Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana
|
|
|
- Lester Baldwin
- 5 years ago
- Views:
Transcription
1 11 Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour a randomised placebocontrolled trial. S Afr Med J 2001;91: Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG 2002;109: Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. Am J Obstet Gynecol 1998;179: Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol 1998;105: Surbek DV, Fehr PM, Hosli I, Holzgreve W. Oral misoprostol for third stage of labor: a randomized placebo-controlled trial. Obstet Gynecol 1999;94: Prasertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. Int J Gynaecol Obstet 2000;71: Hoj L, da Silva D, Hedegaard K, Sandstrom A, Aaby P. Factors associated with maternal mortality in rural Guinea-Bissau. A longitudinal population-based study. BJOG 2002;109: Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana Daniel Chandramohan, Seth Owusu-Agyei, Ilona Carneiro, Timothy Awine, Kwame Amponsa-Achiano, Nathan Mensah, Shabbar Jaffar, Rita Baiden, Abraham Hodgson, Fred Binka, Brian Greenwood Abstract Objective To evaluate the effects of intermittent preventive treatment for malaria in infants (IPTi) with sulfadoxine-pyrimethamine in an area of intense, seasonal transmission. Design Cluster randomised placebo controlled trial, with 96 clusters allocated randomly to sulfadoxine-pyrimethamine or placebo in blocks of eight. Interventions Children received sulfadoxine-pyrimethamine or placebo and one month of iron supplementation when they received DPT-2, DPT-3, or measles vaccinations and at 12 months of age. Main outcome measures Incidence of malaria and of anaemia determined through passive case detection. Results 89% (1103/1242) of children in the placebo group and 88% (1088/1243) in the IPTi group completed follow-up to 24 months of age. The protective efficacy of IPTi against all episodes of malaria was 24.8% (95% confidence interval 14.3% to 34.0%) up to 15 months of age. IPTi had no protective effect against malaria between 16 and 24 months of age (protective efficacy 4.9%, 21.3% to 9.3%). The incidence of high parasite density malaria ( 5000 parasites/μl) was higher in the IPTi group than in the placebo group between 16 and 24 months of age (protective efficacy 19.5%, 39.8% to 2.2%). IPTi reduced hospital admissions with anaemia by 35.1% (10.5% to 52.9%) up to 15 months of age. IPTi had no significant effect on anaemia between 16 and 24 months of age (protective efficacy 6.4%, 76.8% to 35.9%). The relative risk of death up to 15 months of age in the IPTi group was 1.26 (95% confidence interval 0.81 to 1.96; P = 0.31), and from 16 to 24 months it was 1.28 (0.77 to 2.14; P = 0.35). Conclusions Intermittent preventive treatment for malaria with sulfadoxine-pyrimethamine can reduce malaria and anaemia in infants even in seasonal, high transmission areas, but concern exists about possible rebound in the incidence of malaria in the second year of life. Introduction Malaria remains the leading cause of morbidity and mortality in young children in impoverished communities in Africa. It is estimated that 515 (range ) million clinical attacks of falciparum malaria occurred globally in 2002 and that 70% of these cases occurred in Africa. 1 Malaria is one of the major causes of anaemia in children in sub-saharan Africa, accounting for an estimated 18% of the disability adjusted life years lost because of anaemia. 2 The number of deaths attributable to malaria associated anaemia is estimated at to a year. 3 In high intensity transmission areas, the highest burden of malaria and malaria associated anaemia is in infants. 4 Control of malaria and anaemia depends largely on passive case detection and appropriate treatment. However, many children who have malaria parasitaemia, anaemia, or both remain asymptomatic, 5 and chronic asymptomatic parasitaemia increases the risk of severe anaemia. 6 Chemoprophylaxis during the first year of life significantly reduced the incidence of malaria and anaemia in a study in Tanzania. 7 The risk of malaria and anaemia increased in the second year of life after chemoprophylaxis had been stopped, however, suggesting that complete protection against malaria infection during infancy had impaired naturally acquired immunity against the infection. Two recent studies in Tanzania, done in areas with perennial transmission of malaria, showed that administration of a full therapeutic course of an antimalarial at set times during infancy, regardless of whether or not the infant was parasitaemic (intermittent preventive treatment in infants (IPTi)), substantially reduced the incidence of malaria and anaemia. 8 9 The antimalarial was given in A figure and three tables are on bmj.com London School of Hygiene and Tropical Medicine, London WC1E 7HT Daniel Chandramohan clinical senior lecturer Ilona Carneiro lecturer Shabbar Jaffar senior lecturer Brian Greenwood director, Gates Malaria Partnership Kintampo Health Research Centre, Kintampo, Ghana Seth Owusu-Agyei director Navrongo Health Research Centre, Navrongo, Ghana Timothy Awine research officer Kwame Amponsa-Achiano research physician Nathan Mensah data manager Rita Baiden research physician Abraham Hodgson director INDEPTH-Network, Accra, Ghana Fred Binka executive director Correspondence to: D Chandramohan daniel.chandramohan@ lshtm.ac.uk BMJ 2005;331:
2 association with vaccination in one study and at the time of growth monitoring visits in the other. Whether IPTi linked to a child immunisation schedule will have a similar protective effect in areas with intense, seasonal transmission is not known. We aimed to evaluate the effects of IPTi with sulfadoxinepyrimethamine in an area of intense, seasonal transmission in northern Ghana. Methods Study site We did the study in the Kassena-Nankana district in the Upper East region of Ghana, which lies within the open woodland, sub-sahel region of West Africa. Annual rainfall averages 850 mm, with most rain falling during June to October. The entomological inoculation rate was estimated to be 418 infective bites per person per year in , and almost all infective bites occur between June and November, coinciding with the rainy season. 10 The prevalence of parasitaemia in 2-12 month old infants at the end of the dry season in June 2000 was 8.6% (95% confidence interval 6.6% to 11.0%); this increased to 52.8% (48.7% to 56.7%) at the end of the rainy season (November 2000). The prevalence of a packed cell volume < 24% showed a similar seasonal pattern, increasing from 2.4% (1.4% to 3.9%) in the dry season to 12.6% (10.1% to 15.4%) at the end of the rainy season (unpublished data from baseline simple random sample cross sectional surveys). The 14 day adequate clinical and parasitological response rate after treatment with sulfadoxinepyrimethamine for clinical malaria (geometric mean parasite density /μl, range /μl) in children aged 6-59 months was 77.6% (68.9% to 84.8%) in However, the day 14 parasite clearance rate in asymptomatic children aged months (geometric mean parasite density 2595/μl, range /μl) was 94.5% (90.2% to 97.3%) (unpublished data from drug sensitivity study carried out in the IPTi study children in 2004, after all children had completed follow-up at 18 months of age). A demographic surveillance system for monitoring vital events has been operating in this district since The area is divided into four zones, and within each zone households (compounds) are grouped into clusters. On average, a cluster has 100 households. The district contained 244 clusters of households in We restricted the study to the central and southern zones, which had a total of 96 clusters of households. All infants living in these clusters of households without a history of allergy to sulfadoxinepyrimethamine were eligible for enrolment in the study. The study area has one hospital with 140 beds and 11 community health centres that provide outpatient care only. In addition, there are 18 outreach clinics where only expanded programme of immunisation (EPI) and growth monitoring are provided. The study infants were enrolled in these 30 EPI delivery points. Primary objective and sample size The primary objective was to determine the effect of IPTi on the incidence of anaemia. We assumed that the incidence of anaemia would be 25% in the placebo group and 18.7% in the IPTi group and that the coefficient of variation between clusters (k) would be 0.1. With the available 96 clusters, each of 25 children on average, the study had approximately 95% power to detect a statistically significant difference in the incidence of anaemia at the 5% two sided significance level. Randomisation and study drugs We randomly allocated 96 clusters of households to sulfadoxine-pyrimethamine or placebo in blocks of eight clusters by using a computer generated list. To increase blinding, we assigned clusters allocated to sulfadoxine-pyrimethamine or placebo to eight different drug codes (four sulfadoxine-pyrimethamine and four placebo). A statistician at the London School of Hygiene and Tropical Medicine generated and kept safe the randomisation list of clusters and drug codes. The study team and caretakers of study children were blinded to the drug codes. Study infants received half a tablet of sulfadoxine-pyrimethamine or an identical placebo when they received DPT-2 or DPT-3 vaccines and one tablet of sulfadoxine-pyrimethamine or placebo when they received measles vaccine and at 12 months of age, according to the cluster where they lived. In addition to sulfadoxine-pyrimethamine or placebo, all infants received one month s supply of iron supplement (2.5 ml, 15 mg elemental iron, twice weekly for four weeks) when they received DPT-2, DPT-3, or measles vaccines and at 12 months of age. We obtained sulfadoxine-pyrimethamine and placebo from Cosmos Pharmaceuticals, Nairobi. The content and solubility of the sulfadoxine-pyrimethamine tablets were confirmed by solubility testing and high performance liquid chromatography at the London School of Hygiene and Tropical Medicine. Study drugs were crushed and mixed with water and administered by a study field worker at an EPI clinic. Enrolment and follow-up Enrolment of study infants started in September 2000, and follow-up to 24 months of age was completed in June The nature of the trial was explained to caretakers of infants attending the study EPI clinics for DPT-1 vaccine, and they were invited to enrol their infant in the study. After informed written consent had been obtained, infants were enrolled, assigned a unique personal identity number, and allocated to the respective study group according to the cluster where they lived. All study infants were given a photo identity card, and their guardians were asked to bring the card whenever they visited an EPI clinic or other health facility. A field worker visited the households of study infants one or two days before the date when DPT-2 (IPT-1), DPT-3 (IPT-2), and measles (IPT-3) vaccinations were due to remind caretakers to attend an EPI clinic. A similar visit was made shortly before the IPT-4 administration at the age of 12 months was due. Finger prick blood samples for assessing packed cell volume, malaria parasitaemia, and the immune response to EPI vaccines were taken when infants received IPT-1, IPT-3, and IPT-4. Immune responses to EPI vaccines are being tested as part of a larger study commissioned by the IPTi consortium, and these results will be reported elsewhere. We used a morbidity questionnaire to assess all study children who presented with any illness at one of the 11 health centres or at the hospital outpatient 728
