Visiting Professors. Clinical Investigators Review Key Publications and Current Cases in Gynecologic Oncology
|
|
|
- Julianna Gilbert
- 5 years ago
- Views:
Transcription
1 Visiting Professors Clinical Investigators Review Key Publications and Current Cases in Gynecologic Oncology C M E I N F O R M A T I O N TARGET AUDIENCE This activity is intended for gynecologic and medical oncologists, gynecologists and other healthcare providers involved in the treatment of gynecologic cancers. OVERVIEW OF ACTIVITY In 2015 it is anticipated that approximately 98,000 new cases of gynecologic cancer which includes cancer of the ovaries, uterine corpus (endometrial cancer), uterine cervix (cervical cancer), vulva and vagina will be documented in the United States and 30,000 individuals will succumb to these diseases. As with many other tumors, patient outcomes are critically dependent on effective multidisciplinary care. Despite many commonalities, each of these diseases is in fact quite distinct, and in this regard management algorithms employed for each are varied. Existing and emerging multimodality treatment regimens used in the routine management of these diseases necessitate the physician s working knowledge of novel surgical, radiation and systemic therapeutic techniques. Ongoing clinical trials will continue to refine the optimal management of these tumors, and the introduction of innovative, targeted compounds may offer individualized treatment options that provide increased efficacy and improved tolerability. In order to offer optimal patient care including the option of clinical trial participation clinicians who care for patients with gynecologic cancers must be well informed of these advances. These video proceedings from a CME symposium held during the Society of Gynecologic Oncology s 2015 Annual Meeting on Women s Cancer feature discussions with leading researchers with an expertise in gynecologic oncology regarding actual patient cases and related clinical research findings. By providing information on the latest research developments and their potential application to routine practice, this activity is designed to assist gynecologic oncologists and other healthcare providers with the formulation of up-to-date clinical management strategies for various gynecologic cancers. LEARNING OBJECTIVES Employ current clinical guidelines and available data in the selection of treatment options for patients with commonly diagnosed gynecologic cancers. Develop a treatment plan for advanced cervical cancer, incorporating recently approved and investigational agents, and understand and counsel patients about the risks of perforation and fistula associated with anti-angiogenic therapy. Consider clinical investigator perspectives regarding the indications for BRCA mutation testing, and use this information to appropriately select patients with ovarian cancer (OC) for this analysis. Develop an evidence-based algorithm for the initial and long-term treatment of advanced OC considering the role of the recently approved anti-vegf antibody bevacizumab. Understand the rationale for the investigation of PARP inhibitors in OC, and recall the results of studies with olaparib and other similar agents under development for patients with advanced disease. Appreciate the recent approval of olaparib for patients with highly refractory advanced OC, and integrate this agent into the clinical care of appropriate individuals. Develop an understanding of the emerging efficacy data and toxicity profiles of investigational agents in OC to effectively prioritize clinical trial opportunities for appropriate patients. Counsel appropriately selected patients with gynecologic cancers about participation in ongoing clinical trials. Develop strategies for managing complex psychosocial issues affecting patients with advanced cancers, incorporating palliative care specialists and identifying special needs, such as minor children of patients with cancer. ACCREDITATION STATEMENT Research To Practice is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. CREDIT DESIGNATION STATEMENT Research To Practice designates this live activity for a maximum of 1.5 AMA PRA Category 1 Credits. Physicians should claim only the credit commensurate with the extent of their participation in the activity. ResearchToPractice.com/GynOnc15 1
2 HOW TO USE THIS CME ACTIVITY This CME activity consists of a video component. To receive credit, the participant should watch the video, complete the Post-test with a score of 75% or better and fill out the Educational Assessment and Credit Form located at ResearchToPractice.com/GynOnc15/CME. CONTENT VALIDATION AND DISCLOSURES Research To Practice (RTP) is committed to providing its participants with high-quality, unbiased and state-of-theart education. We assess potential conflicts of interest with faculty, planners and managers of CME activities. Real or apparent conflicts of interest are identified and resolved through a conflict of interest resolution process. In addition, all activity content is reviewed by both a member of the RTP scientific staff and an external, independent physician reviewer for fair balance, scientific objectivity of studies referenced and patient care recommendations. FACULTY The following faculty (and their spouses/partners) reported real or apparent conflicts of interest, which have been resolved through a conflict of interest resolution process: Deborah K Armstrong, MD Director, Breast and Ovarian Surveillance Service Professor of Oncology, Gynecology and Obstetrics The Sidney Kimmel Comprehensive Cancer Center The Johns Hopkins University Baltimore, Maryland Advisory Committee: Eisai Inc; Contracted Research: Astex Pharmaceuticals, AstraZeneca Pharmaceuticals LP, Clovis Oncology, Eisai Inc, Genentech BioOncology, Incyte Corporation, MedImmune Inc, VentiRx Pharmaceuticals Inc; Other Remunerated Activities: Abbott Laboratories, Sanofi. Ursula A Matulonis, MD Medical Director and Program Leader Gynecologic Oncology Program Associate Professor of Medicine Harvard Medical School Dana-Farber Cancer Institute Boston, Massachusetts Advisory Committee: Momenta Pharmaceuticals Inc; Speakers Bureau: AstraZeneca Pharmaceuticals LP; Unpaid Consultant: AstraZeneca Pharmaceuticals LP. Bradley J Monk, MD Professor and Director, Division of Gynecologic Oncology Vice Chair, Department of Obstetrics and Gynecology University of Arizona Cancer Center - Phoenix Creighton University School of Medicine at Dignity Health St Joseph s Hospital and Medical Center Phoenix, Arizona Consulting Agreements: Amgen Inc, AstraZeneca Pharmaceuticals LP, Advaxis Inc, Cerulean Pharma Inc, Genentech BioOncology, GlaxoSmithKline, Gradalis Inc, Merck, Roche Laboratories Inc, TESARO Inc, Verastem Inc; Contracted Research: Amgen Inc, Array BioPharma Inc, Genentech BioOncology, Janssen Biotech Inc, Johnson & Johnson Pharmaceuticals, Lilly, Novartis Pharmaceuticals Corporation, TESARO Inc; Speakers Bureau: Genentech BioOncology, Roche Laboratories Inc. Richard T Penson, MD, MRCP Associate Professor, Department of Medicine Harvard Medical School Clinical Director, Medical Gynecologic Oncology Massachusetts General Hospital Boston, Massachusetts Consulting Agreements: Advance Medical - Health Care Management Services, AstraZeneca Pharmaceuticals LP, Genentech BioOncology, Vascular Biogenics Ltd; Contracted Research: Amgen Inc, AstraZeneca Pharmaceuticals LP, Eisai Inc, Endocyte Inc, ImClone Systems, a wholly owned subsidiary of Eli Lilly and Company, Vascular Biogenics Ltd. MODERATOR Dr Love is president and CEO of Research To Practice, which receives funds in the form of educational grants to develop CME activities from the following commercial interests: AbbVie Inc, Amgen Inc, Astellas Scientific and Medical Affairs Inc, AstraZeneca Pharmaceuticals LP, Bayer HealthCare Pharmaceuticals, Biodesix Inc, biotheranostics Inc, Boehringer Ingelheim Pharmaceuticals Inc, Boston Biomedical Pharma Inc, Bristol-Myers Squibb Company, Celgene Corporation, Clovis Oncology, Daiichi Sankyo Inc, Dendreon Corporation, Eisai Inc, Exelixis Inc, Foundation Medicine, Genentech BioOncology, Genomic Health Inc, Gilead Sciences Inc, Incyte Corporation, Janssen Biotech Inc, Jazz Pharmaceuticals Inc, Lilly, Medivation Inc, Merck, Myriad Genetic Laboratories Inc, NanoString Technologies, Novartis Pharmaceuticals Corporation, Novocure, Onyx Pharmaceuticals, an Amgen subsidiary, Pharmacyclics Inc, Prometheus Laboratories Inc, Regeneron Pharmaceuticals, Sanofi, Seattle Genetics, Sigma-Tau Pharmaceuticals Inc, Sirtex Medical Ltd, Spectrum Pharmaceuticals Inc, Taiho Oncology Inc, Takeda Oncology, Teva Oncology, Tokai Pharmaceuticals Inc and VisionGate Inc. RESEARCH TO PRACTICE STAFF AND EXTERNAL REVIEWERS The scientific staff and reviewers for Research To Practice have no real or apparent conflicts of interest to disclose. This educational activity contains discussion of published and/ or investigational uses of agents that are not indicated by the Food and Drug Administration. Research To Practice does not recommend the use of any agent outside of the labeled indications. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings. The opinions expressed are those of the presenters and are not to be construed as those of the publisher or grantors. This activity is supported by educational grants from AbbVie Inc, AstraZeneca Pharmaceuticals LP, Genentech BioOncology and Myriad Genetic Laboratories Inc. Hardware/Software Requirements: A high-speed Internet connection A monitor set to 1280 x 1024 pixels or more Internet Explorer 7 or later, Firefox 3.0 or later, Chrome, Safari 3.0 or later Adobe Flash Player 10.2 plug-in or later Adobe Acrobat Reader (Optional) Sound card and speakers for audio Last review date: July 2015 Expiration date: July 2016 ResearchToPractice.com/GynOnc15 2
