Initiative for Molecular Profiling in Advanced Cancer Therapy (IMPACT) 2: Randomized Study of Biomarker-Based Treatment
|
|
|
- Margery Stokes
- 5 years ago
- Views:
Transcription
1 Initiative for Molecular Profiling in Advanced Cancer Therapy (IMPACT) 2: Randomized Study of Biomarker-Based Treatment Apostolia M. Tsimberidou, MD, PhD Associate Professor Department of Investigational Cancer Therapeutics
2 Precision Medicine A form of medicine that uses information about a person s genes, proteins, and environment to prevent, diagnose, and treat disease NCI, 2011 Personalized Medicine in Cancer Requires: 1. Identification of genetic alterations that drive carcinogenesis 2. Development of drugs that can effectively inhibit the function of the genetic alterations Molecularly targeted therapy to be used consistently and successfully in patients with cancer.
3 Precision Medicine in a Phase I Program Initiative for Molecular Profiling in Advanced Cancer Therapy (IMPACT)
4 Initiative for Molecular Profiling in Advanced Cancer Therapy (IMPACT) Background Imatinib: first FDA-approved tyrosine kinase inhibitor (CML): 2002 Druker et al NEJM 2001;344:1031 BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) Kim ES, Hong WK et al Cancer Discovery 2011;1:42 IMPACT Hypothesis, 2007 Genetic and molecular analyses of patients cancers will enable selection of optimal therapy for each patient Tsimberidou et al, Clin Cancer Res. 2012; 18(22):
5 Tissue Molecular Aberrations No. of pts. Molecular analysis ordered 3,745 % * Adequate tissue available 3, No. of aberrations 0 1, , No. of pts. with aberration 2, * Proportion was calculated for patients whose tissue was analyzed for 1 molecular aberration
6 Molecular Aberrations by Tumor Type (N=3,536)
7 Proportions of Molecular Aberrations (N=3,536) Includes tests performed in 30 patients RET mutations: were tested mostly in patients with medullary thyroid cancer
8 IMPACT 1 (N=3,536): Best Response (RECIST) in Pts. with 1 Molecular Alteration (n=688) Matched Targeted Therapy Non-Matched Therapy Matched TT Non-Matched P Evaluable CR+PR (%) 72 (21) 17 (6) <.0001 CR+PR+SD 6 months (%) 143 (42) 58 (19) <.0001
9 IMPACT 1: Pts. with 1 Molecular Alteration (n=688) Progression-Free Survival Overall Survival Matched TT Non-Matched P No. of patients Median PFS, months <.0001 Median OS, months <.0001
10 Multivariate Analysis for Response Treated Patients with 1 Aberration (N=607) Factor OR* 95% CI P Matched therapy (vs. non-matched) <.0001 No Liver Metastases Albumin ULN (vs. < ULN) LDH ULN (vs. > ULN) * OR = Odds Ratio (>1 is associated with higher response)
11 Multivariate Analysis for TTF Treated Patients with 1 Aberration Factor HR * 95% CI P Matched therapy (vs. non-matched) <.0001 Albumin 3.5 g/dl (vs. < 3.5 g/dl) <.0001 No liver metastases (vs. liver mets) <.0001 LDH ULN (vs. > ULN) Performance status < * HR = Hazard Ratio (<1 is associated with longer TTF)
12 No. of Aberr. Clinical Outcomes by No. of Aberrations and Type of Therapy (Non-Randomized) Therapy N CR+PR+ SD 6 Mo. (%) P TTF, Mo. P OS, Mo. 1 Matched /293 (39) < < Not matched /337 (15) P 2 Matched /82 (26) Not matched 68 10/57 (18) Matched 33 9/26 (35) Not matched 14 3/12 (25)
13 Patients with 1 Aberration. Response by Type of Therapy and BRAF Mutational Status Aberration Type of Therapy No. of treated pts. CR/PR (%) p-value BRAF Matched (33).0009 Non-matched 20 0 (0) Non-BRAF Matched (12).0007 Non-matched (4) Total Matched (19) <.0001 Non-matched (4)
14 2-Month Landmark Analysis: PFS Matched Therapy Non-Matched Therapy Tsimberidou et al, Clin Cancer Res. 2014; 20(18):1-10
15 2-Month Landmark Analysis: Survival Matched Therapy Non-Matched Therapy Tsimberidou et al, Clin Cancer Res. 2014; 20(18):1-10
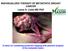
16 Salivary Cancer With BRAF Mutation V600E Treated With Vemurafenib
17 THE WALL STREET JOURNAL. June 6, 2011 Major Shift in War on Cancer The MD Anderson program pooled 1,144 patients in a phase I study after profiling their tumors for mutations that might be targets of the tested drugs. Apostolia Tsimberidou, the researcher who led the study, reported that 40% had mutations in 10 molecular pathways that were targeted by the experimental compounds. Tumors in 27% of those given agents that targeted their mutations responded to treatment compared to 5% for those with unmatched therapies.
18 THE ECONOMIST June 9, 2011 Taking aim sooner If personalized medicine is to achieve its full potential, it should be used earlier on in clinical trials Many scientists believe that matching volunteers' genetic profiles to the drugs being tested will not only be better for the volunteers, but may also speed up the trials, and save millions of dollars in the process. One such is Apostolia-Maria Tsimberidou of the University of Texas's MD Anderson Cancer Centre, in Houston. And her preliminary results, presented this week at a meeting of the American Society of Clinical Oncology in Chicago, suggest she is right.
19 Ongoing Challenges in Implementation of Precision Medicine
20 Challenges: Current Barriers Actual Goal Biopsy for molecular profile Not standard Standard of care CLIA, comprehensive test Not standard Standard of care Bioinformatics Limited Optimized Molecular analysis, results days 1-3 days Clinical trial/targeted agent 10-30% of patients 100% of patients Selection of targeted therapy Subjective Evidence-based Adaptive learning, N of 1 <10% 100%
21 Challenges: Molecular Profiling
22 Challenges: Molecularly Targeted Therapy
23 Primary Mets Ubiquitous Shared Primary R9 Shared Mets M2a,b M1 65% mutations from a single biopsy are heterogeneous not shared by all parts of the tumour Private Gerlinger et al. NEJM, 2012:366,883 Courtesy of Dr. Andrew Futreal
24 Intra-Patient Heterogeneity May Foster Tumor Adaptation and Therapeutic Failure Gerlinger, et al. NEJM. 2012;366:883.
25 Longitudinal Evaluation of Patients Treated Repeatedly with Erlotinib Sequist LV, et al. Sci Transl Med. 2011;3(75ra26):1-12.