3 department. We collected blood samples for malaria parasites from all children who had fever or a history of fever during the previous two days. We used an inpatient morbidity questionnaire to assess children who were admitted to the hospital and took blood samples for microscopy and determination of packed cell volume. Study children were visited at home at 18 months of age to collect finger prick blood samples for assessing packed cell volume and the presence of malaria parasitaemia and again at 23 months of age to assess their health. The study physician certified the cause of death of children who died in the hospital. We assessed cause of death of children who died at home by verbal autopsy. 12 A fieldworker visited a random 20% sample of study children at home within four weeks after administration of IPT dose 1 or dose 2 to assess adherence to administration of iron at home and to inquire about adverse events. Children who had any rash other than scabies, heat rash, or insect bites were referred to the project physician for a clinical assessment. Ethical clearance and analytical plan An independent statistician (Simon Cousens) and epidemiologist (Laura Rodrigues) approved the analytical plan. We cleaned and audited the data before locking the data and unmasking the intervention codes. The primary outcomes, defined in the analytical plan, were the incidence of all episodes of malaria associated fevers (history of fever or temperature 37.5 C plus malaria parasites detected on a blood smear) detected in study children attending the health centres or the hospital and the incidence of anaemia (packed cell volume < 24%) in children admitted to the study hospital for any illness. We stratified analyses by age (2-15 months and months) to assess the effect of IPTi during the intermittent treatment phase (2-15 months) and after the effect of the intervention had ceased (16-23 months). We decided a priori to investigate the effect of sex, urban residence, and mosquito net use on the outcome, including only those covariates that were significantly associated with the outcome (P < 0.1) in the final model. Because of the highly seasonal nature of transmission of malaria at the study site, we compared the effect of IPTi administered during the wet season (July- November) with IPTi administered during the dry season (December-June). We restricted this analysis to 2-12 month old infants. We estimated the protective efficacy of IPTi against malaria, anaemia, and hospital admissions by all combinations of timing of IPTi doses. Statistical methods We analysed data on an intention to treat basis, including all infants who were enrolled, at the time of receipt of DPT-1, regardless of whether they received any doses of IPT. Time at risk started at enrolment, approximately four weeks before the first dose was administered and will, therefore, give a conservative estimate of the effect of IPTi. Thirty episodes of malaria occurred in person years at risk in the placebo group and 30 episodes in person years at risk in the sulfadoxine-pyrimethamine group in the period between enrolment and the first dose received. We analysed data by using random effects Poisson or negative binomial regression models to allow for intracluster correlation at the level of randomisation (household cluster) and to adjust for important covariates. We used Poisson regression to analyse the incidence of anaemia, cerebral malaria, hospital admissions, and death. We used negative binomial regression to analyse the incidence of malaria associated fevers and high density fevers, as these were significantly clustered within individuals. We calculated person time at risk from the date of enrolment to second birthday, date of migration, or date of death. In addition, if a child had received an antimalarial drug during the follow-up period, we subtracted the 28 day posttreatment period from the person time at risk. We analysed data on cross sectional outcomes by using a random effects logistic regression model. Results Between September 2000 and June 2004, 2485 infants were enrolled in the study and followed up until the age of 24 months, death, or migration out of the study area. The number of children per cluster varied but was well balanced between the two groups. It ranged from 2 to 47 (mean 30.1) in the IPTi group and from 7 to 47 (mean 29.5) in the placebo group. The proportions of infants who received drug treatment at different time points and the proportion of children followed up to 24 months of age were similar between the IPTi and control groups (see figure on bmj.com). Table A on bmj.com shows the characteristics of the infants in each arm of the study. Tables 1 and 2 summarise the effects of IPTi on malaria, anaemia, and other outcomes. Incidence of malaria The incidence of malaria was significantly lower in the IPTi group than in the placebo group up to 15 months of age (table 1). The protective efficacy of IPTi against all episodes of malaria was 24.8% (95% confidence interval 14.3% to 34.0%). IPTi had no effect on the incidence of malaria between 16 and 24 months of age (protective efficacy 4.9%, 21.3% to 9.3%). From 2 to 24 months of age IPTi had a protective efficacy of 16.1% (6.4% to 24.8%) (table 2). Kaplan-Meier survival analysis of time to first episode of malaria (figure) suggests that much of the protective effect against malaria occurred after the first and second doses, with some indication of waning of effect with time (log rank χ 2 = 16.4, P = for 0-8 months; log rank χ 2 = 8.86, P = for 9-15 months, and log rank χ 2 = 0.14, P = for months). Although the protective efficacy against all malaria episodes during a one month post-ipti period was not significantly different between the four courses of IPTi, during the second month after IPTi the protective efficacy of IPTi dose 4 was significantly lower than that seen after dose 2 (table 3). Protective efficacy of IPTi against malaria attacks associated with high parasite density (defined a priori as 5000 parasite/μl) up to 15 months of age was similar to the protective efficacy against malaria attacks of any parasite density (23.3%, 10.8% to 34.1%) (table 2). The incidence of high parasite density malaria was significantly higher in the IPTi group than in the placebo group between 16 and 24 months of age (protective efficacy 19.5%, 39.8% to 2.2%). From 729
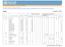
4 Table 1 Outcomes in sulfadoxine-pyrimethamine (SP) and placebo arms by age group 2-15 months months Placebo SP Odds ratio Placebo SP Odds ratio Outcomes Events Rate* Events Rate* (95% CI) Events Rate* Events Rate* (95% CI) Malaria Person years at risk All episodes of malaria (0.66 to 0.86) (0.91 to 1.21) Malaria ( 5000 parasites/μl) (0.66 to 0.89) (1.02 to 1.39) Hospital admissions Person years at risk All causes (0.73 to 1.05) (0.98 to 1.31) All malaria admissions (0.42 to 0.86) (0.54 to 1.56) PCV<24% (0.47 to 0.89) (0.66 to 1.81) PCV<15% (0.26 to 1.21) (0.16 to 2.04) Malaria + PCV<24% (0.28 to 0.77) (0.56 to 2.95) Malaria + PCV<15% (0.21 to 1.95) Cerebral malaria (0.35 to 1.35) (0.33 to 1.99) Deaths Person years at risk All causes (0.82 to 1.99) (0.76 to 2.13) Malaria or anaemia (0.66 to 2.23) (0.54 to 2.53) PCV=packed cell volume. *Per 1000 child years at risk. Intracluster correlation log rank test χ 2 =34.04; P< Intracluster correlation log rank test χ 2 =4.77; P= Intracluster correlation log rank test χ 2 =0.03; P= Intracluster correlation log rank test χ 2 =1.26; P= to 24 months of age IPTi had a protective efficacy of 11.5% (0.0% to 21.6%) against high parasite density malaria. Incidence of anaemia The incidence of all episodes of anaemia (packed cell volume < 24%), identified through passive case detection, was significantly lower in the IPTi group than in the placebo group up to 15 months of age (table 1). The protective efficacy of IPTi against anaemia was 35.1% (10.5% to 52.9%). IPTi had no significant effect on the incidence of anaemia between 16 and 24 months of age (protective efficacy 6.4%, 76.8% to 35.9%). From 2 to 24 months of age IPTi had a protective efficacy of 23.8% ( 1.3% to 42.6%) against anaemia (table 2). The incidence of anaemia associated with malaria parasitaemia was also significantly lower in the IPTi group up to 15 months of age (table 1). The protective efficacy of IPTi against malaria associated anaemia was 53% (22.8% to 71.3%). The incidence of malaria associated anaemia increased in the IPTi group by 26.3% ( 44.9% to 189.8%) between 16 and 24 months of age, but this difference was not statistically significant. From 2 to 24 months of age IPTi had a protective efficacy of 10.5% ( 1.6% to 19.3%) against malaria associated anaemia (table 2). Incidence of severe disease and mortality The incidence of hospital admissions for all causes was 13.2% ( 4.5% to 28.0%) lower in the IPTi group than in the placebo group up to 15 months of age, but this reduction was not statistically significant (table 2). We found no significant difference between groups in the all cause hospital admission rate between 16 and 24 months. The incidence of all cause hospital admissions from 2 to 24 months of age was slightly lower in the IPTi group, but this difference was not statistically significant (9.5%, 7.5% to 23.8%). Table 2 Protective efficacy (95% confidence interval) and number of children protected per 1000 (95% confidence interval), by age group 2-15 months months 2-24 months Outcome Protective efficacy (%)* No protected/1000 Protective efficacy (%)* No protected/1000 Protective efficacy (%)* No protected/1000 Malaria All episodes of malaria 24.9 (14.5 to 34.1) (183.0 to 326.6) 4.9 ( 21.3 to 9.3) 53.0 ( to 39.9) 16.3 (6.7 to 25.0) (88.7 to 202.4) Malaria 5000/μl 23.6 (11.1 to 34.3) (83.6 to 195.9) 18.9 ( 39.1 to 1.7) 98.1 ( to 24.7) 11.9 (0.5 to 22.0) 55.4 (10.8 to 100.0) Hospital admissions All causes 12.7 ( 4.8 to 27.3) 37.5 (3.1 to 78.1) 0.4 ( 31.3 to 23.3) 2.4 ( 40.4 to 45.2) 9.5 ( 7.5 to 23.8) 25.1 (5.3 to 55.4) All malaria admissions 39.8 (14.0 to 57.9) 22.5 (6.2 to 38.7) 8.4 ( 56.0 to 46.2) 3.6 ( 17.5 to 24.7) 31.2 (5.4 to 49.9) 15.9 (3.0 to 28.8) PCV<24% 35.5 (11.2 to 53.1) 23.9 (6.4 to 41.4) 9.0 ( 81.3 to 34.4) 3.0 ( 24.9 to 18.8) 24.2 ( 0.5 to 42.8) 14.5 (0.8 to 28.2) PCV<15% 44.4 ( 20.6 to 74.3) 5.6 (1.7 to 12.9) 43.3 ( to 84.3) 3.9 ( 4.7 to 12.4) 44.1 ( 11.7 to 72.1) 5.0 (0.6 to 10.6) Malaria + PCV<24% 53.3 (23.4 to 71.6) 18.3 (6.6 to 30.0) 28.4 ( to 44.1) 4.1 ( 17.0 to 8.8) 38.8 (5.5 to 60.4) 10.5 (1.6 to 19.3) Malaria + PCV<15% 36.3 ( 94.9 to 79.2) 2.1 ( 2.9 to 7.1) 5.2 (0.1 to 10.4) 57.1 ( 25.9 to 85.4) 3.2 (0.5 to 6.9) Cerebral malaria 31.6 ( 35.3 to 65.4) 4.2 ( 4.1 to 12.5) 18.6 ( 99.2 to 66.7) 5.1 ( 8.5 to 18.8) 26.6 ( 28.6 to 58.1) 4.5 (2.7 to 11.7) Deaths All causes 27.4 ( 98.7 to 18.3) 6.4 ( 18.7 to 5.9) 27.3 ( to 23.9) 9.5 ( 29.4 to 10.3) 27.2 ( 78.1 to 9.1) 7.5 ( 18.1 to 3.1) Malaria 21.4 ( to 34.0) 2.9 ( 12.0 to 6.3) 17.0 ( to 45.9) 2.8 ( 15.9 to 10.4) 19.1 ( 92.0 to 25.1) 2.8 ( 10.4 to 4.7) PCV=packed cell volume. *Adjusted for sex and rural/urban residence; protective efficacy (% reduction in outcomes in IPTi group)=(1 odds ratio)
