In Vivo Colorectal Polyp Analysis Archived Medical Policy
|
|
|
- Wilfred McDowell
- 6 years ago
- Views:
Transcription
1 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided in the applicable contract. Medical technology is constantly evolving, and we reserve the right to review and update Medical Policy periodically. Services Are Considered Investigational Coverage is not available for investigational medical treatments or procedures, drugs, devices or biological products. Based on review of available data, the Company considers in vivo analysis of colorectal polyps to be investigational.* Background/Overview During a colonoscopy or sigmoidoscopy as a screening test for colorectal cancer, the physician must often decide which polyp should be removed for histologic diagnosis. While hyperplastic polyps are considered benign without malignant potential, adenomatous polyps are thought to represent one of the earliest stages in the progression to a malignancy. Identification of these premalignant lesions is considered one of the cornerstones of colorectal cancer prevention. The physician must thus balance the time and potential morbidity of removing all polyps, many of which will be benign, versus removal of those polyps most likely to be adenomatous. Techniques have been developed as adjuncts to colonoscopy that are intended to distinguish between normal and precancerous tissue. FDA or Other Governmental Regulatory Approval U.S. Food and Drug Administration (FDA) The first system developed was based on the observation that benign and malignant tissues emit different patterns and wavelengths of fluorescence after exposure to a laser light. One such device was approved by the U.S. FDA in 2000, the Optical Biopsy System (SpectraScience, Minneapolis MN). This system consists of an optical fiber emitting a laser that is directed against three different regions of the same polyp. The subsequent fluorescent signal is collected, measured, and analyzed by a proprietary system software, and classifies a polyp as "suspicious" (i.e., adenomatous) or "not suspicious" (i.e., hyperplastic). Narrow band imaging (NBI) is another technique that allows visualization of the mucosal surface and capillary vessels and thus may assist in the differentiation of abnormal from normal mucosa during colonoscopy. Two NBI systems are available. The NBI color chip system is used in the United States; in this system a single filter with a 2-band pass characteristic is used to generate central wavelengths at 415 nm (blue) and 540 nm (green and red). The NBI red-green-blue sequential illumination system uses narrow spectra of red, green, and blue light and a video endoscopic system with a frame sequential lighting method. The light source unit consists of a xenon lamp and a rotation disk with 3 optical filters. The rotation Page 1 of 8
2 disk and monochrome charge-coupled device are synchronized and sequentially generate image in 3 optical filter bands. By use of all 3 band images, a single color endoscopic image is synthesized by the video processor. NBI has limited penetration into the mucosal surface and has enhanced visualization of capillary vessels and their fine structure on the surface layer of colonic tissue. The FDA-labeled indication for the Optical Biopsy System reads as follows: "The SpectraScience Optical Biopsy System is indicated for use as an adjunct to lower gastrointestinal endoscopy. The device is intended for the evaluation of polyps less than 1 cm in diameter that the physician has not already elected to remove. The device is only to be used in deciding whether such polyps should be removed (which includes submission for histological examination)." Narrow band imaging received FDA clearance through the 510(k) process in This clearance (K051645) added NBI with the EVIS EXERA 160A System (Olympus Medical Systems Corp) to existing endoscopic equipment. FDA indications are for endoscopic diagnosis, treatment, and video observation. Centers for Medicare and Medicaid Services (CMS) No national coverage determination. Rationale/Source This policy was first developed in February 2002 and focused on the Optical Biopsy System and preliminary data on NBI. Since that time, literature searches have failed to find any new data on the Optical Biopsy System, while narrow band imaging has several randomized, controlled trials. This policy contains sections describing the evidence related to technology developed to improve the diagnosis and differentiation of colorectal polyps. Optical Biopsy System The U.S. FDA approval for the Optical Biopsy System was based on a prospective, nonrandomized Phase II study involving 101 subjects from 5 sites. The data from this trial have not been published in a peerreviewed journal but are available as an FDA summary of safety and effectiveness. Patients who participated in the study had undergone a prior lower gastrointestinal endoscopic procedure with at least 1 polyp identified, and were referred for an additional colonoscopy exam, in which fiberoptic analysis of the polyps was performed. At the time of the colonoscopy, the physicians documented whether or not the polyp was considered hyperplastic or adenomatous, and whether or not they would remove the polyp. The fiberoptic probe was then applied to three different portions of the polyp and a segment of normal adjacent mucosa. The physician did not know the results of the analysis and thus the test did not affect patient treatment. The effectiveness of the analysis was then calculated as its ability to correctly identify adenomatous polyps (i.e., sensitivity) and to correctly identify hyperplastic polyps (i.e., the specificity), either alone or in conjunction with physician assessment. The sensitivity and specificity of the physician assessment alone was 82.7% and 50%, respectively, compared to a combined sensitivity and specificity of Page 2 of 8
