OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices
|
|
|
- George Garrett
- 5 years ago
- Views:
Transcription
1 OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices Ontario Health Technology Advisory Committee January 2012
2 Issue Background The Ontario Health Technology Advisory Committee (OHTAC) met on January 28, 2011 to review the effectiveness, safety, and cost-effectiveness of Internet-based device-assisted remote monitoring systems (RMSs) for therapeutic cardiac implantable electronic devices (CIEDs) based on an evidence-based review by the Medical Advisory Secretariat (MAS). Clinical Indication Sudden cardiac death (SCD) is a major cause of fatalities in developed countries. In Canada each year more than 40,000 people die from a cardiovascular-related cause; approximately half of these deaths are attributable to SCD. Most cases of SCD occur in the general population, typically in those without a known history of heart disease. Most SCDs are caused by cardiac arrhythmia, an abnormal heart rhythm caused by malfunctions of the heart s electrical system. Up to half of patients with significant heart failure (HF) also have advanced conduction or electrical abnormalities. Cardiac arrhythmias are managed by a variety of drugs, ablative procedures, and therapeutic CIEDs. The range of CIEDs includes pacemakers (PMs), implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices. Bradycardia (slow heartbeat) is the main indication for PMs and increased risk for SCD is an indication for ICDs. Heart failure is also a significant health problem and is the most frequent cause of hospitalization in those over 65 years of age. Patients with moderate to severe HF may also have cardiac arrhythmias, although these may be more likely to be related to heart pump or hemodynamic failure. The presence of HF, however, increases the risk of SCD five-fold, regardless of aetiology. Patients with HF who remain highly symptomatic despite optimal drug therapy are sometimes also treated with CRT devices. With an increasing prevalence of age-related conditions such as chronic HF and the expanding indications for ICD therapy, the rate of ICD placement has been dramatically increasing. The appropriate indications for ICD placement, as well as the rate of ICD placement, are increasingly an issue. In addition to the increased ICD placement and the up-front device costs, there is the need for life-long follow-up or surveillance, placing a significant burden on patients and device clinics. In 2007, over 1.6 million CIEDs were implanted in Europe and the United States; that translates to over 5.5 million patient encounters per year if the recommended follow-up practices are considered. A safe and effective RMS could potentially improve the efficiency of long-term follow-up of patients and their CIEDs. The Technology In addition to being therapeutic devices, CIEDs have extensive diagnostic abilities. All CIEDs can be interrogated and reprogrammed during an in-clinic visit using an inductive programming wand. Remote monitoring (RM) would allow patients to transmit information from their CIEDs from the comfort of their own homes. Currently most ICD devices also have the potential to be remotely monitored. Remote monitoring can be used to check system integrity, to alert on arrhythmic episodes, and to potentially replace in-clinic follow-ups and manage disease remotely. Cardiac implantable electronic devices do not currently have the capability of being reprogrammed remotely, although this feature is being tested in pilot settings. Internet-based device-assisted RMSs for CIEDs are intended to function as surveillance systems rather than emergency systems. Currently there are 2 general types of RMSs: those that transmit device diagnostic information automatically and without patient assistance to secure Internet-based registry 2
3 systems, and those that require patient assistance to transmit information. Both systems employ the use of preprogrammed alerts transmitted either automatically or at regular scheduled intervals to patients and/or physicians. All Internet-based device-assisted RMSs have common components. The implanted device is equipped with a microantenna that communicates with a small external device (at bedside or wearable) commonly known as the transmitter. Transmitters are able to interrogate programmed parameters and diagnostic data stored in the patients implant devices. The information transfer to the communicator can occur at preset time intervals, with or without the participation of the patient. The encrypted data are then uploaded to a web-based database registry system on a secure central server. The data processing facilities at the central database, depending on the clinical urgency, can trigger an audible alert for patients or an alert for the physician(s) that can be sent via , fax, text message, or phone. The details are also posted on the secure website for viewing by the physician (or their delegate) at their convenience. Regulatory Status There are currently 4 manufacturers of RMSs in Canada Medtronic Inc., Biotronik, Boston Scientific Corp., and St Jude Medical Inc. that have regulatory approval for remote transmitting ICD devices. Remote monitoring systems are proprietary to the manufacturer of the implant device. Remote monitoring systems for one manufacturer s device will not work with another manufacturer s device and RMSs may not be available for all versions of the manufacturers devices. Further customization of Internet-based device-assisted RMSs also includes details such as the website and application, multiplatform sensors, software algorithms, programming information, and types and methods of alerting patients and/or physicians. The addition of peripherals for monitoring weight and pressure or to communicate with patients through the onsite communicators also varies by manufacturer. Evidence The MAS evidence review was performed to review available evidence on Internet-based device-assisted RMSs for CIEDs published until September The search identified 6 systematic reviews, 7 randomized controlled trials, and 19 reports for 16 cohort studies. The evidence is summarized in the 3 sections below. 1. Effectiveness of Remote Monitoring Systems for CIEDs for Cardiac Arrhythmia and Device Functioning In total, 15 reports on 13 cohort studies involving investigations with 4 different RMSs for CIEDs in cardiology implant clinics were identified in the review. The 4 RMSs were: Care Link Network (Medtronic Inc, Minneapolis, MN, USA); Home Monitoring (Biotronic, Berlin, Germany); House Call 11 (St. Jude Medical Inc., St Pauls, MN, USA); and a manufacturer-independent RMS. Eight of the 15 reports were with the Home Monitoring RMS (12,949 patients), 3 were with the Care Link Network RMS (167 patients), 1 was with the House Call 11 RMS (124 patients), and 1 was with a manufacturer-independent RMS (44 patients). All of the studies, except for 2 in the United States (1 with Home Monitoring and 1 with House Call 11 ), were performed in European countries. The RMSs in the studies were evaluated with different cardiac implant device populations and included ICDs only (6 studies), ICD and CRT devices (3 studies), PM and ICD and CRT devices (4 studies), and PMs only (2 studies). The patient populations were predominately male (range, 52% 87%) in all studies, 3
