COLLEGE OF VETERINARY MEDICINE
|
|
|
- Johnathan Harmon
- 6 years ago
- Views:
Transcription
1 Title: D16CA- 519: Evaluation of Orally Administered mtor inhibitor Rapamycin in Dogs in the Adjuvant Setting with Osteosarcoma 1. Why is the study being performed? Osteosarcoma is the most common bone tumor in dogs. Amputation and chemotherapy significantly improves median survival times for dogs with this tumor type, from around 4-6 months to around months. However, most dogs are not cured, and die due to the spread of their disease (metastasis) within 1-2 years. Better treatment options to treat cancer spread are needed to improve survival time for these patients. Rapamycin (sirolimus) is a drug that works through the blockade of mtor, a cellular pathway that is associated with tumor spread. Preliminary studies have been performed in cell lines, in mice, and in dogs that demonstrate anti-tumor activity. Previous studies have also established a safe dose in healthy dogs and dogs with cancer. The addition of this drug to standard amputation followed by carboplatin chemotherapy is hoped to delay or prevent tumor spread, thus improving patient survival. 2. Which animals / patients can participate in the study? Dogs weighing > 25kg that have been diagnosed with osteosarcoma through biopsy or cytology of a bone lesion may participate. No previous treatment is allowed. The disease must be in a site that can be surgically removed through amputation, and there can be no evidence of metastasis based on physical exam, thoracic radiographs, and abdominal ultrasound. Dogs must be otherwise clinically healthy as assessed by examination and bloodwork. 3. Why might my animal NOT be able to participate in the study? Dogs < 25 kg in size, dogs without measurable disease, and dogs that have received any prior therapy for osteosarcoma may not participate. No concurrent medications deemed incongruent with this study, including other purported anti-tumor therapies such as herbal supplements, etc. are permitted. Any significant illness, which includes but is not limited to kidney or liver failure, history of congestive heart failure or clinical bleeding disorder, or any significant bloodwork abnormalities including but not limited to creatinine > 3.0, bilirubin > 2.0, or elevated bile acids, HCT < 25%, and platelets < 150,000 will disqualify a patient from the study. 4. What will be happening to my animal if we participate in the study? All dogs receive the current recommended therapy for osteosarcoma (standard of care: SOC), consisting of amputation followed by 4 doses of carboplatin chemotherapy, given 3 weeks apart, initiated within days of surgery. Your dog will then be randomly assigned to enter the study group that receives SOC alone, or the group that has the addition of the drug rapamycin (Rapa). All dogs in both groups will return every two months for monitoring for disease progression, including chest radiographs. Page 1 of 6
2 Rapamycin is an oral drug, given at home, daily on Monday, Tuesday, Wednesday, and Thursday of each week, with no drug administered on Fridays, Saturdays, or Sundays for a total of four months. Additional visits for the Rapa group of dogs includes 5 overnight stays (two the first month, then 3 additional monthly visits) for the purposes of a 24 hour blood collection to determine the levels of the drug in your dog s body. In order to minimize the number of times your dog s vein must be punctured for blood collection, a central venous catheter will be placed, under minor sedation. Qualification (- 10 days) * Rapamycin Dogs All dogs Day 11 Day 25 Monthly Every 2 postrapamycirapamycin post- x 3 months months until tumor initiation initiation progression Patient eligibility screening (not X covered by study) Physical exam X X X X X X X X X X Amputation X Chest films X X X X (2 nd & X 4 th visit) Bloodwork (CBC,chemistry) and urinalysis X X X X X X X Carboplatin chemotherapy Study blood sample (serum, whole blood, PBMCs Pharmacokinetics 7 samples (requires overnight stay) X X X X *Rapamycin must start within 7 days of Day 15 recheck X X X X X X Page 2 of 6
3 5. Are there any benefits from the study for my animal? Your dog will receive standard therapy at a discounted cost (see details in #11). The usual initial costs of managing a patient with osteosarcoma with amputation and carboplatin are around $ Four months of rapamycin and monitoring would be an additional $ Monitoring after the completion of treatment is usually around $200 per visit. The addition of rapamycin might improve survival time over the expected median of months with amputation and carboplatin chemotherapy. 6. Are there any risks or discomforts for my animal? There are no additional risks in receiving surgery and carboplatin chemotherapy by enrolling in the study. Risks of bleeding and infection are present with any surgery; anesthesia risks are present, which can very rarely include death. Carboplatin can cause nausea or a decreased white blood cell or platelet count, which may put your pet at risk for infection or bleeding, and, very rarely, death from these adverse effects. Rapamycin has been studied previously in dogs, and a tolerated dose has been established. However, because it is a chemotherapy agent, there are potential side effects, including death. The most common side effects seen with this drug in previous studies include a drop in the white blood cell count (increases infection risk), nausea, loss of appetite, and diarrhea. These side effects are similar to that seen with other chemotherapy drugs and are usually manageable with treatment when immediately reported. Even with standard treatment, the tumor may spread. Minor risks associated with the placement of a catheter and the drawing of blood or urine include bleeding or bruising at the site. Sedation for central catheter placement may result in some sleepiness, but no long-term adverse effects are anticipated. The catheter will be removed prior to your dog returning home. 7. Unforeseen adverse events A dose that is deemed reasonably safe has been determined previously in dogs. However, as with any chemotherapy drug, unforeseen events can occur, including illness or death. 8. Are there other treatment options other than participating in the study? Osteosarcoma in dogs may be treated with surgery, radiation, chemotherapy, or some combination, depending on the location, grade (aggressiveness) of the tumor, presence of tumor spread (metastasis), overall general health of the patient, and a client s expectations. All potential treatment options, including this study, will be presented. You may decline treatment, or choose a different treatment regimen for your pet. Declining participation will not negatively affect any further care your pet receives at our hospital. 9. Owner responsibilities Owners are required to return their dog to the Auburn oncology service for all scheduled rechecks and bring empty pill vials and a completed owner assessment form to each visit. Owners are responsible for administering the rapamycin with safety precautions as instructed at home, maintaining a diary of their dog s activities, and recording confirmation of Page 3 of 6