3 S E L E C T P U B L I C A T I O N S Richard T Penson, MD, MRCP A randomized phase III trial of cisplatin plus paclitaxel with and without NCI-supplied bevacizumab (NSC ) versus the non-platinum doublet, topotecan plus paclitaxel, with and without NCI-supplied bevacizumab, in Stage IVB, recurrent or persistent carcinoma of the cervix. NCT A single arm, single stage phase II trial of GSK and GSK in persistent or recurrent cervical cancer. NCT Basu P et al. ADXS immunotherapy targeting HPV-E7: Final results from a phase 2 study in Indian women with recurrent cervical cancer. Proc ASCO 2014;Abstract Diaz-Padilla I et al. Treatment of metastatic cervical cancer: Future directions involving targeted agents. Crit Rev Oncol Hematol 2013;85(3): Fludeoxyglucose F 18 PET scan, CT scan, and ferumoxtran-10 MRI scan before chemotherapy and radiation therapy in finding lymph node metastasis in patients with locally advanced cervical cancer or high-risk endometrial cancer. NCT Kitagawa R et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The open-label randomized phase III trial JCOG0505. J Clin Oncol 2015;[Epub ahead of print]. Kitagawa R et al. A randomized, phase III trial of paclitaxel plus carboplatin (TC) versus paclitaxel plus cisplatin (TP) in stage IVB, persistent or recurrent cervical cancer: Japan Clinical Oncology Group study (JCOG0505). Proc ASCO 2012;Abstract Penson RT et al. Bevacizumab for advanced cervical cancer: Patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240). Lancet Oncol 2015;16(3): Monk BJ et al. Phase III trial of four cisplatin-containing doublet combinations in Stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol 2009;27:4649. Monk BJ et al. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol 2007;25(20): Obermair A et al. Tumor angiogenesis in Stage IB cervical cancer: Correlation of microvessel density with survival. Am J Obstet Gynecol 1998;178(2): Oxaliplatin in treating patients with recurrent or refractory cervical cancer. NCT Paclitaxel in treating patients with advanced, refractory, or recurrent cervical or vaginal cancer. NCT Phase II evaluation of oxaliplatin in persistent or recurrent squamous cell carcinoma of the cervix. NCT Tewari KS et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370(8): Bradley J Monk, MD A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube, and primary peritoneal carcinoma NCI-supplied agent(s): Bevacizumab (NSC ). NCT Aghajanian C et al. OCEANS: A randomized, double-blinded, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC). Proc ASCO 2011;Abstract LBA5007. AGO-Ovar 17: A prospective randomised phase III trial to evaluate optimal treatment duration of first-line bevacizumab in combination with carboplatin and paclitaxel in patients with primary epithelial ovarian, fallopian tube or peritoneal cancer. NCT AGO-Ovar 2.21: A prospective randomized phase III trial of carboplatin/gemcitabine/bevacizumab versus carboplatin/pegylated liposomal doxorubicin/bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. NCT AURELIA: A multi-center, open-label, randomised, two-arm phase III trial of the effect on progression free survival of bevacizumab plus chemotherapy versus chemotherapy alone in patients with platinum-resistant, epithelial ovarian, fallopian tube or primary peritoneal cancer. NCT Bookman MA, Brady MF. Intraperitoneal chemotherapy: Long-term outcomes revive a long-running debate. J Clin Oncol 2015;33(13): ResearchToPractice.com/GynOnc15 3
4 Burger RA et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365(26): GOG-0213: A phase III randomized controlled clinical trial of carboplatin and paclitaxel (or gemcitabine) alone or in combination with bevacizumab (NSC ) followed by bevacizumab and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer. NCT GOG-0218: A phase III trial of carboplatin and paclitaxel plus placebo versus carboplatin and paclitaxel plus concurrent bevacizumab (NSC ) followed by placebo, versus carboplatin and paclitaxel plus concurrent and extended bevacizumab, in women with newly diagnosed, previously untreated, suboptimal advanced stage epithelial ovarian, primary peritoneal cancer, or fallopian tube cancer. NCT GOG-0262: A phase III trial of every-3-weeks paclitaxel versus dose dense weekly paclitaxel in combination with carboplatin with or without concurrent and consolidation bevacizumab (NSC ) in the treatment of primary stage II, III or IV epithelial ovarian, peritoneal or fallopian tube cancer and ACRIN 6695: Perfusion CT imaging to evaluate treatment response in patients participating in GOG NCT ICON7: A randomised, two-arm, multi-centre gynaecologic cancer InterGroup trial of adding bevacizumab to standard chemotherapy (carboplatin and paclitaxel) in patients with epithelial ovarian cancer. NCT Ledermann JA et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(Suppl 6): MITO16MANGO2b: Multicenter phase III randomized study with second line chemotherapy plus or minus bevacizumab in patients with platinum sensitive epithelial ovarian cancer recurrence after a bevacizumab/chemotherapy first line. NCT OCEANS: A phase III, multicenter, randomized, blinded, placebo-controlled trial of carboplatin and gemcitabine plus bevacizumab in patients with platinum-sensitive recurrent ovary, primary peritoneal, or fallopian tube carcinoma. NCT OCTAVIA: A single-arm phase II clinical study investigating the addition of bevacizumab to carboplatin and weekly paclitaxel as first-line treatment in patients with epithelial ovarian cancer. NCT Oza AM et al. ICON7: Final overall survival results in the GCIG phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. Proc ECC 2013;Abstract 6. Perren TJ et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365(26): Pfisterer J et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 2006;24(29): Pujade-Lauraine E et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32(13): Pujade-Lauraine E et al. AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). Proc ASCO 2012;Abstract LBA5002. ROSIA: Global study to assess the addition of bevacizumab to carboplatin and paclitaxel as front-line treatment of epithelial ovarian cancer, fallopian tube carcinoma or primary peritoneal carcinoma. NCT Tewari D et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A gynecologic oncology group study. J Clin Oncol 2015;33(13): Ursula A Matulonis, MD A cancer research UK phase II proof of principle trial of the activity of the PARP-1 inhibitor, AG , in known carriers of a BRCA 1 or BRCA 2 mutation with locally advanced or metastatic breast or advanced ovarian cancer. NCT A phase 1 dose-escalation study of intraperitoneal (IP) cisplatin, IV/IP paclitaxel, IV bevacizumab, and oral olaparib for newly diagnosed ovarian, primary peritoneal, and fallopian tube cancer. NCT A phase I study of intravenous carboplatin/paclitaxel or intravenous and intraperitoneal paclitaxel/cisplatin in combination with continuous or intermittent/ctep-supplied agent ABT-888 (NSC ) and CTEP-supplied agent bevacizumab (NSC ) in newly diagnosed patients with previously untreated epithelial ovarian, fallopian tube or primary peritoneal cancer. NCT A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, and NCI supplied veliparib (ABT-888), and NCI supplied bevacizumab in recurrent platinum sensitive ovarian, primary peritoneal and fallopian tube cancer. NCT ResearchToPractice.com/GynOnc15 4
5 A phase I/II, open-label, safety, pharmacokinetic, and preliminary efficacy study of oral rucaparib in patients with gbrca mutation ovarian cancer or other solid tumors. NCT A phase 2 pilot study of BMN 673 (talazoparib), an oral PARP inhibitor, in patients with deleterious BRCA1/2 mutation-associated ovarian cancer who have had prior PARP inhibitor treatment. NCT A phase 2 study of olaparib and cediranib for the treatment of recurrent ovarian cancer. NCT A phase 3 randomized double-blind trial of maintenance with niraparib versus placebo in patients with platinum sensitive ovarian cancer. NCT A phase III study comparing single-agent olaparib or the combination of cediranib and olaparib to standard platinum-based chemotherapy in women with recurrent platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer. NCT A proof of concept clinical trial of the combination cediranib-olaparib at the time of disease progression on olaparib in ovarian cancer. NCT ARIEL2: A phase 2, open-label study of rucaparib in patients with platinum-sensitive, relapsed, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer. NCT ARIEL3: Phase 3 study of rucaparib as switch maintenance after platinum in relapsed high grade serous and endometrioid ovarian cancer. NCT Audeh MW et al. Oral poly(adp-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010;376(9737): AVANOVA1 A phase I study of bevacizumab-niraparib combination. AVANOVA2 A 3-arm, phase II randomized study of niraparib and/or niraparib-bevacizumab combination against bevacizumab alone in platinum-sensitive ovarian cancer. NCT Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474(7353): Kaufman B et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33(3): Ledermann J et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15: Ledermann J et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366: Liu JF et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol 2014;15(11): Liu JF et al. PARP inhibitors in ovarian cancer: Current status and future promise. Gynecol Oncol 2014;133(2): Liu JF et al. A Phase 1 trial of the poly(adp-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer 2013;49(14): Matulonis UA et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer and a BRCA mutation: Overall survival adjusted for post-progression PARP inhibitor therapy. Proc SGO 2015;Abstract 14. Pennington KP et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 2014;20(3): Phase I/II study of cediranib and olaparib in combination for treatment of recurrent papillary-serous ovarian, fallopian tube, or peritoneal cancer or for treatment of recurrent triple-negative breast cancer. NCT Ramus SJ, Gayther SA. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol 2009;3(2): SOLO-1: A phase III, randomised, double blind, placebo controlled, multicentre study of olaparib maintenance monotherapy in patients with BRCA mutated advanced (FIGO stage III-IV) ovarian cancer following first line platinum based chemotherapy. NCT Deborah K Armstrong, MD Bogdanova N et al. Hereditary breast cancer: Ever more pieces to the polygenic puzzle. Hered Cancer Clin Pract 2013;11(1):12. GOG-0218: A phase III trial of carboplatin and paclitaxel plus placebo versus carboplatin and paclitaxel plus concurrent bevacizumab (NSC ) followed by placebo, versus carboplatin and paclitaxel plus concurrent and extended ResearchToPractice.com/GynOnc15 5
6 bevacizumab, in women with newly diagnosed, previously untreated, suboptimal advanced stage epithelial ovarian, primary peritoneal cancer, or fallopian tube cancer. NCT GOG-0262: A phase III trial of every-3-weeks paclitaxel versus dose dense weekly paclitaxel in combination with carboplatin with or without concurrent and consolidation bevacizumab (NSC ) in the treatment of primary stage II, III or IV epithelial ovarian, peritoneal or fallopian tube cancer and ACRIN 6695: Perfusion CT imaging to evaluate treatment response in patients participating in GOG NCT Norquist BS et al. Germline mutations in DNA repair genes in women with ovarian, peritoneal, or fallopian tube cancer treated on GOG protocols 218 and 262. Proc SGO 2014;Abstract 10. Rebbeck TR et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst 1999;91(17): Schwartz MD et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 2014;32(7): Tung N et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using nextgeneration sequencing with a 25-gene panel. Cancer 2015;121(1): ResearchToPractice.com/GynOnc15 6
PARP Inhibition in the Management of Ovarian and Other Gynecologic Cancers
 Addressing Current Questions and Controversies: PARP Inhibition in the Management of Ovarian and Other Gynecologic Cancers (Part 1 of a 2-Part Series) CME Information TARGET AUDIENCE This activity is intended
Addressing Current Questions and Controversies: PARP Inhibition in the Management of Ovarian and Other Gynecologic Cancers (Part 1 of a 2-Part Series) CME Information TARGET AUDIENCE This activity is intended
NEW AGENTS AND STRATEGIES IN THE MANAGEMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA
 NEW AGENTS AND STRATEGIES IN THE MANAGEMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA CME Information TARGET AUDIENCE This activity is intended for medical oncologists, hematologists and other healthcare providers
NEW AGENTS AND STRATEGIES IN THE MANAGEMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA CME Information TARGET AUDIENCE This activity is intended for medical oncologists, hematologists and other healthcare providers
New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer
 New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer A Clinical Investigator Think Tank CME Information TARGET AUDIENCE This activity is intended for medical oncologists,
New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer A Clinical Investigator Think Tank CME Information TARGET AUDIENCE This activity is intended for medical oncologists,
Key ASH Presentations Issue 2, Lenalidomide in the Management of Chronic Lymphocytic Leukemia (CLL)
 Key ASH Presentations Issue 2, 2011 Lenalidomide in the Management of Chronic Lymphocytic Leukemia (CLL) For more visit ResearchToPractice.com/5MJCASH2011 CME INFORMATION OVERVIEW OF ACTIVITY The annual
Key ASH Presentations Issue 2, 2011 Lenalidomide in the Management of Chronic Lymphocytic Leukemia (CLL) For more visit ResearchToPractice.com/5MJCASH2011 CME INFORMATION OVERVIEW OF ACTIVITY The annual
CME Information. ResearchToPractice.com/ThoracicCancers17/Nontargeted 1
 CONSENSUS OR CONTROVERSY: Clinical Investigators Provide Perspectives on the Treatment of Metastatic Non-Small Cell Lung Cancer in Patients without Targetable Tumor Mutations CME Information TARGET AUDIENCE
CONSENSUS OR CONTROVERSY: Clinical Investigators Provide Perspectives on the Treatment of Metastatic Non-Small Cell Lung Cancer in Patients without Targetable Tumor Mutations CME Information TARGET AUDIENCE
CME Information. without targetable tumor mutations. ResearchToPractice.com/IASLCNSCLC15 1
 Beyond the Guidelines Clinical Investigators Provide Their Perspectives on Current Treatment Strategies and Ongoing Research in the Management of Non-Small Cell Lung Cancer without Targetable Tumor Mutations
Beyond the Guidelines Clinical Investigators Provide Their Perspectives on Current Treatment Strategies and Ongoing Research in the Management of Non-Small Cell Lung Cancer without Targetable Tumor Mutations
CONSENSUS OR CONTROVERSY: Clinical Investigators Provide Perspectives on Targeted Treatment of Metastatic Non-Small Cell Lung Cancer
 CONSENSUS OR CONTROVERSY: Clinical Investigators Provide Perspectives on Targeted Treatment of Metastatic Non-Small Cell Lung Cancer CME Information TARGET AUDIENCE This activity is intended for hematologists,
CONSENSUS OR CONTROVERSY: Clinical Investigators Provide Perspectives on Targeted Treatment of Metastatic Non-Small Cell Lung Cancer CME Information TARGET AUDIENCE This activity is intended for hematologists,
CME Information. ResearchToPractice.com/AUABladder17 1
 What Urologists Want to Know: Addressing Current Questions and Controversies Regarding the Role of Immune Checkpoint Inhibitors in the Management of Bladder Cancer CME Information TARGET AUDIENCE This
What Urologists Want to Know: Addressing Current Questions and Controversies Regarding the Role of Immune Checkpoint Inhibitors in the Management of Bladder Cancer CME Information TARGET AUDIENCE This
Key ASH Presentations Issue 5, SAVE-ONCO Trial of Thromboprophylactic Treatment with Semuloparin for Venous Thromboembolism
 Key ASH Presentations Issue 5, 2012 SAVE-ONCO Trial of Thromboprophylactic Treatment with Semuloparin for Venous Thromboembolism CME INFORMATION OVERVIEW OF ACTIVITY The annual American Society of Hematology
Key ASH Presentations Issue 5, 2012 SAVE-ONCO Trial of Thromboprophylactic Treatment with Semuloparin for Venous Thromboembolism CME INFORMATION OVERVIEW OF ACTIVITY The annual American Society of Hematology
Critical Pathways in Breast Cancer Treatment
 Critical Pathways in Breast Cancer Treatment Faculty Jenny C Chang, MD Paul E Goss, MD, PhD Kathy D Miller, MD Lawrence N Shulman, MD Dennis J Slamon, MD, PhD Andrew Tutt, MB ChB, PhD Editor Neil Love,
Critical Pathways in Breast Cancer Treatment Faculty Jenny C Chang, MD Paul E Goss, MD, PhD Kathy D Miller, MD Lawrence N Shulman, MD Dennis J Slamon, MD, PhD Andrew Tutt, MB ChB, PhD Editor Neil Love,
Bevacizumab (Bev) with Chemotherapy, Followed by Bev, in the Treatment of Newly Diagnosed and Platinum- Sensitive Recurrent Ovarian Cancer
 SPECIAL EDITION Issue 1, 2011 Bevacizumab (Bev) with Chemotherapy, Followed by Bev, in the Treatment of Newly Diagnosed and Platinum- Sensitive Recurrent Ovarian Cancer For more visit ResearchToPractice.com/5MJCASCO2011
SPECIAL EDITION Issue 1, 2011 Bevacizumab (Bev) with Chemotherapy, Followed by Bev, in the Treatment of Newly Diagnosed and Platinum- Sensitive Recurrent Ovarian Cancer For more visit ResearchToPractice.com/5MJCASCO2011
RVD in Patients with High-Risk, Relapsed/Refractory Multiple Myeloma
 RVD in Patients with High-Risk, Relapsed/Refractory Multiple Myeloma For more visit ResearchToPractice.com/5MJC CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians and basic scientists
RVD in Patients with High-Risk, Relapsed/Refractory Multiple Myeloma For more visit ResearchToPractice.com/5MJC CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians and basic scientists
Meet The Professors: Acute Myeloid Leukemia Edition, 2017
 Meet The Professors: Acute Myeloid Leukemia Edition, 2017 CME Information TARGET AUDIENCE This activity is intended for medical oncologists and other healthcare providers involved in the treatment of acute
Meet The Professors: Acute Myeloid Leukemia Edition, 2017 CME Information TARGET AUDIENCE This activity is intended for medical oncologists and other healthcare providers involved in the treatment of acute
Head & Neck/Thyroid. Recall the efficacy of promising investigational checkpoint inhibitors and EGFR inhibitors being evaluated in SCCHN.