3 96.3% and 33%, respectively. In other words, fiberoptic analysis identified additional adenomatous polyps that the physician had classified as hyperplastic and presumably would not have removed based on visual assessment alone. This increase in sensitivity comes at the price of a decrease in specificity, as more hyperplastic polyps will undergo biopsy. However, according to the FDA, the risk of taking biopsies of additional hyperplastic polyps is minimal. The clinical significance of these results and their effect on patient management is difficult to interpret from the data presented. It is not clear how the physician decided to select additional polyps for fiberoptic analysis (it is not entirely clear whether all polyps were analyzed and then underwent biopsy), or whether the same results could be obtained by simply randomly taking a biopsy of a subset of polyps that were considered hyperplastic on visual assessment. While adenomatous polyps are considered premalignant lesions, the evolution to cancer is a slow process requiring 7 to 8 years, and thus the immediate removal of all adenomatous polyps is not required. In addition, the finding of an adenomatous polyp serves as a marker that the patient should undergo more frequent endoscopic exams. It is well known that the current practice of visual inspection of polyps will certainly miss some adenomatous polyps, but this lack of sensitivity is considered acceptable if at least 1 adenomatous polyp is identified and the patient undergoes more frequent screening. Few data have been published on the SpectraScience Optical Biopsy System since A feasibility study of fiberoptic analysis of normal, adenomatous, and cancerous tissue in 11 patients was published in No additional literature on the Optical Biopsy System was found, but a report in 2006 detailed the results of spectral scattering to different colonic lesions in a small series of 45 patients. Narrow Band Imaging (NBI) Several studies from both outside and inside the United States have evaluated the NBI system. These studies are a mixture of those evaluating its overall detection rates for colonic polyps and those specifically examining its ability to differentiate between neoplastic and non-neoplastic lesions. Data from four randomized trials of NBI versus white-light colonoscopy failed to show any advantage in total detection rate for NBI. Published randomized trials differed from the conventional approach to the assessment of diagnostic tests. In these trials patients were randomized to one test or the other (i.e., they received only one test). In general, when comparing diagnostic tests we would have each patient receive both tests and compare the test results. Adler and colleagues published two trials. The first trial enrolled 401 participants where the majority of the patients (89%) were enrolled for a diagnostic colonoscopy and evaluated by expert endoscopists (> 500 per provider). The second trial enrolled 1,256 participants evaluated with a screening colonoscopy in a private practice setting by six endoscopists with substantial lifetime experience (> 10,000 total colonoscopies). Both trials randomized participants to receive NBI or white-light colonoscopy; neither trial showed a benefit of NBI over white-light for overall polyp detection rate. In a similar study, with the same conclusion, Rex and Page 3 of 8
4 colleagues enrolled 434 participants, in a population split between 60% screening colonoscopy and 40% returning for surveillance. Each participant was randomized to either NBI or white-light colonoscopy. No benefit of NBI for the detection of adenomas was observed over white-light colonoscopy. Kaltenback and colleagues randomized 434 participants to receive both NBI and a white-light colonoscopy, or 2 white-light colonoscopies. Participants were screened by experienced endoscopists. With the first test, all visible polyps were removed, then the second test was performed to pick up any additional missed polyps; from this difference, the polyp miss rate was calculated. The major limitation with this method is that removing polyps with the first test eliminates the opportunity for the second test to miss any polyps that were already removed. NBI did not improve what was termed the neoplasm miss rate compared with white light. Inoue and colleagues, in a randomized, controlled trial of 243 patients in Japan, presented data showing that NBI did improve overall adenoma detection rates over conventional colonoscopy, as well as improving the number of small (< 5 mm) adenomas detected, while the number of patients with at least one adenoma remained the same. Participants in this trial