EXPERIMENTAL PROCEDURES
|
|
|
- David McKenzie
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 284, NO. 47, pp , November 20, by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. The Macrophage Cholesterol Exporter ABCA1 Functions as an Anti-inflammatory Receptor * Received for publication, July 21, 2009, and in revised form, September 7, 2009 Published, JBC Papers in Press, September 25, 2009, DOI /jbc.M Chongren Tang, Yuhua Liu, Peter S. Kessler, Ashley M. Vaughan, and John F. Oram 1 From the Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, University of Washington, Seattle, Washington ATP-binding cassette transporter A1 (ABCA1) is a cell membrane protein that exports excess cholesterol from cells to apolipoprotein (apo) A-I, the major protein in high density lipoproteins. Genetic studies have shown that ABCA1 protects against cardiovascular disease. The interaction of apoa-i with ABCA1 promotes cholesterol removal and activates signaling molecules, such as Janus kinase 2 (JAK2), that optimize the lipid export activity of ABCA1. Here we show that the ABCA1-mediated activation of JAK2 also activates STAT3, which is independent of the lipid transport function of ABCA1. ABCA1 contains two candidate STAT3 docking sites that are required for the apoa-i/abca1/jak2 activation of STAT3. The interaction of apoa-i with ABCA1-expressing macrophages suppressed the ability of lysopolysaccaride to induce the inflammatory cytokines interleukin-1, interleukin-6, and tumor necrosis factor-, which was reversed by silencing STAT3 or ABCA1. Thus, the apoa-i/abca1 pathway in macrophages functions as an anti-inflammatory receptor through activation of JAK2/STAT3. These findings implicate ABCA1 as a direct molecular link between the cardioprotective effects of cholesterol export from arterial macrophages and suppressed inflammation. Two major processes that initiate the formation of atherosclerotic lesions in the artery wall are inflammation and the deposition of excess cholesterol in macrophages. It is believed that both of these events are in response to trapping of sterolrich lipoproteins in the artery, where they undergo oxidation and other modifications to become inflammatory stimuli that recruit and activate macrophages (1). These cells ingest and degrade the modified lipoproteins, leading to intracellular accumulation of cholesterol ester lipid droplets. Population studies have shown an inverse relationship between circulating levels of HDLs and risk for cardiovascular disease, implying that factors associated with HDL metabolism are cardioprotective. One of these factors is ABCA1, which exports cholesterol and phospholipids from cells to lipid-poor apoa-i to generate precursors for HDL particles (2). Because it is highly induced by sterols through nuclear receptors, ABCA1 is expressed in cholesterol-loaded cells, such as macrophages in atherosclerotic lesions (3). Loss-of-function mutations in ABCA1 accelerate atherosclerosis (4), which is * This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL055362, HL085437, and PO1 HL To whom correspondence should be addressed. joram@u. washington.edu. likely to be the result of enhanced accumulation of cholesterolrich macrophages in arteries and the hyper-inflammatory responses of these cells. The cholesterol export function of ABCA1 occurs by a cascade of events involving direct binding of apoa-i to ABCA1, activation of signaling pathways, and solubilization of cholesterol and phospholipid domains formed by ABCA1 on the cell surface (5). We reported previously that incubating ABCA1-transfected baby hamster kidney (BHK) 2 cells with apoa-i or its synthetic mimetic peptides for only minutes dramatically increased autophosphorylation and thus activation of JAK2 by an ABCA1-dependent mechanism. Activation of JAK2 enhanced the apoa-i binding activity of ABCA1 responsible for lipid removal (6, 7). Thus JAK2 plays a feed-forward mechanism for optimizing the lipid export function of ABCA1. Receptor-mediated activation of JAK2 usually stimulates signaling pathways that activate transcription factors called STATs (8). We therefore investigated the possibility that the interaction of apoa-i with ABCA1 also activates one or more STATs. We found that exposing ABCA1 expressing cells to apoa-i increased phosphorylation of STAT3 without affecting STAT1 or STAT5, and this was independent of the lipid export activity of ABCA1. Because STAT3 plays a major role in suppressing inflammatory cytokine production by macrophages (9), a cell-type that expresses high levels of ABCA1 when sterol loaded, we also examined the possibility that the interaction of apoa-i with ABCA1 could suppress macrophage inflammatory cytokine production through STAT3. Results show that incubating apoa-i with activated ABCA1-expressing macrophages suppresses production of inflammatory cytokines IL-1, IL-6, and TNF- by a STAT3-dependent process, raising the possibility that the ABCA1 has a direct anti-inflammatory function in addition to its lipid export activity. EXPERIMENTAL PROCEDURES Antibodies and Reagents STAT3, phospho-stat3, and phosopho-jak2 antibodies were purchased from Cell Signaling; and STAT1, phopsho-stat1, anti-stat5, phospho- STAT5, and JAK2 antibodies were purchased from Santa Cruz. ABCA1 antibody was purchased from Novus. The JAK2 specific inhibitor AG490 was purchased from Sigma, and cell-per- 2 The abbreviations used are: BHK, baby hamster kidney; JAK, Janus kinase; STAT, signal transducers and activators of transcription; IL, interleukin; TNF, tumor necrosis factor; HDL, high density lipoprotein; LDL, low density lipoprotein; WT, wild-type; DMEM, Dulbecco s modified Eagle s medium; sirna, small interfering RNA; RT, reverse transcription; LPS, lipopolysaccharide; P-STAT, phosphorylated STATs; NBD, nucleotide binding domain JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 284 NUMBER 47 NOVEMBER 20, 2009
2 meable STAT3 inhibitor was purchased from Calbiochem. ApoA-I was purified from HDL as described previously (10). Mice C57BL/6 and DBA mice were purchased from Jackson Laboratories. ABCA1 / /DBA mice were a gift from Robert Aiello, Pfizer-Wyeth; STAT3 flox/flox /C57BL/6 mice were a gift from Shizuo Akiro, Osaka University; and Lys-M-Cre/C57BL/6 mice were a gift from Karin Bornfeldt, University of Washington. To generate mice lacking STAT3 in macrophages and neutrophils, STAT3 flox/flox were crossed with Lys-M-Cre mice to generate mice that carried the homozygous STAT3 flox/flox /Lys-M-Cre mice. Control wild-type mice were generated by interbreeding STAT3 wt/wt with Lys-M-Cre mice. ABCA1-transfected Cells and Cell Culture Wild-type and mutant BHK cells expressing mifepristone-inducible human ABCA1 were generated as described previously (11). Control (mock) BHK cells were derived from the same clonal line transfected with plasmids lacking the ABCA1 cdna insert. Sitedirected mutagenesis of ABCA1 was carried out using the QuikChange XL Site-directed mutagenesis kit (Stratagene, CA) (5). Cells were selected against Neozi, and clonal lines expressing