5 Proportion Sulfadoxine-pyrimethamine Placebo 0.25 Average age at administration of doses Age (months) Kaplan-Meier survival estimates of time to first episode of malaria by intervention group A significant reduction in the incidence of hospital admissions for malaria (37.8%, 11.8% to 56.1%) occurred in the IPTi group up to 15 months of age. IPTi had no significant effect on hospital admissions for malaria between 16 and 24 months. From 2 to 24 months of age IPTi had a significant protective effect of 15.9% (3.0% to 28.8%) against hospital admissions for malaria. More deaths occurred in the IPTi group (77) than in the placebo group (61), but this difference was not statistically significant (table 1). The relative risk of death up to 15 months of age in the IPTi group was 1.26 (0.81 to 1.96; P = 0.31), and from 16 to 24 months it was 1.28 (0.77 to 2.14; P = 0.35). There were 32 deaths attributable to malaria in the placebo group and 38 deaths in the IPTi group. This difference was not statistically significant. Prevalence of anaemia and parasitaemia Table B on bmj.com shows the prevalences of anaemia and parasitaemia at approximately 3, 9, 12, and 18 months of age. The mean packed cell volume was significantly higher in the IPTi group than in the control group at 12 months of age, but it was slightly lower in the IPTi group at 18 months. The prevalence of malaria parasitaemia was lower in the IPTi group at 9, 12, and 18 months of age. Effect of malaria transmission season on protective efficacy of IPTi Table 4 shows the protective efficacy of IPTi by number of doses administered during wet and dry seasons in 2-12 month old infants. Children who received IPTi doses 1 and 2 in the wet season and dose 3 in the dry season had the maximum protective effect against malaria (52%) and anaemia (72%). The next best protective efficacy against malaria (37%) was obtained by giving IPTi dose 2 during the wet season and doses 1 and 3 in the dry season. Although the effect was not statistically significant, infants who received all three IPTi doses in the dry season also seemed to have some protection against malaria (24%). Incidence of adverse events The proportions of children who vomited after administration of drugs was similar between the two groups (0.4% in the placebo group versus 0.3% in the sulfadoxine-pyrimethamine group). Among the children who were visited at home within four weeks after administration of IPTi dose 1 (n = 1765, 74%) or dose 2 (n = 214, 9%), 32 (3.3%) children in the placebo group and 27 (2.7%) in the sulfadoxinepyrimethamine group had skin rashes. None of the skin rashes was severe or suggestive of a drug sensitivity reaction. The incidence of outpatient attendance for any illness and hospital admissions due to any severe illness during the month after IPTi doses 1, 2, 3, and 4 was lower in the IPTi group than in the placebo group (table C on bmj.com). However, during the one month period after IPTi dose 1, four deaths occurred in the sulfadoxine-pyrimethamine group compared with none in the placebo group. Similarly, during the month after IPTi dose 2, six deaths occurred in the sulfadoxine-pyrimethamine group compared with none in the placebo group. Causes of these deaths were as follows: malaria (4), diarrhoea disease (1), septicaemia (1), spirit child (probably herbal poisoning) (2), and unknown (2). None of these deaths was considered to be associated with the IPTi drugs by the panel of physicians who reviewed the verbal autopsy questionnaires. Discussion Effect of malaria transmission intensity on IPTi efficacy The protective efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine against malaria and anaemia in infants observed in our study is moderate and substantially lower than that reported from Ifakara, Tanzania. 8 This may be because of the differences in the intensity and pattern of malaria transmission between the two sites. The intensity of transmission is low (entomological inoculation rate 31/year) and perennial in Ifakara, 13 whereas it is very high (418/year) and highly seasonal in Navrongo, our study site. 10 The incidence of malaria in the placebo group was 0.43 episode/child/year in Ifakara compared with 1.0 episode/child/year in Navrongo. Although the protective efficacy of IPTi was higher in Ifakara than in Navrongo, the number of episodes of malaria prevented was similar at the two sites (260 v 255 per 1000 children per year) because the background incidence of malaria was higher in Navrongo. In spite of a higher incidence of malaria, the Table 3 Protective efficacy against malaria during the first and second month after each dose of IPTi Malaria episodes during first month after IPTi Malaria episodes during second month after IPTi Protective efficacy (%) during first month after IPTi (95% CI) Protective efficacy (%) during second month after IPTi (95% CI) IPTi dose Placebo SP Placebo SP No Events PYAR Events PYAR Events PYAR Events PYAR (50 to 89) (69 to 91) 43 (19 to 60) (68 to 87) 27 (4 to 44) (65 to 86) 11 ( 49 to 18) IPTi=intermittent preventive treatment for malaria in infants; PYAR=person years at risk; SP=sulfadoxine-pyrimethamine. 731
6 Table 4 Protective efficacy of IPTi by season of administration in 2-12 month old infants. Values are percentages (95% confidence intervals) Doses administered No of children All episodes of malaria* Malaria episodes with parasite 5000/μl* Anaemia (PCV<24) Hospital admissions for malaria 1, 2, and 3 in dry season ( 7 to 46) 31 ( 6 to 55) 44 ( 122 to 86) 61 ( 91 to 7) 1 in wet, 2 and 3 in dry ( 54 to 37) 13 ( 103 to 38) 67 ( 216 to 97) 2 in wet, 1 and 3 in dry (10 to 56) 24 ( 22 to 52) 17 ( 232 to 79) 105 ( 958 to 60) 3 in wet, 1 and 2 in dry ( 1 to 28) 17 ( 2 to 33) 29 ( 31 to 62) 43 ( 5 to69) 1 and 2 in wet, 3 in dry (37 to 63) 57 (38 to 70) 72 (12 to 91) 57 ( 135 to 92) 1 and 3, 2 and 3, or all three in wet ( 40 to 63) 24 ( 73 to 67) 28 ( 1046 to 4) 1, 2, and 3 in any season (16 to 37) 28 (14 to 39) 43 (11 to 64) 44 (8 to 66) PCV=packed cell volume. *Up to 28 days person time at risk and any episodes excluded after treatment with chloroquine or other antimalarial agent. incidences of anaemia and hospital admissions in the placebo group were lower in Navrongo than in Ifakara (anaemia: 0.07 v 0.11/child/year; hospital admissions: 0.32 v 0.63/child/year). This difference may be due to the fact that the Navrongo study covered a large area (some children lived up to 40 km from the hospital) whereas the study population of Ifakara lived within a 5 km radius of the hospital. The incidences of anaemia and hospital admissions are probably underestimated in both the placebo and IPTi groups in our study, and thus the public health impact of IPTi on these outcomes may also be underestimated; more cases may have been prevented than we were able to detect. Effect of malaria transmission season on IPTi efficacy IPTi dose 2 seemed to have a higher protective efficacy against malaria than IPTi doses 1 and 3. Children who received their IPTi dose 2 in the wet season had 46% protection against malaria between 2 and 12 months of age, whereas those who received this dose in the dry season had only 16% protection. Similarly, children who received dose 1 in the wet season had 42% protection compared with 19% protection in those who received dose 1 in the dry season. Children who received dose 3 in the wet season had only 15% protection compared with 38% protection in those who had dose 3 in the dry season. This is because children who received dose 3 in the dry season had received dose 1, dose 2, or both in the wet season. Children who received IPTi doses 1 and 2 in the wet season and dose 3 in the dry season had the maximum protection against malaria (52%), which is comparable to the protective efficacy of IPTi observed in Ifakara (62%). It seems that offering IPTi with sulfadoxinepyrimethamine at the time of DPT-2 and DPT-3 during the wet season alone would have the maximum benefit, although giving IPTi year round may be beneficial in this area with high seasonal transmission. However, as this study was not designed to detect the efficacy of different dosing schedules, these data need to be interpreted with caution. In Senegal, Cisse et al observed 86% protection against clinical attacks of malaria by administering three doses of sulfadoxinepyrimethamine plus artesunate to children under 5 years old on three occasions during the malaria transmission season, 14 and similar results have been obtained in Mali. 15 Further studies of appropriate dosing schedules for IPTi in high, seasonal transmission areas are needed. Mode of action of IPTi Protection against malaria during the one month period after an IPTi dose is much higher than the overall effect of IPTi from 2 to 15 months of age. This suggests that IPTi using sulfadoxine-pyrimethamine reduces the incidence of malaria and anaemia, at least in part, through its chemoprophylactic effect, which is known to last for about a month. However, the fact that doses 1 and 2 administered during the high transmission season had a higher protective efficacy than dose 3 suggests that reducing the number of infections between 3 and 6 months of age may modulate the immune response and thereby give greater protection for prolonged periods. This hypothesis needs to be tested by using long acting and short acting antimalarials and studying their effect on immune responses. Rebound effect of IPTi The IPTi study from Tanzania did not find a rebound increase in the incidence of malaria when IPTi was stopped and even found some evidence of persistence of protection into the second year of life. 16 However, we found a significant increase in malaria attacks associated with high density parasitaemia and a slight decrease in mean packed cell volume between 16 and 24 months of age when the effect of IPTi had worn off. This could be due to the differences in the level of intensity of transmission. Our finding of a potential rebound effect suggests that a careful monitoring of rebound effect is needed if IPTi is implemented on a large scale. Sulfadoxine-pyrimethamine IPTi did not increase the incidence of common adverse events such as vomiting and skin rash, and the incidence of any illness that needed medical attention was lower in the IPTi group than in the placebo group. However, 10 deaths occurred during the first month after IPTi whereas none occurred in the placebo group in this period. Although none of the deaths was deemed to be caused by IPTi, this trend needs to be carefully monitored in all IPTi studies and implementation programmes. Effect of insecticide treated nets plus IPTi Insecticide treated bed nets reduce the incidence of malaria and anaemia substantially. A meta-analysis showed that nets reduce episodes of malaria by 50% (95% confidence interval 45% to 55%) and increase mean packed cell volume by 1.7% in children aged under 5 years. 17 A combination of nets plus malaria chemoprophylaxis (pyrimethamine-dapsone) reduced the incidence of malaria by 97% (92% to 99%) and increased the mean packed cell volume by 1.2% 732
