Summary of the risk management plan by product
|
|
|
- Charles Page
- 6 years ago
- Views:
Transcription
1 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks Missing information Osteonecrosis of the jaw Local irritation of the upper gastro-intestinal mucosa Oesophageal reactions (such as oesophagitis, oesophageal ulcers and oesophageal erosions, oesophageal stricture) Atypical femoral fractures Use in Pregnant women Use in patients below 18 years of age 1.2 Table of ongoing and planned studies in the post-authorisation Pharmacovigilance Development Plan 1.3 Summary of the Post -authorisation efficacy development plan 1.4 Summary table of risk minimisation measures Safety Concern Routine Risk Minimisation measures Additional Risk Minimisation measures Osteonecrosis of the jaw Mentioned in special warning and precaution for use section 4.4 of SmPC Listed in section 4.8 of SmPC Mentioned in package leaflet. Prescription only medicine. Local irritation of the upper gastro-intestinal mucosa Mentioned in section 4.2 of SmPC Mentioned in special warning and precaution for use section 4.4 of Page 01 (09)
2 SmPC. Listed in section 4.8 Mentioned in package leaflet. Prescription only medicine. Oesophageal reactions (such as oesophagitis, oesophageal ulcers and oesophageal erosions, oesophageal stricture) Mentioned in special warning and precaution for use section 4.4 of SmPC. Listed in section 4.8 of SmPC Mentioned in package leaflet. Prescription only medicine. Atypical femoral fractures Mentioned in special warning and precaution for use section 4.4 of SmPC Listed in section 4.8 of SmPC Mentioned in package leaflet. Prescription only medicine. Use in Pregnant women Mentioned in section 4.6 of SmPC Mentioned in package leaflet. Prescription only medicine. Use in patients below 18 years of age Mentioned in Posology and method of administration in section 4.2 of the SmPC. Mentioned in package leaflet. Prescription only medicine. Page 02 (09)
3 2 Elements for a public summary 2.1 Overview of disease epidemiology (for each indication) Indication/target population Incidence and prevalence Postmenopausal Osteoporosis The incidence of osteoporosis in postmenopausal women continues to increase with progressively aging populations. Currently, it is estimated that over 200 million people worldwide have osteoporosis. The incidence of hip fracture in women aged 50 years or above was calculated and expressed as the annual number of hip fractures per 10,000 women. In the United States and the European Union, about 30% of all postmenopausal women have osteoporosis, and it has been predicted that more than 40% of them will suffer one or more fragility fractures during their remaining lifetime. Demographics of the target population age, sex, race/ethnic origin Risk factors for the disease Main treatment options One out of three postmenopausal women and one out of five men over the age of 50 years will experience osteoporotic fractures. The prevalence of osteoporosis increased with age. Significantly higher among women than among men. It was found that Whites are having more frequencies to get post-menopausal osteoporosis compared followed by Blacks and Hispanics. Early menopause, a maternal history of hip fracture, a fracture after 40 years of age, low body weight, or specific diseases and treatments increase susceptibility to fractures Postmenopausal women are at the greatest risk of developing osteoporosis because of the accelerated loss in bone mass associated with menopause, include advanced age, genetics, lifestyle factors (such as low calcium and vitamin D intake, smoking), thinness, and menopause status. Pharmacological management is the primary prevention of osteoporotic fractures in patients at high risk or secondary prevention in patients who have already sustained a fracture. Management focuses first on non-pharmacologic measures, such as a balanced diet, adequate calcium and vitamin D intake, adequate exercise, smoking cessation, avoidance of excessive alcohol intake, and fall prevention. Page 03 (09)
4 Calcium is a key element in the therapy of osteoporosis. Adequate calcium intake throughout life is essential for optimizing peak bone mass and may affect the rate at which bone is lost later in life. Calcium alone is inadequate to completely inhibit the rapid bone loss that occurs at menopause but is necessary to optimize response to antiresorptive agents. (Calcium carbonate and tribasic calcium phosphate have the greatest percentage of elemental calcium). The two exogenous sources of vitamin D are ergosterol (vitamin D2) from plant sources and cholecalciferol (vitamin D3) from animal sources, such as fish liver oils and fortified milk, presence enhances GI absorption of calcium. Postmenopausal estrogen therapy, taken with or without progestin. And bisphosphonates are compounds that adsorb onto hydroxyapatite crystals. Mortality and morbidity (natural history) Alendronate is approved by the FDA for the prevention of osteoporosis in postmenopausal women. Hip fractures are the most serious, since 10 20% more women die than expected for age within the first year, and the excess mortality is even greater for men. The risk of death is greatest immediately after the fracture and decreases over time. Excess morbidity and mortality caused by osteoporosis-related fractures. 