similar amounts of wild-type (WT) ABCA1, mutants Y924F, Y1990F, or Y924/1990F were used for the experiments. BHK cells were maintained in DMEM with 10% fetal bovine serum until experiments. J774 cells were cultured in DMEM with 10% fetal bovine serum. To induce ABCA1 expression, J774 cells were loaded overnight with 50 g/ml acetylated LDL in the presence of 5 M TO (an LXR agonist that induces ABCA1) in DMEM containing 0.1% fatty acid-free bovine albumin (12). Thioglycollate-elicited peritoneal macrophages were obtained as previously described (13) and cultured in DMEM with 10% fetal bovine serum for 2 h, unseeded cells were washed away, and the medium was changed to DMEM, 0.1% fatty acidfree bovine albumin. To induce ABCA1 expression, cells were loaded with 50 g/ml acetylated LDL overnight. Because noninduced macrophages or BHK cells express very low or no detectable endogenous ABCA1, cells treated with inducers are defined as cells expressing ABCA1. For cytokine expression experiments, cells were pre-treated with or without 10 g/ml of apoa-i in DMEM, 0.1% fatty acid-free bovine albumin for 3 h, washed twice, treated with or without 10 ng/ml LPS for 3 h, and then processed for RNA extractions. Bone marrow macrophages for immunoblots were derived by flushing out mouse femurs and tibias with RPMI 1640, 10% fetal bovine serum and culturing cells for 1 week. Cholesterol Export Cellular cholesterol was labeled with 1 Ci/ml of [ 3 H]cholesterol (PerkinElmer Life Sciences) added to the growth medium 24 h prior to mifepristone incubations. Washed cells were then incubated with DMEM/BSA minus or plus 10 g/ml apoa-i, and medium and cells were assayed for free [ 3 H]cholesterol (14). ApoA-I-mediated cholesterol efflux was calculated as the percent total [ 3 H]cholesterol released into medium after subtraction of values obtained in the absence of apoa-i and was normalized to values for cells expressing wildtype ABCA1. Cells without induced ABCA1 had cholesterol efflux values insignificantly above induced cells incubated in the absence of apoa-i. Immunoblots and Immunoprecipitation Cells were lysed in Tris-HCl buffer (50 mm Tris-HCl, ph 7.4, 120 mm NaCl, 1% Nonidet P-40) supplemented with protease inhibitors (Complete mini; Roche) and phosphatase inhibitors (phosphatase inhibitor mixture II; Calbiochem) and then centrifuged at 15,000 g for 10 min at 4 C. Protein concentration was measured using the Bio-Rad protein assay reagent as instructed. Equal amounts of protein were added per gel lane, resolved by SDS-PAGE gel, transferred to nitrocellulose, and immunoblotted with the indicated antibodies. For immunoprecipitation, g of protein of 1 ml of lysates were incubated with the indicated antibodies (2 4 g) overnight at 4 C followed by incubation for 1 h with Protein A-magnetic beads. Immunocomplexes were then resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Band intensity was quantified from the autoradiograph scans using OptiQuant computer software (Packard Instruments). sirna Thioglycollate-elicited mouse peritoneal macrophages or J774 cells were plated in 24-well plates and maintained in DMEM with 10% (v/v) heat-inactivated fetal bovine serum, penicillin, and streptomycin. Silencer negative control sirna and silencer validated STAT3 sirnas (mstat3 sirna: sense, 5 -AAGGCCGTGGTGCGTGAGAAA-3 ) were purchased from Ambion. Transfection with sirna diluted in Opti-MEM I at a concentration of 100 nmol was performed using Hiperfect transfection reagent (Qiagen) according to their instruction. One day after transfection, medium was changed, and cells were loaded overnight with 50 g/ml of acetylated LDL in DMEM without (peritoneal macrophages) or with 5 M TO (J774 macrophages). Cells were treated with or without 10 g/ml of apoa-i for 3 h, washed twice, and then treated with/without 10 ng/ml of LPS for 3 h. Cells were collected for RT-PCR analyses. RT-PCR The one-step real-time RT-PCR was performed to quantify cytokines mrna expression using the Mx4000 Muliplex Quantitative PCR system (Stratagene, La Jolla, CA) and the TaqMan one-step RT-PCR master reagent kit (ABI), according to the instructions. The amplifications of cytokines and glyceraldehyde-3-phosphate dehydrogenase RNA were run using 100 ng of total RNA retrieved from samples using the Agilent total RNA isolation kit (Agilent Technology). Reactions were prepared in a 96-well optical grade PCR plate in a total of 50 l containing the following components: 100 ng of total RNA, 25 lof2 master mix, 2 units of the reverse transcriptase Super- Script II, and 25 l of TaqMan Gene Expression assay mixture (including primers and probe, from ABI). Thermal cycling conditions consisted of an initial reverse transcription step at 45 C for 30 min, followed by 10 min at 95 C, and then followed by 40 cycles of amplification. Each cycle of amplification consisted of a denaturizing step at 95 C for 30 s and an annealing/extension step at 60 C for 1 min. The IL-1, TNF-, IL-6, glyceraldehyde-3-phosphate dehydrogenase, and 18S primers and probes used for the RT-PCR were purchased from Applied Biosystems and used TaqMan Gene Expression Assays. Data Analyses Results were calculated as mean S.D. of 3 6 values, and statistical differences were calculated by Student s t test. Differences were considered to be significant at p NOVEMBER 20, 2009 VOLUME 284 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 32337
3 FIGURE 2. apoa-i interactions with ABCA1-expressing cells increases phosphorylation of ABCA1-associated STAT3. ABCA1-nonexpressing (, untreated) and -expressing (, induced) transfected BHK cells (Tf-BHKs, induced with mifipristone) (A and B) or J774 macrophages (C and D, induced with the LXR agonist TO901317) were incubated for 1 h without ( ) or with ( ) 10 g/ml of apoa-i; and whole cell lysates (A and C) or immunoprecipitated P-STAT3 (B) or ABCA1 (D) were immunoblotted for phosphorylated STAT3 (P-STAT3) (A and C), phosphorylated STAT1 (P-STAT1) (A), ABCA1 (B), whole STAT3 (D), or P-STAT3 (D). Total STAT3 in BHK cells and J774 macrophages did not change under any treatment conditions. ABCA1 was undetectable in the absence of inducer. FIGURE 1. ApoA-I stimulates ABCA1-bound JAK2 autophosphorylation and ABCA1 tyrosine phosphorylation. ABCA1-transfected BHK cells were treated without (, Mock) or with (, ABCA1) 10nM mifepristone to induce ABCA1; cells were treated without ( ) or with ( )10 g/ml of apoa-i for 15 min (A D) or 1 h(e); JAK2 (A and B) or ABCA1 (C E) were immunoprecipitated from lysates; proteins were resolved by SDS-PAGE and transferred to nitrocellulose; and P-JAK2 (A and D), total JAK2 (B and C), or phosphotyrosine residues (E) were detected by immunoblot analyses. To block JAK2-mediated phosphorylation (E), cells were pre-treated with AG490 ( ) for 30 min prior to the apoa-i incubations. RESULTS Using an antibody specific for the phosphorylated form of JAK2 (P-JAK2), we found that incubating ABCA1-transfected BHK cells with apoa-i for only minutes dramatically increased phosphorylation and thus activation of JAK2, which was not observed in