7 What is already known on this topic Intermittent preventive treatment for malaria in infants with sulfadoxine-pyrimethamine substantially reduced the incidence of malaria and anaemia in Tanzania This was in an area of low, perennial transmission of malaria What this study adds Four courses of sulfadoxine-pyrimethamine given at DPT-2, DPT-3, and measles vaccination and at 12 months of age reduced malaria (25%) and anaemia (35%) up to age 15 months in a high, seasonal transmission area in Ghana The incidence of high parasite density malaria increased 20% when treatment was stopped compared with nets alone. 18 Only 17% of the placebo group and 19% of the IPTi group in our study population had used any form of net. Among the children who used a net, addition of IPTi gave 19% ( 2% to 35%; P = 0.07) protection against malaria compared with children who received the placebo. Those using a net had no rebound effect of high parasite density malaria between 16 and 24 months of age (protective efficacy 4%, 47% to 38%; P = 0.83). These results should be interpreted with caution, however, as this study was not designed to evaluate the combined effect of nets plus IPTi. Distribution of nets is increasingly linked with immunisation, and the coverage of nets in children attending immunisation clinics has increased remarkably from 4% to 94% in a district in Ghana. 19 If this approach to the delivery of nets continues, both nets and IPTi will reach the same population, so assessing the combined effect of nets and IPTi on malaria is important, particularly the possible rebound effect in the second year of life. Any potential rebound effect might be offset by the continued protection offered by nets. On the other hand, the net plus IPTi combination might give almost total protection against malaria infection during infancy and impair naturally acquired immunity, as seen with continuous chemoprophylaxis, 7 and increase the burden of severe malaria later in life. 20 Conclusions More data are needed to decide on the appropriate dose schedule for IPTi in areas with high seasonal transmission and its interaction with insecticide treated bed nets. However, intermittent preventive treatment in infants with sulfadoxine-pyrimethamine linked to the expanded programme of immunisation schedule has the potential to reduce the burden of malaria even in areas with high, seasonal transmission. We thank the Kassena-Nankana district health management team, the project staff, and the study participants for their kind cooperation and support. We also thank Harparkash Kaur for testing the contents and quality of the study drugs. Contributors: DC was involved in protocol development, implementation of the trial, data analysis, and drafting the manuscript; he is the guarantor. SO-A and FB were involved in protocol development, implementation of the trial, and reviewing the manuscript. IC was involved in data analysis and drafting the manuscript. TA and NM were involved in implementation of the trial, data management, and reviewing the manuscript. KA-A, RB, and AH were involved in implementation of the trial and reviewing the manuscript. SJ was involved in randomisation, data analysis, and reviewing the manuscript. BG was involved in protocol development, monitoring of the trial, data analysis, and drafting the manuscript. Funding: This study was funded by a grant from the Department for International Development (DFID) UK (grant No R7602). The analytical plan and the manuscript were not influenced by the DFID. The DFID cannot accept any responsibility for the information and views presented in this manuscript. The subsidiary study of sensitivity of malaria parasites to sulfadoxine-pyrimethamine was funded by the Gates Malaria Partnership. Competing interest statement: None declared. Ethical approval: The study was approved by the ethical committees of the Ministry of Health of Ghana and the London School of Hygiene and Tropical Medicine. 1 Snow R, Guerra C, Noor A, Myint H, Hay S. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005;434: Murray CJ, Lopez AD. Global burden of disease and injury series. Geneva: World Health Organization, 1996: Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anaemia, respiratory distress, hypoglycaemia and complications of pregnancy. Am J Trop Med Hyg 2001;64(1-2 suppl): Schellenberg D, Menendez C, Kahigwa E, Font F, Galindom C, Acosta C, et al. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am J Trop Med Hyg 1999;61: Schellenberg D, Schellenberg JR, Mushi A, Savigny D, Mgalula L, Mbuya C, et al. The silent burden of anaemia in Tanzanian children: a community-based study. Bull World Health Organ 2003;81: Kitua AY, Smith TA, Alonso PL, Urassa H, Masanja H, Kimario J, et al. The role of low level Plasmodium falciparum parasitaemia in anaemia among infants living in an area of intense and perennial transmission. Trop Med Int Health 1997;2: Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 1997;350: Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebocontrolled trial. Lancet 2001;357: Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Ronn AM, et al. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: a randomised placebo-controlled trial. Lancet 2003;361: Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, et al. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health 2004;9: Oduro AR, Anyorigiya T, Hodgson A, Ansah P, Anto F, Ansah NA, et al. A randomized comparative study of chloroquine, amodiaquine and sulphadoxine-pyrimethamine for the treatment of uncomplicated malaria in Ghana. Trop Med Int Health 2005;10: Population, Health and Survival at Indepth sites. Ottawa, Canada: International Development Research Centre, Drakeley C, Schellenberg D, Kihonda J, Sousa CA, Arez AP, Lopes D, et al. An estimation of the entomological inoculation rate for Ifakara: a semiurban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health 2003;8: Cisse B, Sokhna CS, Alexander N, Trape JF, Greenwood B. A double blind, randomised placebo-controlled trial to measure the potential of SIPT with artesunate pus SP to reduce the malaria burden in Niakhar, Senegal. Am J Trop Med Hyg 2004;71(suppl):abstract Dicko A, Sagara I, Sissoko M, Guindo O, Diallo A, Kone M, et al. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children of 6 months to 10 years in Kambili, Mali. Am J Trop Med Hyg 2004;71(suppl):abstract Schellenberg D, Menendez C, Aponte J, Kahigwa E, Tanner M, Mshinda H, et al. Intermittent treatment antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 2005;365: Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004;(2):CD Greenwood BM, Greenwood AM, Bradley AK, Snow RW, Byass P, Hayes RJ, et al. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia. Lancet 1988;i: Grabowsky M, Nobiya T, Ahun M, Donna R, Lengor M, Zimmerman D, et al. Distributing insecticide-treated bednets during measles vaccination: a low-cost means of achieving high and equitable coverage. Bull World Health Organ 2005;83: Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol 2002;52:
Intermittent preventive treatment for malaria in infants: a decision-support tool for sub-saharan Africa
 Ilona Carneiro et al. Intermittent preventive treatment for malaria in African infants Intermittent preventive treatment for malaria in infants: a decision-support tool for sub-saharan Africa Ilona Carneiro,
Ilona Carneiro et al. Intermittent preventive treatment for malaria in African infants Intermittent preventive treatment for malaria in infants: a decision-support tool for sub-saharan Africa Ilona Carneiro,
Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review)
 Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review) Meremikwu MM, Donegan S, Esu E This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Chemoprophylaxis and intermittent treatment for preventing malaria in children (Review) Meremikwu MM, Donegan S, Esu E This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya
 MAJOR ARTICLE Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya Tabitha W. Mwangi, 1 Amanda Ross, 1,2,a Robert W. Snow, 1,2 and Kevin Marsh 2 1 Kenya
MAJOR ARTICLE Case Definitions of Clinical Malaria under Different Transmission Conditions in Kilifi District, Kenya Tabitha W. Mwangi, 1 Amanda Ross, 1,2,a Robert W. Snow, 1,2 and Kevin Marsh 2 1 Kenya
GRADE tables to assist guideline development and recommendations. Plain Language Summary of Results
 Seasonal Malaria Chemoprevention (formally known as Intermittent Preventive Treatment in children) for preventing malaria morbidity in children aged less than 5 years living in areas of marked seasonal
Seasonal Malaria Chemoprevention (formally known as Intermittent Preventive Treatment in children) for preventing malaria morbidity in children aged less than 5 years living in areas of marked seasonal
IPTc BIBLIOGRAPHY. PUBLICATIONS ON IPTc IN CHILDREN IN THE COMMUNITY UNDER FIVE YEARS OF AGE
 IPTc BIBLIOGRAPHY PUBLICATIONS ON IPTc IN CHILDREN IN THE COMMUNITY UNDER FIVE YEARS OF AGE Published 2006 1. Cissé B, Sokhna C,Boulanger D, Milet J, Ba el H, Richardson K, Hallett R, Sutherland C, Simondon
IPTc BIBLIOGRAPHY PUBLICATIONS ON IPTc IN CHILDREN IN THE COMMUNITY UNDER FIVE YEARS OF AGE Published 2006 1. Cissé B, Sokhna C,Boulanger D, Milet J, Ba el H, Richardson K, Hallett R, Sutherland C, Simondon