had a previous positive colonoscopy or positive fecal occult blood test; approximately 80% were undergoing polyp surveillance. All testing was performed at an endoscopy center by 6 experienced endoscopists. Differences in results may be attributed to different study populations and/or differences in the version of NBI system used. One randomized trial addressed both total detection rate and differentiation of neoplastic from nonneoplastic lesions. Pohl and colleagues conducted a randomized multicenter trial of virtual chromoendoscopy with the Fujinon intelligent colour enhancement system (FICE or NBI) versus standard colonoscopy with targeted indigocarmine chromoscopy. This German trial included 764 patients in the final analysis and reported that FICE/NBI was not superior to control for overall adenoma detection rates; it was comparable on the differentiation of neoplastic and non-neoplastic lesions. The sensitivity of FICE/NBI was 92.7% versus 90.4% for the control. Additional data on NBI for the differentiation of neoplastic from nonneoplastic lesions comes from nonrandomized studies of various sizes where the conclusion often is that NBI may be more accurate than conventional colonoscopy for the differentiation of lesions. For example, Hirata evaluated 148 colorectal lesions and concluded that determination of pit patterns of colorectal neoplasia by NBI magnification was nearly the same as that by standard magnification with chromoendoscopy and that NBI can distinguish neoplastic and non-neoplastic lesions without chromoendoscopy. Rastogi and colleagues, after evaluating 100 patients with 236 detected polyps, concluded that NBI without magnification was significantly superior to high-definition white-light colonoscopy for the real-time prediction of adenomas. van den Broek reported sensitivity, specificity, and overall accuracy of NBI for differentiation of 90%, 70%, and 79%, respectively, and while the specificity and overall accuracy were superior to high-resolution endoscopy, endoscopic trimodal imaging and autofluorescence imaging, the test characteristics were disappointing for the diagnostic accuracy for polyp differentiation. These studies only reported on the accuracy of the NBI system in the in vivo evaluation of colonic polyps. None of the studies evaluated the impact of this technology on outcomes including whether or not there would be an improvement in the selection of polyps for removal during colonoscopy. Page 4 of 8
5 In an editorial, Soetikno mentions the need for a user-friendly classification system for use of these devices. The editorial also comments on the need for high-definition recording devices to allow further research. As noted, without these devices, the details of lesions cannot be seen beyond the fleeting moment during the procedure and patterns cannot be fully correlated with pathology. Current technology allows for images to be saved and reviewed at a later time, but development of a standardized system for the classification of the different patterns seen on NBI is needed. While other technologies are under investigation, including chromocolonoscopy, Third Eye Retroscope, and autofluorescence, there is no evidence that current studies of these technologies overcome the issues referenced. Randomized trial data, in which participants receive both screening tests, and histologic confirmation of disease is matched to screening test results for each polyp are required to evaluate this technology. Since the impact of this technology on health outcomes is not known, it is considered investigational. Technology Assessments, Guidelines and Position Statements Neither the U.S. Preventive Services Task Force nor the National Comprehensive Cancer Network (NCCN) mentions NBI in their current policy statements or screening guidelines. The American Gastroenterological Association in 2008 published a technology assessment of image-enhanced endoscopy, which mentions optical and electronic devices potentially playing a role in colon screening in the future, but currently, more data are needed. References 1. Blue Cross and Blue Shield Association, Medical Policy Reference Manual, Fiberoptic Analysis of Colorectal Polyps, , 11: Optical Biopsy System: Summary of Safety and Effectiveness Mayinger B, Jordan M, Horner P et al. Endoscopic light-induced autofluorescence spectroscopy for the diagnosis of colorectal cancer and adenoma. J Photochem Photobiol B. 2003; 70(1): Dhar A, Johnson KS, Novelli MR et al. Elastic scattering spectroscopy for the diagnosis of colonic lesions: initial results of a novel optical biopsy technique. Gastrointest Endosc 2006; 63(2): Inoue T, Murano M, Murano N et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol 2008; 43(1): Matsumoto T, Esaki M, Fujisawa R et al. Chromoendoscopy, narrow-band imaging colonoscopy and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis. Dis Colon Rectum 2009; 52(6): Huneburg R, Lammert F, Rabe C et al. Chromocolonoscopy detects more adenomas than white light colonoscopy or narrow band imaging colonoscopy in hereditary nonpolyposis colorectal cancer screening. Endoscopy 2009; 41(4): Adler A, Pohl H, Papanikolaou IS. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut 2008; 57(1): Adler A, Aschenbeck J 9. Yenerim T et al. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: A prospective randomized trial. Gastroenterology 2009; 136(2): Togashi K, Osawa H, Koinuma K et al. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc 2009; 69(3): Page 5 of 8
