Proposal is to add words or statements in red and delete words or statements with strikethrough.
|
|
|
- Ira Holmes
- 5 years ago
- Views:
Transcription
1 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Psitrn Emissin Tmgraphy (PET) fr Onclgic Applicatins DESCRIPTION Psitrn Emissin Tmgraphy (PET), als called PET imaging r a PET scan, is a frm f nuclear medicine imaging. PET imaging is based n the use f psitrn emitting radinuclide tracers cupled t rganic mlecules such as glucse, ammnia, r water. The radinuclide tracers simultaneusly emit tw high-energy phtns in ppsite directins that can be simultaneusly detected by a PET scanner. The PET scanner cnsists f multiple detectrs that encircle the area f interest. A variety f tracers are used fr PET scanning including xygen-15, nitrgen-13, carbn-11, and flurine-18. The mst cmmnly used raditracer in nclgy imaging is flurine-18 cupled with flurdexyglucse (FDG). FDG has a metablism related t glucse metablism. It has been cnsidered ptentially useful in cancer imaging, since tumr cells shw increased metablism f glucse. Prpsal is t add wrds r statements in red and delete wrds r statements with strikethrugh. POLICY Psitrn emissin tmgraphy (PET) scans fr the nclgical applicatins listed belw are cnsidered medically necessary if the medical apprpriateness criteria are met: Adrenal Tumrs Anal Cancer Bne Cancers Breast Cancer Castleman s Disease Central Nervus System Tumrs Cervical Cancer Esphageal Cancer Gastric Cancer Gastrintestinal/Pancreatic Neurendcrine Cancers Gastrintestinal Strmal Tumr Head and Neck Cancers Hematpietic Stem Cell Transplantatin Hepatcellular Carcinma/Biliary Tumrs Langerhans Cell Histicytsis (LCH) Leukemia (Chrnic Lymphcytic (CLL) Lung Cancers Lymphmas Medullblastma, Supratentrial Primitive Neurectdermal Tumrs, and Pineblastma Melanmas and ther Skin Cancers Metastatic Cancer, Carcinma f Unknwn Primary Site, & Other Types f Cancer Multiple Myelma and Plasmacytmas Neurblastma Ovarian Cancer Pancreatic Cancer Paraneplastic Syndrmes Prstate Cancer Rhabdmysarcma Salivary Gland Cancer Sft Tissue Sarcmas This dcument has been classified as public infrmatin
2 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Testicular and Nnepithelial Ovarian Cancer (Germ Cell Cancer) Thracic Cancers (Other than Esphageal and Lung) Thyrid Cancers Transitinal Cell Cancer Wilms Tumr Psitrn emissin tmgraphy (PET) scans fr the fllwing applicatins are cnsidered investigatinal: General Guidelines When imaging lesins less than 8 mm in size Surveillance imaging (unless specifically stated in the criteria) Distant r diffuse metastatic disease Metastatic disease in the central nervus system (CNS) Fllw-up after lcalized therapy (e.g., radifrequency ablatin, emblizatin, steretactic radiatin) Breast When used fr nn-invasive breast cancers When bvius multi-rgan metastatic disease is present PET mammgraphy When used fr surveillance Esphageal When used fr surveillance Gastrintestinal Nn-invasive carcinmas Carcinmas cntained within a plyp Cln cancer that is cmpletely resected and lymph nde negative Anal margin carcinmas T1 gastric cancers Fllwing cmplete resectin f gastrintestinal strmal tumr (GIST) Liver lesins less than 1 cm in individuals withut a prir histry f cnfirmed malignancy When used fllwing interventinal radilgy prcedures invlving liver lesins, such as radifrequency ablatin (RFA) When used fllwing pstperative adjuvant chemtherapy with the fllwing: Resectin has remved all knwn grss disease Markers are nt elevated When used in the setting f bvius multi-rgan metastatic disease Gyneclgic Cancers Endmetrial cancer Vulvar cancer Vaginal cancer Uterine cancers Nn-invasive cervical cancer External genitalia cancer Head and Neck (Squamus cell carcinmas f the head and neck) When used t determine the need fr neck dissectin fr individual with newly diagnsed head and neck cancer This dcument has been classified as public infrmatin
3 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement When used fr restaging f cancers f the head and neck when surgery is the primary treatment mdality (e.g. cmplete resectin/ radical neck dissectin) When cmplete respnse r prgressin is clearly bvius n physical examinatin When used fr salivary gland tumrs When used as a substitute fr panendscpy Leukemia (acute lymphblastic, acute myelid, and chrnic myelid) Lungs When using serial PET scans t evaluate lung ndules When ther imaging mdalities have cnfirmed skeletal disease metastasis frm ther primary sites (i.e. lung, breast, prstate, renal cell and ther urgenital cancers) Metastatic nn-small cell disease utside chest cavity (e.g., malignant pleural/pericardial effusin) When ther imaging shws extensive staged disease in small cell carcinma Fr restaging fr the fllwing: When surgery was the primary treatment and all knwn tumr was resected When tumrs were initially treated with radiatin therapy as the nly treatment mdality Melanma and Other Skin Cancers Fr rutine surveillance in all skin cancers (including melanma) fr asymptmatic individuals Miscellaneus (Carcinmas f unknwn primary site, sft tissue sarcma, generalized lymphadenpathy and mediastinal abnrmalities/ lymphadenpathy, liver lesins, adrenal lesins and neurendcrine lesins) When used fr surveillance and fr rutine bdy imaging f lymph ndes When used fllwing cmplete resectin When used fr generalized lymphadenpathy and mediastinal abnrmalities prir t histlgic diagnsis Prstate Cancer Primary brain tumrs Fr detectin, initial wrk up r staging fr primary brain tumrs Fr the use f FDG-PET in the evaluatin f metastatic depsits and well-differentiated brain tumrs Salivary Gland Cancers Renal Cell Cancer Skin Cancers (Nn-Melanma) Unless specifically addressed within the plicy criteria (e.g., Merkel cell carcinma) Uterine Cancer MEDICAL APPROPRIATENESS Psitrn emissin tmgraphy (PET) fr nclgical applicatins is cnsidered medically apprpriate if ANY ONE f the fllwing are met: Adrenal Tumrs with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: This dcument has been classified as public infrmatin
4 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Phechrmcytma, paraganglima, r paraganglineurma r adrencrtical carcinma Cntinued suspicin with negative r incnclusive imaging PET results will direct immediate care decisins Restaging r recurrence with ALL f the fllwing met: Cntinued suspicin with negative r incnclusive imaging Anal Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: Stage II - IV squamus cell carcinma f the anal canal Restaging r recurrence with ALL f the fllwing met: Stage III r IV squamus cell carcinma f the anal canal Bipsy prven recurrence Incnclusive cnventinal imaging Hepatcellular Carcinma (HCC) / Biliary Tumrs (Primary) with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: Primary biliary carcinma (bipsy is nt required; liver lesin with AFP >20 is adequate fr imaging) N evidence f metastatic disease by cnventinal imaging T determine if individual is a surgical candidate Bne Sarcma Chrdma with ANY ONE f the fllwing met: Initial wrkup r initial staging with incnclusive cnventinal imaging Restaging r recurrence with ALL f the fllwing met: Cmpletin f raditherapy r every 2 cycles f chemtherapy Incnclusive cnventinal imaging Bne Cancer - Ewing Sarcma and Primitive Neurectdermal Tumrs with ANY ONE f the fllwing met: Initial staging with ALL f the fllwing met: Plain X-ray has been perfrmed Histlgic diagnsis has been established Treatment respnse with ALL f the fllwing met: Distant bne metastasis with ANY ONE f the fllwing met: After every 2 cycles f chemtherapy End f planned chemtherapy Restaging with ANY ONE f the fllwing met: After apprximately 12 weeks f neadjuvant chemtherapy and prir t lcal cntrl surgery t cnfirm the absence f disease prgressin Fllwing lcal cntrl surgery and planned chemtherapy Surveillance with ALL ANY ONE f the fllwing met: Cnventinal imaging incnclusive r suspicius fr recurrence and PET avidity will determine whether bipsy r cntinued bservatin is apprpriate PET will determine whether bipsy r cntinued bservatin is apprpriate Obvius clinical symptms shw strng evidence suggesting recurrence (PET culd replace cnventinal imaging) Restaging after bipsy-cnfirmed recurrence Bne Cancer - Ostegenic Sarcma with ANY ONE f the fllwing met: Initial staging with ALL f the fllwing met: Plain X-ray has been perfrmed This dcument has been classified as public infrmatin