4 with mean ages ranging from 58 to 76 years. One study population was unique in that RMSs were evaluated for ICDs implanted solely for primary prevention in young patients (mean age, 44 years) with Brugada syndrome, which carries an inherited increased genetic risk for sudden heart attack in young adults. Most of the cohort studies reported on the feasibility of RMSs in clinical settings with limited follow-up. In the short follow-up periods of the studies, the majority of the events reported in follow-up were related to the detection of medical events rather than system configuration or device abnormalities. The results of the studies are summarized below: Interrogation of devices on the web platform, both for continuous and scheduled transmissions, was significantly quicker with remote follow-up, both for nurses and physicians. Two studies examined the role of RMSs in following ICD leads under regulatory advisory in a European clinical setting and noted: There were fewer inappropriate shocks in the RM group. Urgent in-office interrogations and surgical revisions were performed within 12 days of remote alerts. Lead fractures were not detected at in-office follow-up; all were detected at remote followup. Quality of life for patients was generally not reported. Patient satisfaction was evaluated in 5 cohort studies, all in short-term follow-up: 1 for the Home Monitoring RMS, 3 for the Care Link RMS, and 1 for the House Call 11 RMS. Patients reported a sense of security from the transmitter, a good relationship with nurses and physicians, positive implications for their health, and satisfaction with RM and organization of services. Although patients reported that the system was easy to implement, many patients reported the need for assistance of a caregiver for their transmission. The majority of patients would recommend RM to other ICD patients. Patients with hearing or other physical or mental conditions hindering the use of the system were generally excluded from studies, but the frequency of this was not reported. Physician satisfaction was evaluated in 3 studies, all with the Care Link RMS: Physicians reported an ease of use and high satisfaction with a generally short-term use of the RMS. Both nurses and physicians reported a high level of satisfaction with the web registry system. Physicians reported being able to address the problems in unscheduled patient transmissions or physician-initiated transmissions remotely and were able to handle the majority of the troubleshooting calls remotely. 2. Effectiveness of RMSs in Heart Failure Patients for Cardiac Arrhythmia and Heart Failure Episodes Remote follow-up of HF patients implanted with ICD or CRT devices, generally managed in specialized HF clinics, was evaluated in 3 small cohort studies: 1 involved the Home Monitoring RMS and 2 involved the Care Link RMS. In these RMSs, in addition to the standard diagnostic features, the cardiac devices continuously assess other variables such as patient activity, mean heart rate, and heart rate variability. Intra-thoracic impedance, a proxy measure for lung fluid overload, was also measured in the 4
5 Care Link studies. The overall diagnostic performance of these measures cannot be evaluated, as the information was not reported for patients who did not experience intra-thoracic impedance threshold crossings or did not undergo interventions. The trial results involve descriptive information on transmissions, alerts, and management of selected small patient groups experiencing high morbidity and hospitalization in the short study periods. 3. Comparative Effectiveness of Remote Monitoring Systems for CIEDs Seven randomized controlled trials (RCTs) comparing RMSs with in-office follow-up for CIEDs were identified: 2 were for PMs and 5 were for ICD/CRT devices. Clinical studies performed in the United States involved both the Care Link RMS and the Home Monitoring RMS, whereas all studies performed in European countries involved only the Home Monitoring RMS. 3A. Randomized Controlled Trials of Remote Monitoring Systems for Pacemakers Two trials, both multicenter RCTs, were conducted in different countries with different pacemaker RMSs and study objectives. The first trial conducted in the United States was designed to examine the ability of Care Link, an Internet-based remote PM interrogation system, to detect clinically actionable events sooner than the current in-office follow-up supplemented with transtelephonic monitoring transmission, a limited form of remote device interrogation. Event rate detection and median time to detection was significantly faster in the RMS group. The second trial, performed in France, was designed to evaluate the ability of the Home Monitoring RMS to shorten post-operative hospitalization while preserving the safety of conventional management of longer hospital stays. The mean duration of hospitalization was shorter in the RM group and mean medical reaction time following discharge was faster in the RM group. 3B. Randomized Controlled Trials Evaluating Remote Monitoring Systems for ICD or CRT Devices The 5 studies evaluating the impact of RMSs with ICD/CRT devices were conducted in the United States and in European countries and involved 2 RMSs, Care Link and Home Monitoring. Three of the trials were smaller pilot investigations and 2 trials were large multicenter studies. Randomized Controlled Trial Pilot Studies The first of the 3 pilot studies evaluated patient satisfaction, achievement of patient outcomes, and the cost-effectiveness of the Care Link RMS compared to quarterly in-office device interrogations with 1- year follow-up. Outcomes between the 2 groups were similar at 12-month follow-up. The second small pilot study examined the impact of RM follow-up with the House Call RMS on work schedules and cost savings in patients randomized to 2 study arms varying in the degree of remote followup. The outcome improvements reported included decreases in device interrogation time, transmission time, time required for data analysis, physician time, and in-clinic wait time. The third pilot study examined the impact of RM with the Home Monitoring system, compared to scheduled trimonthly in-clinic visits, on the number of unplanned visits, total costs, health-related quality of life (SF-36), and overall mortality. A reduction in the number of in-office visits in the RM group was reported, with no differences in hospitalizations and overall mortality. 5
6 Large Multicenter Randomized Controlled Trials The first of 2 large multicenter RCTs comparing RMSs with in-office follow-up involved a study conducted at 102 centers in the United States with the Home Monitoring RMS for ICD devices. The primary objectives of the trial were to determine if remote follow-up could be safely substituted for inoffice clinic follow-up, while still enabling earlier physician detection of clinically actionable events. The key outcomes from the trial were as follows: Adherence to the protocol follow-up schedule was significantly higher in the RM group (P < 0.001). The overall mean number of in-clinic and hospital visits was significantly lower in the RM group than in the in-office follow-up group (2.1 per patient-year vs. 3.8 per patient-year, P < 0.001), representing a 45% visit reduction at 12 months. The median time from onset of first arrhythmia to physician evaluation and the median time to detect clinically asymptomatic arrhythmia events were significantly shorter (P < 0.001) in the RM group for all arrhythmias. System-related problems occurred infrequently in both groups. The adverse event rate (individual and overall) and 12-month cumulative survival were not significantly different between the 2 groups. The second major multicenter RCT evaluating RM involved the Care Link RMS for ICD/CRT devices at 133 sites in the United States and followed patients for 15 months. The primary objective of the trial was to determine whether automatically transmitted physician alerts decreased the time from the occurrence of clinically relevant events to medical decisions. Of the 575 clinical alerts, 246 did not trigger an automatic physician alert. Transmission failures were related to technical issues such as the alert not being programmed or not being reset and/or a variety of patient-related factors. The overall mean time from the clinically relevant event to the clinical decision was significantly faster by 17.4 days in the RM group (P < 0.001). Similar low numbers of events involving low battery and of ventricular fibrillation detection/therapy being turned off were noted in both groups. More alerts however were noted for out-of-range lead impedance in the RM group, and the time to detect these critical events was significantly shorter in the RM group (same day vs. 17 days). The total number of in-office clinic visits was reduced by 38% in the RM group compared to the in-office group (6.27 visits per patient-year vs visits per patient-year). Health care utilization, including cardiovascular-related hospitalizations, emergency department visits, and unscheduled clinic visits, was not significantly higher in the RM group. The overall mean length of hospitalization was significantly (P = 0.002) shorter for those in the RM group, and was shorter both for patients with ICD and CRT implants. The mortality rate was not significantly different between the follow-up groups for ICD or CRT patients. 6
