Assessing the effectiveness of computerised dissemination of the 'NHMRC's Clinical Guidelines for the Management of Early Breast Cancer': final report
|
|
|
- Marilyn Thompson
- 5 years ago
- Views:
Transcription
1 Southern Cross University School of Education 1998 Assessing the effectiveness of computerised dissemination of the 'NHMRC's Clinical Guidelines for the Management of Early Breast Cancer': final report Sallie Newell Southern Cross University Nicole Rankin NSW Cancer Council Rob William Sanson-Fisher University of Newcastle Publication details Newell, S, Rankin, N & Sanson-Fisher, RW 1998, Assessing the effectiveness of computerised dissemination of the 'NHMRC's Clinical Guidelines for the Management of Early Breast Cancer': final report, prepared for NHMRC National Breast Cancer Centre, Wallsend, NSW. epublications@scu is an electronic repository administered by Southern Cross University Library. Its goal is to capture and preserve the intellectual output of Southern Cross University authors and researchers, and to increase visibility and impact through open access to researchers around the world. For further information please contact epubs@scu.edu.au.
2 Assessing the effectiveness of computerised dissemination of the NHMRC s Clinical Guidelines for the Management of Early Breast Cancer. (Medical Oncologists Guidelines Dissemination Project) Final Report December 1998 A Report Prepared for the NHMRC National Breast Cancer Centre Prepared by: Sallie Newell, Nicole Rankin, Rob Sanson-Fisher Cancer Education Research Program (CERP) Locked Mail Bag 10, WALLSEND NSW 2287
3 ACKNOWLEDGEMENTS: This project was funded by the National Breast Cancer Centre and undertaken by the New South Wales Cancer Council Cancer Education Research Program team, directed by Professor Rob Sanson-Fisher to November The views expressed are not necessarily those of the Cancer Council. The authors of this report gratefully acknowledge the involvement of the oncologists who participated in the project: Dr Michael Byrne, Dr Joanna Dewar, Dr Guy van Hazel, Dr Martin Buck (Sir Charles Gairdner Hospital, Perth); Dr Nicholas Wilcken, Professor Paul Harnett, Dr Catherine Crombie, Dr Jo Hornbuckle, (Nepean Hospital, Nepean); Dr Damien Thompson, Dr Helen Juffs, Dr Euan Walpole (Princess Alexandra Hospital, Brisbane); Associate Professor Ian Olver, Dr Dorothy Keefe, Dr Jack Russell, Dr Caroline Smith, Dr Anne Taylor (Royal Adelaide Hospital). We would also like to thank the oncologists who assisted in the development phases of this project: Dr Howard Gurney, Dr Geoff Beadle, Professor Rick Kefford and Professor Martin Tattersall. Our thanks also go to the nursing and administrative staff at each hospital; to the two facilitators based at the Cancer Education Research Program, Kathy Rainbird and Kathy Alder; and to Sally Burrows for her invaluable assistance with analysing the complex dataset from this project. Finally, we are particularly grateful to the women who gave their time to contribute to this study. Trial of Computerised Dissemination of Guidelines Final Report CERP 2
4 Table of Contents INTRODUCTION 4 METHOD 5 Design 5 Outcome measures 5 Process measures 5 The feedback 6 Recruiting the medical oncologists 6 Recruiting the patients 6 RESULTS 7 Quantitative Results 8 Participants Characteristics 8 Level of Diagnosis in accordance with the Guidelines 9 Level of Treatment in accordance with the Guidelines 10 Women s Satisfaction with their Level of Involvement in Treatment Decisions 11 The Adequacy and Appropriateness of Information Provided to Women 12 Appropriateness of patient follow-up care 17 Qualitative Results 17 Acceptability of the Intervention 18 Acceptability to patients 18 Acceptability to hospital staff 18 DISCUSSION 18 REFERENCES 18 APPENDIX 1: Diagnostic And Treatment Information Form 19 APPENDIX 2: Computerised Feedback for Oncologists 20 APPENDIX 3: Diagnostic & Treatment Feedback Sheets 22 Trial of Computerised Dissemination of Guidelines Final Report CERP 3
5 Introduction In 1995, the National Health and Medical Research Council (NHMRC) launched the Clinical Guidelines for the Management of Early Breast Cancer (1). These guidelines aimed to provide breast cancer specialists with evidence-based information and recommendations for best practice in relation to the management of women with early stage breast cancer. It was intended that the NHMRC guidelines be adopted by surgeons, medical oncologists, radiotherapists, general practitioners and other health care providers involved in the management of women with early breast cancer. The next step in the implementation of the NHMRC guidelines is their effective dissemination to relevant health care providers. Relatively little is known about how best to encourage the implementation of clinical practice guidelines. A recent review of the effectiveness of guidelines at affecting clinical practice concluded that more active methods of dissemination, such as the use of computer prompting or reminder and feedback systems were more effective than simple postal dissemination of guidelines (2). However, no previous studies have involved breast cancer care guidelines. Therefore, this project aimed to assess the effectiveness of a computerised method of disseminating the NHMRC Guidelines for the Management of Early Breast Cancer. For the sake of brevity, these will be referred to as "the Guidelines" throughout this report. More specifically, the study aimed to assess the effectiveness of printed feedback from an interactive touchscreen computer survey, completed by patients, at increasing the proportion of medical oncology patients with early breast cancer who are treated in accordance with the Guidelines. The study focused on providing tools to assist medical oncologists in providing a level of care as outlined by the NHMRC, by accessing the recommendations contained in the Guidelines. This study provided an opportunity to explore one strategy which could potentially be effective at implementing guidelines in a clinical setting, while also attempting to ensure all women diagnosed with early breast cancer receive optimal treatment. A subset of the Guidelines considered most relevant to medical oncologists and their patients have been used as outcome measures, indicating the interactive computer program s effectiveness. The acceptability of the interactive computer program, to patients and medical oncologists, was also assessed in this study. Trial of Computerised Dissemination of Guidelines Final Report CERP 4
6 Method Design This project used a multiple baseline design, whereby each oncology unit acted as its own control. The intervention was introduced in each unit at a different time to control for any changes occurring outside of the trial. Each unit nominated a convenient starting date within the multiple baseline schedule. This design was chosen in preference to a randomised, controlled trial because the method of using interactive computers as a means of disseminating guidelines has not been widely studied. Although a randomised, controlled trial would have had greater power to detect changes, it would have necessitated involving a much larger number of medical oncology units and patients in order to assess the effectiveness of the method. Therefore, it was considered prudent to conduct this type of pilot study prior to a full-scale efficacy trial. Each unit was expected to see between five and 15 early breast cancer patients a week, sufficient to allow the detection of any significant changes in treatment practices. The number of women recruited from each medical oncology unit was anticipated to vary, depending on the number of women seen with early breast cancer. Data collection was set to run for 16 weeks: 6 baseline weeks and 10 intervention weeks. This was expected to allow adequate time to collect data for enough patients, given that the majority of breast cancer patients have chemotherapy in three week cycles, allowing multiple opportunities for feedback to be acted on. Outcome measures The process involved in determining the outcome measures required two steps. Firstly, it was necessary to establish which recommendations from the Guidelines could be translated into questions. Once these questions had been determined, questions were grouped appropriately. Although the majority of these questions could be answered by the patients, a subset of questions required input from patients oncologists. This subset included information about patients diagnoses and treatment regimens. This information was collected on standardised forms, produced automatically by the computer at the end of each relevant patient s completion of the computer survey (see Appendix 1). The development of the computer survey and of these forms is described in detail in the baseline report from this study (3). Process measures The acceptability of this intervention, to patients, was assessed using some extra questions included at the end of patients survey the second time they completed it. At the end of the intervention phase, medical oncologists, nursing and reception staff were also asked to complete a brief paper survey giving their perceptions about the acceptability of the intervention. Trial of Computerised Dissemination of Guidelines Final Report CERP 5