5 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Histlgic diagnsis has been established Treatment respnse with ANY ONE f the fllwing met: Distant bne metastasis with ANY ONE the fllwing met: After every 2 cycles f chemtherapy End f planned chemtherapy Restaging with ANY ONE f the fllwing met: After weeks f neadjuvant chemtherapy and prir t lcal cntrl surgery t cnfirm absence f disease prgressin Distant bne metastasis with ANY ONE f the fllwing met: Fllwing lcal cntrl surgery, whle bdy PET/CT every 4 mnths End f planned chemtherapy Breast Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging when CT and /r bne scan are incnclusive Restaging r recurrence with ALL f the fllwing met: Incnclusive CT, MRI, and/r bne scan fr suspected recurrence Further characterizatin is needed t make treatment decisins Stage IV disease with ALL f the fllwing met: Bne metastasis nly site f Stage IV disease (excluding brain mets) Prir bne scan has nt been perfrmed fr serial cmparisn Carcinma f Unknwn Primary Site with ANY ONE f the fllwing met: Carcinma fund in lymph nde r rgan knwn nt t be primary and ANY ONE f the fllwing studies have failed t demnstrate site f primary: CT Chest and Abdmen/Pelvis with cntrast CT Neck with cntrast if cervical r supraclavicular invlvement CT with cntrast f any ther symptmatic site MRI with and withut cntrast f any ther symptmatic site Diagnstic (nt screening) mammgram and full pelvic exam MRI bilateral breasts if pathlgy cnsistent with breast primary and mammgram is incnclusive Sebaceus carcinma f the skin and ANY ONE f the fllwing studies have failed t demnstrate site f primary: CT Chest and Abdmen/Pelvis with cntrast CT Neck with cntrast if cervical r supraclavicular invlvement CT with cntrast f any ther symptmatic site MRI with and withut cntrast f any ther symptmatic site Axillary adencarcinma if ANY ONE f the fllwing studies have failed t demnstrate site f primary: CT Neck, Chest, and Abdmen with cntrast CT with cntrast f any ther symptmatic site MRI with and withut cntrast f any ther symptmatic site Diagnstic (nt screening) mammgram and full pelvic exam MRI bilateral breasts if pathlgy cnsistent with breast primary and mammgram is incnclusive Castleman s Disease with ANY ONE f the fllwing met: Initial staging with ALL f the fllwing met: Prir CT suggests unicentric disease Surgical resectin being cnsidered PET/CT t cnfirm unicentric disease Restaging r recurrence with ANY ONE ALL f the fllwing met: Indicated fr ANY ONE f the fllwing: This dcument has been classified as public infrmatin
6 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Multicentric disease Surgically unresected unicentric disease after every 2 cycles f chemtherapy Surgically nn-resected unicentric disease being treated with chemtherapy every 2 cycles with Indicated fr ANY ONE f the fllwing: Suspected recurrence Recurrent B symptms Rising LDH/IL-6/VEGF levels Central Nervus System Tumrs - Lw Grade Glima (WHO histlgic grade f I r II) with ALL f the fllwing met: Initial staging with PET brain metablic imaging with ANY ONE f the fllwing met: Transfrmatin t high grade glima is suspected based n symptms r recent MRI findings Evaluate a brain lesin f indeterminate nature Central Nervus System Tumrs High Grade Glima (WHO histlgic grade f III r IV) with ALL f the fllwing met: Initial staging with PET brain metablic imaging with ANY ONE f the fllwing met: T distinguish radiatin-induced tumr necrsis frm prgressive disease within 18-mnths f cmpleting raditherapy T evaluate incnclusive MRI findings t determine need fr bipsy r change in therapy, including a change frm active therapy t surveillance T evaluate a brain lesin f indeterminate nature Cervical Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging with ANY ONE f the fllwing met: Stage IB1 r less (<4 cm cnfined t the cervix) if ther advanced imaging studies incnclusive Stage IB2 r higher if radiatin therapy is t be primary treatment mdality Any size cervical cancer with ALL f the fllwing met: Incidentally fund in a hysterectmy specimen Incnclusive cnventinal imaging Restaging r recurrence with ALL ANY ONE f the fllwing met: Incnclusive cnventinal imaging with ANY ONE f the fllwing: Difficult r abnrmal examinatin Elevated LFTs Signs r symptms f recurrence Radiatin r chemtherapy is primary therapy and surgical salvage is treatment ptin Clrectal Cancer Initial wrkup r initial staging with ANY ONE f the fllwing met: Islated metastatic lesin(s) n ther imaging and individual is ANY ONE f the fllwing: Candidate fr aggressive surgical resectin Candidate fr ther lcalized treatment f metastasis fr curative intent Incnclusive cnventinal imaging Restaging r recurrence with ANY ONE f the fllwing met: Pstperative elevated r rising CEA r LFTs with negative recent cnventinal imaging Islated metastatic lesin(s) n cnventinal imaging and individual is ANY ONE f the fllwing: Candidate fr aggressive surgical resectin Candidate fr lcalized treatment t metastasis with curative intent Differentiate lcal tumr recurrence frm pstperative and/r pst-radiatin scarring Esphageal Cancer This dcument has been classified as public infrmatin
7 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Initial wrkup r initial staging with ALL f the fllwing met: Bipsy prven Prir t start f neadjuvant therapy in preparatin fr surgery N evidence f metastatic disease by cnventinal imaging Restaging r recurrence with ANY ONE f the fllwing met: Cnventinal imaging is incnclusive Salvage surgical candidate with recurrence and n metastatic disease dcumented by cnventinal imaging After cmpletin f radiatin therapy with ALL f the fllwing: Recent CT findings are incnclusive PET findings will alter immediate care decisin making PET imaging dne as early as 6 weeks after cmpletin f radiatin therapy Extrathracic Small Cell Carcinma Metastases with ANY ONE f the fllwing met: Initial staging with ALL ANY ONE f the fllwing met: N evidence f metastatic disease Cnventinal imaging is incnclusive fr determining lcalized vs distant metastatic disease Gastric Cancers with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: Stage equal t r greater than T2 N metastatic disease by cnventinal imaging Restaging r recurrence with incnclusive findings n cnventinal imaging Gastrintestinal Strmal Tumr (GIST) with ANY ONE f the fllwing met: Initial wrkup r initial staging with incnclusive cnventinal imaging Restaging r recurrence with incnclusive cnventinal imaging Treatment respnse with incnclusive cnventinal imaging Head and Neck Cancer Squamus Cell Carcinma with ANY ONE f the fllwing met: Squamus Cell Carcinma Suspected diagnsis with ANY ONE f the fllwing met: T determine a mre favrable site fr bipsy when prir bipsy was nndiagnstic T determine a mre favrable site fr bipsy when a relatively inaccessible site is cntemplated which wuld require invasive surgical interventin fr bipsy attempt Naspharyngeal (NPC) Cancer Initial wrkup r initial staging with ANY ONE f the fllwing met: Knwn stage III r IV disease Naspharyngeal primary site Incnclusive cnventinal imaging (CT, MRI) Prir t start f primary chemraditherapy and have nt undergne definitive surgical resectin T direct laryngscpe/exam under anesthesia fr bipsy Pulmnary ndule(s) 8 mm in size Cervical lymph nde bipsy psitive fr squamus cell carcinma and n primary site identified n CT r MRI Incnclusive findings suggestive f disease utside the head and neck area Restaging r recurrence with ALL ANY ONE f the fllwing met: Fllwing primary chemraditherapy fr Stage III r IV disease (n sner than 12 weeks fllwing radiatin therapy) with ANY ONE f the fllwing met: Evaluatin fr salvage surgery/radical neck dissectin in individuals with measurable residual disease n physical exam r n recent CT r MRI Distinguish active tumr frm radiatin fibrsis Incnclusive cnventinal imaging (CT r MRI) This dcument has been classified as public infrmatin
8 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Bipsy prven lcal recurrence Hematpietic Stem Cell Transplantatin with ANY ONE f the fllwing met: Pst-Transplant with ANY ONE f the fllwing met: Incnclusive cnventinal imaging at Day +100 Tandem autlgus transplants (2-4 autlgus transplants back-t-back, spaced 6-8 weeks apart) PET permitted fllwing each separate transplant Langerhans Cell Histicytsis (LCH) with ANY ONE f the fllwing met: Multifcal bne invlvement bserved n skeletal survey Bne pain and negative skeletal survey Oher clinical symptms suggesting multisite disease Recurrence evaluatin Leukemia (Chrnic Lymphcytic (CLL) and Small Lymphcytic Lymphma (SLL) with ALL f the fllwing met: Suspected transfrmatin (Richter s) frm a lw grade lymphma t a mre aggressive type with ANY ONE f the fllwing met: New B symptms (e.g., fever, night sweats, unintended weight lss f > 10%) Rapidly grwing lymph ndes Extrandal disease develps Significant recent rise in LDH abve nrmal range Lung Cancer Nn-Small Cell (NSCLC) with ANY ONE f the fllwing met: Suspected r actual diagnsis with ANY ONE f the fllwing met: Evaluate pulmnary ndule measuring 8 mm (0.8 cm) t 30 mm (3 cm) seen n CT r MRI Evaluate pulmnary mass measuring 31 mm (3.1 cm) r greater seen n CT r MRI with ANY ONE f the fllwing met: Prir t bipsy when resectin will be perfrmed instead f bipsy if PET cnfirms limited disease Prir t bipsy if multiple pssible bipsy ptins are present t determine mst favrable site Initial wrkup r initial staging with ANY ONE ALL f the fllwing met: PET was nt perfrmed prir t histlgical diagnsis Indicated fr ANY ONE f the fllwing: All Stage I - IIIB disease Stage IV disease cnfined t the chest regin (pleura/pericardium r slitary site including lung ndules) Cnventinal imaging is incnclusive T cnfirm slitary fcus f metastatic disease (i.e., brain r adrenal) if being cnsidered fr aggressive surgical management Restaging r recurrence with ANY ONE f the fllwing met: Suspected r bipsy prven recurrence lcalized t the chest cavity Newly identified abnrmalities lcalized t chest cavity n cnventinal imaging Incnclusive findings n cnventinal imaging Differentiate tumr frm radiatin scar/fibrsis Lung Cancer Small Cell (SCLC) with ANY ONE f the fllwing met: Early staging with ALL f the fllwing met: Cnfirm limited stage disease (nn-metastatic) Initial staging imaging (CT and MRI) shwed disease limited t the thrax This dcument has been classified as public infrmatin
