AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
|
|
|
- Clifton Parks
- 5 years ago
- Views:
Transcription
1 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475, 481, 506 actinic keratoses 5-fluorouracil 05 Actinomycin-D. See dactinomycin-d ACT regimen, 475 AC T regimen, 476, AC T 1 trastuzumab regimen, acute leukemias carboplatin and, 87 primary induction acute lymphoblastic leukemia (ALL) asparaginase and, 34 clofarabine, 112 daunorubicin and, 139 idarubicin 29 mercaptopurine 83 methotrexate 86 primary induction T-cell, 303 thioguanine and, 393 acute lymphocytic leukemia cytarabine and, 122 imatinib 37, 238 acute myelogenous leukemia (AML) cytarabine and, 122 daunorubicin and, 139 etoposide phosphate and, 190 hydroxyurea 22 idarubicin 29 mitoxantrone 99 thioguanine and, 393 acute promyelocytic leukemia (APL) arsenic trioxide, 30, 31 tretinoin and, 415 Adcetris. See brentuximab adjuvant treatment to local treatment modalities, 2 3 ADOC regimen, 581 ado-trastuzumab emtansine, 8 10, 487 AD regimen, 576 adrenal steroid inhibitor aminoglutethimide, adrenocortical cancer mitotane 96 adrenolytic agent mitotane, Adriamycin. See doxorubicin Afinitor. See everolimus AG See axitinib AIDA regimen, 525 a-interferon. See interferon-a AI regimen, 577 albumin-bound paclitaxel, alcohol lomustine 71 nilutamide and, 309 procarbazine and, 353 thalidomide and, 389 aldesleukin, alemtuzumab, 17 19, , 457 Alimta. See pemetrexed Alkeran. See melphalan alkylating agents bendamustine, busulfan, carmustine, chlorambucil, , dacarbazine, ifosfamide, lomustine, mechlorethamine, melphalan, streptozocin, thiotepa, ALL. See acute lymphoblastic leukemia (ALL) allopurinol ifosfamide 34 mercaptopurine 83 All- trans -retinoic acid. See tretinoin Aloxi. See palonosetron altretamine, , 457 aluminum hydroxide, capecitabine and, 83 Amethopterin. See methotrexate amifostine cisplatin and, amino-9-b-D-arabinofu- ranosyl-6-methoxy- 9H-purine. See nelarabine aminoglutethimide, , 457 megestrol acetate 77 aminoglycosides cisplatin and, 105 amiodarone AML. See acute myelogenous leukemia (AML)
2 AMN107. See nilotinib amphotericin B carmustine and, 94 cisplatin and, 105 amputees (adult), body surface area of, 450 amsacrine dialysis guidelines, anal cancer fluorouracil 05 neoadjuvant anaplastic astrocytomas adjuvant and, 3 temozolomide and, 383 anaplastic large cell lymphoma brentuximab and, 68 anastrozole, 26 28, 480, 486 adjuvant treatment to local treatment modalities, 3 antacids vismodegib and, 439 anthracyclines trastuzumab and, 412 antiandrogen bicalutamide, enzalutamide, flutamide, nilutamide, anti-angiogenic agent lenalidomide, pomalidomide, thalidomide, antibody-drug conjugate ado-trastuzumab emtansine, 8 10 anticoagulants antidiabetic agents procarbazine and, 353 anti-egfr antibody cetuximab, panitumumab, antiemetic agents aprepitant, , dexamethasone, , 632, 633 diphenhydramine, dolasetron, , 632, 633, 635 dronabinol, , 635 granisetron, 106, lorazepam, metoclopramide, , 632, 634, 635 nabilone, , 635 ondansetron, 106, , 632, 633, overview, 601 palonosetron, , 633, 634 prochlorperazine, , 632, 635 antiestrogen tamoxifen, toremifene, Anti-HER-2-antibody. See pertuzumab; trastuzumab antihistamines diphenhydramine, procarbazine and, 353 antimetabolite azacitidine, capecitabine, cladribine, clofarabine, cytarabine, decitabine, floxuridine, fludarabine, fluorouracil, gemcitabine, hydroxyurea, mercaptopurine, methotrexate, nelarabine, pemetrexed, pralatrexate, thioguanine, antimicrotubule agent albumin-bound paclitaxel, cabazitaxel, docetaxel, eribulin, estramustine, ixabepilone, paclitaxel, vinblastine, vincristine, vinorelbine, antineoplastic agent decitabine, antithymocyte globulin (ATG) 1 cyclosporine regimen, 563 antitumor antibiotic bleomycin, dactinomycin-d, daunorubicin, daunorubicin liposome, doxorubicin, doxorubicin liposome, epirubicin, mitomycin-c, mitoxantrone, antitumor antibiotics idarubicin, anti-vegf antibody bevacizumab, ziv-aflibercept, Anzemet. See dolasetron AP See ponatinib APC8015. See sipuleucel-t APL. See acute promyelocytic leukemia (APL) AP regimen, 506 aprepitant, , Ara-C. See cytarabine area under the curve (AUC), 449 Arimidex. See anastrozole Aromasin. See exemestane aromatase inhibitor letrozole, Arranon. See nelarabine Index 641
3 642 Index arsenic trioxide (As 2 O 3 ), 29 32, 527 Arzerra. See ofatumumab asparaginase, cytarabine and, 124 methotrexate 88 vincristine and, 432 aspirin methotrexate 87 pemetrexed and, 336 pralatrexate and, 350 astemizole astrocytomas, 94 anaplastic, 3, 383 atazanavir A T C, 478 Ativan. See lorazepam ATRA. See tretinoin ATRA regimen, 527 AT regimen, 481 AUC. See area under the curve (AUC) Avastin. See bevacizumab axitinib, azacitidine, 41 43, 527, Aza-2 -deoxycytidine. See decitabine azidothymidine (AZT) hydroxyurea 22 azithromycin vismodegib and, 439 B barbiturates mitotane 96 thalidomide and, 389 basal cell carcinoma regimens, fluorouracil 05 vismodegib and, 439 BAY See sorafenib BAY See regorafenib B-cell chronic lymphocytic leukemia (B-CLL) alemtuzumab and, 17 BCNU. See carmustine BCNU regimen, BEACOPP regimen, 542 Benadryl. See diphenhydramine bendamustine, 44 46, 530, 552 benzodiazepine lorazepam, BEP regimen, 568, 579 bepridil bevacizumab, 47 50, 475, 519 bexarotene, Bexxar. See tositumomab bicalutamide, 54 55, 220 BIC regimen, 492 biliary tract cancer, regimens, biologic response modifier agent ado-trastuzumab emtansine, 8 10 aldesleukin, alemtuzumab, bevacizumab, brentuximab, cetuximab, denileukin difitox, ibritumomab, interferon-a, ipilimumab, lenalidomide, ofatumumab, panitumumab, pertuzumab, pomalidomide, rituximab, sipuleucel-t, thalidomide, tositumomab, trastuzumab, ziv-aflibercept, BIP regimen, 491 Bischloroethylnitrosourea. See carmustine bladder cancer carboplatin and, cisplatin and, 104 docetaxel and, 154 gemcitabine 17 ifosfamide 33 methotrexate 86 mitomycin-c and, 292 neoadjuvant paclitaxel and, 323 thiotepa and, 396 Blenoxane. See bleomycin bleomycin, brentuximab and, 68 cisplatin and, 105 BMP regimen, 561 BMPT regimen, 561 BMS See ixabepilone BMS See dasatinib body surface area (BSA) of amputees (adult), 450 dosage determination from, bone sarcoma
