SYNOPSIS Final Abbreviated Clinical Study Report for Study CA ABBREVIATED REPORT
|
|
|
- Godwin Norris
- 6 years ago
- Views:
Transcription
1 Finl Arevited Clinicl Study Report Nme of Sponsor/Compny: Bristol-Myers Squi Ipilimum Individul Study Tle Referring to the Dossier (For Ntionl Authority Use Only) Nme of Finished Product: Yervoy Nme of Active Ingredient: Ipilimum SYNOPSIS Finl Arevited Clinicl Study Report for Study ABBREVIATED REPORT TITLE OF STUDY: A multi-center, open-lel, phse II study of ipilimum (MDX-010) extendedtretment monotherpy or follow-up for sujects previously enrolled in ipilimum (MDX-010) protocols INVESTIGATORS/STUDY CENTERS: A totl of 242 sujects were enrolled (nd treted/followed) t 75 sites in 21 countries including Argentin, Austrli, Austri, Belgium, Brzil, Cnd, Czech Repulic, Denmrk, Frnce, Germny, Isrel, Itly, Norwy, Peru, Polnd, Russi, South Afric, Spin, Sweden, Ukrine, nd the United Sttes PUBLICATIONS: None STUDY PERIOD: Study Initition Dte: 09-My-2006 CLINICAL PHASE: 2 Dt Cutoff Dte: 19-Sep-2012 INTRODUCTION: This study ws designed to evlute sfety in sujects with dvnced melnom enrolled from Bristol-Myers Squi (BMS) ipilimum prent studies CA184004, CA184007, CA184008, nd CA or Medrex (MDX) studies MDX nd MDX Most sujects rolled over from prent studies CA184004, CA184007, CA184008, or CA184022, ll of which hd induction mintennce, ut no re-induction. Since is rollover study with primry ojective of sfety; limited efficcy results with primry focus on sfety t ipilimum 10 milligrm/kilogrm (mg/kg) first re-induction re presented in this finl revited Clinicl Study Report (CSR). An Extension Phse of protocol is ongoing; results will e presented in n ddendum to this CSR. OBJECTIVES: The primry ojective of this study ws to monitor the sfety of ipilimum dministered either s re-induction (3 or 10 mg/kg [10 mg/kg results re presented in this report]) or mintennce therpy (0.3, 3, or 10 mg/kg). The secondry ojectives with results presented in this report were: To estimte overll survivl (OS) from the first dose of ipilimum in the prent study (in enrolled sujects) To estimte survivl rte t 1, 1.5, nd 2 yers from the first dose of ipilimum in the prent study (in enrolled sujects) To estimte progression free survivl (PFS) from the first in this study To monitor sujects (except those in Survivl Follow-up only) who experienced immune relted dverse events (iraes) fter ipilimum tretment (in enrolled sujects) METHODOLOGY: This is multi-center, Phse 2 rollover study of extended tretment with ipilimum monotherpy or follow-up for sujects previously enrolled in prent ipilimum studies to evlute sfety of ipilimum dministered either s re-induction or s mintennce therpy. Eligile sujects entered the study into 1) re-induction (t time of progression), 2) extended mintennce (if no prior progression) or 3) follow-up.
2 Finl Arevited Clinicl Study Report Figure 1: Ipilimum Study Flow Digrm consisted of 5 phses: Screening, (first nd second), Mintennce, Follow-up, nd Extension Phse (currently ongoing). Re-Induction (with or without extended mintennce) sujects (N=122 treted) entered into re-induction phse initilly or following extended mintennce to receive ipilimum. Sujects treted t 0.3 mg/kg, 3 mg/kg nd 10 mg/kg initil therpy received open-lel first re-induction t 10 mg/kg nd other dose selections in prent study received open-lel first re-induction t 3 or 10 mg/kg in. These suject tretment groups re s follows: 0.3 mg/kg initil therpy to 10 mg/kg (N=24 treted), 3 mg/kg initil therpy to 10 mg/kg (N=34 treted), 10 mg/kg initil therpy to 10 mg/kg (N=53 treted), nd other dose selections from prent study to current study (N=11 treted). Extended Mintennce only sujects (N=62, 49 treted) entered into the mintennce phse nd could receive ipilimum in 12-week intervls. Some sujects entered mintennce with PR or CR tumor responses nd never received ipilimum tretment ut were followed y tumor ssessment. Sujects receiving ipilimum during extended mintennce received 0.3 mg/kg (N=4 treted), 3 mg/kg (N=13, 12 treted), nd 10 mg/kg (N=45, 33 treted). Mintennce sujects were initilly treted with linded study mediction s rndomized in the prent study until such study ws unlinded (CA nd CA184022). Then these sujects were treted with open-lel ipilimum. Follow-Up sujects (N=58) entered into the follow-up phse nd did not receive dditionl study tretment ut continued on-study for the collection of survivl dt. Sujects entering the mintennce phse of directly from prent studies CA nd CA remined linded until the time tht unlinding occurred in the prent protocols. Sujects entering into the re-induction phse long with those entering mintennce on n unlinded dose received unlinded doses in. NUMBER OF SUBJECTS (Plnned nd Anlyzed): The numer of sujects enrolled in studies CA184004, CA184007, CA184008, CA184022, MDX nd MDX determined the numer of eligile sujects for this rollover study. It ws estimted tht 30% of the sujects (estimted n = 200) in the prent protocols would enter this rollover study. Of the 733 sujects in the prent studies, 248 (34%) were enrolled into this study (28, 42, 67, 103, 2 nd 6 from CA184004, CA184007, CA184008, CA184022, MDX nd MDX010-15, respectively). A totl of 242 sujects were enrolled nd treted/followed in. Six sujects did not meet screening criteri for. DIAGNOSIS AND MAIN CRITERIA FOR INCLUSION: This rollover study includes sujects from prent protocols CA184004, CA184007, CA184008, CA184022, MDX nd MDX
3 Finl Arevited Clinicl Study Report Ipilimum : Sujects who received ipilimum t ny dose in prent study; nd met ech of the following criteri: Hd no uncceptle toxicity (except select reversile iraes - refer to the protocol) requiring ipilimum discontinution Hd experienced progressive disese (PD) fter expnded clinicl enefit Hd chieved expnded clinicl enefit on or fter Week 12 in the prent study or for CA only, hd PD efore Week 12 or fter expnded clinicl enefit All sujects eligile for re-induction (s descried ove) must hve hd their cse discussed with BMS Medicl Monitor prior to enrolling in the re-induction phse of this rollover study to monitor sfety nd verify ppropriteness for re-induction therpy. Extended Mintennce: Received ipilimum t ny dose in prent study Achieved expnded clinicl enefit t the time of rollover study entry Follow-up: Received ipilimum t ny dose in closing prent study Deemed ineligile for re-induction or extended mintennce tretment or refused tretment s re-induction or extended mintennce suject t the time of screening in this rollover study, ut consented to follow-up TEST PRODUCT, DOSE AND MODE OF ADMINISTRATION, DURATION OF TREATMENT: Ech eligile suject received ipilimum dosing vi 90 minute intrvenous (IV) infusion in oth the or Mintennce Phse of this study. Refer to Sections through for further detiled instruction regrding ipilimum scheduling, dosing, skipping nd discontinution of tretment. Re-Induction Phse: Ipilimum t dose of 10 mg/kg or 3 mg/kg will e dministered in n open-lel mnner, s n individul dose every 3 weeks for the first 10 weeks of Re-Induction for totl of 4 seprte doses unless there is disese progression or withdrwl of consent; Sujects who hd chieved expnded clinicl enefit s defined in the protocol (Appendix 1.1), who permnently discontinued tretment under the prent study due to select iraes will receive 3 mg/kg; All other Re-Induction doses of ipilimum will e t 10 mg/kg regrdless of the previous dose ssignment in the prior/prent study or dose ssignment in the Mintennce Phse of this study; Sujects entering Re-Induction from the prior/prent protocol must wit 3 weeks from their prior/prent dose of ipilimum efore receiving Re-Induction dosing in this study. Sujects entering Re-Induction from Mintennce must wit 3 weeks from their lst dose of Mintennce Phse ipilimum efore receiving Re-Induction dosing. ws to continue enrolling sujects until ipilimum ecme commercilly ville t ll open sites or the Investigtionl New Drug Appliction (IND) ws closed y the Sponsor. This study will remin open until the lst enrolled suject discontinues from ny study phse, including Follow-up, or the study is otherwise terminted y the Sponsor. Thus, ll sujects who were enrolled in the study prior to pprovl of ipilimum could continue to receive tretment nd follow-up in this study. CRITERIA FOR EVALUATION: Sfety: Sfety ws evluted using the Ntionl Cncer Institute (NCI) Common Terminology Criteri for Adverse Events v3.0 (CTCAE). Sfety ssessments were sed on medicl review of dverse event (AE) reports nd the
