Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
|
|
|
- Rosa Gibson
- 5 years ago
- Views:
Transcription
1 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Last Review: 1/2018 Origination: 9/2002 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for hematopoietic cell transplantation for epithelial ovarian cancer. This is considered investigational. When Policy Topic is covered Not Applicable When Policy Topic is not covered Autologous and allogeneic hematopoietic cell transplantation are considered investigational to treat epithelial ovarian cancer. Description of Procedure or Service Populations Interventions Comparators Outcomes Individuals: Interventions of Comparators of Relevant outcomes include: With advancedstage epithelial Hematopoietic cell Standard Disease specific survival interest are: interest are: Overall survival ovarian cancer transplantation chemotherapy Change in disease regimen status Treatment-related morbidity Treatment-related mortality Use of hematopoietic cell transplantation (HCT) has been investigated for treatment of patients with epithelial ovarian cancer. Hematopoietic cells are infused to restore bone marrow function after cytotoxic doses of chemotherapeutic agents with or without whole body radiotherapy. For individuals who have advanced-stage epithelial ovarian cancer who receive HCT, the evidence includes randomized trials and data from case series and registries. Relevant outcomes are overall survival, disease-specific survival, change in disease status, and treatment related mortality and morbidity. Although some observational studies have reported longer survival in subsets of women
2 with advanced epithelial ovarian cancer than in women treated with standard chemotherapy, none of the randomized trial evidence has shown a benefit from HCT in this population. Overall, the evidence has not shown that HCT improves health outcomes in treating epithelial ovarian cancer, including survival, compared with conventional standard doses of chemotherapy. The evidence is insufficient to determine the effects of the technology on health outcomes. EPITHELIAL OVARIAN CANCER Several types of malignancies can arise in the ovary; epithelial carcinoma is the most common. Epithelial ovarian cancer is the fifth most common cause of cancer death in women. New cases and deaths from ovarian cancer in the United States for 2016 were estimated at 22,280 and 14,240, respectively.(1) Most ovarian cancer patients present with widespread disease, and annual mortality is approximately 65% of the incidence rate. Current management for advanced epithelial ovarian cancer is cytoreductive surgery with chemotherapy. (2) Approximately 75% of patients present with International Federation of Gynecology and Obstetrics stage III to IV ovarian cancer and are treated with paclitaxel plus a platinum analogue, the preferred regimen for newly diagnosed advanced disease.(3,4) Use of platinum and taxanes has improved progression-free survival and overall survival in advanced disease to between 16 and 21 months and 32 and 57 months, respectively.(3) However, cancer recurs in most women and they die of the disease, because chemotherapy drug resistance leads to uncontrolled cancer growth.(4) HEMATOPOIETIC CELL TRANSPLANTATION Hematopoietic cell transplantation (HCT) is a procedure in which hematopoietic stem cells are infused to restore bone marrow function in cancer patients who receive bone-marrow-toxic doses of drugs with or without whole body radiotherapy. Bone marrow stem cells may be obtained from the transplant recipient (autologous HCT) or from a donor (allogeneic HCT). They can be harvested from bone marrow, peripheral blood, or umbilical cord blood and placenta shortly after delivery of neonates. Although cord blood is an allogeneic source, the stem cells in it are antigenically naive and thus are associated with a lower incidence of rejection or graft-versus-host disease. Cord blood is discussed in detail in evidence review in a separate policy. HCT is an established treatment for certain hematologic malignancies; however, its use in solid tumors in adults is largely experimental. HCT for Epithelial Ovarian Cancer HCT has been investigated as a therapy to overcome drug resistance. However, limited data exist on this treatment approach; the ideal patient population and best treatment regimen remain to be established.(4) HCT has been tested in various patient groups with ovarian cancer: to consolidate remission after induction therapy to treat relapse after a durable response to platinum-based chemotherapy
