Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
|
|
|
- Emmeline Sharp
- 6 years ago
- Views:
Transcription
1 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or hematopoietic Primary malignant neoplasms, not lymphatic or hematopoietic Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site Leukemias and lymphomas Specific cancer type , Lung Cancer 174.xx, 175.0, Breast Cancer (warehouse) 185.xx Prostate Cancer 157.xx Pancreatic Cancer 153.xx, 154.0, Colon Cancer Supplementary Table 2. ICD-9-CM codes used to define active cancer Chemotherapy MS-DRGs MS-DRG MS-DRG Title 837 CHEMO W ACUTE LEUKEMIA AS SDX OR W HIGH DOSE CHEMO AGENT W MCC 838 CHEMO W ACUTE LEUKEMIA AS SDX W CC OR HIGH DOSE CHEMO AGENT 839 CHEMO W ACUTE LEUKEMIA AS SDX W/O CC/MCC 846 CHEMOTHERAPY W/O ACUTE LEUKEMIA AS SECONDARY DIAGNOSIS W MCC 847 CHEMOTHERAPY W/O ACUTE LEUKEMIA AS SECONDARY DIAGNOSIS W CC 848 CHEMOTHERAPY W/O ACUTE LEUKEMIA AS SECONDARY DIAGNOSIS W/O CC/MCC Radiation therapy codes CPT code range Description Therapeutic Radiology: Treatment Planning Radiation Therapy Simulation Radiation Physics Services Stereotactic Radiosurgery (SRS) Planning and Delivery Unlisted procedure, medical radiation physics, dosimetry and treatment devices, and special services Radiation Treatment IMRT Delivery Stereoscopic Imaging Guidance Neutron Therapy Radiation Therapy Management Proton Therapy Hyperthermia Treatment Brachytherapy
2 Chemotherapy J codes HCPCS Description Category J8510 Oral busulfan Cytotoxic J8520 Capecitabine, oral, 150 mg Cytotoxic J8521 Capecitabine, oral, 500 mg Cytotoxic J8530 Cyclophosphamide oral 25 MG Cytotoxic J8560 Etoposide oral 50 MG Cytotoxic J8561 Oral everolimus Cytotoxic J8562 Oral fludarabine phosphate Cytotoxic J8565 Gefitinib oral Cytotoxic J8600 Melphalan oral 2 MG Cytotoxic J8610 Methotrexate oral 2.5 MG Cytotoxic J8700 Temozolomide Cytotoxic J8705 Topotecan oral Cytotoxic J8999 Oral prescription drug chemo Cytotoxic J9000 Doxorubicin hcl injection Cytotoxic J9001 Doxorubicin hcl liposome inj Cytotoxic J9002 Doxil injection Cytotoxic J9010 Alemtuzumab injection Biologic J9015 Aldesleukin injection Biologic J9017 Arsenic trioxide injection Cytotoxic J9019 Erwinaze injection Biologic J9020 Asparaginase, NOS Biologic J9025 Azacitidine injection Cytotoxic J9027 Clofarabine injection Cytotoxic J9031 Bcg live intravesical vac Biologic J9033 Bendamustine injection Cytotoxic J9035 Bevacizumab injection Biologic J9040 Bleomycin sulfate injection Cytotoxic J9041 Bortezomib injection Cytotoxic J9042 Brentuximab vedotin inj Biologic J9043 Cabazitaxel injection Cytotoxic J9045 Carboplatin injection Cytotoxic J9047 Injection, carfilzomib, 1 mg Cytotoxic J9050 Carmustine injection Cytotoxic J9055 Cetuximab injection Biologic J9060 Cisplatin 10 MG injection Cytotoxic J9062 Cisplatin 50 MG injection Cytotoxic J9065 Inj cladribine per 1 MG Cytotoxic J9070 Cyclophosphamide 100 MG inj Cytotoxic J9080 Cyclophosphamide 200 MG inj Cytotoxic J9090 Cyclophosphamide 500 MG inj Cytotoxic J9091 Cyclophosphamide 1.0 grm inj Cytotoxic J9092 Cyclophosphamide 2.0 grm inj Cytotoxic J9093 Cyclophosphamide lyophilized Cytotoxic