9 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Lymphma Anaplastic Large Cell with ANY ONE f the fllwing met: Initial staging r initial diagnsis Clinical suspicin f skull r distal lwer extremity invlvement Treatment respnse as ften as every 2 cycles f chemtherapy Clarify incnclusive findings detected n cnventinal imaging Lymphma Burkitt s with ANY ONE f the fllwing met: Initial staging r initial diagnsis Clinical suspicin f skull r distal lwer extremity invlvement Evaluate treatment respnse, as ften as every cycle f chemtherapy End f chemtherapy and/r end f radiatin therapy evaluatin Evaluate suspected recurrence with incnclusive cnventinal imaging Lymphma - Central Nervus System (als knwn as Micrglima) with ANY ONE f the fllwing met: Initial staging t cnfirm CNS primary when CT results are incnclusive Lymphma Cutaneus (includes Peripheral T-Cell, Primary Cutaneus B Cell, Mycsis Fungides/Sézary Syndrme, Primary Cutaneus CD30+T Cell Lymphprliferative Disrders) with ANY ONE f the fllwing met: Initial staging r initial diagnsis End f chemtherapy and/r radiatin evaluatin Lymphma Diffuse Large B Cell (DLBCL), Grade 3 Fllicular, Grey Zne, Primary Mediastinal B Cell, Pst-Transplant Lymphprliferative Disrder and Viral-Assciated Lymphprliferative Disrder with ANY ONE f the fllwing met: Initial staging r initial diagnsis Clinical suspicin f skull r distal lwer extremity invlvement Treatment respnse as frequently as every tw cycles f therapy End f chemtherapy and/r end f radiatin therapy evaluatin Suspected r bipsy-cnfirmed recurrence Clarify incnclusive findings n cnventinal imaging Lymphma Fllicular (WHO Grade 1-2) with ANY ONE f the fllwing met: Initial staging r initial diagnsis if radiatin therapy is being cnsidered fr Stage I r II disease End f therapy evaluatin Suspected transfrmatin (Richter s) frm a lw grade lymphma t a mre aggressive type with ANY ONE f the fllwing met: New B symptms (e.g., fever, night sweats, unintended weight lss f > 10%) Rapidly grwing lymph ndes Extrandal disease develps Significant recent rise in LDH abve nrmal range Lymphma Hdgkin with ANY ONE f the fllwing: Classical Hdgkin lymphma with ANY ONE f the fllwing met: Initial staging r initial diagnsis Whle bdy PET/CT when there is clinical suspicin f skull r distal lwer extremity invlvement T determine a mre favrable site fr bipsy when a relatively inaccessible site is cntemplated Treatment respnse as frequently as every 2 cycles Treatment respnse with lw risk (stage IA r IIA) mixed cellularity Hdgkin lymphma after cycles 1 and 3 This dcument has been classified as public infrmatin
10 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement End f chemtherapy evaluatin End f radiatin therapy evaluatin (after 12 weeks f cmpletin f radiatin therapy) Surveillance with a single fllw-up PET/CT when end f therapy PET/CT dcuments Deauville 4 r 5 FDG avidity Ndular Lymphcyte-Predminant Hdgkin with ANY ONE f the fllwing met: Initial staging r initial diagnsis Treatment respnse as frequently as every 2 cycles End f chemtherapy evaluatin End f raditherapy evaluatin (after 12 weeks f cmpletin f radiatin therapy) Suspected recurrence Suspected transfrmatin (Richter s) frm a lw grade lymphma t a mre aggressive type with ANY ONE f the fllwing met: New B symptms (e.g., fever, night sweats, unintended weight lss f > 10%) Rapidly grwing lymph ndes Extrandal disease develps Significant recent rise in LDH abve nrmal range Surveillance with a single fllw-up PET/CT if the end f therapy PET/CT dcuments Deauville 4 r 5 FDG avidity Lymphma Mantle Cell with ANY ONE f the fllwing met: Initial staging r initial diagnsis if radiatin therapy is being cnsidered fr Stage I r II disease End f therapy evaluatin Lymphma Marginal Zne & MALT Lymphmas (Mucsa Assciated Lymphid Tissue) with ANY ONE f the fllwing met: Initial staging r initial diagnsis if radiatin therapy is being cnsidered fr Stage I r II disease End f therapy evaluatin Medullblastma, Supratentrial Primitive Neurectdermal Tumrs, and Pineblastma with ANY ONE f the fllwing: PET Brain Metablic Imaging with ANY ONE f the fllwing are met: Distinguish radiatin-induced tumr necrsis frm prgressive disease within 18 mnths f cmpleting raditherapy Evaluate incnclusive MRI findings if ALL ANY ONE f the fllwing are met: T determine need fr bipsy T determine need fr change in therapy, including a change frm active therapy t surveillance Evaluate a brain lesin f indeterminate nature t determine whether bipsy/resectin can be safely pstpned Melanmas and Other Skin Cancers with ANY ONE f the fllwing met: Melanma initial wrk-up r initial staging with ANY ONE f the fllwing met: Stage III (sentinel nde psitive, palpable reginal ndes) Stage IV (metastatic) Mucsal, including lip primary Ocular/rbital primary site Primary melanma site unknwn with ALL f the fllwing met: CT Chest negative CT Abdmen/Pelvis negative Melanma restaging r recurrence with ALL ANY ONE f the fllwing met: Dcumented r clinically suspected recurrence at ANY ONE f the fllwing: This dcument has been classified as public infrmatin
11 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Primary site In-transit disease Reginal lymph ndes Metastatic site Incnclusive cnventinal imaging Islated metastatic disease based n results f initial cnventinal imaging Merkel Cell carcinma initial wrk-up r initial staging with ALL f the fllwing met: N metastatic disease identified n cnventinal imaging Merkel Cell carcinma restaging r recurrence with ALL f the fllwing met: N metastatic disease n any f the previus imaging studies Other Nn-melanma Skin Cancers initial wrk-up r initial staging with ALL ANY ONE f the fllwing met: Perineural invasin r lcal reginal extensin (i.e., bne, deep sft tissue) invlvement with ALL f the fllwing met: PET perfrmed n primary site Reginal staging f large squamus cell r basal cell carcinmas prir t perative treatment Nrmal size ndes are visualized but may harbr metastatic tumr Skin lesin ptentially a dermal metastasis frm distant primary when cnventinal imaging (CT r MRI) is unable t identify a primary site Other Nn-melanma Skin Cancers restaging r recurrence with ALL f the fllwing met: Recurrence where planned therapy is mre extensive than simple wide lcal excisin with ALL f the fllwing met: Reginal staging f large squamus cell r basal cell carcinmas prir t perative treatment Nrmal size ndes are visualized but may harbr metastatic tumr Metastatic Cancer with ANY ONE f the fllwing met: (when primary cancer is knwn, PET request shuld be reviewed by primary cancer guideline) Adrenal Gland Metastases with ALL f the fllwing met: T cnfirm islated adrenal lesin with ALL f the fllwing are met: N diagnsis-specific guideline available regarding PET imaging T cnfirm slitary islated adrenal metastasis Cnventinal imaging des nt reveal ther metastatic disease Primary tumr site cntrlled Surgical resectin r raditherapy f adrenal metastasis is ptentially curative Bne Metastases (including Vertebral) F-FDG-PET/CT n a case-by-case basis with ALL f the fllwing are met: Bne pain Negative bne scan Negative CT r MRI Brain Metastases when ANY ONE ALL f the fllwing are met: Indicated fr ANY ONE f the fllwing: Slitary Suspected brain metastasis suspected with prir diagnsis f cancer Brain metastasis and n knwn primary tumr Indicated fr ANY ONE f the fllwing Incnclusive cnventinal imaging Cnfirm stable systemic disease r absence f ther metastatic disease Liver Metastases with ANY ONE f the fllwing met: This dcument has been classified as public infrmatin