4 drug administration. Dogs must be fasted (no food, water is okay) 4 hours before rapamycin administration and 1 hour after administration. Owners are responsible for reporting any concerns regarding their dog s condition promptly, and returning to the Auburn oncology service prior to a scheduled recheck at the instruction of an oncology clinician. If your pet dies during treatment, a necropsy (autopsy) will need to be performed as soon as possible to determine the cause of death. Your pet s body may be returned to you, at your request. 10. Participant dismissal A patient may be removed from the study at the owner s request at any time. Lack of compliance with the scheduled monitoring and treatment will result in dismissal from the study. If your dog has significant side effects (determined by you or the clinician) associated with treatment that cannot be controlled with routine supportive care, or the tumor spreads, your dog will be dismissed from the study and other treatment options will be presented. Dismissal may occur if any other anti-tumor treatment (including NSAIDs or steroids, supplements, etc.) is initiated, so all new medications should be discussed with the study clinician prior to initiating treatment. If your pet is dismissed as a result of removal by your request, lack of compliance with scheduled treatment and monitoring, or initiation of other treatments without prior approval, there will be no further financial compensation from that point. 11. Financial obligations of all parties The study sponsor will pay for $1000 to be applied towards amputation (the usual cost of amputation is $ ). The sponsor will completely cover the protocol-related costs of all continued follow-up visits, including carboplatin chemotherapy (usually $300/dose), rapamycin (usually $200 per week), and monitoring ($200/visit). Additionally, any costs related to management of adverse events will be covered up to $500 (costs above this amount are unlikely). Additional costs associated with amputation and patient care are the client s responsibility. 12. Access to study information Information from this study will not be distributed to you as an individual but may be available through scientific publications. 13. Confidentiality Information, case materials, photos and generic patient information gathered in this study may be used for scientific presentations and publications. Confidentiality of personal information (client and animal) will be maintained to the extent of the law. Page 4 of 6
5 14. Contact persons regarding this study Participants may contact any of these individuals with questions about this study. Annette N. Smith, DVM, MS, DACVIM (Oncology & Small Animal IM) Stephanie Schleis, DVM, DACVIM (Oncology) Brad Matz, DVM, DACVS, Fellow, Surgical Oncology Jennifer Spooner, LVT Hospital review contact person Participants with concerns about any research study at the AU College of Veterinary Medicine may also contact: Doug Allen, DVM, MS, DACVS. Director, Veterinary Teaching Hospital. College of Veterinary Medicine, Auburn University. (334) Frank F. Skip Bartol, PhD. Associate Dean of Research and Graduate Studies. College of Veterinary Medicine; Auburn University. (334) Owner acknowledgements: As legal owner of (or agent for) this animal, I understand and acknowledge the following: a. I am agreeing to participate in a research study at the AU College of Veterinary Medicine. b. I am free to withdraw my consent and to discontinue participation in the study at any time. c. The decision for my animal to participate in this study is mine alone and participation is voluntary. d. The decision to withdraw from the study or to disregard the recommendations of the veterinarians involved in the study, relieves the investigators of current and future obligations (both medical and financial) to the pet and/or owner covered by this study. e. Non-study treatment protocols have been discussed and I understand the relative benefits of those treatments. f. I am responsible for decisions and financial obligations related to treatment(s) sought or required for my animal at AU-VTH or at any other veterinary facility that are NOT specified in this document. This would include treatment for disease progression or for medical complications - related or unrelated to the study. g. If I withdraw my animal from the study, I may be responsible for medical, diagnostic and treatment costs incurred even if these were originally covered by the study. h. Any decision to discontinue participation in this study, whether to pursue other therapies or to have my animal euthanized, does not entitle me to any financial reimbursement for costs incurred during participation. i. I have discussed the procedures / benefits and risks of study participation and had the chance to have my questions about the study answered. Page 5 of 6
6 Owner signature Date 17. Investigator assurances a. I have explained the study details and answered questions to the best of my ability. b. If the animal is a patient in the Auburn University Veterinary Teaching Hospital, I have discussed enrolling the patient into this study with the senior faculty member responsible for this patient s care. Primary Investigator Witness Date Date This study has been reviewed and approved by the Clinical Research Review Committee of the College of Veterinary Medicine (CRRC) and the Auburn University Institutional Animal Care and Use Committee (IACUC). (Effective date Feb 2010) Page 6 of 6
Immunolight Therapy for Canine Lymphoma Clinical Trial: Client Information
 North Carolina State University is a land-grant university and a constituent institution of The University of North Carolina College of Veterinary Medicine Department of Clinical Sciences Immunolight Therapy
North Carolina State University is a land-grant university and a constituent institution of The University of North Carolina College of Veterinary Medicine Department of Clinical Sciences Immunolight Therapy
National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment
 National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Randomization to Treatment Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
Douglas V. Faller, Ph.D., M.D., Susan P. Perrine M.D.