2.2 Summary of treatment benefits As per the evidence-based position statement published by The North American Menopause Society (NAMS) in 2006 recommendations for the prevention/management/treatment of osteoporosis in postmenopausal women as follows: Lifestyle approaches to prevent bone loss and fractures; Nutrition, including adequate intakes of calcium, vitamin D, vitamin K, magnesium, protein, and isoflavones Exercise Fall prevention Smoking cessation Alcohol avoidance Pharmacologic approaches for the prevention and/or treatment of osteoporosis; Estrogen or estrogen plus progestin therapy Page 04 (09)
5 Bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid, etidronate) Selective estrogen-receptor modulators, such as raloxifene Parathyroid hormone 1-34 (teriparatide) Calcitonin Combination therapies (considered, but no recommendation made for or against) Tibolone (not approved in the United States or Canada for osteoporosis prevention) New/experimental therapies: strontium ranelate, parathyroid I-84, bazedoxifene, lasofoxifene, denosumab (considered, but no definitive recommendation made) Bisphosphonates are the first-line drugs for treating postmenopausal women with osteoporosis. They have reduced the risk of vertebral fractures by 40% to 70% and reduced the incidence of nonvertebral fracture, including hip fracture, by about half this amount. Alendronate is comes under the group of bisphosphonates. In osteoporosis the strength of the bones is reduced and this can increase the chances of getting bone fractures. Alendronate can make bones stronger in people with osteoporosis which can reduce the chances of getting bone fractures Unknowns relating to treatment benefits None identified. 2.4 Summary of safety concerns Important identified risks Risk What is known Preventability Bone damage in the jaw (osteonecrosis of the jaw) Osteonecrosis of the jaw generally associated with tooth extraction and/or local infection (including osteomyelitis) has been reported in patients with cancer receiving treatment regimens including primarily intravenously administered bisphosphonates. Many of these patients were also receiving chemotherapy and corticosteroids. Osteonecrosis of the jaw has also been reported in patients with osteoporosis receiving oral bisphosphonates Yes, The following risk factors should be considered when evaluating an individual s risk of developing osteonecrosis of the jaw: - potency of the bisphosphonate (highest for zoledronic acid), route of administration (see above) and cumulative dose - cancer, chemotherapy, radiotherapy, corticosteroids, smoking - a history of dental disease, poor oral hygiene, periodontal disease, invasive dental procedures and poorly fitting dentures Page 05 (09)
6 Risk What is known Preventability A dental examination with appropriate preventive dentistry should be considered prior to treatment with oral bisphosphonates in patients with poor dental status. While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment. During bisphosphonate treatment, all patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups, and report any oral symptoms such as dental mobility, pain, or swelling. Local irritation of the upper gastrointestinal mucosa Alendronate can cause local irritation of the upper gastro-intestinal lining (mucosa). Because there is a potential for worsening of the underlying disease, caution should be used when alendronate is given to patients with active upper gastrointestinal problems, such as difficulty swallowing (dysphagia), oesophageal disease, gastritis, inflammation of the first part of the small intestine (duodenitis), ulcers, or with a recent history (within the previous year) of Yes, - Instructions given in section 4.2 Posology and method of administration of SmPC - Contraindicated in abnormalities of the oesophagus and other factors which delay oesophageal emptying such as stricture or achalasia (a condition which impairs the ability of the oesophageal smooth muscle to Page 06 (09)