7 Conclusion There is limited clinical trial information on the effectiveness of RMSs for PMs compared to ICD devices. However, for RMSs for ICD devices, multiple cohort studies and 2 large multicenter RCTs demonstrated feasibility and significant reductions for in-office clinic follow-ups in the first year post implantation. The detection rates of clinically significant arrhythmia events (and asymptomatic events) were higher and the time to a clinical decision for these events was significantly shorter in remote followup groups than in the in-office follow-up groups. The earlier detection of clinical events in remote followup groups, however, was not associated with lower morbidity or mortality rates. The substitution of almost all the first-year in-office clinic follow-ups with RM was also not associated with increased health care utilization such as emergency department visits or hospitalization. Internet-based device-assisted RMSs involve a new approach to monitoring patients, their disease progression, and their CIEDs. Remote monitoring also has the potential to improve the current postmarket surveillance systems of evolving CIEDs and their ongoing hardware and software modifications. However, the broader issues surrounding the infrastructure, impacts on existing clinical care systems, and regulatory issues need to be considered for implementation of Internet-based RMSs in jurisdictions involving different clinical practices. At this point therefore, there is insufficient information to evaluate the overall impact to the health care system, although the improved efficiency of long-term follow-up for physicians and the time saving and convenience to patients associated with a substitution of in-office follow-up by RM is more certain. Decision Determinants A decision-making framework has been developed by OHTAC that consists of seven guiding principles for decision making, and a decision-making tool, called the Decision Determinants (DD) tool. The evaluation of the four explicit main criteria (overall clinical benefit, value for money, feasibility of adoption into health system, and consistency with expected societal and ethical values) are reported in using 1 of 4 symbols. Based on the evidence reported in the MAS review and the deliberations of OHTAC on January 28 th, 2011 pertaining to this evidence, OHTAC made the following ratings with respect to the decision determinants criteria: Internet-Based Device-Assisted Remote Monitoring for Cardiac Implantable Electronic Devices Overall clinical benefit high Consistency with expected societal and ethical values Value for money moderate moderate/uncertainty Feasibility of adoption into the health system moderate/uncertainty 7
8 OHTAC Recommendations In considering the above ratings, OHTAC took into account: the high burden of cardiac disease, the critical nature of arrhythmia events being monitored, and the high levels of effectiveness and safety of RMSs; the consistency with expected societal and ethical values; the moderate uncertainty of the cost-effectiveness due to limited cost information and the absence of economic studies; the moderate uncertainty of the feasibility of adoption into the health system. Therefore, OHTAC made the following recommendations: 1. Based on high quality evidence that Internet-based device-assisted RMSs for ICDs can safely and effectively be substituted for in-office device clinic follow-up care, OHTAC recommends that these RMSs should be increasingly used in patients for whom access to clinic-mediated monitoring presents a problem for geographic or other reasons. 2. Due to the diversity of implementation, broader organizational issues, and lack of information on the real cost of RMSs, their overall impact to the health care system is uncertain. OHTAC therefore also recommends the establishment of an expert panel with a mandate for forward planning on the broader organizational issues that may influence the direction and potential outcomes of RMSs that are being implemented in clinical practices where efficiencies are being sought for long-term surveillance of a growing patient burden. 8
Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD
 Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD Philippe Mabo University Hospital, Rennes, France ESC congress, Paris 30 August 2011 U642 Disclosures Biotronik: research
Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD Philippe Mabo University Hospital, Rennes, France ESC congress, Paris 30 August 2011 U642 Disclosures Biotronik: research
LATITUDE Patient Management System
 LATITUDE Patient Management System Boston Scientific Corporation Overview Key Benefits Benefits in HF-Management The future of RPM 2 Boston Scientific Corporation LATITUDE Patient Management Overview Patient
LATITUDE Patient Management System Boston Scientific Corporation Overview Key Benefits Benefits in HF-Management The future of RPM 2 Boston Scientific Corporation LATITUDE Patient Management Overview Patient
2009 CPT Codes for Cardiac Device Monitoring
 2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
A Closer Look. LATITUDE NXT Alerts SUMMARY. Alerts. Red Alerts
 A Closer Look SUMMARY Boston Scientific s LATITUDE NXT Patient Management System enables a clinician to periodically monitor patient and device information remotely via a Communicator placed in the patient
A Closer Look SUMMARY Boston Scientific s LATITUDE NXT Patient Management System enables a clinician to periodically monitor patient and device information remotely via a Communicator placed in the patient
Top 5 Things to Know about Pacemakers and ICD s. Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017.
 Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Remote Monitoring of Implantable Cardioverter Defibrillators and Cardiac Resynchronization Therapy Defibrillators
 Cost-Effectiveness Analysis of: Remote Monitoring of Implantable Cardioverter Defibrillators and Cardiac Resynchronization Therapy Defibrillators CADTH Symposium April 17, 2018 Man Wah Yeung, Vania Costa,
Cost-Effectiveness Analysis of: Remote Monitoring of Implantable Cardioverter Defibrillators and Cardiac Resynchronization Therapy Defibrillators CADTH Symposium April 17, 2018 Man Wah Yeung, Vania Costa,
PHYSICIAN S TECHNICAL GUIDE HeartLogic TM Heart Failure Diagnostic Service
 PHYSICIAN S TECHNICAL GUIDE HeartLogic TM Heart Failure Diagnostic Service This feature requires the following external device: 6290 This feature is immediately available for the following pulse generators:
PHYSICIAN S TECHNICAL GUIDE HeartLogic TM Heart Failure Diagnostic Service This feature requires the following external device: 6290 This feature is immediately available for the following pulse generators:
DOWNLOAD OR READ : VALIDITY OF CARDIAC IMPLANTABLE ELECTRONIC DEVICES IN ASSESSING DAILY PHYSICAL ACTIVITY PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : VALIDITY OF CARDIAC IMPLANTABLE ELECTRONIC DEVICES IN ASSESSING DAILY PHYSICAL ACTIVITY PDF EBOOK EPUB MOBI Page 1 Page 2 validity of cardiac implantable electronic devices in assessing
DOWNLOAD OR READ : VALIDITY OF CARDIAC IMPLANTABLE ELECTRONIC DEVICES IN ASSESSING DAILY PHYSICAL ACTIVITY PDF EBOOK EPUB MOBI Page 1 Page 2 validity of cardiac implantable electronic devices in assessing
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
that physicians image Riata and Riata ST leads implanted in patients to assess for externalization or other visible insulation abnormalities.