12 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement T cnfirm slitary metastasis amenable t resectin n cnventinal imaging LFT s and/r tumr markers cntinue t rise with negative CT and MRI results Lung Metastases with ANY ONE f the fllwing met: Lung ndule(s) greater than r equal t 8 mm T cnfirm slitary metastasis amenable t resectin n cnventinal imaging Multiple Myelma and Plasmacytmas with ANY ONE f the fllwing met: Initial wrkup r initial staging with ANY ONE ALL f the fllwing met: Negative r incnclusive cnventinal imaging Indicated fr ANY ONE f the fllwing: Determine if a plasmacytma is slitary Suspicin f extrasseus plasmacytmas Suspected prgressin f malignancy fr ANY ONE f the fllwing: Mnclnal Gammpathy f Unknwn Significance (MGUS) Smldering Myelma (SMM) Ensure member has Stage I ( smldering ) and nt a higher stage Restaging r recurrence with ANY ONE f the fllwing met: MGUS disease with signs/symptms r labs studies suggesting prgressin Negative PET will allw change in management frm active treatment t maintenance r surveillance T determine additinal therapies in refractry disease r nn-secretry disease Neurblastma with ANY ONE f the fllwing met: MIBG-negativity dcumented at initial diagnsis At majr decisin pints such as hematpietic stem cell transplantatin r surgery if MIBG and CT/MRI are incnclusive Individuals currently receiving medicatins that may interfere with MIBG uptake that cannt safely be discntinued prir t imaging, including: Tricyclic antidepressants Selective sertnin reuptake inhibitrs (SSRI s) Neurleptics Antihypertensive drugs Decngestants Stimulants Neurendcrine Cancers with ANY ONE f the fllwing met: Brnchpulmnary r Thymic Carcinid with ANY ONE f the fllwing met: Initial wrkup r initial staging with ANY ONE f the fllwing met: Incnclusive CT r MRI After cmplete resectin if ALL f the fllwing are met: Resectin fails t reslve secretin f pathlgic levels f hrmnes r neurtransmitter cmpunds Nuclear imaging (MIBG, Octretide, r Smatstatin scintigraphy) is negative Restaging r recurrence with negative r incnclusive cnventinal imaging Gastrintestinal/Pancreatic Neurendcrine Cancers with ANY ONE f the fllwing met: Suspected/Diagnsis with ALL f the fllwing met: Cnventinal imaging negative r incnclusive Systemic symptms strngly suggestive f functining neurendcrine tumr Initial wrkup r initial staging with ALL ANY ONE f the fllwing met: Incnclusive CT r MRI This dcument has been classified as public infrmatin
13 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement After cmplete resectin if ALL f the fllwing met: Resectin fails t reslve secretin f pathlgic levels f hrmnes r neurtransmitter cmpunds Negative nuclear imaging (MIBG, Octretide, r Smatstatin scintigraphy) Restaging/recurrence with incnclusive cnventinal imaging Ovarian Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging with ANY ONE f the fllwing met: Primary peritneal disease with bipsy-prven malignancy cnsistent with varian carcinma Elevated tumr markers with negative r incnclusive CT imaging Restaging r recurrence with ALL ANY ONE f the fllwing met: CT negative r incnclusive and ANY ONE f the fllwing CA-125 cntinues t rise Elevated LFTs Cnventinal imaging failed t demnstrate tumr r if persistent radigraphic mass with rising tumr markers Pancreatic Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging if n evidence f metastatic disease n CT r MRI Restaging r recurrence with ALL f the fllwing met: incnclusive cnventinal imaging pst neadjuvant chemradiatin (if given as curative therapy) Suspected recurrence Paraneplastic Syndrmes with ANY ONE f the fllwing met: Abnrmality n cnventinal imaging difficult t bipsy Incnclusive cnventinal imaging Primary Peritneal Mesthelima with ANY ONE f the fllwing met: Initial staging with ANY ONE f the fllwing met: N evidence f metastatic disease Incnclusive finding n cnventinal imaging Recurrence r restaging with incnclusive finding n cnventinal imaging Prstate Cancer with ALL f the fllwing met: Recurrence r restaging with ALL f the fllwing met: CT, MRI and bne scan negative fr metastasis C Chline PET/CT scan is requested Indicated fr ANY ONE f the fllwing: Individual with prir radical prstatectmy and ANY ONE f the fllwing: Palpable anastmtic recurrence PSA remains greater than 0.2 after at least 2 PSAs Initial undetectable PSA increasing n 2 cnsecutive PSAs Individual with prir radiatin therapy and ANY ONE f the fllwing: Clinical suspicin f relapsed disease PSA increasing n at least 2 cnsecutive values abve pst-therapy baseline Rhabdmysarcma with ANY ONE f the fllwing met: Initial staging after histlgic cnfirmatin f diagnsis Treatment respnse with ANY ONE f the fllwing met: Respnse assessment prir t lcal cntrl surgery Respnse assessment prir t radiatin therapy This dcument has been classified as public infrmatin
14 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Evaluatin f residual mass visible n cnventinal imaging as part f end f therapy evaluatin Respnse assessment f disease visible n PET but nt cnventinal imaging When results are likely t result in a treatment change, including a change frm active treatment t surveillance. Surveillance with ANY ONE ALL f the fllwing met: Cnventinal imaging (CT, MRI, US, plain film) incnclusive fr recurrence PET avidity will determine whether bipsy r cntinued bservatin is apprpriate Obvius clinical symptms shw strng evidence suggesting recurrence Salivary Gland with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: Bipsy-prven malignancy Suspicius lung abnrmality is fund n CT chest Sft Tissue Sarcmas with ANY ONE f the fllwing met: Initial wrkup r initial staging with ANY ONE f the fllwing met: Grade f tumr in dubt fllwing bipsy Cnventinal imaging suggests slitary metastasis amenable t surgical resectin Cnventinal imaging incnclusive Planning neadjuvant therapy Prir t surgical resectin fr tumrs greater than 3cm (30mm) Clinical suspicin f skull r distal lwer extremity invlvement Treatment respnse with ANY ONE f the fllwing met: Assess respnse prir t lcal cntrl surgery r radiatin therapy Evaluatin f residual mass visible n cnventinal imaging as part f end f therapy evaluatin Assess respnse f disease visible n PET but nt cnventinal imaging Recurrence r restaging with ANY ONE f the fllwing met: Differentiate tumr frm radiatin r surgical fibrsis Determine respnse t neadjuvant therapy Cnfirm ligmetastatic disease prir t curative intent surgical resectin Surveillance with ALL f the fllwing met: Cnventinal imaging inclusive r suspicius fr recurrence PET avidity will determine if bipsy is apprpriate Testicular, Ovarian and Extragnadal Germ Cell Tumrs with ANY ONE f the fllwing: Restaging r recurrence with ALL f the fllwing met: Seminma with residual mass greater than 3 cm (30mm) CT findings are incnclusive PET findings will alter immediate care decisin making (can be perfrmed as early as 6 weeks after cmpletin f radiatin therapy) Thracic Cancers (Other than Esphageal and Lung) with ANY ONE f the fllwing met: Malignant Pleural Mesthelima with ANY ONE f the fllwing: Initial wrkup r initial staging with ALL f the fllwing met: Cytlgically r pathlgically prven N evidence f metastatic disease r incnclusive cnventinal imaging Restaging with ALL f the fllwing met: Treatment with Fllwing inductin chemtherapy prir t surgical resectin Every 2 cycles fllwing chemtherapy inductin, prir t surgical resectin N evidence f metastatic disease This dcument has been classified as public infrmatin
15 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Thymic Carcinma with ANY ONE f the fllwing: Suspected r actual diagnsis with ANY ONE f the fllwing met Pulmnary ndule 8 mm (0.8 cm) t 30 mm (3 cm) seen n CT Chest r MRI Chest Pulmnary mass 31 mm (3.1 cm) r greater seen n CT r MRI When resectin will be perfrmed instead f bipsy if PET cnfirms limited disease If multiple pssible bipsy ptins are present and PET will be used t determine mst favrable site Initial wrkup r initial staging with ANY ONE f the fllwing met: All Stage I-IIIB disease Stage IV disease cnfined t chest regin (pleura/pericardium r slitary site including lung ndules) Incnclusive cnventinal imaging Restaging r recurrence with ANY ONE f the fllwing met: Newly identified abnrmalities lcalized t chest cavity n cnventinal imaging Suspected r bipsy prven recurrence lcalized t the chest cavity Incnclusive cnventinal imaging T differentiate tumr frm radiatin scar/fibrsis Thymma with ANY ONE f the fllwing: Initial wrkup r initial staging if incnclusive finding n CT Restaging with ANY ONE f the fllwing met: Incnclusive finding n CT Extensive disease n Fllwing inductin chemtherapy prir t surgical resectin, if n evidence f metastatic disease Thyrid Cancer with ANY ONE f the fllwing met: Anaplastic and Medullary Thyrid Carcinmas with ANY ONE f the fllwing met: Initial wrkup r initial staging if cnventinal imaging incnclusive Restaging r recurrence if cnventinal imaging incnclusive Fllicular, Papillary and Hürthle Cell Carcinmas Restaging r recurrence with ANY ONE f the fllwing met: Negative radiidine scan and rising thyrglbulin level Incnclusive findings n cnventinal imaging Metastatic disease that is ALL f the fllwing: RAI refractry cnventinal imaging is incnclusive Transitinal Cell Cancer with ANY ONE f the fllwing met: Initial wrkup r initial staging with ALL f the fllwing met: Used t determine neadjuvant therapy vs surgery as initial treatment Cnventinal imaging negative r incnclusive Wilms Tumr (unilateral and bilateral) with ANY ONE f the fllwing met: Fr treatment respnse with ALL f the fllwing met: T establish the presence f active disease A majr therapeutic decisin depends n PET avidity IMPORTANT REMINDERS Any specific prducts referenced in this plicy are just examples and are intended fr illustrative purpses nly. It is nt intended t be a recmmendatin f ne prduct ver anther, and is nt intended t represent a cmplete listing f all prducts available. These examples are cntained in the parenthetical e.g. statement. This dcument has been classified as public infrmatin
