Hodgkin s Lymphoma (Hodgkin s Disease, HD) PI HD-1 Protocol
|
|
|
- Barnaby Moris Fisher
- 5 years ago
- Views:
Transcription
1 1 Hodgkin s Lymphoma (Hodgkin s Disease, HD) PI HD-1 Protocol NCCN Pacific Island Working Group Clinical Members Scott Macfarlane Peter Bradbeer Jane Skeen Rob Corbett Radhika Sandilya PI HD-1 Updated 30/10/17 Page 1
2 2 TREATMENT OUTLINE 6 cycles of identical treatment for all stages and HD histologies Doxorubicin, Bleomycin, Vinblastine, Dacarbazine (ABVD) Each cycle 28 days. Total therapy duration, 6 months PI HD-1 Updated 30/10/17 Page 2
3 3 TABLE OF CONTENTS Section Page Treatment Outline Aims 4 Primary 4 Secondary Rationale for study design Patient eligibility Exclusions Initial evaluation Registration Treatment Toxicity and Dose Modification Administration of Therapy Completion of Therapy 6 PI HD-1 Updated 30/10/17 Page 3
4 4 1.0 AIMS Primary 1.1 To increase the proportion of children with Hodgkin s Lymphoma who are cured. Secondary 1.2 To assess the ability of Pacific Island health systems to deliver chemotherapy according to a well-recognised HD protocol. 1.3 To assess the ability of Pacific Island health systems to provide supportive care guided by protocol and shared care advice from NZ centres. 2.0 RATIONALE FOR STUDY DESIGN 2.1 Children and young people in the Pacific have not enjoyed the survival of their peers in developed health systems. This has been the result of a number of factors including late or non-diagnosis, treatment toxicity on protocols considered standard in developed health systems and treatment abandonment due to expense and family dislocation. This protocol has been drawn from strategies used by New Zealand and Australian paediatricians to treat children with HD in the past, at a time when the ability to treat and support children with malignant disease was at an early stage of development. An extensive literature supports the selection of ABVD as a historically confirmed protocol suitable for outpatient administration in less developed health systems. 2.2 The protocol should be able to be delivered in its entirety in Fiji. Eligible patients in Samoa and Tonga will be referred to New Zealand for confirmation of diagnosis, staging and commencement on therapy. All patients will be repatriated for ongoing therapy. 3.0 PATIENT ELIGIBILITY 3.1 Newly diagnosed patients with Hodgkin s lymphoma of any age are eligible. All histological types of Hodgkin s Lymphoma are eligible. All stages (IA-IVB) are eligible. 4.0 EXCLUSIONS 4.1 None PI HD-1 Updated 30/10/17 Page 4
5 5 5.0 INITIAL EVALUATION 5.1 Complete history including family history. 5.2 Complete physical examination including careful documentation of size of lymph nodes, spleen and liver size, presence of extra-medullary involvement. 5.3 Chest X-ray. 5.4 CT scan of affected area plus chest and abdomen (if available). 5.5 Full blood and platelet count. 5.6 Bone marrow aspirate and trephine if FBC suggests marrow involvement For morphology and cytochemistry. 6.0 REGISTRATION 6.1 Upon diagnosis, all patients with Hodgkin s Lymphoma will be recorded on the unit registry. 7.0 TREATMENT 7.1 All patients with biopsy proven Hodgkin s receive identical therapy. 7.2 The initial cycle of therapy should be given as soon as practically possible after appropriate supportive care has been given. This will include correction of anaemia (if applicable), treatment of infection and any co-morbidities. 7.3 First Cycle of ABVD Doxorubicin 25mg/m 2 iv days 1 and 15 Bleomycin 10mg/m 2 iv days 1 and 15 (1mg=1000IU) Vinblastine 6 mg/m 2 iv days 1 and 15 Dacarbazine 375mg/m 2 iv days 1 and Second cycle of ABVD starts on day 29. Subsequent cycles are at 28 day intervals until completion of 6 cycles PI HD-1 Updated 30/10/17 Page 5
6 6 8.0 TOXICITY AND DOSE MODIFICATION 8.1 In general, haematological toxicity will result in delay of the subsequent course of chemotherapy rather than dose reduction. (Await recovery of neutrophil count to >1.0x 109/L and platelets to >100x 109/L before giving day 1 therapy each cycle, but do not delay day 15 chemotherapy for blood count alone.) 8.2 If consecutive haematologic toxicity occurs, consider dose reduction by 25%. 8.3 Signs of congestive heart failure are an absolute contraindication for continuing Doxorubicin. Discuss with New Zealand centre. 9.0 ADMINISTRATION OF THERAPY 9.1 Doxorubicin and Vinblastine are vesicant agents and every care must be taken to ensure that extravasation does not occur. This will mean the establishment of reliable peripheral venous access for day 1 and day 15 chemotherapy administration and checking for vascular patency with a flush of normal saline before commencing chemotherapy administration by slow iv push or supervised infusion without the use of an infusion pump COMPLETION OF THERAPY 10.1 Documentation of response including appropriate imaging (where available) will take place 1 month after the 6 th and final cycle of ABVD has been completed Follow-up to document outcome and any treatment toxicity will be documented and will form the basis of a future Hodgkin s Lymphoma treatment strategy. PI HD-1 Updated 30/10/17 Page 6
GERM CELL TUMOUR PI GC-1