cells lacking ABCA1 (Fig. 1A). The total cell content of JAK2 was unaffected by either ABCA1 expression or exposure to apoa-i (Fig. 1B). We carried out co-immunoprecipitation studies to determine whether JAK2 and ABCA1 form a molecular complex. When ABCA1 was induced in transfected BHK cells, JAK2 coimmunoprecipitated with ABCA1 whether or not cells were treated with apoa-i (Fig. 1C). This JAK2 was phosphorylated only when the ABCA1-expressing cells were treated with apoa-i (Fig. 1D). We also found that treating lysates of ABCA1- expressing BHK cells with a P-JAK2 antibody co-precipitated ABCA1 (not shown). Thus, JAK2 constitutively binds to ABCA1, where it is activated by apoa-i. Because JAK2 is a tyrosine kinase, we examined ABCA1 immunoprecipitated from transfected BHK cells for changes in its phosphotyrosine content using an antibody specific for this modified amino acid. Phosphorylated tyrosines were detected in ABCA1 in the basal state, but this increased over 2-fold when cells were incubated with apoa-i (Fig. 1E). Reversal of this increase by AG490 (a JAK2-specific inhibitor) implied that JAK2 was responsible for this effect. These results are consistent with the idea that the interaction of apoa-i with ABCA1- expressing cells rapidly activates JAK2, which in turn phosphorylates ABCA1 tyrosines. Activation of JAK2 through binding of ligands to a wide variety of plasma membrane receptors also activates one or more of a family of transcription factors called STATs (8). We therefore tested the possibility that the interaction of apoa-i with ABCA1 activates a STAT using antibodies specific for different P-STATs. Incubating BHK cells lacking ABCA1 with apoa-i had no effect on phosphorylation of STAT3, STAT1 (Fig. 2A), or STAT5 (not shown). When ABCA1 was induced, however, apoa-i stimulated STAT3 but not STAT1 (Fig. 2A) or STAT5 phosphorylation. This effect was inhibited by AG490 (not shown), implying that it was mediated by JAK2. When lysates of transfected BHK cells were treated with an antibody specific for P-STAT3, ABCA1 was co-immunoprecipitated with P-STAT3 only from cells expressing ABCA1 and incubated with apoa-i (Fig. 2B). We also examined the possibility that apoa-i-mediated activation of STAT3 occurs in cholesterol-loaded macrophages, the cell-type most relevant to ABCA1 cell biology in vivo (3). P-STAT3 levels were markedly increased in J774 macrophages when they were incubated with the LXR agonist TO to induce ABCA1 and then treated with apoa-i (Fig. 2C). Total STAT3 and P-STAT3 were co-immunoprecipitated with ABCA1 only from macrophages that were induced to express ABCA1 and exposed to apoa-i (Fig. 2D). Thus, incubating ABCA1-expressing macrophages with apoa-i recruits STAT3 to ABCA1 where it becomes phosphorylated. Taken together, these results suggest that the interaction of apoa-i with ABCA1-expressing cells rapidly activates JAK2, which creates phosphorylated docking sites on ABCA1 that bind and activate STAT3. The STAT3 docking sites in cytosolic domains of plasma membrane receptors have a highly conserved YXXQ sequence (15). We searched ABCA1 for these motifs and found one in JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 284 NUMBER 47 NOVEMBER 20, 2009
4 each of the large cytosolic loops that contain the nucleotide binding domains (NBDs): at amino acids (YEGQ) in NBD1 and residues (YCPQ) in NBD2. These motifs are highly conserved between ABCA1s of different species (chimpanzee, horse, dog, cow, mouse, rat, chicken, zebrafish, zebra finch, platypus, and tree shrew), and the Tyr-1990 motif is conserved between all ABCAs. We generated cells expressing ABCA1 with phenylalanines substituted for tyrosines in each or both of these YXXQ motifs and selected clonal cell lines that had similar ABCA1-dependent cholesterol efflux activities and ABCA1 protein levels (based on immunoblots) as cells expressing wild-type ABCA1 (Fig. 3A). We compared the ability of apoa-i to stimulate phosphorylation of STAT3 in these different cell lines by scanning autoradiograms of blots probed with P-STAT3 antibody and reprobed with total STAT3 antibody and by calculating the ratios. Exposing apoa-i to BHK cells transfected with wild-type ABCA1 tripled the fraction of STAT3 that was phosphorylated (Fig. 3B). Mutating Tyr-924 slightly decreased this fraction, but mutating Tyr-1990 markedly decreased apoa-i-stimulated phosphorylation of STAT3. Mutating both Tyr-924 and Tyr completely abolished apoa-i-stimulated STAT3 phosphorylation, suggesting some cooperativity between these two sites. Probing immunoprecipitates of STAT3 with an ABCA1 antibody revealed that the apoa-i-stimulated interaction of ABCA1 with STAT3 was completely abolished by the Y924F/ Y1990F mutations (Fig. 3C). These findings implicate these motifs, especially Tyr-1990, as the docking sites on ABCA1 required for STAT3 activation. A lack of effect of the Y924F and Y1990F mutations on cholesterol efflux despite a loss of STAT3 phosphorylation (Fig. 3, A and B) indicates that the ABCA1-dependent activation of STAT3 plays no role in the cholesterol export function of ABCA1. This idea was further supported by studies showing that silencing of STAT3 with a sirna had no effect of apoa-imediated cholesterol or phospholipid efflux (not shown). ABCA1 is induced by sterol-sensing LXR nuclear receptors and thus is expressed at high levels in cells that accumulate cholesterol, such as tissue macrophages. In macrophages, the JAK2/STAT3 signaling pathway has an anti-inflammatory function (9). We therefore tested the effects of apoa-i on expression of anti-inflammatory cytokines by ABCA1-expressing J774 macrophages. For these studies, we loaded cells with cholesterol and treated them with the LXR agonist TO to induce ABCA1 (16), incubated them without or with apoa-i for 3 h, incubated them for an additional 3 h without or with the inflammatory agent LPS in the absence of apoa-i, and measured the levels of TNF-, IL-1, and IL-6 mrna. The purpose of these studies was to determine whether preincubating ABCA1-expressing macrophages with apoa-i affects subsequent cytokine production in response to LPS. In agreement with studies by others (17), we found that pretreating these cells with the LXR agonist alone markedly suppressed the ability of LPS to induce TNF-, IL-1, and IL-6 (not shown). Despite this baseline suppression, however, exposing cells to LPS for 3 h still increased transcription of these cytokines by 2 4-fold (Fig. 4A). Although apoa-i FIGURE 3. Mutating candidate STAT3 docking sites in ABCA1 reduces apoa-i-stimulated STAT3 phosphorylation and ABCA1 association without affecting cholesterol efflux. A, mifepristone-treated BHK cells transfected with wild-type (WT) ABCA1 or ABCA1 containing Y924F, Y1990F, or Y924F/Y1990F substitutions were labeled with [ 3 H]cholesterol and incubated for 2 h without or with 10 g/ml apoa-i, and cholesterol efflux was calculated as the apoa-i-mediated release of [ 3 H]cholesterol into the medium normalized for values for WT cells. A, total ABCA1 protein was detected by immunoblots of ABCA1 