 St. Jude Medical Cardiac Rhythm Management Division 15900 Valley View Court Sylmar, CA 91342-3577 USA Tel 818 362 6822 800 423 5611 www.sjm.com Mark Carlson, MD, MA Chief Medical Officer and Sr. VP Clinical
St. Jude Medical Cardiac Rhythm Management Division 15900 Valley View Court Sylmar, CA 91342-3577 USA Tel 818 362 6822 800 423 5611 www.sjm.com Mark Carlson, MD, MA Chief Medical Officer and Sr. VP Clinical
Newer pacemakers also can monitor your blood temperature, breathing, and other factors and adjust your heart rate to changes in your activity.
 Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
SPORTS AND EXERCISE ADVICE IN PATIENTS WITH ICD AND PPM
 SPORTS AND EXERCISE ADVICE IN PATIENTS WITH ICD AND PPM Rio De Janeiro 2016 Sport and Exercise Cardiology Symposium SBC/SOCERJ ACC Sharlene M. Day, MD Associate Professor, Cardiovascular Medicine Director,
SPORTS AND EXERCISE ADVICE IN PATIENTS WITH ICD AND PPM Rio De Janeiro 2016 Sport and Exercise Cardiology Symposium SBC/SOCERJ ACC Sharlene M. Day, MD Associate Professor, Cardiovascular Medicine Director,
Endpoints When Treating VT/VF in Patients with ICDs Programming Wojciech Zareba, MD, PhD
 Endpoints When Treating VT/VF in Patients with ICDs Programming Wojciech Zareba, MD, PhD Professor of Cardiology/Medicine Director of the Heart Research Follow Up Program, University of Rochester, Rochester,
Endpoints When Treating VT/VF in Patients with ICDs Programming Wojciech Zareba, MD, PhD Professor of Cardiology/Medicine Director of the Heart Research Follow Up Program, University of Rochester, Rochester,
Make the Connection A clinical compendium on the relationship between AF, stroke, and early intervention
 Make the Connection A clinical compendium on the relationship between AF, stroke, and early intervention Data that date back to the 1970 s have illustrated the strong relationship between atrial fibrillation
Make the Connection A clinical compendium on the relationship between AF, stroke, and early intervention Data that date back to the 1970 s have illustrated the strong relationship between atrial fibrillation
Continuous ECG telemonitoring with implantable devices: the expected clinical benefits
 Continuous ECG telemonitoring with implantable devices: the expected clinical benefits C. W. Israel, M.D. Dept. of Cardiology Evangelical Hospital Bielefeld Germany Carsten.Israel@evkb.de Declaration of
Continuous ECG telemonitoring with implantable devices: the expected clinical benefits C. W. Israel, M.D. Dept. of Cardiology Evangelical Hospital Bielefeld Germany Carsten.Israel@evkb.de Declaration of
Device Interrogation- Pacemakers, ICD and Loop Recorders. Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI
 Device Interrogation- Pacemakers, ICD and Loop Recorders Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI Disclosures Consultant: Medtronic Speaker s Bureau: St. Jude Medical
Device Interrogation- Pacemakers, ICD and Loop Recorders Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI Disclosures Consultant: Medtronic Speaker s Bureau: St. Jude Medical
OHTAC Recommendation. Endovascular Laser Treatment for Varicose Veins. Presented to the Ontario Health Technology Advisory Committee in November 2009
 OHTAC Recommendation Endovascular Laser Treatment for Varicose Veins Presented to the Ontario Health Technology Advisory Committee in November 2009 April 2010 Issue Background The Ontario Health Technology
OHTAC Recommendation Endovascular Laser Treatment for Varicose Veins Presented to the Ontario Health Technology Advisory Committee in November 2009 April 2010 Issue Background The Ontario Health Technology
Shock Reduction Strategies Michael Geist E. Wolfson MC
 Shock Reduction Strategies Michael Geist E. Wolfson MC Shock Therapy Thanks, I needed that! Why Do We Need To Reduce Shocks Long-term outcome after ICD and CRT implantation and influence of remote device
Shock Reduction Strategies Michael Geist E. Wolfson MC Shock Therapy Thanks, I needed that! Why Do We Need To Reduce Shocks Long-term outcome after ICD and CRT implantation and influence of remote device
MEDICAL POLICY Cardioverter Defibrillators
 POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF)
 Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF) Martin R Cowie Professor of Cardiology, Imperial College London (Royal Brompton Hospital) London,
Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF) Martin R Cowie Professor of Cardiology, Imperial College London (Royal Brompton Hospital) London,
Peri-operative management of pacemakers and implantable cardiac defibrillators
 Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Heart Failure Challenges and Unmet needs
 Heart Failure Challenges and Unmet needs. Angelo Auricchio, MD FESC Director, Cardiac Electrophysiology Programme, Fondazione Cardiocentro Ticino, Lugano, Switzerland Professor of Cardiology, University
Heart Failure Challenges and Unmet needs. Angelo Auricchio, MD FESC Director, Cardiac Electrophysiology Programme, Fondazione Cardiocentro Ticino, Lugano, Switzerland Professor of Cardiology, University
Quality Standards for the Implantation of Cardiac Rhythm Management Devices. Pan- London Arrhythmia Project Group. Version 3 (18 th July 2011)
 Quality Standards for the Implantation of Cardiac Rhythm Management Devices Pan- London Arrhythmia Project Group Version 3 (18 th July 2011) 1 Standards for Implantation of Permanent Pacemakers (including
Quality Standards for the Implantation of Cardiac Rhythm Management Devices Pan- London Arrhythmia Project Group Version 3 (18 th July 2011) 1 Standards for Implantation of Permanent Pacemakers (including
How is it possible to preview acute HF?