7 Risk What is known Preventability major gastro-intestinal disease such as peptic ulcer, or active gastrointestinal bleeding, or surgery of the upper gastro-intestinal tract other than pyloroplasty (a surgical procedure to widen the opening of the lower portion of the stomach which connect to the duodenum). move food effectively to the stomach). - It is very important that the full dosing instructions are provided to, and understood by the patient. Patients should be informed that failure to follow these instructions may increase their risk of oesophageal problems. The tube that connects your mouth with your stomach damages/injury (Oesophageal reaction) Oesophageal reactions (sometimes severe and requiring hospitalisation), such as oesophagitis, oesophageal ulcers and oesophageal erosions, rarely followed by oesophageal stricture, have been reported in patients receiving alendronate. Physicians should therefore be alert to any signs or symptoms signaling a possible oesophageal reaction and patients should be instructed to discontinue alendronate and seek medical attention if they develop symptoms of oesophageal irritation such as dysphagia, pain on swallowing or central chest (retrosternal) pain, new or worsening heartburn. The risk of severe oesophageal adverse experiences appears to be greater in patients who fail to take alendronate properly and/or who continue to take alendronate after developing symptoms suggestive of oesophageal irritation. In patients with known Barrett's oesophagus, prescribers should consider the benefits and potential risks of alendronate on an individual patient basis. Yes, - Instructions given in section 4.2 Posology and method of administration - It is very important that the full dosing instructions are provided to, and understood by the patient. Patients should be informed that failure to follow these instructions may increase their risk of oesophageal problems. Page 07 (09)
8 Important potential risks Risk Bone fracture (Atypical femoral fractures) What is known (Including reason why it is considered a potential risk) Atypical femoral fractures have been reported with bisphosphonate therapy, primarily in patients receiving longterm treatment for osteoporosis. These fractures occur after minimal or no trauma and some patients experience thigh or groin pain, often associated with imaging features of stress fractures, weeks to months before presenting with a completed femoral fracture. Fracture often occur on both sides ; therefore the opposite femur should be examined in bisphosphonatetreated patients who have sustained a femoral shaft fracture. Poor healing of these fractures has also been reported. Discontinuation of bisphosphonate therapy in patients suspected to have an atypical femur fracture should be considered pending evaluation of the patient, based on an individual benefit risk assessment. During bisphosphonate treatment patients should be advised to report any thigh, hip or groin pain and any patient presenting with such symptoms should be evaluated for an incomplete femur fracture. As per the CMDh atypical femoral fracture as a potential risk following the commission decision on the Article 31 of Directive 2001/83/EC referral concerning bisphosphonates. Missing information Risk What is known Use in pregnant woman There are insufficient data regarding the use of alendronate in pregnant women. Animal studies showed that effects on foetal bone formation at high doses. Alendronate given to pregnant rats caused obstruction of labour related to low blood calcium levels. Use in patients below 18 years of age Alendronate sodium is not recommended for use in children under the age of 18 years due to insufficient data on safety and efficacy in conditions associated with paediatric osteoporosis Page 08 (09)
9 2.5 Summary of additional risk minimisation measures by safety concern Alendronate has a Summary of Product Characteristics (SmPC) which provides physicians, pharmacists and other health care professionals with details on how to use the medicine, the risks and recommendations for minimising them. An abbreviated version of this in lay language is provided in the form of the package leaflet. The measures in these documents are known as routine risk minimisation measures. Alendronate has no additional risk minimisation measures. 2.6 Planned post authorisation development plan 2.7 Summary of changes to the risk management plan over time Major changes to the risk management plan over time Version Date Safety Concerns Comment Page 09 (09)
Elements for a Public Summary
 Ibandronat Stada 50 mg film-coated tablets Ibandronat Stada 150 mg film-coated tablets 3.11.2014, Version V2.1 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary
Ibandronat Stada 50 mg film-coated tablets Ibandronat Stada 150 mg film-coated tablets 3.11.2014, Version V2.1 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary
FOSAMAX (Alendronic acid as alendronate sodium trihydrate)
 CSP - UK/H/PSUR/0070 - March 2012 EUROPEAN UNION CORE SAFETY PROFILE FOSAMAX (Alendronic acid as alendronate sodium trihydrate) 4.2 Posology and method of administration The recommended dosage is one 70
CSP - UK/H/PSUR/0070 - March 2012 EUROPEAN UNION CORE SAFETY PROFILE FOSAMAX (Alendronic acid as alendronate sodium trihydrate) 4.2 Posology and method of administration The recommended dosage is one 70
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer
 Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Patients should receive supplemental calcium and vitamin D, if dietary intake is inadequate (see PRECAUTIONS).
