventil HFA (albuterol sulfate) and Xopenex HFA (levalbuterol tartrate) to be eligible for coverage when the below patient selection criterion is met: Coverage eligibility will be considered for Proventil HFA (albuterol sulfate) or Xopenex HFA (levalbuterol tartrate) when the following criterion is met: There is clinical evidence or patient history that suggests the use of ProAir RespiClick (albuterol sulfate), ProAir HFA (albuterol sulfate) or Ventolin HFA (albuterol sulfate) will be/was ineffective or will/did cause an adverse reaction to the patient. Based on review of available data, the Company considers the use of Proventil HFA (albuterol sulfate) or Xopenex HFA (levalbuterol tartrate) WITHOUT clinical evidence or patient history that suggests the use of ProAir RespiClick (albuterol sulfate), ProAir HFA (albuterol sulfate), or Ventolin HFA (albuterol sulfate) will be/was ineffective or will/did cause an adverse reaction to the patient to be not medically necessary.** Schematic Class Non-Preferred Products Preferred Products Inhaled Corticosteroids Aerospan Alvesco Arnuity Ellipta Flovent Diskus Asmanex Twisthaler Flovent HFA Asmanex HFA QVAR Pulmicort Flexhaler Inhaled Long Acting Beta Arcapta Neohaler Striverdi Respimat Agonists (LABAs) Nebulized Long Acting Beta Agonists (LABAs) Inhaled Corticosteroids/Long Acting Beta Agonists (ICS/LABAs) Inhaled Long Acting Antimuscarinic Agents (LAMAs) Inhaled Long Acting Antimuscarinic Agents/Long Acting Beta Agonists Foradil Aerolizer Brovana Perforomist Dulera Tudorza Pressair Seebri Neohaler Stiolto Respimat Utibron Neohaler Bevespi Aerosphere Serevent Diskus Serevent Diskus Spiriva Respimat Spiriva HandiHaler Anoro Ellipta Striverdi Respimat Incruse Ellipta Advair Diskus Advair HFA Breo Ellipta Symbicort Spiriva Respimat Spiriva HandiHaler Incruse Ellipta Anoro Ellipta Page 5 of 7
6 (LAMA/LABA) Inhaled Short Acting Beta Agonists (SABAs) Proventil HFA Xopenex HFA ProAir RespiClick ProAir HFA Ventolin HFA Background/Overview The various products mentioned in this policy are approved for use in COPD and/or asthma patients, depending on the particular product. Rationale/Source The patient selection criteria presented in this policy takes into consideration clinical evidence or patient history that suggests the preferred products listed in this policy will be ineffective or cause an adverse reaction to the patient. Based on a review of the available data and in the absence of any of the caveats mentioned, there is no advantage of using the non-preferred agents mentioned in this policy over the preferred agents mentioned in this policy. References 1. Advair Diskus [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated April Advair HFA [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated December Aerospan [package insert]. Meda Pharmaceuticals. Somerset, New Jersey. Updated June Alvesco [package insert]. Sunovion Pharmaceutical s, Inc. Marlborough, Massachusetts. January Anoro Ellipta [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated February Arcapta Neohaler [package insert]. Novartis. East Hanover, New Jersey. Updated September Arnuity Ellipta [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated November Asmanex HFA [package insert]. Merck and Company. Whitehouse Station, New Jersey. Updated July Asmanex Twisthaler [package insert]. Merck and Company. Whitehouse Station, New Jersey. September Breo Ellipta [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated July Brovana [package insert]. Sunovion Pharmaceuticals. Marlborough, Massachusetts. Updated February Dulera [package insert]. Merck. Whitehouse Station, New Jersey. Updated July Flovent Diskus [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated May Flovent HFA [package insert]. GlaxoSmithKl ine. Research Triangle Park, North Carolina. Updated July Foradil Aerolizer [package insert]. Merck. Whitehouse Station, New Jersey. Updated September Incruse Ellipta {package insert}. GlaxoSmithKline. Research Triangle Park, North Carolina. Up dated February Perforomist [package insert]. Mylan Specialty. Morgantown, West Virginia. Updated March ProAir HFA [package insert]. Teva. Frazer, Pennsylvania. Updated June ProAir Respiclick [package insert]. Frazer, Pennsylvania. Updated April Proventil HFA [package insert]. Merck. Whitehouse Station, New Jersey. Updated December Pulmicort Flexhaler [package insert[. AstraZeneca. Wilmington, Delaware. Updated July Qvar [package insert]. Teva Respiratory, LLC. Horsham, Pennsylvania. Updated July Seebri Neohaler. [package insert]. Novartis. East Hanover, New Jersey. Updated January Serevent Diskus [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Update unknown. 