from equal amounts of total cell protein from WT and mutant cells resolved on the same gel. B, lysates from equal amounts of whole cell protein from BHK cell lines incubated for 1 h without or with 10 g/ml apoa-i were immunoblotted first with a P-STAT3 antibody and reprobed with a STAT3 antibody. Autoradiograms were scanned and quantified, and the relative amount of STAT3 phosphorylated was calculated as a ratio of P-STAT3 to STAT3 signal for each sample (mean S.D. from four experiments). Total STAT3 did not differ between wild-type and mutant cell lines (not shown). C, whole cell lysates from BHK cells transfected with WT and Y924F/Y1990F were immunoprecipitated with anti-stat3 antibody and immunoblotted with anti-abca1 antibody. had little effect on cytokine mrna levels without LPS treatment, it markedly reduced the stimulatory effects of LPS (Fig. 4A). Thus, the interaction of apolipoproteins with ABCA1-expressing macrophages suppresses LPS-induced inflammatory cytokine production. We used sirna transfections to determine whether apoa-i is exerting its anti-inflammatory effects through STAT3. Results from three experiments showed that transfecting J774 macrophages with STAT3 sirna reduced STAT3 protein levels by 50 60% when compared with cells transfected NOVEMBER 20, 2009 VOLUME 284 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 32339
5 FIGURE 4. ApoA-I inhibits inflammatory cytokine production in macrophages by a STAT3-dependent mechanism. A, J774 macrophages were loaded for 24 h with 50 g/ml of acetyl-ldl-derived cholesterol, incubated for 24 h with 5 g/ml of TO to induce ABCA1, incubated without or with 10 g/ml of apoa-i for 3 h, and treated without or with 10 ng/ml of LPS for 3 h. TNF-, IL-1, and IL-6 mrna levels were measured by RT-PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase mrna levels. Results are mean S.D. of triplicate values from 2 separate experiments representative of four similar experiments. *, p 0.001compared with no apoa-i. AU, arbitrary units (B) J774 (top) or mouse peritoneal (MP, bottom) macrophages were loaded with 50 g/ml of acetyl-ldl-derived cholesterol, transfected with either a scrambled control (Ctrl, 100 nm) or STAT3 sirna ( nm), and J774 macrophages were incubated for 24 h with 5 M TO Cells were incubated for 3 h without or with 10 g/ml of apoa-i, washed, and incubated for 3 h with 10 ng/ml of LPS; and STAT3 levels were measured by immunoblot analyses. C, cholesterol-loaded TO treated J744 (top)ormp(bottom) macrophages were incubated without or with 10 g/ml of apoa-i for 3 h, and treated with 10 ng/ml of LPS for 3 h. TNF- and IL-6 mrna levels were measured and expressed as percentage of no apoa-i values. Results are mean S.D. of triplicate values from two separate experiments representative of four similar experiments. *, p compared with controls. D, cholesterol-loaded peritoneal macrophages were pre-treated with the indicated concentrations of STAT3 phosphorylation (P-STAT3) inhibitor for 2 h and then treated with or without 10 g/ml apoa1 for 1 h. Cell proteins were immunoblotted with P-STAT3 antibody and then re-probed with total STAT3 antibody. E, cholesterol-loaded MP macrophages were pretreated for 2 h without (Ctrl) or with 10 M P-STAT3 inhibitor (Inhib), incubated without or with inhibitor or 10 g/ml of apoa-i for 3 h, and treated with 10 ng/ml LPS for 3 h. TNF- and IL-6 mrna levels were measured and expressed as percentage of no apoa1 values. Results are mean S.D. of quadruplicate values from two separate experiments. *, p compared with controls. with a scrambled sirna (Fig. 4B). The ability of apoa-i to reduce LPS-stimulated expression of TNF- (or IL-6) was partially but significantly (p 0.03) reversed by knocking down STAT3 (Fig. 4C). In this experiment, apoa-i decreased TNF- and IL-6 mrna levels by 46 and 56% in control cells, respectively, and this was reduced to 25 and 26% in STAT3 sirna-transfected cells. These differences are comparable with the degree of silencing of STAT3 by the sirna. These findings support the conclusion that apoa-i suppresses inflammation in J774 macrophages through the JAK2/STAT3 pathway. Because J774 macrophages were relatively resistant to silencing STAT3 with a sirna, we used another macrophage cell line to confirm the involvement of STAT3 in the anti-inflammatory effects of apoa- I. With isolated mouse peritoneal macrophages, transfection with the same STAT3 sirna reduced STAT3 protein levels by 80% (Fig. 4B). Also in contrast to J774 macrophages, we were able to induce ABCA1 to high levels by loading them with acetylated LDL-derived cholesterol without the need to treat them with an LXR agonist (data not shown). As with J774 macrophages, 3-h incubations of cholesterol-loaded peritoneal macrophages with apoa-i inhibited LPS-induced expression of both TNF- and IL-6 (Fig. 4C). Transfecting cells with the STAT3 sirna almost completely reversed the inhibitory effects of apoa-i on LPSinduced expression of TNF- and IL-6 (Fig. 4C). Thus, our results show that apoa-i interactions with two different types of ABCA1- expressing macrophages inhibits inflammatory cytokine production by a STAT3-dependent process. To examine if activation of STAT3 is required for the antiinflammatory effects of apoa-i/ ABCA1 interactions, we treated macrophages with the cell-permeable STAT3 inhibitor peptide (18). This inhibitor markedly reduced STAT3 phosphorylation in choles JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 284 NUMBER 47 NOVEMBER 20, 2009
6 terol-loaded peritoneal macrophages without affecting total STAT3 protein levels (Fig. 4D). Whereas apoa-i reduced subsequent LPS-stimulated production of TNF- and IL-6 by 60% in these cells, these apoa-i effects were reduced to less than 30% in the presence of the STAT3 phosphorylation inhibitor (Fig. 4E). These findings support the idea that STAT3 activation is involved in the anti-inflammatory effects of apoa-i. We used cholesterol-loaded peritoneal macrophages from mice lacking macrophage STAT3 to further confirm a role of STAT3 in the apoa-i-mediated suppression of cytokine production. As an additional control, we compared the effects of apoa-i with those of IL-10, a cytokine receptor system that suppresses macrophage inflammation through STAT3 (9). Because general knock-out of STAT3 is embryonic lethal (19), we generated mice with STAT3 silenced specifically in myeloid cells by breeding STAT3 fl/fl mice with a mouse Lys-M-Cre line, which is under control of the macrophage/granulocyte-specific lysozyme-m promoter. Immunoblots of peritoneal and bone marrow macrophages from these mice showed a complete absence of STAT3 as compared with cells from wild-type animals (STAT3 wt/wt /Lys-M-Cre) or the whole population of white blood cells from STAT3 fl/fl /Lys-M-Cre mice (Fig. 5A). Knocking out STAT3 markedly reduced the ability of apoa-i to suppress cytokine production from cholesterol-loaded peritoneal macrophages (Fig. 5B), supporting the idea that STAT3 plays a critical role in the anti-inflammatory effects of macrophage/apoa-i interactions. The