6 11. Pohl J, Lotterer E, Balzer C et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut 2009; 58(1): van den Broek FJ, Fockens P, Van Eeden S et al. Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps. Clin Gastroenterol Hepatol 2009; 7(3): Hirata M, Tanaka S, Oka S et al. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc 2007; 65(7): Tischendorf JJ, Wasmuth HE, Koch A et al. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy 2007; 39(12): Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut 2008; 57(10): Rex DK. Narrow band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology 2009; 136(4): Rastogi A, Keighley J, Singh V et al. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol 2009; 104(10): Rex DK, Helbig CC. High yields of small flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology 2007; 133(1): Rogart JN, Jain D, Siddiqui UD. Narrow band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc 2008; 68(6): Sikka S, Ringold DA, Jonnalagadda S et al. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopes without optical magnification. Endoscopy 2008; 40(10): Soetikno R, Kaltenbach T. The beginning of a new paradigm in colonoscopy? Gastrointest Endosc 2007; 65(7): Lee MM, Enns R. Narrow band imaging for the detection of neoplastic lesions of the colon. Can J Gastroenterology 2009; 23(1): Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy 2008; 40(6): Matsuda T, Saito Y, Fu KI et al. Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate?--a pilot study. Am J Gastroenterol 2008; 103(8): Kaltenbach T, Sano Y, Friedland S et al. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology 2008; 134(1): Coding The five character codes included in the Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines (BCBSLAMPCG) are obtained from Current Procedural Terminology (CPT ), copyright 2011 by the American Medical Association (AMA). CPT is developed by the AMA as a listing of descriptive terms and five character identifying codes and modifiers for reporting medical services and procedures performed by physician. The responsibility for the content of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines is with Blue Cross and Blue Shield of Louisiana and no endorsement by the AMA is intended or should be implied. The AMA disclaims responsibility for any consequences or liability attributable or related to any use, nonuse or interpretation of information contained in Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. Any use of CPT outside of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines should refer to the most current Current Page 6 of 8
7 Procedural Terminology which contains the complete and most current listing of CPT codes and descriptive terms. Applicable FARS/DFARS apply. CPT is a registered trademark of the American Medical Association. Codes used to identify services associated with this policy may include (but may not be limited to) the following: Code Type Code CPT HCPCS ICD-9 Diagnosis ICD-9 Procedure No code No code No code Policy History 05/19/2002 Medical Director review 06/20/2002 Medical Policy Committee review 06/24/2002 Managed Care Advisory Council approval 07/06/2004 Medical Director review 07/20/2004 Medical Policy Committee review. Format revision. No substance change to policy. 07/26/2004 Managed Care Advisory Council approval 07/07/2006 Format revision, including addition of FDA and or other governmental regulatory approval and rationale/source. Coverage eligibility unchanged. 09/06/2006 Medical Director review 09/20/2006 Medical Policy Committee approval. No changes to policy guidelines. 09/09/2008 Medical Director review 09/17/2008 Medical Policy Committee approval. Title changed to Colorectal Polyp Analysis In Vivo. 09/03/2009 Medical Policy Committee approval. 09/16/2009 Medical Policy Implementation Committee approval. Title change reflects the title of the BCBSA medical policy. 09/09/2010 Medical Policy Committee review 09/15/2010 Medical Policy Implementation Committee approval. Coverage eligibility unchanged. 09/01/2011 Medical Policy Committee review 09/14/2011 Medical Policy Implementation Committee approval. Coverage eligibility unchanged. 09/06/2012 Medical Policy Committee review. Recommend archiving policy. 09/19/2012 Medical Policy Implementation Committee approval. Archived. Next Scheduled Review Date: Archived medical policy Page 7 of 8