 PRODUCT CIRCULAR Tablets Once Weekly 70 mg I. THERAPEUTIC CLASS Bisphosphonates are synthetic analogs of pyrophosphate that bind to the hydroxyapatite found in bone. is a bisphosphonate that acts as a
PRODUCT CIRCULAR Tablets Once Weekly 70 mg I. THERAPEUTIC CLASS Bisphosphonates are synthetic analogs of pyrophosphate that bind to the hydroxyapatite found in bone. is a bisphosphonate that acts as a
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
1. NAME OF THE MEDICINAL PRODUCT 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Alendroninezuur Mylan 70 mg, tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate). Excipient:
1. NAME OF THE MEDICINAL PRODUCT Alendroninezuur Mylan 70 mg, tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate). Excipient:
FOSAMAX 70 mg Merck Sharp & Dhome
 FOSAMAX 70 mg Merck Sharp & Dhome 1. NAME OF THE MEDICINAL PRODUCT Fosamax Once Weekly 70 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains the equivalent of 70 mg alendronic
FOSAMAX 70 mg Merck Sharp & Dhome 1. NAME OF THE MEDICINAL PRODUCT Fosamax Once Weekly 70 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains the equivalent of 70 mg alendronic
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Orifarm Veckotablett 70 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains alendronate sodium trihydrate
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Orifarm Veckotablett 70 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains alendronate sodium trihydrate
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
SPC. Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate). Each tablet contains about 192 mg lactose monohydrate.
 SPC 1. NAME OF THE MEDICINAL PRODUCT Alendronic acid Actavis 70 mg, tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate). Each
SPC 1. NAME OF THE MEDICINAL PRODUCT Alendronic acid Actavis 70 mg, tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate). Each
Package leaflet: Information for the patient. Risedronate sodium 35 mg film-coated tablets. Risedronate sodium
 Package leaflet: Information for the patient Risedronate sodium 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient Risedronate sodium 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Management of postmenopausal osteoporosis
 Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
SUMMARY OF PRODUCT CHARACTERISTICS. Each tablet contains 70 mg alendronic acid (as sodium alendronate trihydrate).
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fostolin Once Weekly 70 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fostolin Once Weekly 70 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 70 mg alendronic acid (as sodium
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 5 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures
 Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 35 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
OSTEOFOS Tablets (Alendronate)
 Published on: 10 Jul 2014 OSTEOFOS Tablets (Alendronate) Composition OSTEOFOS 70 Each uncoated tablet contains: Sodium Alendronate, BP, equivalent to Alendronic Acid... 70 mg OSTEOFOS 35 Each uncoated
Published on: 10 Jul 2014 OSTEOFOS Tablets (Alendronate) Composition OSTEOFOS 70 Each uncoated tablet contains: Sodium Alendronate, BP, equivalent to Alendronic Acid... 70 mg OSTEOFOS 35 Each uncoated
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION should be recommended
 Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Barts Health NHS Trust and local GPs Shared Care Guidelines. DENOSUMAB (Prolia) Post menopausal osteoporosis
 Barts Health NHS Trust and local GPs Shared Care Guidelines Indication: DENOSUMAB (Prolia) Post menopausal osteoporosis DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name
Barts Health NHS Trust and local GPs Shared Care Guidelines Indication: DENOSUMAB (Prolia) Post menopausal osteoporosis DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Bisphosphonates Length of treatment in osteoporosis in primary care- Treatment holiday
 Bisphosphonates Length of treatment in osteoporosis in primary care- Treatment holiday This guidance incorporate National Osteoporosis Guideline Group (NOGG) guidance March 2017 Clinical guideline for
Bisphosphonates Length of treatment in osteoporosis in primary care- Treatment holiday This guidance incorporate National Osteoporosis Guideline Group (NOGG) guidance March 2017 Clinical guideline for
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT
 NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Each film-coated tablet contains 70 mg alendronic acid (as sodium trihydrate).
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Sandoz Veckotablett 70 mg Film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 70
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Sandoz Veckotablett 70 mg Film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 70
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
SUMMARY OF PRODUCT CHARACTERISTICS. Each tablet contains 10 mg alendronic acid (equivalent to mg of sodium alendronate).