25. Spiriva Respimat [package insert]. Boehringer Ingelheim. Ridgefield, Connecticut. Updated June Spiriva Handihaler [package insert]. Boehringer Ingelheim. Ridgefield, Connecticut. Updated January Stiolto Respimat [package insert]. AstraZeneca. Wilmington, Delaware. Upd ated June Striverdi Respimat [package insert]. Boehringer Ingelheim. Ridgefield, Connecticut. Updated June Symbicort [package insert]. AstraZeneca. Wilmington, Delaware. Updated February Tudorza Pressair [package insert]. AstraZeneca. Wil mington, Delaware. Updated March Utibron Neohaler [package insert]. Novartis. East Hanover, New Jersey. Updated January Ventolin HFA [package insert]. GlaxoSmithKline. Research Triangle Park, North Carolina. Updated December Page 6 of 7
7 33. Xopenex HFA [package insert]. Sunovion. Marlborough, Massachusetts. Updated March Bevespi Aerosphere [package insert]. AstraZeneca. Wilmington, Delaware. Updated April Policy History 09/08/2016 Medical Policy Committee review 09/21/2016 Medical Policy Implementation Committee approval. New policy. Next Scheduled Review Date: 09/2017 **Medically Necessary (or Medical Necessity ) - Health care services, treatment, procedures, equipment, drugs, devices, items or supplies that a Provider, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, eva luating, diagnosing or treating an illness, injury, disease or its symptoms, and that are: A. In accordance with nationally accepted standards of medical practice; B. Clinically appropriate, in terms of type, frequency, extent, level of care, site and duration, and considered effective for th e patient's illness, injury or disease; and C. Not primarily for the personal comfort or convenience of the patient, physician or other health care provider, and not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient's illness, injury or disease. For these purposes, nationally accepted standards of medical practice means standards that are based on credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community, Physician Specialty Society recommendations and the views of Physicians practicing in relevant clinical areas and any other relevant factors. Indicated trademarks are the registered trademarks of their respective owners. NOTICE: Medical Policies are scientific based opinions, provided solely for coverage and informational purposes. Medical Policies should not be construed to suggest that the Company recommends, advocates, requires, encourages, or discourages any particular treatment, procedure, or service, or any particular course of treatment, procedure, or service. Page 7 of 7
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
QUANTITY LIMIT CRITERIA. BROVANA (arformoterol tartrate) SEREVENT DISKUS (salmeterol) STRIVERDI RESPIMAT (olodaterol)
 Carelirst. +.V Family of health care plans DRUG CLASS COMBINATIONS QUANTITY LIMIT CRITERIA LONG ACTING BETA2-ADRENERGIC AGONIST, ORAL INHALATION BRAND NAME (generic) LONG-ACTING BETA2-ADRENERGIC AGONISTS:
Carelirst. +.V Family of health care plans DRUG CLASS COMBINATIONS QUANTITY LIMIT CRITERIA LONG ACTING BETA2-ADRENERGIC AGONIST, ORAL INHALATION BRAND NAME (generic) LONG-ACTING BETA2-ADRENERGIC AGONISTS:
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
AIRDUO RESPICLICK (fluticasone-salmeterol) aerosol DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol
 DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
The Medical Letter. on Drugs and Therapeutics
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 07/05/18 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FASENRA (benralizumab)
 FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS
 Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients
 Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients Maren A. McGurran, PharmD, BCPS; Lisa M. Richter, PharmD, BCPS, BCCCP; Nathan D. Leedahl,
Impact of a Comprehensive COPD Therapeutic Interchange Program on 30-Day Readmission Rates in Hospitalized Patients Maren A. McGurran, PharmD, BCPS; Lisa M. Richter, PharmD, BCPS, BCCCP; Nathan D. Leedahl,
COPD Update. Plus New and Improved Products for Inhaled Therapy. Catherine Bourg Rebitch, PharmD, BCACP Clinical Associate Professor
 COPD Update Plus New and Improved Products for Inhaled Therapy Catherine Bourg Rebitch, PharmD, BCACP Clinical Associate Professor Disclosure The presenter has nothing to disclose concerning possible financial
COPD Update Plus New and Improved Products for Inhaled Therapy Catherine Bourg Rebitch, PharmD, BCACP Clinical Associate Professor Disclosure The presenter has nothing to disclose concerning possible financial
STRIVERDI RESPIMAT (olodaterol hcl) aerosol
 STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
Drug Effectiveness Review Project Summary Report
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Clinical Policy: Roflumilast (Daliresp) Reference Number: CP.PMN.46 Effective Date: Last Review Date: 08.18
 Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11.01.11 Last Review Date: 08.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11.01.11 Last Review Date: 08.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
COPD Medications Coverage Summary Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes
 COPD Medications Coverage Summary Drug Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes Ventolin MDI + generics Yes Yes Ventolin Diskus NO NO Yukon Pharmacare/Chronic Disease Program
COPD Medications Coverage Summary Drug Non-Insured Health Benefits Coverage SABA Bricanyl turbuhaler Yes Yes Ventolin MDI + generics Yes Yes Ventolin Diskus NO NO Yukon Pharmacare/Chronic Disease Program
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled Corticosteroid Dose Comparison in Asthma
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
Drug Class Monograph
 Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
MDI Bonanza. Dwayne Griffin, DO
 MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Seebri Neohaler) Reference Number: CP.CPA.150 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Seebri Neohaler) Reference Number: CP.CPA.150 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management. Colleen Sakon, PharmD BCPS September 27, 2018
 Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management Colleen Sakon, PharmD BCPS September 27, 2018 Disclosures I have no actual or potential conflicts of interest 2 Objectives Summarize
Up in FLAMES: Stable Chronic Obstructive Pulmonary Disease (COPD) Management Colleen Sakon, PharmD BCPS September 27, 2018 Disclosures I have no actual or potential conflicts of interest 2 Objectives Summarize
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Asthma/COPD Update with Inhaler Workshop
 Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Medication Acronyms SABA: Short acting
Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Medication Acronyms SABA: Short acting
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
COPD Medicine. No one ever showed me how to use this. Wendy Happel; RRT, COPD Educator Krystal Fedoris; RRT-NPS, BA, COPD Educator
 Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS:
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS: LOB: Respiratory Disorders Medi-Cal REVIEW HISTORY (MONTH/YEAR) 12/17,12/16, 5/15,
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2017 CLASS: LOB: Respiratory Disorders Medi-Cal REVIEW HISTORY (MONTH/YEAR) 12/17,12/16, 5/15,
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE
 REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE ID NUMBER: 0a) Date of Collection / / 0b) Staff Code Instructions: This form should be completed during the participant s clinic visit. 1) Are you regularly
REVISED RESPIRATORY MEDICATION USE QUESTIONNAIRE ID NUMBER: 0a) Date of Collection / / 0b) Staff Code Instructions: This form should be completed during the participant s clinic visit. 1) Are you regularly
Three s Company - The role of triple therapy in chronic obstructive pulmonary
 Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) October 26 th, 2018 Zahava Picado, PharmD PGY1 Pharmacy Resident Central Texas Veterans Healthcare System Zahava.Picado@va.gov
Three s Company - The role of triple therapy in chronic obstructive pulmonary disease (COPD) October 26 th, 2018 Zahava Picado, PharmD PGY1 Pharmacy Resident Central Texas Veterans Healthcare System Zahava.Picado@va.gov
Test Your Inhaler Knowledge
 A Breath of Fresh Air: Updates in COPD Management Jennifer Austin Szwak, PharmD, BCPS, DPLA University of Chicago Medicine The speaker has nothing to disclose Abbreviations COPD: Chronic obstructive pulmonary
A Breath of Fresh Air: Updates in COPD Management Jennifer Austin Szwak, PharmD, BCPS, DPLA University of Chicago Medicine The speaker has nothing to disclose Abbreviations COPD: Chronic obstructive pulmonary
Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline. MedStar Health
 Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
COPD: Treatment Update Property of Presenter. Not for Reproduction. Barry Make, MD Professor of Medicine National Jewish Health
 COPD: Treatment Update Barry Make, MD Professor of Medicine National Jewish Health Disclosures Advisory board, consultant, multi-center trial, research funding, Data Safety Monitoring Board (DSMB), or