8 *Investigational A medical treatment, procedure, drug, device, or biological product is Investigational if the effectiveness has not been clearly tested and it has not been incorporated into standard medical practice. Any determination we make that a medical treatment, procedure, drug, device, or biological product is Investigational will be based on a consideration of the following: A. whether the medical treatment, procedure, drug, device, or biological product can be lawfully marketed without approval of the U.S. Food and Drug Administration (FDA) and whether such approval has been granted at the time the medical treatment, procedure, drug, device, or biological product is sought to be furnished; or B. whether the medical treatment, procedure, drug, device, or biological product requires further studies or clinical trials to determine its maximum tolerated dose, toxicity, safety, effectiveness, or effectiveness as compared with the standard means of treatment or diagnosis, must improve health outcomes, according to the consensus of opinion among experts as shown by reliable evidence, including: 1. Consultation with the Blue Cross and Blue Shield Association technology assessment program (TEC) or other nonaffiliated technology evaluation center(s); 2. credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community; or 3. reference to federal regulations. Indicated trademarks are the registered trademarks of their respective owners. NOTICE: Medical Policies are scientific based opinions, provided solely for coverage and informational purposes. Medical Policies should not be construed to suggest that the Company recommends, advocates, requires, encourages, or discourages any particular treatment, procedure, or service, or any particular course of treatment, procedure, or service. Page 8 of 8
Topic: In Vivo Analysis of Colorectal Polyps Date of Origin: June Section: Medicine Last Reviewed Date: October 2013
 Medical Policy Manual Topic: In Vivo Analysis of Colorectal Polyps Date of Origin: June 2002 Section: Medicine Last Reviewed Date: October 2013 Policy No: 104 Effective Date: January 1, 2014 IMPORTANT
Medical Policy Manual Topic: In Vivo Analysis of Colorectal Polyps Date of Origin: June 2002 Section: Medicine Last Reviewed Date: October 2013 Policy No: 104 Effective Date: January 1, 2014 IMPORTANT
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
In Vivo Analysis of Colorectal Polyps
 Medical Policy Manual Medicine, Policy No. 104 In Vivo Analysis of Colorectal Polyps Next Review: October 2018 Last Review: October 2017 Effective: December 1, 2017 IMPORTANT REMINDER Medical Policies
Medical Policy Manual Medicine, Policy No. 104 In Vivo Analysis of Colorectal Polyps Next Review: October 2018 Last Review: October 2017 Effective: December 1, 2017 IMPORTANT REMINDER Medical Policies
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
vedolizumab (Entyvio )
 vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
Percutaneous Discectomy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Cryosurgical Ablation of Breast Fibroadenomas
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Archived Medical Policy
 Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Monitoring of Regional Cerebral Blood Flow Using an Implanted Cerebral Thermal Perfusion Probe Archived Medical Policy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Vertebral Axial Decompression
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Virtual Colonoscopy/CT Colonography
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
and will be denied as not medically necessary** if not met. This criterion only applies to the initial
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Urinary Metabolite Tests for Adherence to Direct-Acting Antiviral Medications for Hepatitis C Archived Medical Policy
 Urinary Metabolite Tests for Adherence to Direct-Acting Antiviral Medications for Hepatitis C Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Urinary Metabolite Tests for Adherence to Direct-Acting Antiviral Medications for Hepatitis C Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Implantable Hormone Pellets
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2018 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
burosumab (Crysvita )
 burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
sebelipase alfa (Kanuma )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer
 Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Corporate Medical Policy
 Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
denosumab (Prolia ) Policy # Original Effective Date: 07/21/2011 Current Effective Date: 04/19/2017
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Based on review of available data, the Company may consider the use of denosumab (Prolia) for the
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema
 End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
ustekinumab (Stelara )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Peripheral Subcutaneous Field Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Ustekinumab (Stelara )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Nutrient/Nutritional Panel Testing
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Note: Repository Corticotropin Injection (ACTH Gel, H.P. Acthar Gel) is addressed in medical policy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Zurampic, Duzallo (lesinurad Products)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/CT Colonography Chromoendoscopy as an Adjunct to Colonoscopy
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/CT Colonography Chromoendoscopy as an Adjunct to Colonoscopy
Laboratory Tests for Heart Transplant Rejection
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia
 Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia Ralf Kiesslich I. Medical Department Johannes Gutenberg University Mainz, Germany Cumulative cancer risk in ulcerative colitis 0.5-1.0%