26 Molecular Diagnostics and Point of Care Testing. A Key Future Driver in the Healthcare Value Chain Complex biosignature profiling Genomics Proteomics Immunosignatures Signature detection, deconvolution and multivariate analysis Automated, high throughput multiplex assays Novel test formats and devices (POC) New algorithms for complex signal/deconvolution
27 Ethnic Diversity in Drug Effects O'Donnell PH, Dolan ME. Clin Cancer Res. 2009;15:
28 Intratumour Heterogeneity and Predicting Outcome: Myeloma subclone determining final outcome: <1% of population 2 distinct bi allelic deletions of BIRC2/3 Keats et al. Blood
29 Challenges of Cancer Evolution and Heterogeneity Tumor Heterogeneity Supports Evolutionary Fitness Progression to invasive carcinoma Poor Clinical Outcome Tumor Adaptation and Selection for Drug resistance Metastatic growth Tumor Sampling Bias Different tumor biopsies, different results Sites of disease evolve independently Consider Clonal Dominance of Actionable Mutations Mutations present at one site but not another
30 Intra-tumor Heterogeneity: How to Resolve it? Circulating free tumor DNA
31 APOLLO is enabled by adaptive learning Patient Consent, Biospecimen Collection, QC, Banking, Biomolecule Processing Clinical information and data Big Data Warehouse Omics & Research Data Treatment Decisions & Response Assessment Big Data Analytics TCGA/ICGC Pubmed Patent db Social media Research Watson Solutions Insight discovery Clinical decision support Business Analytics Dr. Andrew Futreal
32 CancerLinQ A continuous cycle of learning Richard L. Schilsky. ASCO 2013
33 IMPACT 2 Study: Randomized Study Evaluating Molecular Profiling and Targeted Agents in Metastatic Cancer
34 Precision Medicine: Role of Prospective Studies Precision medicine Encouraging results Challenge: Not well-validated Solution: Prospective randomized controlled trials Challenge: Multiple new tests; too few patients to analyze Solution: Innovative study designs Objective: To assess the effectiveness of molecular profiling to select targeted therapy Tsimberidou et al. ASCO Educational Book 2014: 61-9
35 Randomized Study Evaluating Molecular Profiling and Targeted Agents in Metastatic Cancer (IMPACT 2) Primary Objective To determine whether patients treated with a targeted therapy selected on the basis of mutational analysis of the tumor have longer PFS from the time of randomization than those whose treatment is not selected based on alteration analysis PI: Tsimberidou, AM; Co-PIs: Berry, D; Meric-Bernstam, F NCT Supported by a research grant from Foundation Medicine
36 IMPACT 2. Study Design (I) Metastatic disease (0-3 prior therapies) Tumor biopsy for molecular profiling, 100% If patient can t wait for results of MP (with up to 2 prior therapies), they may receive 1 line of therapy, excluding targeted therapy Targetable molecular aberrations ( 1 aberration) Yes, 50% (n 613) No, 50% (n 613) FDA-approved drugs within labeled indication Yes, 30% (n 184) No, 70% (n 429) Excluded; patient followed for progression but not randomized Is there a clinical trial or commercially available targeted therapy? Yes, 70% (n 300) Randomize
37 IMPACT 2. Study Design (II) 1 Targeted therapy* I Z E 1 If: Progressive disease Toxicity Treatment not selected based on molecular analysis Crossover * Expansion phase of phase I studies or phase II studies
38 Key Inclusion Criteria 1. Metastatic cancer who have had up to three prior treatments (excluding biologics, cytokines or cell therapies). 2. Pathologically confirmed cancer by tumor biopsy and/or fine-needle aspiration. 3. Measurable disease 4. ECOG performance status The patient has biopsy-accessible tumor. 6. Normal organ function 7. Age >/= 18 years
39 Key Exclusion Criteria 1. The patient has received chemotherapy, surgery, or radiotherapy within 3 weeks of initiating study treatment (4 weeks for bevacizumab or investigational drugs) or the patient has not recovered (Grade </= 1) from side effects of the previous therapy (localized palliative radiotherapy within 2 weeks is allowed). 2. Patients to be randomized: Patients for whom an FDA-approved therapy for the tumor type and the molecular aberration is available will be excluded from the randomization. 3. Surgery within 30 days of study entry, excluding diagnostic biopsy.
40 Tumor Board To establish: ordered list of alterations & ordered list of trials/drugs Order: may depend on organ site Each individual Department will determine: trial priority per tumor type & will provide updated lists Meeting/Teleconference: every 2 wks Protocol master database: weekly updates Development of a treatment algorithm
41 IMPACT2: Treatment Assignment An ordered list of possible drugs by alterations and organ site will be established by the study personnel, that includes physicians, clinical decision support investigators and/or tumor board members. Drugs in clinical trials and/or off-label drugs will be added to the drug list as soon as they become available; and they can be assigned to patients.