 Head & Neck/Thyroid C M E I n f o r m a t i o n TARGET AUDIENCE This activity is intended for medical oncologists, hematologyoncology fellows and other healthcare providers involved in the treatment of
Head & Neck/Thyroid C M E I n f o r m a t i o n TARGET AUDIENCE This activity is intended for medical oncologists, hematologyoncology fellows and other healthcare providers involved in the treatment of
New Prognostic Markers in Acute Myeloid Leukemia (AML)
 New Prognostic Markers in Acute Myeloid Leukemia (AML) For more visit ResearchToPractice.com/5MJCMDSAML CME INFORMATION OVERVIEW OF ACTIVITY Acute myeloid leukemia (AML) and the myelodysplastic syndromes
New Prognostic Markers in Acute Myeloid Leukemia (AML) For more visit ResearchToPractice.com/5MJCMDSAML CME INFORMATION OVERVIEW OF ACTIVITY Acute myeloid leukemia (AML) and the myelodysplastic syndromes
CERVICAL/VULVAR CANCER CLINICAL TRIALS
 CERVICAL/VULVAR CANCER CLINICAL TRIALS ALL-COMERS Primary Treatment Locally Advanced Recurrent Cervical GTFB (07-0935) TISSUE BANK ALL GYN TISSUE ETCTN (Phase II) (17-0458) LAO-MD017/#10010 Phase II Study
CERVICAL/VULVAR CANCER CLINICAL TRIALS ALL-COMERS Primary Treatment Locally Advanced Recurrent Cervical GTFB (07-0935) TISSUE BANK ALL GYN TISSUE ETCTN (Phase II) (17-0458) LAO-MD017/#10010 Phase II Study
Targeted Molecular Therapy Gynaecological Cancer Where are we now?
 Targeted Molecular Therapy Gynaecological Cancer Where are we now? 0 T O M D E G R E V E S U B - S P E C I A LT Y F E L L O W G Y N A E C O L O G I C A L O N C O L O G Y U N I V E R S I T Y O F P R E T
Targeted Molecular Therapy Gynaecological Cancer Where are we now? 0 T O M D E G R E V E S U B - S P E C I A LT Y F E L L O W G Y N A E C O L O G I C A L O N C O L O G Y U N I V E R S I T Y O F P R E T
POST-SABCS Issue 4, CALOR Trial of Adjuvant Chemotherapy for Local or Regional Recurrence of Breast Cancer
 POST-SABCS Issue 4, 2013 CALOR Trial of Adjuvant Chemotherapy for Local or Regional Recurrence of Breast Cancer For more visit ResearchToPractice.com/5MJCSABCS2013 CME Information OVERVIEW OF ACTIVITY
POST-SABCS Issue 4, 2013 CALOR Trial of Adjuvant Chemotherapy for Local or Regional Recurrence of Breast Cancer For more visit ResearchToPractice.com/5MJCSABCS2013 CME Information OVERVIEW OF ACTIVITY
THE NEW BIOLOGY OF NON-SMALL CELL LUNG CANCER
 THE NEW BIOLOGY OF NON-SMALL CELL LUNG CANCER CME Information TARGET AUDIENCE This activity is intended for medical oncologists and other healthcare providers involved in the treatment of lung cancer.
THE NEW BIOLOGY OF NON-SMALL CELL LUNG CANCER CME Information TARGET AUDIENCE This activity is intended for medical oncologists and other healthcare providers involved in the treatment of lung cancer.
Oncology Grand Rounds Series:
 Oncology Grand Rounds Series: Part 6 Lymphomas and Chronic Lymphocytic Leukemia CNE Information TARGET AUDIENCE This activity has been designed to meet the educational needs of oncology nurses, nurse practitioners
Oncology Grand Rounds Series: Part 6 Lymphomas and Chronic Lymphocytic Leukemia CNE Information TARGET AUDIENCE This activity has been designed to meet the educational needs of oncology nurses, nurse practitioners
ToGA: Trastuzumab with Standard Chemotherapy for HER2-Positive Advanced Gastric Cancer (GC)
 ToGA: Trastuzumab with Standard Chemotherapy for HER2-Positive Advanced Gastric Cancer (GC) For more visit ResearchToPractice.com/5MJC CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians
ToGA: Trastuzumab with Standard Chemotherapy for HER2-Positive Advanced Gastric Cancer (GC) For more visit ResearchToPractice.com/5MJC CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians
A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG
 A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG Arizona Oncology (US Oncology Network) Professor, Gynecologic Oncology University of Arizona and Creighton
A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG Arizona Oncology (US Oncology Network) Professor, Gynecologic Oncology University of Arizona and Creighton
OVARIAN CANCER CLINICAL TRIALS
 OVARIAN CANCER CLINICAL TRIALS FRONT-LINE THERAPIES STG III, IV PHASE 3 GOG 3015/Roche YO39523 (16-2745) Carbo/Taxol/Bev/Atezolizumab ECOG 0-2 Allows for primary cytoreductive surgery or interval debulking
OVARIAN CANCER CLINICAL TRIALS FRONT-LINE THERAPIES STG III, IV PHASE 3 GOG 3015/Roche YO39523 (16-2745) Carbo/Taxol/Bev/Atezolizumab ECOG 0-2 Allows for primary cytoreductive surgery or interval debulking
Key ASCO Presentations Issue 2, Ipilimumab Monotherapy for Patients with Melanoma and Brain Metastases
 Key ASCO Presentations Issue 2, 2010 Ipilimumab Monotherapy for Patients with Melanoma and Brain Metastases For more visit ResearchToPractice.com/5MJCMT2010 CME INFORMATION OVERVIEW OF ACTIVITY Each year,
Key ASCO Presentations Issue 2, 2010 Ipilimumab Monotherapy for Patients with Melanoma and Brain Metastases For more visit ResearchToPractice.com/5MJCMT2010 CME INFORMATION OVERVIEW OF ACTIVITY Each year,
POST-ASH Issue 4, Results from the Phase III RESPONSE Trial of Ruxolitinib versus Best Available Therapy for Patients with Polycythemia Vera
 POST-ASH Issue 4, 2015 Results from the Phase III RESPONSE Trial of Ruxolitinib versus Best Available Therapy for Patients with Polycythemia Vera For more visit ResearchToPractice.com/5MJCASH2015 CME INFORMATION
POST-ASH Issue 4, 2015 Results from the Phase III RESPONSE Trial of Ruxolitinib versus Best Available Therapy for Patients with Polycythemia Vera For more visit ResearchToPractice.com/5MJCASH2015 CME INFORMATION
S1207 Trial of Adjuvant Endocrine Therapy with or without Everolimus for High-Risk, Hormone Receptor-Positive, HER2-Negative Breast Cancer
 POST-SABCS Issue 1, 2013 S1207 Trial of Adjuvant Endocrine Therapy with or without Everolimus for High-Risk, Hormone Receptor-Positive, HER2-Negative Breast Cancer For more visit ResearchToPractice.com/5MJCSABCS2013
POST-SABCS Issue 1, 2013 S1207 Trial of Adjuvant Endocrine Therapy with or without Everolimus for High-Risk, Hormone Receptor-Positive, HER2-Negative Breast Cancer For more visit ResearchToPractice.com/5MJCSABCS2013
Beyond the Guidelines Investigator Perspectives on Current Clinical Issues and Ongoing Research in the Management of Early and Advanced Breast Cancer
 Beyond the Guidelines Investigator Perspectives on Current Clinical Issues and Ongoing Research in the Management of Early and Advanced Breast Cancer CME Information TARGET AUDIENCE This program is intended
Beyond the Guidelines Investigator Perspectives on Current Clinical Issues and Ongoing Research in the Management of Early and Advanced Breast Cancer CME Information TARGET AUDIENCE This program is intended
Phase II Study of Clofarabine for Older Patients with Treatment-Naïve AML
 Phase II Study of Clofarabine for Older Patients with Treatment-Naïve AML CME INFORMATION OVERVIEW OF ACTIVITY Acute myeloid leukemia (AML) and the myelodysplastic syndromes (MDS) account for approximately
Phase II Study of Clofarabine for Older Patients with Treatment-Naïve AML CME INFORMATION OVERVIEW OF ACTIVITY Acute myeloid leukemia (AML) and the myelodysplastic syndromes (MDS) account for approximately
First-Line Erlotinib with or without Chemotherapy for Never or Light Smokers with Advanced Non-Small Cell Lung Cancer (NSCLC)
