CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
|
|
|
- Emil Thompson
- 6 years ago
- Views:
Transcription
1 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2
2 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives 2
3 Preparation of Carboxylic Acids By Oxidation of Alkanes By Oxidation of Aldehydes and Primary Alcohols By Oxidation of Alkylbenzenes 3
4 By Oxidation of the Benzene Ring By Oxidation of Methyl Ketones (The Haloform Reaction) By Hydrolysis of Cyanohydrins and Other Nitriles Hydrolysis of a cyanohydrin yields an a-hydroxy acid 4
5 Primary alkyl halides can react with cyanide to form nitriles and these can be hydrolyzed to carboxylic acids By Carbonation of Grignard Reagents 5
6 Nucleophilic Addition-Elimination at the Acyl Carbon Recall that aldehydes and ketones undergo nucleophilic addition to the carbon-oxygen double bond The carbonyl group of carboxylic acids and their derivatives undergo nucleophilic addition-elimination The nucleophile reacts at the carbonyl group to form a tetrahedral intermediate The tetrahedral intermediate eliminates a leaving group (L) The carbonyl group is regenerated; the net effect is an acyl substitution 6
7 To undergo nucleophilic addition-elimination the acyl compound must have a good leaving group or a group that can be converted into a good leaving group Acid chlorides react with loss of chloride ion Anhydrides react with loss of a carboxylate ion 7
8 Esters, carboxylic acids and amides generally react with loss of the leaving groups alcohol, water and amine, respectively These leaving groups are generated by protonation of the acyl compound Aldehydes and ketones cannot react by this mechanism because they lack a good leaving group 8
9 Relative Reactivity of Acyl Compounds The relative reactivity of carboxylic acids and their derivatives is as follows: In general, reactivity can be related to the ability of the leaving group (L) to depart Leaving group ability is inversely related to basicity Chloride is the weakest base and the best leaving group Amines are the strongest bases and the worst leaving groups As a general rule, less reactive acyl compounds can be synthesized from more reactive ones Synthesis of more reactive acyl derivatives from less reactive ones is difficult and requires special reagents (if at all possible) 9
10 Acid Chlorides Synthesis of Acid Chlorides Acid chlorides are made from carboxylic acids by reaction with thionyl chloride, phosphorus trichloride or phosphorus pentachloride These reagents work because they turn the hydroxyl group of the carboxylic acid into an excellent leaving group 10
11 Reactions of Acyl Chlorides Acyl chlorides are the most reactive acyl compounds and can be used to make any of the other derivatives Since acyl chlorides are easily made from carboxylic acids they provide a way to synthesize any acyl compound from a carboxylic acid Acyl chlorides react readily with water, but this is not a synthetically useful reaction 11
12 12
13 Carboxylic Acid Anhydrides Synthesis of Carboxylic Acid Anhydrides Acid chlorides react with carboxylic acids to form mixed or symmetrical anhydrides It is necessary to use a base such as pyridine Sodium carboxylates react readily with acid chlorides to form anhydrides 13
14 Cyclic anhydrides with 5- and 6-membered rings can be synthesized by heating the appropriate diacid Reactions of Carboxylic Acid Anhydrides Carboxylic acid anhydrides are very reactive and can be used to synthesize esters and amides Hydrolysis of an anhydride yields the corresponding carboxylic acids 14
15 15
16 Esters Synthesis of Esters: Esterification Acid catalyzed reaction of alcohols and carboxylic acids to form esters is called Fischer esterification Fischer esterification is an equilibrium process Ester formation is favored by use of a large excess of either the alcohol or carboxylic acid Ester formation is also favored by removal of water 16
17 Esterification with labeled methanol gives a product labeled only at the oxygen atom bonded to the methyl group A mechanism consistent with this observation is shown below 17
18 The reverse reaction is acid-catalyzed ester hydrolysis Ester hydrolysis is favored by use of dilute aqueous acid Esters from Acid Chlorides Acid chlorides react readily with alcohols in the presence of a base (e.g. pyridine) to form esters 18
19 Esters from Carboxylic Acid Anhydrides Alcohols react readily with anhydrides to form esters 19