 GERM CELL TUMOUR PI GC-1 NCCN Pacific Island Working Group Clinical Members Scott Macfarlane Peter Bradbeer Jane Skeen Rob Corbett Radhika Sandilya Page 1 2 TREATMENT OUTLINE 6* cycles of identical treatment
GERM CELL TUMOUR PI GC-1 NCCN Pacific Island Working Group Clinical Members Scott Macfarlane Peter Bradbeer Jane Skeen Rob Corbett Radhika Sandilya Page 1 2 TREATMENT OUTLINE 6* cycles of identical treatment
PVACE-BOP (Hodgkin s Lymphoma)
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1 Ondansetron 8mg IV / Oral vinblastine 6mg/m 2 (Max: 10mg) IV Infusion Etoposide 100mg/m 2 IV infusion Patients over 65 years by 15 min infusion
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1 Ondansetron 8mg IV / Oral vinblastine 6mg/m 2 (Max: 10mg) IV Infusion Etoposide 100mg/m 2 IV infusion Patients over 65 years by 15 min infusion
Hodgkin's Lymphoma. Symptoms. Types
 Hodgkin's lymphoma (Hodgkin's disease) usually develops in the lymphatic system, a part of the body's immune system. This system carries disease-fighting white blood cells throughout the body. Lymph tissue
Hodgkin's lymphoma (Hodgkin's disease) usually develops in the lymphatic system, a part of the body's immune system. This system carries disease-fighting white blood cells throughout the body. Lymph tissue
Comparison of Three Radiation Dose Levels after EBVP Regimen in Favorable Supradiaphragmatic Clinical Stages I-II Hodgkin s Lymphoma (HL):
 Comparison of Three Radiation Dose Levels after EBVP Regimen in Favorable Supradiaphragmatic Clinical Stages I-II Hodgkin s Lymphoma (HL): Preliminary Results of the EORTC-GELA H9-F Trial H. Eghbali, P.
Comparison of Three Radiation Dose Levels after EBVP Regimen in Favorable Supradiaphragmatic Clinical Stages I-II Hodgkin s Lymphoma (HL): Preliminary Results of the EORTC-GELA H9-F Trial H. Eghbali, P.
Relapse After Transplant: Next Steps for Patients with Hodgkin Lymphoma
 Hi! My name is Alison Moskowitz. I am an attending at Memorial Sloan Kettering Cancer Center within the Lymphoma Department. I am speaking on behalf of ManagingHodgkinLymphoma.com. I will be discussing
Hi! My name is Alison Moskowitz. I am an attending at Memorial Sloan Kettering Cancer Center within the Lymphoma Department. I am speaking on behalf of ManagingHodgkinLymphoma.com. I will be discussing
NECN CHEMOTHERAPY HANDBOOK PROTOCOL
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
2007 ANNUAL SITE STUDY HODGKIN S LYMPHOMA
 2007 ANNUAL SITE STUDY HODGKIN S LYMPHOMA SUSQUEHANNA HEALTH David B. Nagel, M.D. April 11, 2008 Hodgkin s lymphoma was first described by Thomas Hodgkin in 1832. It remained an incurable malignancy until
2007 ANNUAL SITE STUDY HODGKIN S LYMPHOMA SUSQUEHANNA HEALTH David B. Nagel, M.D. April 11, 2008 Hodgkin s lymphoma was first described by Thomas Hodgkin in 1832. It remained an incurable malignancy until
2.07 Protocol Name: CHOP & Rituximab
 2.07 Protocol Name: CHOP & Rituximab Indication Intermediate and high grade, B-cell non-hodgkins lymphoma expressing CD20. Second or third line therapy for low grade, B cell non- Hodgkins lymphoma expressing
2.07 Protocol Name: CHOP & Rituximab Indication Intermediate and high grade, B-cell non-hodgkins lymphoma expressing CD20. Second or third line therapy for low grade, B cell non- Hodgkins lymphoma expressing
Lymphoma Case Scenario 1
 Lymphoma Case Scenario 1 HISTORY: A 23-year-old healthy female presented with a month-long history of persistent headache of increasing severity. She noted episodic nausea and vomiting in association with
Lymphoma Case Scenario 1 HISTORY: A 23-year-old healthy female presented with a month-long history of persistent headache of increasing severity. She noted episodic nausea and vomiting in association with
Breast Pathway Group Docetaxel in Advanced Breast Cancer
 Breast Pathway Group Docetaxel in Advanced Breast Cancer Indication: First-line palliative treatment, with or without trastuzumab, for advanced breast cancer in patients for whom an anthracycline is not
Breast Pathway Group Docetaxel in Advanced Breast Cancer Indication: First-line palliative treatment, with or without trastuzumab, for advanced breast cancer in patients for whom an anthracycline is not
DA-EPOCH-R (Etoposide/Inpatient)
 DA- (Etoposide/Inp) INDICATION High grade lymphoma. Omit rituximab if CD20 negative. PRE-ASSESSMENT 1. Ensure histology is confirmed prior to administration of chemotherapy and document in notes. 2. Record
DA- (Etoposide/Inp) INDICATION High grade lymphoma. Omit rituximab if CD20 negative. PRE-ASSESSMENT 1. Ensure histology is confirmed prior to administration of chemotherapy and document in notes. 2. Record
2017 Repeat Audit of Red cell and Platelet Transfusion in Adult Haematology Patients
 07 Repeat Audit of Red cell and Platelet Transfusion in Adult Haematology Patients Haematology Audit July 07 The audit was conducted on adults undergoing surgery and who received a transfusion during a
07 Repeat Audit of Red cell and Platelet Transfusion in Adult Haematology Patients Haematology Audit July 07 The audit was conducted on adults undergoing surgery and who received a transfusion during a
Note: There are other bendamustine protocols, ensure this is the correct one for a given patient.