 Key ASCO Presentations Issue 7, 2010 First-Line Erlotinib with or without Chemotherapy for Never or Light Smokers with Advanced Non-Small Cell Lung Cancer (NSCLC) For more visit ResearchToPractice.com/5MJCMT2010
Key ASCO Presentations Issue 7, 2010 First-Line Erlotinib with or without Chemotherapy for Never or Light Smokers with Advanced Non-Small Cell Lung Cancer (NSCLC) For more visit ResearchToPractice.com/5MJCMT2010
NCCN Guidelines for Ovarian Cancer V Meeting on 11/15/17
 OV-1 External request: Submission from Vermillion/ASPiRA Laboratories to consider: Inclusion of the following recommendation in the workup for suspected ovarian cancer: OVA1 and/or Multivariate Index Assay
OV-1 External request: Submission from Vermillion/ASPiRA Laboratories to consider: Inclusion of the following recommendation in the workup for suspected ovarian cancer: OVA1 and/or Multivariate Index Assay
Current Strategies and Ongoing Research in the Management of Advanced Prostate Cancer
 Current Strategies and Ongoing Research in the Management of Advanced Prostate Cancer CME Information TARGET AUDIENCE This activity has been designed to meet the educational needs of medical and radiation
Current Strategies and Ongoing Research in the Management of Advanced Prostate Cancer CME Information TARGET AUDIENCE This activity has been designed to meet the educational needs of medical and radiation
Oncology Grand Rounds Series:
 Oncology Grand Rounds Series: Part 1 Cancer Immunotherapy CNE Information TARGET AUDIENCE This activity has been designed to meet the educational needs of oncology nurses, nurse practitioners and clinical
Oncology Grand Rounds Series: Part 1 Cancer Immunotherapy CNE Information TARGET AUDIENCE This activity has been designed to meet the educational needs of oncology nurses, nurse practitioners and clinical
GOG-172: Survival Outcomes
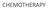 CHEMOTHERAPY GOG-172: Survival Outcomes Progression-Free Survival Overall Survival Proportion Progression-Free 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Rx Group IV IP PF Failed Total 50 160 210 63 142
CHEMOTHERAPY GOG-172: Survival Outcomes Progression-Free Survival Overall Survival Proportion Progression-Free 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Rx Group IV IP PF Failed Total 50 160 210 63 142
Oncology Grand Rounds
 Oncology Grand Rounds Nurse and Physician Investigators Discuss New Agents, Novel Therapies and Actual Cases from Practice Part 4: Non-Small Cell Lung Cancer CNE Information TARGET AUDIENCE This activity
Oncology Grand Rounds Nurse and Physician Investigators Discuss New Agents, Novel Therapies and Actual Cases from Practice Part 4: Non-Small Cell Lung Cancer CNE Information TARGET AUDIENCE This activity
PARP Inhibitors: Patients Selection. Dr. Cristina Martin Lorente Hospital de la Santa Creu i Sant Pau Formigal, June 23th 2016
 PARP Inhibitors: Patients Selection Dr. Cristina Martin Lorente Hospital de la Santa Creu i Sant Pau Formigal, June 23th 2016 OVARIAN CANCER (OC): MULTIPLES DISEASES Different types with different behaviour
PARP Inhibitors: Patients Selection Dr. Cristina Martin Lorente Hospital de la Santa Creu i Sant Pau Formigal, June 23th 2016 OVARIAN CANCER (OC): MULTIPLES DISEASES Different types with different behaviour
Key ASCO Presentations Issue 4, Effects of Zoledronic Acid on Overall Survival in Newly Diagnosed Multiple Myeloma (MM)
 Key ASCO Presentations Issue 4, 2010 Effects of Zoledronic Acid on Overall Survival in Newly Diagnosed Multiple Myeloma (MM) For more visit ResearchToPractice.com/5MJCMT2010 CME INFORMATION OVERVIEW OF
Key ASCO Presentations Issue 4, 2010 Effects of Zoledronic Acid on Overall Survival in Newly Diagnosed Multiple Myeloma (MM) For more visit ResearchToPractice.com/5MJCMT2010 CME INFORMATION OVERVIEW OF
Cómo Incorporar la Terapia Antiangiogénica en el Cáncer de Ovario? XIV Congreso Nacional Salamanca Octubre de 2013 SESION CONTROVERSIA-1 15,45-17H
 Cómo Incorporar la Terapia Antiangiogénica en el Cáncer de Ovario? XIV Congreso Nacional Salamanca Octubre de 2013 SESION CONTROVERSIA-1 15,45-17H Andres Poveda Fundación Instituto Valenciano de Oncología
Cómo Incorporar la Terapia Antiangiogénica en el Cáncer de Ovario? XIV Congreso Nacional Salamanca Octubre de 2013 SESION CONTROVERSIA-1 15,45-17H Andres Poveda Fundación Instituto Valenciano de Oncología
Ovarian Cancer: Implications for the Pharmacist
 Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
2/21/2016. Cancer Precision Medicine: A Primer. Ovarian Cancer Statistics and Standard of Care in 2015 OUTLINE. Background
 Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
POST-ASH Issue 2, Phase I/II Study of the Bruton Tyrosine Kinase Inhibitor Acalabrutinib in Relapsed/ Refractory CLL
 POST-ASH Issue 2, 2016 Phase I/II Study of the Bruton Tyrosine Kinase Inhibitor Acalabrutinib in Relapsed/ Refractory CLL For more visit ResearchToPractice.com/5MJCASH2016 CME Information OVERVIEW OF ACTIVITY
POST-ASH Issue 2, 2016 Phase I/II Study of the Bruton Tyrosine Kinase Inhibitor Acalabrutinib in Relapsed/ Refractory CLL For more visit ResearchToPractice.com/5MJCASH2016 CME Information OVERVIEW OF ACTIVITY
Angiogenesis in Ovarian Cancer
 Angiogenesis in Ovarian Cancer Dr Shibani Nicum Consultant Medical Oncologist and Lead for Gynae- Oncology Oxford University Hospitals Content 1. Epithelial Ovarian Cancer : epidemiology 2. Angiogenesis-normal
Angiogenesis in Ovarian Cancer Dr Shibani Nicum Consultant Medical Oncologist and Lead for Gynae- Oncology Oxford University Hospitals Content 1. Epithelial Ovarian Cancer : epidemiology 2. Angiogenesis-normal
Media Release. Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer. Basel, 08 February 2011
 Media Release Basel, 08 February 2011 Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer Avastin study in recurrent, platinum-sensitive ovarian cancer showed women lived
Media Release Basel, 08 February 2011 Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer Avastin study in recurrent, platinum-sensitive ovarian cancer showed women lived
The Role of PARP Inhibitors in Ovarian Cancer: An Emerging Picture
 The Role of PARP Inhibitors in Ovarian Cancer: An Emerging Picture This satellite symposium took place on 10 th September 2017 as part of the European Society for Medical Oncology (ESMO) Congress in Madrid,
The Role of PARP Inhibitors in Ovarian Cancer: An Emerging Picture This satellite symposium took place on 10 th September 2017 as part of the European Society for Medical Oncology (ESMO) Congress in Madrid,
SPECIAL EDITION Issue 1, 2011
 SPECIAL EDITION Issue 1, 2011 Studies in Advanced NSCLC of Maintenance Pemetrexed and Erlotinib and of BIBW 2992 or MetMAb Targeted Therapy for Acquired Resistance to Erlotinib and Gefitinib For more visit
SPECIAL EDITION Issue 1, 2011 Studies in Advanced NSCLC of Maintenance Pemetrexed and Erlotinib and of BIBW 2992 or MetMAb Targeted Therapy for Acquired Resistance to Erlotinib and Gefitinib For more visit
Update on PARP inhibitors
 Professor of Medicine Harvard Medical School Boston MA Update on PARP inhibitors Ursula Matulonis, M.D. Chief, Division of Gynecologic Oncology Brock-Wilson Family Chair Dana-Farber Cancer Institute History
Professor of Medicine Harvard Medical School Boston MA Update on PARP inhibitors Ursula Matulonis, M.D. Chief, Division of Gynecologic Oncology Brock-Wilson Family Chair Dana-Farber Cancer Institute History