Chromoendoscopy and Endomicroscopy for detecting colonic dysplasia Ralf Kiesslich I. Medical Department Johannes Gutenberg University Mainz, Germany Cumulative cancer risk in ulcerative colitis 0.5-1.0%
The Usefulness Of Narrow Band Imaging Endoscopy For The Real Time Characterization Of Colonic Lesions
 Acta Medica Marisiensis 2016;62(2):182-186 DOI: 10.1515/amma-2016-0004 RESEARCH ARTICLE The Usefulness Of Narrow Band Imaging Endoscopy For The Real Time Characterization Of Colonic Lesions Boeriu Alina
Acta Medica Marisiensis 2016;62(2):182-186 DOI: 10.1515/amma-2016-0004 RESEARCH ARTICLE The Usefulness Of Narrow Band Imaging Endoscopy For The Real Time Characterization Of Colonic Lesions Boeriu Alina
Implantable Hormone Pellets
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Advances in Endoscopic Imaging
 Advances in Endoscopic Imaging SGNA meeting February 20, 2010 Amar R. Deshpande, MD Asst Professor of Medicine Division of Gastroenterology University of Miami Miller School of Medicine Objectives To recognize
Advances in Endoscopic Imaging SGNA meeting February 20, 2010 Amar R. Deshpande, MD Asst Professor of Medicine Division of Gastroenterology University of Miami Miller School of Medicine Objectives To recognize
certolizumab pegol (Cimzia )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Gastric Electrical Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines
 Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
BENEFIT APPLICATION BLUE CARD/NATIONAL ACCOUNT ISSUES
 Medical Policy BCBSA Ref. Policy: 2.01.84 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/Computed
Medical Policy BCBSA Ref. Policy: 2.01.84 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.87 Confocal Laser Endomicroscopy 6.01.32 Virtual Colonoscopy/Computed
Chromoendoscopy as an Adjunct to Colonoscopy
 Chromoendoscopy as an Adjunct to Colonoscopy Policy Number: 2.01.84 Last Review: 1/2018 Origination: 7/2017 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Chromoendoscopy as an Adjunct to Colonoscopy Policy Number: 2.01.84 Last Review: 1/2018 Origination: 7/2017 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Vertebroplasty/Kyphoplasty
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Colon Cancer Screening with Image-Enhanced Endoscopy
 FOCUSED REVIEW SERIES: Endoscopic Screening and Surveillance for Gastrointestinal Cancer Clin Endosc 2014;47:504-508 Print ISSN 2234-2400 / On-line ISSN 2234-2443 http://dx.doi.org/10.5946/ce.2014.47.6.504
FOCUSED REVIEW SERIES: Endoscopic Screening and Surveillance for Gastrointestinal Cancer Clin Endosc 2014;47:504-508 Print ISSN 2234-2400 / On-line ISSN 2234-2443 http://dx.doi.org/10.5946/ce.2014.47.6.504
Medical Policy Coverage Guidelines
 Medical Policy Coverage Guidelines Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc. (collectively referred to as "BCBSLA") have developed these medical policies to serve
Medical Policy Coverage Guidelines Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc. (collectively referred to as "BCBSLA") have developed these medical policies to serve
Sensipar (cinacalcet)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence
 Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions
 Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions
 Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Skin Contact Monochromatic Infrared Energy as a Technique to Treat Cutaneous Ulcers, Diabetic Neuropathy, and Miscellaneous Musculoskeletal Conditions Applies to all products administered or underwritten
Description. Section: Medicine Effective Date: July 15, Subsection: Original Policy Date: September 13, 2012 Subject: Page: 1 of 17
 Page: 1 of 17 Last Review Status/Date: June 2016 Description Chromoendoscopy refers to the application of dyes or stains during endoscopy to enhance tissue differentiation or characterization. When used
Page: 1 of 17 Last Review Status/Date: June 2016 Description Chromoendoscopy refers to the application of dyes or stains during endoscopy to enhance tissue differentiation or characterization. When used
dichlorphenamide (Keveyis )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Epithelial Cell Cytology in Breast Cancer Risk Assessment and High-Risk Patient Management: Ductal Lavage and Fiberoptic Ductoscopy
 Epithelial Cell Cytology in Breast Cancer Risk Assessment and High-Risk Patient Management: Ductal Lavage and Fiberoptic Ductoscopy Applies to all products administered or underwritten by Blue Cross and
Epithelial Cell Cytology in Breast Cancer Risk Assessment and High-Risk Patient Management: Ductal Lavage and Fiberoptic Ductoscopy Applies to all products administered or underwritten by Blue Cross and
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
비확대협대역대장내시경을이용한대장용종의감별진단
 Korean J Gastroenterol Vol. 63 No. 5, 276-282 http://dx.doi.org/10.4166/kjg.2014.63.5.276 pissn 1598-9992 eissn 2233-6869 ORIGINAL ARTICLE 비확대협대역대장내시경을이용한대장용종의감별진단 김봉진, 박무인, 박선자, 문 고신대학교의과대학내과학교실 원, 박은택,
Korean J Gastroenterol Vol. 63 No. 5, 276-282 http://dx.doi.org/10.4166/kjg.2014.63.5.276 pissn 1598-9992 eissn 2233-6869 ORIGINAL ARTICLE 비확대협대역대장내시경을이용한대장용종의감별진단 김봉진, 박무인, 박선자, 문 고신대학교의과대학내과학교실 원, 박은택,
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
COLON: Innovations 3 steps, 3 parts..