4 bortezomib, 60 63, 553, 562 doxorubicin liposome and, 164 Bosulif. See bosutinib bosutinib, 64 66, brain stem glioma, 94 brain tumor carmustine and, lomustine 71 procarbazine and, 353 breast cancer adjuvant treatment to local treatment modalities, 3 ado-trastuzumab emtansine for, 9 albumin-bound paclitaxel for, 12 aminoglutethimide 3 anastrozole 7 capecitabine and, docetaxel and, 154, 155 epirubicin and, 171 etoposide and, 185 exemestane and, fluorouracil 05 fulvestrant 11 gemcitabine 16 ixabepilone 55 lapatinib 58 letrozole 65 megestrol acetate 77 melphalan 80 methotrexate 86 mitomycin-c 92 mitoxantrone 99 neoadjuvant optimal duration of drug administration, 4 paclitaxel and, 323 tamoxifen and, 379 thiotepa and, 396 toremifene and, 402 trastuzumab and, 412 vinorelbine and, 435 brentuximab, 67 69, 553 bleomycin and, 58 bretylium BSA. See body surface area (BSA) bupropion Burkitt s lymphoma primary induction buserelin busulfan, 70 72, 531 Busulfex. See busulfan BV regimen, 519 C cabazitaxel, cabozantinib, CAE regimen, CAF regimen, 477, 481 calcium channel blockers Calvert formula, for carboplatin dose, 87 Campath. See alemtuzumab Camptosar. See irinotecan cancer of unknown primary, cannabinoid dronabinol, nabilone, capecitabine, 80 85, 487, 496, 504, 519, 571 CAP regimen, 472, 506, 557, 580 Caprelsa. See vandetanib carbamazepine cabozantinib and, 78 dasatinib and, 136 erlotinib and, 178 everolimus and, 192 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 toremifene and, 402 carboplatin, albumin-bound paclitaxel for non small cell lung cancer, 12 area under the curve, 449 paclitaxel and, 324 carcinoid tumors streptozocin and, 372 carcinomatous meningitis methotrexate 86 carfilzomib, 90 92, 562 Index 643
5 644 Index carmustine, , 457 Casodex. See bicalutamide CAV alternating with IE regimen, 578 CAV regimen, 539 CBDCA. See carboplatin CC See lenalidomide CCI-779. See temsirolimus CCNU. See lomustine CC regimen, CdA. See cladribine CDDP. See cisplatin CEF regimen, 481 CEL. See chronic eosinophilic leukemia (CEL) cephalosporins methotrexate 87 pralatrexate and, 350 Cerubidine. See daunorubicin cervical cancer ifosfamide 33 mitomycin-c 92 topotecan and, 399 Cesamet. See nabilone cetuximab, 96 99, , 518 CF regimen, 511, 527 adjuvant treatment to local treatment modalities, 2 3 clinical efficacy, obstacles to, 1 combination, 3 4 guidelines (See guidelines, ) neoadjuvant, 2 overview, 1 primary induction, 2 principles of, 1 4 salvage, 468 drugs ado-trastuzumab emtansine, 8 10 albumin-bound paclitaxel, altretamine, arsenic trioxide, asparaginase, axitinib, azacitidine, bendamustine, bleomycin, bortezomib, bosutinib, busulfan, cabazitaxel, cabozantinib, capecitabine, carboplatin, carfilzomib, carmustine, chlorambucil, cisplatin, cladribine, clofarabine, crizotinib, , cytarabine and, dabrafenib, dacarbazine, dactinomycin-d, dasatinib, daunorubicin, daunorubicin liposome, docetaxel, doxorubicin, doxorubicin liposome, emetogenic potential of, epirubicin, eribulin, erlotinib, estramustine, etoposide, etoposide phosphate, everolimus, floxuridine, fludarabine, fluorouracil, gefitinib, gemcitabine, hydroxyurea, idarubicin, ifosfamide, imatinib, ironotecan, ixabepilone, lapatinib, lomustine, mechlorethamine, melphalan, mercaptopurine, methotrexate, mitomycin-c, mitotane, mitoxantrone, nelarabine, nilotinib, omacetaxine mepesuccinate, oxaliplatin, paclitaxel, pazopanib, pemetrexed, ponatinib, pralatrexate, procarbazine, regorafenib, romidepsin, sorafenib, streptozocin, sunitinib, temozolomide, temsirolimus, thioguanine, thiotepa, topotecan, trametinib, vandetanib, vemurafenib, vinblastine, vincristine, vinorelbine, vismodegib, vorinostat, See also specific drugs acute lymphocytic leukemia, acute myelogenous leukemia, anal cancer, basal cell carcinoma, 469 biliary tract cancer,
6 bladder cancer, brain tumor, breast cancer, Burkitt s lymphoma, cancer of unknown primary, carcinoid tumors, cervical cancer, chronic lymphocytic leukemia, chronic myelogenous leukemia, colorectal cancer, endometrial cancer, esophageal cancer, gastric cancer, gastrointestinal stromal tumor, hairy cell leukemia, 531 head and neck cancer, hepatocellular cancer, Hodgkin s lymphoma, Kaposi s sarcoma, lymphoma, malignant melanoma, malignant mesothelioma, multiple myeloma, myelodysplastic syndrome, non-hodgkin s lymphoma, non-small cell lung cancer, osteogenic sarcoma, ovarian cancer, pancreatic cancer, primary CNS lymphoma, prostate cancer, renal cell cancer, small cell lung cancer, soft tissue sarcoma, testicular cancer, thymoma, thyroid cancer, chlorambucil, , Chlorodeoxyadenosine. See cladribine chloroquine chlorpromazine thalidomide and, 389 CHOP regimen, 544, 545 CHOP 1 rituximab regimen, 546 choriocarcinoma primary induction chronic eosinophilic leukemia (CEL) imatinib 38 chronic leukemias chronic lymphocytic leukemia (CLL) bendamustine and, chlorambucil and, 101 cladribine and, 109 fludarabine 02 ofatumumab and, 312 chronic myelogenous leukemia (CML) busulfan and, 71 regimens, cytarabine and, 123 dasatinib and, 136 hydroxyurea 22 idarubicin 29 imatinib 37 interferon-a 42 nilotinib and, 306 omacetaxine mepesuccinate and, 316 thioguanine and, 393 chronic myelomonocytic leukemia azacitidine and, 42 cimetidine carmustine and, 94 epirubicin and, 171 ifosfamide 34 lomustine 71 melphalan 80 tretinoin and, 415 ciprofloxacin Cis-diamminedichloroplatinum,. See cisplatin cisplatin, , 519 bleomycin and, 57 cytarabine and, 123 fludarabine 02 gemcitabine 17 granisetron and, 614 ifosfamide 34 paclitaxel and, 324 vincristine and, 432 vinorelbine and, 435 cladribine, , 526, 529, , 458 clarithromycin cabozantinib and, 78 dasatinib and, 136 erlotinib and, 179 everolimus and, 193 exemestane and, 196 gefitinib 13 Index 645