 INDICATIONS 1 st line treatment for follicular lymphoma with FLIPI score 2 or higher: (NICE TA513- BLUETEQ required) Rituximab refractory follicular lymphoma (progression on R-chemo, R-maintenance or within
INDICATIONS 1 st line treatment for follicular lymphoma with FLIPI score 2 or higher: (NICE TA513- BLUETEQ required) Rituximab refractory follicular lymphoma (progression on R-chemo, R-maintenance or within
NCCP Chemotherapy Protocol. INDICATION ICD10 Protocol Code Hodgkin s Lymphoma C a
 ABVD THERAPY INDICATIONS FOR USE: INDICATION ICD10 Protocol Code Hodgkin s Lymphoma C81 00290a ELIGIBILTY: Indications as above ECOG 0-3. Patients with an ECOG 4 may be considered for treatment at the
ABVD THERAPY INDICATIONS FOR USE: INDICATION ICD10 Protocol Code Hodgkin s Lymphoma C81 00290a ELIGIBILTY: Indications as above ECOG 0-3. Patients with an ECOG 4 may be considered for treatment at the
This is a controlled document and therefore must not be changed or photocopied L.80 - R-CHOP-21 / CHOP-21
 R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
ABVD for Hodgkin s Lymphoma (LYMWOS008/1)
 West of Scotland Cancer Network Chemotherapy Protocol Indication 1 st line treatment of Hodgkin s Lymphoma Eligibility Criteria ABVD for Hodgkin s Lymphoma (LYMWOS008/1) Confirmed diagnosis of Hodgkin
West of Scotland Cancer Network Chemotherapy Protocol Indication 1 st line treatment of Hodgkin s Lymphoma Eligibility Criteria ABVD for Hodgkin s Lymphoma (LYMWOS008/1) Confirmed diagnosis of Hodgkin
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
R-GemOx. Lymphoma group INDICATION. Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative
 R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma
 Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
THORACIC MALIGNANCIES
 THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
North of Scotland Cancer Network Clinical Management Guideline for Cancer of the Ovary
 North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
Haematological malignancies in England Cancers Diagnosed Haematological malignancies in England Cancers Diagnosed
 Northern and Yorkshire Cancer Registry and Information Service Haematological malignancies in England Cancers Diagnosed 2001-2008 Haematological 2001-2008 Malignancies www.nycris.nhs.uk www.nycris.nhs.uk
Northern and Yorkshire Cancer Registry and Information Service Haematological malignancies in England Cancers Diagnosed 2001-2008 Haematological 2001-2008 Malignancies www.nycris.nhs.uk www.nycris.nhs.uk
Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand
 Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand This list provides indications for the majority of adult BMTs that are performed in New Zealand. A small number of BMTs
Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand This list provides indications for the majority of adult BMTs that are performed in New Zealand. A small number of BMTs
PACIFIC ISLAND WORKSTREAM BURKITT LYMPHOMA GUIDELINE. Burkitt Lymphoma MODIFIED LMB PROTOCOL
 PACIFIC ISLAND WORKSTREAM BURKITT LYMPHOMA GUIDELINE For The Treatment of Burkitt Lymphoma MODIFIED LMB PROTOCOL DR ROB CORBETT ASSOCIATE PROFESSOR MICHAEL SULLIVAN Children s Haematology Oncology Centre
PACIFIC ISLAND WORKSTREAM BURKITT LYMPHOMA GUIDELINE For The Treatment of Burkitt Lymphoma MODIFIED LMB PROTOCOL DR ROB CORBETT ASSOCIATE PROFESSOR MICHAEL SULLIVAN Children s Haematology Oncology Centre
LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center
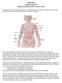 LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center Lymphoma is cancer of the lymphatic system. The lymphatic system is made up of organs all over the body that make up and store cells
LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center Lymphoma is cancer of the lymphatic system. The lymphatic system is made up of organs all over the body that make up and store cells
ABVD. by infusion over 30 minutes. You will have treatment on Day 1 and Day 15 (this is one cycle) for 2, 3 or 6 cycles.
 ABVD ABVD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others it may
ABVD ABVD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others it may
Lymphology 28 (1995) 73-77
 73 Lymphology 28 (1995) 73-77 Lymphological Laboratory (PH), Department of Gynecology, University of Tiibingen, Germany and Fifth Department of Internal Medicine (EZ), Silesian Medical Academy, Poland
73 Lymphology 28 (1995) 73-77 Lymphological Laboratory (PH), Department of Gynecology, University of Tiibingen, Germany and Fifth Department of Internal Medicine (EZ), Silesian Medical Academy, Poland
Lymphoma accounts for 10-20% of all canine cancers and is by far the most common canine blood cancer.