7 The feedback Two forms of feedback were developed for this project. The first type of feedback was an individualised summary feedback sheet that was printed automatically by the computer and placed in the patients files before they saw their oncologists. This feedback, which was based on patients responses to the computer survey, both summarised and highlighted the most important issues for each patient on that particular day. An example of a completed feedback sheet is included in Appendix 2. The second form of feedback, also given to clinicians throughout the intervention phase, was based on the information provided by the oncologists on the diagnostic and treatment information forms mentioned earlier. Using a standardised coding method, a member of the research team checked the diagnostic and treatment information against the Guidelines to determine for each patient whether she was diagnosed and/or treated in accordance with the Guidelines; she was not diagnosed and/or treated in accordance with the Guidelines; it was not possible to determine (from the information provided) if she was diagnosed and/or treated in accordance with the Guidelines. This feedback was then mailed back to the medical oncology unit, within five working days, to be inserted into the patient's file prior to their next visit. An example of this feedback is included in Appendix 3. Recruiting the medical oncologists A convenience sample of oncologists, working in five medical oncology units across Australia, were invited to participate by one of the co-investigators. All five senior oncologists approached accepted the invitation to their Unit to participate and took an active role in the development of the project materials. Recruiting the patients All breast cancer patients attending each participating medical oncology unit received an information letter explaining the project from one of the clinic staff members. The clinic staff member s position varied across the units and was either clinical trial coordinator, nursing unit manager, clinical nurse consultant or receptionist. All breast cancer patients were asked to participate in the study, as many patients would not know if their diagnosis was classified as of an early or advanced stage. The diagnostic and treatment information form was later used to determine and categorise individual patients' disease stages. Generally, patients gave their consent to participate by a series of questions presented on the computer. However, one unit required patients to also sign a paper consent form before completing the survey, due to ethics committee requirements. Consenting breast cancer patients answered the survey on the computer while waiting for their consultation with an oncologist. Trial of Computerised Dissemination of Guidelines Final Report CERP 6
8 A research assistant was present in each unit for the first week of data collection. Their tasks were to liaise with the oncologists and staff in each unit to determine the most appropriate location of the touch screen within the waiting area, and the location of the printer in a secure and convenient location so that the diagnosis information forms and the individualised feedback summary sheets could be immediately inserted into patients' files on completion of the survey. The research assistant then trained the oncologists and the unit staff, using a procedures manual, which detailed the specific instructions for using the computer, including how to deal with minor malfunctions. The patient survey was demonstrated and explained to all staff members. Throughout data collection, contact was maintained on at least a weekly basis with a staff member, nominated as project liaison person, at each unit. Results Throughout the 16 weeks of data collection, a total of 121 separate women with breast cancer completed the computer survey at least once. Of these, 87 completed their first survey during the baseline phase and 34 completed their first survey during the intervention phase. Across the four hospitals, 27 women completed the computer survey more than once: 19 women completed it twice, five women completed it three times, two women completed it four times and one woman completed it six times. Unfortunately, these numbers were considerably less than expected, making traditional time series analyses, as planned, inappropriate. Therefore, the results of this study are presented in two different ways. First, quantitative pre-post comparisons have been conducted, classifying women whose first survey was completed during the baseline phase as the pre-test group and those whose first survey was completed during the intervention phase as the post-test group. These results are summarised in the section entitled Quantitative Results. Although not ideal, due to the small numbers, percentages have been presented in all the tables to enable easier comparisons between the groups. The percentages in each table throughout this section of the results may not add to 100% due to rounding. Second, more qualitative, descriptive analyses were conducted to explore whether any changes occurred in the responses of those women who completed the survey more than once. These results are presented in the section entitled Qualitative Results. Trial of Computerised Dissemination of Guidelines Final Report CERP 7
9 Quantitative Results Participants Characteristics Table 1.1 summarises the demographic characteristics of the participating women. As shown, they were fairly evenly split between those aged less than and those aged more than 50 years. Slightly over three quarters were married or living with a partner; about half had a less than high school level of education; and almost half were in the workforce, with almost a third engaged in home duties. Table 1.1: Patients demographic characteristics (%) Baseline Phase (n=87) Intervention Phase (n=34) Total (n=121) Age years years Mean age (sd) 50.4 years (10.0) 48.7 years (9.6) 49.9 years (9.9) Median age 50 years 47 years 50 years Current marital status Married / living with partner Divorced / separated / widowed / single Level of education Didn t complete high school Year 10 or equivalent Year 12 or equivalent University / trade / technical / other Usual employment situation Employed full-time or part-time Home duties Student Retired / unemployed / unable to work / other Table 1.2 summarises the participating women s disease and past treatment characteristics. As shown, approximately half of the participating women were diagnosed with early-stage breast cancer, according to the Guidelines. However, for one in five participants, the information provided by the oncologists was insufficient to determine the stage of disease at diagnosis. Although a few women were attending for an initial visit with their oncologist, prior to starting treatment, the remaining women were reasonably split between those attending for treatment and those attending for follow-up only. There was a fairly even spread in the length of time since diagnosis. Most women had received surgical treatment, chemotherapy and radiotherapy, with a little over a quarter having had hormone therapy and very few having had ovarian treatment. Trial of Computerised Dissemination of Guidelines Final Report CERP 8
10 Table 1.2: Patients disease and treatment characteristics (%) Baseline Phase (n=87) Intervention Phase (n=34) Total (n=121) Disease stage at diagnosis Early (according to Guidelines) Advanced (according to Guidelines) Insufficient information to determine Reason for visiting oncology unit Initial consultation with oncologist Routine treatment session Follow-up visit Time since breast cancer diagnosis Less than 6 months months 2 years More than 2 years Past treatments Surgery (all types) Chemotherapy Hormone therapy Radiotherapy Ovarian treatment Level of Diagnosis in accordance with the Guidelines The Guidelines provide information on the most widely used information regarding how breast cancers are classified the TNM clinical classification. TNM classifications are utilised to assign stage groupings (ie: Stage I, II, III or IV) to cases of breast cancer. These stage groupings are used to determine whether a woman s diagnosis is generally classified as early or advanced breast cancer. Oncologists responses on the diagnostic and treatment information sheets, and their subsequent staging decisions, were compared against the criteria provided in the Guidelines see Appendix 3 for a summary of these criteria. Table 1.3 summarises the level of agreement between the oncologists staging decisions and the Guidelines during the baseline and intervention phases. The results appear somewhat mixed, with oncologists seeming more likely to stage patients earlier than the Guidelines during the intervention phase but more likely to assign patients the correct general classification (ie: early or advanced). Trial of Computerised Dissemination of Guidelines Final Report CERP 9
11 Table 1.3: The level of agreement between oncologists and the Guidelines regarding patients stage groupings and general classifications, based on their TNM data (%) All Patients Those With Diagnostic Forms Baseline Phase (n=87) Intervention Phase (n=34) Baseline Phase (n=76) Intervention Phase (n=26) Stage Grouping Oncologist agreed with Guidelines Oncologist earlier than Guidelines Oncologist later than Guidelines Insufficient information to determine Diagnostic form not returned General Classification Early, in agreement with Guidelines Advanced, in agreement with Guidelines Early, contrary to Guidelines Advanced, contrary to Guidelines Insufficient information to determine Diagnostic form not returned Level of Treatment in accordance with the Guidelines In addition to explicit staging criteria, the Guidelines also indicate the appropriateness of chemotherapy, hormone therapy and ovarian treatments for patients depending on their menopausal status, node status, oestrogen receptor status and their age see the flowchart in Appendix 3 for a summary of these criteria. Oncologists responses on the diagnostic and treatment information sheets were compared against the recommendations provided in the Guidelines to determine the apparent appropriateness of patients treatments. Table 1.4 summarises the level of agreement between patients treatments and the Guidelines. As the Guidelines refer to women with early breast cancer, the numbers shown are smaller than in other tables, as they represent only these women. Although little change was seen in the proportion of patients treated in accordance with the Guidelines, the overall results were encouragingly high and it is probable that other factors, which we were unable to measure, play a role for many of those patients apparently not treated in accordance with the Guidelines. Trial of Computerised Dissemination of Guidelines Final Report CERP 10