3 J9094 Cyclophosphamide lyophilized Cytotoxic J9095 Cyclophosphamide lyophilized Cytotoxic J9096 Cyclophosphamide lyophilized Cytotoxic J9097 Cyclophosphamide lyophilized Cytotoxic J9098 Cytarabine liposome inj Cytotoxic J9100 Cytarabine hcl 100 MG inj Cytotoxic J9110 Cytarabine hcl 500 MG inj Cytotoxic J9120 Dactinomycin injection Cytotoxic J9130 Dacarbazine 100 mg inj Cytotoxic J9140 Dacarbazine 200 MG inj Cytotoxic J9150 Daunorubicin injection Cytotoxic J9151 Daunorubicin citrate inj Cytotoxic J9155 Degarelix injection Hormonal J9160 Denileukin diftitox inj Biologic J9165 Diethylstilbestrol injection Hormonal J9170 Docetaxel injection Cytotoxic J9171 Docetaxel injection Cytotoxic J9175 Elliotts b solution per ml Not J9178 Inj, epirubicin hcl, 2 mg Cytotoxic J9179 Eribulin mesylate injection Cytotoxic J9181 Etoposide injection Cytotoxic J9182 Etoposide injection Cytotoxic J9185 Fludarabine phosphate inj Cytotoxic J9190 Fluorouracil injection Cytotoxic J9200 Floxuridine injection Cytotoxic J9201 Gemcitabine hcl injection Cytotoxic J9202 Goserelin acetate implant Hormonal J9206 Irinotecan injection Cytotoxic J9207 Ixabepilone injection Cytotoxic J9208 Ifosfamide injection Cytotoxic J9209 Mesna injection Not J9211 Idarubicin hcl injection Cytotoxic J9212 Interferon alfacon-1 inj Biologic J9213 Interferon alfa-2a inj Biologic J9214 Interferon alfa-2b inj Biologic J9215 Interferon alfa-n3 inj Biologic J9216 Interferon gamma 1-b inj Biologic J9217 Leuprolide acetate suspension Hormonal J9218 Leuprolide acetate injection Hormonal J9219 Leuprolide acetate implant Hormonal J9225 Vantas implant Hormonal J9226 Supprelin LA implant Hormonal J9228 Ipilimumab injection Biologic J9230 Mechlorethamine hcl inj Cytotoxic J9245 Inj melphalan hydrochl 50 MG Cytotoxic J9250 Methotrexate sodium inj Cytotoxic
4 J9260 Methotrexate sodium inj Cytotoxic J9261 Nelarabine injection Cytotoxic J9262 Inj, omacetaxine mep, 0.01mg Cytotoxic J9263 Oxaliplatin Cytotoxic J9264 Paclitaxel protein bound Cytotoxic J9265 Paclitaxel injection Cytotoxic J9266 Pegaspargase injection Biologic J9268 Pentostatin injection Cytotoxic J9270 Plicamycin (mithramycin) inj Cytotoxic J9280 Mitomycin injection Cytotoxic J9290 Mitomycin 20 MG inj Cytotoxic J9291 Mitomycin 40 MG inj Cytotoxic J9293 Mitoxantrone hydrochl / 5 MG Cytotoxic J9300 Gemtuzumab ozogamicin inj Biologic J9302 Ofatumumab injection Biologic J9303 Panitumumab injection Biologic J9305 Pemetrexed injection Cytotoxic J9306 Injection, pertuzumab, 1 mg Biologic J9307 Pralatrexate injection Cytotoxic J9310 Rituximab injection Biologic J9315 Romidepsin injection Cytotoxic J9320 Streptozocin injection Cytotoxic J9328 Temozolomide injection Cytotoxic J9330 Temsirolimus injection Cytotoxic J9340 Thiotepa injection Cytotoxic J9350 Topotecan injection Cytotoxic J9351 Topotecan injection Cytotoxic J9354 Inj, ado-trastuzumab emt 1mg Biologic J9355 Trastuzumab injection Biologic J9357 Valrubicin injection Cytotoxic J9360 Vinblastine sulfate inj Cytotoxic J9370 Vincristine sulfate 1 MG inj Cytotoxic J9371 Inj, vincristine sul lip 1mg Cytotoxic J9375 Vincristine sulfate 2 MG inj Cytotoxic J9380 Vincristine sulfate 5 MG inj Cytotoxic J9390 Vinorelbine tartrate inj Cytotoxic J9395 Injection, Fulvestrant Hormonal J9400 Inj, ziv-aflibercept, 1mg Biologic J9600 Porfimer sodium injection Other J9999 Chemotherapy drug Cytotoxic Q2043 Provenge, 50 million autologous CD54+ Biologic cells Q2049 Lipodox 10 mg Cytotoxic Q2050 Doxil 10mg Cytotoxic Q0138 Ferumoxytol Not
5 Outpatient cancer surgeries Procedure code Code description Exc tr-ext mal+marg 0.5 cm/< Exc tr-ext mal+marg cm Exc tr-ext mal+marg cm Exc tr-ext mal+marg cm Exc tr-ext mal+marg cm Exc tr-ext mal+marg >4 cm Exc h-f-nk-sp mal+marg 0.5/< Exc s/n/h/f/g mal+mrg Exc s/n/h/f/g mal+mrg Exc s/n/h/f/g mal+mrg Exc s/n/h/f/g mal+mrg Exc s/n/h/f/g mal+mrg >4 cm Exc f/e/e/n/l mal+mrg 0.5cm< Exc f/e/e/n/l mal+mrg Exc f/e/e/n/l mal+mrg Exc f/e/e/n/l mal+mrg Exc f/e/e/n/l mal+mrg Exc f/e/e/n/l mal+mrg >4 cm Mohs 1 stage Mohs 2 stage Mohs 3 stage Mohs addl stage Mohs addl specimen Mohs 1 stage h/n/hf/g Mohs addl stage Mohs 1 stage t/a/l Mohs addl stage t/a/l Mohs surg addl block Partial mastectomy P-mastectomy w/ln removal Mast radical Mast rad urban type Mast mod rad Partial mastectomy P-mastectomy w/ln removal Mast radical Mast rad urban type Mast mod rad Colonoscopy w/lesion removal Colonoscopy w/lesion removal Mobilization of colon Partial removal of colon Partial removal of colon