COPD: Treatment Update Barry Make, MD Professor of Medicine National Jewish Health Disclosures Advisory board, consultant, multi-center trial, research funding, Data Safety Monitoring Board (DSMB), or
Asthma & COPD Medication Review. Hutchison Disclosures 2/16/2017. Objectives
 Asthma & COPD Medication Review Anna Meador, PharmD, BCACP Assistant Professor/ Pharmacy Director McWhorter School of Pharmacy/ Christ Health Center Amber Hutchison, PharmD, BCPS Assistant Clinical Professor
Asthma & COPD Medication Review Anna Meador, PharmD, BCACP Assistant Professor/ Pharmacy Director McWhorter School of Pharmacy/ Christ Health Center Amber Hutchison, PharmD, BCPS Assistant Clinical Professor
Correct Use of Inhaler Devices
 PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
Asthma/COPD Update with Inhaler Workshop
 Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Asthma Updates Asthma Updates SMART Trial
Asthma/COPD Update with Inhaler Workshop October 8, 2017 Nathan Samsa, DO, Pharm D, RPh, FACOI None Disclosures Agenda Asthma Updates COPD Updates Inhaler Workshop Asthma Updates Asthma Updates SMART Trial
A Patient s Guide to Aerosol Medication Delivery
 A Patient s Guide to Aerosol Medication Delivery 3rd Edition Prepared by: Tim Op t Holt, EdD, RRT, AE-C, FAARC Kimberly Wiles, RRT, CPFT Ellen Becker, PhD, RRT, RRT-NPS, RPFT, AE-C, FAARC Edited by: Timothy
A Patient s Guide to Aerosol Medication Delivery 3rd Edition Prepared by: Tim Op t Holt, EdD, RRT, AE-C, FAARC Kimberly Wiles, RRT, CPFT Ellen Becker, PhD, RRT, RRT-NPS, RPFT, AE-C, FAARC Edited by: Timothy
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless?
 Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
Using Inhaled Corticosteroids as Needed for Asthma: giving patients relief or leaving them breathless? Lindsay Thomas, Pharm.D. PGY2 Ambulatory Care Resident Department of Pharmacotherapy and Pharmacy
Asthma COPD Update 2018
 Asthma COPD Update 2018 Roger Hefflinger, Pharm.D. Clinical Associate Professor ISU COP Clinical Teaching Pharmacist Family Medicine Residency of Idaho In support of improving patient care, Idaho State
Asthma COPD Update 2018 Roger Hefflinger, Pharm.D. Clinical Associate Professor ISU COP Clinical Teaching Pharmacist Family Medicine Residency of Idaho In support of improving patient care, Idaho State
Question I was one of the first dry power devices available in the US Flovent, Serevent and Advair are all available in this device
 What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
University System of Georgia Prior Authorization, Step Therapy and Quantity Limit List (Updated 1/1/2016)
 University System of Georgia, Step Therapy and Quantity Limit List (Updated 1/1/2016) (PA) Your doctor will need to obtain a prior authorization for the drugs listed below, before your prescription drug
University System of Georgia, Step Therapy and Quantity Limit List (Updated 1/1/2016) (PA) Your doctor will need to obtain a prior authorization for the drugs listed below, before your prescription drug
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.PMN.69 Effective Date: 11/15 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.69 Effective Date: 11/15 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Zurampic, Duzallo (lesinurad Products)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Pulmonary Medication Toolkit: Is yours up to date?
 Pulmonary Medication Toolkit: Is yours up to date? Michelle Schymik, PharmD, BCPS Pharmacist for Deaconess Health System Overview Assuming basic knowledge of pulmonary diseases FEEL FREE TO ASK ME ANY
Pulmonary Medication Toolkit: Is yours up to date? Michelle Schymik, PharmD, BCPS Pharmacist for Deaconess Health System Overview Assuming basic knowledge of pulmonary diseases FEEL FREE TO ASK ME ANY
Michelle Zeidler, MD, MS
 7/1/18 Chronic Obstructive Pulmonary Disease: Optimizing Outpatient Care & Reducing Exacerbations Michelle Zeidler, MD, MS Professor of Medicine, Pulmonary, Critical Care Medicine & Sleep Medicine, VA
7/1/18 Chronic Obstructive Pulmonary Disease: Optimizing Outpatient Care & Reducing Exacerbations Michelle Zeidler, MD, MS Professor of Medicine, Pulmonary, Critical Care Medicine & Sleep Medicine, VA
QUANTITY LIMIT CRITERIA
 DRUG CLASS INHALATION BRAND NAME (generic) QUANTITY LIMIT CRITERIA SHORT ACTING BETA2-ADRENERGIC AGONIST ORAL (albuterol inhalation solution) PROAIR HFA PROAIR RESPICLICK PROVENTIL HFA VENTOLIN HFA XOPENEX
DRUG CLASS INHALATION BRAND NAME (generic) QUANTITY LIMIT CRITERIA SHORT ACTING BETA2-ADRENERGIC AGONIST ORAL (albuterol inhalation solution) PROAIR HFA PROAIR RESPICLICK PROVENTIL HFA VENTOLIN HFA XOPENEX
I have no conflicts of interest or financial relationships to disclose!