20 Base-Promoted Hydrolysis of Esters: Saponification Reaction of an ester with sodium hydroxide results in the formation of a sodium carboxylate and an alcohol The mechanism is reversible until the alcohol product is formed Protonation of the alkoxide by the initially formed carboxylic acid is irreversible This step draws the overall equilibrium toward completion of the hydrolysis 20
21 Lactones g- or d-hydroxyacids undergo acid catalyzed reaction to give cyclic esters known as g- or d-lactones, respectively 21
22 Lactones can be hydrolyzed with aqueous base Acidification of the carboxylate product can lead back to the original lactone if too much acid is added 22
23 Amides Synthesis of Amides Amides From Acyl Chlorides Ammonia, primary or secondary amines react with acid chlorides to form amides An excess of amine is added to neutralize the HCl formed in the reaction Carboxylic acids can be converted to amides via the corresponding acid chloride 23
24 Amides from Carboxylic Anhydrides Anhydrides react with 2 equivalents of amine to produce an amide and an ammonium carboxylate Reaction of a cyclic anhydride with an amine, followed by acidification yields a product containing both amide and carboxylic acid functional groups Heating this product results in the formation of a cyclic imide 24
25 Amides from Carboxylic Acids and Ammonium Carboxylates Direct reaction of carboxylic acids and ammonia yields ammonium salts Some ammonium salts of carboxylic acids can be dehydrated to the amide at high temperatures This is generally a poor method of amide synthesis A good way to synthesize an amide is to convert a carboxylic acid to an acid chloride and to then to react the acid chloride with ammonia or an amine 25
26 Hydrolysis of Amides Heating an amide in concentrated aqueous acid or base causes hydrolysis Hydrolysis of an amide is slower than hydrolysis of an ester 26
27 27
28 28
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
1/3/2011. Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Carboxylic Acid Derivatives Reading Study Problems Key Concepts and Skills Lecture Topics: Structures and reactivity of carboxylic acid derivatives
 Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Chapter 20: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
Chapter 19: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution 19.1: Nomenclature of Carboxylic Acid Derivatives (please read)
 problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
Lecture 20. Herman Emil Fischer Nobel Prize 1902 Sugars, Esters and Purines. April 4, Chemistry 328N
 Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
10. CARBOXYLIC ACIDS AND THEIR DERIVATIVES 10.1 Nomenclature of Carboxylic Acids 10.2 Physical Properties of Carboxylic Acids 10.
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
Esters of Carboxylic Acids These are derivatives of carboxylic acids where the hydroxyl group is replaced by an alkoxy group.
 Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
Carboxylic Acids and Carboxylic Acid Deriva3ves. Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on)
 Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Lecture 19. Nucleophilic Acyl Substitution Y - + X - Y X R C X. April 2, Chemistry 328N
 Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
Loudon Chapter 21 Review: Carboxylic Acid Derivatives Jacquie Richardson, CU Boulder Last updated 3/20/2018
 Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Carboxylic Acid Derivatives
 arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
Carboxylic Acids and Nitriles. Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry
 Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Carboxylic Acids and Their Derivatives. Chapter 17. Carboxylic Acids and Their Derivatives
 Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
R O R' Acid anhydride. Acid halide. Carboxylic acid. Ester O O O O. Nitrile Acyl phosphate Thioester. Amide
 Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Physical properties: C L = L. Cl, NH 2, OCH 3, OH, OCR O O O NH 2 CH 3 N(CH 3 ) 2. Sol. in H 2 O
 Lecture Notes hem 51 S. King hapter 22 arboxylic Acids and their Derivatives: Nucleophilic Acyl Substitution I. Structure and Physical Properties: Type 2 carbonyl compounds (carboxylic acids and derivatives)
Lecture Notes hem 51 S. King hapter 22 arboxylic Acids and their Derivatives: Nucleophilic Acyl Substitution I. Structure and Physical Properties: Type 2 carbonyl compounds (carboxylic acids and derivatives)
REACTIONS OF CARBOXYLIC ACID DERIVATIVES WITH NUCLEOPHILES A. Reactions of Acid Chlorides with Nucleophiles
 1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