7 646 Index clarithromycin Cont. imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 vismodegib and, 439 CLL. See chronic lymphocytic leukemia (CLL) clofarabine, , 524, 527 Clolar. See clofarabine clopidogrel (plavix) letrozole 65 CMF regimen, 482 CML. See chronic myelogenous leukemia (CML) CMV regimen, 471 CNOP regimen, 544, 546 CNS lymphoma, primary methotrexate 86 CODOX-M regimen, 550 colon cancer capecitabine and, 82 oxaliplatin and, 319 colorectal cancer adjuvant treatment to local treatment modalities, 3 bevacizumab and, 48 capecitabine and, floxuridine and, fluorouracil 05 ironotecan and, 250, 251 optimal duration of drug administration, 4 oxaliplatin and, 319 panitumumab and, 328 ziv-aflibercept and, 445 combination, 3 4 Cometriq. See cabozantinib Compazine. See prochlorperazine Cosmegen. See dactinomycin-d coumadin mercaptopurine 83 CP regimen, 527, 564 CPT-11. See irinotecan creatinine clearance, determination of, crizotinib, Crockcroft-Gault formula, for creatinine clearance, CTCL. See cutaneous T-cell lymphoma (CTCL) CT regimen, 565 CTX. See cutaneous T-cell lymphoma (CTCL) bexarotene and, 51, 52 denileukin difitox and, 150 fludarabine 02 interferon-a 42 mechlorethamine 74 procarbazine and, 353 vorinostat and, 442 CVP regimen, 527, 543, and busulfan in stem cell transplant, 71 doxorubicin and, 160 epirubicin and, 171 fludarabine 02 mitotane 96 paclitaxel and, 324 rituximab and, 360 tamoxifen and, 379 cyclosporine docetaxel and, 155 melphalan 80 tretinoin and, 415 Cytadren. See aminoglutethimide cytarabine, , 526 fludarabine 02 cytokines aldesleukin, cytarabine and, 124 Cytosine arabinoside. See cytarabine Cytoxan. See CYVADIC regimen, 577 D DAB389. See denileukin difitox dabrafenib, dacarbazine, Dacogen. See decitabine dactinomycin dactinomycin-d, dasatinib, , 524, Daunomycin. See daunorubicin daunorubicin, , 520 daunorubicin liposome, DaunoXome. See daunorubicin liposome DCF regimen, 511
8 DCIS. See ductal carcinoma in situ (DCIS) Decadron. See dexamethasone decitabine, , 527, 562 degarelix, degramont regimen, 496, Demethoxydaunorubicin. See idarubicin denileukin difitox, depsipeptide. See romidepsin dexamethasone, 561, , 632, 633 daunorubicin and, 139 everolimus and, 192 dexrazoxane daunorubicin and, 139 doxorubicin and, 160 dextromethorphan DHAP (salvage regimen), 548 DH FEC regimen, 479 Diaminocyclohexane platinum. See oxaliplatin DIC. See dacarbazine dideoxycytidine (ddc) hydroxyurea 22 dideoxyinosine (ddi) hydroxyurea 22 differentiating agents arsenic trioxide, bexarotene, tretinoin, digoxin carmustine and, 94 cytarabine and, 123 vincristine and, 432 dilantin dasatinib and, 136 erlotinib and, 178 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 diltiazem everolimus and, 193 tretinoin and, 415 diphenhydramine, disopyramide docetaxel, , 487, 493, 513, 517, 520 and abiraterone acetate for prostate cancer, 6 capecitabine and, 82 pertuzumab and, 339 docetaxel AC regimen, 478 dofetilide dolasetron, , 632, 633, 635 Douillard regimen, 497 Doxil. See doxorubicin liposome doxorubicin, , 487, 488, 507, 519, paclitaxel and, 324 doxorubicin liposome, dronabinol, , 635 droperidol DTIC 1 BCNU 1 cisplatin regimen, 555 DTIC-Dome. See dacarbazine ductal carcinoma in situ (DCIS) tamoxifen and, 379 duloxetine DVD regimen, 560 E ECF regimen, 509, 511 ECOG performance scale, 448 ECOG regimen, 569 EC regimen, 539 ECX regimen, 510, 512 Efudex. Se e 5-fluorouracil Ellence. See epirubicin Eloxatin. See oxaliplatin Elspar. See asparaginase embryonal rhabdomyosarcoma primary induction Emcyt. See estramustine Emend. See aprepitant endometrial cancer carboplatin and, megestrol acetate 77 tamoxifen and, 379 ENL. See erythema nodosum leprosum (ENL) enzalutamide, enzymes asparaginase, EOF regimen, 510, 511 EORTC regimen, 467 EOX regimen, 510, 512 EP 1 docetaxel regimen, 536 ependymoma, 94 4 Epi-doxorubicin. See epirubicin epipodophyllotoxin etoposide, etoposide phosphate, Index 647
9 648 Index epirubicin, EPOCH regimen, 546 EPOCH 1 rituximab regimen, 547 epothilone ixabepilone, EP regimen, 488, 536, 538, 579 Erbitux. See cetuximab eribulin, , 488 Erivedge. See vismodegib erlotinib, erythema nodosum leprosum (ENL) thalidomide and, 389 erythromycin bexarotene and, 52 cabozantinib and, 78 dasatinib and, 136 docetaxel and, 155 erlotinib and, 179 everolimus and, 193 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 toremifene and, 402 tretinoin and, 415 vismodegib and, 439 ESHAP (salvage regimen), 548 esophageal cancer regimens, cisplatin and, fluorouracil 05 neoadjuvant paclitaxel and, 323 Estracyte. See estramustine estramustine, estrogen receptor antagonist fulvestrant, Etopophos. See etoposide phosphate etoposide, , 520 cisplatin and, 105 etoposide phosphate, Eulexin. See flutamide EVAP regimen, 542 EVA regimen, 541 everolimus, , 490 Ewing s sarcoma dactinomycin-d, 133 ifosfamide 33 exemestane, , 486 F Fareston. See toremifene Faslodex. See fulvestrant FC regimen, 544 FCR regimen, 527, 544 FEC docetaxel regimen, 477 FEC regimen, 477 FEC-50 regimen, 483 FEC-75 regimen, 483 FEC-100 regimen, 483 Femara. See letrozole FFD French regimen, 508 filgrastim vincristine and, 432 Firmagon. See degarelix FLAG regimen, 526 FLO regimen, 513 FLOX regimen, 496 floxuridine, , , 458 fluconazole everolimus and, 193 ixabepilone 55 Fludara. See fludarabine fludarabine, , 529, 552 alemtuzumab, 17 cytarabine and, , 458 rituximab and, Fluoro-ara-AMP. See fludarabine 5-fluorocytosine cytarabine and, Fluoro-2 -deoxyuridine. See floxuridine 5-fluorouracil, , 505 colorectal cancer and irinotecan, , 458 epirubicin and, 171 hydroxyurea 22 methotrexate 87 pemetrexed and, 336 fluoxetine flutamide, , , 459 FND regimen, 544 FOLFIRI 1 bevacizumab regimen, 503 FOLFIRI 1 cetuximab regimen, 502 FOLFIRINOX regimen, 570 FOLFIRI 1 panitumumab regimen, 503 FOLFIRI regimen, 497 FOLFIRI 1 ziv-aflibercept regimen,