6 44143 Partial removal of colon Partial removal of colon Partial removal of colon Partial removal of colon Partial removal of colon Removal of colon Removal of colon/ileostomy Colectomy w/ileoanal anast Colectomy w/ileoanal anast Removal of colon/ileostomy Removal of colon/ileostomy Colectomy w/ileoanal anast Colectomy w/neo-rectum pouch Removal of colon Laparo partial colectomy Lap colectomy part w/ileum Lap part colectomy w/stoma L colectomy/coloproctostomy L colectomy/coloproctostomy Laparo total proctocolectomy Lap colectomy w/proctectomy Laparo total proctocolectomy Lap mobil splenic fl add-on Total hysterectomy Total hysterectomy Partial hysterectomy Extensive hysterectomy Extensive hysterectomy Removal of pelvis contents Vaginal hysterectomy Vag hyst including t/o Vag hyst w/t/o & vag repair Vag hyst w/urinary repair Vag hyst w/enterocele repair Hysterectomy/revise vagina Hysterectomy/revise vagina Extensive hysterectomy Vag hyst complex Vag hyst incl t/o complex Vag hyst t/o & repair compl Vag hyst w/uro repair compl Vag hyst w/enterocele compl Other codes 00.10, 99.25, 99.28, 99.29, ICD-9 procedure codes for antineoplastic agent injection or infusion
7 Supplementary Table 3. ICD-9-CM codes for outcomes Condition ICD-9-CM codes Ischemic stroke 434, 436 Severe bleeding Intracranial bleeding 430, 431 Gastrointestinal bleeding 455.2, 455.5, 455.8, 456.0, , 530.7, , 531.0, 531.2, 531.4, 531.6, 532.0, 532.2, 532.4, 532.6, 533.0, 533.2, 533.4, 533.6, 534.0, 534.2, 534.4, 534.6, , , , , , , , , , , , , , 569.3, , 578.0, 578.1, Other bleeding , 423.0, 432.0, 432.1, 432.9, 459.0, , , 599.7, 623.8, 626.2, 626.6, 719.1, , 784.7, 784.8, 786.3, VTE 415.1x, 451.1x, 453.2, 453.4x, 453.5x, 453.8, or Asthma , , , , , , , , , , , , , Supplementary Table 4. ICD-9-CM codes used to define comorbidities Condition ICD-9-CM codes Alcoholism 265.2, 291.1, 291.2, 291.3, 291.5, 291.6, 291.7, 291.8, 291.9, 303.0, 303.9, 305.0, 357.5, 425.5, 535.3, 571.0, 571.1, 571.2, 571.3, 980, V11.3 Hematological disorders 280, 281, 282, 283, 284, 285, 286, 287.1, 287.3, 287.4, 287.5, (Coagulopathy, anemia) Dementia 290.x, 294.1, Depression 296.2, 296.3, 296.5, 300.4, 309.x, 311.x Diabetes 250 Heart failure , , , , , , , , , , 425.4, 425.9, 428 Hypertension 401, 402, 403, 404, 405 Kidney disease , , , , , , , , , 582, 583.0, 583.1, 583.2, 583.3, 583.4, 583.5, 583.6, 583.7, 585, 586, 588.0, V42.0, V45.1, V56 Liver disease , , , , , , 070.6, 070.9, 456.0, 456.1, 456.2, 570, 571, 572.2, 572.3, 572.4, 572.5, 572.6, 572.7, 572.8, 573.3, 573.4, 573.8, 573.9, V42.7 Myocardial infarction 410, 412 Peripheral artery disease 440.0, 440.2, 440.9, 443.9