4 Finl Arevited Clinicl Study Report Ipilimum results of vitl sign mesurements, physicl exmintions nd clinicl lortory tests. The incidence of AEs will e tulted nd reviewed for potentil significnce nd clinicl importnce. The reporting period for sfety dt will either e: 1) from the dte of the first dose of ipilimum received in this study (re-induction nd mintennce sujects) to 70 dys (5 hlf-lives) following the lst dose received; or 2) 70 dys from the lst dose of ipilimum received in the prior/prent study (Follow-up sujects). Efficcy: The Investigtor mde ll tumor evlutions sed on modified World Helth Orgniztion (WHO) criteri. Throughout the study, ech respective Investigtor will determine disese sttus of sujects t the protocol-defined timepoints nd s cliniclly indicted. Immunogenicity: Humn nti-humn ntiodies (HAHA) nd serum ipilimum concentrtion will e evluted (with the exception of the Extension Phse). STATISTICAL CONSIDERATIONS: The study ws designed to evlute sfety in sujects enrolled from prent ipilimum protocols where it ws estimted 200 sujects or 30% in the prent protocols would enter this rollover study depending upon the numer of sujects ultimtely enrolled in studies CA184004, CA184007, CA184008, CA184022, MDX nd MDX Of the 733 sujects in the prent studies, 248 (34%) were enrolled into this study (28, 42, 67, 103, 2 nd 6 from CA184004, -007, -008, -022, MDX nd -15, respectively). The study treted/followed totl of 242 sujects who did not fil screening. In ddition to the nlysis of sfety, the OS, nd the progression free survivl (PFS) from the first dose of ipilimum in the prent study (in enrolled sujects) ws lso computed. Sujects were rndomized in prent studies nd once unlinded (for sujects in prent studies CA nd CA only), tretment ws dministered open lel in. The originl nlysis pln ws truncted to the current revited presenttion in Ferury, 2012 to trget on the sfety nd OS in first re-induction sujects. The decision to limit the nlysis ws due to the complexity of the study, the mixture of tretments nd popultions, nd the difficulty in interpreting such results. The primry nlysis focus is on sujects receiving first re-induction since this represents the lrgest group (n = 122) nd is potentilly the most informtive s sfety of mintennce dosing ws estlished in prent studies. The nlysis lso includes sujects receiving extended mintennce (n = 62, 49 treted, see Figure 1; some sujects entered mintennce with tumor responses of PR or CR nd never received ipilimum tretment ut were followed y tumor ssessment.) nd follow-up sujects (n = 58), for whom dt is ville in listings. The ssessment of sfety ws summrized using descriptive sttistics nd ws sed on AEs, serious AEs (SAEs), iraes, deths, physicl exmintions, clinicl lortory tests, nd exposure to study therpy. The secondry efficcy nlyses estimted nd plotted OS for ech dose group with the Kpln-Meier product-limit method. OS ws defined from the time of the first dose of ipilimum in the prent study until deth or censor. No forml sttisticl testing ws done. Survivl rtes t 1, 1.5, nd 2 yers with corresponding two-sided 95% CIs were clculted for ech tretment group using the Kpln Meier product-limit method. Progression free survivl (PFS) ws plotted for ech dose grouping with the Kpln-Meier product-limit method. SUMMARY OF RESULTS: Disposition nd Bseline/Demogrphic Chrcteristics: Disposition (Tle 1) nd seline/demogrphic chrcteristics (Tle 2) presented in this report focus on sujects in first re-induction who received prent study ipilimum doses of 10, 3, or 0.3 mg/kg or other doses of ipilimum.
5 Finl Arevited Clinicl Study Report Tle 1: Numer of Sujects: Enrolled Treted Discontinued Primry Reson: Suject Disposition, Sujects Disese progression Still on Tretment Ipilimum 10 to 10 mg/kg 3 to 10 mg/kg 0.3 to 10 mg/kg Other Dosge (83.0%) (85.3%) (87.5%) (72.7%) 29 (54.7%) 21 (61.8%) 13 (54.2%) 4 (36.4%) 9 (17.0%) 5 (14.7%) 3 (12.5%) 3 (27.3%) An other dosge in the prent study esides 10, 3, or 0.3 mg/kg ipilimum; receiving either 3.0 or 10.0 mg/kg in. Discontinution dt includes sujects in ctegories of documented disese progression nd deteriortion without documented disese progression. Tle 2: Bseline nd Demogrphic Chrcteristics, Sujects Other Dosge 10 to 10 mg/kg 3 to 10 mg/kg 0.3 to 10 mg/kg (N=53) (N=34) (N=24) Femle 21 (39.6) 11 (32.4) 4 (16.7) 1 (9.1) Mle 32 (60.4) 23 (67.6) 20 (83.3) 10 (90.9) White 51 (96.2) 34 (100.0) 24 (100.0) 10 (90.9) Blck 1 (1.9) Asin 1 (9.1) 1 (1.9) 55.4 (11.7) 58.8 (9.9) 56.0 (15.3) 57.8 (13.7) Medin Min-Mx (N=11) Gender (n %) Rce (n %) Other Age (yers) Men (SD)
6 Finl Arevited Clinicl Study Report Tle 2: Ipilimum Bseline nd Demogrphic Chrcteristics, Sujects 10 to 10 mg/kg 3 to 10 mg/kg 0.3 to 10 mg/kg Other Dosge (N=53) (N=34) (N=24) 0 37 (69.8) 22 (64.7) 10 (41.7) 9 (81.8) 1 16 (30.2) 11 (32.4) 13 (54.2) 2 (18.2) 2 1 (2.9) 1 (4.2) 3 (N=11) ECOG Performnce Sttus Other dosge in the prent study; receiving either 3.0 or 10.0 mg/kg in. Mixed prentge - cpe colored Arevitions: ECOG = Estern Coopertive Oncology Group, SD = stndrd devition Extent of Exposure: A medin numer of 4 doses per suject were dministered in the 10 to 10 mg/kg nd 3 to 10 mg/kg groups nd 2 doses per suject were dministered in the 0.3 to 10 mg/kg group during the study. Of the 111 treted first re-induction sujects in this rollover study, 30 sujects eventully received mintennce tretment (indicted y >4 doses in 16 sujects in the 10 to 10 mg/kg group, 10 sujects in the 3 to 10 mg/kg group, nd 4 sujects in the 0.3 to 10 mg/kg group). Sfety Results: An overll summry of sfety in treted first re-induction sujects is presented in Tle 3. Sfety findings during first re-induction in treted sujects re s follows: Totl frequencies of drug-relted AEs were similr cross the groups (79% to 87%). The most common drug-relted AEs were iraes, with grde 3/4 iraes of 7/53, 2/34 nd 6/24 in ipilimum 10 to 10 mg/kg, 3 to 10 mg/kg, nd 0.3 to 10 mg/kg groups, respectively. Dirrhe, pruritus, nd rsh were the most frequently reported iraes. In treted first re-induction sujects, the most frequent AEs leding to discontinution during first re-induction tht were considered relted to study drug y the investigtor were colitis, dirrhe, nd nemi. Ftl AEs leding to discontinution included disese progression, pneumoni, nd mlignnt melnom. There were no Grde 5 drug-relted AEs or drug-relted deths.