10 FOLFOX 4 1 bevacizumab regimen, 501 FOLFOX 1 cetuximab 4 regimen, 502 FOLFOX6 1 cetuximab regimen, FOLFOXIRI regimen, 499 FOLFOX 4 1 panitumumab regimen, 503 FOLFOX regimen, 495, 4 498, 518 FOLFOX regimen, FOLFOX 7 regimen, 498 folic acid supplements methotrexate 87 Folotyn. See pralatrexate FP regimen, 527 FP 1 trastuzumab regimen, 513 FR regimen, FU. See 5-fluorouracil FUDR. See floxuridine fulvestrant, , 486 furosemide cisplatin and, 105 G gastric cancer adjuvant treatment to local treatment modalities, docetaxel and, 154, 155 epirubicin and, 171 etoposide and, fluorouracil 05 mitomycin-c 92 trastuzumab and, 412 gastroesophageal cancer oxaliplatin and, 319 trastuzumab and, 412 gastrointestinal (GI) adenocarcinoma floxuridine and, 198 gastrointestinal stromal tumor (GIST) adjuvant treatment to local treatment modalities, imatinib 37, 238 optimal duration of drug administration, 4 GBM. See glioblastoma multiformae (GBM) GCB regimen, 534 GCP regimen, 489 gefitinib, , 459 GEM-CAP regimen, 569 gemcitabine, , 472, 487, 493, , 459 gemfibrozil bexarotene and, 52 GEMOX regimen, 518 gemtuzumab, 527 Gemzar. See gemcitabine gentamicin cytarabine and, 123 German AIO regimen, German schedule, low dose regimen, 500 germ cell cancer bleomycin and, 57 carboplatin and, 87 dactinomycin-d, 133 etoposide and, 185 etoposide phosphate and, 189 ifosfamide 33 ovarian, 568 primary induction gestational trophoblastic disease dactinomycin-d, 133 methotrexate 86 GITSG regimen, 568 Gleevec. See imatinib Gliadel, 94. See also carmustine glioblastoma, bevacizumab and, 48 glioblastoma multiformae (GBM) carmustine and, 94 temozolomide and, 383 glucocorticoid steroid dexamethasone, GnRH antagonist degarelix, goserelin, , 459 granisetron, , cisplatin and, 106 grapefruit juice grepafloxacin GTX regimen, 569 guidelines, area under the curve, 449 body surface area in adult amputees, 450 creatinine clearance, dialysis of drugs, drug dose determination of, hepatic function and, renal function and, patient factors, 447 performance scales, 448 GVD regimen, 543 GW See lapatinib H hairy cell leukemia regimens, 531 cladribine and, 109 interferon-a 42 Halaven. See eribulin halofantrine haloperidol H2-blockers Index 649
11 650 Index HCC. See hepatocellular cancer (HCC) HDAC inhibitor. See histone deacetylase (HDAC) inhibitor head and neck cancer bleomycin and, 57 carboplatin and, 87 cetuximab and, cisplatin and, 104, 105 docetaxel and, 154, fluorouracil 05 hydroxyurea 22 ifosfamide 33 methotrexate 86 mitomycin-c 92 paclitaxel and, 323 hemangioma interferon-a 42 heparin dacarbazine and, 130 daunorubicin and, 139 epirubicin and, 171 idarubicin 29 mitoxantrone 99 hepatic function, and dosage, hepatocellular cancer (HCC) regimens, hepatoma 5-fluorouracil 05 Herceptin. See trastuzumab Herskovic regimen, Hexalen. See altretamine Hexamethylmelamine. See altretamine histone deacetylase (HDAC) inhibitor romidepsin, vorinostat, HMM. See altretamine Hodgkin s lymphoma bleomycin and, 57 brentuximab and, 68 carmustine and, chlorambucil and, 101 dacarbazine and, 130 etoposide and, 185 gemcitabine 17 ifosfamide 33 lomustine 71 mechlorethamine 74 primary induction procarbazine and, 353 thiotepa and, 396 Hopkins/Yale regimen, 508 hormonal agents abiraterone acetate, 5 7 albumin-bound paclitaxel, aminoglutethimide, anastrozole, bicalutamide, degarelix, enzalutamide, exemestane, flutamide, fulvestrant, goserelin, letrozole, leuprolide, megestrol acetate, nilutamide, tamoxifen, toremifene, H2-receptor inhibitors vismodegib and, HT3 receptor antagonist dolasetron, granisetron, ondansetron, palonosetron, HuMax-CD20. See ofatumumab Hycamtin. See topotecan Hydrea. See hydroxyurea hydrocortisone dacarbazine and, 130 Hydroxydaunorubicin. See doxorubicin hydroxyurea, , 531 cytarabine and, , 459 Hyper-CVAD/MTX-Ara-C regimen, Hyper-CVAD regimen, 523 hypereosinophilic syndrome (HES) imatinib 37, 238 hypomethylating agents azacitidine, decitabine, I ibritumomab, ibritumomab tiuxetan regimen, 552 ibutilide IBW. See ideal body weight (IBW) ICE (salvage regimen), Iclusig. See ponatinib Idamycin. See idarubicin idarubicin, , 459 ideal body weight (IBW), IDEC-Y2B8. See ibritumomab Ifex. See ifosfamide IFL Saltz regimen, IFN-a. See interferon-a IFN 1 DTIC regimen, 555 ifosfamide, cisplatin and, , 459 IL-2. See aldesleukin imatinib, , , 524, 530, , 459
12 Imidazole Carboxamide. See dacarbazine immunomodulatory agent thalidomide, immunomodulatory analog of thalidomide lenalidomide, pomalidomide, immunotherapy aldesleukin, denileukin difitox, interferon-a, sipuleucel-t, indinavir Inlyta. See axitinib interferon-a, , , 459 Interferon-a2a. See interferon- a interleukin-2 453, 459 Intron A. See interferon- a ionizing radiation cytarabine and, 123 IPB regimen, 512 ipilimumab, IP regimen, 512 Iressa. See gefitinib irinotecan, , 475, 493 colorectal cancer and cetuximab, , 459 IROX regimen, 500 Isophosphamide. See ifosfamide isotretinoin 453, 459 Istodax. See romidepsin ITP regimen, 470 itraconazole bexarotene and, 52 busulfan and, 71 cabozantinib and, 78 dasatinib and, 136 everolimus and, 193 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 IVAC regimen, 550 ixabepilone, , , 459 Ixempra. See ixabepilone J Jevtana. See cabazitaxel K Kadcyla. See ado-trastuzumab emtansine Kaposi s sarcoma regimens, daunorubicin liposome and, 143 doxorubicin liposome and, 164 interferon-a 42 paclitaxel and, 323 Karnofsky performance scale, 448 ketoconazole bexarotene and, 52 bortezomib and, 61 cabozantinib and, 78 dasatinib and, 136 docetaxel and, 155 erlotinib and, 179 everolimus and, 193 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 toremifene and, 402 tretinoin and, 415 Kyprolis. See carfilzomib Kytril and Sancuso Transdermal Patch. See granisetron L lansoprazole lapatinib, dialysis guidelines, , 459 Larson regimen, laryngeal cancer neoadjuvant L-Asparaginase. See asparaginase Index 651