8 Supplemental Table 5. Adjusted hazard ratios (95% confidence intervals) stratified by cancer type comparing rivaroxaban users to matched warfarin users for the treatment of non-valvular atrial fibrillation in active cancer patients, MarketScan, Rivaroxaban User Matched Warfarin User HR (95% CI) p-value Breast # Events Person-years # Events Person-years Ischemic stroke ( ) 0.50 Severe bleeding (0.64, 3.11) 0.40 Other bleeding (0.26, 1.59) 0.34 VTE (0.32, 0.94) 0.03 Colon # Events Person-years # Events Person-years Ischemic stroke ( ) 0.34 Severe bleeding (0.20, 2.62) 0.63 Other bleeding (0.11, 1.45) 0.16 VTE (0.21, 0.96) 0.04 Lung # Events Person-years # Events Person-years Ischemic stroke ( ) 0.7 Severe bleeding (0.97, 3.75) 0.06 Other bleeding (1.00, 6.28) 0.05 VTE (0.25, 0.67) Prostate # Events Person-years # Events Person-years Ischemic stroke ( ) 0.25 Severe bleeding (0.02, 1.40) 0.1 Other bleeding (0.11, 1.97) 0.3 VTE (0.13, 0.81) 0.02 Other # Events Person-years # Events Person-years Ischemic stroke (0.32, 2.19) 0.71 Severe bleeding (0.67, 1.71) 0.78 Other bleeding (0.47, 1.32) 0.36 VTE (0.43, 0.82) 0.001
9 Supplemental Table 6. Adjusted hazard ratios (95% confidence intervals) stratified by cancer type comparing dabigatran users to matched warfarin users for the treatment of non-valvular atrial fibrillation in active cancer patients, MarketScan, Dabigatran User Matched Warfarin User HR (95% CI) p-value Breast # Events Person-years # Events Person-years Ischemic stroke (0.25, 1.96) 0.49 Severe bleeding (0.61, 2.93) 0.46 Other bleeding (0.17, 1.01) 0.05 VTE (0.09, 0.46) Colon # Events Person-years # Events Person-years Ischemic stroke (0.46, 4.78) 0.50 Severe bleeding (0.82, 3.20) 0.17 Other bleeding (0.12, 1.47) 0.17 VTE (0.13, 0.75) Lung # Events Person-years # Events Person-years Ischemic stroke (0.62, 6.55) 0.24 Severe bleeding (0.21, 1.92) 0.41 Other bleeding (0.06, 1.16) 0.08 VTE (0.12, 0.50) Prostate # Events Person-years # Events Person-years Ischemic stroke (0.10, 2.65) 0.43 Severe bleeding (0.17, 1.53) 0.23 Other bleeding (0.57, 5.14) 0.34 VTE (0.16, 1.02) 0.06 Other # Events Person-years # Events Person-years Ischemic stroke (0.36, 1.67) 0.51 Severe bleeding (0.58, 1.31) 0.51 Other bleeding (0.45, 1.17) 0.19 VTE (0.20, 0.50) < Adjusted for age, sex, CHA 2DS 2-VASc score, prevalent outcome and high-dimensional propensity score
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Welcome. Coding uidelines Coding Guidelines Coding. Coding Guidelines Coding Guidelines. Contact Us. Edition #1 March 2018
 Coding Guidelines Coding Guidelines uidelines Coding Guidelines Coding Coding Coding Guidelines Guidelines Coding uidelines Coding Guidelines Coding Coding Chemotherapy Guidelines and Radiation Coding
Coding Guidelines Coding Guidelines uidelines Coding Guidelines Coding Coding Coding Guidelines Guidelines Coding uidelines Coding Guidelines Coding Coding Chemotherapy Guidelines and Radiation Coding
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi )
 09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
CPT Service Description Effective Date
 Medical Oncology Program Review Code List 2 nd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of January, April,
Medical Oncology Program Review Code List 2 nd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of January, April,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]
![INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY] INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]](/thumbs/85/92112268.jpg) Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
ANTIEMETICS UTILIZATION MANAGEMENT CRITERIA
 ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
DRUG EXTRAVASATION. Vesicants. Irritants
 DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
DRUG EXTRAVASATION Vesicants Irritants Vesicants Antineoplastic drugs Amsacrine Dactinomycin Daunorubicin Docetaxel (rare) Doxorubicin Epirubicin Idarubicin Mechlorethamine Mitomycin Oxaliplatin (rare)
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Ovarian Cancer. compendia TREATMENT OF
 drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Guidelines for the Management of Extravasation (Version 5 May 2012)
 Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care
 Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
intolerance to, contraindication to, or therapeutic failure on a minimum 3 month trial of Inflectra*
 What s New Medical Pharmaceutical Policy March 2018 Updates MBP 5.0 Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)- Updated policy Remicade (infliximab), Inflectra (infliximab-dyyb)
What s New Medical Pharmaceutical Policy March 2018 Updates MBP 5.0 Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)- Updated policy Remicade (infliximab), Inflectra (infliximab-dyyb)
Supplementary Figures
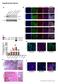 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
Guidelines for the Management of Extravasation
 Guidelines for the Management of Extravasation (Version 5.5-04 Nov 2016) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT
Guidelines for the Management of Extravasation (Version 5.5-04 Nov 2016) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT
Title Developed By. Implementation Contact person(s) Review Date Group Responsible. NICaN Policy: Management of Chemotherapy Extravasation; Version 3
 Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Trust Guideline for Prevention and Control of Chemotherapy and Radiotherapy Induced Nausea and Vomiting in Adults
 A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
The following are J Code requirements
 The following are J Code requirements J Codes 20610 Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) A9579 Injection, gadolinium based
The following are J Code requirements J Codes 20610 Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) A9579 Injection, gadolinium based
StrandAdvantage Tissue-Specific Cancer Genomic Tests. Empowering Crucial First-Line Therapy Decisions for Your Patient
 StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
Clinical Tools and Resources for Self-Study and Patient Education
 Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Corporate Medical Policy
 Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto
 Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
Chemotherapy induced emesis: Are we doing are best? David Warr University of Toronto david.warr@uhn.on.ca Conflict of interest Merck: speakers bureau and consultant Eisai: consultant Outline What is the
Patient-Centered Management of Chemotherapy-Induced Nausea and Vomiting
 Clear and accurate communication between patients and health care teams is the key to successfully controlling chemotherapy-induced nausea and vomiting. Roseate Spoonbill_2005. Photograph courtesy of Henry
Clear and accurate communication between patients and health care teams is the key to successfully controlling chemotherapy-induced nausea and vomiting. Roseate Spoonbill_2005. Photograph courtesy of Henry
ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
FOR A HIGHER DEGREE OF CONFIDENCE
 SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS. > Introduction Manufacturing specialized products in a pharmaceutical manufacturing environment has a specific set of requirements
SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS. > Introduction Manufacturing specialized products in a pharmaceutical manufacturing environment has a specific set of requirements
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Lisa M. Hess Diane Michael Daniel S. Mytelka Julie Beyrer Astra M. Liepa Steven Nicol
 Gastric Cancer (2016) 19:607 615 DOI 10.1007/s10120-015-0486-z ORIGINAL ARTICLE Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective
Gastric Cancer (2016) 19:607 615 DOI 10.1007/s10120-015-0486-z ORIGINAL ARTICLE Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective
Job title: Consultant Pharmacist/Advanced Practice Pharmacist
 Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
Recognising and reducing the risk of chemotherapy extravasation
 Recognising and reducing the risk of chemotherapy extravasation When chemotherapy drugs leak from the veins it can cause serious injury to the patient, greatly heighten their fears of undergoing future
Recognising and reducing the risk of chemotherapy extravasation When chemotherapy drugs leak from the veins it can cause serious injury to the patient, greatly heighten their fears of undergoing future
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
Antiemesis. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version Continue
 Table of Contents Clinical in Oncology Version 2.2010 Continue www.nccn.org Version 2.2010, 04/07/2010 2010 National Comprehensive Cancer Network, Inc. All rights reserved. These guidelines and this illustration
Table of Contents Clinical in Oncology Version 2.2010 Continue www.nccn.org Version 2.2010, 04/07/2010 2010 National Comprehensive Cancer Network, Inc. All rights reserved. These guidelines and this illustration
STEP 2: Identification of recurrence (local, regional or distant metastases) based on electronic health record algorithm using automated databases:
 Appendix 1. Specifications for identifying subsequent breast cancer STEP 1: Identification of second primary breast cancer Identification based on key variables from Cancer Registry including date of diagnosis;
Appendix 1. Specifications for identifying subsequent breast cancer STEP 1: Identification of second primary breast cancer Identification based on key variables from Cancer Registry including date of diagnosis;
Patient 1: Patient 2:
 Appendix A Compiled by Dr. Raymond Ngeh and Dr. Robert Luk Clinical notes and PET/CT scan images of eleven patients: 1. Middle age woman has cancer of the pancreas in the body of the gland. After just
Appendix A Compiled by Dr. Raymond Ngeh and Dr. Robert Luk Clinical notes and PET/CT scan images of eleven patients: 1. Middle age woman has cancer of the pancreas in the body of the gland. After just
SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS
 SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS Manufacturing specialized products in a pharmaceutical
SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS Manufacturing specialized products in a pharmaceutical
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
ONCOLOGY AND HAEMATOLOGY TREATMENT CENTRE Quality System Guidelines For The Safe Delivery of Systemic Anti Cancer Therapy
 Title Document Type Issue no Safe Delivery of Systemic Anti-Cancer Therapy Guideline Clinical Governance Support Team Use Issue date May 2014 Review date May 2016 Distribution Prepared by Developed by
Title Document Type Issue no Safe Delivery of Systemic Anti-Cancer Therapy Guideline Clinical Governance Support Team Use Issue date May 2014 Review date May 2016 Distribution Prepared by Developed by
Introduction to Antineoplastic Prescribing
 Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
Drug Name. J0129 Injection, abatacept (Orencia ), 10 mg Effective 01/01/2014. J0178 Injection, aflibercept (Eylea ), 1 mg Effective 04/01/2015
 J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
PHARMACY PEARLS CHANIN WRIGHT, PHARMD
 PHARMACY PEARLS CHANIN WRIGHT, PHARMD OBJECTIVES From the pearls heard, discuss with someone near you What is something you can apply to your setting Traumatic brain injury in children Goals Correct and