12 Table 1.4: The level of agreement between patients treatment regimens and the Guidelines, based on their menopausal, node and receptor status (%) Baseline Phase (n=47) Intervention Phase (n=17) Chemotherapy Correctly given Correctly not given 6 18 Incorrectly given 6 6 Incorrectly not given 2 0 Insufficient information to determine Hormone Therapy Correctly given Correctly not given Incorrectly given Incorrectly not given 6 6 Insufficient information to determine Ovarian Treatment Correctly given 2 0 Correctly not given Incorrectly given 2 0 Incorrectly not given 0 0 Insufficient information to determine Women s Satisfaction with their Level of Involvement in Treatment Decisions The Guidelines state that all women should be encouraged to participate in decisions about their treatment but indicate that women have the right to delegate all the decision-making responsibility to their doctors. Table 1.5 suggests a drop in women s satisfaction with their level of involvement during the intervention phase, with a tendency for them to feel less involved than they would like to be. Table 1.5: Women s satisfaction with their level of involvement in treatment decisions % women in treatment & F/U Baseline Phase (n=83) Intervention Phase (n=29) Happy with level of involvement Wanted to be MORE involved 7 21 Wanted to be LESS involved 0 0 Unsure 1 0 Trial of Computerised Dissemination of Guidelines Final Report CERP 11
13 The Adequacy and Appropriateness of Information Provided to Women The Guidelines also make recommendations about a wide range of information that should be available to women, including information about their diagnosis, potential treatments, the possible psychosocial impact of their illness and the availability of support services. The Guidelines also state that such information should be provided in a way and at a level that is appropriate for each patient. Therefore, this section reports on women s levels of satisfaction with both the amount and the nature of the information they received in relation to the range of issues raised within the Guidelines. Given the broad range of issues covered, not all questions were relevant for all patients. Therefore, the type and number of women answering each question is indicated within each table. The rationale for each issue is discussed and referenced in the baseline report (3). Table 1.6 summarises women s retrospective assessments of the initial information they received about their treatment in the oncology unit they were visiting. Table 1.6: Women s satisfaction with the amount of initial information in this department Baseline Phase Intervention Phase Treatment patients only (n=48) (n=14) Information about the aims of treatment Happy with amount of information Wanted MORE information 8 7 Wanted LESS information 2 0 Didn t get any information 0 0 Follow-up patients only (n=34) (n=15) Information about possible treatments Happy with amount of information Wanted MORE information Wanted LESS information 0 0 Didn t get any information 0 0 Information about the likely health outcomes Happy with amount of information Wanted MORE information 6 20 Wanted LESS information 3 0 Didn t get any information 3 7 Information about the possible side effects Happy with amount of information Wanted MORE information 21 0 Wanted LESS information 0 0 Didn t get any information 3 20 Trial of Computerised Dissemination of Guidelines Final Report CERP 12
14 Baseline Phase Intervention Phase Treatment & follow-up patients (n=82) (n=29) Information about the involved in treatment Happy with amount of information Wanted MORE information 9 14 Wanted LESS information 2 7 Didn t get any information 0 0 Overall information level Happy with amount of information Wanted MORE information Wanted LESS information 0 0 Didn t get any information 1 3 Table 1.7 summarises the satisfaction of women attending for follow-up visits regarding the amount of information received during their treatment about a range of support services. Table 1.7: Women s satisfaction with the amount of information received during treatment Baseline Phase Intervention Phase Follow-up patients only (n=34) (n=15) Information about support groups Happy with amount of information Happy - didn t want any information 9 13 Wanted MORE or SOME information Information about social workers Happy with information received Happy - didn t want any information Wanted MORE or SOME information 21 7 Information about counsellor or psychologists Happy with amount of information Happy - didn t want any information Wanted MORE or SOME information Information about breast cancer support services Happy with amount of information Happy - didn t want any information 3 20 Wanted MORE or SOME information Information about the goals of follow-up Happy with amount of information Happy - didn t want any information 3 19 Wanted MORE or SOME information NB: totals may not add to 100% as some women could not recall their level of satisfaction Trial of Computerised Dissemination of Guidelines Final Report CERP 13
15 Table 1.8 summarises women s responses regarding their current levels of need for help with information about a range of issues. Table 1.8: Women s current levels of need for information Baseline Phase Intervention Phase Treatment patients only (n=48) (n=14) Information about the pros & cons of hormone therapies GREAT or SOME need for information No need - happy with current information Issue not a concern Information about the pros & cons of chemotherapy GREAT or SOME need for information No need - happy with current information Issue not a concern 4 0 Information about the pros & cons of clinical trials GREAT or SOME need for information No need - happy with current information Issue not a concern Follow-up patients only (n=34) (n=16) Information about meaning for other women in family GREAT or SOME need for information No need - happy with current information Issue not a concern 12 0 Information about how often need check-ups GREAT or SOME need for information No need - happy with current information Issue not a concern 3 0 Information about whether future tests will be needed GREAT or SOME need for information No need - happy with current information Issue not a concern 3 0 Treatment & follow-up patients (n=84) (n=30) Information about the causes of breast cancer GREAT or SOME need for information No need - happy with current information Issue not a concern 5 0 Information about extent of breast cancer in community GREAT or SOME need for information No need - happy with current information Issue not a concern 6 13 Trial of Computerised Dissemination of Guidelines Final Report CERP 14
16 Baseline Phase Intervention Phase Treatment & follow-up patients (n=84) (n=30) Information about their type of breast cancer GREAT or SOME need for information No need - happy with current information Issue not a concern 0 7 Information about their chance of the cancer returning GREAT or SOME need for information No need - happy with current information Issue not a concern 1 0 Information about their chance of being cured GREAT or SOME need for information No need - happy with current information Issue not a concern 1 1 The Guidelines also make some recommendations regarding communication between oncologists and patients general practitioners and about the provision of information to women in the form of written treatment and follow-up plans and in the form of tape recordings of important parts of consultations. Table 1.9 summarises the oncologists performance in relation to these issues. Table 1.9: Oncologists use of other communication channels Baseline Phase Intervention Phase Treatment patients only (n=48) (n=14) Got a written treatment plan? Yes No and would like one No and don t want one Got a tape recording of consultation? Yes 2 7 No and would like one No and don t want one GP informed about treatment? Yes No or unsure and don t want them informed 2 14 No or unsure and would like one 17 7 Don t have regular GP 2 0 Trial of Computerised Dissemination of Guidelines Final Report CERP 15
17 Table 1.10 summarises women s current need for help with a variety of issues. Table 1.10: Women s current levels of need for help Baseline Phase Intervention Phase Treatment patients only (n=48) (n=14) With caring for children, elderly parents or spouse Need LOTS or SOME help No need - happy with current help Issue not a concern With finding out about financial assistance available Need LOTS or SOME help No need - happy with current help Issue not a concern With somewhere to stay near the hospital Need LOTS or SOME help 0 0 No need - happy with current help Issue not a concern With any special clothing, such as prostheses Need LOTS or SOME help 6 8 No need - happy with current help Issue not a concern Follow-up patients only (n=34) (n=15) With finding out how to lie a healthier lifestyle Need LOTS or SOME help No need - happy with current help Issue not a concern 12 7 With being able to talk to others about experiences Need LOTS or SOME help No need - happy with current help Issue not a concern With any long term effects on family relationships Need LOTS or SOME help No need - happy with current help Issue not a concern Treatment & follow-up patients (n=82) (n=30) With finding out how to meet a counsellor Need LOTS or SOME help No need - happy with current help Issue not a concern Trial of Computerised Dissemination of Guidelines Final Report CERP 16
18 Baseline Phase Intervention Phase Treatment & follow-up patients (n=82) (n=30) With finding out how to join a support group Need LOTS or SOME help No need - happy with current help Issue not a concern With finding out about the BCSS Need LOTS or SOME help No need - happy with current help Issue not a concern With dealing with the possible long term effects for them Need LOTS or SOME help No need - happy with current help Issue not a concern 9 3 With feeling able to express their thoughts & feelings Need LOTS or SOME help No need - happy with current help Issue not a concern 12 7 Appropriateness of patient follow-up care The Guidelines also recommends a minimal follow-up schedule that should be observed for women who have completed chemotherapy or hormone therapy. In summary, it states that a history and examination should be undertaken according to the amount of time since treatment was completed: 0-1 years: every 3 months; 1-5 years: every six months; after 5 years: every year. Table 1.11 provides information on how often the patients attending for follow-up had presented for a follow-up consultation with a medical oncologist. Table 1.11: The proportion of women who had a follow-up consultation and length of time To come but was 97% ok at baseline so little change expected. Qualitative Results To come will look at what changes, if any have occurred in the responses of those people having completed the survey more than once. Trial of Computerised Dissemination of Guidelines Final Report CERP 17