 New development in AF/HF Session How is it possible to preview acute HF? Gabriele Zanotto MD Interventional Cardiology Unit ULSS 9 Scaligera - Veneto Personal disclosures EP Lab activity with Biotronik
New development in AF/HF Session How is it possible to preview acute HF? Gabriele Zanotto MD Interventional Cardiology Unit ULSS 9 Scaligera - Veneto Personal disclosures EP Lab activity with Biotronik
MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection
 POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
pat hways Medtech innovation briefing Published: 24 May 2016 nice.org.uk/guidance/mib64
 pat hways CareLink network service for remote monitoring of people with cardiac devices Medtech innovation briefing Published: 24 May 2016 nice.org.uk/guidance/mib64 Summary The CareLink network service
pat hways CareLink network service for remote monitoring of people with cardiac devices Medtech innovation briefing Published: 24 May 2016 nice.org.uk/guidance/mib64 Summary The CareLink network service
Comparison of clinical trials evaluating cardiac resynchronization therapy in mild to moderate heart failure
 HOT TOPIC Cardiology Journal 2010, Vol. 17, No. 6, pp. 543 548 Copyright 2010 Via Medica ISSN 1897 5593 Comparison of clinical trials evaluating cardiac resynchronization therapy in mild to moderate heart
HOT TOPIC Cardiology Journal 2010, Vol. 17, No. 6, pp. 543 548 Copyright 2010 Via Medica ISSN 1897 5593 Comparison of clinical trials evaluating cardiac resynchronization therapy in mild to moderate heart
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter
 Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS
 NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
Inappropriate electrical shocks: Tackling the beast
 ESC Paris 2011 Inappropriate electrical shocks: Tackling the beast Gerhard Hindricks University of Leipzig Heart Center Dept. of Electrophysiology ESC Paris 2011 Inappropriate electrical shocks: Tackling
ESC Paris 2011 Inappropriate electrical shocks: Tackling the beast Gerhard Hindricks University of Leipzig Heart Center Dept. of Electrophysiology ESC Paris 2011 Inappropriate electrical shocks: Tackling
NEED A DEFIBRILLATOR? NOW YOU HAVE OPTIONS.
 NEED A DEFIBRILLATOR? NOW YOU HAVE OPTIONS. EXPLORE YOUR OPTIONS. ALL DEFIBRILLATORS ARE NOT THE SAME. Sudden cardiac arrest is a serious and lifethreatening medical emergency caused by a type of irregular
NEED A DEFIBRILLATOR? NOW YOU HAVE OPTIONS. EXPLORE YOUR OPTIONS. ALL DEFIBRILLATORS ARE NOT THE SAME. Sudden cardiac arrest is a serious and lifethreatening medical emergency caused by a type of irregular
OHTAC Recommendation: Twenty-Four-Hour Ambulatory Blood Pressure Monitoring in Hypertension. Ontario Health Technology Advisory Committee
 OHTAC Recommendation: Twenty-Four-Hour Ambulatory Blood Pressure Monitoring in Hypertension Ontario Health Technology Advisory Committee May 2012 Background Hypertension in Canada Hypertension occurs when
OHTAC Recommendation: Twenty-Four-Hour Ambulatory Blood Pressure Monitoring in Hypertension Ontario Health Technology Advisory Committee May 2012 Background Hypertension in Canada Hypertension occurs when
Remote patient management using implantable devices
 DOI 10.1007/s10840-011-9548-2 Remote patient management using implantable devices Colin Movsowitz & Suneet Mittal Received: 22 September 2010 / Accepted: 20 January 2011 # Springer Science+Business Media,
DOI 10.1007/s10840-011-9548-2 Remote patient management using implantable devices Colin Movsowitz & Suneet Mittal Received: 22 September 2010 / Accepted: 20 January 2011 # Springer Science+Business Media,
Sudden death from abnormal heart rhythm: Am I at risk?
 DISCLAIMER: The views and opinions expressed in this presentation are those of the author. The slides in this presentation are prepared as talking points. It is possible that key substantive elements were
DISCLAIMER: The views and opinions expressed in this presentation are those of the author. The slides in this presentation are prepared as talking points. It is possible that key substantive elements were
Cardiac implantable electronic devices (CIEDs) in children include pacemakers and implantable cardioverter defibrillators (ICDs).
 Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
HF and CRT: CRT-P versus CRT-D
 HF and CRT: CRT-P versus CRT-D Andrew E. Epstein, MD Professor of Medicine, Cardiovascular Division University of Pennsylvania Chief, Cardiology Section Philadelphia VA Medical Center Philadelphia, PA
HF and CRT: CRT-P versus CRT-D Andrew E. Epstein, MD Professor of Medicine, Cardiovascular Division University of Pennsylvania Chief, Cardiology Section Philadelphia VA Medical Center Philadelphia, PA
PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS
 PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS DR SUSAN CORCORAN CARDIOLOGIST ONCE UPON A TIME.. Single chamber pacemakers Programmed at 70/min VVI 70 UNIPOLAR SYSTEMS A Unipolar Pacing
PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS DR SUSAN CORCORAN CARDIOLOGIST ONCE UPON A TIME.. Single chamber pacemakers Programmed at 70/min VVI 70 UNIPOLAR SYSTEMS A Unipolar Pacing
ESC Stockholm Arrhythmias & pacing
 ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
Everyone Needs a Device Christopher L. Fellows, MD, FACC, FHRS Virginia Mason Medical Center Seattle, Wa.