42 Impact2 PA Current Status (I) Patients (as of 4/9/15) No. of Patients Screened 131 Consented 37 Completed Molecular Testing 27 Pending Molecular Testing 1 Screen Fails/Withdrew Consent 4 Inadequate Cells 3 Pending Biopsy 2
43 Impact2 PA Current Status (II) Patients (as of 4/9/15) No. of Patients No Actionable Mutations 12 Randomized 8 Pending Initiation of Treatment 0 Treated 7 Pts with PFS event or discontinued 2 Not Treated (No financial clearance) 1 Pending Randomization 0
44 Precision Medicine Other Clinical Trials
45 NSCLC: BATTLE-2 PI: Papadimitrakopoulou, V NCT
46 BATTLE-2: Stage 1. Results Randomized Arm 8-wk disease-control rate, % (Evaluable, n=167) All KRASwild KRASmutated Eval. type Erlotinib Erlotinib+AKT inhibitor AKT+MEK inhibitors Sorafenib Papadimitrakopoulou et al, ASCO 2014, Abstr. 8042
47 SWOG-1400: A biomarker-driven, multi-arm phase II/III registration protocol in squamous cell lung cancer second-line therapy PI: Papadimitrakopoulou, V NCT
48 WINTHER Trial
49
50 SHIVA: A Randomized Phase II Trial Comparing Therapy Based on Tumor Molecular Profiling Versus Conventional Therapy in Patients With Refractory Cancer Patients with refractory cancer (all tumor types) Informed consent signed Tumor biopsy NGS+ Cytoscan HD +IHC Imatinib Everolimus Sorafenib Erlotinib Dasatinib Lapatinib Trastuzumab Vemurafenib Tamoxifen Letrozole Abiraterone Targeted therapy based on molecular profiling Bioinformatics Informed consent signed R Endpoint: PFS Non eligible patient Molecular biology board Eligible patient Conventional therapy at physicians' discretion Cross-over NO Specific therapy available YES PI: Le Tourneau, C Institut Curie
51 Is Molecular Profiling Practice Changing? Potential for improving patient care Molecular profiling is used to guide treatment decisions Increasing complexity of molecular diagnosis Tumor heterogeneity predicts clinical outcomes ASCO s CancerLinQ Standardized procedures and decision support systems Innovative clinical trial design Discovery of novel targeted agents Understanding and prevention of drug resistance Tsimberidou, Best of ASCO Boston 2013
52 Conclusions Clinical trials in precision medicine are feasible Multiple trials are ongoing Innovative studies: correlative scientific queries Prospective innovative trials with adaptive design will accelerate process of drug approval (reduced cost, time, number of patients) Immune mechanisms, proteomics, transcriptome and epigenetic changes; Horizontal and vertical changes in tumor biology need to be considered Optimal big data bioinformatic analyses are necessary to implement precision medicine
53 Simplified Schema of Molecular Pathways EGF lapatinib erlotinib PTEN TGFα HGF IGF-1 HER2 EGFR1 cmet IGF-1R PI3K RAS KRAS Extracellular Fluid Cytoplasm EML4/ALK crizotinib NRAS AKT1 CRAF BRAF vemurafenib everolimus mtor MEK1 temsirolimus trametinib dabrafenib ERK Proliferation Survival Nucleus
54 IMPACT 1 Acknowledgments Authors: AM Tsimberidou, Y Ye, J Wheler, G Falchook, D Hong, A Naing, S Fu, R Kurzrock, S Piha-Paul, F Janku, R Zinner, S Moulder, V Subbiah, D Karp, P Hwu, M Kies, VA Miller, R Broaddus, K Hess, S Wen, D Berry Patients, Their Families IMPACT 2 Donald Berry, Ph.D., Professor of Biostatistics Richard Schilsky, M.D., Chief Medical Officer, ASCO Waun Ki Hong, M.D., Division Head, Cancer Medicine John Mendelsohn, M.D., Director, IPCT Funda Meric, M.D., Chair, ICT All collaborators and referring physicians from multiple Departments and Divisions, MD Anderson Sponsor: Foundation Medicine
55 Questions?
Implementing Precision Medicine in Oncology: The IMPACT Clinical Trials at MD Anderson Cancer Center
 Implementing Precision Medicine in Oncology: The IMPACT Clinical Trials at MD Anderson Cancer Center Apostolia M. Tsimberidou, MD, PhD Professor Department of Investigational Cancer Therapeutics Disclosures
Implementing Precision Medicine in Oncology: The IMPACT Clinical Trials at MD Anderson Cancer Center Apostolia M. Tsimberidou, MD, PhD Professor Department of Investigational Cancer Therapeutics Disclosures
Design considerations for Phase II trials incorporating biomarkers
 Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD
 Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
Clinical utility of precision medicine in oncology
 Clinical utility of precision medicine in oncology Prof. Christophe Le Tourneau, MD, PhD Institut Curie Paris & Saint-Cloud France Head, Department of Drug Development and Innovation (D 3 i) INSERM U900
Clinical utility of precision medicine in oncology Prof. Christophe Le Tourneau, MD, PhD Institut Curie Paris & Saint-Cloud France Head, Department of Drug Development and Innovation (D 3 i) INSERM U900
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets
 Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
K-Ras signalling in NSCLC
 Targeting the Ras-Raf-Mek-Erk pathway Egbert F. Smit MD PhD Dept. Pulmonary Diseases Vrije Universiteit VU Medical Centre Amsterdam, The Netherlands K-Ras signalling in NSCLC Sun et al. Nature Rev. Cancer
Targeting the Ras-Raf-Mek-Erk pathway Egbert F. Smit MD PhD Dept. Pulmonary Diseases Vrije Universiteit VU Medical Centre Amsterdam, The Netherlands K-Ras signalling in NSCLC Sun et al. Nature Rev. Cancer
Looking Beyond the Standard-of- Care : The Clinical Trial Option
 1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
Should novel molecular therapies replace old knowledge of clinical tumor biology?
 Should novel molecular therapies replace old knowledge of clinical tumor biology? Danai Daliani, M.D. Director, 1 st Oncology Clinic Euroclinic of Athens Cancer Treatments Localized disease Surgery XRT
Should novel molecular therapies replace old knowledge of clinical tumor biology? Danai Daliani, M.D. Director, 1 st Oncology Clinic Euroclinic of Athens Cancer Treatments Localized disease Surgery XRT
CUP: Treatment by molecular profiling
 CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
Precision Medicine Lessons from meta-analyses of 70,253 patients
 Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
New Drug development and Personalized Therapy in The Era of Molecular Medicine
 New Drug development and Personalized Therapy in The Era of Molecular Medicine Ramesh K. Ramanathan MD Virginia G. Piper Cancer Center Translational Genomics Research Institute Scottsdale, AZ Clinical
New Drug development and Personalized Therapy in The Era of Molecular Medicine Ramesh K. Ramanathan MD Virginia G. Piper Cancer Center Translational Genomics Research Institute Scottsdale, AZ Clinical
Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges
 Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges Frédérique Nowak - 21 october 2015 "Putting Science into Standards event:
Implementation of nation-wide molecular testing in oncology in the French Health care system : quality assurance issues & challenges Frédérique Nowak - 21 october 2015 "Putting Science into Standards event:
Comprehensive Genomic Profiling, in record time. Accurate. Clinically Proven. Fast.
 Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Melanoma: From Chemotherapy to Targeted Therapy and Immunotherapy. What every patient needs to know. James Larkin
 Melanoma: From Chemotherapy to Targeted Therapy and Immunotherapy What every patient needs to know James Larkin Melanoma Therapy 1846-2017 Surgery 1846 Cytotoxic Chemotherapy 1946 Checkpoint Inhibitors
Melanoma: From Chemotherapy to Targeted Therapy and Immunotherapy What every patient needs to know James Larkin Melanoma Therapy 1846-2017 Surgery 1846 Cytotoxic Chemotherapy 1946 Checkpoint Inhibitors
EGFR inhibitors in NSCLC
 Suresh S. Ramalingam, MD Associate Professor Director of Medical Oncology Emory University i Winship Cancer Institute EGFR inhibitors in NSCLC Role in 2nd/3 rd line setting Role in first-line and maintenance
Suresh S. Ramalingam, MD Associate Professor Director of Medical Oncology Emory University i Winship Cancer Institute EGFR inhibitors in NSCLC Role in 2nd/3 rd line setting Role in first-line and maintenance
I. Diagnosis of the cancer type in CUP
 Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
Basket Trials: Features, Examples, and Challenges
 : Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
: Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
Lung Cancer Case. Since the patient was symptomatic, a targeted panel was sent. ALK FISH returned in 2 days and was positive.
 Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader
 Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD
 INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD A vision for Combining proteomic mapping with genomic analysis of the metastatic lesion 57 year old women TNBC locally recurrent
INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD A vision for Combining proteomic mapping with genomic analysis of the metastatic lesion 57 year old women TNBC locally recurrent
ALCHEMIST. Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials
 ALCHEMIST Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials What is ALCHEMIST? ALCHEMIST is 3 integrated trials testing targeted therapy in early stage lung cancer: l A151216:
ALCHEMIST Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials What is ALCHEMIST? ALCHEMIST is 3 integrated trials testing targeted therapy in early stage lung cancer: l A151216:
1.Basis of resistance 2.Mechanisms of resistance 3.How to overcome resistance. 13/10/2017 Sara Redaelli
 Dott.ssa Sara Redaelli 13/10/2017 1.Basis of resistance 2.Mechanisms of resistance 3.How to overcome resistance Tumor Heterogeneity: Oncogenic Drivers in NSCLC The Promise of Genotype-Directed Therapy
Dott.ssa Sara Redaelli 13/10/2017 1.Basis of resistance 2.Mechanisms of resistance 3.How to overcome resistance Tumor Heterogeneity: Oncogenic Drivers in NSCLC The Promise of Genotype-Directed Therapy
Targeted/Immunotherapy & Molecular Profiling State-of-the-art in Cancer Care
 Targeted/Immunotherapy & Molecular Profiling State-of-the-art in Cancer Care Manmeet Ahluwalia, MD, FACP Miller Family Endowed Chair in Neuro-Oncology Director Brain Metastasis Research Program Cleveland
Targeted/Immunotherapy & Molecular Profiling State-of-the-art in Cancer Care Manmeet Ahluwalia, MD, FACP Miller Family Endowed Chair in Neuro-Oncology Director Brain Metastasis Research Program Cleveland
Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD. Mount Carrigain 2/4/17
 Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD Mount Carrigain 2/4/17 Histology Adenocarcinoma: Mixed subtype, acinar, papillary, solid, micropapillary, lepidic
Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD Mount Carrigain 2/4/17 Histology Adenocarcinoma: Mixed subtype, acinar, papillary, solid, micropapillary, lepidic
Evidenze cliniche nel trattamento del RCC
 Criteri di scelta nel trattamento sistemico del carcinoma renale Evidenze cliniche nel trattamento del RCC Alessandro Morabito Unità Sperimentazioni Cliniche Istituto Nazionale Tumori di Napoli Napoli,
Criteri di scelta nel trattamento sistemico del carcinoma renale Evidenze cliniche nel trattamento del RCC Alessandro Morabito Unità Sperimentazioni Cliniche Istituto Nazionale Tumori di Napoli Napoli,
Metastatic NSCLC: Expanding Role of Immunotherapy. Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian
 Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018
 Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Targeted Agent and Profiling Utilization Registry
 Targeted Agent and Profiling Utilization Registry Richard L. Schilsky, MD, FACP, FASCO Senior Vice President and Chief Medical Officer American Society of Clinical Oncology Disclosure Information Richard
Targeted Agent and Profiling Utilization Registry Richard L. Schilsky, MD, FACP, FASCO Senior Vice President and Chief Medical Officer American Society of Clinical Oncology Disclosure Information Richard
Targeted Agents as Maintenance Therapy. Karen Kelly, MD Professor of Medicine UC Davis Cancer Center
 Targeted Agents as Maintenance Therapy Karen Kelly, MD Professor of Medicine UC Davis Cancer Center Disclosures Genentech Advisory Board Maintenance Therapy Defined Treatment Non-Progressing Patients Drug
Targeted Agents as Maintenance Therapy Karen Kelly, MD Professor of Medicine UC Davis Cancer Center Disclosures Genentech Advisory Board Maintenance Therapy Defined Treatment Non-Progressing Patients Drug
Precision Genetic Testing in Cancer Treatment and Prognosis
 Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
2/21/2016. Cancer Precision Medicine: A Primer. Ovarian Cancer Statistics and Standard of Care in 2015 OUTLINE. Background
 Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
Strategies to Overcome Clinical, Regulatory, and Financial Challenges in Implementation of Personalized Medicine
 CLINICAL TRIALS Strategies to Overcome Clinical, Regulatory, and Financial Challenges in Implementation of Personalized Medicine CHAIR Richard L. Schilsky, MD American Society of Clinical Oncology Alexandria,