19 Acceptability of the Intervention Acceptability to patients To come Acceptability to hospital staff To come To come when all results explored in more depth. Discussion References 1. National Health and Medical Research Council. Clinical Practice Guidelines: The Management of Early Breast Cancer. Australian Government Publishing Service Davis D, Thomson M, Oxman A, Haynes R. Evidence for the effectiveness of CME. A review of 50 randomised controlled trials. JAMA 1992;268: Rankin N, Newell S, Sanson-Fisher RW, Girgis A. Assessing the Effectiveness of Computerised Dissemination of the NHMRC Clinical Guidelines for the Management of Early Breast Cancer : Baseline Report. Report prepared for the NHMRC National Breast Cancer Centre, July Trial of Computerised Dissemination of Guidelines Final Report CERP 18
20 Appendix 1: Diagnostic and Treatment Information Form Medical Oncologists Dissemination Trial PATIENT DIAGNOSIS RECORD SHEET - (BREAST CANCER PATIENTS ONLY) PATIENT SURNAME: EXAMPLE Date of Birth: 01/10/50 FIRST INITIAL: A TODAY S DATE: 17/7/98 Date treatment commenced in this department : Completed: Please circle the relevant answer for each point I. General classification: EARLY ADVANCED II. Menopausal status at diagnosis: PRE PERI POST III. Node: POSITIVE NEGATIVE IV. Oestrogen receptor: POSITIVE NEGATIVE Treatment History/Status NO YES If YES, please specify: surgery 0 0 mastectomy 0 breast conserving surgery 0 chemotherapy 0 0 regime cycle hormone therapy 0 0 tamoxifen other radiotherapy 0 0 ovarian therapy 0 0 details, if known: details, if known: TNM Classification - please circle T Categories TX T0 Tis T1 T1a T1b T1c T2 T3 T4 T4a T4b T4c T4d N Categories NX N0 N1 N2 N3 M Categories MX M0 M1 Stage Groupings - please circle Stage 0 Stage I Stage IIA Stage IIB Stage IIIA Stage IIIB Stage IV Trial of Computerised Dissemination of Guidelines Final Report CERP 19
21 Appendix 2: Computerised Feedback to Oncologists Medical Oncologists Dissemination Trial PATIENT FEEDBACK SHEET - (BREAST CANCER PATIENTS ONLY) PATIENT SURNAME: EXAMPLE Date of Birth: 01/10/50 FIRST INITIAL: A TODAY S DATE: 17/7/98 Patient is attending for a treatment consultation This patient is currently undergoing: chemotherapy hormone therapy 1. Patient preferences and satisfaction information wanted about treatment choices? satisfaction with involvement in treatment choices? satisfaction with information about steps of treatment? satisfaction with amount of info so far? satisfaction with detail about aims of treatment? 2. Information needs the chances of being cured the causes of breast cancer your type of breast cancer the extent of breast cancer in the community the chances of having a recurrence the benefits and side effects of hormone therapies the benefits and side effects of chemotherapy the pros and cons of taking part in a clinical trial 3. Current need for help with finding out how to join a support group any special clothing needed finding out about the breast cancer support service dealing with the possible long term effects for you feeling able to express your thoughts and feelings caring for children, elderly parents, or spouse finding out about how to meet with a counsellor finding out about financial assistance that may be available 4. Other factors wants GP informed of treatment? want a written treatment plan prepared by the doctor? want a tape recording of the doctor explaining treatment? want a chance to ask questions? satisfied with choices satisfied with involvement satisfied would like more information satisfied high need low need no need low need no need low need no need high need no need low need high need high need no need low need no need high need GP already informed would like written treatment plan doesn't want tape Very important Trial of Computerised Dissemination of Guidelines Final Report CERP 20
22 PHYSICAL SYMPTOMS SYMPTOM Session: 27/6/98 nausea vomiting tiredness constipation diarrhoea hair loss loss of appetite skin rash metallic taste in mouth mouth sores Today s Session: 17/7/98 hot flushes Double tick indicates that the patient found this side effect debilitating ANXIETY & DEPRESSION LEVELS Key: (0-7: low level; 8-10 borderline level; clinical level) HADS Score Score Anxiety Depression Visit Trial of Computerised Dissemination of Guidelines Final Report CERP 21
23 Appendix 3: Diagnostic & Treatment Feedback Sheets Patient s Details Patient s Name: Date of birth: Date seen: On their last Patient Diagnosis Record Sheet, the following information was recorded about this patient s diagnosis: TNM code: T= N= M= missing Disease stage: 0 I IIA IIB IIIA IIIB IV missing The Guidelines classification, based on TNM data 0 I IIA IIB IIIA IIIB IV can t tell Do you agree with the Guidelines? yes no can t tell General classification: early missing advanced early can t tell advanced yes no can t tell The indication of the patient s disease stage IS / IS NOT in accordance with the Guidelines / CAN T BE DETERMINED due to missing information. According to the Guidelines: Stage T code N code M code Stage 0 Tis N0 M0 Stage I T1 N0 M0 Stage IIA T0 N1 M0 T1 N1 M0 T2 N0 M0 Stage IIB T2 N1 M0 T3 N0 M0 Stage IIIA T0 N2 M0 T1 N2 M0 T2 N2 M0 T3 N1, N2 M0 Stage IIIB T4 Any N M0 Any T N3 M0 Stage IV Any T Any N M1 The indication of the patient s general classification IS / IS NOT in accordance with the Guidelines / CAN T BE DETERMINED due to missing information. According to the Guidelines: Early = T1 or T2 AND N0 or N1 AND M0 Advanced = T3 or T4 OR N2 OR M1 Trial of Computerised Dissemination of Guidelines Final Report CERP 22
24 Medical Oncologists Dissemination Trial: Treatment Feedback (for patients with early breast cancer only) On the last Patient Diagnosis Record Sheet, the following was noted about this patient s treatment history: Menopausal status: pre peri post missing Node status: +ve -ve unclear missing Receptor status: +ve -ve unclear missing Age: < 65 years 65+ years Systemic treatments: chemotherapy: none CMF(P) doxorubicin & cyclophosphamide other missing hormone therapy: none tamoxifen other missing ovarian therapy: none some missing The patient s indicated treatment IS / IS NOT in accordance with the Guidelines / CAN T BE DETERMINED due to missing information. The flowchart on the back of this page summarises the NHMRC s recommendations regarding chemotherapy, hormone therapy and ovarian therapy, based on each patient s menopausal status, node status, oestrogen receptor status and, in some cases, their age. Trial of Computerised Dissemination of Guidelines Final Report CERP 23
25 Decision Flowchart of Appropriate Systemic Therapies: Early Breast Cancer Patients Only 1 Menopausal status pre post Node status Node status +ve -ve +ve -ve Receptor status Receptor status Receptor status +ve -ve +ve -ve +ve -ve +ve -ve Patient s age Patient s age < < Prognostic features* Prognostics* Prognostics* good poor good poor good poor good poor C or O C only none C or O none C only C & H H only C & H C only H only C only none C only Treatment Key: C = chemotherapy (CMF or doxorubicin & cyclophosphamide), H = hormone therapy (tamoxifen), O = ovarian ablation * Prognostic features: Poor prognostic features are defined as tumours > 20mm; or tumours 11-20mm with additional features such as oestrogen and progesterone receptor negativity, vessel space invasion or high histological grade. 1. NHMRC. The Management of Early Breast Cancer: Clinical Practice Guidelines. 1995
Are touchscreen computer surveys acceptable to medical oncology patients?
 Southern Cross University epublications@scu School of Education 1997 Are touchscreen computer surveys acceptable to medical oncology patients? Sallie Newell Southern Cross University Rob William Sanson-Fisher
Southern Cross University epublications@scu School of Education 1997 Are touchscreen computer surveys acceptable to medical oncology patients? Sallie Newell Southern Cross University Rob William Sanson-Fisher
Combined chemotherapy and Radiotherapy for Patients with Breast Cancer and Extensive Nodal Involvement.
 Combined chemotherapy and Radiotherapy for Patients with Breast Cancer and Extensive Nodal Involvement. Ung O, Langlands A, Barraclough B, Boyages J. J Clin Oncology 13(2) : 435-443, Feb 1995 STUDY DESIGN
Combined chemotherapy and Radiotherapy for Patients with Breast Cancer and Extensive Nodal Involvement. Ung O, Langlands A, Barraclough B, Boyages J. J Clin Oncology 13(2) : 435-443, Feb 1995 STUDY DESIGN
Breast Cancer Network Australia Breast Care Nurse Breast Reconstruction Survey September 2011
 Breast Cancer Network Australia Breast Care Nurse Breast Reconstruction Survey September 2011 This project was undertaken with the support of Cancer Australia through the Building Cancer Support Networks
Breast Cancer Network Australia Breast Care Nurse Breast Reconstruction Survey September 2011 This project was undertaken with the support of Cancer Australia through the Building Cancer Support Networks
Hormonal Therapies for Breast Cancer. Westmead Breast Cancer Institute
 Hormonal Therapies for Breast Cancer Westmead Breast Cancer Institute Hormonal (endocrine) therapies for breast cancer (also called anti-hormone treatment ) Hormonal therapy is used to treat breast cancers
Hormonal Therapies for Breast Cancer Westmead Breast Cancer Institute Hormonal (endocrine) therapies for breast cancer (also called anti-hormone treatment ) Hormonal therapy is used to treat breast cancers
How is primary breast cancer treated? This booklet is for anyone who has primary breast cancer and wants to know more about how it is treated.