A Randomized Controlled Trial of Extended Intermittent Preventive Antimalarial Treatment in Infants
 MAJOR ARTICLE A Randomized Controlled Trial of Extended Intermittent Preventive Antimalarial Treatment in Infants Robin Kobbe, 1 Christina Kreuzberg, 1 Samuel Adjei, 1,3 Benedicta Thompson, 3 Iris Langefeld,
MAJOR ARTICLE A Randomized Controlled Trial of Extended Intermittent Preventive Antimalarial Treatment in Infants Robin Kobbe, 1 Christina Kreuzberg, 1 Samuel Adjei, 1,3 Benedicta Thompson, 3 Iris Langefeld,
Downloaded from:
 Abdulla, S; Armstrong Schellenberg, JRM; Nathan, R; Mukasa, O; Marchant, T; Smith, T; Tanner, M; Lengeler, C (2001) Impact on malaria morbidity of a programme supplying insecticide treated nets in children
Abdulla, S; Armstrong Schellenberg, JRM; Nathan, R; Mukasa, O; Marchant, T; Smith, T; Tanner, M; Lengeler, C (2001) Impact on malaria morbidity of a programme supplying insecticide treated nets in children
Harry Tagbor 1, Matthew Cairns 2, Emmanuel Nakwa 1, Edmund Browne 1, Badu Sarkodie 3, Helen Counihan 4, Sylvia Meek 4 and Daniel Chandramohan 2
 Tropical Medicine and International Health doi:10.1111/j.1365-3156.2010.02699.x volume 16 no 3 pp 280 289 march 2011 The clinical impact of combining intermittent preventive treatment with home management
Tropical Medicine and International Health doi:10.1111/j.1365-3156.2010.02699.x volume 16 no 3 pp 280 289 march 2011 The clinical impact of combining intermittent preventive treatment with home management
Severe Anemia in Young Children After High and Low Malaria Transmission Seasons in the Kassena- Nankana District of Northern Ghana
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 2000 Severe Anemia in Young Children After High and Low Malaria Transmission
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 2000 Severe Anemia in Young Children After High and Low Malaria Transmission
Options for the Delivery of Intermittent Preventive Treatment for Malaria to Children: A Community Randomised Trial
 Options for the Delivery of Intermittent Preventive Treatment for Malaria to Children: A Community Randomised Trial Margaret Kweku 1,3, Jayne Webster 1, Martin Adjuik 2, Samuel Abudey 3, Brian Greenwood
Options for the Delivery of Intermittent Preventive Treatment for Malaria to Children: A Community Randomised Trial Margaret Kweku 1,3, Jayne Webster 1, Martin Adjuik 2, Samuel Abudey 3, Brian Greenwood
NAVRONGO HEALTH INFORMING HEALTH POLICY THROUGH RESEARCH
 NAVRONGO HEALTH RESEARCH CENTRE INFORMING HEALTH POLICY THROUGH RESEARCH Strategic Location of Ghana DSS Sites Burkina Faso Cote d'ivoire Togo Dangbe West Kassena-Nankana Kintampo 50 0 50 100 150 200 250
NAVRONGO HEALTH RESEARCH CENTRE INFORMING HEALTH POLICY THROUGH RESEARCH Strategic Location of Ghana DSS Sites Burkina Faso Cote d'ivoire Togo Dangbe West Kassena-Nankana Kintampo 50 0 50 100 150 200 250
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital
 Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
Cases of Severe Malaria and Cerebral Malaria in Apam Catholic Hospital and Manhiya District Hospital CL Barba, MSIV Charles Drew University of Medicine and Science Los Angeles, CA QUESTION What pediatric
A Systematic Review and Meta-Analysis of the Efficacy and Safety of Intermittent Preventive Treatment of Malaria in Children (IPTc)
 A Systematic Review and Meta-Analysis of the Efficacy and Safety of Intermittent Preventive Treatment of Malaria in Children (IPTc) Anne L. Wilson*, on behalf of the IPTc Taskforce " London School of Hygiene
A Systematic Review and Meta-Analysis of the Efficacy and Safety of Intermittent Preventive Treatment of Malaria in Children (IPTc) Anne L. Wilson*, on behalf of the IPTc Taskforce " London School of Hygiene
Navrongo DSS area Kassena-Nankana District. Kilometre
 NAVRONGO HDSS, GHANA Site Map Upper East Region G H A N A Navrongo DSS area Kassena-Nankana District Ghana 0 25 50 Kilometre 0000 5050 100 Kilometre Location of Navrongo HDSS (Monitored Population 149,000)
NAVRONGO HDSS, GHANA Site Map Upper East Region G H A N A Navrongo DSS area Kassena-Nankana District Ghana 0 25 50 Kilometre 0000 5050 100 Kilometre Location of Navrongo HDSS (Monitored Population 149,000)
Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up. Dr. Badara Cissé Med.
 Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up Dr. Badara Cissé Med. epidemiologist Objectives Define the concept and recall natural history Summarise
Seasonal Malaria Chemo-prevention (SMC) From Doctoral Research to Health Policy and Regional Scaling-Up Dr. Badara Cissé Med. epidemiologist Objectives Define the concept and recall natural history Summarise
WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011
 WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011 Background Report of the Technical consultation on Seasonal Malaria Chemoprevention (SMC) / Chimio-prévention saisonnière
WHO/GMP TECHNICAL EXPERT GROUP ON PREVENTIVE CHEMOTHERAPY, Geneva 4-6 May 2011 Background Report of the Technical consultation on Seasonal Malaria Chemoprevention (SMC) / Chimio-prévention saisonnière
Health care related factors associated with severe malaria in children in Kampala, Uganda
 Health care related factors associated with severe malaria in children in Kampala, Uganda *Byakika-Kibwika P 1, Ndeezi G 2, Kamya MR 1 1 Makerere University, School fo Medicine, Department of Medicine,
Health care related factors associated with severe malaria in children in Kampala, Uganda *Byakika-Kibwika P 1, Ndeezi G 2, Kamya MR 1 1 Makerere University, School fo Medicine, Department of Medicine,
International Journal for Equity in Health
 International Journal for Equity in Health BioMed Central Research Malaria control in Malawi: are the poor being served? Don P Mathanga 1,2 and Cameron Bowie* 1 Open Access Address: 1 Department of Community
International Journal for Equity in Health BioMed Central Research Malaria control in Malawi: are the poor being served? Don P Mathanga 1,2 and Cameron Bowie* 1 Open Access Address: 1 Department of Community
Executive summary... 1 Background... 3 EPI Serology Study Design... 5 Results Pooled Analysis... 12
 WHO Advisory Committee on serological responses to Expanded Programme on Immunization vaccines in infants receiving Intermittent Preventive Treatment for malaria (IPTi) FINAL REPORT October 8, 2009 Contents
WHO Advisory Committee on serological responses to Expanded Programme on Immunization vaccines in infants receiving Intermittent Preventive Treatment for malaria (IPTi) FINAL REPORT October 8, 2009 Contents
Seasonal Malaria Chemoprevention
 Seasonal Malaria Chemoprevention with sulfadoxine pyrimethamine plus amodiaquine in children a field guide Seasonal Malaria Chemoprevention with sulfadoxine pyrimethamine plus amodiaquine in children
Seasonal Malaria Chemoprevention with sulfadoxine pyrimethamine plus amodiaquine in children a field guide Seasonal Malaria Chemoprevention with sulfadoxine pyrimethamine plus amodiaquine in children
Ending Malaria in Nigeria: The WHO Agenda
 Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Downloaded from:
 Sokhna, C; Cisse, B; B, elh; Milligan, P; Hallett, R; Sutherland, C; Gaye, O; Boulanger, D; Simondon, K; Simondon, F; Targett, G; Lines, J; Greenwood, B; Trape, JF (2008) A trial of the efficacy, safety
Sokhna, C; Cisse, B; B, elh; Milligan, P; Hallett, R; Sutherland, C; Gaye, O; Boulanger, D; Simondon, K; Simondon, F; Targett, G; Lines, J; Greenwood, B; Trape, JF (2008) A trial of the efficacy, safety
Downloaded from:
 Dicko, A; Barry, A; Dicko, M; Diallo, AI; Tembine, I; Dicko, Y; Dara, N; Sidibe, Y; Santara, G; Conar, T; Chandramohan, D; Cousens, S; Milligan, PJ; Diallo, DA; Doumbo, OK; Greenwood, B (2011) Malaria
Dicko, A; Barry, A; Dicko, M; Diallo, AI; Tembine, I; Dicko, Y; Dara, N; Sidibe, Y; Santara, G; Conar, T; Chandramohan, D; Cousens, S; Milligan, PJ; Diallo, DA; Doumbo, OK; Greenwood, B (2011) Malaria
Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1
 Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1 Part I: Evaluation of the current list Proposed grouping from the March 2007 meeting 6.5.3 Antimalarial medicines 6.5.3.1 For curative
Antimalarials in the WHO Essential Drugs List for Children Reviewer No.1 Part I: Evaluation of the current list Proposed grouping from the March 2007 meeting 6.5.3 Antimalarial medicines 6.5.3.1 For curative
REPORT OF THE TECHNICAL CONSULTATION BACKGROUND
 Defining and validating a measure of parasite resistance to sulfadoxinepyrimethamine (SP) that would be indicative of the protective efficacy of SP for intermittent preventive treatment in infancy (SP-IPTi)
Defining and validating a measure of parasite resistance to sulfadoxinepyrimethamine (SP) that would be indicative of the protective efficacy of SP for intermittent preventive treatment in infancy (SP-IPTi)
Implementing the Abuja Declaration and Plan of Action: the journey so far
 Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa
 DOI 1.1186/s12936-15-839-4 RESEARCH Open Access Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa Matthew E Cairns
DOI 1.1186/s12936-15-839-4 RESEARCH Open Access Seasonality in malaria transmission: implications for case management with long acting artemisinin combination therapy in sub Saharan Africa Matthew E Cairns
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018
 Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Malaria Control in Togo
 Malaria Control in Togo Introduction * Dr Kodjo Morgah In Collaboration with Dr Koubagnine Takpa (Director, EPI) Dr Jérome Agbekou (DPC/WHO/Togo) Dr Stephan Tohon (ICP/MAL/WA) Dr Jean Pierre E. Batchassi
Malaria Control in Togo Introduction * Dr Kodjo Morgah In Collaboration with Dr Koubagnine Takpa (Director, EPI) Dr Jérome Agbekou (DPC/WHO/Togo) Dr Stephan Tohon (ICP/MAL/WA) Dr Jean Pierre E. Batchassi
abstract n engl j med 374;26 nejm.org June 30,
 The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
The new england journal of medicine established in 1812 June 30, 2016 vol. 374 no. 26 Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children Ally Olotu, Ph.D., Gregory Fegan, Ph.D.,
public facility in the same area context of AMFm
 A CASE CONTROL STUDY OF FACTORS INFLUENCING CARE SEEKING FOR MALARIA IN CHEMICAL SHOPS INVOLVED IN THE DANGME WEST CLUSTER RANDOMIZED TRIAL OFVRAPID DIAGNOSTIC TESTS FOR MALARIA (Dangme CommRDT Study)
A CASE CONTROL STUDY OF FACTORS INFLUENCING CARE SEEKING FOR MALARIA IN CHEMICAL SHOPS INVOLVED IN THE DANGME WEST CLUSTER RANDOMIZED TRIAL OFVRAPID DIAGNOSTIC TESTS FOR MALARIA (Dangme CommRDT Study)
38 Current Concepts in
 38 Current Concepts in Management of Falciparum Malaria Abstract: Artemisinin based Combination Therapy (ACT) is the preferred agent to treat drug resistance uncomplicated Plasmodium Falciparum (PF) Malaria.