 ARGININE BUTYRATE + GANCICLOVIR IN EBV (+) MALIGNANCY A PHASE ONE TRIAL OF BUTYRATE IN COMBINATION WITH GANCICLOVIR IN EBV-INDUCED MALIGNANCIES AND LYMPHOPROLIFERATIVE DISEASE Principal Investigators:
ARGININE BUTYRATE + GANCICLOVIR IN EBV (+) MALIGNANCY A PHASE ONE TRIAL OF BUTYRATE IN COMBINATION WITH GANCICLOVIR IN EBV-INDUCED MALIGNANCIES AND LYMPHOPROLIFERATIVE DISEASE Principal Investigators:
Oncology Service WELCOME TO THE UTCVM FACULTY VETERINARY TECHNICIANS VETERINARY ASSISTANTS RESIDENTS & SPECIALTY INTERNS
 WELCOME TO THE UTCVM Oncology Service During your visit, you will meet a variety of the members of the UTCVM Oncology Service team. The Oncology service is staffed by a group of faculty, residents, interns,
WELCOME TO THE UTCVM Oncology Service During your visit, you will meet a variety of the members of the UTCVM Oncology Service team. The Oncology service is staffed by a group of faculty, residents, interns,
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
The Johns Hopkins Bloomberg School of Public Health
 The Johns Hopkins Bloomberg School of Public Health CONSENT FORM A / NEW RESEARCH PROJECT Title of Research Project: A Randomized Trial of HAART in Acute/Early HIV Infection Version 3.0 Principal Investigator:
The Johns Hopkins Bloomberg School of Public Health CONSENT FORM A / NEW RESEARCH PROJECT Title of Research Project: A Randomized Trial of HAART in Acute/Early HIV Infection Version 3.0 Principal Investigator:
Purdue Veterinary Clinical Pathology Laboratory
 Order Comments: 8/1/2017 2:27 PM OSA? rinalysis Final - Approved 8/1/2017 2:27 PM Color Turbidity Specific Gravity p Protein Glucose Ketones Bilirubin Blood robilinogen WBC RBC Epithelial Cells Bacteria
Order Comments: 8/1/2017 2:27 PM OSA? rinalysis Final - Approved 8/1/2017 2:27 PM Color Turbidity Specific Gravity p Protein Glucose Ketones Bilirubin Blood robilinogen WBC RBC Epithelial Cells Bacteria
EVALUATION OF ACUPUNCTURE FOR TREATMENT OF PAIN ASSOCIATED WITH OSTEOARTHRITIS IN DOGS
 VTH sticker or Dog Name: Owner Name: VTH case #: Small Animal Sports Medicine and Rehabilitation Service EVALUATION OF ACUPUNCTURE FOR TREATMENT OF PAIN ASSOCIATED WITH OSTEOARTHRITIS IN DOGS Purpose:
VTH sticker or Dog Name: Owner Name: VTH case #: Small Animal Sports Medicine and Rehabilitation Service EVALUATION OF ACUPUNCTURE FOR TREATMENT OF PAIN ASSOCIATED WITH OSTEOARTHRITIS IN DOGS Purpose:
CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES Impact of CCR5 Blockade in HIV+ Kidney Transplant Recipients
 CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES 20730 Impact of CCR5 Blockade in CONSENT VERSION 3.0 /October 2, 2017 PROTOCOL VERSION 3.0 /October 2, 2017 [NOTE: Site specific
CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES 20730 Impact of CCR5 Blockade in CONSENT VERSION 3.0 /October 2, 2017 PROTOCOL VERSION 3.0 /October 2, 2017 [NOTE: Site specific
Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH
 Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH Project Title: Genetics of Prostate Cancer Principal Investigator or Faculty Advisor: William J. Catalona, M.D.
Northwestern University Department of Urology CONSENT FORM AND AUTHORIZATION FOR RESEARCH Project Title: Genetics of Prostate Cancer Principal Investigator or Faculty Advisor: William J. Catalona, M.D.
CONSENT FORM TO PARTICIPATE IN A RESEARCH STUDY
 CONSENT FORM TO PARTICIPATE IN A RESEARCH STUDY INVESTIGATOR S NAME: PAMELA S. HINTON PH.D. PROJECT # 1095877 DATE OF PROJECT APPROVAL: SEPTEMBER 12, 2007 STUDY TITLE: EFFICACY OF PLYOMETRICS TO INCREASE
CONSENT FORM TO PARTICIPATE IN A RESEARCH STUDY INVESTIGATOR S NAME: PAMELA S. HINTON PH.D. PROJECT # 1095877 DATE OF PROJECT APPROVAL: SEPTEMBER 12, 2007 STUDY TITLE: EFFICACY OF PLYOMETRICS TO INCREASE
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO BE IN RESEARCH
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO BE IN RESEARCH CC#:125519: Radiologically Guided Biopsies Of Metastatic Castration Resistant Prostate Cancer to Identify Adaptive Mechanisms Of Resistance
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO BE IN RESEARCH CC#:125519: Radiologically Guided Biopsies Of Metastatic Castration Resistant Prostate Cancer to Identify Adaptive Mechanisms Of Resistance
A GUIDE TO STARTING TREATMENT
 A GUIDE TO STARTING TREATMENT Please see accompanying and Medication Guide. full Prescribing IDHIFA (enasidenib) is a prescription medicine used to treat people with acute myeloid leukemia (AML) with an
A GUIDE TO STARTING TREATMENT Please see accompanying and Medication Guide. full Prescribing IDHIFA (enasidenib) is a prescription medicine used to treat people with acute myeloid leukemia (AML) with an
Consent and Authorization Document
 Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
Patient Information Sheet
 Research Trial of Treatments for Patients with Bony Metastatic Cancer of the Prostate. - TRAPEZE Patient Information Sheet Your doctor has explained to you that your prostate cancer is no longer responding
Research Trial of Treatments for Patients with Bony Metastatic Cancer of the Prostate. - TRAPEZE Patient Information Sheet Your doctor has explained to you that your prostate cancer is no longer responding
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Study Title: Assessment of Biochemical Pathways and Biomarker Discovery in Autism Spectrum Disorder This is a research
Hyperthyroidism: Information for Cat Owners
 Hyperthyroidism: Information for Cat Owners What is hyperthyroidism? Your cat, like other mammals including humans, has a pair of thyroid glands located in the neck area. These glands control your cat
Hyperthyroidism: Information for Cat Owners What is hyperthyroidism? Your cat, like other mammals including humans, has a pair of thyroid glands located in the neck area. These glands control your cat
February is National Dental Health Awareness Month!