CLINICAL TRIALS Strategies to Overcome Clinical, Regulatory, and Financial Challenges in Implementation of Personalized Medicine CHAIR Richard L. Schilsky, MD American Society of Clinical Oncology Alexandria,
Fifteenth International Kidney Cancer Symposium
 The following presentation should not be regarded as an endorsement of a particular product/drug/technique by the speaker. The presentation topics were assigned to the speakers by the scientific committee
The following presentation should not be regarded as an endorsement of a particular product/drug/technique by the speaker. The presentation topics were assigned to the speakers by the scientific committee
7/6/2015. Cancer Related Deaths: United States. Management of NSCLC TODAY. Emerging mutations as predictive biomarkers in lung cancer: Overview
 Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
IRESSA (Gefitinib) The Journey. Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca
 IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective
 Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
NSCLC 2 nd and further line therapies. Egbert F. Smit MD PhD. Dept. Thoracic Oncology, Netherlands Cancer Institute
 NSCLC 2 nd and further line therapies Egbert F. Smit MD PhD. Dept. Thoracic Oncology, Netherlands Cancer Institute e.smit@nki.nl ESMO Guidelines 2016: Treatment of Stage IV nonsquamous NSCLC at progression
NSCLC 2 nd and further line therapies Egbert F. Smit MD PhD. Dept. Thoracic Oncology, Netherlands Cancer Institute e.smit@nki.nl ESMO Guidelines 2016: Treatment of Stage IV nonsquamous NSCLC at progression
RT +/- Surgery. Concurrent ChemoRT +/- Surgery
 Molecular targeted approaches to head and neck cancer Lillian L. Siu Department of Medical Oncology & Hematology Princess Margaret Hospital, University of Toronto Locally Advanced HNSCC Locally Advanced
Molecular targeted approaches to head and neck cancer Lillian L. Siu Department of Medical Oncology & Hematology Princess Margaret Hospital, University of Toronto Locally Advanced HNSCC Locally Advanced
Update on Targeted Therapy in Melanoma
 Update on Targeted Therapy in Melanoma Seville June 2013 James Larkin FRCP PhD London UK Overview What are the targets in melanoma? BRAF / KIT / NRAS / GNAQ / MEK DNA / microtubules CTLA4 / PD1 / PDL1
Update on Targeted Therapy in Melanoma Seville June 2013 James Larkin FRCP PhD London UK Overview What are the targets in melanoma? BRAF / KIT / NRAS / GNAQ / MEK DNA / microtubules CTLA4 / PD1 / PDL1
Development of Rational Drug Combinations for Oncology Indications - An Industry Perspective
 Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
ARIAD Pharmaceuticals, Inc.
 ARIAD Pharmaceuticals, Inc. June 8, 2016 David Sachs Non-small cell lung cancer 1 ARIAD clinical trial patient Some of the statements in this presentation constitute forward looking statements under the
ARIAD Pharmaceuticals, Inc. June 8, 2016 David Sachs Non-small cell lung cancer 1 ARIAD clinical trial patient Some of the statements in this presentation constitute forward looking statements under the
Nivolumab: esperienze italiane nel carcinoma polmonare avanzato
 NSCLC avanzato: quali novità nel 2018? Negrar, 30 Ottobre 2018 Nivolumab: esperienze italiane nel carcinoma polmonare avanzato Francesco Grossi UOC Oncologia Medica Fondazione IRCCS Ca Granda Ospedale
NSCLC avanzato: quali novità nel 2018? Negrar, 30 Ottobre 2018 Nivolumab: esperienze italiane nel carcinoma polmonare avanzato Francesco Grossi UOC Oncologia Medica Fondazione IRCCS Ca Granda Ospedale
Molecular Panel Testing of Cancers to Identify Targeted Therapies
 Molecular Panel Testing of Cancers to Identify Targeted Therapies Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively
Molecular Panel Testing of Cancers to Identify Targeted Therapies Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making
 Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer
 Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy
 Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy 1 st st International Oncological Conference Wrocław, October 6 th, 2012 Dr. Frank Kischkel Individualized Cancer Therapy:
Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy 1 st st International Oncological Conference Wrocław, October 6 th, 2012 Dr. Frank Kischkel Individualized Cancer Therapy:
Colorectal Cancer in the Coming Years: What Can We Expect?
 Colorectal Cancer in the Coming Years: What Can We Expect? Clara Montagut, MD, PhD Hospital Universitari del Mar, Barcelona, Spain Memorial Sloan Kettering Cancer Center, New York, United States What Are
Colorectal Cancer in the Coming Years: What Can We Expect? Clara Montagut, MD, PhD Hospital Universitari del Mar, Barcelona, Spain Memorial Sloan Kettering Cancer Center, New York, United States What Are
Design of Clinical Trials with Molecularly Targeted Therapies. Gilberto Schwartsmann
 Design of Clinical Trials with Molecularly Targeted Therapies Gilberto Schwartsmann What are the basics of clinical trial design? Presented By Jordan Berlin at 2016 ASCO Annual Meeting Advances in molecular
Design of Clinical Trials with Molecularly Targeted Therapies Gilberto Schwartsmann What are the basics of clinical trial design? Presented By Jordan Berlin at 2016 ASCO Annual Meeting Advances in molecular
Targeted Therapies in Melanoma
 Mutations and Targets Targeted Therapies in Melanoma ckit NRAS
Mutations and Targets Targeted Therapies in Melanoma ckit NRAS
When patients fail on molecular targeted therapy: what to do in 2013
 When patients fail on molecular targeted therapy: what to do in 2013 For 3 rd APASAL HCC conference on 23 Nov 2013 Dr. Stephen L. Chan Department of Clinical Oncology The Chinese University of Hong Kong
When patients fail on molecular targeted therapy: what to do in 2013 For 3 rd APASAL HCC conference on 23 Nov 2013 Dr. Stephen L. Chan Department of Clinical Oncology The Chinese University of Hong Kong
Supplementary Online Content
 Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic Therapy
 Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy
 Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy Oncogenic drivers in NSCLC Certain tumours arise as a result of aberrant activation
Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy Oncogenic drivers in NSCLC Certain tumours arise as a result of aberrant activation
WINTHER: GUSTAVE ROUSSY GUSTAVE ROUSSY. NOM DU DOCUMENT / Date
 WINTHER Study Jean-Charles Soria, Razelle Kurzrock, Josep Tabernero, Apostolia Tsimberidou, Jordi Rodon, Raanan Berger, Amir Onn, Gerald Batist, Eitan Rubin, Yohann Loriot, Catherine Bresson, Vladimir
WINTHER Study Jean-Charles Soria, Razelle Kurzrock, Josep Tabernero, Apostolia Tsimberidou, Jordi Rodon, Raanan Berger, Amir Onn, Gerald Batist, Eitan Rubin, Yohann Loriot, Catherine Bresson, Vladimir
Personalized Genetics
 Personalized Genetics Understanding Your Genetic Test Results Tracey Evans, MD September 29, 2017 Genetics 101 Punnett Square Genetic Pedigree 2 Genetics 101 Punnett Square Genetic Pedigree 3 It s not
Personalized Genetics Understanding Your Genetic Test Results Tracey Evans, MD September 29, 2017 Genetics 101 Punnett Square Genetic Pedigree 2 Genetics 101 Punnett Square Genetic Pedigree 3 It s not
Page. Objectives: Hormone Therapy Resistance: Challenges and Opportunities. Research Support From Merck
 Hormone Therapy Resistance: Challenges and Opportunities Pamela. N. Munster, MD University of California, San Francisco Financial Disclosures Research Support From Merck Objectives: Understanding the current
Hormone Therapy Resistance: Challenges and Opportunities Pamela. N. Munster, MD University of California, San Francisco Financial Disclosures Research Support From Merck Objectives: Understanding the current
2 nd line Therapy and Beyond NSCLC. Alan Sandler, M.D. Oregon Health & Science University
 2 nd line Therapy and Beyond NSCLC Alan Sandler, M.D. Oregon Health & Science University Treatment options for advanced or metastatic (stage IIIb/IV) NSCLC Suitable for chemotherapy Diagnosis Unsuitable/unwilling
2 nd line Therapy and Beyond NSCLC Alan Sandler, M.D. Oregon Health & Science University Treatment options for advanced or metastatic (stage IIIb/IV) NSCLC Suitable for chemotherapy Diagnosis Unsuitable/unwilling
Dr C K Kwan. Associate Consultant, Queen Elizabeth Hospital, HKSAR Honorary member, Targeted Therapy Team, ICR, UK
 HA Convention 2013 Dr C K Kwan MBChB, FRCR, FHKCR, FHKAM, MPM, CPI, MAppMgt(Hlth) Associate Consultant, Queen Elizabeth Hospital, HKSAR Honorary member, Targeted Therapy Team, ICR, UK To personalize cancer
HA Convention 2013 Dr C K Kwan MBChB, FRCR, FHKCR, FHKAM, MPM, CPI, MAppMgt(Hlth) Associate Consultant, Queen Elizabeth Hospital, HKSAR Honorary member, Targeted Therapy Team, ICR, UK To personalize cancer
European consortium study on the availability of anti-neoplastic medicines
 European consortium study on the availability of anti-neoplastic medicines Nathan I Cherny Alexandru ENIU, MD, PhD Norman Levan Chair in Humanistic Chair, Emerging Countries Committee Medicine Department
European consortium study on the availability of anti-neoplastic medicines Nathan I Cherny Alexandru ENIU, MD, PhD Norman Levan Chair in Humanistic Chair, Emerging Countries Committee Medicine Department
Targeted therapy in NSCLC: do we progress? Prof. Dr. V. Surmont. Masterclass 27 september 2018
 Targeted therapy in NSCLC: do we progress? Prof. Dr. V. Surmont Masterclass 27 september 2018 Outline Introduction EGFR TKI ALK TKI TKI for uncommon driver mutations Take home messages The promise of
Targeted therapy in NSCLC: do we progress? Prof. Dr. V. Surmont Masterclass 27 september 2018 Outline Introduction EGFR TKI ALK TKI TKI for uncommon driver mutations Take home messages The promise of
Targeted therapies for advanced non-small cell lung cancer. Tom Stinchcombe Duke Cancer Insitute
 Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
Contemporary Chemotherapy-Based Strategies for First-Line Metastatic Breast Cancer
 Contemporary Chemotherapy-Based Strategies for First-Line Metastatic Breast Cancer Hope S. Rugo, MD Professor of Medicine Director, Breast Oncology and Clinical Trials Education University of California
Contemporary Chemotherapy-Based Strategies for First-Line Metastatic Breast Cancer Hope S. Rugo, MD Professor of Medicine Director, Breast Oncology and Clinical Trials Education University of California
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Heather Wakelee, M.D.
 Heather Wakelee, M.D. Assistant Professor of Medicine, Oncology Stanford University Sponsored by Educational Grant Support from Adjuvant (Post-Operative) Lung Cancer Chemotherapy Heather Wakelee, M.D.
Heather Wakelee, M.D. Assistant Professor of Medicine, Oncology Stanford University Sponsored by Educational Grant Support from Adjuvant (Post-Operative) Lung Cancer Chemotherapy Heather Wakelee, M.D.
CDx in oncology Prof. Christophe Le Tourneau, MD, PhD FEAM Geneva September 27, 2018
 CDx in oncology Prof. Christophe Le Tourneau, MD, PhD Institut Curie Paris & Saint-Cloud France Head, Department of Drug Development and Innovation (D 3 i) INSERM U900 Research unit Versailles Saint-Quentin-en-Yvelines
CDx in oncology Prof. Christophe Le Tourneau, MD, PhD Institut Curie Paris & Saint-Cloud France Head, Department of Drug Development and Innovation (D 3 i) INSERM U900 Research unit Versailles Saint-Quentin-en-Yvelines
Have Results of Recent Randomized Trials Changed the Role of mtor Inhibitors?
 Have Results of Recent Randomized Trials Changed the Role of mtor Inhibitors? Bernard Escudier Institut Gustave Roussy Villejuif, France EIKCS Lyon April 2015 What is the current role of mtor inhibitors?
Have Results of Recent Randomized Trials Changed the Role of mtor Inhibitors? Bernard Escudier Institut Gustave Roussy Villejuif, France EIKCS Lyon April 2015 What is the current role of mtor inhibitors?