38 Current Concepts in Management of Falciparum Malaria Abstract: Artemisinin based Combination Therapy (ACT) is the preferred agent to treat drug resistance uncomplicated Plasmodium Falciparum (PF) Malaria.
t r a i n i n g m a n u a l
 training manual Acknowledgements The SMC toolkit was produced by Medicines for Malaria Venture (MMV). MMV gratefully acknowledges the following partners who contributed to the technical content and development
training manual Acknowledgements The SMC toolkit was produced by Medicines for Malaria Venture (MMV). MMV gratefully acknowledges the following partners who contributed to the technical content and development
Risk of malaria in Pregnancy and under-five (5) children in densely populated communities in Kumasi, Ghana
 P P 1 2 International Journal of Scientific Engineering and Applied Science (IJSEAS) Volume-2, Issue-9, September 2016 Risk of malaria in Pregnancy and under-five (5) children in densely populated communities
P P 1 2 International Journal of Scientific Engineering and Applied Science (IJSEAS) Volume-2, Issue-9, September 2016 Risk of malaria in Pregnancy and under-five (5) children in densely populated communities
WG: Vaccinations and child survival Focus:
 WG: Vaccinations and child survival Focus: Monitoring childhood interventions including routine services and campaigns => To find possible changes in policy Why is that necessary? Paradox: All interventions
WG: Vaccinations and child survival Focus: Monitoring childhood interventions including routine services and campaigns => To find possible changes in policy Why is that necessary? Paradox: All interventions
Fighting Harder and Smarter Against Malaria. Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010
 Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
Gametocytaemia after Drug Treatment of Asymptomatic Plasmodium falciparum
 Gametocytaemia after Drug Treatment of Asymptomatic Plasmodium falciparum Samuel Dunyo 1, Paul Milligan 2*, Tansy Edwards 2, Colin Sutherland 2, Geoffrey Targett 2, Margaret Pinder 1 1 Medical Research
Gametocytaemia after Drug Treatment of Asymptomatic Plasmodium falciparum Samuel Dunyo 1, Paul Milligan 2*, Tansy Edwards 2, Colin Sutherland 2, Geoffrey Targett 2, Margaret Pinder 1 1 Medical Research
Protocol Synopsis. Administrative information
 Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Outcome of Presumptive Ukwaja K. et al 179
 Outcome of Presumptive Ukwaja K. et al 179 ORIGINAL ARTICLE OUTCOME OF PRESUMPTIVE VERSUS RAPID DIAGNOSTIC TESTS-BASED MANAGEMENT OF CHILDHOOD MALARIA PNEUMONIA OVERLAP IN URBAN NIGERIA: A PILOT QUASI-
Outcome of Presumptive Ukwaja K. et al 179 ORIGINAL ARTICLE OUTCOME OF PRESUMPTIVE VERSUS RAPID DIAGNOSTIC TESTS-BASED MANAGEMENT OF CHILDHOOD MALARIA PNEUMONIA OVERLAP IN URBAN NIGERIA: A PILOT QUASI-
Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis
 Comfort et al. Malaria Journal 2014, 13:383 RESEARCH Open Access Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis Alison B Comfort
Comfort et al. Malaria Journal 2014, 13:383 RESEARCH Open Access Association between malaria control and paediatric blood transfusions in rural Zambia: an interrupted time-series analysis Alison B Comfort
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations
 WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
THE EFFECTS OF HAEMOGLOBINOPATHIES AND G6PD DEFICIENCY ON MALARIA AMONG CHILDREN OF THE KINTAMPO NORTH MUNICIPALITY OF GHANA
 THE EFFECTS OF HAEMOGLOBINOPATHIES AND G6PD DEFICIENCY ON MALARIA AMONG CHILDREN OF THE KINTAMPO NORTH MUNICIPALITY OF GHANA Presenter: Amoako Nicholas 1 Supervisors: Dr. Kwaku Poku Asante 1, Dr. Bimi
THE EFFECTS OF HAEMOGLOBINOPATHIES AND G6PD DEFICIENCY ON MALARIA AMONG CHILDREN OF THE KINTAMPO NORTH MUNICIPALITY OF GHANA Presenter: Amoako Nicholas 1 Supervisors: Dr. Kwaku Poku Asante 1, Dr. Bimi
Statistical Analysis Plan (SAP)
 Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Malaria DR. AFNAN YOUNIS
 Malaria DR. AFNAN YOUNIS Objectives: Epidemiology of malaria Clinical picture Mode of transmission Risk factors Prevention and control Malaria is a life-threatening disease caused by Plasmodium parasites
Malaria DR. AFNAN YOUNIS Objectives: Epidemiology of malaria Clinical picture Mode of transmission Risk factors Prevention and control Malaria is a life-threatening disease caused by Plasmodium parasites
Cost Effectiveness Analysis: Malaria Vector Control In Kenya
 THE BUDGET FOCUS A Publication of the IEA Budget Information Programme Issue No. 28 November 2011 Cost Effectiveness Analysis: Malaria Vector Control In Kenya Malaria in Kenya is a major epidemic and is
THE BUDGET FOCUS A Publication of the IEA Budget Information Programme Issue No. 28 November 2011 Cost Effectiveness Analysis: Malaria Vector Control In Kenya Malaria in Kenya is a major epidemic and is
Malaria preventive measures, health care seeking behaviour and malaria burden in different epidemiological settings in Sudan
 Tropical Medicine and International Health doi:10.1111/j.1365-3156.2009.02394.x volume 14 no 12 pp 1488 1495 december 2009 Malaria preventive measures, health care seeking behaviour and malaria burden
Tropical Medicine and International Health doi:10.1111/j.1365-3156.2009.02394.x volume 14 no 12 pp 1488 1495 december 2009 Malaria preventive measures, health care seeking behaviour and malaria burden
DEATH AUDIT OF DEATHS DUE TO MALARIA AT LG HOSPITAL, AHMEDABAD (GUJARAT) DURING THE YEAR 2011
 ORIGINAL ARTICLE DEATH AUDIT OF DEATHS DUE TO MALARIA AT LG HOSPITAL, AHMEDABAD (GUJARAT) DURING THE YEAR 2011 Vyas Sheetal 1, Solanki Anand 2, Joshi Urvish 2, Nayak Himanshu 2 1 Professor and Head, 2
ORIGINAL ARTICLE DEATH AUDIT OF DEATHS DUE TO MALARIA AT LG HOSPITAL, AHMEDABAD (GUJARAT) DURING THE YEAR 2011 Vyas Sheetal 1, Solanki Anand 2, Joshi Urvish 2, Nayak Himanshu 2 1 Professor and Head, 2
In several African countries in sub-saharan Africa, malaria is the leading cause of death in children under five.