 Everyone Needs a Device 2018 Christopher L. Fellows, MD, FACC, FHRS Virginia Mason Medical Center Seattle, Wa. CIED therapy The heart as an electrical organ History of pacing and defibrillation What is
Everyone Needs a Device 2018 Christopher L. Fellows, MD, FACC, FHRS Virginia Mason Medical Center Seattle, Wa. CIED therapy The heart as an electrical organ History of pacing and defibrillation What is
OHTAC Recommendation. KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer
 OHTAC Recommendation KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer Presented to the Ontario Health Technology Advisory Committee in August, 2010 December 2010 Issue Background In February
OHTAC Recommendation KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer Presented to the Ontario Health Technology Advisory Committee in August, 2010 December 2010 Issue Background In February
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING. SEEQ External Cardiac Monitor (ECM) System. From the leaders who brought you Reveal LINQ ICM
 November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
Cardiac Rhythm Management Coder 2017
 Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life
 Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
NHS. Implantable cardioverter defibrillators (ICDs) for arrhythmias. National Institute for Health and Clinical Excellence. Issue date: January 2006
 NHS National Institute for Health and Clinical Excellence Issue date: January 2006 Implantable cardioverter defibrillators (ICDs) for arrhythmias Understanding NICE guidance information for people with
NHS National Institute for Health and Clinical Excellence Issue date: January 2006 Implantable cardioverter defibrillators (ICDs) for arrhythmias Understanding NICE guidance information for people with
FREQUENTLY ASKED QUESTIONS MONITORING
 Core Devices Pacemakers (PM) Implantable Cardioverter-Defibrillators (ICD) Cardiac Resynchronization Therapy (CRT) devices OptiVol Fluid Status Monitoring FREQUENTLY ASKED QUESTIONS MONITORING SEPTEMBER
Core Devices Pacemakers (PM) Implantable Cardioverter-Defibrillators (ICD) Cardiac Resynchronization Therapy (CRT) devices OptiVol Fluid Status Monitoring FREQUENTLY ASKED QUESTIONS MONITORING SEPTEMBER
MADIT-RIT: Simple programming change averts most inappropriate ICD therapy
 Print MADIT-RIT: Simple programming change averts most inappropriate ICD therapy NOV 6, 2012 Steve Stiles Los Angeles, CA - A large randomized trial has identified specific programming criteria for implantable
Print MADIT-RIT: Simple programming change averts most inappropriate ICD therapy NOV 6, 2012 Steve Stiles Los Angeles, CA - A large randomized trial has identified specific programming criteria for implantable
POWERFULLY SIMPLE CARDIAC MONITORING
 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry (MCT) System From the leaders who brought you Reveal LINQ ICM MEDTRONIC CARDIAC DIAGNOSTIC & MONITORING SYSTEMS TRANSFORM Transform your
POWERFULLY SIMPLE CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry (MCT) System From the leaders who brought you Reveal LINQ ICM MEDTRONIC CARDIAC DIAGNOSTIC & MONITORING SYSTEMS TRANSFORM Transform your
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist
 Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist LOTS!!! This presentation confines itself to the situation in the North West. The views expressed are my
Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist LOTS!!! This presentation confines itself to the situation in the North West. The views expressed are my
AdvaMed Medtech Value Assessment Framework in Practice
 AdvaMed Medtech Value Assessment Framework in Practice Application of the Medtech Value Assessment Framework to Cymedica s e-vive System Value Framework Overview In response to the growing need to demonstrate
AdvaMed Medtech Value Assessment Framework in Practice Application of the Medtech Value Assessment Framework to Cymedica s e-vive System Value Framework Overview In response to the growing need to demonstrate
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France
 How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
Pediatric pacemakers & ICDs:
 Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Defibrillation threshold testing should no longer be performed: contra
 Defibrillation threshold testing should no longer be performed: contra Andreas Goette St. Vincenz-Hospital Paderborn Dept. of Cardiology and Intensive Care Medicine Germany No conflict of interest to disclose
Defibrillation threshold testing should no longer be performed: contra Andreas Goette St. Vincenz-Hospital Paderborn Dept. of Cardiology and Intensive Care Medicine Germany No conflict of interest to disclose
Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use?
 Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use? Helmut Pürerfellner, MD, Assoc. Prof. Saint Elisabeth s Sisters Hospital Academic Teaching Center Linz/Austria
Implantable Cardiac Monitors for Atrial Fibrillation (AF) Detection: Ready for Routine Use? Helmut Pürerfellner, MD, Assoc. Prof. Saint Elisabeth s Sisters Hospital Academic Teaching Center Linz/Austria
Current clinical evidence for remote patient management
 Europace (2013) 15, i6 i10 doi:10.1093/europace/eut119 Current clinical evidence for remote patient management Laurence Guédon-Moreau 1 *, Philippe Mabo 2,3, and Salem Kacet 1 1 Hôpital Cardiologique,
Europace (2013) 15, i6 i10 doi:10.1093/europace/eut119 Current clinical evidence for remote patient management Laurence Guédon-Moreau 1 *, Philippe Mabo 2,3, and Salem Kacet 1 1 Hôpital Cardiologique,
The protection you need - without touching your heart
 The S ICD System The protection you need - without touching your heart listen to your heart Patient information You have options An implantable defibrillator, commonly known as an ICD, is a device designed
The S ICD System The protection you need - without touching your heart listen to your heart Patient information You have options An implantable defibrillator, commonly known as an ICD, is a device designed
Cardiac Rhythm Management Coder 2018
 Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Tech Corner. ATP in the Fast VT zone
 Tech Corner ATP in the Fast VT zone NOTE: PLEASE NOTE THAT THE FOLLOWING INFORMATION IS A GENERAL DESCRIPTION OF THE FUNCTION. DETAILS AND PARTICULAR CASES ARE NOT DESCRIBED IN THE ARTICLE. FOR ADDITIONAL
Tech Corner ATP in the Fast VT zone NOTE: PLEASE NOTE THAT THE FOLLOWING INFORMATION IS A GENERAL DESCRIPTION OF THE FUNCTION. DETAILS AND PARTICULAR CASES ARE NOT DESCRIBED IN THE ARTICLE. FOR ADDITIONAL
Emergency Department Management of Patients with Implantable Cardioverter Defibrillators
 IAEM Clinical Guideline Emergency Department Management of Patients with Implantable Cardioverter Defibrillators Version 1 June 2014 DISCLAIMER IAEM recognises that patients, their situations, Emergency
IAEM Clinical Guideline Emergency Department Management of Patients with Implantable Cardioverter Defibrillators Version 1 June 2014 DISCLAIMER IAEM recognises that patients, their situations, Emergency
Interactive Simulator for Evaluating the Detection Algorithms of Implantable Defibrillators
 22 March 2002 Interactive Simulator for Evaluating the Detection Algorithms of Implantable Defibrillators F. HINTRINGER, O. PACHINGER Division of Cardiology, Department for Internal Medicine, University
22 March 2002 Interactive Simulator for Evaluating the Detection Algorithms of Implantable Defibrillators F. HINTRINGER, O. PACHINGER Division of Cardiology, Department for Internal Medicine, University
Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator
 Europace (2008) 10, 351 357 doi:10.1093/europace/eun010 Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator Hein Heidbüchel*, Pieter
Europace (2008) 10, 351 357 doi:10.1093/europace/eun010 Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator Hein Heidbüchel*, Pieter
12 th International Winter Arrhythmia School Collingwood, Ontario February 7, Dr. Derek Yung. MD, FRCPC
 12 th International Winter Arrhythmia School Collingwood, Ontario February 7, 2015 Dr. Derek Yung. MD, FRCPC Presenter: Dr. Derek Yung Relationships with industry: Educational material provided by Biotronik,
12 th International Winter Arrhythmia School Collingwood, Ontario February 7, 2015 Dr. Derek Yung. MD, FRCPC Presenter: Dr. Derek Yung Relationships with industry: Educational material provided by Biotronik,
Flowchart for ICD patients undergoing Surgery or procedures involving diathermy/magnetic fields
 Flowchart for ICD patients undergoing Surgery or procedures involving diathermy/magnetic fields Identify patient with an ICD at POAC. Notify Cardiac Physiologist that patient is due to have surgery and
Flowchart for ICD patients undergoing Surgery or procedures involving diathermy/magnetic fields Identify patient with an ICD at POAC. Notify Cardiac Physiologist that patient is due to have surgery and
2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac
 2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac Electrophysiology Martin S. Green, Chair, CHRS Education Committee Peter
2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac Electrophysiology Martin S. Green, Chair, CHRS Education Committee Peter
Ambulatory Cardiac Monitoring Modalities
 Ambulatory Cardiac Monitoring Modalities NAVIGATING THE CHOICES MKT0390.03 LEARNING OBJECTIVES Identify the Clinical Applications for Each Modality Define and Distinguish Between the Various Monitoring
Ambulatory Cardiac Monitoring Modalities NAVIGATING THE CHOICES MKT0390.03 LEARNING OBJECTIVES Identify the Clinical Applications for Each Modality Define and Distinguish Between the Various Monitoring
G Lin, R F Rea, S C Hammill, D L Hayes, P A Brady
 Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA Correspondence to: Dr Peter A Brady, MD, FRCP, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905, USA; brady.peter@mayo.edu Accepted
Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA Correspondence to: Dr Peter A Brady, MD, FRCP, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905, USA; brady.peter@mayo.edu Accepted
A SIMPLE SOLUTION FOR CARDIAC MONITORING
 A SIMPLE SOLUTION FOR CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry System Actual size. NOW THERE S AN EXTERNAL HEART MONITOR THAT S AS SIMPLE TO WEAR AS A BANDAGE It s called the SEEQ Mobile Cardiac
A SIMPLE SOLUTION FOR CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry System Actual size. NOW THERE S AN EXTERNAL HEART MONITOR THAT S AS SIMPLE TO WEAR AS A BANDAGE It s called the SEEQ Mobile Cardiac
How to set up a device clinic for heart failure patients Dr C Sridevi
 How to set up a device clinic for heart failure patients Dr C Sridevi Consultant Cardiologist /Electro physiologist CARE Hospitals, CARE Foundation Need to set up a device clinic for heart failure patients
How to set up a device clinic for heart failure patients Dr C Sridevi Consultant Cardiologist /Electro physiologist CARE Hospitals, CARE Foundation Need to set up a device clinic for heart failure patients
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
UNLOCK THE ANSWER. The Reveal LINQ TM System lets your physician learn about your heart while you live your life.
 UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
Point-of-Care Hemoglobin A1c Testing: OHTAC Recommendation
 Point-of-Care Hemoglobin A1c Testing: OHTAC Recommendation Ontario Health Technology Advisory Committee July 2014 Point-of-Care Hemoglobin A 1c Testing: OHTAC Recommendation. July 2014; pp. 1 11 Suggested
Point-of-Care Hemoglobin A1c Testing: OHTAC Recommendation Ontario Health Technology Advisory Committee July 2014 Point-of-Care Hemoglobin A 1c Testing: OHTAC Recommendation. July 2014; pp. 1 11 Suggested
Mahmoud Suleiman MD. On behalf of the Israeli ICD Registry Scientific Committee. Jan 11, National ICD Registry
 The Israeli ICD Registry- Update Mahmoud Suleiman MD On behalf of the Israeli ICD Registry Scientific Committee Jan 11, 2013 Jul 2010-Dec 2012 Total Number of Procedures N=5280 New Implants N=3448 Generator
The Israeli ICD Registry- Update Mahmoud Suleiman MD On behalf of the Israeli ICD Registry Scientific Committee Jan 11, 2013 Jul 2010-Dec 2012 Total Number of Procedures N=5280 New Implants N=3448 Generator
Wearable Cardioverter-Defibrillators
 Wearable Cardioverter-Defibrillators Policy Number: 2.02.15 Last Review: 12/2018 Origination: 10/1988 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Wearable Cardioverter-Defibrillators Policy Number: 2.02.15 Last Review: 12/2018 Origination: 10/1988 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Antiarrhythmic Drugs and Ablation in Patients with ICD and Shocks
 Antiarrhythmic Drugs and Ablation in Patients with ICD and Shocks Alireza Ghorbani Sharif, MD Interventional Electrophysiologist Tehran Arrhythmia Clinic January 2016 Recurrent ICD shocks are associated
Antiarrhythmic Drugs and Ablation in Patients with ICD and Shocks Alireza Ghorbani Sharif, MD Interventional Electrophysiologist Tehran Arrhythmia Clinic January 2016 Recurrent ICD shocks are associated
Pacemaker and ICD Interrogation
 Pacemaker and ICD Interrogation To receive the maximum benefit from your pacemaker, you will need to have regular follow-up appointments to ensure that it is working properly. This follow up can be arranged
Pacemaker and ICD Interrogation To receive the maximum benefit from your pacemaker, you will need to have regular follow-up appointments to ensure that it is working properly. This follow up can be arranged
Response of Right Ventricular Size to Treatment with Cardiac Resynchronization Therapy and the Risk of Ventricular Tachyarrhythmias in MADIT-CRT
 Response of Right Ventricular Size to Treatment with Cardiac Resynchronization Therapy and the Risk of Ventricular Tachyarrhythmias in MADIT-CRT Heart Rhythm Society (May 11, 2012) Colin L. Doyle, BA,*
Response of Right Ventricular Size to Treatment with Cardiac Resynchronization Therapy and the Risk of Ventricular Tachyarrhythmias in MADIT-CRT Heart Rhythm Society (May 11, 2012) Colin L. Doyle, BA,*
ICD Diagnostics: Making the most of it
 ICD Basics and Beyond ICD Diagnostics: Making the most of it Dulce Obias-Manno RN, BSN, MHSA, FHRS, CCDS, CEPS Cardiac Arrhythmia Center, Device Clinic Washington Hospital Center, Washington DC Objectives
ICD Basics and Beyond ICD Diagnostics: Making the most of it Dulce Obias-Manno RN, BSN, MHSA, FHRS, CCDS, CEPS Cardiac Arrhythmia Center, Device Clinic Washington Hospital Center, Washington DC Objectives
A Closer Look Product Education at a glance
 A Closer Look Product Education at a glance an ICD or CRT-D When Atrial Information is Not Used SUMMARY Boston Scientific dual-chamber ICDs and multi-chamber CRT-Ds will respond to atrial sensing regardless
A Closer Look Product Education at a glance an ICD or CRT-D When Atrial Information is Not Used SUMMARY Boston Scientific dual-chamber ICDs and multi-chamber CRT-Ds will respond to atrial sensing regardless
09/05/2018. True/False: All pacemakers have defibrillation capabilities.