 How is primary breast cancer treated? This booklet is for anyone who has primary breast cancer and wants to know more about how it is treated. How is primary breast cancer treated? Part 1 the treatment
How is primary breast cancer treated? This booklet is for anyone who has primary breast cancer and wants to know more about how it is treated. How is primary breast cancer treated? Part 1 the treatment
Phyllodes tumours: borderline and malignant
 Phyllodes tumours: borderline and malignant This booklet is for people who would like more information about borderline or malignant phyllodes tumours. It describes what they are, the symptoms, how a diagnosis
Phyllodes tumours: borderline and malignant This booklet is for people who would like more information about borderline or malignant phyllodes tumours. It describes what they are, the symptoms, how a diagnosis
Breast Cancer Treatment
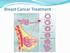 Breast Cancer Treatment Treatment 2 aspects 1. Treatment of the breast itself: Local Treatment 2. Treatment of the whole body = Systemic treatment Local Treatment Surgery +/- Radiation Usually: a. Breast
Breast Cancer Treatment Treatment 2 aspects 1. Treatment of the breast itself: Local Treatment 2. Treatment of the whole body = Systemic treatment Local Treatment Surgery +/- Radiation Usually: a. Breast
surgery choices For Women with Early-Stage Breast Cancer family EDUCATION PATIENT
 surgery choices For Women with Early-Stage Breast Cancer PATIENT & family EDUCATION U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute As a woman with
surgery choices For Women with Early-Stage Breast Cancer PATIENT & family EDUCATION U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute As a woman with
Development and pilot testing of a comprehensive support package for bowel cancer survivors
 Development and pilot testing of a comprehensive support package for bowel cancer survivors Michael Jefford, Carl Baravelli, Megan Rogers, Penelope Schofield, Kerryann Lotfi-Jam, Meinir Krishnasamy, Carmel
Development and pilot testing of a comprehensive support package for bowel cancer survivors Michael Jefford, Carl Baravelli, Megan Rogers, Penelope Schofield, Kerryann Lotfi-Jam, Meinir Krishnasamy, Carmel
It can also be used to try to preserve fertility during chemotherapy (see page 4). Goserelin as a treatment for breast cancer
 Goserelin (Zoladex) This booklet explains what goserelin is, when it may be prescribed, how it works and what side effects may occur. Goserelin is the generic (non branded) name of the drug and how it
Goserelin (Zoladex) This booklet explains what goserelin is, when it may be prescribed, how it works and what side effects may occur. Goserelin is the generic (non branded) name of the drug and how it
 Understanding ovarian cancer An information sheet for women with ovarian cancer, their family and friends If you or someone you love has recently been diagnosed with ovarian cancer, you re sure to have
Understanding ovarian cancer An information sheet for women with ovarian cancer, their family and friends If you or someone you love has recently been diagnosed with ovarian cancer, you re sure to have
BREAST CANCER PATHOLOGY
 BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
I MAY NOT HAVE ALL THE ANSWERS BUT AT LEAST I HAVE THE QUESTIONS TO GET THE PROPER. care guidelines
 I MAY NOT HAVE ALL THE ANSWERS BUT AT LEAST I HAVE THE QUESTIONS TO GET THE PROPER care guidelines Hi, Being diagnosed with breast cancer is tough at any age, but being diagnosed when you re young makes
I MAY NOT HAVE ALL THE ANSWERS BUT AT LEAST I HAVE THE QUESTIONS TO GET THE PROPER care guidelines Hi, Being diagnosed with breast cancer is tough at any age, but being diagnosed when you re young makes
Southern Cross University Sallie Newell Southern Cross University Rob William Sanson-Fisher University of Newcastle
 Southern Cross University epublications@scu School of Education 1998 How well do medical oncologists' perceptions reflect their patients' reported physical and psychosocial problems? data from a survey
Southern Cross University epublications@scu School of Education 1998 How well do medical oncologists' perceptions reflect their patients' reported physical and psychosocial problems? data from a survey
Australians and mental health How have things changed?
 Australians and mental health How have things changed? Research study into the attitudes of Australians towards mental health for World Mental Health Day, 10 October 2016. Prepared for Flourish Australia
Australians and mental health How have things changed? Research study into the attitudes of Australians towards mental health for World Mental Health Day, 10 October 2016. Prepared for Flourish Australia
Supportive Care Audit Mercy Hospital for Women - Heidelberg
 Supportive Care Audit 2013-2014 Mercy Hospital for Women - Heidelberg Melissa Shand Service Improvement Facilitator NEMICS July 2015 Acknowledgments Mandy Byrne NEMICS Cancer and Data Information Analyst
Supportive Care Audit 2013-2014 Mercy Hospital for Women - Heidelberg Melissa Shand Service Improvement Facilitator NEMICS July 2015 Acknowledgments Mandy Byrne NEMICS Cancer and Data Information Analyst
Radiotherapy to one side of the mouth and neck
 Clinical Oncology Radiotherapy to one side of the mouth and neck Consent information for patients Radiotherapy may be given on its own or with chemotherapy (separate information will be given about chemotherapy).
Clinical Oncology Radiotherapy to one side of the mouth and neck Consent information for patients Radiotherapy may be given on its own or with chemotherapy (separate information will be given about chemotherapy).
Trialling computer touch-screen technology to assess psychological distress in patients with gynaecological cancer
 Trialling computer touch-screen technology to assess psychological distress in patients with gynaecological cancer Georgia Halkett 1, Moyez Jiwa 2, Pauline Tanner 3, Céline Fournier 1, Paul Katris 4 1-WA
Trialling computer touch-screen technology to assess psychological distress in patients with gynaecological cancer Georgia Halkett 1, Moyez Jiwa 2, Pauline Tanner 3, Céline Fournier 1, Paul Katris 4 1-WA
This report summarizes the stakeholder feedback that was received through the online survey.
 vember 15, 2016 Test Result Management Preliminary Consultation Online Survey Report and Analysis Introduction: The College s current Test Results Management policy is under review. This review is being
vember 15, 2016 Test Result Management Preliminary Consultation Online Survey Report and Analysis Introduction: The College s current Test Results Management policy is under review. This review is being
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Docetaxel-EC PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Docetaxel-EC PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM FERRERI / IELSG PROTOCOL. Patient s first names. Date of birth
 Page 1 of 6 FORM FERRERI / IELSG PROTOCOL Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital NHS
Page 1 of 6 FORM FERRERI / IELSG PROTOCOL Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital NHS
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CHOP 21 +/- RITUXIMAB. Patient s first names.
 Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CHOP 21 +/- RITUXIMAB Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CHOP 21 +/- RITUXIMAB Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: BEP (5 day) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: BEP (5 day) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: FEC-P PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP: MALE
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: FEC-P PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP: MALE
Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC - CP) Pilot results
 Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC - CP) Pilot results Outline 1. EPIC-CP Background 2. EPIC-CP Pilots - Phase I and II 3. EPIC Pilot Phase II Results 2 EPIC-CP Background
Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC - CP) Pilot results Outline 1. EPIC-CP Background 2. EPIC-CP Pilots - Phase I and II 3. EPIC Pilot Phase II Results 2 EPIC-CP Background
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM ABVD. Patient s first names.
 Patient identifier/label: Page 1 of 6 Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital NHS number
Patient identifier/label: Page 1 of 6 Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital NHS number
Breast cancer. In this fact sheet: Breast cancer: English
 Breast cancer: English Breast cancer This information is about breast cancer and treatments for breast cancer. Any words that are underlined are explained in the word list at the end. If you have any questions,
Breast cancer: English Breast cancer This information is about breast cancer and treatments for breast cancer. Any words that are underlined are explained in the word list at the end. If you have any questions,
SURVEY ON THE EXPERIENCE AND NEEDS OF PATIENTS
 SURVEY ON THE EXPERIENCE AND NEEDS OF PATIENTS LIVING WITH OESOPHAGEAL OR GASTRIC CANCER I am writing to you on behalf of EuropaColon, a not-for-profit organization established in 00, dedicated to saving
SURVEY ON THE EXPERIENCE AND NEEDS OF PATIENTS LIVING WITH OESOPHAGEAL OR GASTRIC CANCER I am writing to you on behalf of EuropaColon, a not-for-profit organization established in 00, dedicated to saving
Health Bites Breast Cancer. Breast Cancer. Normal breast
 Health Bites Breast Cancer Breast Cancer Normal breast The normal breast tissue varies in size and shape. The breasts rest in front of the rib cage. The breasts are made up of fatty tissue, milk ducts
Health Bites Breast Cancer Breast Cancer Normal breast The normal breast tissue varies in size and shape. The breasts rest in front of the rib cage. The breasts are made up of fatty tissue, milk ducts
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Carboplatin-Etoposide PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Carboplatin-Etoposide PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
Audit. Public Health Monitoring Report on 2006 Data. National Breast & Ovarian Cancer Centre and Royal Australasian College of Surgeons.