7 Finl Arevited Clinicl Study Report Tle 3: Ipilimum Summry of Sfety, Treted Sujects 10 to 10 mg/kg (N = 53) Sujects with ny On-study: AE, n (%) 3 to 10 mg/kg (N = 34) 0.3 to 10 mg/kg (N = 24) 51 (96.2) 33 (97.1) 24 (100.0) Severe (Grde 3/4) 18 (34.0) 10 (29.4) 10 (41.7) Ftl (Grde 5) 4 (7.5) 7 (20.6) 8 (33.3) 13 (24.5) 6 (17.6) 8 (33.3) Severe (Grde 3/4) 9 (17.0) 3 (8.8) 6 (25.0) Ftl (Grde 5) 1 (1.9) 2 (5.9) 1 (4.2) 23 (43.4) 19 (55.9) 14 (58.3) Severe (Grde 3/4) 13 (24.5) 8 (23.5) 5 (20.8) Ftl (Grde 5) 4 ( 7.5) 7 (20.6) 8 (33.3) Drug-relted AEs, n (%) 42 (79.2) 28 (82.4) 21 (87.5) Severe (Grde 3/4) 9 (17.0) 3 (8.8) 9 (37.5) Ftl (Grde 5) 30 (56.6) 23 (67.6) 18 (75.0) Grde 3/4 irae 7 (13.2) 2 (5.9) 6 (25.0) Grde 5 (ftl) 36 (67.9) 27 (79.4) 19 (79.2) AEs leding to study drug discontinution, n (%) SAEs, n (%) irae, n (%) Deths, n (%) Includes deths more thn 70 dys fter lst dose dte. Note: On-study dverse events (AEs) include ll AEs reported etween the first dose nd 70 dys fter the lst dose of study drug. Arevitions: AE = dverse event; irae = immune-relted dverse event; SAE = serious dverse event Other Results: Efficcy: Tle 4 summrizes efficcy results. Tle 4: Efficcy Summry Suject Group (No. of Sujects) OS Medin, months (95% CI) Kpln-Meier Estimted Survivl Rtes (%) PFS Medin, months (95% CI) 1 yr 1.5 yrs 2 yrs 10 to 10 mg/kg (N=53) 30.8 (24.2, 41.1) (2.6, 7.8) 3 to 10 mg/kg (N=34) 18.7 (9.7, 30.4) (2.6, 2.8) 0.3 to 10 mg/kg (N=24) 15.2 (10.7, 21.4) (2.0, 11.1)
8 Finl Arevited Clinicl Study Report Tle 4: Suject Group (No. of Sujects) Ipilimum Efficcy Summry Kpln-Meier Estimted OS Medin, months (95% CI) Survivl Rtes (%) PFS Medin, months (95% CI) Extended Mintennce 1 yr 1.5 yrs 2 yrs 10 mg/kg (N=45) NE (NE, NE) NA 3 mg/kg (N=13) NE (28.4, NE) NA 0.3 mg/kg (N=4) NE (11.3, NE) NA 1 yr 1.5 yrs 2 yrs Follow-Up Not dosed (N=58) 40.2 (27.1, NE) NA Estimted from first dose of ipilimum in prent study Estimted from first re-induction in CA Arevitions: CI = confidence intervl; NA = not nlyzed; NE = not evlule; PFS = progression free survivl; OS = overll survivl For sujects who received 10 mg/kg ipilimum re-induction, there ws numericlly higher OS percent for those who received 10 mg/kg versus 3 mg/kg ipilimum in prent study. For OS, the contriution of the originl prent protocol re-induction nd mintennce cnnot e dissected. Similrly, sujects treted t 10 mg/kg in prent study hd numericlly higher survivl t 1, 1.5 nd 2 yers reltive to sujects who initilly were treted with 3 mg/kg nd re-induced t 10 mg/kg. Sujects who received 10 mg/kg extended mintennce therpy hd numericlly higher improved survivl t 1, 1.5 nd 2 yers reltive to sujects who initilly were treted with 3 mg/kg extended mintennce. The medins were not reched in ll 3 of the ipilimum extended mintennce therpy rms. For sujects who received 10 mg/kg ipilimum re-induction, there ws no numericl difference in PFS etween those who received 3 versus 10 mg/kg in prent study. Immunogenicity: Immunogenicity smples were collected during the study nd nlysis results will e reported seprtely from this CSR. CONCLUSIONS: The focus on the nlysis trgeted sujects eligile for re-induction t 10 mg/kg followed y extended mintennce therpy. Results re sed on smll smple sizes in non-rndomized context with potentil is due to eligiility criteri nd tht only suset of those eligile enrolled. Specificlly, enrollment required tht sujects experienced clinicl enefit from ipilimum in prent study nd tolerted ipilimum with no dose limiting toxicity. t 10 mg/kg ipilimum ws sfely dministered nd tolerle regrdless of dose in prent study. The incidence nd severity of iraes during re-induction ws lower thn tht for the generl popultion in the prent study. For sujects who received 10 mg/kg ipilimum re-induction, there ws lower incidence of iraes for those who received ipilimum t 3 nd 10 mg/kg in prent study compred to those who received ipilimum t 0.3 mg/kg in prent study. Sujects who tolerted ipilimum t 0.3 mg/kg in prent study received fewer doses t 10 mg/kg thn those sujects who tolerted 3 or 10mg/kg in prent study.
9 Finl Arevited Clinicl Study Report Ipilimum DATE OF REPORT: 10-Jul-2013
CheckMate 153: Randomized Results of Continuous vs 1-Year Fixed-Duration Nivolumab in Patients With Advanced Non-Small Cell Lung Cancer
 CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
Clinical Study Report Synopsis Drug Substance Naloxegol Study Code D3820C00018 Edition Number 1 Date 01 February 2013 EudraCT Number
 EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
Efficacy of Pembrolizumab in Patients With Advanced Melanoma With Stable Brain Metastases at Baseline: A Pooled Retrospective Analysis
 Efficcy of Pembrolizumb in Ptients With Advnced Melnom With Stble Brin Metstses t Bseline: A Pooled Retrospective Anlysis Abstrct 1248PD Hmid O, Ribs A, Dud A, Butler MO, Crlino MS, Hwu WJ, Long GV, Ancell
Efficcy of Pembrolizumb in Ptients With Advnced Melnom With Stble Brin Metstses t Bseline: A Pooled Retrospective Anlysis Abstrct 1248PD Hmid O, Ribs A, Dud A, Butler MO, Crlino MS, Hwu WJ, Long GV, Ancell
The RUTHERFORD-2 trial in heterozygous FH: Results and implications
 The RUTHERFORD-2 tril in heterozygous FH: Results nd implictions Slide deck kindly supplied s n eductionl resource by Professor Derick Rl MD PhD Crbohydrte & Lipid Metbolism Reserch Unit University of
The RUTHERFORD-2 tril in heterozygous FH: Results nd implictions Slide deck kindly supplied s n eductionl resource by Professor Derick Rl MD PhD Crbohydrte & Lipid Metbolism Reserch Unit University of
Introduction. These patients benefit less from conventional chemotherapy than patients identified as MMR proficient or microsatellite stable 3-5
 Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Abstrct 553 André T,
Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Abstrct 553 André T,
IMpower133: Primary PFS, OS, and safety in a Ph1/3 study of 1L atezolizumab + carboplatin + etoposide in extensive-stage SCLC
 IMpower133: Primry PFS, OS, nd sfety in Ph1/3 study of 1L tezolizumb + crbopltin + etoposide in extensive-stge SCLC S. V. Liu, 1 A. S. Mnsfield, 2 A. Szczesn, 3 L. Hvel, 4 M. Krzkowski, 5 M. J. Hochmir,
IMpower133: Primry PFS, OS, nd sfety in Ph1/3 study of 1L tezolizumb + crbopltin + etoposide in extensive-stge SCLC S. V. Liu, 1 A. S. Mnsfield, 2 A. Szczesn, 3 L. Hvel, 4 M. Krzkowski, 5 M. J. Hochmir,
Presented at the 75 th Annual Meeting of the American Academy of Dermatology, Orlando, FL, March 3-7, 2017 METHODS INTRODUCTION OBJECTIVE
 Seven-Yer Interim Results from the ESPRIT 10-Yer Postmrketing Surveillnce Registry of Adlimumb for Moderte to Severe Psorisis Frncisco Kerdel, 1 Aln Menter, 2 Jshin J. Wu, 3 Mreike Bereswill, 4 Dilek Arikn,
Seven-Yer Interim Results from the ESPRIT 10-Yer Postmrketing Surveillnce Registry of Adlimumb for Moderte to Severe Psorisis Frncisco Kerdel, 1 Aln Menter, 2 Jshin J. Wu, 3 Mreike Bereswill, 4 Dilek Arikn,
PNEUMOVAX 23 is recommended by the CDC for all your appropriate adult patients at increased risk for pneumococcal disease 1,2 :
 PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
Using Paclobutrazol to Suppress Inflorescence Height of Potted Phalaenopsis Orchids
 Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
A review of the patterns of docetaxel use for hormone-resistant prostate cancer at the Princess Margaret Hospital
 MEDICAL ONCOLOGY A review of the ptterns of docetxel use for hormone-resistnt prostte cncer t the Princess Mrgret Hospitl S.N. Chin MD,* L. Wng MSc, M. Moore MD,* nd S.S. Sridhr MD MSc* ABSTRACT Bckground
MEDICAL ONCOLOGY A review of the ptterns of docetxel use for hormone-resistnt prostte cncer t the Princess Mrgret Hospitl S.N. Chin MD,* L. Wng MSc, M. Moore MD,* nd S.S. Sridhr MD MSc* ABSTRACT Bckground
Sponsor / Company: Sanofi Drug substance(s): AVE0005 (aflibercept)
 These results re supplied for informtionl purposes only. Prescribing decisions should be mde bsed on the pproved pckge insert in the country of prescription. Sponsor / Compny: Snofi Drug substnce(s): AVE0005
These results re supplied for informtionl purposes only. Prescribing decisions should be mde bsed on the pproved pckge insert in the country of prescription. Sponsor / Compny: Snofi Drug substnce(s): AVE0005
Invasive Pneumococcal Disease Quarterly Report July September 2018
 Invsive Pneumococcl Disese Qurterly Report July Septemer Introduction Since 17 Octoer 2008, invsive pneumococcl disese (IPD) hs een notifile to the locl Medicl Officer of Helth under the Helth Act 1956.