 Lymphoma What is cancer? Cancer is the uncontrolled growth of a small population of abnormal cells. These abnormal cells form by a mutation during the normal division cycle and are able to escape detection
Lymphoma What is cancer? Cancer is the uncontrolled growth of a small population of abnormal cells. These abnormal cells form by a mutation during the normal division cycle and are able to escape detection
SOUTH THAMES CHILDREN S CANCER NETWORK GROUP. REFERRAL PROTOCOLS AND DIAGNOSIS AND STAGING PROTOCOLS October 2014
 SOUTH THAMES CHILDREN S CANCER NETWORK GROUP REFERRAL PROTOCOLS AND DIAGNOSIS AND STAGING PROTOCOLS October 2014 Contents 1. Leukaemia Referral, Diagnostic and Staging Guidelines 2. Lymphoma Referral,
SOUTH THAMES CHILDREN S CANCER NETWORK GROUP REFERRAL PROTOCOLS AND DIAGNOSIS AND STAGING PROTOCOLS October 2014 Contents 1. Leukaemia Referral, Diagnostic and Staging Guidelines 2. Lymphoma Referral,
R-GDP: Rituximab, Gemcitabine, Dexamethasone &Cisplatin
 : Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
: Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma
 Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer
 Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for patients in whom anthracyclines are contraindicated or inappropriate Regimen
Breast Pathway Group TC (Docetaxel / Cyclophosphamide) in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for patients in whom anthracyclines are contraindicated or inappropriate Regimen
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
Study Title The SACS trial - Phase II Study of Adjuvant Therapy in CarcinoSarcoma of the Uterus
 Study Title The SACS trial - Phase II Study of Adjuvant Therapy in CarcinoSarcoma of the Uterus Investigators Dr Bronwyn King, Peter MacCallum Cancer Centre Dr Linda Mileshkin, Peter MacCallum Cancer Centre
Study Title The SACS trial - Phase II Study of Adjuvant Therapy in CarcinoSarcoma of the Uterus Investigators Dr Bronwyn King, Peter MacCallum Cancer Centre Dr Linda Mileshkin, Peter MacCallum Cancer Centre
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
TEMSIROLIMUS in renal cell cancer
 Systemic Anti Cancer Treatment Protocol TEMSIROLIMUS in renal cell cancer PROTOCOL REF: MPHARTEMS (Version No: 1.0) Approved for use in: First-line treatment of adult patients with advanced renal cell
Systemic Anti Cancer Treatment Protocol TEMSIROLIMUS in renal cell cancer PROTOCOL REF: MPHARTEMS (Version No: 1.0) Approved for use in: First-line treatment of adult patients with advanced renal cell
(R) CHOEP. May be used for stage IA - IV Diffuse Large B Cell non-hodgkin lymphoma in combination with rituximab.
 (R) CHOEP Indication Treatment of stage IA - IV T cell non-hodgkin lymphoma as an alternative to CHOP in younger, fitter patients with normal LDH level. May be used for stage IA - IV Diffuse Large B Cell
(R) CHOEP Indication Treatment of stage IA - IV T cell non-hodgkin lymphoma as an alternative to CHOP in younger, fitter patients with normal LDH level. May be used for stage IA - IV Diffuse Large B Cell
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Elderly Patients with Hodgkin s Lymphoma: FIL experience. Massimo Federico University of Modena and Reggio Emilia
 Elderly Patients with Hodgkin s Lymphoma: FIL experience Massimo Federico University of Modena and Reggio Emilia RESULTS OF VbMp CHEMOTHERAPY REGIMEN IN ELDERLY PATIENTS WITH HODGKIN DISEASE: THE GISL
Elderly Patients with Hodgkin s Lymphoma: FIL experience Massimo Federico University of Modena and Reggio Emilia RESULTS OF VbMp CHEMOTHERAPY REGIMEN IN ELDERLY PATIENTS WITH HODGKIN DISEASE: THE GISL
R-GDP: Rituximab, Gemcitabine, Dexamethasone &Cisplatin
 : Rituximab, &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma. TREATMENT INTENT Palliative or curative depending on context.
: Rituximab, &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma. TREATMENT INTENT Palliative or curative depending on context.