 National Breast & Ovarian Cancer Centre and Royal Australasian College of Surgeons Audit Public Health Monitoring Report on 2006 Data November 2009 Prepared by: Australian Safety & Efficacy Register of
National Breast & Ovarian Cancer Centre and Royal Australasian College of Surgeons Audit Public Health Monitoring Report on 2006 Data November 2009 Prepared by: Australian Safety & Efficacy Register of
The Accessible Information Standard - guidance for practices
 The Accessible Information Standard - guidance for practices The Accessible Information Standard guidance for practices The Accessible Information Standard (AIS) known officially as SCCI1605 Accessible
The Accessible Information Standard - guidance for practices The Accessible Information Standard guidance for practices The Accessible Information Standard (AIS) known officially as SCCI1605 Accessible
Not Equal: Follow-up workshop
 Not Equal: Follow-up workshop As part of our ongoing work to ensure the voices of Deaf people are heard, on 23rd March we held a further workshop to bring commissioners and providers of Health and Social
Not Equal: Follow-up workshop As part of our ongoing work to ensure the voices of Deaf people are heard, on 23rd March we held a further workshop to bring commissioners and providers of Health and Social
Breast Cancer in Younger Women. Westmead Breast Cancer Institute
 Breast Cancer in Younger Women Westmead Breast Cancer Institute Breast cancer in younger women Only 6% of breast cancers in Australia develop in women under the age of 40. In women aged 35 39 only 65 women
Breast Cancer in Younger Women Westmead Breast Cancer Institute Breast cancer in younger women Only 6% of breast cancers in Australia develop in women under the age of 40. In women aged 35 39 only 65 women
A Guide for Women with Breast Cancer
 INFORMATION SHEET The sheet has information about diagnosis, treatment, practical support and the emotional impact of breast cancer. We hope this information sheet will answer some of your questions and
INFORMATION SHEET The sheet has information about diagnosis, treatment, practical support and the emotional impact of breast cancer. We hope this information sheet will answer some of your questions and
Computerised Tomography Pulmonary Angiography (CTPA)
 Computerised Tomography Pulmonary Angiography (CTPA) Radiology Department Patient information leaflet This leaflet tells you about having a CT Pulmonary Angiography (CTPA) scan. If you have any questions
Computerised Tomography Pulmonary Angiography (CTPA) Radiology Department Patient information leaflet This leaflet tells you about having a CT Pulmonary Angiography (CTPA) scan. If you have any questions
iprevent Breast Cancer Risk Assessment and Risk Management Decision Support Tool Kelly Anne Phillips MBBS MD FRACP
 iprevent Breast Cancer Risk Assessment and Risk Management Decision Support Tool Click to edit Master subtitle style Kelly Anne Phillips MBBS MD FRACP Medical Oncologist, Head Peter Mac Breast and Ovarian
iprevent Breast Cancer Risk Assessment and Risk Management Decision Support Tool Click to edit Master subtitle style Kelly Anne Phillips MBBS MD FRACP Medical Oncologist, Head Peter Mac Breast and Ovarian
Patient identifier/label: Page 1 of 7 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM EC-T: EPIRUBICIN / CYCLOPHOSPHAMIDE - DOCETAXEL
 Patient identifier/label: Page 1 of 7 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM EC-T: EPIRUBICIN / CYCLOPHOSPHAMIDE - DOCETAXEL Patient s surname/family name Patient s first names Date of birth
Patient identifier/label: Page 1 of 7 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM EC-T: EPIRUBICIN / CYCLOPHOSPHAMIDE - DOCETAXEL Patient s surname/family name Patient s first names Date of birth
Breast Magnetic Resonance Imaging (MRI) Westmead Breast Cancer Institute
 Breast Magnetic Resonance Imaging (MRI) Westmead Breast Cancer Institute What is breast MRI? Breast MRI is a technique that uses a magnetic field to create an image of the breast tissue, using hundreds
Breast Magnetic Resonance Imaging (MRI) Westmead Breast Cancer Institute What is breast MRI? Breast MRI is a technique that uses a magnetic field to create an image of the breast tissue, using hundreds
PatientsLikeMe IOM Survey. Paul Wicks, PhD.
 PatientsLikeMe IOM Survey Paul Wicks, PhD. Outline Rationale Methods Results Participants Health Care Providers Data Sharing Beliefs Care Coordination Reasons for Joining PatientsLikeMe Sharing on PatientsLikeMe
PatientsLikeMe IOM Survey Paul Wicks, PhD. Outline Rationale Methods Results Participants Health Care Providers Data Sharing Beliefs Care Coordination Reasons for Joining PatientsLikeMe Sharing on PatientsLikeMe
Wales Cancer Patient Experience. Survey Aneurin Bevan University Health Board. Published January 2014
 Wales Cancer Patient Experience Survey 2013 Aneurin Bevan University Health Published January 2014 The Wales Cancer Patient Experience Survey was undertaken by Quality Health on behalf of the Welsh Government
Wales Cancer Patient Experience Survey 2013 Aneurin Bevan University Health Published January 2014 The Wales Cancer Patient Experience Survey was undertaken by Quality Health on behalf of the Welsh Government
Patients preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile?
 British Journal of Cancer (2005) 93, 1319 1323 All rights reserved 0007 0920/05 $30.00 www.bjcancer.com Patients preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile?
British Journal of Cancer (2005) 93, 1319 1323 All rights reserved 0007 0920/05 $30.00 www.bjcancer.com Patients preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile?
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Nintedanib-Docetaxel PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Nintedanib-Docetaxel PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CISPLATIN + ETOPOSIDE ORAL/INTRAVENOUS
 Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CISPLATIN + ETOPOSIDE ORAL/INTRAVENOUS Patient s surname/family name Patient s first names Date of birth Hospital
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CISPLATIN + ETOPOSIDE ORAL/INTRAVENOUS Patient s surname/family name Patient s first names Date of birth Hospital
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Ductal carcinoma in situ (DCIS)
 Ductal carcinoma in situ (DCIS) This booklet is for people who would like more information about ductal carcinoma in situ (DCIS). It describes what DCIS is, the symptoms, how a diagnosis is made and possible
Ductal carcinoma in situ (DCIS) This booklet is for people who would like more information about ductal carcinoma in situ (DCIS). It describes what DCIS is, the symptoms, how a diagnosis is made and possible
INFORMATION BROCHURE - ALLOCATE
 INFORMATION BROCHURE - ALLOCATE AustraLian Ovarian Cancer Assortment Trial Principal Investigator: Prof Michael Quinn, Royal Women s Hospital This Participant Information Sheet and Consent Form is 7 pages
INFORMATION BROCHURE - ALLOCATE AustraLian Ovarian Cancer Assortment Trial Principal Investigator: Prof Michael Quinn, Royal Women s Hospital This Participant Information Sheet and Consent Form is 7 pages
Re-audit of Radiotherapy Waiting Times 2005
 Abstract Re-audit of Radiotherapy Waiting Times 2005 E. Summers, M Williams Royal College of Radiologists, 38 Portland Place, London W1B 4JQ, UK Aim: To determine current waiting times for radiotherapy
Abstract Re-audit of Radiotherapy Waiting Times 2005 E. Summers, M Williams Royal College of Radiologists, 38 Portland Place, London W1B 4JQ, UK Aim: To determine current waiting times for radiotherapy
Defining quality in ovarian cancer services: the patient perspective
 Defining quality in ovarian cancer services: the patient perspective 1 Contents Introduction... 3 Awareness and early diagnosis... 4 Information and support... 5 Treatment and care... 6 Living with and
Defining quality in ovarian cancer services: the patient perspective 1 Contents Introduction... 3 Awareness and early diagnosis... 4 Information and support... 5 Treatment and care... 6 Living with and
Ascertaining and reporting interval cancers in BreastScreen Aotearoa: A protocol NATIONAL SCREENING UNIT (NSU) MINISTRY OF HEALTH
 Ascertaining and reporting interval cancers in BreastScreen Aotearoa: A protocol NATIONAL SCREENING UNIT (NSU) MINISTRY OF HEALTH Dr. Simon Baker National Screening Unit Ministry of Health October 2005
Ascertaining and reporting interval cancers in BreastScreen Aotearoa: A protocol NATIONAL SCREENING UNIT (NSU) MINISTRY OF HEALTH Dr. Simon Baker National Screening Unit Ministry of Health October 2005
Clinical Oncology Radiotherapy to the larynx (4 week treatment) Consent information for patients
 Clinical Oncology Radiotherapy to the larynx (4 week treatment) Consent information for patients Radiotherapy treatment is used for early larynx cancer. We are aiming to cure the cancer and it is successful
Clinical Oncology Radiotherapy to the larynx (4 week treatment) Consent information for patients Radiotherapy treatment is used for early larynx cancer. We are aiming to cure the cancer and it is successful
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM CYTARABINE (HIGH DOSE)
 Patient identifier/label: Page 1 of 6 CYTARABINE Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital
Patient identifier/label: Page 1 of 6 CYTARABINE Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas Hospital King s College Hospital Lewisham Hospital
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Gemcitabine- Carboplatin PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Gemcitabine- Carboplatin PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
UNC Family Health Study
 Health Cognition & Behavior Lab Person County Pilot Study on HPV Vaccination (2006) Updated 04/30/2010 This study was conducted with women (n=146) in two healthcare facilities in Person County (a rural
Health Cognition & Behavior Lab Person County Pilot Study on HPV Vaccination (2006) Updated 04/30/2010 This study was conducted with women (n=146) in two healthcare facilities in Person County (a rural
Hello and welcome to Patient Power sponsored by UCSF Medical Center. I m Andrew Schorr.