 COLON: Innovations 3 steps, 3 parts.. Detection: I see an abnormality (usually a polyp) Characterization: Is this abnormality neoplastic? (for example: an adenoma) Treatment: it is neoplastic. Can I treat
COLON: Innovations 3 steps, 3 parts.. Detection: I see an abnormality (usually a polyp) Characterization: Is this abnormality neoplastic? (for example: an adenoma) Treatment: it is neoplastic. Can I treat
natalizumab (Tysabri )
 natalizumab (Tysabri ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
natalizumab (Tysabri ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
Accurate endoscopic determination of the histology of
 GASTROENTEROLOGY 2013;144:81 91 Real-Time Optical Biopsy of Colon Polyps With Narrow Band Imaging in Community Practice Does Not Yet Meet Key Thresholds for Clinical Decisions URI LADABAUM, 1,2 ANN FIORITTO,
GASTROENTEROLOGY 2013;144:81 91 Real-Time Optical Biopsy of Colon Polyps With Narrow Band Imaging in Community Practice Does Not Yet Meet Key Thresholds for Clinical Decisions URI LADABAUM, 1,2 ANN FIORITTO,
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
droxidopa (Northera )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Treatment of Hepatitis C with ombitasvir, paritaprevir, and ritonavir (Technivie )
 Treatment of Hepatitis C with ombitasvir, paritaprevir, and ritonavir (Technivie ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO
Treatment of Hepatitis C with ombitasvir, paritaprevir, and ritonavir (Technivie ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
How to characterize dysplastic lesions in IBD?
 How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
Cologuard Screening for Colorectal Cancer
 Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Baroreflex Stimulation Devices
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Accuracy for optical diagnosis of colorectal polyps in clinical practice
 1130-0108/2015/107/5/255-261 Revista Española de Enfermedades Digestivas Copyright 2015 Arán Ediciones, S. L. Rev Esp Enferm Dig (Madrid Vol. 107, N.º 5, pp. 255-261, 2015 ORIGINAL PAPERS Accuracy for
1130-0108/2015/107/5/255-261 Revista Española de Enfermedades Digestivas Copyright 2015 Arán Ediciones, S. L. Rev Esp Enferm Dig (Madrid Vol. 107, N.º 5, pp. 255-261, 2015 ORIGINAL PAPERS Accuracy for
C1 Esterase Inhibitor (Cinryze )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
INTRODUCTION. Key Words: Gastroesophageal reflux; Agreement; Experience. ORiginal Article
 Gut and Liver, Vol. 8, No. 2, March 2014, pp. 154-159 ORiginal Article Endoscopic Experience Improves Interobserver Agreement in the Grading of Esophagitis by Los Angeles Classification: Conventional Endoscopy
Gut and Liver, Vol. 8, No. 2, March 2014, pp. 154-159 ORiginal Article Endoscopic Experience Improves Interobserver Agreement in the Grading of Esophagitis by Los Angeles Classification: Conventional Endoscopy
sipuleucel-t (Provenge )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication
 UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
Narrow Band Imaging and Autofluorescence Imaging for the Detection and Optical Diagnosis of Colorectal Polyps
 Narrow Band Imaging and Autofluorescence Imaging for the Detection and Optical Diagnosis of Colorectal Polyps Kelvin Teck-Joo Thia, MBBS, MRCP, Chris San-Choon Kong, MBBS, MRCP, Choon-Jin Ooi, MRCP, FRCP
Narrow Band Imaging and Autofluorescence Imaging for the Detection and Optical Diagnosis of Colorectal Polyps Kelvin Teck-Joo Thia, MBBS, MRCP, Chris San-Choon Kong, MBBS, MRCP, Choon-Jin Ooi, MRCP, FRCP
Devices To Improve Colon Polyp Detection
 Devices To Improve Colon Polyp Detection ACG/VGS Regional Postgraduate Course Sep 10-11, 2016 Williamsburg, VA VIVEK KAUL, MD, FACG Segal-Watson Professor of Medicine Chief, Division of Gastroenterology
Devices To Improve Colon Polyp Detection ACG/VGS Regional Postgraduate Course Sep 10-11, 2016 Williamsburg, VA VIVEK KAUL, MD, FACG Segal-Watson Professor of Medicine Chief, Division of Gastroenterology
Imaging and Advanced Technology
 Imaging and Advanced Technology Narrow-Band Versus White-Light High Definition Television Endoscopic Imaging for Screening Colonoscopy: A Prospective Randomized Trial ANDREAS ADLER,* JENS ASCHENBECK, TIMUR
Imaging and Advanced Technology Narrow-Band Versus White-Light High Definition Television Endoscopic Imaging for Screening Colonoscopy: A Prospective Randomized Trial ANDREAS ADLER,* JENS ASCHENBECK, TIMUR
Systems Pathology in Prostate Cancer
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
Treatment of Hepatitis C with simeprevir (Olysio ) PLUS sofosbuvir (Sovaldi ) Archived Medical Policy
 Treatment of Hepatitis C with simeprevir (Olysio ) PLUS sofosbuvir (Sovaldi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Treatment of Hepatitis C with simeprevir (Olysio ) PLUS sofosbuvir (Sovaldi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
eltrombopag (Promacta )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Predict, Resect and discard : Yes we can! (at least in some hands)
 Diminutive polyps : Real time endoscopic histology Predict, Resect and discard : Yes we can! (at least in some hands) Robert Benamouzig Hôpital Avicenne AP-HP & Paris 13 University France Why it is important?