13 652 Index lenalidomide, , 530, 553, , 459 leptomeningeal carcinomatosis cytarabine and, 123 letrozole, , 480, 486 adjuvant treatment to local treatment modalities, 3 leucovorin capecitabine and, 83 floxuridine and, fluorouracil 06 methotrexate 87 pemetrexed and, 336 pralatrexate and, 350 leukemia acute lymphoblastic, 2, 34, 112 acute promyelocytic, 30, 31 B-CLL, 17 chronic lymphocytic ( See chronic lymphocytic leukemia (CLL)) chronic myelomonocytic, 42 hairy cell, 109 T-cell prolymphocytic, 18 Leukeran. See chlorambucil leuprolide, , 459 Leustatin. See cladribine levodopa procarbazine and, 353 LHRH agonist goserelin, leuprolide, lidocaine dacarbazine and, 130 Linker regimen, lomustine, , 459 lorazepam, L-PAM. See melphalan Lupron. See leuprolide LV 5 FU 2 1 mitomycin-c regimen, 501 LY See pemetrexed lymphoma anaplastic large cell, 68 Burkitt s, etoposide and, 185 Hodgkin s ( See Hodgkin s lymphoma) mantle cell, 61 non-hodgkin s (See non-hodgkin s lymphoma) peripheral T-cell, 350 primary CNS ( See CNS lymphoma, primary) T-cell lymphoblastic, 303 Lysodren. See mitotane M MACOP-B, 547 MAGIC Trial for esophageal cancer, 508 magnesium hydroxide, capecitabine and, 83 Magrath Protocol, MAID regimen, 577 malignant melanoma. See melanoma, malignant mantle cell lymphoma bortezomib and, 61 lenalidomide 61 Marinol. See dronabinol Matulane. See procarbazine Mayo Clinic schedule, 495, 500 m-bacod, MCV regimen, 472 MDS. See myelodysplastic syndromes (MDS) mechlorethamine, , 459 medulloblastoma, 94 Megace. See megestrol acetate megestrol acetate, , 459 Mekinist. See trametinib melanoma, malignant aldesleukin and, 15 regimens, dacarbazine and, 130 interferon-a 42 ipilimumab 45 temozolomide and, 383 trametinib and, 409 melphalan, , , 459 meningeal leukemia methotrexate 86 meperidine procarbazine and, mercaptopurine, , 459 doxorubicin and, 160 mesna cisplatin and, 105 mesoridazine mesothelioma, malignant regimens, pemetrexed and, 335 methadone methotrexate, , 518, 554 asparaginase and, 34 cisplatin and, 105 cytarabine and, , fluorouracil 06 vincristine and, 432
14 metoclopramide, , 632, 634, 635 mfolfox 7 regimen, 495, 498 MINE (salvage regimen), 549 Mini-BEAM regimen, 542 Mitomycin. See mitomycin-c mitomycin-c, , doxorubicin and, 160 vinorelbine and, 435 mitotane, , 460 mitoxantrone, , 460 fludarabine 02 monoclonal antibody alemtuzumab, bevacizumab, brentuximab, cetuximab, ibritumomab, ipilimumab, ofatumumab, panitumumab, pertuzumab, rituximab, tositumomab, trastuzumab, ziv-aflibercept, MOPP/ABVD hybrid regimen, 541 MOPP regimen, motion sickness diphenhydramine and, 607 moxifloxacin 6-MP. See 6-mercaptopurine MPL regimen, 559 MP regimen, 558 MPT regimen, 559 MTX. See methotrexate multiple myeloma bortezomib and, 61 carfilzomib and, 91 carmustine and, 94 regimens, doxorubicin liposome and, 164 interferon-a 42 lenalidomide 61 melphalan 80 pomalidomide and, 342 thalidomide and, 389 Mustargen. See mechlorethamine Mutamycin. See mitomycin-c MVAC regimen, 471 myelodysplastic syndromes (MDS) azacitidine and, decitabine and, 146 idarubicin 29 imatinib 37, 238 lenalidomide 61 thalidomide and, 389 myeloproliferative disease (MPD) imatinib 37, 238 myelosuppressive drugs carboplatin and, 87 thiotepa and, 396 Myleran. See busulfan N nabilone, , 635 natural product arsenic trioxide, Navelbine. See vinorelbine nefazodone nelarabine, , , 460 nelfinavir neoadjuvant, 2 nephrotoxic agents streptozocin and, 372 neuroblastoma dacarbazine and, 130 Nexavar. See sorafenib Nilandron. See nilutamide nilotinib, , 514, 524, nilutamide, 220, , 460 Nitrogen mustard. See mechlorethamine N-Methylhydrazine. See procarbazine Nolvadex. See tamoxifen nonclassic alkylating agent altretamine, procarbazine, temozolomide, non-hodgkin s lymphoma bendamustine and, 45 bleomycin and, 57 carmustine and, chlorambucil and, 101 cisplatin and, 104 cladribine and, 109 Index 653
15 654 Index non-hodgkin s lymphoma Cont. cytarabine and, 123 etoposide and, 185 fludarabine 02 gemcitabine 17 ibritumomab 25 ifosfamide 33 interferon-a 42 lomustine 71 mechlorethamine 74 methotrexate 86 mitoxantrone 99 ofatumumab and, 312 primary induction procarbazine and, 353 rituximab and, 360 thiotepa and, 396 tositumomab and, 405 non-small cell lung cancer (NSCLC) adjuvant treatment to local treatment modalities, 3 albumin-bound paclitaxel for, 12 bevacizumab and, 48, 49 carboplatin and, 87 regimens, cisplatin and, 104, 105 docetaxel and, 154, 155 erlotinib and, 178 etoposide and, 185 etoposide phosphate and, 189 gefitinib 13 gemcitabine and, 216, 217 ifosfamide 33 ironotecan 51 mitomycin-c 92 neoadjuvant paclitaxel and, 323 pemetrexed and, 335 vinorelbine and, 435 nonsteroidal antiinflammatory drugs (NSAIDs) pemetrexed and, 336 pralatrexate and, 350 nonsteroidal aromatase inhibitor anastrozole, nontaxane eribulin, Novantrone. See mitoxantrone NSAIDs. See nonsteroidal anti-inflammatory drugs (NSAIDs) O octreotide, 490 ofatumumab, , , 460 omacetaxine mepesuccinate, Oncovin. See vincristine ondansetron, , 632, 633, cisplatin and, 106 Ontak. See denileukin difitox OSI-774. See erlotinib osteogenic sarcoma adjuvant treatment to local treatment modalities, ifosfamide 33 methotrexate 86 neoadjuvant ovarian cancer altretamine 1 carboplatin and, cisplatin and, 104 and, 118 docetaxel and, 154 doxorubicin liposome and, 164 etoposide and, fluorouracil 05 gemcitabine 16 hydroxyurea 22 melphalan 80 paclitaxel and, 323 thiotepa and, 396 topotecan and, 399 vinorelbine and, 435 oxaliplatin, capecitabine and, , 460 oxygen, bleomycin and, 57 P paclitaxel, , 473, 487, 493, 507, 510, , 520 albumin-bound, carboplatin and, 88 cisplatin and, 105 dialysis guidelines, , 460 vincristine and, 432 palonosetron, , 633, 634 pancreatic cancer erlotinib and, fluorouracil 05 gemcitabine 16, 217 mitomycin-c 92 oxaliplatin and, 319 streptozocin and, 372 pancreatic neuroendocrine tumors (PNET) panitumumab, , , 460 Paraplatin. See carboplatin Parkinsonism diphenhydramine and, 607