 The Integrated Approach to Treating Cancer Symptoms Webcast March 1, 2012 Michael Rabow, M.D. Please remember the opinions expressed on Patient Power are not necessarily the views of UCSF Medical Center,
The Integrated Approach to Treating Cancer Symptoms Webcast March 1, 2012 Michael Rabow, M.D. Please remember the opinions expressed on Patient Power are not necessarily the views of UCSF Medical Center,
Young Adult Cancer Service in south-east London
 Young Adult Cancer Service in south-east London Contents p.3 About our service p.5 Meet the young adult cancer team p.12 Peer support p.12 Young adult research p.13 Useful web links p.14 Notes p.16 Contact
Young Adult Cancer Service in south-east London Contents p.3 About our service p.5 Meet the young adult cancer team p.12 Peer support p.12 Young adult research p.13 Useful web links p.14 Notes p.16 Contact
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Niraparib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Niraparib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Irinotecan- Capecitabine (CAPIRI) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier)
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Irinotecan- Capecitabine (CAPIRI) PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier)
Treatment of oligometastatic NSCLC
 Treatment of oligometastatic NSCLC Jarosław Kużdżał Department of Thoracic Surgery Jagiellonian University Collegium Medicum, John Paul II Hospital, Cracow New idea? 14 NSCLC patients with solitary extrathoracic
Treatment of oligometastatic NSCLC Jarosław Kużdżał Department of Thoracic Surgery Jagiellonian University Collegium Medicum, John Paul II Hospital, Cracow New idea? 14 NSCLC patients with solitary extrathoracic
Standard Reporting Template
 Appendix Seven Annual Report Template Standard Reporting Template London Region [North Central & East/North West/South London] Area Team 2017/2018 Patient Participation Enhanced Service Reporting Template
Appendix Seven Annual Report Template Standard Reporting Template London Region [North Central & East/North West/South London] Area Team 2017/2018 Patient Participation Enhanced Service Reporting Template
Victorian Paediatric Oncology Situational Analysis & Workforce Requirements
 - Victorian Paediatric Oncology Situational Analysis & Workforce Requirements 2012-2026 SUMMARY REPORT May 2013 1 Contents Executive summary...3 1. Introduction...6 2. Project method...8 2.1 Estimating
- Victorian Paediatric Oncology Situational Analysis & Workforce Requirements 2012-2026 SUMMARY REPORT May 2013 1 Contents Executive summary...3 1. Introduction...6 2. Project method...8 2.1 Estimating
Trends in Cancer Survival in NSW 1980 to 1996
 Trends in Cancer Survival in NSW 19 to 1996 Xue Q Yu Dianne O Connell Bruce Armstrong Robert Gibberd Cancer Epidemiology Research Unit Cancer Research and Registers Division The Cancer Council NSW August
Trends in Cancer Survival in NSW 19 to 1996 Xue Q Yu Dianne O Connell Bruce Armstrong Robert Gibberd Cancer Epidemiology Research Unit Cancer Research and Registers Division The Cancer Council NSW August
OVERDETECTION INFORMATION IN A BREAST CANCER SCREENING DECISION AID
 OVERDETECTION INFORMATION IN A BREAST CANCER SCREENING DECISION AID RANDOMISED CONTROLLED TRIAL Jolyn Hersch SYDNEY MEDICAL SCHOOL Screening and Test Evaluation Program (STEP) Sydney School of Public Health
OVERDETECTION INFORMATION IN A BREAST CANCER SCREENING DECISION AID RANDOMISED CONTROLLED TRIAL Jolyn Hersch SYDNEY MEDICAL SCHOOL Screening and Test Evaluation Program (STEP) Sydney School of Public Health
GOVERNING BODY MEETING IN VITRO FERTILISATION (IVF) AND ASSISTED CONCEPTION CONSULTATION. Matt Rangué, Chief Nurse, NHS Southend CCG
 AGENDA ITEM 5. GOVERNING BODY MEETING IN VITRO FERTILISATION (IVF) AND ASSISTED CONCEPTION CONSULTATION Date of the meeting 1 st February 2018 Author Sponsoring Governing Body Member Purpose of Report
AGENDA ITEM 5. GOVERNING BODY MEETING IN VITRO FERTILISATION (IVF) AND ASSISTED CONCEPTION CONSULTATION Date of the meeting 1 st February 2018 Author Sponsoring Governing Body Member Purpose of Report
for healthcare professionals
 Fertility toolkit for healthcare professionals We ve developed this toolkit for setting up a fertility referral pathway for young women with breast cancer at the point of diagnosis. We want to help improve
Fertility toolkit for healthcare professionals We ve developed this toolkit for setting up a fertility referral pathway for young women with breast cancer at the point of diagnosis. We want to help improve
Centre for Research on Ageing [influencing policy improving practice enhancing quality of life]
![Centre for Research on Ageing [influencing policy improving practice enhancing quality of life] Centre for Research on Ageing [influencing policy improving practice enhancing quality of life]](/thumbs/73/69177132.jpg) Centre for Research on Ageing [influencing policy improving practice enhancing quality of life] Associate Professor Barbara Horner (PhD) Director, Centre for Research on Ageing, Faculty of Health Sciences.
Centre for Research on Ageing [influencing policy improving practice enhancing quality of life] Associate Professor Barbara Horner (PhD) Director, Centre for Research on Ageing, Faculty of Health Sciences.
CBT in the Treatment of Persistent Insomnia in Patients with Cancer
 CBT in the Treatment of Persistent Insomnia in Patients with Cancer Colin A Espie University of Glasgow Sleep Centre Sackler Institute of Psychobiological Research University of Glasgow Scotland UK Outline
CBT in the Treatment of Persistent Insomnia in Patients with Cancer Colin A Espie University of Glasgow Sleep Centre Sackler Institute of Psychobiological Research University of Glasgow Scotland UK Outline
Chemotherapy Suite: Ward [Mon - Fri 2pm - 4pm] Your oncologist s secretary:...
![Chemotherapy Suite: Ward [Mon - Fri 2pm - 4pm] Your oncologist s secretary:... Chemotherapy Suite: Ward [Mon - Fri 2pm - 4pm] Your oncologist s secretary:...](/thumbs/85/92602023.jpg) Coping, now your chemotherapy is finishing [ovarian cancer] Patient Information Series PI 41a East and North Hertfordshire NHS Trust 2 Contacts Chemotherapy Suite:... 020 3826 2236 [Mon - Fri, 8.00am -
Coping, now your chemotherapy is finishing [ovarian cancer] Patient Information Series PI 41a East and North Hertfordshire NHS Trust 2 Contacts Chemotherapy Suite:... 020 3826 2236 [Mon - Fri, 8.00am -
GETTING A GRIP. A Report Into Breast Health Understanding Among Women In Australia
 GETTING A GRIP A Report Into Breast Health Understanding Among Women In Australia Based on independent research carried out by AMR for the McGrath Foundation October 2016 FOREWORD With the sheer number
GETTING A GRIP A Report Into Breast Health Understanding Among Women In Australia Based on independent research carried out by AMR for the McGrath Foundation October 2016 FOREWORD With the sheer number
Computed Tomography Renal Scan (Haematuria Pathway)
 Computed Tomography Renal Scan (Haematuria Pathway) Radiology Department Patient information leaflet This leaflet tells you about having a CT Renal scan. This procedure is also sometimes called a Haematuria
Computed Tomography Renal Scan (Haematuria Pathway) Radiology Department Patient information leaflet This leaflet tells you about having a CT Renal scan. This procedure is also sometimes called a Haematuria
Assessing the Risk: Protecting the Child
 Assessing the Risk: Protecting the Child Impact and Evidence briefing Key findings is an assessment service for men who pose a sexual risk to children and are not in the criminal justice system. Interviews
Assessing the Risk: Protecting the Child Impact and Evidence briefing Key findings is an assessment service for men who pose a sexual risk to children and are not in the criminal justice system. Interviews
also finds that only 9% of the elderly patients had stage 0 breast cancer, much lower than the 12% in the cohort.