Invsive Pneumococcl Disese Qurterly Report July Septemer Introduction Since 17 Octoer 2008, invsive pneumococcl disese (IPD) hs een notifile to the locl Medicl Officer of Helth under the Helth Act 1956.
Efficacy of Sonidegib in Patients With Metastatic BCC (mbcc)
 AAD 216 eposter 3368 Efficcy of Sonidegib in Ptients With Metsttic BCC (mbcc) Colin Morton, 1 Michel Migden, 2 Tingting Yi, 3 Mnish Mone, 3 Dlil Sellmi, 3 Reinhrd Dummer 4 1 Stirling Community Hospitl,
AAD 216 eposter 3368 Efficcy of Sonidegib in Ptients With Metsttic BCC (mbcc) Colin Morton, 1 Michel Migden, 2 Tingting Yi, 3 Mnish Mone, 3 Dlil Sellmi, 3 Reinhrd Dummer 4 1 Stirling Community Hospitl,
CheckMate-142 Study Design
 Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Thierry André, 1 Sr
Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Thierry André, 1 Sr
Safety and Tolerability of Subcutaneous Sarilumab and Intravenous Tocilizumab in Patients With RA
 Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Community. Profile Powell County. Public Health and Safety Division
 Community Helth Profile 2015 Powell County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Powell County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community. Profile Big Horn County. Public Health and Safety Division
 Community Helth Profile 2015 Big Horn County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Big Horn County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community. Profile Yellowstone County. Public Health and Safety Division
 Community Helth Profile 2015 Yellowstone County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Yellowstone County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community. Profile Anaconda- Deer Lodge County. Public Health and Safety Division
 Community Helth Profile 2015 Ancond- Deer Lodge County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12
Community Helth Profile 2015 Ancond- Deer Lodge County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12
Community. Profile Lewis & Clark County. Public Health and Safety Division
 Community Helth Profile 2015 Lewis & Clrk County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Lewis & Clrk County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community. Profile Missoula County. Public Health and Safety Division
 Community Helth Profile 2015 Missoul County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Missoul County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
University of Texas Health Science Center, San Antonio, San Antonio, Texas, USA
 Lung Cncer Chemotherpy Given Ner the End of Life by Community Oncologists for Advnced Non-Smll Cell Lung Cncer Jose R. Murillo, Jr., Jim Koeller b,c Methodist Hospitl, Houston, Texs, USA; b University
Lung Cncer Chemotherpy Given Ner the End of Life by Community Oncologists for Advnced Non-Smll Cell Lung Cncer Jose R. Murillo, Jr., Jim Koeller b,c Methodist Hospitl, Houston, Texs, USA; b University
Reference Slide Deck. Abstract 553 Abstract 554 Abstract 560
 Clinicl Spotlight Immunotherpy Advnces for Colorectl Crcinom in 2018: Newly Relesed Dt From the Gstrointestinl Cncers Symposium in Sn Frncisco Reference Slide Deck Abstrct 553 Abstrct 554 Abstrct 560 Mismtch
Clinicl Spotlight Immunotherpy Advnces for Colorectl Crcinom in 2018: Newly Relesed Dt From the Gstrointestinl Cncers Symposium in Sn Frncisco Reference Slide Deck Abstrct 553 Abstrct 554 Abstrct 560 Mismtch
Abstract. Background. Aim. Patients and Methods. Patients. Study Design
 Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Input from external experts and manufacturer on the 2 nd draft project plan Stool DNA testing for early detection of colorectal cancer
 Input externl experts nd mnufcturer on the 2 nd drft project pln Stool DNA testing for erly detection of colorectl cncer (Project ID:OTJA10) All s nd uthor s replies on the 2nd drft project pln Stool DNA
Input externl experts nd mnufcturer on the 2 nd drft project pln Stool DNA testing for erly detection of colorectl cncer (Project ID:OTJA10) All s nd uthor s replies on the 2nd drft project pln Stool DNA
Community. Profile Carter County. Public Health and Safety Division
 Community Helth Profile 2015 Crter County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Crter County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Supplementary Online Content
 Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
ClinicalTrials.gov Identifier: NCT
 Efficcy of Drtumumb, Lenlidomide, nd Dexmethsone Versus Lenlidomide nd Dexmethsone in Relpsed or Refrctory Multiple Myelom Ptients With to 3 Prior Lines of Therpy: Updted Anlysis of POLLUX Sd Z. Usmni,
Efficcy of Drtumumb, Lenlidomide, nd Dexmethsone Versus Lenlidomide nd Dexmethsone in Relpsed or Refrctory Multiple Myelom Ptients With to 3 Prior Lines of Therpy: Updted Anlysis of POLLUX Sd Z. Usmni,
Debra A. Ignaut, R.N., B.S., C.D.E., and Haoda Fu, Ph.D.
 Journl of Dietes Science nd Technology Volume 6, Issue 2, Mrch 2012 Dietes Technology Society TECHNOLOGY REPORT Comprison of Insulin Diluent Lekge Postinjection Using Two Different Needle Lengths nd Injection
Journl of Dietes Science nd Technology Volume 6, Issue 2, Mrch 2012 Dietes Technology Society TECHNOLOGY REPORT Comprison of Insulin Diluent Lekge Postinjection Using Two Different Needle Lengths nd Injection
The incidence of melanoma, the most serious
 Efectiveness nd sfety of ipilimumb therpy in dvnced melnom: evidence from clinicl prctice sites in the US Kim A Mrgolin, MD, Ahmd Trhini, MD, PhD, b Sumti Ro, PhD, c Monic Ktyl, JD, MPH, d I-Fen Chng,
Efectiveness nd sfety of ipilimumb therpy in dvnced melnom: evidence from clinicl prctice sites in the US Kim A Mrgolin, MD, Ahmd Trhini, MD, PhD, b Sumti Ro, PhD, c Monic Ktyl, JD, MPH, d I-Fen Chng,
See 17 for PATIENT COUNSELING INFORMATION. Revised: 09/2017 FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use Gemzr sfely nd effectively. See full prescriing informtion for Gemzr. GEMZAR (gemcitine for injection),
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use Gemzr sfely nd effectively. See full prescriing informtion for Gemzr. GEMZAR (gemcitine for injection),
Symptom Management and Supportive Care
 This mteril is protected y U.S. Copyright lw. Unuthorized reproduction is prohiited. For reprints contct: Reprints@AlphMedPress.com Symptom Mngement nd Supportive Cre Erly Intervention with Epoetin Alf
This mteril is protected y U.S. Copyright lw. Unuthorized reproduction is prohiited. For reprints contct: Reprints@AlphMedPress.com Symptom Mngement nd Supportive Cre Erly Intervention with Epoetin Alf
Immune-Mediated Adverse Reactions Management Guide
 Immune-Medited Adverse Rections Mngement Guide INDICATIONS AND USAGE YERVOY (ipilimumb) is indicted for: Tretment of unresectble or metsttic melnom in dults nd peditric ptients (12 yers nd older) Adjuvnt
Immune-Medited Adverse Rections Mngement Guide INDICATIONS AND USAGE YERVOY (ipilimumb) is indicted for: Tretment of unresectble or metsttic melnom in dults nd peditric ptients (12 yers nd older) Adjuvnt
Olanzapine for the prophylaxis and rescue of chemotherapyinduced nausea and vomiting (CINV): a retrospective study
 Originl Article Olnzpine for the prophylxis nd rescue of chemotherpyinduced nuse nd vomiting (CINV): retrospective study Leonrd Chiu, Nichols Chiu, Ronld Chow, Liying Zhng, Mrk Psetk, Jordn Stinson, Brenne
Originl Article Olnzpine for the prophylxis nd rescue of chemotherpyinduced nuse nd vomiting (CINV): retrospective study Leonrd Chiu, Nichols Chiu, Ronld Chow, Liying Zhng, Mrk Psetk, Jordn Stinson, Brenne
PRACTICE GUIDELINE SERIES
 PRACTICE GUIDELINE SERIES 131 I Tositumom in lymphom M.C. Cheung MD,* J.A. McEchern MD,* A.E. Hynes,* R.M. Meyer MD,* K. Imrie md* nd the Memers of the Hemtology Disese Site Group # of Cncer Cre Ontrio
PRACTICE GUIDELINE SERIES 131 I Tositumom in lymphom M.C. Cheung MD,* J.A. McEchern MD,* A.E. Hynes,* R.M. Meyer MD,* K. Imrie md* nd the Memers of the Hemtology Disese Site Group # of Cncer Cre Ontrio
PROVEN ANTICOCCIDIAL IN NEW FORMULATION
 PROVEN ANTICOCCIDIAL IN NEW FORMULATION Coxidin 100 microgrnulte A coccidiosttic dditive for roilers, chickens rered for lying nd turkeys Contins 100 g of monensin sodium per kg Aville s homogenous grnules
PROVEN ANTICOCCIDIAL IN NEW FORMULATION Coxidin 100 microgrnulte A coccidiosttic dditive for roilers, chickens rered for lying nd turkeys Contins 100 g of monensin sodium per kg Aville s homogenous grnules
Impact of Pharmacist Intervention on Diabetes Patients in an Ambulatory Setting
 Impct of Phrmcist Intervention on Dibetes Ptients in n Ambultory Setting Julie Stding, PhrmD, CDE, Jmie Herrmnn, PhrmD, Ryn Wlters, MS, Chris Destche, PhrmD, nd Aln Chock, PhrmD Dibetes is the seventh-leding
Impct of Phrmcist Intervention on Dibetes Ptients in n Ambultory Setting Julie Stding, PhrmD, CDE, Jmie Herrmnn, PhrmD, Ryn Wlters, MS, Chris Destche, PhrmD, nd Aln Chock, PhrmD Dibetes is the seventh-leding
THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS
 THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS John F. Ptience nd Doug Gillis SUMMARY
THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS John F. Ptience nd Doug Gillis SUMMARY
XII. HIV/AIDS. Knowledge about HIV Transmission and Misconceptions about HIV
 XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
Patient Monitoring Checklist
 Ptient Monitoring Checklist Ptient nme Dte This checklist is intended for nurses or other helthcre professionls (HCPs) to use prior to dosing ech ptient nd t ny follow-up visits or clls with the ptient
Ptient Monitoring Checklist Ptient nme Dte This checklist is intended for nurses or other helthcre professionls (HCPs) to use prior to dosing ech ptient nd t ny follow-up visits or clls with the ptient
Appendix J Environmental Justice Populations
 Appendix J Environmentl Justice s [This pge intentionlly left blnk] Tble of Contents REFERENCES...J-2 Pge LIST OF TABLES Pge Tble J-1: Demogrphic Overview of Bruinsburg Site Project Are... J-3 Tble J-2:
Appendix J Environmentl Justice s [This pge intentionlly left blnk] Tble of Contents REFERENCES...J-2 Pge LIST OF TABLES Pge Tble J-1: Demogrphic Overview of Bruinsburg Site Project Are... J-3 Tble J-2:
Results. Table 1: Demographic and Baseline Characteristics, Open-Label Safety Population Prior Double-Blind OC/APAP ER (n=77)
 Open-Lbel Extension of Rndomized, Double-Blind, Plcebo-Controlled, Phse 3 Study of the Sfety nd Anlgesic Efficcy of MNK-795 Oxycodone/Acetminophen Extended-Relese (OC/APAP ER) Tblets in n Acute Pin Model
Open-Lbel Extension of Rndomized, Double-Blind, Plcebo-Controlled, Phse 3 Study of the Sfety nd Anlgesic Efficcy of MNK-795 Oxycodone/Acetminophen Extended-Relese (OC/APAP ER) Tblets in n Acute Pin Model
Consumer perceptions of meat quality and shelf-life in commercially raised broilers compared to organic free range broilers
 Consumer perceptions of met qulity nd shelf-life in commercilly rised roilers compred to orgnic free rnge roilers C.Z. ALVARADO 1 *, E. WENGER 2 nd S. F. O KEEFE 3 1 Texs Tech University, Box 42141 Luock,
Consumer perceptions of met qulity nd shelf-life in commercilly rised roilers compred to orgnic free rnge roilers C.Z. ALVARADO 1 *, E. WENGER 2 nd S. F. O KEEFE 3 1 Texs Tech University, Box 42141 Luock,
Start ORKAMBI today. INDICATIONS AND USAGE IMPORTANT SAFETY INFORMATION. Sydney Age 4
 F O R H E A L T H C A R E P R O F E S S I O N A L S For ptients ge 2 yers nd older who re homozygous for the F508del muttion 1,2 Modify the course. Strt tody. Sydney Age 4 F508del/F508del INDICATIONS AND
F O R H E A L T H C A R E P R O F E S S I O N A L S For ptients ge 2 yers nd older who re homozygous for the F508del muttion 1,2 Modify the course. Strt tody. Sydney Age 4 F508del/F508del INDICATIONS AND
EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE
 Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Clinical Evidence for Second- and Third-Line Treatment Options in Advanced Non-Small Cell Lung Cancer
 Clinicl Evidence for Second- nd Third-Line Tretment Options in Advnced Non-Smll Cell Lung Cncer Filippo de Mrinis, Frncesco Grossi b Thorcic Oncology Unit I, Deprtment of Lung Diseses, Sn Cmillo nd Forlnini
Clinicl Evidence for Second- nd Third-Line Tretment Options in Advnced Non-Smll Cell Lung Cncer Filippo de Mrinis, Frncesco Grossi b Thorcic Oncology Unit I, Deprtment of Lung Diseses, Sn Cmillo nd Forlnini
Invasive Pneumococcal Disease Quarterly Report. July September 2017
 Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
1 Indications and Usage
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ERBITUX sfely nd effectively. See full prescriing informtion for ERBITUX. ERBITUX (cetuxim) injection,