ALCL 99. International protocol for the treatment of childhood anaplastic large cell lymphoma
 ALCL 99 International protocol for the treatment of childhood anaplastic large cell lymphoma November 1999 1. BACKGROUND...4 1.1. RESULTS OF THE DIFFERENT EUROPEAN NATIONAL PROTOCOLS...4 1.1.1. BFM studies...4
ALCL 99 International protocol for the treatment of childhood anaplastic large cell lymphoma November 1999 1. BACKGROUND...4 1.1. RESULTS OF THE DIFFERENT EUROPEAN NATIONAL PROTOCOLS...4 1.1.1. BFM studies...4
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 16 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Paclitaxel and Trastuzumab Breast Cancer
 Systemic Anti Cancer Treatment Protocol Paclitaxel and Trastuzumab Breast Cancer PROTOCOL REF: MPHAPTRBR (Version No: 1.0) Approved for use in: HER2 positive breast cancer. For adjuvant use in T1 or T2
Systemic Anti Cancer Treatment Protocol Paclitaxel and Trastuzumab Breast Cancer PROTOCOL REF: MPHAPTRBR (Version No: 1.0) Approved for use in: HER2 positive breast cancer. For adjuvant use in T1 or T2
Liposomal Doxorubicin (CAELYX) Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Liposomal Doxorubicin (CAELYX) Gynaecological Cancer PROCTOCOL REF: OPHAGYNCAE (Version No: 1.0) Approved for use in: Advanced ovarian cancer second/third line treatment
Systemic Anti Cancer Treatment Protocol Liposomal Doxorubicin (CAELYX) Gynaecological Cancer PROCTOCOL REF: OPHAGYNCAE (Version No: 1.0) Approved for use in: Advanced ovarian cancer second/third line treatment
Haematology and Haematological Oncology
 Haematology and Haematological Oncology Updated on 23 Feb 2017 I) OBJECTIVES 1. To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism
Haematology and Haematological Oncology Updated on 23 Feb 2017 I) OBJECTIVES 1. To provide a broad training and in-depth experience at a level sufficient for trainees to acquire competence and professionalism
CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated
 INDICATION CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated TREATMENT INTENT Curative or Disease Modification. PRE-ASSESSMENT 1. Ensure histology is confirmed
INDICATION CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated TREATMENT INTENT Curative or Disease Modification. PRE-ASSESSMENT 1. Ensure histology is confirmed
Lymphoma. What is cancer? What are signs that my cat has lymphoma. How is Lymphoma diagnosed?
 What is cancer? Lymphoma Cancer is the uncontrolled growth of a small population of abnormal cells. These abnormal cells form by a mutation during the normal division cycle and are able to escape detection
What is cancer? Lymphoma Cancer is the uncontrolled growth of a small population of abnormal cells. These abnormal cells form by a mutation during the normal division cycle and are able to escape detection
Advanced stage HL The old and new match: BEACOPP
 27.03.2015 1 Advanced stage HL The old and new match: BEACOPP Peter Borchmann German Hodgkin Study Group University of Cologne, Germany Which answer is wrong? For patients with advanced stage HL, treatment
27.03.2015 1 Advanced stage HL The old and new match: BEACOPP Peter Borchmann German Hodgkin Study Group University of Cologne, Germany Which answer is wrong? For patients with advanced stage HL, treatment
EC (Epirubicin Cyclophosphamide) Adjuvant/Neo-adjuvant regimen
 y Systemic Anti Cancer Treatment Protocol EC (Epirubicin Cyclophosphamide) Adjuvant/Neo-adjuvant regimen PROTOCOL REF: MPHAECANBR (Version No: 1.0) Approved for use in: ER positive, HER2 negative ( Luminal
y Systemic Anti Cancer Treatment Protocol EC (Epirubicin Cyclophosphamide) Adjuvant/Neo-adjuvant regimen PROTOCOL REF: MPHAECANBR (Version No: 1.0) Approved for use in: ER positive, HER2 negative ( Luminal
National Cancer Intelligence Network Trends in incidence and outcome for haematological cancers in England:
 National Cancer Intelligence Network Trends in incidence and outcome for haematological cancers in England: 2001-2010 Trends in incidence and outcome for haematological cancers in England: 2001-2010 About
National Cancer Intelligence Network Trends in incidence and outcome for haematological cancers in England: 2001-2010 Trends in incidence and outcome for haematological cancers in England: 2001-2010 About
Radiation and Hodgkin s Disease: A Changing Field. Sravana Chennupati Radiation Oncology PGY-2
 Radiation and Hodgkin s Disease: A Changing Field Sravana Chennupati Radiation Oncology PGY-2 History of Present Illness 19 yo previously healthy male college student began having pain in his R shoulder
Radiation and Hodgkin s Disease: A Changing Field Sravana Chennupati Radiation Oncology PGY-2 History of Present Illness 19 yo previously healthy male college student began having pain in his R shoulder
North of Scotland Cancer Network Clinical Management Guideline for Oropharyngeal Cancer
 Nth of Scotland Cancer Netwk Clinical Management Guideline f Oropharyngeal Cancer UNCONTROLLED WHEN PRINTED Based on NHST CMG with further extensive consultation within NOSCAN DOCUMENT CONTROL Original
Nth of Scotland Cancer Netwk Clinical Management Guideline f Oropharyngeal Cancer UNCONTROLLED WHEN PRINTED Based on NHST CMG with further extensive consultation within NOSCAN DOCUMENT CONTROL Original
If unqualified, Complete remission is considered to be Haematological complete remission
 Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Lymphoma is a cancer that develops in the white blood cells (lymphocytes) of the lymphatic system, which is part of the body's immune system.
 Scan for mobile link. Lymphoma Lymphoma is a cancer that develops in the white blood cells of the lymphatic system. Symptoms may include enlarged lymph nodes, unexplained weight loss, fatigue, night sweats
Scan for mobile link. Lymphoma Lymphoma is a cancer that develops in the white blood cells of the lymphatic system. Symptoms may include enlarged lymph nodes, unexplained weight loss, fatigue, night sweats
NB. This version combines both previous inpatient and ambulatory protocols. See DRUG REGIMEN section for details.