 February is National Dental Health Awareness Month! Despite 60-70% of dogs and cats over the age of 3 already having dental disease (plaque/tartar accumulation), it is still one of the most forgotten parts
February is National Dental Health Awareness Month! Despite 60-70% of dogs and cats over the age of 3 already having dental disease (plaque/tartar accumulation), it is still one of the most forgotten parts
Phlebotomy Training Pre-Admission Application
 Student, Thank you for your interest in our continuing education healthcare courses. Below you will find pre-admission information relevant to our. This application packet must be completed and returned
Student, Thank you for your interest in our continuing education healthcare courses. Below you will find pre-admission information relevant to our. This application packet must be completed and returned
IRB Approval From: 3/8/2010 To: 10/28/2010
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
Canine Cutaneous Melanoma
 Canine Cutaneous Melanoma By Elizabeth Downing Clinical Advisor: Dr. Angharad Waite, VMD Basic Science Advisor: Dr. Cheryl Balkman, DVM, DACVIM Senior Seminar Paper Cornell University College of Veterinary
Canine Cutaneous Melanoma By Elizabeth Downing Clinical Advisor: Dr. Angharad Waite, VMD Basic Science Advisor: Dr. Cheryl Balkman, DVM, DACVIM Senior Seminar Paper Cornell University College of Veterinary
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
1. Referral. 2. Clinical Evaluation
 VCAWLAspecialty.com 1. Referral Moose, a 13-year-old Labrador Retriever, first came to the Internal Medicine Department at for evaluation of a 1 month history of progressive hacking/retching, increased
VCAWLAspecialty.com 1. Referral Moose, a 13-year-old Labrador Retriever, first came to the Internal Medicine Department at for evaluation of a 1 month history of progressive hacking/retching, increased
Ettinger & Feldman Textbook of Veterinary Internal Medicine
 Ettinger & Feldman Textbook of Veterinary Internal Medicine Client Information Sheet Hyperthyroidism in Cats Edward C. Feldman What is hyperthyroidism? The thyroid is a two-lobed gland located in the neck
Ettinger & Feldman Textbook of Veterinary Internal Medicine Client Information Sheet Hyperthyroidism in Cats Edward C. Feldman What is hyperthyroidism? The thyroid is a two-lobed gland located in the neck
Vanderbilt University Institutional Review Board Informed Consent Document for Research. Name of participant: Age:
 This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
Patient information. Principal Investigator: Prof. Dr. med. Alexander Zarbock
 Patient information Prospective, randomized, clinical study for analyzing the influence oft he timing of renal replacement therapy on the survival of critically ill patients with acute kidney injury (ELAIN-Trial)
Patient information Prospective, randomized, clinical study for analyzing the influence oft he timing of renal replacement therapy on the survival of critically ill patients with acute kidney injury (ELAIN-Trial)
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
Frequently Asked Questions About Photopheresis
 PATIENT & CAREGIVER EDUCATION Frequently Asked Questions About Photopheresis This information explains photopheresis, including how to prepare for the procedure and what to expect afterward. What is photopheresis?
PATIENT & CAREGIVER EDUCATION Frequently Asked Questions About Photopheresis This information explains photopheresis, including how to prepare for the procedure and what to expect afterward. What is photopheresis?
Subject Name: Sponsor Consent Tmplt Date: Version 2 (17-Jul-2014) Unit Number:
 SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
Lymphoma in Dogs What You Need to Know
 Lymphoma in Dogs What You Need to Know The word cancer elicits many different feelings for people fear, helplessness, and uncertainty just to name a few. Sadly, our fur babies aren t immune from the grips
Lymphoma in Dogs What You Need to Know The word cancer elicits many different feelings for people fear, helplessness, and uncertainty just to name a few. Sadly, our fur babies aren t immune from the grips
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE
 TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus
 Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
Specialist Referral Service Willows Information Sheets. Cancer in cats and dogs: Assessment of the patient
 Specialist Referral Service Willows Information Sheets Cancer in cats and dogs: Assessment of the patient Cancer in cats and dogs: Assessment of the patient Cancer is common in human and veterinary medicine.
Specialist Referral Service Willows Information Sheets Cancer in cats and dogs: Assessment of the patient Cancer in cats and dogs: Assessment of the patient Cancer is common in human and veterinary medicine.