 Paul G. Tarasi M.D. Staff Anesthesiologist Allegheny Health Network Adjunct Clinical Assistant Professor of Anesthesiology Temple University School of Medicine Describe different types of implanted cardiac
Paul G. Tarasi M.D. Staff Anesthesiologist Allegheny Health Network Adjunct Clinical Assistant Professor of Anesthesiology Temple University School of Medicine Describe different types of implanted cardiac
Assessment of Defibrillation Threshold upon Implantable Cardioverter-Defibrillator implant in Relation to patient s prognosis
 Assessment of Defibrillation Threshold upon Implantable Cardioverter-Defibrillator implant in Relation to patient s prognosis Investigator: Keiko Saito, MD Mentor: Yuji Saito, MD, PhD, FACP, FACC Department
Assessment of Defibrillation Threshold upon Implantable Cardioverter-Defibrillator implant in Relation to patient s prognosis Investigator: Keiko Saito, MD Mentor: Yuji Saito, MD, PhD, FACP, FACC Department
How Wireless Remote Monitoring Improves Clinical Benefits;
 How Wireless Remote Monitoring Improves Clinical Benefits; A CLINICAL CASE STUDY Suneet Mittal, M.D., The Arrhythmia Institute at Valley Hospital, Ridgewood, NJ; New York, New York; E. Martin Kloosterman,
How Wireless Remote Monitoring Improves Clinical Benefits; A CLINICAL CASE STUDY Suneet Mittal, M.D., The Arrhythmia Institute at Valley Hospital, Ridgewood, NJ; New York, New York; E. Martin Kloosterman,
Zoll Medical--LifeVest:
 Zoll Medical--LifeVest: Territory Manager: Sunny Brown Cell: (818) 916-6520 Objectives Why the LifeVest device exist Review indications for Wearable Cardioverter Defibrillator (WCD) use Give a brief description
Zoll Medical--LifeVest: Territory Manager: Sunny Brown Cell: (818) 916-6520 Objectives Why the LifeVest device exist Review indications for Wearable Cardioverter Defibrillator (WCD) use Give a brief description
2017 AHA/ACC/HRS Ventricular Arrhythmias and Sudden Cardiac Death Guideline. Top Ten Messages. Eleftherios M Kallergis, MD, PhD, FESC
 2017 AHA/ACC/HRS Ventricular Arrhythmias and Sudden Cardiac Death Guideline Top Ten Messages Eleftherios M Kallergis, MD, PhD, FESC Cadiology Department - Heraklion University Hospital No actual or potential
2017 AHA/ACC/HRS Ventricular Arrhythmias and Sudden Cardiac Death Guideline Top Ten Messages Eleftherios M Kallergis, MD, PhD, FESC Cadiology Department - Heraklion University Hospital No actual or potential
UnitedHealthcare Medicare Advantage Cardiology Prior Authorization Program
 Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD
 CLINICAL SUMMARY LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and
CLINICAL SUMMARY LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and
Cardiac Resynchronization Therapy with Defibrillation (CRT-D)
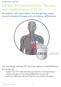 A decision aid for Cardiac Resynchronization Therapy with Defibrillation (CRT-D) For patients with heart failure who are getting cardiac resynchronization therapy and considering defibrillation CRT-D See
A decision aid for Cardiac Resynchronization Therapy with Defibrillation (CRT-D) For patients with heart failure who are getting cardiac resynchronization therapy and considering defibrillation CRT-D See
ACD Heart failure - biventricular pacing (cardiac resynchronisation)
 Mr Christopher Feinmann Project Manager NICE Mid-City Place 71 High Holborn London WC1V 6NA 18 December 2006 Dear Mr Feinmann Re: NICE Appraisal Consultation Document: Cardiac resynchronisation therapy
Mr Christopher Feinmann Project Manager NICE Mid-City Place 71 High Holborn London WC1V 6NA 18 December 2006 Dear Mr Feinmann Re: NICE Appraisal Consultation Document: Cardiac resynchronisation therapy
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
Friedman, Rott, Wokhlu, Asirvatham, Hayes 201. Figure 65.7 Shortening of the AV interval during pacing.
 Friedman, Rott, Wokhlu, Asirvatham, Hayes 201 Figure.7 Shortening of the AV interval during pacing. 202 A Case-Based Approach to Pacemakers, ICDs, and Cardiac Resynchronization Figure.8 is obtained from
Friedman, Rott, Wokhlu, Asirvatham, Hayes 201 Figure.7 Shortening of the AV interval during pacing. 202 A Case-Based Approach to Pacemakers, ICDs, and Cardiac Resynchronization Figure.8 is obtained from
Implantable Cardioverter Defibrillator Therapy in MADIT II Patients with Signs and Symptoms of Heart Failure
 Implantable Cardioverter Defibrillator Therapy in MADIT II Patients with Signs and Symptoms of Heart Failure Wojciech Zareba Postinfarction patients with left ventricular dysfunction are at increased risk
Implantable Cardioverter Defibrillator Therapy in MADIT II Patients with Signs and Symptoms of Heart Failure Wojciech Zareba Postinfarction patients with left ventricular dysfunction are at increased risk
Prevention of sudden cardiac death: With an emphasis on sudden cardiac death from ventricular arrhythmias
 Prevention of sudden cardiac death: With an emphasis on sudden cardiac death from ventricular arrhythmias The Toronto ACS Summit Toronto, March 1, 2014 Andrew C.T. Ha, MD, MSc, FRCPC Cardiac Electrophysiology
Prevention of sudden cardiac death: With an emphasis on sudden cardiac death from ventricular arrhythmias The Toronto ACS Summit Toronto, March 1, 2014 Andrew C.T. Ha, MD, MSc, FRCPC Cardiac Electrophysiology
BETTER HEART FAILURE MANAGEMENT FROM THE COMFORT OF YOUR HOME
 BETTER HEART FAILURE MANAGEMENT FROM THE COMFORT OF YOUR HOME Staying Ahead of Heart Failure with the CardioMEMS HF System The CardioMEMS HF System A UNIQUE APPROACH TO HEART FAILURE MANAGEMENT THAT CAN
BETTER HEART FAILURE MANAGEMENT FROM THE COMFORT OF YOUR HOME Staying Ahead of Heart Failure with the CardioMEMS HF System The CardioMEMS HF System A UNIQUE APPROACH TO HEART FAILURE MANAGEMENT THAT CAN
Silvia G Priori MD PhD
 The approach to the cardiac arrest survivor Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Salvatore Maugeri Pavia, Italy AND Leon Charney Division of Cardiology, Cardiovascular Genetics
The approach to the cardiac arrest survivor Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Salvatore Maugeri Pavia, Italy AND Leon Charney Division of Cardiology, Cardiovascular Genetics