 DA- INDICATION High grade lymphoma. Omit rituximab if CD20 negative. NB. This version combines both previous inpatient and ambulatory protocols. See DRUG REGIMEN section for details. TREATMENT INTENT Curative
DA- INDICATION High grade lymphoma. Omit rituximab if CD20 negative. NB. This version combines both previous inpatient and ambulatory protocols. See DRUG REGIMEN section for details. TREATMENT INTENT Curative
If unqualified, Complete remission is considered to be Haematological complete remission
 Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Diagnosis and patient pathway in lymphomas
 The Royal Marsden Diagnosis and patient pathway in lymphomas Dr Ian Chau Consultant Medical Oncologist The Royal Marsden Hospital London & Surrey Change Presentation title and date in Footer dd.mm.yyyy
The Royal Marsden Diagnosis and patient pathway in lymphomas Dr Ian Chau Consultant Medical Oncologist The Royal Marsden Hospital London & Surrey Change Presentation title and date in Footer dd.mm.yyyy
Abstracting Hematopoietic Neoplasms
 CASE 1: LYMPHOMA PHYSICAL EXAMINATION 43yo male with a history of lower gastrointestinal bleeding and melena undergoing colonoscopy and biopsy to rule out neoplasm versus inflammation. Patient had no other
CASE 1: LYMPHOMA PHYSICAL EXAMINATION 43yo male with a history of lower gastrointestinal bleeding and melena undergoing colonoscopy and biopsy to rule out neoplasm versus inflammation. Patient had no other
Louisa Fleure. Advanced Prostate Cancer Clinical Nurse Specialist. Guys and St Thomas NHS Trust
 Louisa Fleure Advanced Prostate Cancer Clinical Nurse Specialist Guys and St Thomas NHS Trust The classification of advanced prostate cancer The incidence of patients presenting with, or developing advanced
Louisa Fleure Advanced Prostate Cancer Clinical Nurse Specialist Guys and St Thomas NHS Trust The classification of advanced prostate cancer The incidence of patients presenting with, or developing advanced
Relapsed/Refractory Hodgkin Lymphoma
 Relapsed/Refractory Hodgkin Lymphoma Anas Younes, MD Chief, Lymphoma Service Memorial Sloan-Kettering Cancer Center New York, New York, United States Case Study 32-year-old woman was diagnosed with stage
Relapsed/Refractory Hodgkin Lymphoma Anas Younes, MD Chief, Lymphoma Service Memorial Sloan-Kettering Cancer Center New York, New York, United States Case Study 32-year-old woman was diagnosed with stage
TRANSFUSION OF BLOOD COMPONENTS ADMINISTRATION. All blood components are administered according to BOP DHB Policy and NZBS Guidelines.
 STANDARDS All blood components are administered according to BOP DHB Policy and NZBS Guidelines. EQUIPMENT IV administration set with 260 micron filter either integrated blood filter; or add on blood filter
STANDARDS All blood components are administered according to BOP DHB Policy and NZBS Guidelines. EQUIPMENT IV administration set with 260 micron filter either integrated blood filter; or add on blood filter
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
The Lymphomas. An overview..
 The Lymphomas An overview.. Peter Anglin MD, FRCPC, MBA Stronach Regional Cancer Centre Newmarket, ON The lymphomas are an important part of the history of medicine 1666 Magpighi publishes first recorded
The Lymphomas An overview.. Peter Anglin MD, FRCPC, MBA Stronach Regional Cancer Centre Newmarket, ON The lymphomas are an important part of the history of medicine 1666 Magpighi publishes first recorded
Herceptin IV (Trastuzumab) and Paclitaxel Cumbria, Northumberland, Tyne & Wear Area Team
 DRUG ADMINISTRATION SCHEDULE Given as a three weekly schedule Day Drug Daily Dose Route Diluent & Rate On first cycle 250mls Normal Saline Herceptin IV 8 mg/kg Infusion only 90 mins On other 250mls Normal
DRUG ADMINISTRATION SCHEDULE Given as a three weekly schedule Day Drug Daily Dose Route Diluent & Rate On first cycle 250mls Normal Saline Herceptin IV 8 mg/kg Infusion only 90 mins On other 250mls Normal
Indium-111 Zevalin Imaging
 Indium-111 Zevalin Imaging Background: Most B lymphocytes (beyond the stem cell stage) contain a surface antigen called CD20. It is possible to kill these lymphocytes by injecting an antibody to CD20.
Indium-111 Zevalin Imaging Background: Most B lymphocytes (beyond the stem cell stage) contain a surface antigen called CD20. It is possible to kill these lymphocytes by injecting an antibody to CD20.