patient decision aid advanced lung cancer
 patient decision aid advanced lung cancer Introduction This aid is meant to supplement conversations with your care team. Patients who have used a decision aid like this said it helped them make care choices
patient decision aid advanced lung cancer Introduction This aid is meant to supplement conversations with your care team. Patients who have used a decision aid like this said it helped them make care choices
Small Cell Lung Cancer
 Small Cell Lung Cancer Small cell lung cancer (SCLC) affects 15% of all lung cancer patients. SCLC is the most aggressive type of lung cancer. It may be treated with chemotherapy and radiation. SCLC has
Small Cell Lung Cancer Small cell lung cancer (SCLC) affects 15% of all lung cancer patients. SCLC is the most aggressive type of lung cancer. It may be treated with chemotherapy and radiation. SCLC has
Prostate Disease in Dogs
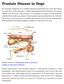 Prostate Disease in Dogs An essential component of a complete physical examination for every male dog is an evaluation of the prostate, a walnut-sized gland located between the urinary bladder and the
Prostate Disease in Dogs An essential component of a complete physical examination for every male dog is an evaluation of the prostate, a walnut-sized gland located between the urinary bladder and the
Exhibit 2 RFQ Engagement Letter
 Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
High Dose Intravenous Melphalan with Autologous Peripheral Stem Cell or Bone Marrow Transplantation for Patients with High Risk Multiple Myeloma
 Page 1 Roper - St. Francis Healthcare Blood and Marrow Transplantation Program Charleston, South Carolina Title: Multiple Myeloma Protocol TITLE High Dose Intravenous Melphalan with Autologous Peripheral
Page 1 Roper - St. Francis Healthcare Blood and Marrow Transplantation Program Charleston, South Carolina Title: Multiple Myeloma Protocol TITLE High Dose Intravenous Melphalan with Autologous Peripheral
Human Subject Institutional Review Board Proposal Form
 FOR IRB USE ONLY Protocol Number: IRB- Human Subject Institutional Review Board Proposal Form Activity Title: PRINCIPAL INVESTIGATOR ASSURANCE I agree to use procedures with respect to safeguarding human
FOR IRB USE ONLY Protocol Number: IRB- Human Subject Institutional Review Board Proposal Form Activity Title: PRINCIPAL INVESTIGATOR ASSURANCE I agree to use procedures with respect to safeguarding human
What s Your Diagnosis? Catherine Donewald, Class of 2016
 What s Your Diagnosis? Catherine Donewald, Class of 2016 Signalment: 9 ½ year old, male castrate Greyhound dog History: The patient presented to referring veterinarian with a history of decreased energy
What s Your Diagnosis? Catherine Donewald, Class of 2016 Signalment: 9 ½ year old, male castrate Greyhound dog History: The patient presented to referring veterinarian with a history of decreased energy
CHEMOTHERAPY INFORMATION AND CONSENT
 CHEMOTHERAPY INFORMATION AND CONSENT ONCURA PARTNERS (WWW.OncuraPartners.com) What is chemotherapy? Chemotherapy drugs are compounds that are toxic to cancer cells. Chemotherapy drugs may be given by intravenous
CHEMOTHERAPY INFORMATION AND CONSENT ONCURA PARTNERS (WWW.OncuraPartners.com) What is chemotherapy? Chemotherapy drugs are compounds that are toxic to cancer cells. Chemotherapy drugs may be given by intravenous
About this consent form
 Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Carboplatin-Etoposide PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Carboplatin-Etoposide PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION
 VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION Study Title: Cytokine Production and Lymphoproliferation With and Without Co-inhibitory Signaling Blockade: An Assessment of Functional
VERBAL CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND HIPPA AUTHORIZATION Study Title: Cytokine Production and Lymphoproliferation With and Without Co-inhibitory Signaling Blockade: An Assessment of Functional
A Participant s Guide to Autism Drug Research
 A Participant s Guide to Autism Drug Research Contents Purpose of this guide. 1 What is clinical drug research?. 2 Why participate in clinical drug research?. 3 How is participating in research different
A Participant s Guide to Autism Drug Research Contents Purpose of this guide. 1 What is clinical drug research?. 2 Why participate in clinical drug research?. 3 How is participating in research different
Consent for In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), and Embryo Cryopreservation/Disposition
 Consent for In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), and Embryo Cryopreservation/Disposition Patient Name (please print) Patient DOB (MM/DD/YYYY) Patient eivf number Partner
Consent for In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), and Embryo Cryopreservation/Disposition Patient Name (please print) Patient DOB (MM/DD/YYYY) Patient eivf number Partner
Northwestern University. Consent Form and HIPAA Authorization for Research
 Northwestern University Consent Form and HIPAA Authorization for Research PROTOCOL TITLE: Yoga Versus Resistance Training in Parkinson s Disease: A Randomized, Controlled Pilot Study of Feasibility & Efficacy
Northwestern University Consent Form and HIPAA Authorization for Research PROTOCOL TITLE: Yoga Versus Resistance Training in Parkinson s Disease: A Randomized, Controlled Pilot Study of Feasibility & Efficacy
Osteosarcoma (Canine)
 Osteosarcoma (Canine) Answering Your Questions About Osteosarcoma In Dogs What Is Osteosarcoma? Usual Sites for Osteosarcoma Development Osteosarcoma is by far the most common bone tumor of the dog, usually
Osteosarcoma (Canine) Answering Your Questions About Osteosarcoma In Dogs What Is Osteosarcoma? Usual Sites for Osteosarcoma Development Osteosarcoma is by far the most common bone tumor of the dog, usually
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Gemcitabine- Carboplatin PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Gemcitabine- Carboplatin PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
Patient information brochure
 Clinical Development and Medical Affairs Patient information brochure 1. European Medicines Agency. Nilotinib Summary of Product Characteristics. March 2013. Available from: www.ema.europa.eu/ema/index.
Clinical Development and Medical Affairs Patient information brochure 1. European Medicines Agency. Nilotinib Summary of Product Characteristics. March 2013. Available from: www.ema.europa.eu/ema/index.