ability of IL-10 to inhibit LPSinduced cytokine production was completely absent in cells lacking STAT3, consistent with IL-10 acting exclusively through STAT3. These findings show that apoa-i mimics IL- 10 in suppressing inflammatory cytokine production by LPStreated macrophages. We also confirmed a role of ABCA1 in the apoa-i-mediated suppression of cytokine production by measuring the effects of apoa-i on cholesterol-loaded peritoneal macrophages from ABCA1 / mice. The ability of apoa-i to suppress production of TNF- and IL-6 was absent in macrophages lacking ABCA1 (Fig. 5C), indicating a complete requirement for this transporter for the anti-inflammatory effects of apoa-i. DISCUSSION We showed previously that the interaction of apoa-i and its mimetic peptides with ABCA1-expressing cells rapidly activates JAK2, and this enhances the direct interaction of apoa-i with ABCA1 required for lipid removal from the cells (6, 7). Here we show that this interaction also activates the transcription factor STAT3, and this is independent of the lipid transport activity of ABCA1. STAT3 has an anti-inflammatory function in macrophages (9), a major ABCA1-expressing cell type, and we found that the interaction of apoa-i with cholesterolloaded macrophages suppressed LPS-induced production of inflammatory cytokines. Silencing STAT3 or ABCA1 in macrophages reduced the ability of apoa-i to inhibit cytokine production, indicating a critical role of these two proteins in the anti-inflammatory effects of apoa-i. These findings show that the interaction of apoa-i with ABCA1-expressing macrophages activates a JAK2/STAT3 pathway that has anti-inflammatory activity. FIGURE 5. Loss of anti-inflammatory activity of apoa-i in macrophages from myeloid-specific STAT3 and ABCA1 knock-out mice. A, STAT3 immunoblots of peritoneal macrophages (PM), bone marrow macrophages (BMM), or white blood cells (WBC) from STAT3 fl/fl /LYS-M-CRE (KO) or STAT3 wt/wt /LYS- M-CRE mice (WT). B, cholesterol-loaded macrophages from WT or myloidspecific STAT3 KO mice were pretreated for 3 h with apoa-i or 10 ng/ml of IL-10 and incubated for 3 h with LPS, and cytokine mrna levels were measured. Results are the mean S.D. from six values representing two experiments. *, p compared with WT. C, cholesterol-loaded macrophages from ABCA1 / mice were pretreated for 3 h with apoa-i and incubated for 3 h with LPS, and cytokine mrna levels were measured. Results are the mean S.D. from six values representing two experiments. *, p compared with controls. The two intracellular loops of ABCA1 (NBDs) each contain a tetrameric amino acid motif (YXXQ) previously shown to create STAT3 docking sites in cytokine receptors (15). Mutating these sites completely abolished the ability of apoa-i to activate and bind STAT3 without affecting the cholesterol export function of ABCA1, indicating that these sites are responsible for the apoa-i-mediated STAT3 activation. Remarkably, the site in NBD2 lies in a pentamer (GYPCQ) that has the identical sequence as the prototypic STAT3 docking site originally described for the gp130 subunit of cytokine receptors (15). Mutating this site had the largest effect of preventing apoa-i from activating STAT3. NOVEMBER 20, 2009 VOLUME 284 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 32341
7 Whereas the JAK2/STAT3 pathway is oncogenic and proinflammatory in many cell types (20), it has an anti-inflammatory function in macrophages. Constitutive expression of active STAT3 in cultured macrophages nearly abolishes LPS-induced inflammatory cytokine production (9). Selective silencing of STAT3 in mouse macrophages and neutrophils increases susceptibility to endotoxic shock and promotes chronic entercolitis (21 23). The anti-inflammatory cytokine IL-10 suppresses production of inflammatory cytokines in macrophages through STAT3, and IL-10 knock-out mice have chronic inflammation and increased atherosclerosis (24, 25). Here we found that pretreating ABCA1-expressing macrophages with apoa-i suppressed subsequent LPS-induced production of the inflammatory cytokines TNF-, IL-1, and IL-6. ApoA-I therefore primes ABCA1-expressing macrophages to resist LPS-induced inflammation. This effect of apoa-i was reduced by knocking down STAT3 with an sirna, silencing STAT3 in myeloid-specific STAT3 knock-out mice, or inhibiting STAT3 phosphorylation, indicating a role for STAT3 in the anti-inflammatory effects of apoa-i. A role for ABCA1 was confirmed by results showing that apoa-i lost its anti-inflammatory activity in macrophages from ABCA1 / mice. Silencing STAT3 in macrophages did not completely reverse the ability of apoa-i to suppress LPS-induced cytokine production, indicating that some of the anti-inflammatory effects of apoa-i are independent of STAT3. Based on studies with macrophages lacking STAT3, it was estimated that 65% of the effects of apoa-i on LPS-induced inflammatory cytokine production could be attributed to STAT3. It has been shown that the cholesterol export activity of apoa-i can also inhibit macrophage inflammatory cytokine production by disrupting sterol-rich membrane rafts (26 30). It is therefore likely that apoa-i/abca1 interactions can suppress macrophage inflammation by multiple mechanisms. The availability of mutant forms of ABCA1 that lack the ability to activate STAT3 but retain lipid export activity will allow us to examine the contribution of these different mechanisms to the overall anti-inflammatory effects of apoa-i. There is controversy about whether or not selective deletion of macrophage ABCA1 modulates atherosclerosis. Transplantation of Abca1-deficient bone marrow into mice significantly reduced atherosclerosis (31, 32), but myeloid-specific silencing of ABCA1 in whole animals had no effect on atherosclerosis (33). One explanation for these discrepancies might be related to the macrophage-like cell types that lack ABCA1 in the atherosclerotic lesions. Of particular interest is the fact that the myeloid-specific ablation of ABCA1 had little effect on the dendritic cell ABCA1, a cell type that may play an important role in atherogenesis (34). Loading macrophages with cholesterol induces a dendritic cell phenotype (35), and STAT3 negatively regulates dendritic cell function (36). It is also not known if overexpression of ABCA1 in macrophages could have an antiinflammatory effect. Taken together, the current and previous studies (5 7) show that interaction of apoa-i with ABCA1-expressing cells rapidly activates JAK2, which in turn activates two independent pathways: lipid export from cells and STAT3-mediated transcription (Fig. 6). The observation that ABCA1 contains active FIGURE 6. A model for the ABCA1/JAK2-dependent branch pathway for activation of cholesterol export and STAT3-mediated suppression of inflammatory cytokine production by macrophages. A, ABCA1 intrinsically forms cell-surface lipid domains enriched with phospholipids (PL) and free cholesterol (FC). B, apoa-i binds to ABCA1, stimulating autophosphorylation (activation) of associated JAK2. C, the activated JAK2 both enhances