Carboxylic acid derivatives
 Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Chapter 20 Carboxylic Acids. Introduction
 hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
DERIVATIVES OF CARBOXYLIC ACIDS
 13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
Ch. 21: CARBOXYLIC ACID DERIVATIVES AND NUCLEOPHILIC ACYL SUBSTITUTION REACTIONS Nomenclature of Carboxylic Acid Derivatives:
 h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
Functional Derivatives of Carboxylic Acids
 Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Carboxylic Acid Derivatives Carboxylic acid derivatives. Acyl chloride Acid anhydride Ester Amide Nucleophilic acyl substitution 19.1 Nomenclature
Carbonyl Chemistry VI + C O C. 1pm In Geology Room 112. Exam is Monday 11am-1pm. Chemistry /06/02
 arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
Lecture Notes Chemistry Mukund P. Sibi Lecture 31 Reactions at the Alpha-Carbon of Carbonyl Compounds
 Lecture Notes hemistry 342-2008 Mukund P. Sibi eactions at the Alpha-arbon of arbonyl ompounds Enolates are nucleophilic and undergo reaction with electrophiles. For example, one can do halogenation under
Lecture Notes hemistry 342-2008 Mukund P. Sibi eactions at the Alpha-arbon of arbonyl ompounds Enolates are nucleophilic and undergo reaction with electrophiles. For example, one can do halogenation under
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
Carboxylic Acids, Esters and Acyl Chlorides
 R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves
 Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
CH 3 C H 3 O. anhydride acid. ester amide. O acid O. amide. acid. amide. acid. nitriles
 C 21: Carboxylic Acid Derivatives Topics: aming Interconversion of Acid Derivatives eactions of each functional group Connections: anhydride acid ester amide acid ester amide acid amide 2 acid nitriles
C 21: Carboxylic Acid Derivatives Topics: aming Interconversion of Acid Derivatives eactions of each functional group Connections: anhydride acid ester amide acid ester amide acid amide 2 acid nitriles
13. Carboxylic Acids (text )
 2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 21. Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions شیمی آلی 2
 Chapter 21. Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry
Chapter 21. Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry
Identifying Functional Groups. (Chapter 2 in the Klein text)
 Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Chapter 21. Carboxylic Acid Derivatives. and Nucleophilic Acyl Substitution. Reactions. - many carboxylic acid derivatives are known:
 hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives
 CHM 201 (Elements of Organic Chemistry) Dr. Virgil Lee Cal Poly Pomona Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives resonance stabilized OH group donates electron density to carbonyl
CHM 201 (Elements of Organic Chemistry) Dr. Virgil Lee Cal Poly Pomona Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives resonance stabilized OH group donates electron density to carbonyl
Carboxylic Acids and Their Derivatives
 arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
Chemistry Chapter 19
 hemistry 2100 hapter 19 arboxyl Derivatives In this chapter, we study three classes of compounds derived from carboxylic acids; anhydrides, esters, and amides. Each is related to a carboxyl group by loss
hemistry 2100 hapter 19 arboxyl Derivatives In this chapter, we study three classes of compounds derived from carboxylic acids; anhydrides, esters, and amides. Each is related to a carboxyl group by loss
Paper 9: ORGANIC CHEMISTRY-III (Reaction Mechanism-2) Module17: Reduction by Metal hydrides Part-II CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Chem 263 Nov 26, 2013 O R' alkyl. acid. ethyl. acetic acid. ethyl acetate ethyl ethanoate
 hem 263 ov 26, 2013 arboxylic Acids and Derivatives omenclature Esters Systematic names for esters are derived by first giving the name of the alkyl group attached to the oxygen, and then identifying the
hem 263 ov 26, 2013 arboxylic Acids and Derivatives omenclature Esters Systematic names for esters are derived by first giving the name of the alkyl group attached to the oxygen, and then identifying the
Chem 263 B6 Notes March 30, 2006 Demo-In-Class: O
 hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
hem 263 B6 otes March 30, 2006 Demo-In-lass: + 2 carbon dioxide carbonic acid arbon dioxide ( 2 ) is a solid at -78. It is dry ice. When it is added to water, we made carbonated water (as in soda pop).