Targeted Treatment of Non-Small Cell Lung Cancer
 Targeted Treatment of Non-Small Cell Lung Cancer Current Algorithms and New Agents CME Information TARGET AUDIENCE This activity is intended for hematologists, medical oncologists and other healthcare
Targeted Treatment of Non-Small Cell Lung Cancer Current Algorithms and New Agents CME Information TARGET AUDIENCE This activity is intended for hematologists, medical oncologists and other healthcare
Controversies in the Management of Advanced Ovarian Cancer
 안녕하세요 Controversies in the Management of Advanced Ovarian Cancer Mansoor R. Mirza Nordic Society of Gynaecological Oncology (NSGO) & Rigshospitalet Copenhagen University Hospital, Denmark Primary Debulking
안녕하세요 Controversies in the Management of Advanced Ovarian Cancer Mansoor R. Mirza Nordic Society of Gynaecological Oncology (NSGO) & Rigshospitalet Copenhagen University Hospital, Denmark Primary Debulking
Tarceva Trial EORTC 55041
 Tarceva Trial EORTC 55041 Primary Chemotherapy Tarceva consolidation 2 years Control Patients closed / 835 Leading Participating EORTC AGO-AUSTRIA, ANZGOG, GINECO, MRC/NCIC, MANGO Randomised trial on Erlotinib
Tarceva Trial EORTC 55041 Primary Chemotherapy Tarceva consolidation 2 years Control Patients closed / 835 Leading Participating EORTC AGO-AUSTRIA, ANZGOG, GINECO, MRC/NCIC, MANGO Randomised trial on Erlotinib
New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer
 ISSUE 1 2015 New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer Proceedings from a Clinical Investigator Think Tank F A C U L T Y David P Carbone, MD, PhD Mark G Kris,
ISSUE 1 2015 New Biological Insights and Recent Therapeutic Advances in the Management of Lung Cancer Proceedings from a Clinical Investigator Think Tank F A C U L T Y David P Carbone, MD, PhD Mark G Kris,
SOLO-1. Dott.ssa Elisabetta Sanna U.O.C. Ginecologia Oncologica- AOB Cagliari Direttore: Dott. Antonio Macciò
 SOLO-1 maintenance therapy in patients with newly diagnosed advanced ovarian cancer following platinum-based chemotherapy Dott.ssa Elisabetta Sanna U.O.C. Ginecologia Oncologica- AOB Cagliari Direttore:
SOLO-1 maintenance therapy in patients with newly diagnosed advanced ovarian cancer following platinum-based chemotherapy Dott.ssa Elisabetta Sanna U.O.C. Ginecologia Oncologica- AOB Cagliari Direttore:
Practical Guidance and Strategies for PARP Inhibition. Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy
 Practical Guidance and Strategies for PARP Inhibition Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy Clinical Data Maintenance therapy : BRCA-mutated or all
Practical Guidance and Strategies for PARP Inhibition Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy Clinical Data Maintenance therapy : BRCA-mutated or all
Update on PARP inhibitors: opportunities and challenges in cancer therapy
 Update on PARP inhibitors: opportunities and challenges in cancer therapy Vanda Salutari Unità di Ginecologia Oncologica Fondazione Policlinico Universitario A. Gemelli vanda.salutari@policlinicogemelli.it
Update on PARP inhibitors: opportunities and challenges in cancer therapy Vanda Salutari Unità di Ginecologia Oncologica Fondazione Policlinico Universitario A. Gemelli vanda.salutari@policlinicogemelli.it
Current state of upfront treatment for newly diagnosed advanced ovarian cancer
 Current state of upfront treatment for newly diagnosed advanced ovarian cancer Ursula Matulonis, M.D. Associate Professor of Medicine, HMS Program Leader, Medical Gyn Oncology Dana-Farber Cancer Institute
Current state of upfront treatment for newly diagnosed advanced ovarian cancer Ursula Matulonis, M.D. Associate Professor of Medicine, HMS Program Leader, Medical Gyn Oncology Dana-Farber Cancer Institute
Second Opinion An Interactive Case-Based Discussion on the Management of Early and Advanced Breast Cancer
 Second Opinion An Interactive Case-Based Discussion on the Management of Early and Advanced Breast Cancer Proceedings from a CME Satellite Symposium at the 30 th Annual San Antonio Breast Cancer Symposium
Second Opinion An Interactive Case-Based Discussion on the Management of Early and Advanced Breast Cancer Proceedings from a CME Satellite Symposium at the 30 th Annual San Antonio Breast Cancer Symposium
Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies
 Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies Uterus Study N Eligibility Regimen RR (No. of Responses) Median OS Grade 3/4 Toxicities Nimeiri et al[42] Total:
Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies Uterus Study N Eligibility Regimen RR (No. of Responses) Median OS Grade 3/4 Toxicities Nimeiri et al[42] Total:
ACRIN Gynecologic Committee
 ACRIN Gynecologic Committee Fall Meeting 2010 ACRIN Abdominal Committee Biomarkers & Endpoints in Ovarian Cancer Trials Robert L. Coleman, MD Professor and Vice Chair, Clinical Research Department of Gynecologic
ACRIN Gynecologic Committee Fall Meeting 2010 ACRIN Abdominal Committee Biomarkers & Endpoints in Ovarian Cancer Trials Robert L. Coleman, MD Professor and Vice Chair, Clinical Research Department of Gynecologic
TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER
 TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER Sandro Pignata, MD, PhD Sabrina Chiara Cecere, MD Uro-Gynecological Department, Division of Medical Oncology, IRCCS National Cancer
TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER Sandro Pignata, MD, PhD Sabrina Chiara Cecere, MD Uro-Gynecological Department, Division of Medical Oncology, IRCCS National Cancer
Late recurrent epithelial ovarian cancer
 Late recurrent epithelial ovarian cancer Dominic Richards University of Cape Town and New Somerset Hospital Gynaecological Oncology Unit September 2016 LATE RECURRENT EPITHELIAL OVARIAN CANCER Background
Late recurrent epithelial ovarian cancer Dominic Richards University of Cape Town and New Somerset Hospital Gynaecological Oncology Unit September 2016 LATE RECURRENT EPITHELIAL OVARIAN CANCER Background
Medicina de precisión en cáncer de ovario: Determinación de BRCA germinal y somático
 Medicina de precisión en cáncer de ovario: Determinación de BRCA germinal y somático Dra. Cristina Martin Lorente Hospital de la Santa Creu i Sant Pau. Barcelona Introduction Ovarian cancer is the fifth
Medicina de precisión en cáncer de ovario: Determinación de BRCA germinal y somático Dra. Cristina Martin Lorente Hospital de la Santa Creu i Sant Pau. Barcelona Introduction Ovarian cancer is the fifth
Progress Update June 2017 Lay Summary Funding: $6,000,000 Grant Funded: July 2015 Dream Team Members Dream Team Leader:
 SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
Virtual Journal Club. Ovarian Cancer. Reference Slides. Platinum-Sensitive Recurrent Ovarian Cancer: Making the Most of Emerging Targeted Therapies
 Virtual Journal Club Ovarian Cancer Reference Slides Platinum-Sensitive Recurrent Ovarian Cancer: Making the Most of Emerging Targeted Therapies Mansoor R. Mirza, MD Copenhagen University Hospital Rigshospitalet
Virtual Journal Club Ovarian Cancer Reference Slides Platinum-Sensitive Recurrent Ovarian Cancer: Making the Most of Emerging Targeted Therapies Mansoor R. Mirza, MD Copenhagen University Hospital Rigshospitalet
Current Medical Oncology Approaches to Gynecologic Cancers. Mihaela Cristea, MD Associate Professor Medical Oncology