the interaction of apoa-i with ABCA1 required for lipid interactions and creates STAT3 docking sites that promote JAK2-mediated phosphorylation of STAT3. D, the lipid-bound apoa-i solubilizes the ABCA1-formed lipid domains to generate nascent HDL particles, and the phosphorylated STAT3 is translocated to the nucleus where it regulates transcription events that suppress LPS-mediated production of inflammatory cytokines. STAT3 docking sites that allow it to function as a signaling receptor is a unique property among cell membrane transporters. One novel feature of ABCA1 compared with other eucharyotic transporters is that its export function requires direct molecular interactions between the transporter and the acceptor of the transported cargo. This creates the opportunity for ligand/receptor-like interactions to regulate both transport activity and signaling pathways. Studies of human HDL deficiencies and mouse models have shown that loss-of-function mutations in ABCA1 increase cardiovascular disease (4, 31). This has been attributed to an impaired removal of excess cholesterol from macrophages by HDL apolipoproteins (37), which would lead to accumulation of sterol-laden macrophages in the artery wall. Mice lacking ABCA1 in all tissues or selectively in macrophages have an enhanced inflammatory response to LPS (26, 38, 39), implying that macrophage ABCA1 has an anti-inflammatory function in vivo and that this may contribute to its cardioprotective effects. Studies of isolated macrophages from these mice suggest that this may be secondary to the inability of ABCA1 to export lipids JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 284 NUMBER 47 NOVEMBER 20, 2009
8 from cells, which modifies plasma membrane lipid rafts and enhances LPS signaling (27). Our studies show that ABCA1 can also directly suppress LPS-induced inflammatory cytokine production by macrophages through a STAT3 pathway that is independent of cholesterol export. This novel dual function of ABCA1 may have evolved as part of the innate immune system, particularly as a component of the resolution phase of inflammation (40). Activated macrophages at sites of inflammation release inflammatory cytokines and phagocytose apoptotic cells, which contain cholesterol-rich membranes. An unchecked accumulation of cholesterol in macrophages can be cytotoxic and evoke exaggerated inflammatory responses (26, 41), thus promoting sustained tissue damage. The induction of ABCA1 by the accumulating cholesterol would divert the cholesterol from cells into the HDL pathway and prevent cytotoxicity and excessive inflammation. In parallel, the induced ABCA1 would resolve the inflammatory response through activation of JAK2/STAT3. The same process is likely to occur in atherosclerotic lesions, except that the major source of macrophage-engulfed cholesterol would be sterol-rich lipoproteins trapped in the artery wall. Thus, ABCA1 may be a direct link between the cardioprotective effects of cholesterol export and anti-inflammation. These findings also raise the interesting possibility that ABCA1 might have a protective role in other inflammatory disorders characterized by the local accumulation of macrophages. Acknowledgment We thank Dr. Renee Leboeuf for expertise and enthusiasm for this study. REFERENCES 1. Williams, K. J., and Tabas, I. (1995) Arterioscler. Thromb. Vasc. Biol. 15, Oram, J. F., and Heinecke, J. W. (2005) Physiol. Rev. 85, Passarelli, M., Tang, C., McDonald, T. O., O Brien, K. D., Gerrity, R. G., Heinecke, J. W., and Oram, J. F. (2005) Diabetes 54, Singaraja, R. R., Brunham, L. R., Visscher, H., Kastelein, J. J., and Hayden, M. R. (2003) Arterioscler. Thromb. Vasc. Biol. 23, Vaughan, A. M., Tang, C., and Oram, J. F. (2009) J. Lipid Res. 50, Tang, C., Vaughan, A. M., Anantharamaiah, G. M., and Oram, J. F. (2006) J. Lipid Res. 47, Tang, C., Vaughan, A. M., and Oram, J. F. (2004) J. Biol. Chem. 279, Aaronson, D. S., and Horvath, C. M. (2002) Science 296, Williams, L. M., Sarma, U., Willets, K., Smallie, T., Brennan, F., and Foxwell, B. M. (2007) J. Biol. Chem. 282, Mendez, A. J., Anantharamaiah, G. M., Segrest, J. P., and Oram, J. F. (1994) J. Clin. Invest. 94, Vaughan, A. M., and Oram, J. F. (2003) J. Lipid Res. 44, Wang, Y., Kurdi-Haidar, B., and Oram, J. F. (2004) J. Lipid Res. 45, Renard, C. B., Kramer, F., Johansson, F., Lamharzi, N., Tannock, L. R., von Herrath, M. G., Chait, A., and Bornfeldt, K. E. (2004) J. Clin. Invest. 114, Oram, J. F., Lawn, R. M., Garvin, M. R., and Wade, D. P. (2000) J. Biol. Chem. 275, Stahl, N., Farruggella, T. J., Boulton, T. G., Zhong, Z., Darnell, J. E., Jr., and Yancopoulos, G. D. (1995) Science 267, Schwartz, K., Lawn, R. M., and Wade, D. P. (2000) Biochem. Biophys. Res. Commun. 274, Zelcer, N., and Tontonoz, P. (2006) J. Clin. Invest. 116, Buettner, C., Pocai, A., Muse, E. D., Etgen, A. M., Myers, M. G., Jr., and Rossetti, L. (2006) Cell Metab. 4, Takeda, K., Noguchi, K., Shi, W., Tanaka, T., Matsumoto, M., Yoshida, N., Kishimoto, T., and Akira, S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, Bromberg, J. F., Wrzeszczynska, M. H., Devgan, G., Zhao, Y., Pestell, R. G., Albanese, C., and Darnell, J. E., Jr. (1999) Cell 98, Matsukawa, A., Takeda, K., Kudo, S., Maeda, T., Kagayama, M., and Akira, S. (2003) J. Immunol. 171, Matsukawa, A., Kudo, S., Maeda, T., Numata, K., Watanabe, H., Takeda, K., Akira, S., and Ito, T. (2005) J. Immunol. 175, Takeda, K., Clausen, B. E., Kaisho, T., Tsujimura, T., Terada, N., Förster, I., and Akira, S. (1999) Immunity 10, Murray, P. J. (2006) Curr. Opin. Pharmacol. 6, Potteaux, S., Esposito, B., van Oostrom, O., Brun, V., Ardouin, P., Groux, H., Tedgui, A., and Mallat, Z. (2004) Arterioscler. Thromb. Vasc. Biol. 24, Zhu, X., Lee, J. Y., Timmins, J. M., Brown, J. M., Boudyguina, E., Mulya, A., Gebre, A. K., Willingham, M. C., Hiltbold, E. M., Mishra, N., Maeda, N., and Parks, J. S. (2008) J. Biol. Chem. 283, Koseki, M., Hirano, K., Masuda, D., Ikegami, C., Tanaka, M., Ota, A., Sandoval, J. C., Nakagawa-Toyama, Y., Sato, S. B., Kobayashi, T., Shimada, Y., Ohno-Iwashita, Y., Matsuura, F., Shimomura, I., and Yamashita, S. (2007) J. Lipid Res. 48, Yvan-Charvet, L., Welch, C., Pagler, T. A., Ranalletta, M., Lamkanfi, M., Han, S., Ishibashi, M., Li, R., Wang, N., and Tall, A. R. (2008) Circulation 118, Murphy, A. J., Woollard, K. J., Hoang, A., Mukhamedova, N., Stirzaker, R. A., McCormick, S. P., Remaley, A. T., Sviridov, D., and Chin-Dusting, J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, Tellier, E., Canault, M., Poggi, M., Bonardo, B., Nicolay, A., Alessi, M. C., Nalbone, G., and Peiretti, F. (2008) J. Cell. Physiol. 214, Aiello, R. J., Brees, D., Bourassa, P. A., Royer, L., Lindsey, S., Coskran, T., Haghpassand, M., and Francone, O. L. (2002) Arterioscler. Thromb. Vasc. Biol. 22, van Eck, M., Bos, I. S., Kaminski, W. E., Orsó, E., Rothe, G., Twisk, J., Böttcher, A., Van Amersfoort, E. S., Christiansen-Weber, T. A., Fung- Leung, W. P., Van Berkel, T. J., and Schmitz, G. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, Brunham, L. R., Singaraja, R. R., Duong, M., Timmins, J. M., Fievet, C., Bissada, N., Kang, M. H., Samra, A., Fruchart, J. C., McManus, B., Staels, B., Parks, J. S., and Hayden, M. R. (2009) Arterioscler. Thromb. Vasc. Biol. 29, Galkina, E., and Ley, K. (2009) Annu. Rev. Immunol. 27, Cho, H. J., Shashkin, P., Gleissner, C. A., Dunson, D., Jain, N., Lee, J. K., Miller, Y., and Ley, K. (2007) Physiol. Genomics 29, Sun, Y., Chin, Y. E., Weisiger, E., Malter, C., Tawara, I., Toubai, T., Gatza, E., Mascagni, P., Dinarello, C. A., and Reddy, P. (2009) J. Immunol. 182, Singaraja, R. R., Visscher, H., James, E. R., Chroni, A., Coutinho, J. M., Brunham, L. R., Kang, M. H., Zannis, V. I., Chimini, G., and Hayden, M. R. (2006) Circ. Res. 99, Francone, O. L., Royer, L., Boucher, G., Haghpassand, M., Freeman, A., Brees, D., and Aiello, R. J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, Aiello, R. J., Brees, D., and Francone, O. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, Serhan, C. N., and Savill, J. (2005) Nat. Immunol. 6, Feng, B., Yao, P. M., Li, Y., Devlin, C. M., Zhang, D., Harding, H. P., Sweeney, M., Rong, J. X., Kuriakose, G., Fisher, E. A., Marks, A. R., Ron, D., and Tabas, I. (2003) Nat. Cell Biol. 5, NOVEMBER 20, 2009 VOLUME 284 NUMBER 47 JOURNAL OF BIOLOGICAL CHEMISTRY 32343
ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation
 ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation Ashley M. Vaughan, Chongren Tang, and John F. Oram 1 Division of Metabolism,
ABCA1 mutants reveal an interdependency between lipid export function, apoa-i binding activity, and Janus kinase 2 activation Ashley M. Vaughan, Chongren Tang, and John F. Oram 1 Division of Metabolism,
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplemental Material. Results
 Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
Supplemental Material Results Fractionation of mouse plasma by high-resolution SEC. APOA1 eluted as a single major peak in fractions 16 of plasma (the apparent size of mature, lipidated HDL) when it was
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice
 Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice Chongren Tang, 1 Yuhua Liu, Wendy Yang, Carl Storey, Tim S. McMillen, Barbara A. Houston, Jay W. Heinecke,
Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice Chongren Tang, 1 Yuhua Liu, Wendy Yang, Carl Storey, Tim S. McMillen, Barbara A. Houston, Jay W. Heinecke,
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Online Data Supplement. Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2
 Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Stewart et al. CD36 ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer
 NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT) and NQO1-null (NQO1-/-) mice.
 competes with 20S proteasome for binding with C/EBP leading to its stabilization and Relative mrna levels Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT)
competes with 20S proteasome for binding with C/EBP leading to its stabilization and Relative mrna levels Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT)
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL
 ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL Ashley M. Vaughan 1 and John F. Oram Department of Medicine, Division of Metabolism, Endocrinology,
ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL Ashley M. Vaughan 1 and John F. Oram Department of Medicine, Division of Metabolism, Endocrinology,
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington
 HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
HDL and Its Galaxy of Proteins: What Do they Do? Jay Heinecke, University of Washington Disclosures NIH AHA Insilicos Consultant Merck Research Support GSK Research Support Pfizer Research Support Corcept
Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine
 Int J Clin Exp Med 2015;8(9):16374-16378 www.ijcem.com /ISSN:1940-5901/IJCEM0010359 Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine Hongming Zhang
Int J Clin Exp Med 2015;8(9):16374-16378 www.ijcem.com /ISSN:1940-5901/IJCEM0010359 Original Article Regulation of macrophage cholesterol efflux and liver X receptor α activation by nicotine Hongming Zhang
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL
 SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL Laurent Yvan-Charvet, 1, * Tamara A. Pagler,* Nan Wang,* Takafumi Senokuchi,* May Brundert, Hongna Li,* Franz Rinninger,
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Supplementary Figure 1
 Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Macrophages are the major source of apolipoprotein E
 Distinct Cellular Loci for the ABCA1-Dependent and ABCA1-Independent Lipid Efflux Mediated by Endogenous Apolipoprotein E Expression Zhi H. Huang, Michael L. Fitzgerald, Theodore Mazzone Objective Macrophage
Distinct Cellular Loci for the ABCA1-Dependent and ABCA1-Independent Lipid Efflux Mediated by Endogenous Apolipoprotein E Expression Zhi H. Huang, Michael L. Fitzgerald, Theodore Mazzone Objective Macrophage
Validation & Assay Performance Summary
 Validation & Assay Performance Summary LanthaScreen IGF-1R GripTite Cells Cat. no. K1834 Modification Detected: Phosphorylation of Multiple Tyr Residues on IGF-1R LanthaScreen Cellular Assay Validation
Validation & Assay Performance Summary LanthaScreen IGF-1R GripTite Cells Cat. no. K1834 Modification Detected: Phosphorylation of Multiple Tyr Residues on IGF-1R LanthaScreen Cellular Assay Validation
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Epidemiological studies have revealed an inverse correlation
 LXR-Induced Redistribution of ABCG1 to Plasma Membrane in Macrophages Enhances Cholesterol Mass Efflux to HDL Nan Wang, Mollie Ranalletta, Fumihiko Matsuura, Felix Peng, Alan R. Tall Objectives This study
LXR-Induced Redistribution of ABCG1 to Plasma Membrane in Macrophages Enhances Cholesterol Mass Efflux to HDL Nan Wang, Mollie Ranalletta, Fumihiko Matsuura, Felix Peng, Alan R. Tall Objectives This study
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by. activating Syk and promoting MyD88 and TRIF degradation via cbl-b
 Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C y pathway
 Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C y pathway Yutong Wang and John F. Oram 1 Department of Medicine, University of Washington, Seattle, WA 98195 Abstract
Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C y pathway Yutong Wang and John F. Oram 1 Department of Medicine, University of Washington, Seattle, WA 98195 Abstract
CHAPTER Cir. Res. 2010; 107(12): e
 CHAPTER 5 Enhanced foam cell formation, atherosclerotic lesion development, and inflammation by combined deletion of ABCA1 and SR-BI in bone marrow-derived cells in LDL receptor knockout mice on Western-type
CHAPTER 5 Enhanced foam cell formation, atherosclerotic lesion development, and inflammation by combined deletion of ABCA1 and SR-BI in bone marrow-derived cells in LDL receptor knockout mice on Western-type
The relationship between coronary artery disease and low
 Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p.