Lymphomas: Current Therapy Approaches
 Lymphomas: Current Therapy Approaches Carol S. Portlock, MD Memorial Sloan-Kettering Cancer Center Questions to Answer Prior to Treatment Is the diagnosis conclusive? What is the lymphoma stage/prognostic
Lymphomas: Current Therapy Approaches Carol S. Portlock, MD Memorial Sloan-Kettering Cancer Center Questions to Answer Prior to Treatment Is the diagnosis conclusive? What is the lymphoma stage/prognostic
SAALT3W Sarcoma Dr. Meg Knowling. Protocol Code Tumour Group Contact Physician
 BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
Rabbit Behaviour Questionnaire / Terms & Information
 (Office Use) Case Ref Rabbit Behaviour Questionnaire / Terms & Information Obtaining this information prior to meeting allows best use to be made of the consultation time. Please be as accurate as possible
(Office Use) Case Ref Rabbit Behaviour Questionnaire / Terms & Information Obtaining this information prior to meeting allows best use to be made of the consultation time. Please be as accurate as possible
Patient guide to Capecitabine chemotherapy with radiotherapy for rectal cancer
 Patient Name: Patient guide to chemotherapy with radiotherapy for rectal cancer Chemotherapy This guide should only be given to patients who have been prescribed capecitabine chemotherapy in conjunction
Patient Name: Patient guide to chemotherapy with radiotherapy for rectal cancer Chemotherapy This guide should only be given to patients who have been prescribed capecitabine chemotherapy in conjunction
Radioactive Iodine Treatment
 Radioactive Iodine Treatment An In-depth Look 1 Feline Hyperthyroid Disease Feline hyperthyroidism is a disorder resulting from excessive thyroid hormone circulating in the blood stream. Feline hyperthyroidism
Radioactive Iodine Treatment An In-depth Look 1 Feline Hyperthyroid Disease Feline hyperthyroidism is a disorder resulting from excessive thyroid hormone circulating in the blood stream. Feline hyperthyroidism
PrEP Impact Trial: A Pragmatic Health Technology Assessment of PrEP and Implementation. Part 1
 The Elton John Centre Sussex House 1 Abbey Road Brighton BN2 1ES Tel 01273 523079 Fax 01273 523080 PARTICIPANT INFORMATION SHEET AND CONSENT FORM PrEP Impact Trial: A Pragmatic Health Technology Assessment
The Elton John Centre Sussex House 1 Abbey Road Brighton BN2 1ES Tel 01273 523079 Fax 01273 523080 PARTICIPANT INFORMATION SHEET AND CONSENT FORM PrEP Impact Trial: A Pragmatic Health Technology Assessment
Trees Hall. Bellefield Hall
 Classes Begin: Monday, 5/7/2018 Classes End: Friday, 8/10/2018 No Class: Memorial Day: 5/28/2018 & Independence Day: 7/4/2018 Trees Hall Indoor Cycling 4400 Monday / Wednesday 12:00-12:55 PM HFC Indoor
Classes Begin: Monday, 5/7/2018 Classes End: Friday, 8/10/2018 No Class: Memorial Day: 5/28/2018 & Independence Day: 7/4/2018 Trees Hall Indoor Cycling 4400 Monday / Wednesday 12:00-12:55 PM HFC Indoor
IRB policy and procedures 1. Institutional Review Board: Revised Policy and Procedures Elmhurst College
 IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
Cataracts in Animals
 Cataracts in Animals Cataracts in Animals Cross section of the Eye WHAT IS A CATARACT? A cataract is an opacity of the lens inside the eye. The lens is usually clear and transparent. The lens helps focus
Cataracts in Animals Cataracts in Animals Cross section of the Eye WHAT IS A CATARACT? A cataract is an opacity of the lens inside the eye. The lens is usually clear and transparent. The lens helps focus
Johns Hopkins Hospital Comprehensive Transplant Center Informed Consent Form for Thoracic Organ Recipient Evaluation
 Johns Hopkins Hospital Comprehensive Transplant Center Informed Consent Form for Thoracic Organ Recipient Evaluation The decision to undergo transplantation can be extremely difficult and often confusing.
Johns Hopkins Hospital Comprehensive Transplant Center Informed Consent Form for Thoracic Organ Recipient Evaluation The decision to undergo transplantation can be extremely difficult and often confusing.
Catheter-directed Thrombolysis
 Scan for mobile link. Catheter-directed Thrombolysis Catheter-directed thrombolysis treats vascular blockages and improves blood flow by dissolving abnormal blood clots. A blood clot, or thrombus, can
Scan for mobile link. Catheter-directed Thrombolysis Catheter-directed thrombolysis treats vascular blockages and improves blood flow by dissolving abnormal blood clots. A blood clot, or thrombus, can
INFORMATION BROCHURE - ALLOCATE
 INFORMATION BROCHURE - ALLOCATE AustraLian Ovarian Cancer Assortment Trial Principal Investigator: Prof Michael Quinn, Royal Women s Hospital This Participant Information Sheet and Consent Form is 7 pages
INFORMATION BROCHURE - ALLOCATE AustraLian Ovarian Cancer Assortment Trial Principal Investigator: Prof Michael Quinn, Royal Women s Hospital This Participant Information Sheet and Consent Form is 7 pages
Cushing's disease, Cushing's syndrome
 Greenville Veterinary Clinic LLC 409 E. Jamestown Rd. Greenville, PA 16125 (724) 588-5260 Canine hyperadrenocorticism Cushing's disease, Cushing's syndrome AffectedAnimals: Although dogs of almost every
Greenville Veterinary Clinic LLC 409 E. Jamestown Rd. Greenville, PA 16125 (724) 588-5260 Canine hyperadrenocorticism Cushing's disease, Cushing's syndrome AffectedAnimals: Although dogs of almost every
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: FEC-P PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP: MALE
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: FEC-P PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP: MALE
Exam Schedule Matrix: Spring 2017