 Current Medical Oncology Approaches to Gynecologic Cancers Mihaela Cristea, MD Associate Professor Medical Oncology Nothing to disclose DISCLOSURE Ovarian Cancer Objectives: a. To discuss new FDA approved
Current Medical Oncology Approaches to Gynecologic Cancers Mihaela Cristea, MD Associate Professor Medical Oncology Nothing to disclose DISCLOSURE Ovarian Cancer Objectives: a. To discuss new FDA approved
FoROMe Lausanne 6 février Anita Wolfer MD-PhD Cheffe de clinique Département d Oncologie, CHUV
 FoROMe Lausanne 6 février 2014 Anita Wolfer MD-PhD Cheffe de clinique Département d Oncologie, CHUV Epithelial Ovarian Cancer (EOC) Epidemiology Fifth most common cancer in women and forth most common
FoROMe Lausanne 6 février 2014 Anita Wolfer MD-PhD Cheffe de clinique Département d Oncologie, CHUV Epithelial Ovarian Cancer (EOC) Epidemiology Fifth most common cancer in women and forth most common
New Treatments for Early Ovarian Cancer. Jonathan Ledermann UCL Cancer Institute University College London
 New Treatments for Early Ovarian Cancer Jonathan Ledermann UCL Cancer Institute University College London Lucerne Oct 213 Progression-free survival in first-line trials of platinum-based chemotherapy 1998
New Treatments for Early Ovarian Cancer Jonathan Ledermann UCL Cancer Institute University College London Lucerne Oct 213 Progression-free survival in first-line trials of platinum-based chemotherapy 1998
Granulosa Cell Tumor Monitoring and Treatment. Outline: 1. Surgery 2. Adjuvant 3. Chemo 4. Hormonal 5. Investigational. Whole Genome Sequencing
 Potential Conflicts of Interest capped at a level befitting an academic role Lo B. Serving Two Masters 2010;362:669-671 Granulosa Cell Tumor Monitoring and Treatment Richard T. Penson, MD, MRCP Clinical
Potential Conflicts of Interest capped at a level befitting an academic role Lo B. Serving Two Masters 2010;362:669-671 Granulosa Cell Tumor Monitoring and Treatment Richard T. Penson, MD, MRCP Clinical
OVAIRES PROTOCOLES PTES PHASE DESCRIPTION
 SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG II PRÉVENTION A Randomized Phase II Double-Blind Placebo-Controlled Trials of Acetylsalicylic
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG II PRÉVENTION A Randomized Phase II Double-Blind Placebo-Controlled Trials of Acetylsalicylic
Dissecting the Decision
 Dissecting the Decision Investigators Discuss the Available Data and Clinical Factors That Shape the Management of Non-Small Cell Lung Cancer CME Information TARGET AUDIENCE This activity is intended for
Dissecting the Decision Investigators Discuss the Available Data and Clinical Factors That Shape the Management of Non-Small Cell Lung Cancer CME Information TARGET AUDIENCE This activity is intended for
THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER. HLC143A June Usha Nagavarapu Project Analyst ISBN: X
 THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER HLC143A June 2013 Usha Nagavarapu Project Analyst ISBN: 1-56965-431-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301
THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER HLC143A June 2013 Usha Nagavarapu Project Analyst ISBN: 1-56965-431-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301
PARP inhibitors for breast cancer
 PARP inhibitors for breast cancer Mark Robson, MD Memorial Sloan Kettering Cancer Center Agenda Mechanism of action Clinical studies Resistance mechanisms Future directions Poly (ADP-ribose) Polymerases
PARP inhibitors for breast cancer Mark Robson, MD Memorial Sloan Kettering Cancer Center Agenda Mechanism of action Clinical studies Resistance mechanisms Future directions Poly (ADP-ribose) Polymerases
Adil Daud, MD Mario Sznol, MD Omid Hamid, MD Kim Margolin, MD. 2 Audio CDs
 DOU 2014 V OL 4 ISSUE 1 Systemic Management of Malignant Melanoma and Basal Cell Carcinoma Bridging the Gap between Research and Patient Care F A C U L T Y I N T E R V I E W S Adil Daud, MD Mario Sznol,
DOU 2014 V OL 4 ISSUE 1 Systemic Management of Malignant Melanoma and Basal Cell Carcinoma Bridging the Gap between Research and Patient Care F A C U L T Y I N T E R V I E W S Adil Daud, MD Mario Sznol,
G ASTROINTESTINA L C A NCER TUMOR PA NEL
 G ASTROINTESTINA L C A NCER TUMOR PA NEL Clinical Investigators Provide Their Perspectives on Current Cases and Clinical Issues in the Management of Colorectal, Gastric and Pancreatic Cancer CME Information
G ASTROINTESTINA L C A NCER TUMOR PA NEL Clinical Investigators Provide Their Perspectives on Current Cases and Clinical Issues in the Management of Colorectal, Gastric and Pancreatic Cancer CME Information
Real-Life Decisions. Proceedings from a Case-Based Symposium on the Management of Early and Advanced Breast Cancer
 Real-Life Decisions Proceedings from a Case-Based Symposium on the Management of Early and Advanced Breast Cancer Featuring Faculty Perspectives on New Data Sets Presented at the 33 rd Annual San Antonio
Real-Life Decisions Proceedings from a Case-Based Symposium on the Management of Early and Advanced Breast Cancer Featuring Faculty Perspectives on New Data Sets Presented at the 33 rd Annual San Antonio
Ovarian Cancer: New insights into biology and treatment
 Ovarian Cancer: New insights into biology and treatment 2018 Master Class Course Ursula A. Matulonis, MD Director, Gynecologic Oncology Brock-Wilson Family Chair Dana-Farber Cancer Institute Professor
Ovarian Cancer: New insights into biology and treatment 2018 Master Class Course Ursula A. Matulonis, MD Director, Gynecologic Oncology Brock-Wilson Family Chair Dana-Farber Cancer Institute Professor
10/24/14. Grand Rounds in Ovarian Cancer: Standards of Care and Novel Treatment Approaches. Disclosure. Learning Objectives
 10/24/14 Grand Rounds in Ovarian Cancer: Standards of Care and Novel Treatment Approaches Jessica Gahres, PA-C Memorial Sloan Kettering Cancer Center Don S. Dizon, MD Massachusetts General Hospital Cancer
10/24/14 Grand Rounds in Ovarian Cancer: Standards of Care and Novel Treatment Approaches Jessica Gahres, PA-C Memorial Sloan Kettering Cancer Center Don S. Dizon, MD Massachusetts General Hospital Cancer
An Audio Review Journal for Surgeons Bridging the Gap between Research and Patient Care
 BCU SURGEONS 2017 VOL 15 ISSUE 1 An Audio Review Journal for Surgeons Bridging the Gap between Research and Patient Care FACULTY INTERVIEWS William J Gradishar, MD Tari King, MD Joseph A Sparano, MD Seema
BCU SURGEONS 2017 VOL 15 ISSUE 1 An Audio Review Journal for Surgeons Bridging the Gap between Research and Patient Care FACULTY INTERVIEWS William J Gradishar, MD Tari King, MD Joseph A Sparano, MD Seema
Title Cancer Drug Phase Status
 Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
SPECIAL EDITION Issue 2, 2011
 SPECIAL EDITION Issue 2, 2011 Benefits of Early ASCT for Newly Diagnosed Multiple Myeloma (MM) and the Incidence of Second Primary Cancer with Lenalidomide Maintenance in the Treatment of MM For more visit
SPECIAL EDITION Issue 2, 2011 Benefits of Early ASCT for Newly Diagnosed Multiple Myeloma (MM) and the Incidence of Second Primary Cancer with Lenalidomide Maintenance in the Treatment of MM For more visit
In 2017, an estimated 22,240 women will
 OVARIAN CANCER Ovarian cancer remains the most deadly gynecologic malignancy in the United States. What are the practice implications of recent research results on screening, neoadjuvant chemotherapy,