3 to treat tumors that relapse after less than 6 months to treat refractory tumors. REGULATORY STATUS The U.S. Food and Drug Administration (FDA) regulates human cells and tissues intended for implantation, transplantation, or infusion through the Center for Biologics Evaluation and Research, under Code of Federal Regulation (CFR) title 21, parts 1270 and Hematopoietic stem cells are included in these regulations. Rationale This evidence review was originally created in December 1999 and has been updated regularly with literature searches of the MEDLINE database. The most recent update covered the period through November 9, HEMATOPOIETIC CELL TRANSPLANTATION FOR EPITHELIAL OVARIAN CANCER This evidence review was originally based on a 1998 TEC Assessment that reached the following conclusions(5): Data were unavailable from randomized controlled trials for any of the patient groups studied. Thus, the Assessment was able to compare outcomes only indirectly, using separate studies of high-dose chemotherapy (HDC) and conventional dose regimens.(5) Although some results reported after HDC appeared encouraging, indirect comparisons did not permit conclusions. In previously untreated patients, reported response rates suggested that HDC increased objective response rates compared with patients given conventionaldose chemotherapy. However, this comparison was flawed by age bias and by differences in performance status and other baseline characteristics of patients included in the 2 sets of studies. Response duration and survival data were unavailable for comparison.(5) Treatment-related mortality was greater after HDC. In previously treated patients, objective response rates after HDC also were reportedly higher than after conventional-dose regimens. Subgroup analyses showed higher response rates among platinum-sensitive, optimally debulked patients. Minimum values of the ranges reported across studies for median response duration and survival after HDC were similar to those reported after conventional-dose chemotherapy. However, the maxima for these ranges suggested improved response duration and overall survival (OS) after HDC. In contrast, data from the Autologous Blood and Marrow Transplant Registry did not show similarly high survival for comparable subgroups. Comparison with conventional-dose chemotherapy was again biased due to differences in age distributions, performance status, and other baseline characteristics of patients included in studies of high-dose or conventional chemotherapies.(5) The 1998 TEC Assessment did not identify any studies reporting outcomes of allogeneic transplants for patients with ovarian cancer. A separate 1999 TEC
4 Assessment evaluated the use of HDC with allogeneic stem cell support as salvage therapy after a failed prior course of HDC with autologous stem cell support.(6) There were no data on outcomes of this strategy as therapy for epithelial ovarian cancer. Experience with hematopoietic cell transplantation (HCT) in epithelial ovarian cancer is primarily derived from registry data and phase 2 trials.(7-10) Many registry patients were treated after relapse and others in nonrandomized trials using HDC as first-line treatment. Case selection and retrospective review make interpretation of registry and nonrandomized data difficult.(3) Survival analyses from registry data and clinical trials have suggested a possible benefit in treating ovarian cancer patients with HCT. Randomized Controlled Trials In 2007, Mobus et al reported on a randomized, phase 3 trial that included 149 patients with untreated ovarian cancer who were randomized, after debulking surgery, to standard chemotherapy or sequential HDC and peripheral blood stem cell support.(3) This was the first randomized trial comparing HDC with standard chemotherapy as first-line treatment of ovarian cancer, and investigators found no statistically significant differences in progression-free survival (PFS) or OS between the 2 treatments. The study was powered such that a sample of 208 patients would be needed to detect an absolute improvement of 15% in PFS with a power of 80% and a 1-sided α of 5%. Median patient age was 50 years (range, years) and International Federation of Gynecology and Obstetrics (FIGO) stage was IIB or IIC in 4%, stage III in 78%, and stage IV in 17%. Seventy-six percent of patients in the HDC arm received all scheduled chemotherapy cycles. After a median follow-up of 38 months, PFS was 20.5 months in the standard chemotherapy arm and 29.6 months in the HDC arm (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.56 to 1.26; p=0.40). Median OS was 62.8 months in the standard chemotherapy arm and 54.4 months in the HDC arm (HR=1.17; 95% CI, 0.71 to 1.94; p=0.54). In 2008, Papadimitriou et al reported on an RCT comparing the use of HDC with stem cell support as consolidation therapy in patients with advanced epithelial ovarian cancer (FIGO stage IIC-IV).