Chapter 15. Alcohols, Diols, and Thiols. B. Sources: there are two principal sources of simple aliphatic alcohols
 Chapter 15 Alcohols, Diols, and Thiols Chapter 15 suggested problems: 17, 19, 24, 28, 37, 39 I. Introduction A. The relevance of alcohols (from M&B: 497): "If an organic chemist were allowed to choose
Chapter 15 Alcohols, Diols, and Thiols Chapter 15 suggested problems: 17, 19, 24, 28, 37, 39 I. Introduction A. The relevance of alcohols (from M&B: 497): "If an organic chemist were allowed to choose
Esterification. Preparation of β-d-glucose pentaacetate. Dr. Zerong Wang at UHCL. Table of contents
 Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
Organic Chemistry. Chapter 23. Hill, Petrucci, McCreary & Perry 4 th. Ed. Alkane to Substituent Group methane CH 4 methyl CH 3
 hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
ORGANIC SYNTHESIS VIA ENOLATES
 1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
1 ORGANIC SYNTHESIS VIA ENOLATES Aldehydes and ketones undergo nucleophilic addition reaction at the carbonyl group. Further, α-hydrogen containing compounds are acidic in nature. In addition to carbonyl
Carboxylic Acids and Derivatives. Decarboxylation R H + CO 2. R OH Reaction type: Elimination. H H Malonic acid. Mechanism:
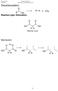 rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
REVIEW IN CARBOXYLIC ACIDS AND ITS DERIVATIVES
 IASET: International Journal of Agricultural & Bio-Chemical Science (IASET: IJABS) ISSN(P): Applied; ISSN(E): Applied Vol. 1, Issue 1, Jan - Jun 2017, 49-66 IASET REVIEW IN CARBXYLIC ACIDS AND ITS DERIVATIVES
IASET: International Journal of Agricultural & Bio-Chemical Science (IASET: IJABS) ISSN(P): Applied; ISSN(E): Applied Vol. 1, Issue 1, Jan - Jun 2017, 49-66 IASET REVIEW IN CARBXYLIC ACIDS AND ITS DERIVATIVES
Chem 263 Dec 1, 2016
 Chem 263 Dec 1, 2016 eactivity of Carboxylic acid Derivatives More eactive S 2 ' ' + a ' 2 - M + Less eactive Example: Acid chloride to anhydride Since an acid chloride is more reactive than an anhydride,
Chem 263 Dec 1, 2016 eactivity of Carboxylic acid Derivatives More eactive S 2 ' ' + a ' 2 - M + Less eactive Example: Acid chloride to anhydride Since an acid chloride is more reactive than an anhydride,
Chemistry 1120 Exam 1 Study Guide
 Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Chem 263 Nov 21, 2013
 hem 263 Nov 21, 2013 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
hem 263 Nov 21, 2013 arbohydrates- emiacetal Formation You know from previous lectures that carbonyl compounds react with all kinds of nucleophiles. ydration and hemiacetal formation are typical examples.
Lecture'11:'February'21,'2013 Reac&ons*of*Deriva&ves*( )
 CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
Nu: - Addition or Nu: - Acyl Substitution?
 12. Apply nucleophilic addition and elimination concepts to nucleophilic acyl substution reactions of acids and derivatives (focus on esters and amides) In Class problems: 1. The reactive site of aldehydes,
12. Apply nucleophilic addition and elimination concepts to nucleophilic acyl substution reactions of acids and derivatives (focus on esters and amides) In Class problems: 1. The reactive site of aldehydes,
Chemistry 212 Fall Semester 1996 Examination #2
 Chemistry 212 Fall Semester 1996 Examination #2 University of Missouri Columbia Prof. Rainer Glaser Wednesday, October 16, 1996 103 Schlundt Hall, 8:40-9:30 featuring Carboxylic Acids and Carboxylic Acid
Chemistry 212 Fall Semester 1996 Examination #2 University of Missouri Columbia Prof. Rainer Glaser Wednesday, October 16, 1996 103 Schlundt Hall, 8:40-9:30 featuring Carboxylic Acids and Carboxylic Acid
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Oxidizing Alcohols. Questions. Prediction. Analysis. Safety Precautions. Materials. Conclusions. Procedure. 74 MHR Unit 1 Organic Chemistry
 xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
H 3 C OCH 3 3 C N(CH 3 ) 2 H 3 C H H 3 C CH 3. ketone. pk a = 9 H H. 1,3-keto ester pk a = 11
 hapter 21: Ester Enolates 21.1: Ester α ydrogens and Their pk a s. The α-protons of s are less acidic that ketones and aldehydes. Typical pk a s of carbonyl compounds (α-protons): aldehydes 17 ketones
hapter 21: Ester Enolates 21.1: Ester α ydrogens and Their pk a s. The α-protons of s are less acidic that ketones and aldehydes. Typical pk a s of carbonyl compounds (α-protons): aldehydes 17 ketones
ORGANIC AND BIOORGANIC CHEMISTRY
 P. GERGELY Department os Medical Chemistry Medical and Health Science Center University of Debrecen ORGANIC AND BIOORGANIC CHEMISTRY FÓR MEDICAL STUDENTS THIRD EDITION University of Debrecen Medical and
P. GERGELY Department os Medical Chemistry Medical and Health Science Center University of Debrecen ORGANIC AND BIOORGANIC CHEMISTRY FÓR MEDICAL STUDENTS THIRD EDITION University of Debrecen Medical and