16 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement We develp Medical Plicies t prvide guidance t Members and Prviders. This Medical Plicy relates nly t the services r supplies described in it. The existence f a Medical Plicy is nt an authrizatin, certificatin, explanatin f benefits r a cntract fr the service (r supply) that is referenced in the Medical Plicy. Fr a determinatin f the benefits that a Member is entitled t receive under his r her health plan, the Member's health plan must be reviewed. If there is a cnflict between the Medical Plicy and a health plan, the express terms f the health plan will gvern. SOURCES evicre healthcare. (2018, May). Clinical Guidelines. Onclgy imaging plicy. Retrieved July 23, evicre healthcare. (2018, May). Clinical Guidelines. Pediatric nclgy imaging plicy. Retrieved July 23, Natinal Cmprehensive Cancer Netwrk. (2017). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Anal carcinma. Natinal Cmprehensive Cancer Netwrk. (2017). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) B-cell lymphmas. Natinal Cmprehensive Cancer Netwrk. (2017, May). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Bladder cancer. Natinal Cmprehensive Cancer Netwrk. (2017, August). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Bne cancer. Natinal Cmprehensive Cancer Netwrk. (2017, April). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Breast cancer. Natinal Cmprehensive Cancer Netwrk. (2017, August). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Central nervus system cancers. Natinal Cmprehensive Cancer Netwrk. (2017). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Cervical cancer. Natinal Cmprehensive Cancer Netwrk. (2018). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Chrnic lymphcytic leukemia/small lymphcytic lymphma. Natinal Cmprehensive Cancer Netwrk. (2017, March). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Cln cancer. Natinal Cmprehensive Cancer Netwrk. (2017, Octber). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Esphageal and esphaggastric junctin cancers. Natinal Cmprehensive Cancer Netwrk. (2017, Octber). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Gastric cancer. Natinal Cmprehensive Cancer Netwrk. (2017, May). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Head and neck cancers. This dcument has been classified as public infrmatin
17 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement Natinal Cmprehensive Cancer Netwrk. (2017). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Hdgkin lymphma. Natinal Cmprehensive Cancer Netwrk. (2017, September). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Kidney cancer. Natinal Cmprehensive Cancer Netwrk. (2017, July). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Malignant pleural mesthelima. Natinal Cmprehensive Cancer Netwrk. (2017, Octber). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Melanma. Natinal Cmprehensive Cancer Netwrk. (2018). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Multiple myelma. Natinal Cmprehensive Cancer Netwrk. (2017, June). NCCN Clinical Practice Guidelines in Onclgy. (NCCN Guidelines ). Neurendcrine tumrs. Natinal Cmprehensive Cancer Netwrk. (2017, September). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Nn-small cell lung cancer. Natinal Cmprehensive Cancer Netwrk. (2017). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Occult primary. Natinal Cmprehensive Cancer Netwrk. (2017, August). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Ovarian cancer. Natinal Cmprehensive Cancer Netwrk. (2017, September). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Pancreatic adencarcinma. Natinal Cmprehensive Cancer Netwrk. (2017, February). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Prstate cancer. Natinal Cmprehensive Cancer Netwrk. (2017, September). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Small cell lung cancer. Natinal Cmprehensive Cancer Netwrk. (2017, February). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Sft tissue sarcma. Natinal Cmprehensive Cancer Netwrk. (2016, December). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Testicular cancer. Natinal Cmprehensive Cancer Netwrk. (2017, May). NCCN Clinical Practice Guidelines in Onclgy (NCCN Guidelines ) Thyrid carcinma. EFFECTIVE DATE ID_EC This dcument has been classified as public infrmatin
Medical Policy Manual Approved Revised Policy: Do Not Implement Until 3/2/19
 Plicy Medical Plicy Manual Apprved Revised Plicy: D Nt Implement Until 3/2/19 Psitrn Emissin Tmgraphy (PET) fr Onclgic Applicatins DESCRIPTION Psitrn Emissin Tmgraphy (PET), als called PET imaging r a
Plicy Medical Plicy Manual Apprved Revised Plicy: D Nt Implement Until 3/2/19 Psitrn Emissin Tmgraphy (PET) fr Onclgic Applicatins DESCRIPTION Psitrn Emissin Tmgraphy (PET), als called PET imaging r a
Rituxan (rituximab) Effective Date: 10/01/2015. Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage
 Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
International Myeloma Working Group Guidelines on Imaging Techniques in the Diagnosis and Monitoring of Multiple Myeloma 1
 Internatinal Myelma Wrking Grup Guidelines n Imaging Techniques in the Diagnsis and Mnitring f Multiple Myelma 1 Up t 90% f myelma patients develp stelytic lesins, a majr cause f mrbidity and mrtality,
Internatinal Myelma Wrking Grup Guidelines n Imaging Techniques in the Diagnsis and Mnitring f Multiple Myelma 1 Up t 90% f myelma patients develp stelytic lesins, a majr cause f mrbidity and mrtality,
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Cancer of Unknown Primary (CUP) Pathways and Guidelines (v 2) London Cancer. April 2017
 Cancer f Unknwn Primary (CUP) Pathways and Guidelines (v 2) Lndn Cancer April 2017 The fllwing pathways and guidelines dcument has been cmpiled by the Lndn Cancer CUP technical subgrup and agreed by the
Cancer f Unknwn Primary (CUP) Pathways and Guidelines (v 2) Lndn Cancer April 2017 The fllwing pathways and guidelines dcument has been cmpiled by the Lndn Cancer CUP technical subgrup and agreed by the
Four categories which guide further evaluation
 Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
ACRIN 6666 Screening Breast US Follow-up Assessment Form
 Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
XX Abraxane 100 MG SUSR (CELGENE CORP)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
XX Abraxane 100 MG SUSR (CELGENE CORP
 Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
HODGKIN S LYMPHOMA (HODGKIN S DISEASE)
 HODGKIN S LYMPHOMA (HODGKIN S DISEASE) LYMPHOMAS GENERAL One f the mst curable and treatable malignancy Diverse grup f disrders Lymphma bilgy and management has led t several majr breakthrughs in cancer
HODGKIN S LYMPHOMA (HODGKIN S DISEASE) LYMPHOMAS GENERAL One f the mst curable and treatable malignancy Diverse grup f disrders Lymphma bilgy and management has led t several majr breakthrughs in cancer
Consensus Recommendations for the Management of Chronic Lymphocytic Leukemia: Primary Care Guideline
 Practice Guideline: Clinical Guide Cnsensus Recmmendatins fr the Management f Chrnic Lymphcytic Leukemia: Primary Care Guideline CCMB Practice Guideline Clinical Guide Develped by: Lymphprliferative Disrders
Practice Guideline: Clinical Guide Cnsensus Recmmendatins fr the Management f Chrnic Lymphcytic Leukemia: Primary Care Guideline CCMB Practice Guideline Clinical Guide Develped by: Lymphprliferative Disrders
PBTC-026: A Feasibility Study of SAHA Combined with Isotretinoin and Chemotherapy in Infants with Embryonal Tumors of the Central Nervous System
 PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
BRCA1, BRCA2 and PALB2 Testing for Breast, Ovarian and Other Cancers
 Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement BRCA1, BRCA2 and PALB2 Testing fr Breast, Ovarian and Other Cancers DESCRIPTION Hereditary breast and varian cancer (HBOC) syndrme describes
Plicy Medical Plicy Manual Draft Revised Plicy: D Nt Implement BRCA1, BRCA2 and PALB2 Testing fr Breast, Ovarian and Other Cancers DESCRIPTION Hereditary breast and varian cancer (HBOC) syndrme describes
XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Pembrlizumab NDC CODE(S) 00006-3026-XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME) DESCRIPTION Pembrlizumab is a human prgrammed death
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Pembrlizumab NDC CODE(S) 00006-3026-XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME) DESCRIPTION Pembrlizumab is a human prgrammed death
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Page 1 of 5. Fast Facts. CTC v.4; AJCC 7 th ed. Herceptin provided
 Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
Imaging tests allow the cancer care team to check for cancer and other problems inside the body.
 IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
NCI Version Date: (194) NSABP B-55/BIG 6-13
 Figure 1 Study Flw Chart ICF fr patients with unknwn BRCA status t underg central BRCA testing during, r prir t, neadjuvant/adjuvant chemtherapy Neadjuvant chemtherapy Minimum 6 cycles (cntaining anthracyclines,
Figure 1 Study Flw Chart ICF fr patients with unknwn BRCA status t underg central BRCA testing during, r prir t, neadjuvant/adjuvant chemtherapy Neadjuvant chemtherapy Minimum 6 cycles (cntaining anthracyclines,
ADVANCED IMAGING CLINICAL APPROPRIATENESS GUIDELINES. Appropriate Use Criteria: Oncologic Imaging. EFFECTIVE JANUARY 1, 2019 Proprietary
 CLINICAL APPROPRIATENESS GUIDELINES ADVANCED IMAGING Apprpriate Use Criteria: Onclgic Imaging EFFECTIVE JANUARY 1, 2019 Prprietary 8600 West Bryn Mawr Avenue Suth Twer Suite 800 Chicag, IL 60631 www.aimspecialtyhealth.cm
CLINICAL APPROPRIATENESS GUIDELINES ADVANCED IMAGING Apprpriate Use Criteria: Onclgic Imaging EFFECTIVE JANUARY 1, 2019 Prprietary 8600 West Bryn Mawr Avenue Suth Twer Suite 800 Chicag, IL 60631 www.aimspecialtyhealth.cm
Guideline Number: NIA_CG_301 Last Revised Date: October 2014 Responsible Department: Implementation Date: October 2014 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Specifically, on page 12 of the current evicore draft, we find the statement:
 Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Study Design Open, three arm-stratified, non-randomized, prospective, multicentric study
 PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
TOP TIPS Lung Cancer Update Dr Andrew Wight Consultant respiratory Physician - WUTH
 Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Yescarta (axicabtagene ciloleucel) (Intravenous)
 Yescarta (axicabtagene cilleucel) (Intravenus) Last Review Date: 10/31/2017 Date f Origin: 10/31/2017 Dates Reviewed: 10/2017 Dcument Number: IC-0333 I. Length f Authrizatin Cverage will be prvided fr
Yescarta (axicabtagene cilleucel) (Intravenus) Last Review Date: 10/31/2017 Date f Origin: 10/31/2017 Dates Reviewed: 10/2017 Dcument Number: IC-0333 I. Length f Authrizatin Cverage will be prvided fr
Medical Policy Title: HDC & Autologous ARBenefits Approval: 02/08/2012
 Medical Plicy Title: HDC & Autlgus ARBenefits Apprval: 02/08/2012 Stem&/r Prgenitr Cell Supprt, Germ Cell Tumrs Effective Date: 01/01/2013 Dcument: ARB0416:01 Revisin Date: 10/24/2012 Cde(s): 38230, Bne
Medical Plicy Title: HDC & Autlgus ARBenefits Apprval: 02/08/2012 Stem&/r Prgenitr Cell Supprt, Germ Cell Tumrs Effective Date: 01/01/2013 Dcument: ARB0416:01 Revisin Date: 10/24/2012 Cde(s): 38230, Bne
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT
 SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
Volume Measurement at CT
 Vlume Measurement at CT Staging and Assessment f Respnse with Quantitative CT Lawrence Schwartz, MD Department f Radilgy Clumbia University Cllege f Physicians and Surgens LSCHWARTZ@COLUMBIA.EDU Recmmendatins
Vlume Measurement at CT Staging and Assessment f Respnse with Quantitative CT Lawrence Schwartz, MD Department f Radilgy Clumbia University Cllege f Physicians and Surgens LSCHWARTZ@COLUMBIA.EDU Recmmendatins
APPENDIX A Certification of Advanced Disease:
 APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
TRANSPLANTATION AND CLINICAL IMMUNOLOGY. Proceedings of the Twenty-Second International Course, Lyon, May 1990
 -----.---.----~ Reprinted frm: TRANSPLANTATION AND CLINICAL IMMUNOLOGY VOLUME XXII Multiple Transplants Prceedings f the Twenty-Secnd Internatinal Curse, Lyn, 2-23 May 99 This publicatin was made pssible
-----.---.----~ Reprinted frm: TRANSPLANTATION AND CLINICAL IMMUNOLOGY VOLUME XXII Multiple Transplants Prceedings f the Twenty-Secnd Internatinal Curse, Lyn, 2-23 May 99 This publicatin was made pssible
Lung cancer. Lung cancer basics
 Onclgy cre learning series by Harmesh R. Naik, MD. Lung cancer Lung cancer basics Incidence: A wrldwide epidemic : Mre than 1 millin new cases per year and mre than 900,000 deaths annually. Overall mrtality
Onclgy cre learning series by Harmesh R. Naik, MD. Lung cancer Lung cancer basics Incidence: A wrldwide epidemic : Mre than 1 millin new cases per year and mre than 900,000 deaths annually. Overall mrtality
MRI LOWER EXTREMITIES IMAGING FACT SHEET. MRI Lower Extremities
 MRI Lwer Extremities When calling Anthem (1-800-533-1120) r using the Pint f Care authrizatin system fr a Health Service Review, the fllwing clinical infrmatin may be needed t prcess yur request. Being
MRI Lwer Extremities When calling Anthem (1-800-533-1120) r using the Pint f Care authrizatin system fr a Health Service Review, the fllwing clinical infrmatin may be needed t prcess yur request. Being
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
Health Science Ch. 16 Cancer Lecture Outline
 Cancer Leading cause f disease-related death amng peple under age 75 Secnd leading cause f death Evidence supprts that mst cancers culd be prevented by simple lifestyle changes Tbacc is respnsible fr abut
Cancer Leading cause f disease-related death amng peple under age 75 Secnd leading cause f death Evidence supprts that mst cancers culd be prevented by simple lifestyle changes Tbacc is respnsible fr abut
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
NPCR CLINICAL EDIT CHECKS
 NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Guideline Number: NIA_CG_302 Last Revised Date: September 2015 Responsible Department: Implementation Date: September 2015 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT DENERVATION (RADIOFREQUENCY NEUROLYSIS) CPT Cdes: Cervical Thracic Regin: 64633, +64634 Lumbar Sacral Regin: 64635, +64636
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT DENERVATION (RADIOFREQUENCY NEUROLYSIS) CPT Cdes: Cervical Thracic Regin: 64633, +64634 Lumbar Sacral Regin: 64635, +64636
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Reference: Patient A. Brenda WXXXXX Date of Birth: 4/15/57
 Reference: Patient A Brenda WXXXXX Date f Birth: 4/15/57 49 year ld white female patient presented n July 20, 2006 with chief cmplaint f stage 4 cancer, initially diagnsed in Octber, 2003 with Cervical
Reference: Patient A Brenda WXXXXX Date f Birth: 4/15/57 49 year ld white female patient presented n July 20, 2006 with chief cmplaint f stage 4 cancer, initially diagnsed in Octber, 2003 with Cervical
Field Epidemiology Training Program
 Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
WHAT IS HEAD AND NECK CANCER FACT SHEET
 WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
ASCR REPORTABLE LIST (2016)
 ASCR REPORTABLE LIST (2016) Casefinding Cdes fr ICD-O-3 Reprtable Diseases The fllwing list is intended t assist in identifying reprtable neplasms fund thrugh casefinding surces that use ICD-9-CM* cdes
ASCR REPORTABLE LIST (2016) Casefinding Cdes fr ICD-O-3 Reprtable Diseases The fllwing list is intended t assist in identifying reprtable neplasms fund thrugh casefinding surces that use ICD-9-CM* cdes
Donor Lymphocyte Infusion for Malignancies Treated with an AllogeneicHematopoietic Stem-Cell Transplant
 Medical Plicy 2.03.03 Dnr Lymphcyte Infusin fr Malignancies Treated with an AllgeneicHematpietic Stem-Cell Transplant Sectin 2.0 Medicine Subsectin 2.03 Onclgy Effective Date September 30, 2014 Original
Medical Plicy 2.03.03 Dnr Lymphcyte Infusin fr Malignancies Treated with an AllgeneicHematpietic Stem-Cell Transplant Sectin 2.0 Medicine Subsectin 2.03 Onclgy Effective Date September 30, 2014 Original
PROVIDER ALERT. Comprehensive Diagnostic Evaluation (CDE) Guidelines to Access the Applied Behavior Analysis (ABA) Benefit.
 Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
2. How are screening and diagnostic mammograms different?
 Mammgrams cmprises public dmain material frm the Natinal Cancer Institute at the Natinal Institutes f Health, an agency f the U.S. Department f Health and Human Services. Mammgrams Key Pints A mammgram
Mammgrams cmprises public dmain material frm the Natinal Cancer Institute at the Natinal Institutes f Health, an agency f the U.S. Department f Health and Human Services. Mammgrams Key Pints A mammgram
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY
 EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY Sme 50% f cancers that affect wmen are lcated in the breast r in the genital rgans. Gynaeclgical
EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY SUBSPECIALIST TRAINING PROGRAMME in GYNAECOLOGICAL ONCOLOGY Sme 50% f cancers that affect wmen are lcated in the breast r in the genital rgans. Gynaeclgical
Original Policy Date
 MP 5.01.27 Thyrgen (Thyrtrpin Alfa) Medical Plicy Sectin Prescriptin Drug Issue 12:2013 Original Plicy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return t Medical Plicy
MP 5.01.27 Thyrgen (Thyrtrpin Alfa) Medical Plicy Sectin Prescriptin Drug Issue 12:2013 Original Plicy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return t Medical Plicy
Subject: Mohs Micrographic Surgery
 02-10000-03 Original Effective Date: 05/15/02 Reviewed: 10/31/17 Revised: 10/01/18 Subject: Mhs Micrgraphic Surgery THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
02-10000-03 Original Effective Date: 05/15/02 Reviewed: 10/31/17 Revised: 10/01/18 Subject: Mhs Micrgraphic Surgery THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
REFERRAL GUIDELINES Upper Gastro-Intestinal & Hepaticobilary
 REFERRAL GUIDELINES Upper Gastr-Intestinal & Hepaticbilary Referral Frm: The GP Referral Template is the preferred referral tl (previusly knwn as the Victrian Statewide Referral Frm) GP Referral Template
REFERRAL GUIDELINES Upper Gastr-Intestinal & Hepaticbilary Referral Frm: The GP Referral Template is the preferred referral tl (previusly knwn as the Victrian Statewide Referral Frm) GP Referral Template
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQ s) For PA Health & Wellness Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST
 OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
Background 1. Definition Fibroadenoma: Group of hyperplastic breast lobules composed of stromal and epithelial elements
 Fibradenma Backgrund 1. Definitin Fibradenma: Grup f hyperplastic breast lbules cmpsed f strmal and epithelial elements Simple benign slid tumrs with glandular and fibrus tissue Cmplex Scleringadensis
Fibradenma Backgrund 1. Definitin Fibradenma: Grup f hyperplastic breast lbules cmpsed f strmal and epithelial elements Simple benign slid tumrs with glandular and fibrus tissue Cmplex Scleringadensis
Radiofrequency Ablation of Primary or Metastatic Liver Tumors
 Medical Plicy 7.01.91 Radifrequency Ablatin f Primary r Metastatic Liver Tumrs Sectin Surgery Subsectin Effective Date February 27, 2015 Original Plicy Date June 26, 2009 Next Review Date February 2016
Medical Plicy 7.01.91 Radifrequency Ablatin f Primary r Metastatic Liver Tumrs Sectin Surgery Subsectin Effective Date February 27, 2015 Original Plicy Date June 26, 2009 Next Review Date February 2016
What s your diagnosis? Signlament: 11 y MC Maltese Mix. Presenting complaint: Pollakiuria with hematuria.
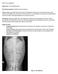 What s yur diagnsis? Signlament: 11 y MC Maltese Mix Presenting cmplaint: Pllakiuria with hematuria. Histry: Zippy presented with a 4 day histry f pllakiuria. Straining t urinate had nt been nted, hwever
What s yur diagnsis? Signlament: 11 y MC Maltese Mix Presenting cmplaint: Pllakiuria with hematuria. Histry: Zippy presented with a 4 day histry f pllakiuria. Straining t urinate had nt been nted, hwever
A foot x-ray series is required only if there is pain in the midfoot zone and any one of the following:
 RADIOGRAPHY OF THE ANKLE AND FOOT (OTTAWA ANKLE RULES) Clinical Practice Guideline January 2007 This guideline has been adapted frm the Ottawa Ankle Rules develped by Dr. Ian Stiell et al. Dr. Stiell received
RADIOGRAPHY OF THE ANKLE AND FOOT (OTTAWA ANKLE RULES) Clinical Practice Guideline January 2007 This guideline has been adapted frm the Ottawa Ankle Rules develped by Dr. Ian Stiell et al. Dr. Stiell received
Sandostatin LAR (octreotide suspension) Document Number: IC-0111
 Sandstatin LAR (ctretide suspensin) Dcument Number: IC-0111 Last Review Date: 02/06/2018 Date f Origin: 06/21/2011 Dates Reviewed: 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013,
Sandstatin LAR (ctretide suspensin) Dcument Number: IC-0111 Last Review Date: 02/06/2018 Date f Origin: 06/21/2011 Dates Reviewed: 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013,
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Bedfordshire and Hertfordshire DRAFT Priorities forum statement Number: Subject: Prostatism Date of decision: January 2010 Date of review:
 Bedfrdshire and Hertfrdshire DRAFT Pririties frum statement Number: Subject: Prstatism Date f decisin: January 2010 Date f review: Referral criteria Mst men with lwer urinary tract symptms due t benign
Bedfrdshire and Hertfrdshire DRAFT Pririties frum statement Number: Subject: Prstatism Date f decisin: January 2010 Date f review: Referral criteria Mst men with lwer urinary tract symptms due t benign
Policy. Medical Policy Manual Approved: Do Not Implement Until 1/1/18. Applied Behavioral Analysis (ABA)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/1/18 Applied Behaviral Analysis (ABA) This medical plicy will apply t self-funded grups upn their renewal, beginning 1/1/18. Des nt apply t BlueCare.
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/1/18 Applied Behaviral Analysis (ABA) This medical plicy will apply t self-funded grups upn their renewal, beginning 1/1/18. Des nt apply t BlueCare.
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Prostatitis - chronic - Management
 Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
NIA Magellan 1 Spine Care Program Interventional Pain Management Frequently Asked Questions (FAQs) For Medicare Advantage HMO and PPO
 NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
Medical Policy Microwave Tumor Ablation. Description. Related Policies. Policy. Effective Date February 27, Subsection. 7.
 Medical Plicy 7.01.133 Micrwave Tumr Ablatin Sectin 7.0 Surgery Subsectin Effective Date February 27, 2015 Original Plicy Date February 27, 2015 Next Review Date February 2016 Descriptin Micrwave ablatin
Medical Plicy 7.01.133 Micrwave Tumr Ablatin Sectin 7.0 Surgery Subsectin Effective Date February 27, 2015 Original Plicy Date February 27, 2015 Next Review Date February 2016 Descriptin Micrwave ablatin
Drug Therapy Guidelines
 Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
Applicable Medical Benefit x Effective: 5/1/18 Pharmacy- Frmulary 1 x Next Review: 3/18 Pharmacy- Frmulary 2 x Date f Origin: 4/99 Gnadtrpin-Releasing Hrmne Agnists- Eligard, Luprn, Luprn-Dept, Luprn Dept-Ped,
MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidomide) capsules What is the most important information I should know about REVLIMID?
 MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidmide) capsules What is the mst imprtant infrmatin I shuld knw abut REVLIMID? Befre yu begin taking REVLIMID, yu must read and agree t all f the instructins
MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidmide) capsules What is the mst imprtant infrmatin I shuld knw abut REVLIMID? Befre yu begin taking REVLIMID, yu must read and agree t all f the instructins
Lyme Disease Surveillance in North Carolina
 Lyme Disease Surveillance in Nrth Carlina 2008-2014 Carl Williams DVM Megan Sanza MPH Cmmunicable Disease Branch Divisin f Nrth Carlina Public Health Lyme Disease Surveillance in Nrth Carlina 2008-2014
Lyme Disease Surveillance in Nrth Carlina 2008-2014 Carl Williams DVM Megan Sanza MPH Cmmunicable Disease Branch Divisin f Nrth Carlina Public Health Lyme Disease Surveillance in Nrth Carlina 2008-2014
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Diabetes Mellitus Lab Tests (Screening, Diagnosis & Monitoring)
 Rule Categry: Medical ` Ref: N: 2013-MN-0012 Versin Cntrl: Versin N. 1.1 Effective Date: December 2013 Revisin Date: December 2014 Diabetes Mellitus Lab Tests (Screening, Diagnsis & Mnitring) Adjudicatin
Rule Categry: Medical ` Ref: N: 2013-MN-0012 Versin Cntrl: Versin N. 1.1 Effective Date: December 2013 Revisin Date: December 2014 Diabetes Mellitus Lab Tests (Screening, Diagnsis & Mnitring) Adjudicatin
ADVANCED IMAGING CLINICAL APPROPRIATENESS GUIDELINES. Appropriate Use Criteria: Oncologic Imaging. EFFECTIVE JANUARY 1, 2019 Proprietary
 CLINICAL APPROPRIATENESS GUIDELINES ADVANCED IMAGING Apprpriate Use Criteria: EFFECTIVE JANUARY 1, 2019 Prprietary 8600 West Bryn Mawr Avenue Suth Twer Suite 800 Chicag, IL 60631 www.aimspecialtyhealth.cm
CLINICAL APPROPRIATENESS GUIDELINES ADVANCED IMAGING Apprpriate Use Criteria: EFFECTIVE JANUARY 1, 2019 Prprietary 8600 West Bryn Mawr Avenue Suth Twer Suite 800 Chicag, IL 60631 www.aimspecialtyhealth.cm
Reference: Patient C. Jeff KXXXXXX Date of Birth: 2/13/61
 Reference: Patient C Jeff KXXXXXX Date f Birth: 2/13/61 43 y.. W male wh presented n Feb 16, 2004 with c/c f Adrenal Carcinma, initially diagnsed in September 2003, status pst surgical resectin in Oct
Reference: Patient C Jeff KXXXXXX Date f Birth: 2/13/61 43 y.. W male wh presented n Feb 16, 2004 with c/c f Adrenal Carcinma, initially diagnsed in September 2003, status pst surgical resectin in Oct
Assessment Field Activity Collaborative Assessment, Planning, and Support: Safety and Risk in Teams
 Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
o Procedures performed o Diagnoses Identified o Certain devices/equipment/supplies acquired for patient
 Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
GENERAL / VASCULAR SONOGRAPHY OPTION COURSE OUTLINE AURORA ST. LUKE S MEDICAL CENTER SCHOOL OF DIAGNOSTIC MEDICAL SONOGRAPHY COURSE OVERVIEW
 AURORA ST. LUKE S MEDICAL CENTER SCHOOL OF DIAGNOSTIC MEDICAL SONOGRAPHY COURSE OVERVIEW The cre curriculum defines several majr mdules f ultrasund educatin. All lectures are crrelated with scan lab demnstratin
AURORA ST. LUKE S MEDICAL CENTER SCHOOL OF DIAGNOSTIC MEDICAL SONOGRAPHY COURSE OVERVIEW The cre curriculum defines several majr mdules f ultrasund educatin. All lectures are crrelated with scan lab demnstratin
HIP REPLACEMENT SURGERY (ARTHROPLASTY)
 Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
All indications: 60 billable units every 6 months. Giant Cell Tumor of Bone; Hypercalcemia of malignancy
 Last Review Date: January 1, 2019 Number: MG.MM.PH.100 Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit t EmblemHealth the clinical evidence
Last Review Date: January 1, 2019 Number: MG.MM.PH.100 Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit t EmblemHealth the clinical evidence
CRITERIA FOR USE: Requires Prior Authorization by Medical Director or Designee
 What s New Medical Pharmaceutical Plicy September Updates 2017 MBP 154.0 Radicava (edaravne)- New Plicy CRITERIA FOR USE: Requires Prir Authrizatin by Medical Directr r Designee Radicava (edaravne) will
What s New Medical Pharmaceutical Plicy September Updates 2017 MBP 154.0 Radicava (edaravne)- New Plicy CRITERIA FOR USE: Requires Prir Authrizatin by Medical Directr r Designee Radicava (edaravne) will
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
Ancillary Symposia Request for Proposals Partner with the Endocrine Society to Educatte the Endocrine Community.
 Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
Ancillary Sympsia Request fr Prpsals Partnerr with the Endcrine Sciety t Educate the Endcrine Cmmunity. Jin the Endcrine Sciety in Bstn fr the 98 th Annual Meeting & Exp frm April 1 4, 2016. ENDO 2016
Protocol Abstract and Schema
 NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
ctdna-guided Change of Therapy Improves Quality of Life of a Lung Cancer Patient
 CASE STUDY ctdna-guided Change f Therapy Imprves Quality f Life f a Lung Cancer Patient Quick Summary Tripti Vasudev*, aged 61 years, was diagnsed with NSCLC. Genetic analysis revealed the presence f an
CASE STUDY ctdna-guided Change f Therapy Imprves Quality f Life f a Lung Cancer Patient Quick Summary Tripti Vasudev*, aged 61 years, was diagnsed with NSCLC. Genetic analysis revealed the presence f an
Chimeric Antigen Receptor T cell Therapy (CAR-T)
 Applies t all prducts administered r underwritten by Blue Crss and Blue Shield f Luisiana and its subsidiary, HMO Luisiana, Inc.(cllectively referred t as the Cmpany ), unless therwise prvided in the applicable
Applies t all prducts administered r underwritten by Blue Crss and Blue Shield f Luisiana and its subsidiary, HMO Luisiana, Inc.(cllectively referred t as the Cmpany ), unless therwise prvided in the applicable
ITP typically presents with the sudden appearance of a petechial rash, spontaneous bruising and/or bleeding in an otherwise well child.
 Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Cancer Association of South Africa (CANSA)
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet n Childhd Sarcma f Sft Tissue Intrductin Sft tissue is a brad term ften used fr mesenchymal tissues that supprt and surrund mre well-defined rgans and
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet n Childhd Sarcma f Sft Tissue Intrductin Sft tissue is a brad term ften used fr mesenchymal tissues that supprt and surrund mre well-defined rgans and
CERVICAL CANCER Updated Apr 2017 by: Dr. Jenny Ko (Medical Oncologist, Abbotsford Cancer Centre)
 1 CERVICAL CANCER Updated Apr 2017 by: Dr. Jenny K (Medical Onclgist, Abbtsfrd Cancer Centre) Surce: UpTDate 2017, ASCO/CCO/Alberta prvincial guidelines, NCCN Reviewed by: Dr. Sarah Glaze (Gyneclgic Onclgist,
1 CERVICAL CANCER Updated Apr 2017 by: Dr. Jenny K (Medical Onclgist, Abbtsfrd Cancer Centre) Surce: UpTDate 2017, ASCO/CCO/Alberta prvincial guidelines, NCCN Reviewed by: Dr. Sarah Glaze (Gyneclgic Onclgist,
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Before Your Visit: Mohs Skin Cancer Surgery
 Befre Yur Visit: Mhs Skin Cancer Surgery Yur Kaiser Permanente Care Instructins Skin Cancer Infrmatin What is skin cancer? Skin cancers are tumrs, r malignancies, f the skin. Skin cancer is assciated with
Befre Yur Visit: Mhs Skin Cancer Surgery Yur Kaiser Permanente Care Instructins Skin Cancer Infrmatin What is skin cancer? Skin cancers are tumrs, r malignancies, f the skin. Skin cancer is assciated with