(4) Patients who achieved first complete remission after conventional chemotherapy were randomized to receive or not high-dose melphalan and autologous HCT. Eighty patients were enrolled in the trial. Of 37 patients allocated to HDC, 11 (30%) did not receive the treatment either due to refusal or failure of peripheral blood stem cell mobilization. In an intention-to-treat analysis, there were no significant differences between the 2 arms in time-to-disease progression (p=0.059) or OS (p=0.38). Observational Comparative Studies In 2012, Sabatier et al retrospectively reviewed 163 patients with advanced or metastatic ([FIGO stage IIIC or IV) epithelial ovarian cancer who were treated at a single institution in France.(11) All patients received cytoreductive surgery and combination platinum plus taxane chemotherapy. Investigators compared median PFS and OS between 60 patients who received subsequent HDC with autologous
5 HCT support and 103 patients who did not. HDC regimens varied, but all contained alkylating agents. At a median follow-up of 47.5 months, PFS in the high-dose and the standard chemotherapy groups was 20.1 and 18.1 months, respectively (p not reported). OS was 47.3 and 41.3 months, respectively (p=0.29). In prespecified subgroup analyses, median PFS was significantly longer in women younger than age 50 years who received HDC (81.7 months) than in women who received standard chemotherapy (11 months; p=0.02); in women older than 50 years, median PFS did not differ statistically between groups (17.9 months vs 18.3 months, respectively; p=0.81). Similarly, median OS was significantly longer in women younger than age 50 years who received HDC (54.6 months) than in women who received standard chemotherapy (36 months; p=0.05), but not in women older than 50 years (49.5 months vs 42 months, respectively; p not reported). The authors recommended further study of HDC with autologous HCT support in patients younger than 50 years. SUMMARY OF EVIDENCE For individuals who have advanced-stage epithelial ovarian cancer who receive hematopoietic cell transplantation (HCT), the evidence includes randomized trials and data from case series and registries. Relevant outcomes are overall survival, disease-specific survival, change in disease status, and treatment related mortality and morbidity. Although some observational studies have reported longer survival in subsets of women with advanced epithelial ovarian cancer than in women treated with standard chemotherapy, none of the randomized trial evidence has shown an benefit from HCT in this population. Overall, the evidence has not shown that HCT improves health outcomes in treating epithelial ovarian cancer, including survival, compared with conventional standard doses of chemotherapy. The evidence is insufficient to determine the effects of the technology on health outcomes. SUPPLEMENTAL INFORMATION PRACTICE GUIDELINES AND POSITION STATEMENTS Current National Comprehensive Cancer Network guidelines (v ) do not address hematopoietic cell transplantation for ovarian cancer for patients either with newly diagnosed or with relapsed or refractory disease.(2) U.S. PREVENTIVE SERVICES TASK FORCE RECOMMENDATIONS Not applicable. MEDICARE NATIONAL COVERAGE The Centers for Medicare and Medicaid Services currently have the following national noncoverage decision on autologous stem cell transplantation [AuSCT]: Insufficient data exist to establish definite conclusions regarding the efficacy of AuSCT for the following condition[s]: Solid tumors (other than neuroblastoma). (12)
6 ONGOING AND UNPUBLISHED CLINICAL TRIALS A search of ClinicalTrials.gov in January 2017 did not identify any ongoing or unpublished trials that would likely influence this review. References 1. American Cancer Society. Cancer Facts & Figures Atlanta, Ga: American Cancer Society; Accessed February 28, (NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer. Version Accessed January 7, Mobus V, Wandt H, Frickhofen N, et al. Phase III trial of high-dose sequential chemotherapy with peripheral blood stem cell support compared with standard dose chemotherapy for firstline treatment of advanced ovarian cancer: intergroup trial of the AGO-Ovar/AIO and EBMT. J Clin Oncol. Sep ;25(27): PMID Papadimitriou C, Dafni U, Anagnostopoulos A, et al. High-dose melphalan and autologous stem cell transplantation as consolidation treatment in patients with chemosensitive ovarian cancer: results of a single-institution randomized trial. Bone Marrow Transplant. Mar 2008;41(6): PMID Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). High-dose chemotherapy with autologous stem-cell support for epithelial ovarian cancer. TEC Assessments. 1998;Volume 13:Tab Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). Salvage high-dose chemotherapy with allogeneic stem cell support for relapse following high-dose chemotherapy with autologous stem cell support for non-lymphoid solid tumors. TEC Assessments. 1999;Volume 14:Tab Donato ML, Aleman A, Champlin RE, et al. Analysis of 96 patients with advanced ovarian carcinoma treated with high-dose chemotherapy and autologous stem cell transplantation. Bone Marrow Transplant. Jun 2004;33(12): PMID Ledermann JA, Herd R, Maraninchi D, et al. High-dose chemotherapy for ovarian carcinoma: long-term results from the Solid Tumour Registry of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol. May 2001;12(5): PMID Stiff PJ, Bayer R, Kerger C, et al. High-dose chemotherapy with autologous transplantation for persistent/relapsed ovarian cancer: a multivariate analysis of survival for 100 consecutively treated patients. J Clin Oncol. Apr 1997;15(4): PMID Stiff PJ, Veum-Stone J, Lazarus HM, et al. High-dose chemotherapy and autologous stem-cell transplantation for ovarian cancer: an autologous blood and marrow transplant registry report. Ann Intern Med. Oct ;133(7): PMID Sabatier R, Goncalves A, Bertucci F, et al. Are there candidates for high-dose chemotherapy in ovarian carcinoma? J Exp Clin Cancer Res. 2012;31:87. PMID Services CfMaM. National Coverage Determination (NCD) for STEM CELL Transplantation ( ). 2010; Version 5: pennsylvania&keyword=stem+cell&keywordlookup=title&keywordsearchtype=and&bc=gaaaaba aaaaa&. Accessed January 7, Billing Coding/Physician Documentation Information Management of recipient hematopoietic progenitor cell donor search and cell acquisition Blood-derived hematopoietic progenitor cell harvesting for transplantation, per collection; allogenic Blood-derived hematopoietic progenitor cell harvesting for transplantation, per collection; autologous Transplant preparation of hematopoietic progenitor cells; thawing of previously frozen harvest, without washing Transplant preparation of hematopoietic progenitor cells; thawing of
7 previously frozen harvest, with washing Transplant preparation of hematopoietic progenitor cells; specific cell depletion within harvest, T cell depletion Transplant preparation of hematopoietic progenitor cells; tumor cell depletion Transplant preparation of hematopoietic progenitor cells; red blood cell removal Transplant preparation of hematopoietic progenitor cells; platelet depletion Transplant preparation of hematopoietic progenitor cells; plasma (volume) depletion Transplant preparation of hematopoietic progenitor cells; cell concentration in plasma, mononuclear, or buffy coat layer Bone marrow; aspiration only Bone marrow; biopsy, needle or trocar Bone marrow harvesting for transplantation; allogeneic Bone marrow harvesting for transplantation; autologous Hematopoietic progenitor cell (HPC); allogeneic transplantation per donor Bone marrow transplantation; autologous Bone marrow or blood-derived peripheral cell transplantation; allogeneic donor lymphocyte Infusions Q0083 Chemotherapy administration by other than infusion technique only (e.g., subcutaneous, intramuscular, push), per visit Q0084 Chemotherapy administration by infusion technique only, per visit Q0085 Chemotherapy administration by both infusion technique and other technique(s) (e.g., subcutaneous, intramuscular, push), per visit S2140 Cord blood harvesting for transplantation, allogeneic S2142 Cord blood-derived cell transplantation, allogeneic S2150 Bone marrow or blood-derived peripheral cell harvesting and transplantation, allogeneic or autologous, including pheresis, high-dose chemotherapy, and 28 days of post-transplant care (including drugs; hospitalization; medical surgical, diagnosis and emergency services) C56.0- C56.9 ICD10 Codes: Investigational for all relevant diagnoses Malignant neoplasm of ovary code range Policy Implementation/Update Information 9/1/02 New policy added to the Surgery and Transplant sections. 9/1/03 No policy statement changes. 9/1/04 No policy statement changes. 9/1/05 No policy statement changes. 9/1/06 No policy statement changes. 9/1/07 No policy statement changes. 9/1/08 No policy statement changes. 9/1/09 No policy statement changes. High-dose chemotherapy removed from