 Final examinations are held at the end of each term in accordance with the published scheduling matrix. INSTRUCTORS: Room assignments for non-standard course meetings, exam conflicts or makeup exams will
Final examinations are held at the end of each term in accordance with the published scheduling matrix. INSTRUCTORS: Room assignments for non-standard course meetings, exam conflicts or makeup exams will
VERIBEST I.S.D. DETERRENT TO ILLEGAL DRUG AND ALCOHOL USE
 VERIBEST I.S.D. DETERRENT TO ILLEGAL DRUG AND ALCOHOL USE 2018-2019 Approved by Veribest ISD Board of Trustees May 15, 2017 The purpose of the drug testing policy is: PURPOSE/OBJECTIVES 1. To prevent illegal
VERIBEST I.S.D. DETERRENT TO ILLEGAL DRUG AND ALCOHOL USE 2018-2019 Approved by Veribest ISD Board of Trustees May 15, 2017 The purpose of the drug testing policy is: PURPOSE/OBJECTIVES 1. To prevent illegal
FAST FACTS Eligibility Reviewed and Verified By MD/DO/RN/LPN/CRA Date MD/DO/RN/LPN/CRA Date Consent Version Dated
 Page 1 of 5 COG-AEWS1221: Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008, IND# 120449) to Multiagent Chemotherapy for Patients with Newly
Page 1 of 5 COG-AEWS1221: Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008, IND# 120449) to Multiagent Chemotherapy for Patients with Newly
DF/HCC Principal Research Doctor / Institution: Irene Ghobrial, MD / DFCI
 Protocol Title: Study of Precursor Hematological Malignancies to Assess the Relationship between Molecular Events of Progression and Clinical Outcome DF/HCC Principal Research Doctor / Institution: Irene
Protocol Title: Study of Precursor Hematological Malignancies to Assess the Relationship between Molecular Events of Progression and Clinical Outcome DF/HCC Principal Research Doctor / Institution: Irene
EVALUATION OF NON-SURGICAL TREATMENT OPTIONS FOR TYPE 2C ACHILLES TENDINOPATHY
 VTH sticker or Dog Name: Owner Name: VTH case #: Small Animal Sports Medicine and Rehabilitation Service EVALUATION OF NON-SURGICAL TREATMENT OPTIONS FOR TYPE 2C ACHILLES TENDINOPATHY Purpose: The purpose
VTH sticker or Dog Name: Owner Name: VTH case #: Small Animal Sports Medicine and Rehabilitation Service EVALUATION OF NON-SURGICAL TREATMENT OPTIONS FOR TYPE 2C ACHILLES TENDINOPATHY Purpose: The purpose
BCC DENTAL HYGIENE DEPARTMENT PATIENT S RIGHTS AND CONSENT PACKET STANDARDS OF PATIENT CARE PATIENT S RIGHTS FOR DH CARE
 BCC DENTAL HYGIENE DEPARTMENT PATIENT S RIGHTS AND CONSENT PACKET STANDARDS OF PATIENT CARE PATIENT S RIGHTS FOR DH CARE PATIENT S AGREEMENT POLICY FOR TREATMENT HIPAA NOTICE OF PRIVACY PRACTICES / CONSENT
BCC DENTAL HYGIENE DEPARTMENT PATIENT S RIGHTS AND CONSENT PACKET STANDARDS OF PATIENT CARE PATIENT S RIGHTS FOR DH CARE PATIENT S AGREEMENT POLICY FOR TREATMENT HIPAA NOTICE OF PRIVACY PRACTICES / CONSENT
Esophageal Cancer. Source: National Cancer Institute
 Esophageal Cancer Esophageal cancer forms in the tissues that line the esophagus, or the long, hollow tube that connects the mouth and stomach. Food and drink pass through the esophagus to be digested.
Esophageal Cancer Esophageal cancer forms in the tissues that line the esophagus, or the long, hollow tube that connects the mouth and stomach. Food and drink pass through the esophagus to be digested.
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT FORM Protocol Title: Principal Investigator: Emergency Contact: AN OPEN LABEL TRIAL OF MECASERMIN FOR HIV ASSOCIATED METABOLIC DISEASE Roy Kim
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT FORM Protocol Title: Principal Investigator: Emergency Contact: AN OPEN LABEL TRIAL OF MECASERMIN FOR HIV ASSOCIATED METABOLIC DISEASE Roy Kim
You are the parent or guardian granting permission for a child in this study.
 Please check one of the following: You are an adult participant in this study. You are the parent or guardian granting permission for a child in this study. Print child s name here: The following information
Please check one of the following: You are an adult participant in this study. You are the parent or guardian granting permission for a child in this study. Print child s name here: The following information
PROVIDER AGREEMENT PROVIDER PROFILE OFFICE PROFILE
 SOUTHWEST PREFERRED DENTAL ORGANIZATION PROVIDER AGREEMENT PROVIDER PROFILE OFFICE PROFILE Southwest Preferred Dental Organization, Inc. 777 East Missouri Avenue, Suite 121 Phoenix, AZ 85014 This Provider
SOUTHWEST PREFERRED DENTAL ORGANIZATION PROVIDER AGREEMENT PROVIDER PROFILE OFFICE PROFILE Southwest Preferred Dental Organization, Inc. 777 East Missouri Avenue, Suite 121 Phoenix, AZ 85014 This Provider
When Your Pet s Diagnosis is Cancer... there is hope. Advanced Medicine Compassionate Care Expert Advice Well-being
 Our practice is a partner with The Veterinary Cancer Center (The VCC) in Norwalk, CT. The VCC is a specialized veterinary practice dedicated to the diagnosis and treatment of cancer in animals. Our partnership
Our practice is a partner with The Veterinary Cancer Center (The VCC) in Norwalk, CT. The VCC is a specialized veterinary practice dedicated to the diagnosis and treatment of cancer in animals. Our partnership
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: BEP (5 day) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: BEP (5 day) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
LUNG CANCER CLINICAL TRIALS
 UNDERSTANDING SERIES LUNG CANCER CLINICAL TRIALS 1-800-298-2436 LungCancerAlliance.org A GUIDE FOR THE PATIENT THE BASICS ABOUT CLINICAL TRIALS WITH MORE INFORMATION YOU CAN MAKE BETTER CHOICES. The decision
UNDERSTANDING SERIES LUNG CANCER CLINICAL TRIALS 1-800-298-2436 LungCancerAlliance.org A GUIDE FOR THE PATIENT THE BASICS ABOUT CLINICAL TRIALS WITH MORE INFORMATION YOU CAN MAKE BETTER CHOICES. The decision