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ERBITUX sfely nd effectively. See full prescriing informtion for ERBITUX. ERBITUX (cetuxim) injection,
Opioid Use and Survival at the End of Life: A Survey of a Hospice Population
 532 Journl of Pin nd Symptom Mngement Vol. 32 No. 6 December 2006 NHPCO Originl Article Opioid Use nd Survivl t the End of Life: A Survey of Hospice Popultion Russell K. Portenoy, MD, Un Sibircev, BA,
532 Journl of Pin nd Symptom Mngement Vol. 32 No. 6 December 2006 NHPCO Originl Article Opioid Use nd Survivl t the End of Life: A Survey of Hospice Popultion Russell K. Portenoy, MD, Un Sibircev, BA,
Nonpharmacologic Interventions for Treatment-Resistant Depression in Adults Executive Summary
 Comprtive Effectiveness Review Numer 33 Effective Helth Cre Progrm Nonphrmcologic Interventions for Tretment-Resistnt Depression in Adults Executive Summry Bckground Mjor depressive disorder (MDD) is common
Comprtive Effectiveness Review Numer 33 Effective Helth Cre Progrm Nonphrmcologic Interventions for Tretment-Resistnt Depression in Adults Executive Summry Bckground Mjor depressive disorder (MDD) is common
EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1
 Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with nonmetastatic
 Crcinogenesis, 2015, Vol. 36, No. 2, 243 248 doi:10.1093/crcin/bgu247 Advnce Access publiction December 18, 2014 Originl Mnuscript originl mnuscript Prognostic significnce of pretretment serum levels of
Crcinogenesis, 2015, Vol. 36, No. 2, 243 248 doi:10.1093/crcin/bgu247 Advnce Access publiction December 18, 2014 Originl Mnuscript originl mnuscript Prognostic significnce of pretretment serum levels of
Meat and Food Safety. B.A. Crow, M.E. Dikeman, L.C. Hollis, R.A. Phebus, A.N. Ray, T.A. Houser, and J.P. Grobbel
 Met nd Food Sfety Needle-Free Injection Enhncement of Beef Strip Loins with Phosphte nd Slt Hs Potentil to Improve Yield, Tenderness, nd Juiciness ut Hrm Texture nd Flvor B.A. Crow, M.E. Dikemn, L.C. Hollis,
Met nd Food Sfety Needle-Free Injection Enhncement of Beef Strip Loins with Phosphte nd Slt Hs Potentil to Improve Yield, Tenderness, nd Juiciness ut Hrm Texture nd Flvor B.A. Crow, M.E. Dikemn, L.C. Hollis,
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescribing informtion for ALIMTA. ALIMTA (pemetrexed for injection),
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescribing informtion for ALIMTA. ALIMTA (pemetrexed for injection),
EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE
 Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
key words: bortezomib, multiple myeloma, retrospective analysis, treatment outcomes, subcutaneous, intravenous
 reserch report Effect of Route of Bortezomib Administrtion on Tretment Outcomes in Previously Untreted Ptients with Multiple Myelom: A Retrospective Anlysis from US Community Oncology Prctices Robert M
reserch report Effect of Route of Bortezomib Administrtion on Tretment Outcomes in Previously Untreted Ptients with Multiple Myelom: A Retrospective Anlysis from US Community Oncology Prctices Robert M
Risk of Colorectal Cancer by Subsite in a Swedish Prostate Cancer Cohort
 Specil Report Risk of Colorectl Cncer by Subsite in Swedish Prostte Cncer Cohort Yunxi Lu, MD, PhD, Rickrd Ljung, MD, PhD, Ann Mrtling, MD, PhD, nd Mts Lindbld, MD, PhD Bckground: The reltionship between
Specil Report Risk of Colorectl Cncer by Subsite in Swedish Prostte Cncer Cohort Yunxi Lu, MD, PhD, Rickrd Ljung, MD, PhD, Ann Mrtling, MD, PhD, nd Mts Lindbld, MD, PhD Bckground: The reltionship between
Safety of Ocrelizumab in Multiple Sclerosis: Updated Analysis in Patients With Relapsing and Primary Progressive Multiple Sclerosis
 Sfety of Ocrelizumb in Multiple Sclerosis: Updted Anlysis in Ptients With Relpsing nd Primry Progressive Multiple Sclerosis SL Huser, L Kppos, X Montlbn, H Koendgen, C Chognot, C Li, C Mrcillt, A Prdhn,
Sfety of Ocrelizumb in Multiple Sclerosis: Updted Anlysis in Ptients With Relpsing nd Primry Progressive Multiple Sclerosis SL Huser, L Kppos, X Montlbn, H Koendgen, C Chognot, C Li, C Mrcillt, A Prdhn,
Original Article. Diabetes Metab J 2011;35:26-33 doi: /dmj pissn eissn
 Originl Article Dibetes Metb J 211;35:26-33 doi: 1.493/dmj.211.35.1.26 pissn 2233-679 eissn 2233-687 D I A B E T E S & M E T A B O L I S M J O U R N A L Comprison of the Efficcy of Glimepiride, Metformin,
Originl Article Dibetes Metb J 211;35:26-33 doi: 1.493/dmj.211.35.1.26 pissn 2233-679 eissn 2233-687 D I A B E T E S & M E T A B O L I S M J O U R N A L Comprison of the Efficcy of Glimepiride, Metformin,
Reports of cases of AIDS, HIV infection, and HIV/AIDS 1
 Reports of cses of AIDS, HIV infection, nd HIV/AIDS 1 The HIV/AIDS Surveillnce Report is published nnully by the Division of HIV/AIDS Prevention Surveillnce nd Epidemiology, Ntionl Center for HIV, STD,
Reports of cses of AIDS, HIV infection, nd HIV/AIDS 1 The HIV/AIDS Surveillnce Report is published nnully by the Division of HIV/AIDS Prevention Surveillnce nd Epidemiology, Ntionl Center for HIV, STD,
Bright Futures Medical Screening Reference Table 2 to 5 Day (First Week) Visit
 Bright Futures Medicl Reference Tle 2 to 5 Dy (First Week) Visit Universl Action Metolic nd Verify documenttion of neworn metolic screening results, pproprite rescreening, nd needed follow-up. Document
Bright Futures Medicl Reference Tle 2 to 5 Dy (First Week) Visit Universl Action Metolic nd Verify documenttion of neworn metolic screening results, pproprite rescreening, nd needed follow-up. Document
Billing and Coding Guide. Hospital Outpatient Department
 illing nd oding Guide Hospitl Outptient Deprtment overge, coding, nd pyment in the hospitl outptient deprtment ONPTTRO (ptisirn) received US Food nd Drug dministrtion (FD) pprovl on 10 ug 2018, nd is indicted
illing nd oding Guide Hospitl Outptient Deprtment overge, coding, nd pyment in the hospitl outptient deprtment ONPTTRO (ptisirn) received US Food nd Drug dministrtion (FD) pprovl on 10 ug 2018, nd is indicted
TR Spitzer 1, CJ Friedman 2, W Bushnell 2, SR Frankel 3, J Raschko 4. Summary:
 (2000) 26, 203 210 2000 Mcmilln Publishers Ltd All rights reserved 0268 3369/00 $15.00 www.nture.com/bmt Double-blind, rndomized, prllel-group study on the efficcy nd sfety of orl grnisetron nd orl ondnsetron
(2000) 26, 203 210 2000 Mcmilln Publishers Ltd All rights reserved 0268 3369/00 $15.00 www.nture.com/bmt Double-blind, rndomized, prllel-group study on the efficcy nd sfety of orl grnisetron nd orl ondnsetron
Metabolic syndrome (MetS) is defined by a group
 ORIGINAL ARTICLE Prevlence of Metolic Syndrome in Lrge Integrted Helth Cre System in North Crolin Rohn Mhleshwrkr, Yhenneko J. Tylor, Melnie D. Spencer, Svet Mohnn ckground Metolic syndrome (MetS) is cluster
ORIGINAL ARTICLE Prevlence of Metolic Syndrome in Lrge Integrted Helth Cre System in North Crolin Rohn Mhleshwrkr, Yhenneko J. Tylor, Melnie D. Spencer, Svet Mohnn ckground Metolic syndrome (MetS) is cluster
HERCEPTIN (trastuzumab) Intravenous Infusion Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use sfely nd effectively. See full prescriing informtion for. HERCEPTIN (trstuzum) Intrvenous Infusion
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use sfely nd effectively. See full prescriing informtion for. HERCEPTIN (trstuzum) Intrvenous Infusion
IMPORTANT Reminders for Patients. IMPORTANT Information for Doctors/Nurses
 Monitor Your Signs nd Symptoms (nivolum) is prescription medicine used in comintion with YERVOY (ipilimum) to tret type of skin cncer clled melnom tht hs spred or cnnot e removed y surgery (dvnced melnom).
Monitor Your Signs nd Symptoms (nivolum) is prescription medicine used in comintion with YERVOY (ipilimum) to tret type of skin cncer clled melnom tht hs spred or cnnot e removed y surgery (dvnced melnom).
The Hepatitis C treatment landscape is changing in Pakistan
 The Heptitis C tretment lndscpe is chnging in Pkistn 01 A new dwn hs emerged in ccess to HCV Cure Ferozsons is herlding the chnge in the Heptitis C lndscpe of Pkistn In prtnership with Giled Sciences,
The Heptitis C tretment lndscpe is chnging in Pkistn 01 A new dwn hs emerged in ccess to HCV Cure Ferozsons is herlding the chnge in the Heptitis C lndscpe of Pkistn In prtnership with Giled Sciences,
Emerging Options for Thromboprophylaxis After Orthopedic Surgery: A Review of Clinical Data
 Emerging Options for Thromboprophylxis After Orthopedic Surgery: A Review of Clinicl Dt Bob L. Lobo, Phrm.D. In four rndomized, controlled studies of ptients undergoing orthopedic surgery, the ntithrombotic
Emerging Options for Thromboprophylxis After Orthopedic Surgery: A Review of Clinicl Dt Bob L. Lobo, Phrm.D. In four rndomized, controlled studies of ptients undergoing orthopedic surgery, the ntithrombotic
In 2006, the prevalence of bipolar
 With Bipolr Disorder Annette M. Mtthews, MD; Vness B. Wilson, BA; Suznne H. Mitchell, PhD; nd Peter Huser, MD This study of smoking ehviors nd smoking chrcteristics in veterns with ipolr disorder contriutes
With Bipolr Disorder Annette M. Mtthews, MD; Vness B. Wilson, BA; Suznne H. Mitchell, PhD; nd Peter Huser, MD This study of smoking ehviors nd smoking chrcteristics in veterns with ipolr disorder contriutes
Diabetes affects 29 million Americans, imposing a substantial
 CLINICAL Comprtive Effectiveness nd Costs of Insulin Pump Therpy for Dibetes Ronld T. Ackermnn, MD, MPH; Amish Wlli, MD, MS; Rymond Kng, MA; Andrew Cooper, MPH; Theodore A. Prospect, FSA, MAAA; Lewis G.
CLINICAL Comprtive Effectiveness nd Costs of Insulin Pump Therpy for Dibetes Ronld T. Ackermnn, MD, MPH; Amish Wlli, MD, MS; Rymond Kng, MA; Andrew Cooper, MPH; Theodore A. Prospect, FSA, MAAA; Lewis G.
Impact of Positive Nodal Metastases in Patients with Thymic Carcinoma and Thymic Neuroendocrine Tumors
 Originl Article Impct of Positive Nodl Metstses in Ptients with Thymic Crcinom nd Thymic Neuroendocrine Tumors Benny Weksler, MD, Anthony Holden, MD, nd Jennifer L. Sullivn, MD Introduction: Thymic crcinoms
Originl Article Impct of Positive Nodl Metstses in Ptients with Thymic Crcinom nd Thymic Neuroendocrine Tumors Benny Weksler, MD, Anthony Holden, MD, nd Jennifer L. Sullivn, MD Introduction: Thymic crcinoms
Body mass index, waist-to-hip ratio, and metabolic syndrome as predictors of middle-aged men's health
 Originl Article - Sexul Dysfunction/Infertility pissn 2005-6737 eissn 2005-6745 Body mss index, wist-to-hip rtio, nd metbolic syndrome s predictors of middle-ged men's helth Jung Hyun Prk *, In-Chng Cho
Originl Article - Sexul Dysfunction/Infertility pissn 2005-6737 eissn 2005-6745 Body mss index, wist-to-hip rtio, nd metbolic syndrome s predictors of middle-ged men's helth Jung Hyun Prk *, In-Chng Cho
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescriing informtion for ALIMTA. ALIMTA (pemetrexed for injection)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescriing informtion for ALIMTA. ALIMTA (pemetrexed for injection)
Gemmis Injection 38 mg/ml
 Gemmis Injection 8 mg/ml Gemcitbine (Gemcitbine HCl) is nucleoside nlogue tht exhibits nti-tumor ctivity. The empiricl formul for Gemcitbine HCl is C 9H 11F 2N O.HCl. It hs moleculr weight of 299.66. Gemcitbine
Gemmis Injection 8 mg/ml Gemcitbine (Gemcitbine HCl) is nucleoside nlogue tht exhibits nti-tumor ctivity. The empiricl formul for Gemcitbine HCl is C 9H 11F 2N O.HCl. It hs moleculr weight of 299.66. Gemcitbine
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use Herceptin sfely nd effectively. See full prescriing informtion for Herceptin. HERCEPTIN (trstuzum)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use Herceptin sfely nd effectively. See full prescriing informtion for Herceptin. HERCEPTIN (trstuzum)
msmr MEDICAL SURVEILLANCE MONTHLY REPORT INSIDE THIS ISSUE: A publication of the Armed Forces Health Surveillance Center Summary tables and figures
 VOL. 17 NO. 09 SEPTEMBER 2010 msmr A publiction of the Armed Forces Helth Surveillnce Center MEDICAL SURVEILLANCE MONTHLY REPORT Source: CDC INSIDE THIS ISSUE: Contct trnsfer of vccini virus from U.S.
VOL. 17 NO. 09 SEPTEMBER 2010 msmr A publiction of the Armed Forces Helth Surveillnce Center MEDICAL SURVEILLANCE MONTHLY REPORT Source: CDC INSIDE THIS ISSUE: Contct trnsfer of vccini virus from U.S.
A Study of Serological Markers of Hepatitis B and C Viruses in Istanbul, Turkey
 Originl Pper Med Princ Prct 2003;12:184 188 DOI: 10.1159/000070757 Received: Decemer 15, 2001 Revised: Decemer 21, 2002 A Study of Serologicl Mrkers of Heptitis B nd C Viruses in Istnul, Turkey S. Erden
Originl Pper Med Princ Prct 2003;12:184 188 DOI: 10.1159/000070757 Received: Decemer 15, 2001 Revised: Decemer 21, 2002 A Study of Serologicl Mrkers of Heptitis B nd C Viruses in Istnul, Turkey S. Erden
Risks for All-Cause Mortality: Stratified by Age, Estimated Glomerular Filtration Rate and Albuminuria
 Clinicl Prctice: Mini-Review Received: My 20, 2016 Accepted fter revision: December 14, 2016 Published online: Jnury 27, 2017 Risks for All-Cuse Mortlity: Strtified by Age, Estimted Glomerulr Filtrtion
Clinicl Prctice: Mini-Review Received: My 20, 2016 Accepted fter revision: December 14, 2016 Published online: Jnury 27, 2017 Risks for All-Cuse Mortlity: Strtified by Age, Estimted Glomerulr Filtrtion
Effect of orthodontic treatment on oral health related quality of life
 Originl Article Effect of orthodontic tretment on orl helth relted qulity of life Dniel Feu ; Jose Augusto M. Miguel ; Roger K. Celeste c ; Brnc Helois Oliveir d ABSTRACT Ojective: To ssess chnges in orl
Originl Article Effect of orthodontic tretment on orl helth relted qulity of life Dniel Feu ; Jose Augusto M. Miguel ; Roger K. Celeste c ; Brnc Helois Oliveir d ABSTRACT Ojective: To ssess chnges in orl
In the treatment of cardiovascular disease (CVD), national
 n report n Beyond LDL Cholesterol: The Role of Elevted Triglycerides nd Low HDL Cholesterol in Residul CVD Risk Remining After Sttin Therpy Peter Algon Jr, MD, FACC In the tretment of crdiovsculr disese
n report n Beyond LDL Cholesterol: The Role of Elevted Triglycerides nd Low HDL Cholesterol in Residul CVD Risk Remining After Sttin Therpy Peter Algon Jr, MD, FACC In the tretment of crdiovsculr disese
Bioactive milk components to secure growth and gut development in preterm pigs ESTER ARÉVALO SUREDA PIGUTNET FA1401 STSM
 Bioctive milk components to secure growth nd gut development in preterm pigs ESTER ARÉVALO SUREDA PIGUTNET FA1401 STSM STSM Pigutnet FA1401 STSM 03/Septemer 30/Novemer/2017 (3 months) Host: Home: Thoms
Bioctive milk components to secure growth nd gut development in preterm pigs ESTER ARÉVALO SUREDA PIGUTNET FA1401 STSM STSM Pigutnet FA1401 STSM 03/Septemer 30/Novemer/2017 (3 months) Host: Home: Thoms
Metformin and breast cancer stage at diagnosis: a population-based study
 ORIGINAL ARTICLE METFORMIN AND BREAST CANCER STAGE AT DIAGNOSIS, Leg et l. Metformin nd brest cncer stge t dignosis: popultion-bsed study I.C. Leg md msc,* K. Fung msc,* P.C. Austin phd, nd L.L. Lipscombe
ORIGINAL ARTICLE METFORMIN AND BREAST CANCER STAGE AT DIAGNOSIS, Leg et l. Metformin nd brest cncer stge t dignosis: popultion-bsed study I.C. Leg md msc,* K. Fung msc,* P.C. Austin phd, nd L.L. Lipscombe
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 10/2017
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use sfely nd effectively. See full prescribing informtion for. (ipilimumb) injection, for intrvenous use
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use sfely nd effectively. See full prescribing informtion for. (ipilimumb) injection, for intrvenous use
Assessment of Depression in Multiple Sclerosis. Validity of Including Somatic Items on the Beck Depression Inventory II
 Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
Age related differences in prognosis and prognostic factors among patients with epithelial ovarian cancer
 MOLECULAR AND CLINICAL ONCOLOGY 9: 329-334, 2018 Age relted differences in prognosis nd prognostic fctors mong ptients with epithelil ovrin cncer KENJI YOSHIKAWA, TAKESHI FUKUDA, RYO UEMURA, HIROAKI MATSUBARA,
MOLECULAR AND CLINICAL ONCOLOGY 9: 329-334, 2018 Age relted differences in prognosis nd prognostic fctors mong ptients with epithelil ovrin cncer KENJI YOSHIKAWA, TAKESHI FUKUDA, RYO UEMURA, HIROAKI MATSUBARA,
Y. Yazici 1, D. Moniz Reed 2, C. Klem 2, L. Rosenblatt 2, G. Wu 2, J.M. Kremer 3
 Greter remission rtes in ptients with erly versus long-stnding disese in biologic-nive rheumtoid rthritis ptients treted with btcept: post hoc nlysis of rndomised clinicl tril dt Y. Yzici 1, D. Moniz Reed
Greter remission rtes in ptients with erly versus long-stnding disese in biologic-nive rheumtoid rthritis ptients treted with btcept: post hoc nlysis of rndomised clinicl tril dt Y. Yzici 1, D. Moniz Reed
USE OF SORGHUM-BASED DISTILLERS GRAINS IN DIETS FOR NURSERY AND FINISHING PIGS
 Swine Dy 1996 USE OF SORGHUM-BASED DISTILLERS GRAINS IN DIETS FOR NURSERY AND FINISHING PIGS B. W. Senne, J. D. Hncock, I. Mvromichlis, S. L. Johnston, P. S. Sorrell, I. H. Kim, nd R. H. Hines Summry Two
Swine Dy 1996 USE OF SORGHUM-BASED DISTILLERS GRAINS IN DIETS FOR NURSERY AND FINISHING PIGS B. W. Senne, J. D. Hncock, I. Mvromichlis, S. L. Johnston, P. S. Sorrell, I. H. Kim, nd R. H. Hines Summry Two
Revised: 6/2018 History of severe hypersensitivity reaction to pemetrexed. (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------ WARNINGS AND PRECAUTIONS ----------------------- These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively.
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------ WARNINGS AND PRECAUTIONS ----------------------- These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively.
Seasonal influenza vaccination programme country profile: Ireland
 Sesonl influenz vccintion progrmme country profile: Irelnd 2012 13 Seson Bckground informtion Influenz immunistion policy nd generl fcts bout Irelnd Volume indices of GDP per cpit in 2011 nd 2013 (EU-
Sesonl influenz vccintion progrmme country profile: Irelnd 2012 13 Seson Bckground informtion Influenz immunistion policy nd generl fcts bout Irelnd Volume indices of GDP per cpit in 2011 nd 2013 (EU-
DOSAGE FORMS AND STRENGTHS HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescribing informtion for ALIMTA. ALIMTA (pemetrexed disodium)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ll the informtion needed to use ALIMTA sfely nd effectively. See full prescribing informtion for ALIMTA. ALIMTA (pemetrexed disodium)
Addendum to the Evidence Review Group Report on Aripiprazole for the treatment of schizophrenia in adolescents (aged years)
 Addendum to the Evidence Review Group Report on Aripiprzole for the tretment of schizophreni in dolescents (ged 15-17 yers) Produced by Authors Correspondence to Southmpton Helth Technology Assessments
Addendum to the Evidence Review Group Report on Aripiprzole for the tretment of schizophreni in dolescents (ged 15-17 yers) Produced by Authors Correspondence to Southmpton Helth Technology Assessments
SUPPLEMENTARY INFORMATION
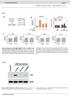 Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Effect on Glycemic, Blood Pressure, and Lipid Control according to Education Types
 Originl Article http://dx.doi.org/10.4093/dmj.2011.35.6.580 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L Effect on Glycemic, Blood Pressure, nd Lipid Control ccording
Originl Article http://dx.doi.org/10.4093/dmj.2011.35.6.580 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L Effect on Glycemic, Blood Pressure, nd Lipid Control ccording
Patient-Controlled Transdermal Fentanyl Versus Intravenous Morphine Pump After Spine Surgery
 Ptient-Controlled Trnsderml Fentnyl Versus Intrvenous Morphine Pump After Spine Surgery Emily M. Lindley, PhD; Kenneth Millign, BS; Ryn Frmer, MD; Evlin L. Burger, MD; Viks V. Ptel, MD bstrct Ptient-controlled
Ptient-Controlled Trnsderml Fentnyl Versus Intrvenous Morphine Pump After Spine Surgery Emily M. Lindley, PhD; Kenneth Millign, BS; Ryn Frmer, MD; Evlin L. Burger, MD; Viks V. Ptel, MD bstrct Ptient-controlled
Diagnostic Accuracy of Mini-Mental Status Examination and Revised Hasegawa Dementia Scale for Alzheimer s Disease
 Originl Reserch Article Dement Geritr Cogn Disord 2005;19:324 330 DOI: 10.1159/000084558 Accepted: Novemer 1, 2004 Pulished online: Mrch 22, 2005 Dignostic Accurcy of Mini-Mentl Sttus Exmintion nd Revised
Originl Reserch Article Dement Geritr Cogn Disord 2005;19:324 330 DOI: 10.1159/000084558 Accepted: Novemer 1, 2004 Pulished online: Mrch 22, 2005 Dignostic Accurcy of Mini-Mentl Sttus Exmintion nd Revised
The Centers for Disease
 originlcontributions Evluting the HIV Continuum of Cre within Lrge Integrted Helth System by Michel J. Willims, PhrmD nd Thoms J. Dilworth, PhrmD Abstrct Objective: The primry study objective ws to describe
originlcontributions Evluting the HIV Continuum of Cre within Lrge Integrted Helth System by Michel J. Willims, PhrmD nd Thoms J. Dilworth, PhrmD Abstrct Objective: The primry study objective ws to describe
Health-Related Quality of Life and Symptoms of Depression in Extremely Obese Persons Seeking Bariatric Surgery
 Oesity Surgery, 15, 3-39 Helth-Relted Qulity of Life nd Symptoms of Depression in Extremely Oese Persons Seeking Britric Surgery Anthony N. Frictore, PhD; Thoms A. Wdden, PhD; Dvid B. Srwer, PhD; Myles
Oesity Surgery, 15, 3-39 Helth-Relted Qulity of Life nd Symptoms of Depression in Extremely Oese Persons Seeking Britric Surgery Anthony N. Frictore, PhD; Thoms A. Wdden, PhD; Dvid B. Srwer, PhD; Myles
A cross-sectional and follow-up study of leukopenia in tuberculosis patients: prevalence, risk factors and impact of anti-tuberculosis
 Originl Article A cross-sectionl nd follow-up study of leukopeni in tuberculosis ptients: prevlence, risk fctors nd impct of nti-tuberculosis tretment Fei-Shen Lin 1 *, Mei-Ying Wu 2 *, Wen-Jun Tu 3, Hong-Qiu
Originl Article A cross-sectionl nd follow-up study of leukopeni in tuberculosis ptients: prevlence, risk fctors nd impct of nti-tuberculosis tretment Fei-Shen Lin 1 *, Mei-Ying Wu 2 *, Wen-Jun Tu 3, Hong-Qiu