 I have no conflicts of interest or financial relationships to disclose! Timothy R. Hudd BS, PharmD, RPh, AE-C Associate Professor of Pharmacy Practice MCPHS University Boston, MA Clinical Pharmacist Primary
I have no conflicts of interest or financial relationships to disclose! Timothy R. Hudd BS, PharmD, RPh, AE-C Associate Professor of Pharmacy Practice MCPHS University Boston, MA Clinical Pharmacist Primary
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Indacaterol/Glycopyrrolate (Utibron Neohaler) Reference Number: CP.PMN.147 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important
Clinical Policy: Indacaterol/Glycopyrrolate (Utibron Neohaler) Reference Number: CP.PMN.147 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
COPD Update: Focus on Intensifying LABA, LAMA and ICS Therapy
 Update: Focus on Intensifying LABA, LAMA and ICS Therapy B.C. Provincial Academic Detailing Service February 2017 Background In Canada, approximately 20 inhaled medications are approved to treat Chronic
Update: Focus on Intensifying LABA, LAMA and ICS Therapy B.C. Provincial Academic Detailing Service February 2017 Background In Canada, approximately 20 inhaled medications are approved to treat Chronic
Chronic obstructive pulmonary disease (COPD) is a condition characterized by
 PUBLISHING STAFF SPECIAL REPORT: COPD EDITOR-IN-CHIEF Troy Trygstad, PharmD, PhD, MBA EDITORIAL EDITORIAL DIRECTOR Kirk McKay DIGITAL MANAGING EDITOR Colleen Hall SENIOR EDITOR Melissa Lauro SENIOR EDITOR
PUBLISHING STAFF SPECIAL REPORT: COPD EDITOR-IN-CHIEF Troy Trygstad, PharmD, PhD, MBA EDITORIAL EDITORIAL DIRECTOR Kirk McKay DIGITAL MANAGING EDITOR Colleen Hall SENIOR EDITOR Melissa Lauro SENIOR EDITOR
Pharmacist CE LESSON. Breathe easy: with asthma/copd 1 MAY 2016
 to this lesson. By Clark Kebodeaux, Pharm.D., BCACP, assistant professor pharmacy practice and science, University of Kentucky College of Pharmacy Author Disclosures: Clark Kebodeaux and the DSN editorial
to this lesson. By Clark Kebodeaux, Pharm.D., BCACP, assistant professor pharmacy practice and science, University of Kentucky College of Pharmacy Author Disclosures: Clark Kebodeaux and the DSN editorial
Individualizing and Optimizing Asthma Care. CFW 2018 Friday, April 13, 9:45 11:15 am Renaissance Austin Hotel in Austin, Texas
 Individualizing and Optimizing Asthma Care CFW 2018 Friday, April 13, 9:45 11:15 am Renaissance Austin Hotel in Austin, Texas Speaker Disclosure Dr. Hawkins has disclosed that he has no actual or potential
Individualizing and Optimizing Asthma Care CFW 2018 Friday, April 13, 9:45 11:15 am Renaissance Austin Hotel in Austin, Texas Speaker Disclosure Dr. Hawkins has disclosed that he has no actual or potential
Commissioner for the Department for Medicaid Services Selections for Preferred Products
 Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner for the Department for
Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner for the Department for
End Stage COPD Guidance Document
 End Stage COPD Guidance Document Suggested Guidelines for the Determination of Hospice Eligibility A patient with severe chronic pulmonary disease that meets the following criteria may be eligible for
End Stage COPD Guidance Document Suggested Guidelines for the Determination of Hospice Eligibility A patient with severe chronic pulmonary disease that meets the following criteria may be eligible for
COPD The New Epidemic. Peter Lin MD CCFP Director Primary Care Initiatives Canadian Heart Research Centre
 COPD The New Epidemic Peter Lin MD CCFP Director Primary Care Initiatives Canadian Heart Research Centre Conflict Disclosure Information Speaker: Dr. Peter Lin Title of Talk: COPD The New Epidemic Financial