OVARIAN CANCER Ovarian cancer remains the most deadly gynecologic malignancy in the United States. What are the practice implications of recent research results on screening, neoadjuvant chemotherapy,
SPECIAL EDITION Issue 2, 2011
 SPECIAL EDITION Issue 2, 2011 First-Line Therapy for CML-CP with Nilotinib or Dasatinib Compared to Imatinib and the Incidence of Treatment- Emergent BCR-ABL Mutations in Patients Who Received Nilotinib
SPECIAL EDITION Issue 2, 2011 First-Line Therapy for CML-CP with Nilotinib or Dasatinib Compared to Imatinib and the Incidence of Treatment- Emergent BCR-ABL Mutations in Patients Who Received Nilotinib
Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant Medical Oncologist
 Title: Systemic Anti-Cancer Therapy (SACT) Guidelines for Ovarian Cancer Author(s) Ownership: Approval by: Operational Date: Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant
Title: Systemic Anti-Cancer Therapy (SACT) Guidelines for Ovarian Cancer Author(s) Ownership: Approval by: Operational Date: Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant
New Developments in Ovarian Cancer
 New Developments in Ovarian Cancer Daniela Matei, MD Professor Gynecology Oncology Northwestern University Feinberg School of Medicine Robert H Lurie Comprehensive Cancer Center Outline Recent and ongoing
New Developments in Ovarian Cancer Daniela Matei, MD Professor Gynecology Oncology Northwestern University Feinberg School of Medicine Robert H Lurie Comprehensive Cancer Center Outline Recent and ongoing
Histology: Its Influence on Therapeutic Decision Making
 Histology: Its Influence on Therapeutic Decision Making Mark A. Socinski, MD Professor of Medicine and Thoracic Surgery Director, Lung Cancer Section, Division of Hematology/Oncology Co-Director, UPMC
Histology: Its Influence on Therapeutic Decision Making Mark A. Socinski, MD Professor of Medicine and Thoracic Surgery Director, Lung Cancer Section, Division of Hematology/Oncology Co-Director, UPMC
trial update clinical
 trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
Carcinosarcoma Trial rial in s a in rare malign rare mali ancy
 Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
VISITING PROFESSORS. CME Information
 VISITING PROFESSORS Clinical Investigators Provide Their Perspectives on Current Cases and Emerging Research in the Management of Hodgkin and Non-Hodgkin Lymphomas and Multiple Myeloma CME Information
VISITING PROFESSORS Clinical Investigators Provide Their Perspectives on Current Cases and Emerging Research in the Management of Hodgkin and Non-Hodgkin Lymphomas and Multiple Myeloma CME Information
An Audio Review Journal for Nurses Bridging the Gap between Research and Patient Care
 ONU VOL 4 ISSUE 1 LUNG CANCER EDITION An Audio Review Journal for Nurses Bridging the Gap between Research and Patient Care FACULTY INTERVIEWS David R Spigel, MD Mollie Reed, MSN, RN, ACNP-BC Lecia V Sequist,
ONU VOL 4 ISSUE 1 LUNG CANCER EDITION An Audio Review Journal for Nurses Bridging the Gap between Research and Patient Care FACULTY INTERVIEWS David R Spigel, MD Mollie Reed, MSN, RN, ACNP-BC Lecia V Sequist,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA91: Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for the treatment of advanced ovarian
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA91: Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for the treatment of advanced ovarian
ESMO SUMMIT AFRICA. Latest evidence and current standard of care in advanced ovarian cancer. C.Sessa. Cape Town February 2018
 ESMO SUMMIT AFRICA Latest evidence and current standard of care in advanced ovarian cancer C.Sessa IOSI, Bellinzona, CH Cape Town 14-16 February 2018 CONFLICT OF INTEREST DISCLOSURE None Ovarian carcinoma
ESMO SUMMIT AFRICA Latest evidence and current standard of care in advanced ovarian cancer C.Sessa IOSI, Bellinzona, CH Cape Town 14-16 February 2018 CONFLICT OF INTEREST DISCLOSURE None Ovarian carcinoma
Treatment of Recurrent Ovarian Cancer
 Treatment of Recurrent Ovarian Cancer Mihaela Cristea, MD Associate Professor Medical Oncology, City of Hope November 11, 2016 No disclosures Financial Disclosure Epithelial Ovarian Cancer Subtypes and
Treatment of Recurrent Ovarian Cancer Mihaela Cristea, MD Associate Professor Medical Oncology, City of Hope November 11, 2016 No disclosures Financial Disclosure Epithelial Ovarian Cancer Subtypes and
GOG212: Taxane Maintenance
 GOG212: Taxane Maintenance Epithelial Ovarian or Primary Peritoneal Cancer Optimal or Suboptimal Cytoreduction Clinical C with normal CA125, no symptoms, normal CT Primary Carboplatin and Paclitaxel (or
GOG212: Taxane Maintenance Epithelial Ovarian or Primary Peritoneal Cancer Optimal or Suboptimal Cytoreduction Clinical C with normal CA125, no symptoms, normal CT Primary Carboplatin and Paclitaxel (or
RESEARCH ARTICLE. Kuanoon Boupaijit, Prapaporn Suprasert* Abstract. Introduction. Materials and Methods
 RESEARCH ARTICLE Survival Outcomes of Advanced and Recurrent Cervical Cancer Patients Treated with Chemotherapy: Experience of Northern Tertiary Care Hospital in Thailand Kuanoon Boupaijit, Prapaporn Suprasert*
RESEARCH ARTICLE Survival Outcomes of Advanced and Recurrent Cervical Cancer Patients Treated with Chemotherapy: Experience of Northern Tertiary Care Hospital in Thailand Kuanoon Boupaijit, Prapaporn Suprasert*
Medical Therapies in Ovarian Cancer The Arabic Perspectives. Mezghani Bassem -Tunisia
 Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
Changing Treatment Paradigms with Immunotherapy and Targeted Therapy in Advanced Non-Small-Cell Lung Cancer and Head & Neck Cancer
 Saturday, October 27, 2018 7:00 AM - 3:45 PM Changing Treatment Paradigms with Immunotherapy and Targeted Therapy in Advanced Non-Small-Cell Lung Cancer and Head & Neck Cancer Program Director John V.
Saturday, October 27, 2018 7:00 AM - 3:45 PM Changing Treatment Paradigms with Immunotherapy and Targeted Therapy in Advanced Non-Small-Cell Lung Cancer and Head & Neck Cancer Program Director John V.
National Horizon Scanning Centre. Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer. December 2007
 Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need?
 Herzog and Monk Gynecologic Oncology Research and Practice (2017) 4:13 DOI 10.1186/s40661-017-0050-0 REVIEW Open Access Bringing new medicines to women with epithelial ovarian cancer: what is the unmet
Herzog and Monk Gynecologic Oncology Research and Practice (2017) 4:13 DOI 10.1186/s40661-017-0050-0 REVIEW Open Access Bringing new medicines to women with epithelial ovarian cancer: what is the unmet
17 th ESO-ESMO Masterclass in clinical Oncology
 17 th ESO-ESMO Masterclass in clinical Oncology Cervical and endometrial Cancer Cristiana Sessa IOSI Bellinzona, Switzerland Berlin, March 28 th, 2018 Presenter Disclosures None Cervical Cancer Estimated
17 th ESO-ESMO Masterclass in clinical Oncology Cervical and endometrial Cancer Cristiana Sessa IOSI Bellinzona, Switzerland Berlin, March 28 th, 2018 Presenter Disclosures None Cervical Cancer Estimated
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Lynparza. Lynparza (olaparib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.52 Subject: Lynparza Page: 1 of 4 Last Review Date: September 15, 2017 Lynparza Description Lynparza
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.52 Subject: Lynparza Page: 1 of 4 Last Review Date: September 15, 2017 Lynparza Description Lynparza