8 policy title. 9/1/10 No policy statement changes. 9/1/11 No policy statement changes. 9/1/12 No policy statement changes. 9/1/13 No policy statement changes. 1/1/15 No policy statement changes. 1/1/16 No policy statement changes. 3/1/16 No policy statement changes. 1/1/17 No policy statement changes. 1/1/18 Removed Stem from polity title. No change to policy statement. State and Federal mandates and health plan contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. The medical policies contained herein are for informational purposes. The medical policies do not constitute medical advice or medical care. Treating health care providers are independent contractors and are neither employees nor agents Blue KC and are solely responsible for diagnosis, treatment and medical advice. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, photocopying, or otherwise, without permission from Blue KC.
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer
 Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Regence. Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT REMINDER
 Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer
 Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer Policy Number: 7.01.121 Last Review: 2/2018 Origination: 8/2006 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer Policy Number: 7.01.121 Last Review: 2/2018 Origination: 8/2006 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Policy. not covered Sipuleucel-T. Considerations Sipuleucel-T. Description Sipuleucel-T. be medically. Sipuleucel-T. covered Q2043.
 Cellular Immunotherapy forr Prostate Cancer Policy Number: 8.01.53 Origination: 11/2010 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for cellular immunotherapy for prostate
Cellular Immunotherapy forr Prostate Cancer Policy Number: 8.01.53 Origination: 11/2010 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for cellular immunotherapy for prostate
Hematopoietic Cell Transplantation for Breast Cancer
 Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Biofeedback as a Treatment of Headache
 Biofeedback as a Treatment of Headache Policy Number: 2.01.29 Last Review: 7/2018 Origination: 7/2008 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
Biofeedback as a Treatment of Headache Policy Number: 2.01.29 Last Review: 7/2018 Origination: 7/2008 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
Vertebral Axial Decompression
 Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2017 Origination: 11/2005 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2017 Origination: 11/2005 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors (GCT)
 Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors (GCT) Policy Number: 8.01.35 Last Review: 7/2017 Origination: 7/2002 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas
Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors (GCT) Policy Number: 8.01.35 Last Review: 7/2017 Origination: 7/2002 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer
 Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer Policy Number: 7.01.121 Last Review: 2/2019 Origination: 8/2006 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
Saturation Biopsy for Diagnosis and Staging and Management of Prostate Cancer Policy Number: 7.01.121 Last Review: 2/2019 Origination: 8/2006 Next Review: 8/2019 Policy Blue Cross and Blue Shield of Kansas
HEMATOPOIETIC CELL TRANSPLANTATION FOR EPITHELIAL OVARIAN CARCINOMA
 CARCINOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
CARCINOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Vertebral Axial Decompression
 Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2018 Origination: 11/2005 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2018 Origination: 11/2005 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CNS Embryonal Tumors File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cns_embryonal_tumors
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CNS Embryonal Tumors File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cns_embryonal_tumors
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Poteligeo (mogamulizmuab-kpkc)
 Poteligeo (mogamulizmuab-kpkc) Policy Number: 5.02.556 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Poteligeo
Poteligeo (mogamulizmuab-kpkc) Policy Number: 5.02.556 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Poteligeo
Intracellular Micronutrient Analysis
 Intracellular Micronutrient Analysis Policy Number: 2.04.73 Last Review: 1/2019 Origination: 1/2013 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Intracellular Micronutrient Analysis Policy Number: 2.04.73 Last Review: 1/2019 Origination: 1/2013 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Placental and Umbilical Cord Blood as a Source of Stem Cells
 Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Avastin (bevacizumab)
 Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
Dynamic Spinal Visualization and Vertebral Motion Analysis
 Dynamic Spinal Visualization and Vertebral Motion Analysis Policy Number: 6.01.46 Last Review: 2/2019 Origination: 2/2006 Next Review: 2/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Dynamic Spinal Visualization and Vertebral Motion Analysis Policy Number: 6.01.46 Last Review: 2/2019 Origination: 2/2006 Next Review: 2/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Nutrient/Nutritional Panel Testing
 Nutrient/Nutritional Panel Testing Policy Number: 2.04.136 Last Review: 10/2017 Origination: 10/2015 Next Review: 10/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Nutrient/Nutritional Panel Testing Policy Number: 2.04.136 Last Review: 10/2017 Origination: 10/2015 Next Review: 10/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Antigen Leukocyte Antibody Test
 Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2018 Origination: 4/2014 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2018 Origination: 4/2014 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Clinical Policy: Donor Lymphocyte Infusion
 Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
HEMATOPOIETIC CELL TRANSPLANTATION FOR SOLID TUMORS OF CHILDHOOD
 CHILDHOOD Non-Discrimination Statement Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices drugs are dependent upon
CHILDHOOD Non-Discrimination Statement Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices drugs are dependent upon
Clinical Policy: Donor Lymphocyte Infusion Reference Number: CP.MP.101 Last Review Date: 11/17
 Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
Populations Interventions Comparators Outcomes Individuals: With previously untreated germ cell tumors
 Hematopoietic Cell Transplantation in the Treatment of Germ Cell (80135) (Formerly Hematopoietic Stem Cell Transplantation in the Treatment of Germ Cell ) Medical Benefit Effective Date: 04/01/13 Next
Hematopoietic Cell Transplantation in the Treatment of Germ Cell (80135) (Formerly Hematopoietic Stem Cell Transplantation in the Treatment of Germ Cell ) Medical Benefit Effective Date: 04/01/13 Next
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia
 Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Vosevi (sofosbuvir/velpatasvir/voxilaprevir)
 Vosevi (sofosbuvir/velpatasvir/voxilaprevir) Policy Number: 5.01.646 Last Review: 10/2017 Origination: 10/2017 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Vosevi (sofosbuvir/velpatasvir/voxilaprevir) Policy Number: 5.01.646 Last Review: 10/2017 Origination: 10/2017 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA
 HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
Antigen Leukocyte Antibody Test
 Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2014 Origination: 4/2014 Next Review: 4/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2014 Origination: 4/2014 Next Review: 4/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA
 STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Donor Lymphocyte Infusion for Malignancies Treated with an Allogeneic Hematopoietic Stem-Cell Transplant
 Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2019 Origination: 4/2014 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2019 Origination: 4/2014 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2018 Origination: 7/2013 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
Subject: Chemoresistance and Chemosensitivity Assays
 05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
MP Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
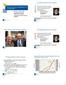 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Ingrezza (valbenazine)
 Ingrezza (valbenazine) Policy Number: 5.01.635 Last Review: 7/2018 Origination: 07/2017 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Ingrezza
Ingrezza (valbenazine) Policy Number: 5.01.635 Last Review: 7/2018 Origination: 07/2017 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Ingrezza
Hematopoietic Stem-Cell Transplantation for Breast Cancer
 Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
Corporate Medical Policy
 White Blood Cell Growth Factors Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: white_blood_cell_growth_factors 9/2016 4/2017 4/2018 6/2017 Description of
White Blood Cell Growth Factors Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: white_blood_cell_growth_factors 9/2016 4/2017 4/2018 6/2017 Description of
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Solid Tumors of File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_solid_tumors_childhood
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Solid Tumors of File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_solid_tumors_childhood
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors
 Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors Policy Number: Original Effective Date: MM.07.020 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 4/27/2018 Section:
Hematopoietic Cell Transplantation in the Treatment of Germ Cell Tumors Policy Number: Original Effective Date: MM.07.020 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 4/27/2018 Section:
HEMATOPOIETIC CELL TRANSPLANTATION FOR CHRONIC MYELOID LEUKEMIA
 LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
Corporate Medical Policy. Policy Effective February 23, 2018
 Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2018 Origination: 4/2014 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2018 Origination: 4/2014 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Medical Policy. MP Placental and Umbilical Cord Blood as a Source of Stem Cells
 Medical Policy MP 7.01.50 BCBSA Ref. Policy: 7.01.50 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies 8.01.20 Hematopoietic Cell Transplantation for Non- Hodgkin Lymphomas
Medical Policy MP 7.01.50 BCBSA Ref. Policy: 7.01.50 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies 8.01.20 Hematopoietic Cell Transplantation for Non- Hodgkin Lymphomas
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2014 Origination: 12/2001 Next Review: 6/2015 Policy Blue Cross
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2014 Origination: 12/2001 Next Review: 6/2015 Policy Blue Cross
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE
 BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
Hematopoietic Cell Transplantation in the Treatment of Germ- Cell Tumors
 Medical Policy Manual Transplant, Policy No. 45.38 Hematopoietic Cell Transplantation in the Treatment of Germ- Cell Tumors Next Review: August 2018 Last Review: December 2017 Effective: January 1, 2018
Medical Policy Manual Transplant, Policy No. 45.38 Hematopoietic Cell Transplantation in the Treatment of Germ- Cell Tumors Next Review: August 2018 Last Review: December 2017 Effective: January 1, 2018
Clinical Policy: Pegfilgrastim (Neulasta) Reference Number: CP.CPA.127 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Neulasta) Reference Number: CP.CPA.127 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Neulasta) Reference Number: CP.CPA.127 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Reference: NHS England 1602
 Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Electrical Stimulation for Scoliosis
 Electrical Stimulation for Scoliosis Policy Number: 1.01.509 Last Review: 8/2017 Origination: 8/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Electrical Stimulation for Scoliosis Policy Number: 1.01.509 Last Review: 8/2017 Origination: 8/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Hematopoietic Stem-Cell Transplantation for Breast Cancer
 Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/22/2013 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/22/2013 Section: Transplants
Induction Therapy & Stem Cell Transplantation for Myeloma
 Induction Therapy & Stem Cell Transplantation for Myeloma William Bensinger, MD Professor of Medicine, Division of Oncology University of Washington School of Medicine Director, Autologous Stem Cell Transplant
Induction Therapy & Stem Cell Transplantation for Myeloma William Bensinger, MD Professor of Medicine, Division of Oncology University of Washington School of Medicine Director, Autologous Stem Cell Transplant
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 10/1/2014 Most Recent Review Date (Revised): 7/25/2017 Effective Date: 9/1/2017 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 10/1/2014 Most Recent Review Date (Revised): 7/25/2017 Effective Date: 9/1/2017 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Surveillance report Published: 17 March 2016 nice.org.uk
 Surveillance report 2016 Ovarian Cancer (2011) NICE guideline CG122 Surveillance report Published: 17 March 2016 nice.org.uk NICE 2016. All rights reserved. Contents Surveillance decision... 3 Reason for
Surveillance report 2016 Ovarian Cancer (2011) NICE guideline CG122 Surveillance report Published: 17 March 2016 nice.org.uk NICE 2016. All rights reserved. Contents Surveillance decision... 3 Reason for
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
HEMATOPOIETIC CELL TRANSPLANTATION FOR PRIMARY AMYLOIDOSIS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Takhzyro (lanadelumab-flyo)
 Takhzyro (lanadelumab-flyo) Policy Number: 5.01.675 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Takhzyro
Takhzyro (lanadelumab-flyo) Policy Number: 5.01.675 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Takhzyro
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Systems Pathology in Prostate Cancer
 Systems Pathology in Prostate Cancer Policy Number: 2.04.64 Last Review: 8/2014 Origination: 8/2010 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Systems Pathology in Prostate Cancer Policy Number: 2.04.64 Last Review: 8/2014 Origination: 8/2010 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Corporate Medical Policy
 Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 4/27/2018 Section: Transplants
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 4/27/2018 Section: Transplants
Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer
 Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Saturation Biopsy for Diagnosis, Staging, and Management of Prostate Cancer Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: 1.01.30 Artificial Pancreas Device Systems 7.03.12 Autologous Islet transplantation Allogeneic Pancreas Transplant Description
FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: 1.01.30 Artificial Pancreas Device Systems 7.03.12 Autologous Islet transplantation Allogeneic Pancreas Transplant Description
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast
 Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast Policy Number: 7.01.153 Last Review: 12/2018 Origination: 6/2015 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast Policy Number: 7.01.153 Last Review: 12/2018 Origination: 6/2015 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
Hematopoietic Stem-Cell Transplantation for CNS Embryonal Tumors and Ependymoma
 Hematopoietic Stem-Cell Transplantation for CNS Embryonal Tumors and Ependymoma Policy Number: 8.01.28 Last Review: 7/2014 Origination: 7/2002 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas
Hematopoietic Stem-Cell Transplantation for CNS Embryonal Tumors and Ependymoma Policy Number: 8.01.28 Last Review: 7/2014 Origination: 7/2002 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas
EBMT2008_22_44:EBMT :29 Pagina 454 CHAPTER 30. HSCT for Hodgkin s lymphoma in adults. A. Sureda
 EBMT2008_22_44:EBMT2008 6-11-2008 9:29 Pagina 454 * CHAPTER 30 HSCT for Hodgkin s lymphoma in adults A. Sureda EBMT2008_22_44:EBMT2008 6-11-2008 9:29 Pagina 455 CHAPTER 30 HL in adults 1. Introduction
EBMT2008_22_44:EBMT2008 6-11-2008 9:29 Pagina 454 * CHAPTER 30 HSCT for Hodgkin s lymphoma in adults A. Sureda EBMT2008_22_44:EBMT2008 6-11-2008 9:29 Pagina 455 CHAPTER 30 HL in adults 1. Introduction
Protocol. Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
Protocol. Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) (Formerly Hematopoietic Stem Cell Transplantation for Hodgkin Lymphoma) Medical Benefit Effective Date: 04/01/18 Next Review Date:
Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) (Formerly Hematopoietic Stem Cell Transplantation for Hodgkin Lymphoma) Medical Benefit Effective Date: 04/01/18 Next Review Date:
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Sargramostim (Leukine) Reference Number: CP.PHAR.295 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Clinical Policy: Sargramostim (Leukine) Reference Number: CP.PHAR.295 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Clinical Policy: Sargramostim (Leukine) Reference Number: CP.PHAR.295
 Clinical Policy: (Leukine) Reference Number: CP.PHAR.295 Effective Date: 12/16 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Leukine) Reference Number: CP.PHAR.295 Effective Date: 12/16 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Hematopoietic Stem-Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Stem-Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/27/2016 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/27/2016 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective
= Medical Policy. MP Hematopoietic Cell Transplantation for Primary Amyloidosis
 = Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
= Medical Policy MP 8.01.42 BCBSA Ref. Policy: 8.01.42 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Therapy Related Policies 7.01.50 Placental and Umbilical Cord Blood as a Source of Stem
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 CAE of Malignancy with MRI of the Breast Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Computer-Aided Evaluation of Malignancy with Magnetic
CAE of Malignancy with MRI of the Breast Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Computer-Aided Evaluation of Malignancy with Magnetic