16 paroxetine pazopanib, , 460 PCB regimen, 534 PCE regimen, 488 PCR regimen, 527 PCV regimen, 473, 474 PD regimen, 560 PEB regimen, 57, 488, pegasparaginase 455, 460 Peginterferon-a2b. See interferon- a pemetrexed, , 473, , 460 penicillins methotrexate 87 pralatrexate and, 350 pentamidine pentostatin, 530 performance scales, 448 peripheral T-cell lymphoma (PTCL) pralatrexate and, 350 Perjeta. See pertuzumab perphenazine pertuzumab, , 460 PF 1 cetuximab regimen, 516 PF-larynx preservation, 517 PF regimen, 516 P-glycoprotein (Pgp) inhibitors nilotinib and, 306 vismodegib and, 439 phenobarbital bexarotene and, 52 busulfan and, 71 cabozantinib and, 78 chlorambucil and, 101 dacarbazine and, 130 dasatinib and, 136 doxorubicin and, 160 erlotinib and, 178 everolimus and, 192 exemestane and, 196 gefitinib 13 ifosfamide 33 imatinib 38 interferon-a 42 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 paclitaxel and, 324 temsirolimus and, 386 toremifene and, 402 tretinoin and, 415 phenothiazine prochlorperazine, Phenylalanine mustard. See melphalan phenytoin bexarotene and, 52 busulfan and, 71 cabozantinib and, 78 capecitabine and, 83 carmustine and, 94 chlorambucil and, 101 cisplatin and, 105 dacarbazine and, 130 doxorubicin and, 160 ifosfamide 33 interferon-a 42 methotrexate 87 mitotane 96 paclitaxel and, 324 streptozocin and, 372 temsirolimus and, 386 toremifene and, 402 vinorelbine and, 435 pimozide Platinol. See cisplatin platinum analogs carboplatin, cisplatin, oxaliplatin, platinum-based erlotinib and, 179 PLX4032. See vemurafenib PNET. See pancreatic neuroendocrine tumors (PNET) polycythemia vera hydroxyurea 22 melphalan 80 pomalidomide, , 460 Pomalyst. See pomalidomide ponatinib, , , 460 PONV. See postoperative nausea and vomiting (PONV) posaconazole Index 655
17 656 Index postoperative nausea and vomiting (PONV) aprepitant and, 603 dolasetron and, 609 granisetron and, 614 pralatrexate, , , 460 prednisone, 529 pregnancy, use of agents in, primary induction, 2 probenecid idarubicin 29 methotrexate 87 pralatrexate and, 350 procainamide procarbazine, , , 460 prochlorperazine, , 632, 635 progestational agents megestrol acetate, Proleukin. See aldesleukin ProMACE/CytaBOM regimen, 548 prostate cancer abiraterone acetate for, 6 aminoglutethimide 3 bicalutamide and, degarelix and, 149 docetaxel and, 154, 155 enzalutamide and, 168 estramustine and, 182 flutamide 09 goserelin 19 leuprolide 67 mitoxantrone 99 nilutamide and, 309 paclitaxel and, 323 sipuleucel-t and, 367 proteasome inhibitor bortezomib, carfilzomib, protein synthesis inhibitor omacetaxine mepesuccinate, proton pump inhibitors methotrexate 87 vismodegib and, 439 Provenge. See sipuleucel-t PTCL. See peripheral T-cell lymphoma (PTCL) Purinethol. See 6-mercaptopurine PVB regimen, 579 Q quinidine R RAD001. See everolimus radiation therapy bleomycin and, 58 cisplatin and, 105 docetaxel and, 155 gemcitabine 17 paclitaxel and, 323 R-CHOP regimen, R-CHOP- 14 regimen, 546 refractory anemia azacitidine and, 42 Reglan. See metoclopramide regorafenib, , , 461 renal cell cancer aldesleukin and, 15 bevacizumab and, interferon-a 42 megestrol acetate 77 temsirolimus and, 386 reserpine thalidomide and, 389 retinoid bexarotene, tretinoin, retinoid X receptor (RXR), 51 Revlimid. See lenalidomide R-FCM regimen, 545 R-GemOx (salvage regimen), 549 rhabdomyosarcoma dactinomycin-d, 133 embryonal, 2 RICE (salvage regimen), 549 rifampicin ixabepilone 55 lapatinib 58 letrozole 65 rifampin bexarotene and, 52 cabozantinib and, 78 dasatinib and, 136 erlotinib and, 178 everolimus and, 192 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 nilotinib and, 306 temsirolimus and, 386 tretinoin and, 415 ritonavir
18 Rituxan. See rituximab rituximab, , 529, 552 and B-cell non-hodgkin s lymphoma, , 461 ibritumomab 25 RMPT regimen, 561 Roferon Interferon-a2b. See interferon-a romidepsin, , 553 Roswell Park schedule, high dose regimen, 500 RTOG chemoradiation regimen, RTOG/ECOG regimen, 467 Rubidomycin. See daunorubicin RVD regimen, 560 S salvage, 468 saquinavir sertraline SGN-35. See brentuximab signal transduction inhibitor axitinib, bosutinib, cabozantinib, crizotinib, dabrafenib, dasatinib, erlotinib, everolimus, gefitinib, imatinib, lapatinib, nilotinib, pazopanib, ponatinib, regorafenib, sorafenib, sunitinib, temsirolimus, trametinib, vandetanib, vemurafenib, vismodegib, sipuleucel-t, Thioguanine. See thioguanine SKI-606. See bosutinib skin cancer. See also cutaneous T-cell lymphoma (CTCL); melanoma, malignant bleomycin and, 57 5-fluorouracil 05 small cell lung cancer carboplatin and, cisplatin and, 104 docetaxel and, 154 etoposide and, 185 etoposide phosphate and, 189 ifosfamide 33 ironotecan 51 paclitaxel and, 323 topotecan and, 399 sodium thiosulfate mechlorethamine 74 soft tissue sarcoma (STS) dacarbazine and, 130 gemcitabine 17 ifosfamide 33 sorafenib, , 514, , 461 sotalol sparfloxacin Sprycel. See dasatinib squamous cell carcinomas bleomycin and, 57 St. John s Wort bortezomib and, 62 cabozantinib and, 78 dasatinib and, 136 erlotinib and, everolimus and, 192 exemestane and, 196 gefitinib 13 imatinib 38 ironotecan 51 ixabepilone 55 lapatinib 58 letrozole 65 nilotinib and, 306 temsirolimus and, 386 Stanford regimen, Stanford V regimen, 541 stem cell transplant busulfan with in, 71 carboplatin dose during, 87 steroidal aromatase inactivator exemestane, Index 657
19 658 Index steroids melphalan 80 mitotane 96 streptozocin and, 372 STI571. See imatinib Stivarga. See regorafenib streptozocin, , 461 Streptozotocin. See streptozocin STS. See soft tissue sarcoma (STS) SU See sunitinib substance P/NK1 receptor antagonist aprepitant, substituted benzamide metoclopramide, sulfamethoxazole pralatrexate and, 350 sulfinpyrazone idarubicin 29 sunitinib, , 490, , 461 Sutent. See sunitinib Synribo. See omacetaxine mepesuccinate T TAC regimen, Tafinlar. See dabrafenib T-ALL. See T-cell acute lymphoblastic leukemia (T-ALL) tamoxifen, , 480, 486 adjuvant treatment to local treatment modalities, 3 455, 461 Tarceva. See erlotinib Targretin. See bexarotene Tasigna. See nilotinib taxanes albumin-bound paclitaxel, cabazitaxel, docetaxel, paclitaxel, trastuzumab and, 412 Taxol. See paclitaxel Taxotere. See docetaxel T-cell acute lymphoblastic leukemia (T-ALL) nelarabine and, 303 T-cell lymphoblastic lymphoma (T-LBL) nelarabine and, 303 T-cell prolymphocytic leukemia alemtuzumab and, 18 TCH regimen, 479, 484 TC regimen, 476 telithromycin Temodar. See temozolomide temozolomide, , 475, 554 dialysis guidelines, , 461 temsirolimus, dialysis guidelines, teratogenic potential, agents, terfenadine testicular cancer bleomycin and, cisplatin and, 104, 105 etoposide and, 185 etoposide phosphate and, 189 ifosfamide 33 thalidomide, , , 461 Thalomid. See thalidomide thiazide diuretics toremifene and, 402 thiethylperazine, 632 thioguanine, , 461 Thioplex. See thiotepa thioridazine thiotepa, , 461 thrombocytosis hydroxyurea 22 thymidine floxuridine and, fluorouracil 06 methotrexate 87 pemetrexed and, 336 pralatrexate and, 350 thymoma regimens, thyroid cancer cabozantinib and, 78 regimens, ticlopidine TIC regimen, 515 TIP (salvage regimen), 580 TIP regimen, 515 T-LBL. See T-cell lymphoblastic lymphoma (T-LBL) topoisomerase II inhibitor etoposide, etoposide phosphate,