 TECHNICAL SEMINAR - MALARIA SLIDE 1 Technical Seminar - Malaria Malaria is an extremely important cause of mortality in different parts of world. In this technical seminar, I ll discuss the rationale for
TECHNICAL SEMINAR - MALARIA SLIDE 1 Technical Seminar - Malaria Malaria is an extremely important cause of mortality in different parts of world. In this technical seminar, I ll discuss the rationale for
Downloaded from:
 Konat, AT; Yaro, JB; Oudraogo, AZ; Diarra, A; Gansan, A; Soulama, I; Kangoy, DT; Kabor, Y; Oudraogo, E; Oudraogo, A; Tiono, AB; Oudraogo, IN; Chandramohan, D; Cousens, S; Milligan, PJ; Sirima, SB; Greenwood,
Konat, AT; Yaro, JB; Oudraogo, AZ; Diarra, A; Gansan, A; Soulama, I; Kangoy, DT; Kabor, Y; Oudraogo, E; Oudraogo, A; Tiono, AB; Oudraogo, IN; Chandramohan, D; Cousens, S; Milligan, PJ; Sirima, SB; Greenwood,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
 Malaria Policy Advisory Committee Meeting 5 7 March 2015, Geneva, Switzerland Background document for Session 4 Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
Malaria Policy Advisory Committee Meeting 5 7 March 2015, Geneva, Switzerland Background document for Session 4 Manuscripts for the WHO Evidence Review Group for malaria in pregnancy (MiP-ERG), July 2015
Effect of Adding Azithromycin to Seasonal Malaria Chemoprevention
 Original Article Effect of Adding Azithromycin to Seasonal Malaria D. Chandramohan, A. Dicko, I. Zongo, I. Sagara, M. Cairns, I. Kuepfer, M. Diarra, A. Barry, A. Tapily, F. Nikiema, S. Yerbanga, S. Coumare,
Original Article Effect of Adding Azithromycin to Seasonal Malaria D. Chandramohan, A. Dicko, I. Zongo, I. Sagara, M. Cairns, I. Kuepfer, M. Diarra, A. Barry, A. Tapily, F. Nikiema, S. Yerbanga, S. Coumare,
Analysis of partial and complete protection in malaria cohort studies
 Cairns et al. Malaria Journal 2013, 12:355 RESEARCH Analysis of partial and complete protection in malaria cohort studies Open Access Matthew E Cairns 1*, Kwaku Poku Asante 2, Seth Owusu-Agyei 2, Daniel
Cairns et al. Malaria Journal 2013, 12:355 RESEARCH Analysis of partial and complete protection in malaria cohort studies Open Access Matthew E Cairns 1*, Kwaku Poku Asante 2, Seth Owusu-Agyei 2, Daniel
Tanzania s Progress in Combating Malaria: Achievement and Challenges
 Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
Downloaded from:
 Hill, N; Lenglet, A; Arnz, AM; Carneiro, I (2007) Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised
Hill, N; Lenglet, A; Arnz, AM; Carneiro, I (2007) Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised
Clinical Analysis Report For Study Sample Study
 Clinical Analysis Report For Study Sample Study Automated report generated by WWARN May 9, 2014 Falciparum class org.wwarn.csr.clinicalstudyreport Version 1.020140508-1558 1 Contents 1 Executive Summary
Clinical Analysis Report For Study Sample Study Automated report generated by WWARN May 9, 2014 Falciparum class org.wwarn.csr.clinicalstudyreport Version 1.020140508-1558 1 Contents 1 Executive Summary
in control group 7, , , ,
 Q1 Rotavirus is a major cause of severe gastroenteritis among young children. Each year, rotavirus causes >500,000 deaths worldwide among infants and very young children, with 90% of these deaths occurring
Q1 Rotavirus is a major cause of severe gastroenteritis among young children. Each year, rotavirus causes >500,000 deaths worldwide among infants and very young children, with 90% of these deaths occurring
PROGRESS REPORT ON CHILD SURVIVAL: A STRATEGY FOR THE AFRICAN REGION. Information Document CONTENTS
 29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
Summary World Malaria Report 2010
 Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Diagnostic accuracy of physician review, expert algorithms and data-derived algorithms in adult verbal autopsies
 International Epidemiological Association 1999 Printed in Great Britain International Journal of Epidemiology 1999;28:1081 1087 Diagnostic accuracy of physician review, expert algorithms and data-derived
International Epidemiological Association 1999 Printed in Great Britain International Journal of Epidemiology 1999;28:1081 1087 Diagnostic accuracy of physician review, expert algorithms and data-derived
MALARIA A MAJOR CAUSE OF CHILD DEATH AND POVERTY IN AFRICA
 MALARIA A MAJOR CAUSE OF CHILD DEATH AND POVERTY IN AFRICA CONTROLLING THE MALARIA BURDEN IN AFRICA KEY ACTIONS FOR UNICEF Strengthen UNICEF input to evidence-based antenatal services Forge partnership
MALARIA A MAJOR CAUSE OF CHILD DEATH AND POVERTY IN AFRICA CONTROLLING THE MALARIA BURDEN IN AFRICA KEY ACTIONS FOR UNICEF Strengthen UNICEF input to evidence-based antenatal services Forge partnership
Women by area: Urban F Women by area: Rural F
 Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
Malaria Burden Estimation Evidence Review Group (MBE-ERG)
 Malaria Burden Estimation Evidence Review Group (MBE-ERG) Peter Smith - Chair MBE-ERG (with thanks to Kathryn Andrews for drafting slides) MPAC meeting 13 th March, 2013 MBE-ERG: Terms of Reference To
Malaria Burden Estimation Evidence Review Group (MBE-ERG) Peter Smith - Chair MBE-ERG (with thanks to Kathryn Andrews for drafting slides) MPAC meeting 13 th March, 2013 MBE-ERG: Terms of Reference To
Invest in the future, defeat malaria
 Invest in the future, defeat malaria Malaria is caused by parasites from the genus Plasmodium, which are spread to people by infected mosquitoes. There are five species of Plasmodium that can infect humans.
Invest in the future, defeat malaria Malaria is caused by parasites from the genus Plasmodium, which are spread to people by infected mosquitoes. There are five species of Plasmodium that can infect humans.
Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania
 Tanzania Health Research Bulletin (2004), Vol. 6, No. 2 37 Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania L.E.G. MBOERA 1, M.L. KAMUGISHA 2, V. BARONGO
Tanzania Health Research Bulletin (2004), Vol. 6, No. 2 37 Community knowledge, perceptions and practices on malaria in Mpwapwa District, central Tanzania L.E.G. MBOERA 1, M.L. KAMUGISHA 2, V. BARONGO
Against intervention No recommendation Strong Conditional Conditional Strong. For intervention. High Moderate Low Very low
 Draft recommendation: Consider using MDA as an additional tool for the elimination of malaria in low prevalence island or nonisland settings where the risk of imported malaria is low Balance of desirable
Draft recommendation: Consider using MDA as an additional tool for the elimination of malaria in low prevalence island or nonisland settings where the risk of imported malaria is low Balance of desirable
RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation:
 RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation: Results from a phase 3 randomized controlled trial in Lilongwe, Malawi Larry Han Senior Honors Thesis Department of
RTS,S/AS01 malaria vaccine efficacy and its interaction with seasonal precipitation: Results from a phase 3 randomized controlled trial in Lilongwe, Malawi Larry Han Senior Honors Thesis Department of
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Fernandes S, Sicuri E, Kayentao K, et al. Cost-effectiveness
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Fernandes S, Sicuri E, Kayentao K, et al. Cost-effectiveness
The use of non-prescribed anti-malarial drugs for the treatment of malaria in the Bolgatanga municipality, northern Ghana
 Malaria Journal This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. The use of non-prescribed
Malaria Journal This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. The use of non-prescribed
Overview of Malaria Epidemiology in Ethiopia
 Overview of Malaria Epidemiology in Ethiopia Wakgari Deressa, PhD School of Public Health Addis Ababa University Symposium on Neuro-infectious Disease United Nations Conference Center, AA February 28,
Overview of Malaria Epidemiology in Ethiopia Wakgari Deressa, PhD School of Public Health Addis Ababa University Symposium on Neuro-infectious Disease United Nations Conference Center, AA February 28,
Mass drug Administration When Is It Useful
 Mass drug Administration When Is It Useful Kwaku Poku Asante, MD MPH PhD Kintampo Health Research Centre, Ghana Malaria Control and Elimination 8-9 December 2016, Congress Center Basel, Switzerland MDA
Mass drug Administration When Is It Useful Kwaku Poku Asante, MD MPH PhD Kintampo Health Research Centre, Ghana Malaria Control and Elimination 8-9 December 2016, Congress Center Basel, Switzerland MDA
INTERMITTENT PREVENTIVE TREATMENT OF MALARIA IN PREGNANCY WITH SULPHADOXINE/PYRIMETHAMINE
 INTERMITTENT PREVENTIVE TREATMENT OF MALARIA IN PREGNANCY WITH SULPHADOXINE/PYRIMETHAMINE WHO EVIDENCE REVIEW GROUP WHO, Geneva, July 9 th 11 th 2012 MPAC MEETING September 11 th - 13 th 2012 CURRENT WHO
INTERMITTENT PREVENTIVE TREATMENT OF MALARIA IN PREGNANCY WITH SULPHADOXINE/PYRIMETHAMINE WHO EVIDENCE REVIEW GROUP WHO, Geneva, July 9 th 11 th 2012 MPAC MEETING September 11 th - 13 th 2012 CURRENT WHO
Maternal, Child and Reproductive Health Initiative
 Maternal, Child and Reproductive Health Initiative Maternal, Child and Reproductive Health Initiative The Maternal, Child and Reproductive Health (MCRH) Initiative works in developing countries to improve
Maternal, Child and Reproductive Health Initiative Maternal, Child and Reproductive Health Initiative The Maternal, Child and Reproductive Health (MCRH) Initiative works in developing countries to improve
Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
 Tropical Medicine and International Health doi:10.1111/j.1365-3156.2007.01977.x volume 13 no 1 pp 83 90 january 2008 Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
Tropical Medicine and International Health doi:10.1111/j.1365-3156.2007.01977.x volume 13 no 1 pp 83 90 january 2008 Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment
Interpretation of the World Malaria Report Country Profile
 Interpretation of the World Malaria Report Country Profile Acknowledgements This presentation was developed to help explain the components of the World Malaria Report Country Profile. The 2017 World Malaria
Interpretation of the World Malaria Report Country Profile Acknowledgements This presentation was developed to help explain the components of the World Malaria Report Country Profile. The 2017 World Malaria
Prompt and Effective Treatment of Malaria through Integrated Services. Dr G.N Ntadom Case Management Branch, NMEP
 Prompt and Effective Treatment of Malaria through Integrated Services Dr G.N Ntadom Case Management Branch, NMEP Case Management Branch of the NMEP Introduction Case Management Branch under the NMEP is
Prompt and Effective Treatment of Malaria through Integrated Services Dr G.N Ntadom Case Management Branch, NMEP Case Management Branch of the NMEP Introduction Case Management Branch under the NMEP is
Monitoring and Evaluation Reference Group (MERG) GUIDANCE NOTE
 BACKGROUND Monitoring and Evaluation Reference Group (MERG) GUIDANCE NOTE Assessing the Impact of Malaria Control Activities on Mortality among African Children Under 5 Years of Age The Roll Back Malaria
BACKGROUND Monitoring and Evaluation Reference Group (MERG) GUIDANCE NOTE Assessing the Impact of Malaria Control Activities on Mortality among African Children Under 5 Years of Age The Roll Back Malaria
Key Messages for World Malaria Day 2009
 INFORMATION RBM/WG/2009/INF.12 10 APR 2009 Draft document General distribution English Only Key Messages for World Malaria Day 2009 Counting Malaria Out to Reaching the 2010 Targets On the occasion of
INFORMATION RBM/WG/2009/INF.12 10 APR 2009 Draft document General distribution English Only Key Messages for World Malaria Day 2009 Counting Malaria Out to Reaching the 2010 Targets On the occasion of
Europe PMC Funders Group Author Manuscript Trop Med Int Health. Author manuscript; available in PMC 2012 December 13.