PHARMACY PEARLS CHANIN WRIGHT, PHARMD OBJECTIVES From the pearls heard, discuss with someone near you What is something you can apply to your setting Traumatic brain injury in children Goals Correct and
Disclosure. Objectives. Objectives. Oncologic Emergency. Classification of Emergencies 1 3/2/2015
 Disclosure Oncologic Emergencies and Acute Supportive Care of the Critically Ill Oncology Patient By: Kimberly Regis, Pharm.D. Broward Health Medical Center Pharmacy Resident 2014-2015 I do not have a
Disclosure Oncologic Emergencies and Acute Supportive Care of the Critically Ill Oncology Patient By: Kimberly Regis, Pharm.D. Broward Health Medical Center Pharmacy Resident 2014-2015 I do not have a
DRUG PROPERTIES YOU NEED TO KNOW
 jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT
 CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT OF ETRAVASATION Policy for the management of extravasation Contents Page number Introduction 3 Definition 3 Prevention 3 Follow up 6 Signs and symptoms
CHEMOTHERAPY NETWORK GROUP POLICY FOR THE MANAGEMENT OF ETRAVASATION Policy for the management of extravasation Contents Page number Introduction 3 Definition 3 Prevention 3 Follow up 6 Signs and symptoms
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
ECN Protocol Book. Guidelines on the management of extravasation. ECN_Protocol_Book_extravasation guidelines_2
 ECN Protocol Book Guidelines on the management of extravasation Name of person presenting document: Reason for document development: Names of development team: Specify groups of staff to whom the document
ECN Protocol Book Guidelines on the management of extravasation Name of person presenting document: Reason for document development: Names of development team: Specify groups of staff to whom the document
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS
 H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
Index. derm.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acitretin and cutaneous T-cell lymphomas, 716 719, 722, 725 ADCC. See Antibody-dependent cell-mediated cytotoxicity. Adjunctive therapies and
Note: Page numbers of article titles are in boldface type. A Acitretin and cutaneous T-cell lymphomas, 716 719, 722, 725 ADCC. See Antibody-dependent cell-mediated cytotoxicity. Adjunctive therapies and
Exposure Control Plan for Cytotoxic Substances
 Exposure Control Plan Department of www.rms.ubc.ca UBC-RMS-OHS-ECP-17-001 Effective date: December 8, 2017 Review date: NA Supersedes: NA 1. PURPOSE Exposure Control Plan for Cytotoxic Substances The University
Exposure Control Plan Department of www.rms.ubc.ca UBC-RMS-OHS-ECP-17-001 Effective date: December 8, 2017 Review date: NA Supersedes: NA 1. PURPOSE Exposure Control Plan for Cytotoxic Substances The University
REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Pediatr Blood Cancer 2011;57:191 198 REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients L. Lee Dupuis, MScPhm, ACPR, FCSHP,
Pediatr Blood Cancer 2011;57:191 198 REVIEW Guideline for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients L. Lee Dupuis, MScPhm, ACPR, FCSHP,
Supportive care session 1:
 Board review in oncology pharmacy 2013 Managing Disease or Treatment Related Complication Supportive care session 1: Chemotherapy induced-nausea and vomiting Suthan Chanthawong, B. Pharm, RPh. Objectives
Board review in oncology pharmacy 2013 Managing Disease or Treatment Related Complication Supportive care session 1: Chemotherapy induced-nausea and vomiting Suthan Chanthawong, B. Pharm, RPh. Objectives