About this consent form. Why is this research study being done? Partners HealthCare System Research Consent Form
 Protocol Title: Gene Sequence Variants in Fibroid Biology Principal Investigator: Cynthia C. Morton, Ph.D. Site Principal Investigator: Cynthia C. Morton, Ph.D. Description of About this consent form Please
Protocol Title: Gene Sequence Variants in Fibroid Biology Principal Investigator: Cynthia C. Morton, Ph.D. Site Principal Investigator: Cynthia C. Morton, Ph.D. Description of About this consent form Please
INFORMED CONSENT REQUIREMENTS AND EXAMPLES
 Office of Research Compliance INFORMED CONSENT REQUIREMENTS AND EXAMPLES No investigator may involve a human being as a subject in research unless the investigator has obtained the legally effective informed
Office of Research Compliance INFORMED CONSENT REQUIREMENTS AND EXAMPLES No investigator may involve a human being as a subject in research unless the investigator has obtained the legally effective informed
What You Need to Know About ARZERRA (ofatumumab)
 Starting your treatment for chronic lymphocytic leukemia (CLL) What You Need to Know About ARZERRA (ofatumumab) Approved Use ARZERRA is a prescription medication used: With a chemotherapy drug called chlorambucil
Starting your treatment for chronic lymphocytic leukemia (CLL) What You Need to Know About ARZERRA (ofatumumab) Approved Use ARZERRA is a prescription medication used: With a chemotherapy drug called chlorambucil
BRAJACTT. Protocol Code. Breast. Tumour Group. Dr. Karen Gelmon. Contact Physician
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine
 BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Docetaxel-EC PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Docetaxel-EC PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v )
 UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v. 11.2012) Project Title: The Impact of Achilles Tightness on Lower Etremity Injuries in Adolescent Athletes Principal Investigator:
UNIVERSITY HOSPITALS CASE MEDICAL CENTER CONSENT FOR INVESTIGATIONAL STUDIES (v. 11.2012) Project Title: The Impact of Achilles Tightness on Lower Etremity Injuries in Adolescent Athletes Principal Investigator:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy
 A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts
A5272 Version 2.0, 5/19/11: Oral HPV Shedding and Oral Warts After Initiation of Antiretroviral Therapy CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts
Personal Training Intake Form
 Personal Training Intake Form Name: Date: Cell Phone: Office Phone: E-Mail: USC Affiliation: STUDENT ALUMNI FACULTY/STAFF FACULTY/STAFF SPOUSE Sex: Male Female Age: Trainer preference (if any): How many
Personal Training Intake Form Name: Date: Cell Phone: Office Phone: E-Mail: USC Affiliation: STUDENT ALUMNI FACULTY/STAFF FACULTY/STAFF SPOUSE Sex: Male Female Age: Trainer preference (if any): How many
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
IRB Review Points to Consider September 2016
 POINTS TO CONSIDER Principal investigators 1. Does the principal investigator have the appropriate qualifications, experience, and facilities to ensure that all aspects of the project and follow-up will
POINTS TO CONSIDER Principal investigators 1. Does the principal investigator have the appropriate qualifications, experience, and facilities to ensure that all aspects of the project and follow-up will
JMC House Advisor Application Information Packet
 JMC House Advisor 2018 2019 Application Information Packet TO: All House Advisor (HA) Candidates FROM: Dr. Pat Danylyshyn-Adams, Director of Residence Life Dr. Sonia Rosado and LeRoy Ford, Assistant Directors
JMC House Advisor 2018 2019 Application Information Packet TO: All House Advisor (HA) Candidates FROM: Dr. Pat Danylyshyn-Adams, Director of Residence Life Dr. Sonia Rosado and LeRoy Ford, Assistant Directors
Sorafenib (Nexavar ) ( sor-af-e-nib )
 Sorafenib (Nexavar ) ( sor-af-e-nib ) How the drug is given: by mouth Purpose: To stop the growth of cancer cells in kidney cancer, liver cancer, and other cancers How to take the drug by mouth Take on
Sorafenib (Nexavar ) ( sor-af-e-nib ) How the drug is given: by mouth Purpose: To stop the growth of cancer cells in kidney cancer, liver cancer, and other cancers How to take the drug by mouth Take on
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Doctor office visit checklist
 Doctor office visit checklist Before the visit Gather your questions Identify symptoms Check your loved one s file Call to confirm appointment Take a list of any medicines the patient is currently taking
Doctor office visit checklist Before the visit Gather your questions Identify symptoms Check your loved one s file Call to confirm appointment Take a list of any medicines the patient is currently taking
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY
 UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Epileptic Encephalopathies: Clinical and Genetic Predictors of Outcomes and Therapeutic Insights This is a research study.
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO CONSENT TO PARTICIPATE IN A RESEARCH STUDY Epileptic Encephalopathies: Clinical and Genetic Predictors of Outcomes and Therapeutic Insights This is a research study.
Information for patients
 Information for patients Selective Internal Radiation Therapy (SIRT) with Yttrium-90 Micro-spheres Leading Interventional Oncology Network Contents Introduction 3 What to tell the doctor 3 What is Selective
Information for patients Selective Internal Radiation Therapy (SIRT) with Yttrium-90 Micro-spheres Leading Interventional Oncology Network Contents Introduction 3 What to tell the doctor 3 What is Selective