 Europe PMC Funders Group Author Manuscript Published in final edited form as: Trop Med Int Health. 2005 June ; 10(6): 530 536. doi:10.1111/j.1365-3156.2005.01439.x. Clinical algorithms for malaria diagnosis
Europe PMC Funders Group Author Manuscript Published in final edited form as: Trop Med Int Health. 2005 June ; 10(6): 530 536. doi:10.1111/j.1365-3156.2005.01439.x. Clinical algorithms for malaria diagnosis
Estimating the potential public health impact of seasonal malaria chemoprevention in African children
 Received 16 Feb 212 Accepted 3 Apr 212 Published 6 Jun 212 DOI: 1.138/ncomms1879 Estimating the potential public health impact of seasonal malaria chemoprevention in African children Matthew Cairns 1,
Received 16 Feb 212 Accepted 3 Apr 212 Published 6 Jun 212 DOI: 1.138/ncomms1879 Estimating the potential public health impact of seasonal malaria chemoprevention in African children Matthew Cairns 1,
Profile: The Mbita Health and Demographic Surveillance System.
 Profile: The Mbita Health and Demographic Surveillance System. Figure 1. Map of Mbita HDSS showing the 4 locations. Brief Introduction The Mbita Health and Demographic Surveillance System (Mbita HDSS)
Profile: The Mbita Health and Demographic Surveillance System. Figure 1. Map of Mbita HDSS showing the 4 locations. Brief Introduction The Mbita Health and Demographic Surveillance System (Mbita HDSS)
Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group
 Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Charles Newton Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group Specific Aims prevalence of active convulsive
Studies of Epilepsy Epidemiology in Demographic Sites (SEEDS) Charles Newton Kenya Medical Research Institute/WellcomeTrust Kilifi, Kenya INDEPTH SEEDS Group Specific Aims prevalence of active convulsive
Issue 8: January December 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
Epidemiology and control profile of malaria in. Sierra Leone 2017 Supplement
 Epidemiology and control profile of malaria in Sierra Leone 2017 Supplement About this supplement In 2015, the Sierra Leone National Malaria Control Programme released a comprehensive malaria control profile
Epidemiology and control profile of malaria in Sierra Leone 2017 Supplement About this supplement In 2015, the Sierra Leone National Malaria Control Programme released a comprehensive malaria control profile
Gradual acquisition of immunity to severe malaria with increasing
 Gradual acquisition of immunity to severe malaria with increasing exposure Jamie T Griffin 1*, T Déirdre Hollingsworth 2,3,4*, Hugh Reyburn 5, Chris J Drakeley 5, Eleanor M Riley 5, Azra C Ghani 1 1 MRC
Gradual acquisition of immunity to severe malaria with increasing exposure Jamie T Griffin 1*, T Déirdre Hollingsworth 2,3,4*, Hugh Reyburn 5, Chris J Drakeley 5, Eleanor M Riley 5, Azra C Ghani 1 1 MRC
Thesis: Community-based studies on the epidemiology, treatment and prevention of malaria in an area of intense malaria transmission in western Kenya
 Summary and discussion Thesis: Community-based studies on the epidemiology, treatment and prevention of malaria in an area of intense malaria transmission in western Kenya Anja Terlouw 16 October 2003
Summary and discussion Thesis: Community-based studies on the epidemiology, treatment and prevention of malaria in an area of intense malaria transmission in western Kenya Anja Terlouw 16 October 2003
Malaria: A Global Perspective and Prospects for Elimination. Rima Shretta
 Malaria: A Global Perspective and Prospects for Elimination Rima Shretta Background Spread by the female anopheles mosquito; caused by plasmodium parasite: P. falciparum, P. vivax, P. ovale, P. malariae,
Malaria: A Global Perspective and Prospects for Elimination Rima Shretta Background Spread by the female anopheles mosquito; caused by plasmodium parasite: P. falciparum, P. vivax, P. ovale, P. malariae,
A Simple Dose Regimen of Artesunate and Amodiaquine Based on Arm Span- or Age Range for Childhood Falciparum Malaria: A Preliminary Evaluation
 JOURNAL OF TROPICAL PEDIATRICS, VOL. 58, NO. 4, 2012 A Simple Dose Regimen of Artesunate and Amodiaquine Based on Arm Span- or Age Range for Childhood Falciparum Malaria: A Preliminary Evaluation by Akintunde
JOURNAL OF TROPICAL PEDIATRICS, VOL. 58, NO. 4, 2012 A Simple Dose Regimen of Artesunate and Amodiaquine Based on Arm Span- or Age Range for Childhood Falciparum Malaria: A Preliminary Evaluation by Akintunde
Managing malaria. Scenarios. Scenario 1: Border provinces, Cambodia. Key facts. Your target region: Cambodia /Thailand border
 Managing malaria Scenario 1: Border provinces, Cambodia You have been made responsible for three border provinces in Cambodia; Koh Kong Province, Perah Vihear Province and Sampovloun operational district.
Managing malaria Scenario 1: Border provinces, Cambodia You have been made responsible for three border provinces in Cambodia; Koh Kong Province, Perah Vihear Province and Sampovloun operational district.
Cerebral malaria in children
 Cerebral malaria in children M. Chiara Stefanini Catholic University - Rome Malaria: epidemiology Global distribution of malaria transmission risk,, 2003 World malaria report, WHO, 2005 Estimated incidence
Cerebral malaria in children M. Chiara Stefanini Catholic University - Rome Malaria: epidemiology Global distribution of malaria transmission risk,, 2003 World malaria report, WHO, 2005 Estimated incidence
Symptoms of Malaria. Young children, pregnant women, immunosuppressed and elderly travellers are particularly at risk of severe malaria.
 Preventing Malaria 1 Malaria is the world s most prevalent parasitic disease, accounting for an estimated 216 million cases with 655,000 deaths annually. Many people acquire malaria during travel to tropical
Preventing Malaria 1 Malaria is the world s most prevalent parasitic disease, accounting for an estimated 216 million cases with 655,000 deaths annually. Many people acquire malaria during travel to tropical
Malaria. Edwin J. Asturias, MD
 Malaria Edwin J. Asturias, MD Associate Professor of Pediatrics and Epidemiology Director for Latin America Center for Global Health, Colorado School of Public Health Global Health and Disasters Course
Malaria Edwin J. Asturias, MD Associate Professor of Pediatrics and Epidemiology Director for Latin America Center for Global Health, Colorado School of Public Health Global Health and Disasters Course
World Health Organization Global Fund concept note development WHO POLICY BRIEF
 World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization,
World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization,
Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC.
 Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC October 11-15, 2010 Pre-Course Test 1. You are preparing for an elective
Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC October 11-15, 2010 Pre-Course Test 1. You are preparing for an elective
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
Sponsor / Company: Sanofi Drug substance(s): artesunate plus amodiaquine Title of the study:
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
RESEARCH. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial
 Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial Peter Aaby, professor, 1,2 Cesário L Martins, clinician, 1 May-Lill Garly,
Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial Peter Aaby, professor, 1,2 Cesário L Martins, clinician, 1 May-Lill Garly,
Evaluation of Malaria Surveillance System in Hooghly district of West Bengal - India
 Original Research Article Evaluation of Malaria Surveillance System in Hooghly district of West Bengal - India Dan Amitabha 1,*, Kunal Kanti De 2, Pasi A R 3, Jalaluddeen M 4, Roy Bibhash 5 1 Airport Health
Original Research Article Evaluation of Malaria Surveillance System in Hooghly district of West Bengal - India Dan Amitabha 1,*, Kunal Kanti De 2, Pasi A R 3, Jalaluddeen M 4, Roy Bibhash 5 1 Airport Health
Malaria Endemicity Among Pregnant Women Attending Antenatal Clinic in the University of Calabar Teaching Hospital, Calabar, Nigeria.
 Malaria Endemicity Among Pregnant Women Attending Antenatal Clinic in the University of Calabar Teaching Hospital, Calabar, Nigeria. B. E. MONJOL 1* and M. F. USEH 2 1 Microbiology Department, University
Malaria Endemicity Among Pregnant Women Attending Antenatal Clinic in the University of Calabar Teaching Hospital, Calabar, Nigeria. B. E. MONJOL 1* and M. F. USEH 2 1 Microbiology Department, University