20 topoisomerase I inhibitor ironotecan, topotecan, topotecan, , 493, 507, , 461 toremifene, , 486 Torisel. See temsirolimus tositumomab, TPF induction followed by carboplatin 1 radiation therapy, TPF regimen, 515 trametinib, , 461 trastuzumab, , , 461 doxorubicin and, 160 pertuzumab and, 339 Treanda. See bendamustine tretinoin, , 461 tricyclic antidepressants procarbazine and, 353 Triethylenethiophosphoramide. See thiotepa trimethroprim pralatrexate and, 350 trimetrexate 5-fluorouracil 06 Trisenox. See arsenic trioxide (As 2 O 3 ) troleandomycin trophoblastic neoplasms cisplatin and, 104 Tykerb. See lapatinib U unclassified therapeutic agent lenalidomide, pomalidomide, thalidomide, V VAB-6 regimen, 579 VAD regimen, 559 vandetanib, VCR. See vincristine Vectibix. See panitumumab VeIP (salvage regimen), Velban. See vinblastine Velcade. See bortezomib vemurafenib, VePesid. See etoposide verapamil everolimus and, 193 ixabepilone 55 tretinoin and, 415 vermurafenib dialysis guidelines, 464 Vesanoid. See tretinoin Vidaza. See azacitidine vinblastine, dialysis guidelines, , 461 vinca alkaloid vinblastine, vincristine, vinorelbine, vincristine, asparaginase and, , 461 vinorelbine, , 487, 518, , 461 VIP (salvage regimen), 580 VIP regimen, 581 vismodegib, vismodegih, 469 vistonuridine (PN401 ) floxuridine and, fluorouracil 06 vitamin A supplements tretinoin and, 415 voriconazole vorinostat, , 461 Votrient. See pazopanib VP-16. See etoposide VP regimen, 517 W Waldenstrom s macroglobulinemia chlorambucil and, 101 warfarin bicalutamide and, 55 capecitabine and, 83 erlotinib and, 179 etoposide and, 185 etoposide phosphate and, 189 flutamide 09 gefitinib 13 ifosfamide 34 imatinib and, lapatinib 58 letrozole 65 methotrexate and, 287 mitotane 96 nilutamide and, 309 tamoxifen and, 379 toremifene and, 402 vemurafenib and, 423 vorinostat and, 442 Index 659
21 Wilms tumor adjuvant treatment to local treatment modalities, 3 dactinomycin-d, 133 primary induction X Xalkori. See crizotinib XELIRI regimen, Xeloda. See capecitabine XELOX 1 bevacizumab regimen, 501 XELOX 1 radiation therapy, 468, XELOX regimen, 82, 496, 499 XP 1 trastuzumab regimen, 512 Xtandi. See enzalutamide Y Yervoy. See ipilimumab Z Zaltrap. S ee ziv-aflibercept Zanosar. See streptozocin ZD1839. See gefitinib Zelboraf. See vemurafenib Zevalin. See ibritumomab ziv-aflibercept, Zofran. See ondansetron Zoladex. See goserelin Zolinza. See vorinostat Zytiga. See abiraterone acetate 660 Index
22 Index 661
23 662 Index
24 Index 663
25 664 Index
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Formulary Chemotherapy Agents: (Current as of 6/2018) Therapeutic Class
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
Genetics in Cancer Therapy. Raju Kucherlapati, Ph.D. Harvard Medical School
 Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Overview of Cancer. Laura Bingell RN Transition Center Nurse for MFP (607)
 Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
ANTIEMETICS UTILIZATION MANAGEMENT CRITERIA
 ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS
 H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
Introduction to Antineoplastic Prescribing
 Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Ovarian Cancer. compendia TREATMENT OF
 drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
DRUG PROPERTIES YOU NEED TO KNOW
 jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Bladder Cancer (Urothelial) Pathways
 Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
ALKYLATING AGENTS (22)
 Serving the United States, Canada and North Central America MEMBER OF TEXAS HEALTH CARE ENTITIES A PRIVATE MEMBERSHIP MEDICAL ASSOCIATION 3105 MAIN STREET, ROWLETT, TX 75088 TEL: 214-299-9449 office@rgccusa.com
Serving the United States, Canada and North Central America MEMBER OF TEXAS HEALTH CARE ENTITIES A PRIVATE MEMBERSHIP MEDICAL ASSOCIATION 3105 MAIN STREET, ROWLETT, TX 75088 TEL: 214-299-9449 office@rgccusa.com
Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi )
 09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
ORAL ONCOLOGY CRITERIA
 ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
Cancer drug approvals for paediatric indications (n=43)
 Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
2. Date of most recent breast cancer diagnosis / / 3. Pathlogy: Padget s Phyllodes Tubular Medullary Mucinous
 Breast Cancer Patients: Begin on page 1, then skip to page 3 Lymphoma Patients: Begin on page 2 1. Newly diagnosed with Breast carcinoma Stage: (includes inflammatory breast and newly diagnosed recurrent
Breast Cancer Patients: Begin on page 1, then skip to page 3 Lymphoma Patients: Begin on page 2 1. Newly diagnosed with Breast carcinoma Stage: (includes inflammatory breast and newly diagnosed recurrent
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
University of Groningen. Health economics of targeted cancer therapies Mihajlovic, Jovan
 University of Groningen Health economics of targeted therapies Mihajlovic, Jovan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Health economics of targeted therapies Mihajlovic, Jovan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
Bladder Cancer Pathways (Urothelial)
 Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
DRUGS YOU NEED TO KNOW
 jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Supplementary Table 1. Summary of FDA approval data for rare cancer indications from December 1987 to May 2011
 Rare Cancer : Lessons from FDA s by Gaddipati et al. Supplementary Table 1 Summary of FDA approval data for rare cancer indications from December 1987 to May 2011 1. mitoxantrone hydrochloride (NOVANTRONE
Rare Cancer : Lessons from FDA s by Gaddipati et al. Supplementary Table 1 Summary of FDA approval data for rare cancer indications from December 1987 to May 2011 1. mitoxantrone hydrochloride (NOVANTRONE
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
ORAL ONCOLOGY CRITERIA
 ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
CancerPACT Cancer Patients Alliance for Clinical Trials
 TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Salinas Valley Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Salinas Valley Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
FREEDOM OF INFORMATION SOUTH EAST SCOTLAND CANCER NETWORK
 Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
BLOOD AND LYMPH CANCERS
 BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
Systemic Anticancer Therapy Drug Interactions Table
 Systemic Anticancer Therapy Drug Interactions Table 1 This guide is intended to cover the most common interactions involving Systemic Anticancer therapies, e.g. cytotoxic agents, monoclonal antibodies
Systemic Anticancer Therapy Drug Interactions Table 1 This guide is intended to cover the most common interactions involving Systemic Anticancer therapies, e.g. cytotoxic agents, monoclonal antibodies
Supplementary Figures
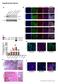 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS. MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS
 ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
Cancer Drugs CANCER DRUGS NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS
 Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
Fundamentals of Pharmacology for Veterinary Technicians Chapter 20
 Figure 20-1 Figure 20-2 Figure 20-3A Figure 20-3B Figure 20-4 Table 20-1 Antineoplastic Agents Used for Chemotherapy in Animals Drug Type Drug Category Examples Cell-Cycle Effect CCNS Alkylating agents:
Figure 20-1 Figure 20-2 Figure 20-3A Figure 20-3B Figure 20-4 Table 20-1 Antineoplastic Agents Used for Chemotherapy in Animals Drug Type Drug Category Examples Cell-Cycle Effect CCNS Alkylating agents:
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment
 Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
2. Mitotic Spindle Inhibitors (modulators of tubulin polymerisation) 3. Antimetabolites (anti-folates, pyrimidine and purine analogues)
 CANCER DRUG CLASSES The classes of drugs currently used in the cancer clinic are 1. DNA Binding Agents (intercalating and alkylating agents) 2. Mitotic Spindle Inhibitors (modulators of tubulin polymerisation)
CANCER DRUG CLASSES The classes of drugs currently used in the cancer clinic are 1. DNA Binding Agents (intercalating and alkylating agents) 2. Mitotic Spindle Inhibitors (modulators of tubulin polymerisation)
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting
 Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
Chemotherapy Agents 101
 Chemotherapy Agents 101 Jumana Ashy, Pharm D, BCOP Judith Misas, PharmD Candidate 2018 Jeremy Hutchinson, PharmD Sady Castance, PharmD September 12, 2017 1 Learning Objectives At the end of the presentation
Chemotherapy Agents 101 Jumana Ashy, Pharm D, BCOP Judith Misas, PharmD Candidate 2018 Jeremy Hutchinson, PharmD Sady Castance, PharmD September 12, 2017 1 Learning Objectives At the end of the presentation
4. Shown below is the structure of doxorubicin (Adriamycin). What is true about this agent?
 Midterm 2: 3 points each (except final question worth 1 point 1. A useful regimen for treating colorectal cancer is FOLFIRI. What is true about this regimen? A. The regimen includes folinic acid, 5-fluorouracil
Midterm 2: 3 points each (except final question worth 1 point 1. A useful regimen for treating colorectal cancer is FOLFIRI. What is true about this regimen? A. The regimen includes folinic acid, 5-fluorouracil
Hazard Meds: Navigating the Warning Beacons
 Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
Melanoma. compendia. Associated ICD-9-CM Codes: Drug & Administration
 Drug & Administration compendia Treatment of Melanoma With each publication, ManagedCare Oncology s Drug & Administration Compendia highlights a single medication or a group of medications that could be
Drug & Administration compendia Treatment of Melanoma With each publication, ManagedCare Oncology s Drug & Administration Compendia highlights a single medication or a group of medications that could be
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
CancerPACT Cancer Patients Alliance for Clinical Trials
 TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Sacramento Area Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
Domperidone: Drug Interactions & Cautions (Mayo Clinic)
 Domperidone: Drug Interactions & Cautions (Mayo Clinic) Before Using In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you
Domperidone: Drug Interactions & Cautions (Mayo Clinic) Before Using In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you
Leukemia. Treatment of. compendia. Associated ICD-9-CM Codes: Drug & Administration. managedcareoncology.com
 Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
ANTIEMETIC GUIDELINES: MASCC/ESMO
 Open Issues for CINV Do we reliably measure that? Do we control nausea optimally? Are guidelines useful for oral therapies related nausea and vomiting? Breakthrough and refractory nausea and vomiting:
Open Issues for CINV Do we reliably measure that? Do we control nausea optimally? Are guidelines useful for oral therapies related nausea and vomiting? Breakthrough and refractory nausea and vomiting:
Objectives. Principles of Cancer Treatment. Cancer Treatment Modalities. Cancer Treatment Modalities
 Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