Topic 4.5 COMPOUNDS CONTAINING THE CARBONYL GROUP. Aldehydes and Ketones Carboxylic Acids and their Salts Esters Acyl Chlorides and Acid Anhydrides
 Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
Topic 4.5 MPUNDS NTAINING TE ARBNYL GRUP Aldehydes and Ketones arboxylic Acids and their Salts Esters Acyl hlorides and Acid Anhydrides ALDEYDES AND KETNES 1. Introduction Aldehydes and ketones are collectively
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
Alcohol aldehydes cetones and carboxylic acids
 Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
AS Describe aspects of organic chemistry. COLLATED POLYMER QUESTIONS - polyesters, polyamides and peptides
 o Brain Too Small EMISTRY AS 90698 Describe aspects of organic chemistry LLATED PLYMER QUESTIS - polyesters, polyamides and peptides (2011:2) ylon 6,6 is a polymer with the following structure: (a) (b)
o Brain Too Small EMISTRY AS 90698 Describe aspects of organic chemistry LLATED PLYMER QUESTIS - polyesters, polyamides and peptides (2011:2) ylon 6,6 is a polymer with the following structure: (a) (b)
(iii). Decarboxylation (iv)kolbe Electrolysis
 ALKANOIC ACID/CARBOXYLIC ACID Contains carboxyl functional group COOH Two functional groups are contained in carboxyl- carbonyl (C=O)and hydroxyl (-OH) Saturated aliphatic alkanoic acids have general formula
ALKANOIC ACID/CARBOXYLIC ACID Contains carboxyl functional group COOH Two functional groups are contained in carboxyl- carbonyl (C=O)and hydroxyl (-OH) Saturated aliphatic alkanoic acids have general formula
A carboxylic acid is an organic compound that contains a carboxyl group, COOH
 1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
1.6 Carboxylic Acids, Esters and Fats Carboxylic Acids A carboxylic acid is an organic compound that contains a carboxyl group, COOH These compounds are weak acids. Citrus fruits, crabapples, rhubarb,
Ex17. Analgesics, TLC Analysis. Analgesics. The Experiment. Part A. Carboxylic Acids. Part B. Willow Bark Esters & Esterification
 Ex17 Analgesics, TLC Analysis Analgesics Carboxylic Acids Structure Properties Willow Bark Esters & Esterification The Experiment Part A Use TLC to Separate Compounds Part B Determine Elution Solvents
Ex17 Analgesics, TLC Analysis Analgesics Carboxylic Acids Structure Properties Willow Bark Esters & Esterification The Experiment Part A Use TLC to Separate Compounds Part B Determine Elution Solvents
ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES. Jack DeRuiter
 ESTES AD ELATED ABYLI AID DEIVATIVES I. Structure and Preparation Jack Deuiter Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl () portion of the acid function with
ESTES AD ELATED ABYLI AID DEIVATIVES I. Structure and Preparation Jack Deuiter Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl () portion of the acid function with
Carboxylic Acids and Esters
 24 Carboxylic Acids and Esters The sour tang in fruit juice comes from carboxylic acids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and
24 Carboxylic Acids and Esters The sour tang in fruit juice comes from carboxylic acids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and
TOPIC 4. CARBOXYLIC ACIDS AND THEIR DERIVATES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON (Chapter 17)
 L TPI 4. ABXYLI AIDS AND TEI DEIVATES: NULEPILI ADDITIN-ELIMINATIN AT TE AYL ABN (hapter 17) BJETIVES 1. Name carboxylic acids and acid derivatives: acyl chlorides, anhydrides, esters, amides and nitriles
L TPI 4. ABXYLI AIDS AND TEI DEIVATES: NULEPILI ADDITIN-ELIMINATIN AT TE AYL ABN (hapter 17) BJETIVES 1. Name carboxylic acids and acid derivatives: acyl chlorides, anhydrides, esters, amides and nitriles
3/27/2011. Chapter 8 Reactions of Alkenes and Alkynes. Alkene Addition Reactions. 8.1 Preparing Alkenes: A Preview of Elimination Reactions
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 8 Reactions of Alkenes and Alkynes Richard Morrison University of Georgia, Athens Alkene Addition Reactions Alkene addition reactions Addition
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 8 Reactions of Alkenes and Alkynes Richard Morrison University of Georgia, Athens Alkene Addition Reactions Alkene addition reactions Addition
Chapter 16 and GHW#6 Questions. Carboxylic Acids, Esters, and Other Acid Derivatives
 Chapter 16 and GHW#6 Questions Carboxylic Acids, Esters, and Other Acid Derivatives Bonding Characteristics of Carboxylic Acids A carboxylic acid has functional a carboxyl group. A carboxyl group is a
Chapter 16 and GHW#6 Questions Carboxylic Acids, Esters, and Other Acid Derivatives Bonding Characteristics of Carboxylic Acids A carboxylic acid has functional a carboxyl group. A carboxyl group is a
Ch14. Carboxylic Acids. Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.