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alendratol 10mg, tabletten 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 mg alendronic acid (equivalent to 10.884mg
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alendratol 10mg, tabletten 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 mg alendronic acid (equivalent to 10.884mg
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
New Developments in Osteoporosis: Screening, Prevention and Treatment
 Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis. Skeletal System
 Osteoporosis Introduction Osteoporosis is a very common bone disease that causes bone to become weak. Bone weakness can lead to fractures of the spine, hip, and wrist from simple falls or even a sneeze
Osteoporosis Introduction Osteoporosis is a very common bone disease that causes bone to become weak. Bone weakness can lead to fractures of the spine, hip, and wrist from simple falls or even a sneeze
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
Dorset Pathway for the use of bisphosphonates for post-menopausal women with breast cancer. Summary
 Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Norsed Septimum 35 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 35 mg risedronate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Norsed Septimum 35 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 35 mg risedronate
Greater Manchester Interface Prescribing Group Shared Care Template
 Greater Manchester Interface Prescribing Group Shared Care Template Shared Care Guideline the use of Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Author(s)/Originator(s):
Greater Manchester Interface Prescribing Group Shared Care Template Shared Care Guideline the use of Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Author(s)/Originator(s):
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR XGEVA. Notes
 For prevention of skeletal-related events XGEVA is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors. XGEVA is not indicated for the prevention
For prevention of skeletal-related events XGEVA is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors. XGEVA is not indicated for the prevention
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 30 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALENDRONATE SODIUM AND COLECALCIFEROL, MSD 70 mg/2800 IU tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ALENDRONATE SODIUM AND COLECALCIFEROL, MSD 70 mg/2800 IU tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays. Suzanne Morin MD FRCP FACP McGill University May 2014
 Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Advanced medicine conference. Monday 20 Tuesday 21 June 2016
 Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Osteoporosis. Definition
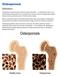 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
MEDICATION GUIDE. Boniva (bon-ee-va) (ibandronate sodium) Tablets
 MEDICATION GUIDE Boniva (bon-ee-va) (ibandronate sodium) Tablets Read the Medication Guide that comes with BONIVA before you start taking it and each time you get a refill. There may be new information.
MEDICATION GUIDE Boniva (bon-ee-va) (ibandronate sodium) Tablets Read the Medication Guide that comes with BONIVA before you start taking it and each time you get a refill. There may be new information.
Package leaflet: Information for the patient. Optinate 5 mg film-coated tablets risedronate sodium
 Package leaflet: Information for the patient Optinate 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the patient Optinate 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Updates in Osteoporosis. I have no conflicts of interest. What Would You Do? Mrs. C. What s New in Osteoporosis. Page 1
 Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Osteoporosis in Men Wendy Rosenthal PharmD. This program has been brought to you by PharmCon
 Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Risontel Once a Week 35 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains 35 mg risedronate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Risontel Once a Week 35 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains 35 mg risedronate
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Optinate 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg risedronate sodium
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Optinate 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 5 mg risedronate sodium
Package leaflet: Information for the user. Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid
 Package leaflet: Information for the user Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it
Package leaflet: Information for the user Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it
Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Alendronat ratiopharm Veckotablett 70 mg tablets. Alendronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Alendronat ratiopharm Veckotablett 70 mg tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Alendronat ratiopharm Veckotablett 70 mg tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains
Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets
 Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets Read the Medication Guide that comes with ACTONEL before you start taking it and each time you get a refill. There may be new information.
Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets Read the Medication Guide that comes with ACTONEL before you start taking it and each time you get a refill. There may be new information.
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS
 New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
New 2010 Osteoporosis Guidelines: What you and your health provider need to know QUESTIONS&ANSWERS Wednesday, December 1, 2010 1:00 p.m. to 2:00 p.m. ET 1. I m 55 years old. I ve been taking Fosavance
Produktinformationen för Alendronat ratiopharm Veckotablett 70 mg veckotablett MTnr 21346, gäller vid det tillfälle då läkemedlet godkändes.
 Produktinformationen för Alendronat ratiopharm Veckotablett 70 mg veckotablett MTnr 21346, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet
Produktinformationen för Alendronat ratiopharm Veckotablett 70 mg veckotablett MTnr 21346, gäller vid det tillfälle då läkemedlet godkändes. Informationen kommer inte att uppdateras eftersom läkemedlet
PACKAGE LEAFLET: INFORMATION FOR THE USER. Alendronic Acid 10 mg Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Alendronic Acid 10 mg Tablets (Alendronic Acid) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER Alendronic Acid 10 mg Tablets (Alendronic Acid) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
EU RISK MANAGEMENT PLAN (EU RMP)
 EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
SUMMARY OF PRODUCT CHARACTERISTICS. Each film-coated tablet contains 150 mg ibandronic acid (as ibandronate sodium monohydrate).