Bilateral Chest X-Ray Shadowing and Bilateral leg lesions - A case of Pulmonary Kaposi Sarcoma
 Article ID: WMC005047 ISSN 2046-1690 Bilateral Chest X-Ray Shadowing and Bilateral leg lesions - A case of Pulmonary Kaposi Sarcoma Peer review status: No Corresponding Author: Dr. Mohammad Fawad Khattak,
Article ID: WMC005047 ISSN 2046-1690 Bilateral Chest X-Ray Shadowing and Bilateral leg lesions - A case of Pulmonary Kaposi Sarcoma Peer review status: No Corresponding Author: Dr. Mohammad Fawad Khattak,
Candidates must answer ALL questions
 Time allowed: Three hours. Part 1 examination Haematology: First paper Tuesday 22 March 2016 Candidates must answer ALL questions Question 1: General Haematology A 16 year old non-european is referred
Time allowed: Three hours. Part 1 examination Haematology: First paper Tuesday 22 March 2016 Candidates must answer ALL questions Question 1: General Haematology A 16 year old non-european is referred
Practical Application of PET adapted Therapy in Hodgkin Lymphoma
 Practical Application of PET adapted Therapy in Hodgkin Lymphoma Matthew Matasar, MD Lymphoma and Adult BMT Services Director, Lymphoma Survivorship Clinic Memorial Sloan Kettering Cancer Center New York,
Practical Application of PET adapted Therapy in Hodgkin Lymphoma Matthew Matasar, MD Lymphoma and Adult BMT Services Director, Lymphoma Survivorship Clinic Memorial Sloan Kettering Cancer Center New York,
PET-adapted therapies in the management of younger patients (age 60) with classical Hodgkin lymphoma
 PET-adapted therapies in the management of younger patients (age 60) with classical Hodgkin lymphoma Ryan Lynch MD Assistant Professor, University of Washington Assistant Member, Fred Hutchinson Cancer
PET-adapted therapies in the management of younger patients (age 60) with classical Hodgkin lymphoma Ryan Lynch MD Assistant Professor, University of Washington Assistant Member, Fred Hutchinson Cancer
Proton or Photon RT for Retroperitoneal Sarcomas
 Principle Investigator Thomas F. DeLaney, M.D. Contact Additional Info Institution Thomas DeLaney, MD 617-726-6876 tdelaney@partners.org www.clinicaltrials.gov NCT01659203 Massachusetts General Hospital
Principle Investigator Thomas F. DeLaney, M.D. Contact Additional Info Institution Thomas DeLaney, MD 617-726-6876 tdelaney@partners.org www.clinicaltrials.gov NCT01659203 Massachusetts General Hospital
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x 4 (3-weekly) in Early Breast Cancer
 Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Part 1 examination. Haematology: First paper. Tuesday 20 March Candidates must answer all questions. Each question is worth a total of 25 marks.
 Part 1 examination Haematology: First paper Tuesday 20 March 2018 Candidates must answer all questions. Each question is worth a total of 25 marks. Time allowed: 3 hours Question 1: General Haematology
Part 1 examination Haematology: First paper Tuesday 20 March 2018 Candidates must answer all questions. Each question is worth a total of 25 marks. Time allowed: 3 hours Question 1: General Haematology
CEPP for Diffuse Large B Cell Lymphoma (LYMWOS005/1)
 West of Scotland Cancer Network Chemotherapy Protocol CEPP for Diffuse Large B Cell Lymphoma (LYMWOS005/1) Indication First line treatment of Diffuse Large B Cell Lymphoma (DLBCL) in patients who are unfit
West of Scotland Cancer Network Chemotherapy Protocol CEPP for Diffuse Large B Cell Lymphoma (LYMWOS005/1) Indication First line treatment of Diffuse Large B Cell Lymphoma (DLBCL) in patients who are unfit
Desmoid Tumors: Understanding Treatment Options in Breelyn A. Wilky, MD Associate Professor Sarcoma Program, Division of Oncology
 Desmoid Tumors: Understanding Treatment Options in 2019 Breelyn A. Wilky, MD Associate Professor Sarcoma Program, Division of Oncology A real desmoid story 35 year old female physician who noted abdominal
Desmoid Tumors: Understanding Treatment Options in 2019 Breelyn A. Wilky, MD Associate Professor Sarcoma Program, Division of Oncology A real desmoid story 35 year old female physician who noted abdominal
Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma
 Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Breast Pathway Group EC x 4: Epirubicin & Cyclophosphamide in Early Breast Cancer
 Breast Pathway Group EC x 4: & in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for moderate to high risk breast cancer Regimen details: 90 mg/m 2 IV Day 1 600 mg/m 2 IV Day 1 Administration:
Breast Pathway Group EC x 4: & in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for moderate to high risk breast cancer Regimen details: 90 mg/m 2 IV Day 1 600 mg/m 2 IV Day 1 Administration:
Paclitaxel Gastric Cancer
 Systemic Anti Cancer Treatment Handbook Paclitaxel Gastric Cancer PROTOCOL REF: MPHAUGIPAC (Version No: 1.0) Approved for use in: Second line treatment of locally advanced and metastatic gastric / gastro-oesophageal
Systemic Anti Cancer Treatment Handbook Paclitaxel Gastric Cancer PROTOCOL REF: MPHAUGIPAC (Version No: 1.0) Approved for use in: Second line treatment of locally advanced and metastatic gastric / gastro-oesophageal
Printed by Martina Huckova on 10/3/2011 3:04:43 PM. For personal use only. Not approved for distribution. Copyright 2011 National Comprehensive
 NCCN Categories of Evidence and Consensus Category 1: The recommendation is based on high-level evidence (e.g. randomized controlled trials) and there is uniform NCCN consensus. Category 2A: The recommendation
NCCN Categories of Evidence and Consensus Category 1: The recommendation is based on high-level evidence (e.g. randomized controlled trials) and there is uniform NCCN consensus. Category 2A: The recommendation
Acute: Symptoms that start and worsen quickly but do not last over a long period of time.