COPD The New Epidemic Peter Lin MD CCFP Director Primary Care Initiatives Canadian Heart Research Centre Conflict Disclosure Information Speaker: Dr. Peter Lin Title of Talk: COPD The New Epidemic Financial
Respiratory Inhalers. Identification Guide Version 3
 Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe
 APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
The Latest Medications A Pharmacological Update for RTs
 The Latest Medications A Pharmacological Update for RTs Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Georgia State University Objectives
The Latest Medications A Pharmacological Update for RTs Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Georgia State University Objectives
Cinqair (reslizumab injection for intravenous use)
 Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
THE COPD PRESCRIBING TOOL
 THE COPD PRESCRIBING TOOL Revised edition, 2017 www.bpac.org.nz/copd CLASSIFICATION The COPD prescribing tool This tool provides pharmacological treatment options for patients with COPD based on their
THE COPD PRESCRIBING TOOL Revised edition, 2017 www.bpac.org.nz/copd CLASSIFICATION The COPD prescribing tool This tool provides pharmacological treatment options for patients with COPD based on their
Objectives. Speaker has no relationship to disclose. Sneezes, Wheezes, and Respiratory Diseases: An update on Asthma, Allergic Rhinitis,
 Sneezes, Wheezes, and Respiratory Diseases: An update on Asthma, Allergic Rhinitis, and COPD Amelie Hollier, DNP, FNP-BC, FAANP Advanced Practice Education Associates Objectives Evaluation of medications,
Sneezes, Wheezes, and Respiratory Diseases: An update on Asthma, Allergic Rhinitis, and COPD Amelie Hollier, DNP, FNP-BC, FAANP Advanced Practice Education Associates Objectives Evaluation of medications,
MEDICAID QUANTITY LIMIT DRUG LIST
 MEDICAID QUANTITY LIMIT DRUG LIST PH51-R-02162018 Brand Name Generic Name Dosage Form Tier Quantity Limit Details Cambia Diclofenac Potassium PACK Tier 2 QL: 9 per 30 days Fentanyl (12 Mcg/Hr, 25 Mcg/
MEDICAID QUANTITY LIMIT DRUG LIST PH51-R-02162018 Brand Name Generic Name Dosage Form Tier Quantity Limit Details Cambia Diclofenac Potassium PACK Tier 2 QL: 9 per 30 days Fentanyl (12 Mcg/Hr, 25 Mcg/
New and Novel Medications for Respiratory Care
 New and Novel Medications for Respiratory Care JASON MOORE, PHARM.D. BCCCP CLINICAL STAFF PHARMACIST STORMONT-VAIL HEALTH Objectives Quick overview of the newest FDA-approved repiratory-related medications
New and Novel Medications for Respiratory Care JASON MOORE, PHARM.D. BCCCP CLINICAL STAFF PHARMACIST STORMONT-VAIL HEALTH Objectives Quick overview of the newest FDA-approved repiratory-related medications
Chronic Obstructive Pulmonary Disease
 Quality Department Guidelines for Clinical Care Ambulatory COPD Guideline Team Team Leader Davoren A Chick, MD General Medicine Team Members Paul J Grant, MD General Medicine R Van Harrison, PhD Learning
Quality Department Guidelines for Clinical Care Ambulatory COPD Guideline Team Team Leader Davoren A Chick, MD General Medicine Team Members Paul J Grant, MD General Medicine R Van Harrison, PhD Learning
Take My Breath Away: COPD Update. Jason Henderson D.O. Warren Clinic Pulmonary & Critical Care
 Take My Breath Away: Update Jason Henderson D.O. Warren Clinic Pulmonary & Critical Care Objectives 1. Recognize clinical signs and symptoms associated with chronic bronchitis and emphysema. 2. Describe
Take My Breath Away: Update Jason Henderson D.O. Warren Clinic Pulmonary & Critical Care Objectives 1. Recognize clinical signs and symptoms associated with chronic bronchitis and emphysema. 2. Describe