Diminutive polyps : Real time endoscopic histology Predict, Resect and discard : Yes we can! (at least in some hands) Robert Benamouzig Hôpital Avicenne AP-HP & Paris 13 University France Why it is important?
Ingestible ph and Pressure Capsule
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
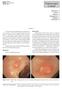 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
Vesicular Monoamine Transporter Type 2 Inhibitors: deutetrabenazine (Austedo ), tetrabenazine (Xenazine ), valbenazine (Ingrezza )
 Vesicular Monoamine Transporter Type 2 Inhibitors: deutetrabenazine (Austedo ), tetrabenazine (Xenazine ), valbenazine (Ingrezza ) Applies to all products administered or underwritten by Blue Cross and
Vesicular Monoamine Transporter Type 2 Inhibitors: deutetrabenazine (Austedo ), tetrabenazine (Xenazine ), valbenazine (Ingrezza ) Applies to all products administered or underwritten by Blue Cross and
Policy Specific Section: March 1, 2005 January 30, 2015
 Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Chromoendoscopy - Should It Be Standard of Care in IBD?
 Chromoendoscopy - Should It Be Standard of Care in IBD? John F. Valentine, MD, FACG Professor of Medicine Division of Gastroenterology, Hepatology and Nutrition University of Utah What is the point of
Chromoendoscopy - Should It Be Standard of Care in IBD? John F. Valentine, MD, FACG Professor of Medicine Division of Gastroenterology, Hepatology and Nutrition University of Utah What is the point of
Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis
 Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis Bret A. Lashner, M.D. Professor of Medicine Director,
Chromoendoscopy or Narrow Band Imaging with Targeted biopsies Should be the Cancer Surveillance Endoscopy Procedure of Choice in Ulcerative Colitis Bret A. Lashner, M.D. Professor of Medicine Director,
Proteomics-Based Testing for the Evaluation of Ovarian (Adnexal) Masses
 Proteomics-Based Testing for the Evaluation of Ovarian (Adnexal) Masses Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Proteomics-Based Testing for the Evaluation of Ovarian (Adnexal) Masses Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Colon Polyps: Detection, Inspection and Characteristics
 Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
Linked color imaging improves the visibility of colorectal polyps: a video study
 Original article Linked color imaging improves the visibility of colorectal polyps: a video study Authors Naohisa Yoshida 1,YujiNaito 1,TakaakiMurakami 1, Ryohei Hirose 1,KiyoshiOgiso 1,YutakaInada 1,
Original article Linked color imaging improves the visibility of colorectal polyps: a video study Authors Naohisa Yoshida 1,YujiNaito 1,TakaakiMurakami 1, Ryohei Hirose 1,KiyoshiOgiso 1,YutakaInada 1,
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Emerging Interventions in Endoscopy. Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital
 Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
hydroxyprogesterone caproate Injection (Makena )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study
 Original article 245 Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study Authors Institution Douglas K. Rex, Kyle Hardacker, Margaret MacPhail,
Original article 245 Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study Authors Institution Douglas K. Rex, Kyle Hardacker, Margaret MacPhail,
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2018
Quality ID #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2018
2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY. MEASURE TYPE: Process
 Measure. #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2017
Measure. #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2017
Philip Chiu Associate Professor Department of Surgery, Prince of Wales Hospital The Chinese University of Hong Kong
 Application of Chromoendoscopy, NBI and AFI in Esophagus why, who, and how? Philip Chiu Associate Professor Department of Surgery, Prince of Wales Hospital The Chinese University of Hong Kong Cancer of
Application of Chromoendoscopy, NBI and AFI in Esophagus why, who, and how? Philip Chiu Associate Professor Department of Surgery, Prince of Wales Hospital The Chinese University of Hong Kong Cancer of
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
cysteamine Delayed Release Capsules (Procysbi )
 cysteamine Delayed Release Capsules (Procysbi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred
cysteamine Delayed Release Capsules (Procysbi ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred
pegloticase (Krystexxa )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