 Cancer Glossary Acute: Symptoms that start and worsen quickly but do not last over a long period of time. Adjuvant therapy: Treatment given after the main treatment. It usually refers to chemotherapy,
Cancer Glossary Acute: Symptoms that start and worsen quickly but do not last over a long period of time. Adjuvant therapy: Treatment given after the main treatment. It usually refers to chemotherapy,
NCCP Chemotherapy Regimen
 INDICATIONS FOR USE: Azacitidine i INDICATION ICD10 Regimen Code *Reimbursement Status Intermediate-1 and low risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System
INDICATIONS FOR USE: Azacitidine i INDICATION ICD10 Regimen Code *Reimbursement Status Intermediate-1 and low risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System
Breast Pathway Group Epirubicin & Cyclophosphamide x 4 followed by Carboplatin & Paclitaxel x 4 for Early Breast Cancer
 Breast Pathway Group Epirubicin & Cyclophosphamide x 4 followed by Carboplatin & Paclitaxel x 4 for Early Breast Cancer Indication: Neoadjuvant therapy for patients with BRCA1/2 mutations EC Regimen details:
Breast Pathway Group Epirubicin & Cyclophosphamide x 4 followed by Carboplatin & Paclitaxel x 4 for Early Breast Cancer Indication: Neoadjuvant therapy for patients with BRCA1/2 mutations EC Regimen details:
NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary)
 NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary) Reviewed by Dr. Michelle Geddes (Staff Hematologist, University of Calgary) and Dr.
NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary) Reviewed by Dr. Michelle Geddes (Staff Hematologist, University of Calgary) and Dr.
Cisplatin Doxorubicin Sarcoma
 Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
Weekly Cisplatin + Radiotherapy - Interlace study -
 Weekly Cisplatin + Radiotherapy - Interlace study - A phase III multicentre trial of weekly induction chemotherapy followed by standard chemoradiation versus standard chemoradiation alone in patients with
Weekly Cisplatin + Radiotherapy - Interlace study - A phase III multicentre trial of weekly induction chemotherapy followed by standard chemoradiation versus standard chemoradiation alone in patients with
North of Scotland Cancer Network Clinical Management Guideline for Carcinoma of the Uterine Cervix
 THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
Gemcitabine & Cisplatin
 Gemcitabine & Cisplatin Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Advanced
Gemcitabine & Cisplatin Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Advanced
WHAT ARE PAEDIATRIC CANCERS
 WHAT ARE PAEDIATRIC CANCERS INTRODUCTION Childhood cancers are RARE 0.5% of all cancers in the West Overall risk that a child will develop cancer during first 15 years of life is 1 in 450 and 1 in 600
WHAT ARE PAEDIATRIC CANCERS INTRODUCTION Childhood cancers are RARE 0.5% of all cancers in the West Overall risk that a child will develop cancer during first 15 years of life is 1 in 450 and 1 in 600
ABVD. by infusion over 30 minutes. You will have treatment on Day 1 and Day 15 of a 28 day cycle. You will have 2, 3 or 6 cycles.
 ABVD ABVD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others it may
ABVD ABVD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others it may
Guidelines for management of Pediatric Hodgkin Lymphoma
 Guidelines for management of Pediatric Hodgkin Lymphoma Section of Leukemia/Lymphoma Department of Pediatric Hematology/Oncology King Faisal Specialist Hospital and Research Center Riyadh TABLE OF CONTENTS
Guidelines for management of Pediatric Hodgkin Lymphoma Section of Leukemia/Lymphoma Department of Pediatric Hematology/Oncology King Faisal Specialist Hospital and Research Center Riyadh TABLE OF CONTENTS
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by
UNMH Hematology/Oncology Clinical Privileges
 o Initial privileges (initial appointment) o Renewal of privileges (reappointment) o Expansion of privileges (modification) All new applicants must meet the following requirements as approved by the UNMH
o Initial privileges (initial appointment) o Renewal of privileges (reappointment) o Expansion of privileges (modification) All new applicants must meet the following requirements as approved by the UNMH
Aggressive NHL and Hodgkin Lymphoma. Dr. Carolyn Faught November 10, 2017
 Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
Pre-operative assessment of patients for cytoreduction and HIPEC
 Pre-operative assessment of patients for cytoreduction and HIPEC Washington Hospital Center Washington, DC, USA Ovarian Cancer Surgery New Strategies Bergamo, Italy May 5, 2011 Background Cytoreductive
Pre-operative assessment of patients for cytoreduction and HIPEC Washington Hospital Center Washington, DC, USA Ovarian Cancer Surgery New Strategies Bergamo, Italy May 5, 2011 Background Cytoreductive
Louisa Fleure. Advanced Prostate Cancer Clinical Nurse Specialist. Guys and St Thomas NHS Trust
 Louisa Fleure Advanced Prostate Cancer Clinical Nurse Specialist Guys and St Thomas NHS Trust The classification of advanced prostate cancer The incidence of patients presenting with, or developing advanced
Louisa Fleure Advanced Prostate Cancer Clinical Nurse Specialist Guys and St Thomas NHS Trust The classification of advanced prostate cancer The incidence of patients presenting with, or developing advanced
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113)
 Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