DEVELOPMENT OF DRUG DEVICE COMBINATION PRODUCTS I. INHALED THERAPEUTIC PRODUCTS IN THE US MARKET Rohinton Toddywala, Ph.D., MBA
 DEVELOPMENT OF DRUG DEVICE COMBINATION PRODUCTS I. INHALED THERAPEUTIC PRODUCTS IN THE US MARKET Rohinton Toddywala, Ph.D., MBA INTRODUCTION The benefits of inhaled therapy for the treatment of lung and
DEVELOPMENT OF DRUG DEVICE COMBINATION PRODUCTS I. INHALED THERAPEUTIC PRODUCTS IN THE US MARKET Rohinton Toddywala, Ph.D., MBA INTRODUCTION The benefits of inhaled therapy for the treatment of lung and
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Balanced information for better care. Helping patients with COPD breathe easier
 Balanced information for better care Helping patients with COPD breathe easier COPD is the third-leading cause of death in the U.S., following cancer and heart disease 1 FIGURE 1. Women now have a higher
Balanced information for better care Helping patients with COPD breathe easier COPD is the third-leading cause of death in the U.S., following cancer and heart disease 1 FIGURE 1. Women now have a higher
Take My Breath Away COPD Update. Take My Breath Away COPD Update 4/16/16. Juliann Horne, PharmD, PhC, BCACP
 Take My Breath Away COPD Update Juliann Horne, PharmD, PhC, BCACP jmhorne@salud.unm.edu April 16, 2016 Take My Breath Away COPD Update Juliann Horne, PharmD, PhC, BCACP jmhorne@salud.unm.edu April 16,
Take My Breath Away COPD Update Juliann Horne, PharmD, PhC, BCACP jmhorne@salud.unm.edu April 16, 2016 Take My Breath Away COPD Update Juliann Horne, PharmD, PhC, BCACP jmhorne@salud.unm.edu April 16,
1.* Dosage. A. Adults
 Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Global Strategy for the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline. MedStar Health
 Global Strategy f the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
Global Strategy f the Diagnosis, Management and Prevention of COPD 2016 Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions
MANAGING ASTHMA. Nancy Davis, RRT, AE-C
 MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
COPD. Understanding. An educational health series from National Jewish Health IN THIS ISSUE. What is COPD? 2. How is COPD Managed?
 Understanding COPD The Mount Sinai National Jewish Health Respiratory Institute was formed by the nation s leading respiratory hospital National Jewish Health, based in Denver, and top ranked academic
Understanding COPD The Mount Sinai National Jewish Health Respiratory Institute was formed by the nation s leading respiratory hospital National Jewish Health, based in Denver, and top ranked academic
Formulary Medical Necessity Program
 BENEFIT APPLICATION Formulary Medical Necessity Program DRUG POLICY Benefit determinations are based on the applicable contract language in effect at the time the services were rendered. Exclusions, limitations
BENEFIT APPLICATION Formulary Medical Necessity Program DRUG POLICY Benefit determinations are based on the applicable contract language in effect at the time the services were rendered. Exclusions, limitations
Common Drug Review Pharmacoeconomic Review Report
 Common Drug Review Pharmacoeconomic Review Report January 2018 Drug umeclidinium bromide (Incruse Ellipta) Indication Listing request Dosage form(s) Manufacturer Indicated for long-term, once daily maintenance
Common Drug Review Pharmacoeconomic Review Report January 2018 Drug umeclidinium bromide (Incruse Ellipta) Indication Listing request Dosage form(s) Manufacturer Indicated for long-term, once daily maintenance
Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists. Learning Objectives.
 Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists Learning Objectives Identify the risk factors for COPD and the clinical features that differentiate
Incorporating Newer Therapies and Strategies to Improve COPD Outcomes: A Practical Guide for Pharmacists Learning Objectives Identify the risk factors for COPD and the clinical features that differentiate
primer on inhalers and nebulizers
 CREDIT: 2.0 Continuing Education EARN CE CREDIT FOR THIS ACTIVITY AT WWW.DRUGTOPICS.COM AN ONGOING CE PROGRAM OF THE UNIVERSITY OF CONNECTICUT SCHOOL OF PHARMACY AND DRUG TOPICS educational objectives
CREDIT: 2.0 Continuing Education EARN CE CREDIT FOR THIS ACTIVITY AT WWW.DRUGTOPICS.COM AN ONGOING CE PROGRAM OF THE UNIVERSITY OF CONNECTICUT SCHOOL OF PHARMACY AND DRUG TOPICS educational objectives
dichlorphenamide (Keveyis )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
COPD Robert Schilz DO, PhD Pulmonary, Critical Care and Sleep Medicine University Hospitals Case Medical Center
 COPD 2018 GOLD 2017 Report Global Initiative for Chronic Obstructive Lung D isease COPYRIGHTED MATERIAL- DO NOT COPY OR DISTRIBUTE GLOBAL STRATEGY FOR THE DIAGNOSIS, MANAGEMENT, AND PREVENTION OF CHRONIC
COPD 2018 GOLD 2017 Report Global Initiative for Chronic Obstructive Lung D isease COPYRIGHTED MATERIAL- DO NOT COPY OR DISTRIBUTE GLOBAL STRATEGY FOR THE DIAGNOSIS, MANAGEMENT, AND PREVENTION OF CHRONIC
4/3/2018 BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018
 BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018 Approach to treatment List of medication Abbreviations When to use medications Pharmokinetics Pharmacodynamics Step wise therapy both for acute and chronic
BRANDY BURGESS, APRN-CNS, MSN-RN APRIL 12-14, 2018 Approach to treatment List of medication Abbreviations When to use medications Pharmokinetics Pharmacodynamics Step wise therapy both for acute and chronic
Combination Beta2-Agonist/Corticosteroid Inhalers
 Combination Beta2-Agonist/Corticosteroid Inhalers Policy Number: 5.01.572 Last Review: 7/2017 Origination: 6/2014 Next Review: 7/2018 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Combination Beta2-Agonist/Corticosteroid Inhalers Policy Number: 5.01.572 Last Review: 7/2017 Origination: 6/2014 Next Review: 7/2018 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Diabetes Fortamet (metformin ER), Glumetza (metformin ER) generic of Glucophage XR (metformin ER) preferred
 Pennsylvania Employees Benefit Trust Fund (PEBTF) and n-medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
Pennsylvania Employees Benefit Trust Fund (PEBTF) and n-medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
Pulmonary Disease Aerosol Delivery Devices A Guide for Physicians, Nurses, Pharmacists, and Other Health Care Professionals
 Pulmonary Disease Aerosol Delivery Devices A Guide for Physicians, Nurses, Pharmacists, and Other Health Care Professionals 3rd Edition Karen L. Gregory, DNP, APRN, CNS, RRT, AE-C, FAARC Lori Wilken, PharmD,
Pulmonary Disease Aerosol Delivery Devices A Guide for Physicians, Nurses, Pharmacists, and Other Health Care Professionals 3rd Edition Karen L. Gregory, DNP, APRN, CNS, RRT, AE-C, FAARC Lori Wilken, PharmD,
COPD A Breath of Fresh Air. Clare Hawkins, MD, FAAFP SW Regional Medical Officer Aspire Healthcare/Anthem
 COPD A Breath of Fresh Air Clare Hawkins, MD, FAAFP SW Regional Medical Officer Aspire Healthcare/Anthem Speaker Disclosure Dr. Hawkins has disclosed that he has no actual or potential conflict of interest
COPD A Breath of Fresh Air Clare Hawkins, MD, FAAFP SW Regional Medical Officer Aspire Healthcare/Anthem Speaker Disclosure Dr. Hawkins has disclosed that he has no actual or potential conflict of interest
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Sensipar (cinacalcet)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Reference Guide for Caring for Pediatric Patients with Asthma
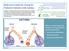 Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Pharmacy Medical Policy Asthma and Chronic Obstructive Pulmonary Disease Medication Management
 Pharmacy Medical Policy Asthma and Chronic Obstructive Pulmonary Disease Medication Management Table of Contents Policy: Commercial Information Pertaining to All Policies Endnotes Policy: Medicare References
Pharmacy Medical Policy Asthma and Chronic Obstructive Pulmonary Disease Medication Management Table of Contents Policy: Commercial Information Pertaining to All Policies Endnotes Policy: Medicare References
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
and will be denied as not medically necessary** if not met. This criterion only applies to the initial
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Pain Oral-Intranasal Fentanyl (Abstral, Actiq, Fentora, Lazanda, Onsolis, Subsys)
 Pennsylvania Employees Benefit Trust Fund (PEBTF) and n- Medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
Pennsylvania Employees Benefit Trust Fund (PEBTF) and n- Medicare Eligible Retired Employees Health Program (REHP), Step Therapy and Quantity Limit List Your doctor needs to get prior authorization for