 a. b. c. d. e. f. g. h. i. j. k. l. 2.5 2 1.5 1.5 IL-1β 12 8 6 4 2 IL-1β 9 8 7 6 4 3 3 2.9 IL-1β m. n. o. p. 1.8 1.6 1.4 1.2 1.8.6.4.2 6h LPS 2 15 1 5 6h LPS 2 6h LPS 6 4 3 6h LPS Supplementary Figure
a. b. c. d. e. f. g. h. i. j. k. l. 2.5 2 1.5 1.5 IL-1β 12 8 6 4 2 IL-1β 9 8 7 6 4 3 3 2.9 IL-1β m. n. o. p. 1.8 1.6 1.4 1.2 1.8.6.4.2 6h LPS 2 15 1 5 6h LPS 2 6h LPS 6 4 3 6h LPS Supplementary Figure
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid
 Supplementary Results BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia Oliver Hantschel*, Wolfgang Warsch*, Eva Eckelhart*, Ines Kaupe, Florian Grebien, Kay-Uwe Wagner, Giulio
Supplementary Results BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia Oliver Hantschel*, Wolfgang Warsch*, Eva Eckelhart*, Ines Kaupe, Florian Grebien, Kay-Uwe Wagner, Giulio
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Cardiology, CardioVascular Center University Hospital Zurich Zurich, Switzerland
 Modification of by the lipoxidation product malondialdehyde leads to LOX-1 dependent activation of endothelial PKCbeta-2 and adverse endothelial effects of in patients with coronary artery disease Christian
Modification of by the lipoxidation product malondialdehyde leads to LOX-1 dependent activation of endothelial PKCbeta-2 and adverse endothelial effects of in patients with coronary artery disease Christian
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i
 A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i Nan Wang, Wengen Chen, Patrick Linsel-Nitschke, Laurent O. Martinez, Birgit Agerholm-Larsen, David
A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i Nan Wang, Wengen Chen, Patrick Linsel-Nitschke, Laurent O. Martinez, Birgit Agerholm-Larsen, David
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation
 Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Curriculum Vitae. Research Officer in Baker IDI Heart & Diabetes Institute from Aug
 Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
Curriculum Vitae Yi Fu ( 付毅 ) (PhD, MBBS) Baker IDI Heart and Diabetes Institute 75 Commercial Road, Prahran, Melbourne, VIC 3004 Australia Phone: +61 3 8532 1288 Email: yi.fu@bakeridi.edu.au PROFILE Research
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Quantitative Real-Time PCR was performed as same as Materials and Methods.
 Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway
 Related Commentary, page 1226 Research article HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway Fumihiko Matsuura,
Related Commentary, page 1226 Research article HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoe- and ABCG1-dependent pathway Fumihiko Matsuura,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Receptors Functions and Signal Transduction- L4- L5
 Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
Supplementary Figures. T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ
 Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
William C. Comb, Jessica E. Hutti, Patricia Cogswell, Lewis C. Cantley, and Albert S. Baldwin
 Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
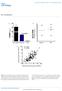 DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
DOI: 10.1038/ncb2210 b. ICAM1 ng ml -1 P = 0.0001 Small RNA (15-30nts) ng ml -1 Cell Lysate Exosome HDL Plasma HDL Normal Human HDL mirnas R = 0.45 P < 0.0001 Normal Human Exosome mirnas Figure S1. Characterization
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Nature Medicine doi: /nm.3150
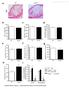 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
The role of the ABCA1 transporter and cholesterol efflux in familial hypoalphalipoproteinemia
 The role of the ABCA1 transporter and cholesterol efflux in familial hypoalphalipoproteinemia G. Kees Hovingh,* Michel J. A. van Wijland, Alison Brownlie, Radjesh J. Bisoendial,* Michael R. Hayden,** John
The role of the ABCA1 transporter and cholesterol efflux in familial hypoalphalipoproteinemia G. Kees Hovingh,* Michel J. A. van Wijland, Alison Brownlie, Radjesh J. Bisoendial,* Michael R. Hayden,** John
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway.
 MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille, France
 LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
CA Tel.: ; Fax: ; ucsd.edu.
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 10, pp. 6300 6311, March 7, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. The Phosphatase PHLPP
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 283, NO. 10, pp. 6300 6311, March 7, 2008 2008 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. The Phosphatase PHLPP
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
c) Macrophages and B cells present antigens to helper T_-cells. (Fill in blanks.) 2 points
 Question 1 You are an immunologist who wants to make the big bucks. You decide to leave the world of science and get a job as a script-consultant on a new medical drama (ER-like) show. You test the writers
Question 1 You are an immunologist who wants to make the big bucks. You decide to leave the world of science and get a job as a script-consultant on a new medical drama (ER-like) show. You test the writers
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy. Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L.
 Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
The New Gold Standard for Lipoprotein Analysis. Advanced Testing for Cardiovascular Risk
 The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
Peli1 negatively regulates T-cell activation and prevents autoimmunity
 Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice
 SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
2013 W. H. Freeman and Company. 12 Signal Transduction
 2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
TD-BF01: Innate immunity to microorganisms
 TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras
 Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer. Application Note
 Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Data Sheet. Notch Pathway Reporter Kit Catalog # 60509
 Data Sheet Notch Pathway Reporter Kit Catalog # 60509 6042 Cornerstone Court W, Ste B Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. NOTCH signaling
Data Sheet Notch Pathway Reporter Kit Catalog # 60509 6042 Cornerstone Court W, Ste B Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. NOTCH signaling
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