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和
 資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
Negative Trials in RCC: Where Did We Go Wrong? Can We Do Better?
 Negative Trials in RCC: Where Did We Go Wrong? Can We Do Better? 9 th European Kidney Cancer Symposium, Dublin, April 2014 Tim Eisen Tim Eisen - Disclosures Company Research Support Advisory Board Trial
Negative Trials in RCC: Where Did We Go Wrong? Can We Do Better? 9 th European Kidney Cancer Symposium, Dublin, April 2014 Tim Eisen Tim Eisen - Disclosures Company Research Support Advisory Board Trial
OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER
 & OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
& OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
The Really Important Questions Current Immunotherapy Trials are Not Answering
 The Really Important Questions Current Immunotherapy Trials are Not Answering David McDermott, MD Beth Israel Deaconess Medical Center Dana Farber/Harvard Cancer Center Harvard Medical School PD-1 Pathway
The Really Important Questions Current Immunotherapy Trials are Not Answering David McDermott, MD Beth Israel Deaconess Medical Center Dana Farber/Harvard Cancer Center Harvard Medical School PD-1 Pathway
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Carcinoma de Tiroide: Teràpies Diana
 Carcinoma de Tiroide: Teràpies Diana Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology THYROID CANCER:
Carcinoma de Tiroide: Teràpies Diana Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology THYROID CANCER:
Lung Cancer Update 2016 BAONS Oncology Care Update
 Lung Cancer Update 2016 BAONS Oncology Care Update Matthew Gubens, MD, MS Assistant Professor Chair, Thoracic Oncology Site Committee UCSF Helen Diller Family Comprehensive Cancer Center Disclosures Consulting
Lung Cancer Update 2016 BAONS Oncology Care Update Matthew Gubens, MD, MS Assistant Professor Chair, Thoracic Oncology Site Committee UCSF Helen Diller Family Comprehensive Cancer Center Disclosures Consulting
7th November, Translational Science: how to move from biology to clinical applications
 7th November, 2014 Translational Science: how to move from biology to clinical applications 1 Translational science: How to move from biology to clinical applications Translational cancer genomics and
7th November, 2014 Translational Science: how to move from biology to clinical applications 1 Translational science: How to move from biology to clinical applications Translational cancer genomics and
Molecular Targets in Lung Cancer
 Molecular Targets in Lung Cancer Robert Ramirez, DO, FACP Thoracic and Neuroendocrine Oncology November 18 th, 2016 Disclosures Consulting and speaker fees for Ipsen Pharmaceuticals, AstraZeneca and Merck
Molecular Targets in Lung Cancer Robert Ramirez, DO, FACP Thoracic and Neuroendocrine Oncology November 18 th, 2016 Disclosures Consulting and speaker fees for Ipsen Pharmaceuticals, AstraZeneca and Merck
MEDICAL POLICY. SUBJECT: MOLECULAR PANEL TESTING OF CANCERS TO IDENTIFY TARGETED THERAPIES (Excluding NSCLC and CRC) EFFECTIVE DATE: 12/21/17
 MEDICAL POLICY SUBJECT: MOLECULAR PANEL TESTING OF PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MOLECULAR PANEL TESTING OF PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Personalized Cancer Medicine. Conceptual,Organizational,Financial Challenges
 Personalized Cancer Medicine Conceptual,Organizational,Financial Challenges SHANGHAI 6 July, 2012 What is breast cancer? The old perception The New Family of Diseases Perception / Molecular Portraits What
Personalized Cancer Medicine Conceptual,Organizational,Financial Challenges SHANGHAI 6 July, 2012 What is breast cancer? The old perception The New Family of Diseases Perception / Molecular Portraits What
A View to the Future: The Development of Targeted Therapy for Melanoma. Michael Davies, M.D., Ph.D.
 A View to the Future: Science to Survivorship Symposium September 26, 2009 The Development of Targeted Therapy for Melanoma Michael Davies, M.D., Ph.D. Assistant Professor, Melanoma Medical Oncology How
A View to the Future: Science to Survivorship Symposium September 26, 2009 The Development of Targeted Therapy for Melanoma Michael Davies, M.D., Ph.D. Assistant Professor, Melanoma Medical Oncology How
In 2014, the National Cancer Institute will launch a series of
 NCI S PRECISION MEDICINE INITIATIVES FOR THE NEW NCTN National Cancer Institute s Precision Medicine Initiatives for the New National Clinical Trials Network Jeffrey Abrams, MD, Barbara Conley, MD, Margaret
NCI S PRECISION MEDICINE INITIATIVES FOR THE NEW NCTN National Cancer Institute s Precision Medicine Initiatives for the New National Clinical Trials Network Jeffrey Abrams, MD, Barbara Conley, MD, Margaret
Big data vs. the individual liver from a regulatory perspective
 Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
Antiangiogenic Agents in NSCLC Where are we? Which biomarkers? VEGF Is the Only Angiogenic Factor Present Throughout the Tumor Life Cycle
 Antiangiogenic Agents in NSCLC Where are we? Which biomarkers? Martin Reck Department e t of Thoracic c Oncology ogy Hospital Grosshansdorf Germany VEGF Is the Only Angiogenic Factor Present Throughout
Antiangiogenic Agents in NSCLC Where are we? Which biomarkers? Martin Reck Department e t of Thoracic c Oncology ogy Hospital Grosshansdorf Germany VEGF Is the Only Angiogenic Factor Present Throughout
Best Practices in the Treatment and Management of Metastatic Melanoma. Melanoma
 Best Practices in the Treatment and Management of Metastatic Melanoma Philip Friedlander MD PhD Director of Melanoma Medical Oncology Program Assistant Professor Division of Hematology Oncology Assistant
Best Practices in the Treatment and Management of Metastatic Melanoma Philip Friedlander MD PhD Director of Melanoma Medical Oncology Program Assistant Professor Division of Hematology Oncology Assistant
Neratinib in patients with HER2-mutant, metastatic cervical cancer: findings from the phase 2 SUMMIT basket trial
 Neratinib in patients with HER2-mutant, metastatic cervical cancer: findings from the phase 2 SUMMIT basket trial Anishka D Souza 1, Lynda D. Roman 1, Cristina Saura 2, Irene Braña 2, Geoffrey I. Shapiro
Neratinib in patients with HER2-mutant, metastatic cervical cancer: findings from the phase 2 SUMMIT basket trial Anishka D Souza 1, Lynda D. Roman 1, Cristina Saura 2, Irene Braña 2, Geoffrey I. Shapiro
Emerging Biomarkers of VEGF and mtor Inhibitors in 2015
 Emerging Biomarkers of VEGF and mtor Inhibitors in 2015 Laurence Albiges Institut Gustave Roussy, France Fourteenth International Kidney Cancer Symposium Miami, Florida, USA November 6-7, 2015 www.kidneycancersymposium.com
Emerging Biomarkers of VEGF and mtor Inhibitors in 2015 Laurence Albiges Institut Gustave Roussy, France Fourteenth International Kidney Cancer Symposium Miami, Florida, USA November 6-7, 2015 www.kidneycancersymposium.com
The oncologist s point of view: the promise and challenges of increasing options for targeted therapies in NSCLC
 The oncologist s point of view: the promise and challenges of increasing options for targeted therapies in NSCLC Egbert F. Smit Department of Thoracic Oncology, Netherlands Cancer Institute, and Department
The oncologist s point of view: the promise and challenges of increasing options for targeted therapies in NSCLC Egbert F. Smit Department of Thoracic Oncology, Netherlands Cancer Institute, and Department
Evolution of Pathology
 1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
POLICY The use of expanded cancer molecular panels for selecting targeted cancer treatment is considered investigational.