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ibandronate Bluefish 150 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 150 mg ibandronic
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ibandronate Bluefish 150 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 150 mg ibandronic
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Osteoporosis Care Sheet
 Osteoporosis Care Sheet Serial no Keynote for Osteoporosis Care Not to emphasize side effect too much. No need to explain too many things at the first counseling. Support patient's self care! 1) Diagnosis
Osteoporosis Care Sheet Serial no Keynote for Osteoporosis Care Not to emphasize side effect too much. No need to explain too many things at the first counseling. Support patient's self care! 1) Diagnosis
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Part VI: Summary of the risk management plan by product
 Part VI: Summary of the risk management plan by product VI.1 Elements for summary tables in the EPAR VI.1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Hepatotoxic
Part VI: Summary of the risk management plan by product VI.1 Elements for summary tables in the EPAR VI.1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Hepatotoxic
Updates in Osteoporosis
 Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Download slides:
 Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Osteoporosis/Fracture Prevention Clinical Practice Guidelines
 NATIONAL CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinical Practice Guidelines Reviewed/Approved by the National Guideline Directors September 2017 Next Review/Approval: September
NATIONAL CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinical Practice Guidelines Reviewed/Approved by the National Guideline Directors September 2017 Next Review/Approval: September
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
ALENDRONATE SODIUM FOSAMAX
 PRODUCT CIRCULAR PHL-MK0217-T-032010 ALENDRONATE SODIUM FOSAMAX Tablets Anti-Osteoporosis NAME AND STRENGTH OF ACTIVE INGREDIENT Each tablet of ALENDRONATE SODIUM (FOSAMAX) contains either 13.05 or 91.37
PRODUCT CIRCULAR PHL-MK0217-T-032010 ALENDRONATE SODIUM FOSAMAX Tablets Anti-Osteoporosis NAME AND STRENGTH OF ACTIVE INGREDIENT Each tablet of ALENDRONATE SODIUM (FOSAMAX) contains either 13.05 or 91.37
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Package leaflet: Information for the patient. Alendronic Acid 70 mg Tablets. sodium alendronate
 Package leaflet: Information for the patient Alendronic Acid 70 mg Tablets sodium alendronate Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Alendronic Acid 70 mg Tablets sodium alendronate Read all of this leaflet carefully before you start taking this medicine because it contains important information
Index. Rheum Dis Clin N Am 32 (2006) Note: Page numbers of article titles are in boldface type.
 Rheum Dis Clin N Am 32 (2006) 775 780 Index Note: Page numbers of article titles are in boldface type. A AACE (American Association of Clinical Endocrinologists), bone mineral density recommendations of,
Rheum Dis Clin N Am 32 (2006) 775 780 Index Note: Page numbers of article titles are in boldface type. A AACE (American Association of Clinical Endocrinologists), bone mineral density recommendations of,
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Effective Health Care
 Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 9 1. NAME OF THE MEDICINAL PRODUCT Optinate 30 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 30 mg risedronate
SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 9 1. NAME OF THE MEDICINAL PRODUCT Optinate 30 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 30 mg risedronate
Learning Objectives. Controversies in Osteoporosis Prevention and Management. Etiology. Presenter Disclosure Information. Epidemiology.
 12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
How to treat osteoporosis With what and for how long?
 How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
Le linee guida: Indicazioni terapeutiche
 Le linee guida: Indicazioni terapeutiche Ranuccio Nuti Department of Medicine, Surgery and Neurosciences University of Siena, Italy 5.0 NON-PHARMACOLOGICAL MEASURES FOR OSTEOPOROSIS PREVENTION AND TREATMENT
Le linee guida: Indicazioni terapeutiche Ranuccio Nuti Department of Medicine, Surgery and Neurosciences University of Siena, Italy 5.0 NON-PHARMACOLOGICAL MEASURES FOR OSTEOPOROSIS PREVENTION AND TREATMENT
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
The Osteoporosis Center at St. Luke s Hospital
 The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ADROVANCE 70 mg/2,800 IU tablets ADROVANCE 70 mg/5,600 IU tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION ADROVANCE 70
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ADROVANCE 70 mg/2,800 IU tablets ADROVANCE 70 mg/5,600 IU tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION ADROVANCE 70