 Ch14 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.0 Nick DeMello, PhD. 2007-2015 Ch14 Carboxylic Acids & Esters Carboxylic
Ch14 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make more powerful functional groups. version 1.0 Nick DeMello, PhD. 2007-2015 Ch14 Carboxylic Acids & Esters Carboxylic
Chapter 15 Alcohols, Diols, and Thiols
 Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
CELLULAR METABOLISM. Metabolic pathways can be linear, branched, cyclic or spiral
 CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chem 239 C: Chapter 19 Carboxylic Acid Derivatives Goals: Description, Nomenclature Reactivity of Carboxylic Acid Derivatives Nucleophylic Acyl
 Chem 239 C: Chapter 19 Carboxylic Acid Derivatives Goals: Description, Nomenclature Reactivity of Carboxylic Acid Derivatives Nucleophylic Acyl Substitution Acyl Chlorides Carboxylic Acid Anhydrides, Esters
Chem 239 C: Chapter 19 Carboxylic Acid Derivatives Goals: Description, Nomenclature Reactivity of Carboxylic Acid Derivatives Nucleophylic Acyl Substitution Acyl Chlorides Carboxylic Acid Anhydrides, Esters
Hydrolytic transformations involving amide-, ester bonds are the easiest to perform
 Hydrolytic reactions Hydrolytic transformations involving amide-, ester bonds are the easiest to perform Proteins used for these reactions are proteases, esterases or lipases. A favourite class of enzymes
Hydrolytic reactions Hydrolytic transformations involving amide-, ester bonds are the easiest to perform Proteins used for these reactions are proteases, esterases or lipases. A favourite class of enzymes
6. The catalytic mechanism of arylsulfatase A and its theoretical investigation
 6. The catalytic mechanism of arylsulfatase A and its theoretical investigation When the crystal structure of arylsulfatase A was solved, a remarkable structural analogy to another hydrolytic enzyme, the
6. The catalytic mechanism of arylsulfatase A and its theoretical investigation When the crystal structure of arylsulfatase A was solved, a remarkable structural analogy to another hydrolytic enzyme, the
MahaAbuAjamieh. BahaaNajjar. MamoonAhram
 7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
KMnO 4 1 O 4'' Apigenin. 1 In the following reactions draw the structures of products B and C. 1. NaH/DMF 2. excess MeI. acetic anhydride(excess)
 Problem 26: hemical and Stereochemical Structure of oniine oniine is a toxic compound found in the plant hemlock (conium maculatum), with which the ancient Greek philosopher Socrates was poisoned. oniine
Problem 26: hemical and Stereochemical Structure of oniine oniine is a toxic compound found in the plant hemlock (conium maculatum), with which the ancient Greek philosopher Socrates was poisoned. oniine
Carboxylic Acids. Carboxylic acid groups are always terminal groups with a carbonyl carbon also bound to a hydroxyl For example:
 Carboxylic Acids The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon. Structure of Carboxyl Carbon is sp 2 hybridized. Bond angles are close to 120. O-H eclipsed
Carboxylic Acids The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon. Structure of Carboxyl Carbon is sp 2 hybridized. Bond angles are close to 120. O-H eclipsed
Carbon s unique bonding pattern arises from the hybridization of the electrons.
 Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Chapter 22 Carbohydrates
 Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Chapter 22 Carbohydrates Introduction Classification of Carbohydrates Carbohydrates have the general formula C x (H 2 O) y Carbohydrates are defined as polyhydroxy aldehydes or ketones or substances that
Ch07. Carboxylic Acids. Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0
 Ch07 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check
Ch07 Carboxylic Acids Combining the hydroxyl and carbonyl functional groups. To make organic acids. version 1.0 Nick DeMello, PhD. 2007-2015 Important Dates This Wednesday: - Lab Checkout (you must check
ANSWERS BIOCHEMISTRY CARBOHYDRATES
 1. Fructose ANSWERS BIOCEMISTRY CARBOYDRATES 2. Sucrose or maltose 3. (C 6 10 O 5 ) n 4. Monosaccharides are the simplest carbohydrates which cannot be further hydrolysed. 5. D(-) fructofuranose structure:
1. Fructose ANSWERS BIOCEMISTRY CARBOYDRATES 2. Sucrose or maltose 3. (C 6 10 O 5 ) n 4. Monosaccharides are the simplest carbohydrates which cannot be further hydrolysed. 5. D(-) fructofuranose structure:
Chemistry B11 Chapters 13 Esters, amides and carbohydrates
 Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Chapters 13 Esters, amides and carbohydrates Esters: esters are derived from carboxylic acids (the hydrogen atom in the carboxyl group of carboxylic acid is replaced by an alkyl group). The functional
Page 2. Q1.Which one of the following is not a correct statement about vitamin C, shown below? It is a cyclic ester.
 Q1.Which one of the following is not a correct statement about vitamin, shown below? It is a cyclic ester. It can form a carboxylic acid on oxidation. It decolourises a solution of bromine in water. It
Q1.Which one of the following is not a correct statement about vitamin, shown below? It is a cyclic ester. It can form a carboxylic acid on oxidation. It decolourises a solution of bromine in water. It
Dow Haltermann Custom Processing The World of Hydrogen Cyanide Chemistry
 Dow altermann Custom Processing The World of ydrogen Cyanide Chemistry Sharing Your Vision esourcing Your Success Specialists in ydrogen Cyanide Chemistry With Dow s acquisition of the ampshire Chemical
Dow altermann Custom Processing The World of ydrogen Cyanide Chemistry Sharing Your Vision esourcing Your Success Specialists in ydrogen Cyanide Chemistry With Dow s acquisition of the ampshire Chemical
where R doesn t have to equal R or R
 hem 263 Nov 24, 2016 arboxylic Acids and Derivatives arboxylic acids are very important compounds in nature and serve as building blocks for preparing related derivatives such as esters and amides. The
hem 263 Nov 24, 2016 arboxylic Acids and Derivatives arboxylic acids are very important compounds in nature and serve as building blocks for preparing related derivatives such as esters and amides. The
Experiment 15: Fischer Esterification and Combinatorial Chemistry Phill Rasnick Introduction
 28 November 2012 Experiment 15: Fischer Esterification and Combinatorial Chemistry Phill Rasnick Introduction Esterification reactions, which produce esters, have proven to have great importance in organic
28 November 2012 Experiment 15: Fischer Esterification and Combinatorial Chemistry Phill Rasnick Introduction Esterification reactions, which produce esters, have proven to have great importance in organic
Esters. What intermolecular forces do you think esters have? δ + CH 3
 Esters What intermolecular forces do you think esters have? ow will these intermolecular forces affect their: Melting and boiling points compared to alkanes Solubility in water δ 3 δ + 3 Dipole dipole
Esters What intermolecular forces do you think esters have? ow will these intermolecular forces affect their: Melting and boiling points compared to alkanes Solubility in water δ 3 δ + 3 Dipole dipole
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Chapter 44. Typical reactions of various functional groups Introducing organic reactions Typical reactions of alkanes
 Chapter 44 Typical reactions of various functional groups 44.1 Introducing organic reactions 44.2 Typical reactions of alkanes 44.3 Typical reactions of alkenes 44.4 Typical reactions of haloalkanes 44.5
Chapter 44 Typical reactions of various functional groups 44.1 Introducing organic reactions 44.2 Typical reactions of alkanes 44.3 Typical reactions of alkenes 44.4 Typical reactions of haloalkanes 44.5