 Medical Policy BCBSA Ref. Policy: 2.04.115 Last Review: 10/18/2018 Effective Date: 10/18/2018 Section: Medicine Related Policies 2.04.93 Genetic Cancer Susceptibility Panels Using Next- Generation Sequencing
Medical Policy BCBSA Ref. Policy: 2.04.115 Last Review: 10/18/2018 Effective Date: 10/18/2018 Section: Medicine Related Policies 2.04.93 Genetic Cancer Susceptibility Panels Using Next- Generation Sequencing
Targeted Therapy in Advanced Renal Cell Carcinoma
 Targeted Therapy in Advanced Renal Cell Carcinoma Brian I. Rini, M.D. Department of Solid Tumor Oncology Glickman Urologic and Kidney Institute Cleveland Clinic Taussig Cancer Institute Cleveland, Ohio
Targeted Therapy in Advanced Renal Cell Carcinoma Brian I. Rini, M.D. Department of Solid Tumor Oncology Glickman Urologic and Kidney Institute Cleveland Clinic Taussig Cancer Institute Cleveland, Ohio
State of the art for radiotherapy of SCCHN
 State of the art for radiotherapy of SCCHN Less side effects Cured More organ & function preservation Head & neck cancer = 42 000 new cases / year in Europe Not cured Local failure Distant failure More
State of the art for radiotherapy of SCCHN Less side effects Cured More organ & function preservation Head & neck cancer = 42 000 new cases / year in Europe Not cured Local failure Distant failure More
Changing demographics of smoking and its effects during therapy
 Changing demographics of smoking and its effects during therapy Egbert F. Smit MD PhD. Dept. Pulmonary Diseases, Vrije Universiteit Medical Centre, Amsterdam, The Netherlands Smoking prevalence adults
Changing demographics of smoking and its effects during therapy Egbert F. Smit MD PhD. Dept. Pulmonary Diseases, Vrije Universiteit Medical Centre, Amsterdam, The Netherlands Smoking prevalence adults
NCI Community Cancer Centers Program
 NCI Community Cancer Centers Program BIOSPECIMENS SURVIVORSHIP Andrew L. Salner, MD FACR Director, Helen & Harry Gray Cancer Center Hartford Hospital Hartford, CT Hartford Hospital 865 bed community and
NCI Community Cancer Centers Program BIOSPECIMENS SURVIVORSHIP Andrew L. Salner, MD FACR Director, Helen & Harry Gray Cancer Center Hartford Hospital Hartford, CT Hartford Hospital 865 bed community and
Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma
 Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
Response and resistance to BRAF inhibitors in melanoma
 Response and resistance to BRAF inhibitors in melanoma Keith T. Flaherty, M.D. Massachusetts General Hospital Cancer Center Disclosures Roche/Genentech: consultant GlaxoSmithKline: consultant BRAF mutations
Response and resistance to BRAF inhibitors in melanoma Keith T. Flaherty, M.D. Massachusetts General Hospital Cancer Center Disclosures Roche/Genentech: consultant GlaxoSmithKline: consultant BRAF mutations
Tumor Gene Sequencing: Ready for a Revolution?
 Tumor Gene Sequencing: Ready for a Revolution? Philippe Aftimos, M.D. Clinical Pharmacology Unit Institut Jules Bordet Brussels, Belgium ICBD Antwerp February 7 th, 2015 IHC: Historical Biomarkers Cold
Tumor Gene Sequencing: Ready for a Revolution? Philippe Aftimos, M.D. Clinical Pharmacology Unit Institut Jules Bordet Brussels, Belgium ICBD Antwerp February 7 th, 2015 IHC: Historical Biomarkers Cold
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
On population diversity in clinical trials A few remarks. Prof. Olaf M. Dekkers Dept clinical epidemiology & internal medicine LUMC
 On population diversity in clinical trials A few remarks Prof. Olaf M. Dekkers Dept clinical epidemiology & internal medicine LUMC Thinking about population diversity in trials Design What populations
On population diversity in clinical trials A few remarks Prof. Olaf M. Dekkers Dept clinical epidemiology & internal medicine LUMC Thinking about population diversity in trials Design What populations
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Design of a Disease-Specific Master Protocol
 Design of a Disease-Specific Master Protocol Design of a Disease-Specific Master Protocol Roy Herbst, MD/PhD Yale Cancer Center Modernizing Clinical Trial Process Some of the current challenges of drug
Design of a Disease-Specific Master Protocol Design of a Disease-Specific Master Protocol Roy Herbst, MD/PhD Yale Cancer Center Modernizing Clinical Trial Process Some of the current challenges of drug