 Hong Kong Breast Cancer Registry Report No. 8 Subanalysis: Elders with Breast Cancer Tend to Delay Seeking Medical Care and Present with a Later Cancer Stage (Press Release 30 September 2016) As Hong Kong
Hong Kong Breast Cancer Registry Report No. 8 Subanalysis: Elders with Breast Cancer Tend to Delay Seeking Medical Care and Present with a Later Cancer Stage (Press Release 30 September 2016) As Hong Kong
Name: QI&CPD#: Mobile Ph: Clinic Ph: Women s Health. Workbook Triennium
 Name: QI&CPD#: Clinic Ph: Mobile Ph: Email: Women s Health Workbook 2014 2016 Triennium What You Need To Do For category 1 points (40 category 1 points) 1. Select 4 of the articles provided, read them
Name: QI&CPD#: Clinic Ph: Mobile Ph: Email: Women s Health Workbook 2014 2016 Triennium What You Need To Do For category 1 points (40 category 1 points) 1. Select 4 of the articles provided, read them
Oncotype DX testing in node-positive disease
 Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
1. What is fulvestrant?
 Talk to our breast care nurses 0808 800 6000 1. What is fulvestrant? 2. How does fulvestrant work? 3. When might fulvestrant be prescribed? 4. How is fulvestrant given? 5. How long will I be given fulvestrant
Talk to our breast care nurses 0808 800 6000 1. What is fulvestrant? 2. How does fulvestrant work? 3. When might fulvestrant be prescribed? 4. How is fulvestrant given? 5. How long will I be given fulvestrant
Improving services for upper GI (OG) cancer Application template (Version 2)
 Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
Adult Patient Information and Consent Form
 The ROAM Trial Radiation versus Observation following surgical resection of Atypical Meningioma: a randomised controlled trial
The ROAM Trial Radiation versus Observation following surgical resection of Atypical Meningioma: a randomised controlled trial
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Dabrafenib-Trametinib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Dabrafenib-Trametinib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL
4 Call our Helpline on
 Anastrozole This booklet explains what anastrozole is, how it works, when it may be prescribed, the benefits of taking it and the side effects you may experience. This information is by Breast Cancer
Anastrozole This booklet explains what anastrozole is, how it works, when it may be prescribed, the benefits of taking it and the side effects you may experience. This information is by Breast Cancer
Clinical Trials: Improving Care of Women with Breast Cancer Through Research
 Clinical Trials: Improving Care of Women with Breast Cancer Through Research Som Mukherjee MD, MSc, FRCP(C) Life After Breast Cancer October 17, 2013 Breast Cancer Is research making a difference? Waging
Clinical Trials: Improving Care of Women with Breast Cancer Through Research Som Mukherjee MD, MSc, FRCP(C) Life After Breast Cancer October 17, 2013 Breast Cancer Is research making a difference? Waging
Evaluation of two phases of an alcohol and cancer education campaign: A
 Evaluation of two phases of an alcohol and cancer education campaign: A partnership between Cancer Council Western Australia and The Western Australian Drug and Alcohol Office Terry Slevin, Education and
Evaluation of two phases of an alcohol and cancer education campaign: A partnership between Cancer Council Western Australia and The Western Australian Drug and Alcohol Office Terry Slevin, Education and
The 7th Edition of TNM in Lung Cancer.
 10th European Conference Perspectives in Lung Cancer. Brussels, March 2009. The 7th Edition of TNM in Lung Cancer. Peter Goldstraw, Consultant Thoracic Surgeon, Royal Brompton Hospital, Professor of Thoracic
10th European Conference Perspectives in Lung Cancer. Brussels, March 2009. The 7th Edition of TNM in Lung Cancer. Peter Goldstraw, Consultant Thoracic Surgeon, Royal Brompton Hospital, Professor of Thoracic
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Pazopanib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Pazopanib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
4 Call our Helpline on
 Exemestane This booklet explains what exemestane is, how it works, when it may be prescribed, the benefits of taking it and the side effects you may experience. This information is by Breast Cancer Care.
Exemestane This booklet explains what exemestane is, how it works, when it may be prescribed, the benefits of taking it and the side effects you may experience. This information is by Breast Cancer Care.
FAQ-Protocol 3. BRCA mutation carrier guidelines Frequently asked questions
 ULast updated: 09/02/2015 Protocol 3 BRCA mutation carrier guidelines Frequently asked questions UQ: How accurate are the remaining lifetime and 5 year breast cancer risks in the table? These figures are
ULast updated: 09/02/2015 Protocol 3 BRCA mutation carrier guidelines Frequently asked questions UQ: How accurate are the remaining lifetime and 5 year breast cancer risks in the table? These figures are
Workforce Analysis: Children and Young People s Mental Health and Wellbeing Wider system
 Workforce Analysis: Children and Young People s Mental Health and Wellbeing Wider system This questionnaire is aimed at any member of the workforce supporting the mental health and wellbeing for children
Workforce Analysis: Children and Young People s Mental Health and Wellbeing Wider system This questionnaire is aimed at any member of the workforce supporting the mental health and wellbeing for children
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Hersch J, Barratt A, Jansen J, et al. Use of
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Hersch J, Barratt A, Jansen J, et al. Use of
AVELEY MEDICAL CENTRE & THE BLUEBELL SURGERY
 AVELEY MEDICAL CENTRE & THE BLUEBELL SURGERY Drs Leighton, Ahrin, Beroiz, Munro, Saluja, Ruiz-Gutierrez and George Aveley Medical Centre, 22 High Street, Aveley, Essex, RM15 4AD The Bluebell Surgery, Darenth
AVELEY MEDICAL CENTRE & THE BLUEBELL SURGERY Drs Leighton, Ahrin, Beroiz, Munro, Saluja, Ruiz-Gutierrez and George Aveley Medical Centre, 22 High Street, Aveley, Essex, RM15 4AD The Bluebell Surgery, Darenth
Inflammatory breast cancer. This booklet describes what inflammatory breast cancer is, the symptoms, how it is diagnosed and how it may be treated.
 Inflammatory breast cancer This booklet describes what inflammatory breast cancer is, the symptoms, how it is diagnosed and how it may be treated. 2 Call our Helpline on 0808 800 6000 Introduction We hope
Inflammatory breast cancer This booklet describes what inflammatory breast cancer is, the symptoms, how it is diagnosed and how it may be treated. 2 Call our Helpline on 0808 800 6000 Introduction We hope
Title: What 'outliers' tell us about missed opportunities for TB control: a cross-sectional study of patients in Mumbai, India
 Author's response to reviews Title: What 'outliers' tell us about missed opportunities for TB control: a cross-sectional study of patients in Authors: Anagha Pradhan (anp1002004@yahoo.com) Karina Kielmann
Author's response to reviews Title: What 'outliers' tell us about missed opportunities for TB control: a cross-sectional study of patients in Authors: Anagha Pradhan (anp1002004@yahoo.com) Karina Kielmann
The Swinburne National Technology and Society Monitor. Australian Centre for Emerging Technologies and Society 2006 Monitor
 The Swinburne National Technology and Society Monitor Australian Centre for Emerging Technologies and Society Monitor Table of Contents Swinburne National Technology and Society Monitor Executive Summary...
The Swinburne National Technology and Society Monitor Australian Centre for Emerging Technologies and Society Monitor Table of Contents Swinburne National Technology and Society Monitor Executive Summary...
Annual Report April 2016 March 2017
 Patient Reference Group Annual Report April 2016 March 2017 This report covers all of the following 6 components as set out in the PRG DES Guidelines document: Component 1 - Develop a PRG and the description
Patient Reference Group Annual Report April 2016 March 2017 This report covers all of the following 6 components as set out in the PRG DES Guidelines document: Component 1 - Develop a PRG and the description
Patient Outcomes in Pain Management. Enterprise One Pain Management Service Mid Year Report
 Patient Outcomes in Pain Management Pain Management Service 2017 Mid Year Report 1 July 2016 30 June 2017 About the electronic Persistent Pain Outcomes Collaboration (eppoc) eppoc is a program which aims
Patient Outcomes in Pain Management Pain Management Service 2017 Mid Year Report 1 July 2016 30 June 2017 About the electronic Persistent Pain Outcomes Collaboration (eppoc) eppoc is a program which aims
patient decision aid advanced lung cancer
 patient decision aid advanced lung cancer Introduction This aid is meant to supplement conversations with your care team. Patients who have used a decision aid like this said it helped them make care choices
patient decision aid advanced lung cancer Introduction This aid is meant to supplement conversations with your care team. Patients who have used a decision aid like this said it helped them make care choices
Information for Patients Receiving Radiation Therapy: Breast Cancer or Ductal Carcinoma in Situ (DCIS) of the Breast
 Patient & Family Guide 2018 Information for Patients Receiving Radiation Therapy: Breast Cancer or Ductal Carcinoma in Situ (DCIS) of the Breast www.nscancercare.ca Information for Patients Receiving Radiation
Patient & Family Guide 2018 Information for Patients Receiving Radiation Therapy: Breast Cancer or Ductal Carcinoma in Situ (DCIS) of the Breast www.nscancercare.ca Information for Patients Receiving Radiation
Treatment choices for someone with Stage 5 kidney disease are:
 Information for patients about advanced kidney disease Dialysis and non-dialysis treatments DOCUMENT PREPARED FOR This information is to help you understand some key issues about dialysis; it is designed
Information for patients about advanced kidney disease Dialysis and non-dialysis treatments DOCUMENT PREPARED FOR This information is to help you understand some key issues about dialysis; it is designed
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY:
 PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Axitinib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
PATIENT AGREEMENT TO SYSTEMIC ANTI- CANCER THERAPY: Axitinib PATIENT DETAILS PATIENT S SURNAME/FAMILY NAME: PATIENT S FIRST NAME(S): DATE OF BIRTH: NHS NUMBER: (or other identifier) HOSPITAL NAME/STAMP:
