Statistical analysis plan for the Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM)
|
|
|
- Scarlett Smith
- 5 years ago
- Views:
Transcription
1 Young et al. Trials (2017) 18:455 DOI /s y UPDATE Statistical analysis plan for the Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM) Open Access Grace J. Young 1,2, Amanda L. Lewis 1, J. Athene Lane 1,2, Helen L. Winton 1, Marcus J. Drake 3,4* and Peter S. Blair 1,2 Abstract Background: Current management for men with lower urinary tract symptoms (LUTS) is a pathway that results in prostate surgery in a significant proportion. While helpful in relieving benign prostatic obstruction (BPO), surgery may be ineffective for men suffering from difficulties not relating to BPO. The UPSTREAM trial started recruitment in October 2014 with the aim of establishing whether a care pathway including urodynamics (a diagnostic tool for BPO and thus an indication of whether surgery is needed) is no worse for men, in terms of symptomatic outcome, than one without (routine care). Methods/design: This analysis plan outlines the main outcomes of the study and specific design choices, such as non-inferiority margins. The trial is currently recruiting in 26 hospitals across the UK, randomising men to either urodynamics or routine care, with recruitment set to end on the 31 December All outcomes will be measured 18 months after randomisation to allow sufficient time for surgical procedures and recovery. The primary outcome is based on a non-inferiority design with a margin of 1 point on the International Prostate Symptom Score (IPSS) scale. The key secondary outcome for this trial is surgery rate per arm, which is estimated to be at least 18% lower in the urodynamics arm. Surgery rates, adverse events, flow rate, urinary symptoms and sexual symptoms are secondary outcomes to be assessed for superiority. This is an update to the UPSTREAM protocol, which has already been published in this journal. Discussion: This a priori statistical analysis plan aims to reduce reporting bias by allowing access to the trial s objectives and plans in advance of recruitment end. The results of the trial are expected to be published soon after the trial end date of 30 September Trial registration: ISRCTN registry, ISRCTN Registered on 8 April Keywords: Statistical analysis plan, Randomised controlled trial, Urodynamics, Lower urinary tract symptoms, Non-inferiority trial * Correspondence: marcus.drake@bristol.ac.uk 3 North Bristol NHS Trust, Bristol Urological Institute, Level 3, Learning and Research Building, Southmead Hospital, Bristol BS10 5NB, UK 4 Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK Full list of author information is available at the end of the article The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Young et al. Trials (2017) 18:455 Page 2 of 7 Introduction The Urodynamics for Prostate Surgery Trial (UP- STREAM) is a two-arm trial, which randomises men with bothersome lower urinary tract symptoms (LUTS), for whom surgeons would consider offering surgery, between two treatment pathways. The intervention arm is a slightly invasive care pathway to see if surgery is needed based on urodynamic tests with multichannel cystometry while the control arm is a care pathway without urodynamics tests (current routine care). The UPSTREAM trial is a pragmatic, randomised controlled trial with a non-inferiority primary outcome. More details concerning the trial s rationale can be found in the published protocol [1]. Briefly, the primary aim of the UPSTREAM trial is to establish whether a treatment pathway for LUTS that includes urodynamics is no worse for men, in terms of symptom burden, than current routine care (without urodynamics). Research suggests that the majority (90%) of men aged 50 to 80 years suffer from at least one lower urinary tract symptom. These symptoms increase with age and relate to a spectrum of urinary problems both in the storage phase (increased daytime urinary frequency, nocturia, urgency, incontinence) and the voiding phase (slow stream, intermittency, hesitancy, straining and dribbling) [2]. Voiding LUTS may be caused by either bladder dysfunction caused by poor expulsion strength of bladder muscle or benign prostate obstruction (BPO). Prostate surgery such as transurethral resection of the prostate (TURP) is an invasive procedure which sometimes causes unpleasant adverse events, such as incontinence and difficulties with sexual function [1]. A large proportion of men suffering from BPO have improved LUTS after surgery; however, for those with bladder dysfunction this procedure is deemed unnecessary and potentially harmful [1]. Estimates from hospital audit data suggest that between 18% and 28% of the men currently undergoing prostate surgery for LUTS do not have BPO; such men are at risk of undergoing potentially unnecessary surgery [1]. Urodynamics is a diagnostic test that can ascertain whether BPO is present and therefore indicate whether the patient may benefit from surgery or not [3]. It is anticipated that use of this diagnostic tool will reduce surgery rates (the key secondary outcome) and this, along with the primary outcome, International Prostate Symptom Score (IPSS) score, will be measured 18 months after randomisation. The trial will cover many aspects of the men s treatment and recovery with a clinical, cost-effectiveness and qualitative analysis. If the intervention group yield a similar symptom burden to those under usual care and the surgery rates are reduced then this may warrant consideration of the test becoming a more prominent feature in the assessment pathway for men with LUTS. This analysis plan was written and finalised by the trial team during the recruitment period for UP- STREAM. Original drafts began in January 2015 with a version finalised in September Content has been approved by the Independent Data Monitoring Committee (IDMC) and Trial Steering Committee (TSC) chairs. Although one DMC report had already been created and presented prior to this final version, no 18-month data were available and all statisticians and members of the DMC remained blinded to the trial arm allocation. No formal statistical analyses have been conducted thus far. Methods/design The IPSS score The primary outcome will be measured using the IPSS, originally known as the American Urological Association symptom index for benign prostatic hyperplasia [4]. The scale ranges from 0 to 35, where higher scores indicate more severe symptoms. It comprises seven sections scored on a scale of 0 to 5 with 0 referring to not at all and 5 referring to almost always. These seven sections are incomplete emptying, frequency, intermittency, urgency, weak stream, straining and nocturia. To put this into perspective a recent study found men with voiding symptoms, storage symptoms and no symptoms to have IPSS scores of 16.8, 14.6 and 8.5, respectively [5]. The IPSS questionnaire also includes a quality of life (QoL) measure which asks how patients would feel if their urinary conditions remained the same for the rest of their life. This ranges from 0 to 6 where 0 is delighted and 6 is terrible. The IPSS questionnaire is used at baseline, 6, 12 and 18 months. For the sensitivity analysis, if men are missing a baseline questionnaire, 6-month scores may substitute baseline scores if men have not received treatment before 6 months. If men are missing 18-month scores then the 12-month scores may substitute 18-month scores if men have received all allocated treatment by this time point. Objective This trial investigates the research question: Is a care pathway including urodynamics no worse for men, in terms of symptom outcome, than routine care (without urodynamics) at 18 months after randomisation. The null hypothesis is that the routine care is superior to urodynamics while the alternative hypothesis is that urodynamics is non-inferior to routine care. The IPSS score will be used to calculate this:
3 Young et al. Trials (2017) 18:455 Page 3 of 7 H 0 : μ routine care μ urodynamics 1 H 1 : μ routine care μ urodynamics > 1 For example, if the mean IPSS score for the urodynamics arm is 17 at 18 months and the mean score for the routine care arm is 15, the urodynamics arm will be considered inferior. Conversely, if the IPSS score for the urodynamics and routine care arms were 17.5 and 17, respectively, then urodynamics would be considered non-inferior. As a main secondary outcome it will also establish whether inclusion of urodynamics reduces rates of bladder outlet surgery, compared with routine care. All analyses will be based on the questionnaire and consultation data collected at the 18-month followup consultation. This time frame should allow enough time for the urodynamics procedure, transurethral resection of the prostate, additional treatments and recovery from these procedures. Non-inferiority margin Non-inferiority trials are particularly helpful when you are testing whether a new treatment is no worse than the current routine treatment by more than an acceptable amount. This is often the case when investigators believe that the new treatment may offer other advantages over the comparator treatment, e.g. safety, costs [6]. To fully appreciate the reasoning behind choosing to conduct a non-inferiority trial it is important to understand the research questions behind the primary and secondary outcomes. The key secondary outcome will be the proportion of men who have surgery. In the event that this is reduced, as anticipated in the urodynamics arm, this could reduce unnecessary surgical interventions for men who are suffering from LUTS which is not attributable to BPO. Should this be the case, it is then important to establish whether this change does not worsen patient symptoms. With this in mind, a non-inferiority approach was chosen to establish whether including urodynamics leads to outcomes which are not inferior (rather than equivalent) to a pathway without urodynamics. For the primary outcome, a difference in LUTS score of 1 point (on the IPSS scale) was considered non-inferior. The trial team felt that a 1 point non-inferiority margin was appropriate with the following justification: A difference of 3 points and 0.5 points on the total IPSS score and QoL IPSS score, respectively, indicates a minimally clinical important difference (MCID) for the overall urinary condition [7]. While this is the case for the overall IPSS score, a difference of < 3 may involve substantial changes in symptom bother associated with a certain subscale [8], especially in relation to storage-type LUTS. Given this MCID the team felt that a substantial yet conservative estimate was needed below this figure. One void per night does not generally prove a problem for patients whereas two or more is considered substantially bothersome by most patients [9]. Given that a 1-point difference on the IPSS scale could indicate a difference in nocturia of 2 to 1 the team considered this to be a significant turning point on the IPSS scale. The trial team felt that a 1-point difference was a conservative estimate and, given this, would avoid false claims of non-inferiority. This margin will be explored using the bothersome measures in the International Consultation on Incontinence Modular Questionnaire Male Lower Urinary Tract Symptoms (ICIQ-MLUTS) [10]. Sample size calculation The sample size calculation was constructed from both the primary and key secondary outcomes. For the primary outcome, IPSS score at 18 months, the trial needed to detect a non-inferiority margin of 1 point. Using STATA v14.1 [11], calculating a one-sided t test with a common standard deviation of 5, mean difference of 1, 80% power and 5% alpha level, the required sample size was 310 men per arm. It was estimated that 20% attrition would occur due to withdrawals and losses to follow up. Adjusting for this led to an increased sample size of 388 per arm, 776 in total. The key secondary outcome of surgery rate per arm was a superiority hypothesis where the team had anticipated a difference in surgery rates between the arms. Data from hospital audit indicated that 73 83% of men presenting with LUTS were having surgery [1]. Had the urodynamics test been conducted on the same men, the data indicate that only 60% would have had surgery based on the prevalence of impaired bladder contractility contraindicating surgery. In order to show an absolute risk reduction in surgery from 73% to 60% in the intervention arm (relative risk reduction = 18%), a total of 291 men would be needed in each group. This calculation was based on a two-sided 5% significance level and 90% power. Inflating the sample size to account for 20% attrition increased the sample size to 364 per arm, 728 in total. Thus to have sufficient power for both hypotheses the trial aimed to recruit 800 men in total, around 400 men per arm. Baseline characteristics Baseline characteristics will be compared between the two arms by reporting relevant summary statistics in order to determine whether any potentially influential
4 Young et al. Trials (2017) 18:455 Page 4 of 7 imbalance occurred, by chance, between the two arms. Characteristics will be reported as means (SD), medians (IQR) or proportions (percentage) depending on the nature and distribution of the data. P values will not be reported for differences between the two groups at baseline, since appropriate randomisation methods will have accounted for this. Therefore, any differences identified would be due to chance such that a significant p value would in reality be representative of a type 1 error (a rejection of the null hypothesis of no relationship when it is in fact true). Large differences at baseline (more than half a SD for continuous variables or 10% for categorical variables) will be investigated in a sensitivity analysis. The following baseline measures will be recorded and compared: age, centre, ethnicity, Index of Multiple Deprivation (based on postcode), comorbidities, digital rectal examination (DRE) findings (e.g. benign enlargement), maximum flow rate (ml/s), post void residual volume (ml), voided volume (ml), additional tests (e.g. cystoscopy), IPSS and QoL score, ICIQ-MLUTS and International Consultation on Incontinence Questionnaire - Sexual Matters associated with Lower Urinary Tract Symptoms (ICIQ-MLUTSsex). Primary analyses The primary analysis will be conducted under the intention to treat (ITT) principle using a linear regression model. To test the robustness of the results we will use the per-protocol principle in a sensitivity analysis. The dependent variable will be the overall IPSS at 18 months post randomisation. The tested null hypothesis is that a treatment pathway with urodynamics is inferior to a treatment pathway without urodynamics (routine care). The mean difference will be presented with the 95% confidence interval, adjusted for the baseline IPSS score and centre. The difference will be calculated as the mean IPSS score in routine care minus the mean IPSS score in urodynamics. After adjustment for centre and baseline IPSS score, urodynamics will be classed as non-inferior if the lower band of the 95% confidence interval lies above minus one. Should the lower confidence interval band exceed both minus one and zero then we may test whether urodynamics is superior to routine care, as testing for non-inferiority before superiority does not require a statistical penalty for multiple testing [12]. Secondary analyses Similar to the primary analysis model, all secondary analyses will be on an intention to treat (ITT) basis, adjusting for centre and baseline measures (if applicable) for the following outcomes: 1. Surgery rate at 18 months (key secondary): using logistic regression, we will be estimating the difference in surgery rates between the groups. It was hypothesised that the urodynamics procedure would reduce unnecessary further intervention and therefore surgery rate, in the intervention arm. 2. Adverse events due to testing and/or treatment at 18 months: all adverse events will be compared between the groups at 18 months; including treatment-related and unrelated adverse events and deaths. The number of cases of acute urinary retention will also be examined. These will be analysed according to the number of events altogether and the number of events per patient. Logistic and ordinal logistic regression will be used to compare the groups. 3. Measures from the ICIQ [10] (ICIQ-MLUTS, ICIQ- MLUTSsex) and IPSS QoL at 18 months: alongside the IPSS score, the IPSS QoL and ICIQ measures will offer a more detailed analysis of LUTS in terms of severity, bother and impact on quality of life. We will calculate voiding and incontinence scores along with the proportion of men with a high frequency of voids during the day and night. We will also look at the proportion of men with sexual dysfunction and the effects this has on their lives. Linear and logistic regression will be used for continuous and binary outcomes respectively, adjusting for centre and respective baseline measures. Non-parametric techniques may be employed if model assumptions are not met. 4. Maximum urinary flow rate (Qmax) at 18 months: the urinary flow will be measured at the 18-month follow-up clinic appointment and analysed using linear regression, adjusting for centre and baseline Qmax. Adjusted for baseline measures this will give an indication of how well a man s urinary symptoms have improved (or worsened), at the end of their treatment follow up. There will be additional cost-effectiveness and qualitative analysis outcomes not mentioned in this statistical analysis plan that will present measures on QoL. Sensitivity analyses Sensitivity analyses will be utilised to ensure that the primary analysis model results are robust to appropriate adjustments and imputations. Pre-specified sensitivity analyses include: 1. Per-protocol analysis: the per-protocol analysis allows assessment of treatment effect among those who received the treatment that they were assigned to. This analysis will include patients who received
5 Young et al. Trials (2017) 18:455 Page 5 of 7 the treatment they were assigned to (compliers). Therefore, from those randomised to urodynamics, if a patient did not receive the procedure they will be removed from this analysis. Patients who received urodynamics despite being randomised to the routine-care arm will also be excluded. For non-inferiority trials it is recommended that both ITT and per-protocol analyses are reported [13]. 2. Complier average causal effect (CACE) analysis: the CACE analysis allows unbiased assessment of treatment effect, after separating the intervention arm into compliers and non-compliers. Alternative approaches may be considered if the contamination rate is high [14]. 3. Mixed-effects model: through the reviewing process of this analysis plan, it was strongly recommended that the team use a mixed effects repeated measures model (MMRM). The trial team anticipates that missing data will be missing at random (MAR) and therefore feel that MMRM is appropriate to evaluate the difference in treatments, while accounting for missing IPSS scores [15]. 4. Imputation using 6 and 12 month scores: for those with missing baseline scores, the 6-month IPSS score may be used as a substitute unless any treatment has been received prior to the 6-month questionnaire. Similarly, for those with missing 18-month scores, the 12-month IPSS score may be used as a substitute. If the primary outcome has more than 15% missing data (which could benefit from supplementation from 6 and 12 month data) we may consider this model for our main primary analysis. 5. Imputation for missing data: missing data for the primary outcome, assumed to be MAR will be imputed under conservative assumptions and the effect of missing data investigated. If the data, as anticipated, is MAR the trial team will consider an approach such as multiple imputation by chained equations (MICE). In the event that the data appear to be missing not at random (MNAR), then alternative approaches, such as pattern mixture model (PMM) will be adopted. The handling of missing data will follow the principles specified in the European Medicines Agency guidelines [16] and any changes to the methods described here will be fully justified in the study report and publication. For the imputation model adopted, a pre-specified random seed of 648 has been chosen. 6. Adjustment for clinically important confounders: the primary analysis and key secondary analysis will be adjusted for centre, age, comorbidities and symptom severity (all collected at baseline) that were pre-specified as clinically important by the investigators. 7. Adjustment for imbalance at baseline between the arms: to ensure that the groups are balanced in terms of unfavourable baseline characteristics, we will adjust for any imbalance at baseline. A difference of 0.5 SD or 10% between the descriptive data will be considered an imbalance. 8. Adjustment for time between surgery and the 18 month time point: the primary outcome - IPSS score at 18 months - is measured 18 months after randomisation. It was originally hoped that this would allow a 6-month post-surgery gap to allow us to see the long-term effects of surgery. However, given the nature of surgery waiting lists we will be adjusting for time since surgery as a sensitivity analysis to avoid spurious results caused by symptoms retained from recent surgery. If any of the sensitivity analyses prove important they may be used for the secondary outcomes. Subgroup analyses Formal tests of interaction between the dichotomised variables and treatment pathway will be carried out to test whether treatment effect differ between different patient groups. Interaction tests are likely to be underpowered, therefore emphasis will be placed on the point estimates and confidence intervals generated, rather than any associated p values. These will be at the 10% alpha level and interpreted as hypothesis generating, as any formal testing will be unreliable. They will be applied to the primary analysis (IPSS score) and the main secondary analysis (surgery rates), including (but not limited to): 1. Age (above and below the median) 2. Flow rate (>12 ml/s vs. 12 ml/s) 3. Maximum voided volume (<200 ml vs. 200 ml), measured in the baseline bladder diary 4. Storage dysfunction: nocturia (yes vs. no) 5. Severity of storage LUTS (more substantial vs. less substantial) Presentation of figures and tables When publishing the trial results, Figure 1 will depict the trial flow (Consolidated Standards of Reporting Trials (CONSORT)) diagram, identifying the numbers of patients who were ineligible, who declined, withdrew, were lost to follow up or were excluded from the analysis (e.g. due to missing questionnaire data). Table 1 will offer proportions (percentage), means (SD) or medians (IQR) of the baseline covariates for those in the UP- STREAM trial, by arm. If adequate data are collected we will also compare baseline characteristics between those who entered the study and those who declined or were
6 Young et al. Trials (2017) 18:455 Page 6 of 7 ineligible. The primary, secondary, sensitivity and subgroup analyses will all be presented in tables with difference estimates, confidence intervals and p values. It is anticipated that different centres may interpret urodynamic results differently, potentially affecting surgery rates. Therefore, estimates will be adjusted for the baseline measure in the question and the centre. Adverse events will be differentiated by arm and by relation to treatment, and graded using the Clavien-Dindo [17] classification for surgery-based events. An independent reviewer will assess the assigned relation to treatment and the Clavien-Dindo scoring of surgery-related adverse events. Discussion Following on from the protocol this paper aims to add a layer of specificity to the UPSTREAM trial, to prevent biased and misleading statistical inferences. However, in the event that the analytical tests we have pre-specified are not appropriate for the final data, then alternative approaches may be considered and full justification given. When UPSTREAM comes to publication, an unbiased and informed overview of pathways for men with LUTS should result. In order to do this it is necessary to be sure that the conclusions drawn are robust and not due to statistical multiplicity. The trial team has defined, a priori, both a primary and key secondary outcome on which they based their sample size. We are not powered to test our secondary analyses and will therefore interpret any p values with due caution. This field of medicine is relatively unexplored, offering numerous but potentially sceptical observers, therefore the primary analysis details have been set out in advance of recruitment end. Differences between the protocol and the statistical analysis plan This analysis plan is very similar to the protocol published in December last year with only a few minor changes. In the protocol abstract we stated that the aim of the trial was to determine whether a care pathway not including invasive urodynamics is no worse for men in terms of symptom outcome than one in which it is included, at 18 months after randomisation. Given that this a non-inferiority trial and urodynamics is the intervention we are testing, this should have read determine whether a care pathway including invasive urodynamics is no worse for men in terms of symptom outcome than one in which it is not included. However, our noninferiority margin has not changed and we are still classing a one-point difference on the IPSS scale as noninferior, as mentioned in the Statistics and data analysis section of the published trial protocol. Originally in the protocol paper it was stated that randomisation would be stratified by centre. UPSTREAM in fact, uses a simple randomisation approach whereby centres utilise an automated web/telephone randomisation system provided by the Bristol Randomised Trials Collaboration (BRTC). Since publishing the protocol paper the trial has also received funding for a 6-month extension, which will now mean that recruitment will end in December and the trial will officially end in September Analysis of primary and secondary outcomes remains unchanged. However, the subgroup analyses have been updated and some sensitivity analyses added. Originally, we had included factors that could only be found in the urodynamics arm (e.g. whether or not the patient was suffering from BPO). After careful consideration these were moved to exploratory analyses. All subgroups listed in this update can be found in both arms of the study and will therefore be used in an interaction term in the primary and key secondary analyses. Trial status This manuscript was submitted before recruitment ended (14 December 2016) and underwent minor revisions, based on reviewers feedback, after recruitment had ceased (23 of May 2017). Abbreviations BPO: Benign prostatic obstruction; BRTC: Bristol Randomised Trials Collaboration; CACE: Complier average causal effect; CONSORT: Consolidated Standards of Reporting Trials; DRE: Digital rectal examination; ICIQ: International Consultation on Incontinence Questionnaire; ICIQ- MLUTS: International Consultation on Incontinence Questionnaire Male Lower Urinary Tract Symptoms; ICIQ-MLUTSsex: International Consultation on Incontinence Questionnaire Sexual Matters associated with Male Lower Urinary Tract Symptoms; IDMC: Independent Data Monitoring Committee; IPSS: International Prostate Symptom Score; IQR: Interquartile range; ITT: Intention to treat; LUTS: Lower urinary tract symptoms; MAR: Missing at random; MCID: Minimally clinically important difference; MICE: Multiple imputation by chained equations; MMRM: Mixed-effects model for repeated measures; MNAR: Missing not at random; PMM: Pattern mixture model; Qmax: Maximum urinary flow rate; QoL: Quality of life; SD: Standard deviation; TSC: Trial Steering Committee; TURP: Transurethral resection of the prostate; UK: United Kingdom; UPSTREAM: Urodynamics for Prostate Surgery Trial: Randomised Evaluation of Assessment Methods Acknowledgements The authors acknowledge with thanks the contributions of Prof Paul Abrams, Prof Christopher Chapple, Prof Charis Glazener, Dr Jeremy Horwood, Dr J. Athene Lane, Mr John McGrath, Dr Sian Noble, Prof Robert Pickard, Mr Gordon Taylor, Mr Kwame Agyapong Ansu, Mrs Lyndsey Johnson, and Mrs Samantha Clarke to the UPSTREAM study. Funding This project was funded by the National Institute for Health Research HTA programme (project number 12/140/01). This study was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration (BRTC), a UKCRC Registered Clinical Trials Unit in receipt of National Institute for Health Research CTU support funding. Availability of data and materials Not applicable.
7 Young et al. Trials (2017) 18:455 Page 7 of 7 Department of Health disclaimer The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. Authors contributions MJD is the Principal Investigator of UPSTREAM; he conceived the study, participated in its design and coordination and helped develop the analysis plan. JAL is a member of the trial management team and has contributed to the design of the trial. ALL is the trial manager for UPSTREAM and developed the trial protocol and manages the coordination of the study. HLW also contributes to the coordination of the study. PSB and GJY developed the statistical analysis plan which forms the basis of this update. All authors read and approved the final manuscript. Ethics approval and consent to participate The UPSTREAM trial will obtain informed consent from all participants in the study. The Chief Investigator (CI) obtained approval from the South Central Oxford B Research Ethics Committee (14/SC/0237). The study must be submitted for site-specific assessment (SSA) at each participating National Health Service (NHS) Trust. The CI will require a copy of the Trust Research and Development (R&D) approval letter before accepting participants into the study from that Trust. The study will be conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964 and later revisions. All 26 centres in UPSTREAM are approved by a centralised ethics committee (REC reference: 14/SC/0237). Full details of all participating hospitals and supporting NHS Trusts that provided local R&D approval can be found in the Additional file of the protocol paper [1]. Consent for publication Not applicable. controlled trials on drugs: a systematic review. Plos One. 2010;5(10):art. no. e Barry MJ, Cantor A, Roehrborn CG, Group CS. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. J Urol. 2013;189: Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TLJ, Guyatt GH, Auvinen A, Tikkinen KAO. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol. 2014;65: Tikkinen KAO, Johnson TM, Tammela TLJ, Sintonen H, Haukka J, Huhtala H, Auvinen A. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010;57: Abrams P, Avery K, Gardener N, Donovan J, Board IA. The International Consultation on Incontinence Modular Questionnaire: J Urol. 2006;175: StataCorp. Stata Statistical Software: Release Schumi J, Wittes JT. Through the looking glass: understanding noninferiority. Trials. 2011;12: Shah PB. Intention-to-treat and per-protocol analysis. Can Med Assoc J. 2011;183: Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med. 1997;16: Siddiqui O, Hung HMJ, O'Neill R. MMRM vs. LOCF: A comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19: European Medicines Agency. Guideline on missing data in confirmatory clinical Trials Dindo D, Demartines N, Clavien PA. Classification of surgical complications - A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240: Competing interests The authors declare that they have no competing interests. Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Author details 1 Population Health Sciences, Bristol Medical School, University of Bristol, Canynge Hall, 39 Whatley Road, Bristol BS8 2PS, UK. 2 Bristol Randomised Trials Collaboration (BRTC), University of Bristol, Canynge Hall, 39 Whatley Road, Bristol BS8 2PS, UK. 3 North Bristol NHS Trust, Bristol Urological Institute, Level 3, Learning and Research Building, Southmead Hospital, Bristol BS10 5NB, UK. 4 Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK. Received: 14 December 2016 Accepted: 21 September 2017 References 1. Bailey K, Abrams P, Blair PS, Chapple C, Glazener C, Horwood J, Lane JA, McGrath J, Noble S, Pickard R, et al. Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM) for diagnosis and management of bladder outlet obstruction in men: study protocol for a randomised controlled trial. Trials. 2015;16: Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155: Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol. 2005;7 Suppl 6:S Barry MJ, Fowler FJ, Oleary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett ATK, Blaivas JG, Wein AJ. The American-Urological-Association Symptom Index for Benign Prostatic Hyperplasia. J Urol. 1992;148: Liao CH, Chung SD, Kuo HC. Diagnostic value of International Prostate Symptom Score voiding-to-storage subscore ratio in male lower urinary tract symptoms. Int J Clin Pract. 2011;65: Wangge G, Klungel OH, Roes KCB, de Boer A, Hoes AW, Knol MJ. Room for improvement in conducting and reporting non-inferiority randomized Submit your next manuscript to BioMed Central and we will help you at every step: We accept pre-submission inquiries Our selector tool helps you to find the most relevant journal We provide round the clock customer support Convenient online submission Thorough peer review Inclusion in PubMed and all major indexing services Maximum visibility for your research Submit your manuscript at
University of Bristol - Explore Bristol Research
 Drake, M., Lewis, A. L., & Lane, A. (2016). Urodynamic Testing for Men with Voiding Symptoms Considering Interventional Therapy: The Merits of a Properly Constructed Randomised Trial. European Urology,
Drake, M., Lewis, A. L., & Lane, A. (2016). Urodynamic Testing for Men with Voiding Symptoms Considering Interventional Therapy: The Merits of a Properly Constructed Randomised Trial. European Urology,
Diagnostic approach to LUTS in men. Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center
 Diagnostic approach to LUTS in men Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center Classification of LUTS Storage symptoms Voiding symptoms Post micturition
Diagnostic approach to LUTS in men Prof Dato Dr. Zulkifli Md Zainuddin Consultant Urologist / Head Of Urology Unit UKM Medical Center Classification of LUTS Storage symptoms Voiding symptoms Post micturition
ISSN: (Print) (Online) Journal homepage:
 Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/iaan19 CHANGE IN INTERNATIONAL PROSTATE SYMPTOM SCORE AFTER TRANSURETHRAL
Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/iaan19 CHANGE IN INTERNATIONAL PROSTATE SYMPTOM SCORE AFTER TRANSURETHRAL
Shrestha A, Chalise PR, Sharma UK, Gyawali PR, Shrestha GK, Joshi BR. Department of Surgery, TU Teaching Hospital, Maharajgunj, Kathmandu, Nepal
 Original Article Intravesical Prostatic Protrusion is better than Prostate Volume in Predicting Symptom Severity in Benign Prostatic Hyperplasia: A Prospective Clinical Study Shrestha A, Chalise PR, Sharma
Original Article Intravesical Prostatic Protrusion is better than Prostate Volume in Predicting Symptom Severity in Benign Prostatic Hyperplasia: A Prospective Clinical Study Shrestha A, Chalise PR, Sharma
Effect of Transurethral Resection of the Prostate Based on the Degree of Obstruction Seen in Urodynamic Study
 www.kjurology.org http://dx.doi.org/10.4111/kju.2013.54.12.840 Voiding Dysfunction/Female Urology Effect of Transurethral Resection of the Prostate Based on the Degree of Obstruction Seen in Urodynamic
www.kjurology.org http://dx.doi.org/10.4111/kju.2013.54.12.840 Voiding Dysfunction/Female Urology Effect of Transurethral Resection of the Prostate Based on the Degree of Obstruction Seen in Urodynamic
The patient, your co-pilot in assessing LUTS
 The patient, your co-pilot in assessing LUTS Frank Van der Aa Leuven, Belgium This symposium is supported by Astellas Pharma Europe Ltd., including speaker honoraria and production of materials the slides
The patient, your co-pilot in assessing LUTS Frank Van der Aa Leuven, Belgium This symposium is supported by Astellas Pharma Europe Ltd., including speaker honoraria and production of materials the slides
Bailey et al. Trials (2015) 16:567 DOI /s
 Bailey et al. Trials (2015) 16:567 DOI 10.1186/s13063-015-1087-1 STUDY PROTOCOL Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM) for diagnosis and management
Bailey et al. Trials (2015) 16:567 DOI 10.1186/s13063-015-1087-1 STUDY PROTOCOL Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM) for diagnosis and management
NOTE: This policy is not effective until April 1, Transurethral Water Vapor Thermal Therapy of the Prostate
 NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
50% of men. 90% of men PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS. Want more information? What are the symptoms?
 PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
INJINTERNATIONAL. Original Article INTRODUCTION
 Official Journal of Korean Continence Society / Korean Society of Urological Research / The Korean Children s Continence and Enuresis Society / The Korean Association of Urogenital Tract Infection and
Official Journal of Korean Continence Society / Korean Society of Urological Research / The Korean Children s Continence and Enuresis Society / The Korean Association of Urogenital Tract Infection and
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Systematic review of the diagnostic performance of non-invasive tests in diagnosing bladder outlet obstruction or detrusor underactivity
PROSPERO International prospective register of systematic reviews Systematic review of the diagnostic performance of non-invasive tests in diagnosing bladder outlet obstruction or detrusor underactivity
NIH Public Access Author Manuscript J Urol. Author manuscript; available in PMC 2010 May 4.
 NIH Public Access Author Manuscript Published in final edited form as: J Urol. 2009 December ; 182(6): 2819 2824. doi:10.1016/j.juro.2009.08.086. Intravesical Prostatic Protrusion in Men in Olmsted County,
NIH Public Access Author Manuscript Published in final edited form as: J Urol. 2009 December ; 182(6): 2819 2824. doi:10.1016/j.juro.2009.08.086. Intravesical Prostatic Protrusion in Men in Olmsted County,
RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA
 American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
Can men with prostates sized 80 ml or larger be managed conservatively?
 Original Article - Lower Urinary Tract Dysfunction Investig Clin Urol 2017;58:359-364. pissn 2466-0493 eissn 2466-054X Can men with prostates sized 80 ml or larger be managed conservatively? Alvin Lee,
Original Article - Lower Urinary Tract Dysfunction Investig Clin Urol 2017;58:359-364. pissn 2466-0493 eissn 2466-054X Can men with prostates sized 80 ml or larger be managed conservatively? Alvin Lee,
Dichotomizing partial compliance and increased participant burden in factorial designs: the performance of four noncompliance methods
 Merrill and McClure Trials (2015) 16:523 DOI 1186/s13063-015-1044-z TRIALS RESEARCH Open Access Dichotomizing partial compliance and increased participant burden in factorial designs: the performance of
Merrill and McClure Trials (2015) 16:523 DOI 1186/s13063-015-1044-z TRIALS RESEARCH Open Access Dichotomizing partial compliance and increased participant burden in factorial designs: the performance of
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH
 MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
APPENDIX CLINICAL INPUT RESPONSES
 CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
Strategies for handling missing data in randomised trials
 Strategies for handling missing data in randomised trials NIHR statistical meeting London, 13th February 2012 Ian White MRC Biostatistics Unit, Cambridge, UK Plan 1. Why do missing data matter? 2. Popular
Strategies for handling missing data in randomised trials NIHR statistical meeting London, 13th February 2012 Ian White MRC Biostatistics Unit, Cambridge, UK Plan 1. Why do missing data matter? 2. Popular
Worthington et al. Trials (2017) 18:179 DOI /s
 Worthington et al. Trials (2017) 18:179 DOI 10.1186/s13063-017-1916-5 STUDY PROTOCOL Open Access A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral
Worthington et al. Trials (2017) 18:179 DOI 10.1186/s13063-017-1916-5 STUDY PROTOCOL Open Access A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral
Patient Information. Lower Urinary Tract Symptoms (LUTS) and Diagnosis of BPE
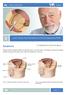 Patient Information English 32 Lower Urinary Tract Symptoms (LUTS) and Diagnosis of BPE Symptoms The underlined terms are listed in the glossary. Benign prostatic enlargement (BPE) can affect the way you
Patient Information English 32 Lower Urinary Tract Symptoms (LUTS) and Diagnosis of BPE Symptoms The underlined terms are listed in the glossary. Benign prostatic enlargement (BPE) can affect the way you
Increasing Awareness, Diagnosis, and Treatment of BPH, LUTS, and EP
 Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
Introduction to Enlarged Prostate E. David Crawford, MD Professor of Surgery (Urology) and Radiation Oncology Head, Urologic Oncology E. David Crawford Endowed Chair in Urologic Oncology University of
VOIDING DYSFUNCTION IN ELDERLY MALE CURRENT STATUS
 VOIDING DYSFUNCTION IN ELDERLY MALE CURRENT STATUS DR. FRANCIS LEE Voiding dysfunction Storage Emptying Common voiding dysfunction in elderly male Emptying BPH Storage Incontinence Overactive bladder Post-prostatectomy
VOIDING DYSFUNCTION IN ELDERLY MALE CURRENT STATUS DR. FRANCIS LEE Voiding dysfunction Storage Emptying Common voiding dysfunction in elderly male Emptying BPH Storage Incontinence Overactive bladder Post-prostatectomy
The Enlarged Prostate Symptoms, Diagnosis and Treatment
 The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics. Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital
 Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital 01/02/2018 Lower Urinary Tract Symptoms LUTS - one of
Lower Urinary Tract Symptoms (LUTS) and Nurse-Led Clinics Sean Diver Urology Advanced Nurse Practitioner candidate Letterkenny University Hospital 01/02/2018 Lower Urinary Tract Symptoms LUTS - one of
BLISTER STATISTICAL ANALYSIS PLAN Version 1.0
 The Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Study EudraCT number: 2007-006658-24 ISRCTN: 13704604 BLISTER STATISTICAL ANALYSIS PLAN Version 1.0 Version Date Comments 0.1 13/01/2011 First
The Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Study EudraCT number: 2007-006658-24 ISRCTN: 13704604 BLISTER STATISTICAL ANALYSIS PLAN Version 1.0 Version Date Comments 0.1 13/01/2011 First
α-blocker Monotherapy and α-blocker Plus 5-Alpha-Reductase Inhibitor Combination Treatment in Benign Prostatic Hyperplasia; 10 Years Long-Term Results
 www.kjurology.org http://dx.doi.org/10.4111/kju.2012.53.4.248 Voiding Dysfunction α-blocker Monotherapy and α-blocker Plus 5-Alpha-Reductase Inhibitor Combination Treatment in Benign Prostatic Hyperplasia;
www.kjurology.org http://dx.doi.org/10.4111/kju.2012.53.4.248 Voiding Dysfunction α-blocker Monotherapy and α-blocker Plus 5-Alpha-Reductase Inhibitor Combination Treatment in Benign Prostatic Hyperplasia;
Health authorities are asking for PRO assessment in dossiers From rejection to recognition of PRO
 UNDERSTANDING AND ADDRESSING POTENTIAL BIAS IN PATIENT-REPORTED OUTCOMES FROM CLINICAL TRIALS ISPOR Barcelona Workshop Tuesday 13 November 14:00-15:00 Prof. Olivier Chassany EA 7334, Patient-Centered Outcomes
UNDERSTANDING AND ADDRESSING POTENTIAL BIAS IN PATIENT-REPORTED OUTCOMES FROM CLINICAL TRIALS ISPOR Barcelona Workshop Tuesday 13 November 14:00-15:00 Prof. Olivier Chassany EA 7334, Patient-Centered Outcomes
BPH: a present and future perspective on health impact
 BPH: a present and future perspective on health impact Burden of disease in men with moderate LUTS Dalibor Pacík This presentation is financially supported by GlaxoSmithKline. CZ/DUTT/0019/12 Men with
BPH: a present and future perspective on health impact Burden of disease in men with moderate LUTS Dalibor Pacík This presentation is financially supported by GlaxoSmithKline. CZ/DUTT/0019/12 Men with
Chapter 4: Research and Future Directions
 Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Diagnosis and Mangement of Nocturia in Adults
 Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Diagnosis and Mangement of Nocturia in Adults Christopher Chapple Professor of Urology Sheffield Teaching Hospitals University of Sheffield Sheffield Hallam University UK 23 rd October 2015 Terminology
Male LUTS, OAB, Sex: natural history. JR Sathiya
 Male LUTS, OAB, Sex: natural history JR Sathiya Definitions Newer concepts of LUTs Natural history of BPH Prevalence of LUTs Definition BPH- represents a histologic diagnosis that refers to the proliferation
Male LUTS, OAB, Sex: natural history JR Sathiya Definitions Newer concepts of LUTs Natural history of BPH Prevalence of LUTs Definition BPH- represents a histologic diagnosis that refers to the proliferation
A unit of International Journal Foundation Page I 96
 Occupational distribution of Patients with LUTS Single tertiary center experience. Part -4 (Medical Science) Chapter-III August/Vol.4.0/Issue-II ISSN NO : 2456-1045 ISSN CODE: 2456-1045 (Online) (ICV-MDS
Occupational distribution of Patients with LUTS Single tertiary center experience. Part -4 (Medical Science) Chapter-III August/Vol.4.0/Issue-II ISSN NO : 2456-1045 ISSN CODE: 2456-1045 (Online) (ICV-MDS
CHAPTER 6. M.D. Eckhardt, G.E.P.M. van Venrooij, T.A. Boon. hoofdstuk :49 Pagina 89
 hoofdstuk 06 19-12-2001 09:49 Pagina 89 Urethral Resistance Factor (URA) Versus Schäfer s Obstruction Grade and Abrams-Griffiths (AG) Number in the Diagnosis of Obstructive Benign Prostatic Hyperplasia
hoofdstuk 06 19-12-2001 09:49 Pagina 89 Urethral Resistance Factor (URA) Versus Schäfer s Obstruction Grade and Abrams-Griffiths (AG) Number in the Diagnosis of Obstructive Benign Prostatic Hyperplasia
PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA
 St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
Management of LUTS. Simon Woodhams February 2012
 Management of LUTS Simon Woodhams February 2012 The management of lower urinary tract symptoms (LUTS) in men Implementing NICE guidance May 2010 NICE clinical guideline 97 Background Lower urinary tract
Management of LUTS Simon Woodhams February 2012 The management of lower urinary tract symptoms (LUTS) in men Implementing NICE guidance May 2010 NICE clinical guideline 97 Background Lower urinary tract
Impact of smoking on Lower Urinary Tract Symptoms (LUTS) - Single tertiary centre experience
 International Journal of Scientific and Research Publications, Volume 6, Issue 5, May 2016 119 Impact of smoking on Lower Urinary Tract Symptoms (LUTS) - Single tertiary centre experience AUB Pethiyagoda
International Journal of Scientific and Research Publications, Volume 6, Issue 5, May 2016 119 Impact of smoking on Lower Urinary Tract Symptoms (LUTS) - Single tertiary centre experience AUB Pethiyagoda
The ICS- BPH Study: uroflowmetry, lower urinary tract symptoms and bladder outlet obstruction
 British Journal of Urology (1998), 82, 619 623 The ICS- BPH Study: uroflowmetry, lower urinary tract symptoms and bladder outlet obstruction J.M. REYNARD1, Q. YANG2, J.L. DONOVAN3,T.J. PETERS3, W. SCHAFER4,
British Journal of Urology (1998), 82, 619 623 The ICS- BPH Study: uroflowmetry, lower urinary tract symptoms and bladder outlet obstruction J.M. REYNARD1, Q. YANG2, J.L. DONOVAN3,T.J. PETERS3, W. SCHAFER4,
Benign Prostatic Hyperplasia (BPH):
 Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Prostatic Urethral Lift
 Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
All about the Prostate
 MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
Title of Research Thesis:
 Eastern Michigan University By Fatai Osinowo Adviser s Name: Dr. Stephen Sonstein, PhD Title of Research Thesis: A sub-analyses from the Benign Prostatic Hyperplasia (BPH) Registry and Patient survey:
Eastern Michigan University By Fatai Osinowo Adviser s Name: Dr. Stephen Sonstein, PhD Title of Research Thesis: A sub-analyses from the Benign Prostatic Hyperplasia (BPH) Registry and Patient survey:
URODYNAMICS IN MALE LUTS: NECESSARY OR WASTE OF TIME?
 URODYNAMICS IN MALE LUTS: NECESSARY OR WASTE OF TIME? Andrea Tubaro, MD, FEBU Chairman Department of Urology Sant Andrea Hospital Sapienza University of Rome, Italy Disclosures Consultant, paid speaker,
URODYNAMICS IN MALE LUTS: NECESSARY OR WASTE OF TIME? Andrea Tubaro, MD, FEBU Chairman Department of Urology Sant Andrea Hospital Sapienza University of Rome, Italy Disclosures Consultant, paid speaker,
REPORTS. Clinical and Economic Outcomes in Patients Treated for Enlarged Prostate
 Clinical and Economic Outcomes in Patients Treated for Enlarged Prostate Michael James Naslund, MD, MBA; Muta M. Issa, MD, MBA; Amy L. Grogg, PharmD; Michael T. Eaddy, PharmD, PhD; and Libby Black, PharmD
Clinical and Economic Outcomes in Patients Treated for Enlarged Prostate Michael James Naslund, MD, MBA; Muta M. Issa, MD, MBA; Amy L. Grogg, PharmD; Michael T. Eaddy, PharmD, PhD; and Libby Black, PharmD
Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients
 Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2018/10 Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients N. Narayanamoorthy,
Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2018/10 Evaluation of Sexual Dysfunction in Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Patients N. Narayanamoorthy,
Guidance Document for Claims Based on Non-Inferiority Trials
 Guidance Document for Claims Based on Non-Inferiority Trials February 2013 1 Non-Inferiority Trials Checklist Item No Checklist Item (clients can use this tool to help make decisions regarding use of non-inferiority
Guidance Document for Claims Based on Non-Inferiority Trials February 2013 1 Non-Inferiority Trials Checklist Item No Checklist Item (clients can use this tool to help make decisions regarding use of non-inferiority
LONG-TERM SAFETY AND EFFICACY OF TAMSULOSIN FOR THE TREATMENT OF LOWER URINARY TRACT SYMPTOMS ASSOCIATED WITH BENIGN PROSTATIC HYPERPLASIA
 0022-5347/03/1702-0498/0 Vol. 170, 498 502, August 2003 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2003 by AMERICAN UROLOGICAL ASSOCIATION DOI: 10.1097/01.ju.0000076140.68657.fd LONG-TERM SAFETY
0022-5347/03/1702-0498/0 Vol. 170, 498 502, August 2003 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2003 by AMERICAN UROLOGICAL ASSOCIATION DOI: 10.1097/01.ju.0000076140.68657.fd LONG-TERM SAFETY
The Evolution of Combination Therapy. US men eligible for BPH treatment * with projected population changes
 The Management of BPH & The Impact of Combination Therapy Results Combination of Avodart and Tamsulosin (CombAT) Medical Therapy of Prostate Symptoms (MTOPS) Dr. Jack Barkin, md, fics, facs, dabu, Mcert
The Management of BPH & The Impact of Combination Therapy Results Combination of Avodart and Tamsulosin (CombAT) Medical Therapy of Prostate Symptoms (MTOPS) Dr. Jack Barkin, md, fics, facs, dabu, Mcert
Evidence-based guidelines in lower urinary tract symptoms secondary to benign prostatic hyperplasia and variation in care
 REVIEW C URRENT OPINION Evidence-based guidelines in lower urinary tract symptoms secondary to benign prostatic hyperplasia and variation in care Seth A. Strope Purpose of review Guidelines have been developed
REVIEW C URRENT OPINION Evidence-based guidelines in lower urinary tract symptoms secondary to benign prostatic hyperplasia and variation in care Seth A. Strope Purpose of review Guidelines have been developed
Understanding noninferiority trials
 Review article http://dx.doi.org/10.3345/kjp.2012.55.11.403 Korean J Pediatr 2012;55(11):403-407 eissn 1738-1061 pissn 2092-7258 Understanding noninferiority trials Seokyung Hahn, PhD Department of Medicine,
Review article http://dx.doi.org/10.3345/kjp.2012.55.11.403 Korean J Pediatr 2012;55(11):403-407 eissn 1738-1061 pissn 2092-7258 Understanding noninferiority trials Seokyung Hahn, PhD Department of Medicine,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water vapour ablation for lower urinary tract symptoms caused by
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water vapour ablation for lower urinary tract symptoms caused by
Statistical Analysis Plans
 Statistical Analysis Plans PROFESSOR CARROL GAMBLE UNIVERSITY OF LIVERPOOL Clinical Trials Lots of different types of clinical trials Various interventions Pharmaceutical/drugs regulated Regulated environment
Statistical Analysis Plans PROFESSOR CARROL GAMBLE UNIVERSITY OF LIVERPOOL Clinical Trials Lots of different types of clinical trials Various interventions Pharmaceutical/drugs regulated Regulated environment
Title 1 Descriptive title identifying the study design, population, interventions, and, if applicable, trial acronym
 SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
Overview. Methods of assessment. Assessment of LUTS. Patient Assessment and Bladder Diary 15/05/2017. How are LUTS and quality of life (QoL) assessed?
 Overview How are LUTS and quality of life (QoL) assessed? Patient Assessment and Bladder Diary Patient centred questionnaire evaluation ICIQ Bladder Diary Nikki Cotterill Assessment of LUTS Methods of
Overview How are LUTS and quality of life (QoL) assessed? Patient Assessment and Bladder Diary Patient centred questionnaire evaluation ICIQ Bladder Diary Nikki Cotterill Assessment of LUTS Methods of
Clinical audit for occupational therapy intervention for children with autism spectrum disorder: sampling steps and sample size calculation
 DOI 10.1186/s13104-015-1247-0 CORRESPONDENCE Open Access Clinical audit for occupational therapy intervention for children with autism spectrum disorder: sampling steps and sample size calculation Scott
DOI 10.1186/s13104-015-1247-0 CORRESPONDENCE Open Access Clinical audit for occupational therapy intervention for children with autism spectrum disorder: sampling steps and sample size calculation Scott
Alpha antagonists from initial concept to routine clinical practice
 european urology 50 (2006) 635 642 available at www.sciencedirect.com journal homepage: www.europeanurology.com Editorial 50th Anniversary Alpha antagonists from initial concept to routine clinical practice
european urology 50 (2006) 635 642 available at www.sciencedirect.com journal homepage: www.europeanurology.com Editorial 50th Anniversary Alpha antagonists from initial concept to routine clinical practice
Further data analysis topics
 Further data analysis topics Jonathan Cook Centre for Statistics in Medicine, NDORMS, University of Oxford EQUATOR OUCAGS training course 24th October 2015 Outline Ideal study Further topics Multiplicity
Further data analysis topics Jonathan Cook Centre for Statistics in Medicine, NDORMS, University of Oxford EQUATOR OUCAGS training course 24th October 2015 Outline Ideal study Further topics Multiplicity
THE ACONTRACTILE BLADDER - FACT OR FICTION?
 THE ACONTRACTILE BLADDER - FACT OR FICTION? Jacob Golomb Department of Urology Chaim Sheba Medical Center Tel Hashomer NEUROGENIC UNDERACTIVE DETRUSOR Central (complete/incomplete): Spinal cord injury-
THE ACONTRACTILE BLADDER - FACT OR FICTION? Jacob Golomb Department of Urology Chaim Sheba Medical Center Tel Hashomer NEUROGENIC UNDERACTIVE DETRUSOR Central (complete/incomplete): Spinal cord injury-
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
Catherine A. Welch 1*, Séverine Sabia 1,2, Eric Brunner 1, Mika Kivimäki 1 and Martin J. Shipley 1
 Welch et al. BMC Medical Research Methodology (2018) 18:89 https://doi.org/10.1186/s12874-018-0548-0 RESEARCH ARTICLE Open Access Does pattern mixture modelling reduce bias due to informative attrition
Welch et al. BMC Medical Research Methodology (2018) 18:89 https://doi.org/10.1186/s12874-018-0548-0 RESEARCH ARTICLE Open Access Does pattern mixture modelling reduce bias due to informative attrition
Protocol Development: The Guiding Light of Any Clinical Study
 Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
Takashi Yagisawa 1,2*, Makiko Mieno 1,3, Norio Yoshimura 1,4, Kenji Yuzawa 1,5 and Shiro Takahara 1,6
 Yagisawa et al. Renal Replacement Therapy (2016) 2:68 DOI 10.1186/s41100-016-0080-9 POSITION STATEMENT Current status of kidney transplantation in Japan in 2015: the data of the Kidney Transplant Registry
Yagisawa et al. Renal Replacement Therapy (2016) 2:68 DOI 10.1186/s41100-016-0080-9 POSITION STATEMENT Current status of kidney transplantation in Japan in 2015: the data of the Kidney Transplant Registry
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Torsion bottle, a very simple, reliable, and cheap tool for a basic scoliosis screening
 Romano and Mastrantonio Scoliosis and Spinal Disorders (2018) 13:4 DOI 10.1186/s13013-018-0150-6 RESEARCH Open Access Torsion bottle, a very simple, reliable, and cheap tool for a basic scoliosis screening
Romano and Mastrantonio Scoliosis and Spinal Disorders (2018) 13:4 DOI 10.1186/s13013-018-0150-6 RESEARCH Open Access Torsion bottle, a very simple, reliable, and cheap tool for a basic scoliosis screening
Overview. Methods of assessment. Assessment of LUTS. Methods of self-report assessment. Why use self-report instruments 11/12/2015
 Overview How are LUTS and quality of life (QoL) assessed? Patient Assessment and Bladder Diary Patient-centred questionnaire evaluation ICIQ-Bladder Diary Nikki Cotterill and Alan Uren Assessment of LUTS
Overview How are LUTS and quality of life (QoL) assessed? Patient Assessment and Bladder Diary Patient-centred questionnaire evaluation ICIQ-Bladder Diary Nikki Cotterill and Alan Uren Assessment of LUTS
Quality standard Published: 18 September 2013 nice.org.uk/guidance/qs45
 Lower urinary tract symptoms in men Quality standard Published: 18 September 2013 nice.org.uk/guidance/qs45 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Lower urinary tract symptoms in men Quality standard Published: 18 September 2013 nice.org.uk/guidance/qs45 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Implementation of estimands in Novo Nordisk
 Implementation of estimands in Novo Nordisk Søren Andersen Helle Lynggaard Biostatistics, Novo Nordisk A/S DSBS meeting 26 October 2017 2 Agenda Overview of implementation process Cross-functional working
Implementation of estimands in Novo Nordisk Søren Andersen Helle Lynggaard Biostatistics, Novo Nordisk A/S DSBS meeting 26 October 2017 2 Agenda Overview of implementation process Cross-functional working
Title: Intention-to-treat and transparency of related practices in randomized, controlled trials of anti-infectives
 Author s response to reviews Title: Intention-to-treat and transparency of related practices in randomized, controlled trials of anti-infectives Authors: Robert Beckett (rdbeckett@manchester.edu) Kathryn
Author s response to reviews Title: Intention-to-treat and transparency of related practices in randomized, controlled trials of anti-infectives Authors: Robert Beckett (rdbeckett@manchester.edu) Kathryn
Key words: Lower Urinary Tract Symptoms (LUTS), Prostatic Hyperplasia, Alpha-1 Adrenoceptor Antagonists, Tamsulosin, Terazosin.
 The Professional Medical Journal DOI: 10.17957/TPMJ/17.4102 ORIGINAL PROF-4102 PROSTATIC HYPERPLASIA; COMPARISON BETWEEN TAMSULOSIN AND TERAZOSIN FOR EFFICACY IN MEDICAL MANAGEMENT OF LOWER URINARY TRACT
The Professional Medical Journal DOI: 10.17957/TPMJ/17.4102 ORIGINAL PROF-4102 PROSTATIC HYPERPLASIA; COMPARISON BETWEEN TAMSULOSIN AND TERAZOSIN FOR EFFICACY IN MEDICAL MANAGEMENT OF LOWER URINARY TRACT
Overview. Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia. Iain McAuley September 15, 2014
 Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy (Protocol
Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy (Protocol
What should we consider before surgery? BPH with bladder dysfunction. Inje University Sanggye Paik Hospital Sung Luck Hee
 What should we consider before surgery? BPH with bladder dysfunction Inje University Sanggye Paik Hospital Sung Luck Hee Diagnostic tests in three categories Recommendation: there is evidence to support
What should we consider before surgery? BPH with bladder dysfunction Inje University Sanggye Paik Hospital Sung Luck Hee Diagnostic tests in three categories Recommendation: there is evidence to support
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
NEED A SAMPLE SIZE? How to work with your friendly biostatistician!!!
 NEED A SAMPLE SIZE? How to work with your friendly biostatistician!!! BERD Pizza & Pilots November 18, 2013 Emily Van Meter, PhD Assistant Professor Division of Cancer Biostatistics Overview Why do we
NEED A SAMPLE SIZE? How to work with your friendly biostatistician!!! BERD Pizza & Pilots November 18, 2013 Emily Van Meter, PhD Assistant Professor Division of Cancer Biostatistics Overview Why do we
Cone-beam CT findings during prostate artery embolization for benign prostatic hyperplasia-induced lower urinary tract symptoms: a case report
 Chen et al. BMC Urology (2017) 17:120 DOI 10.1186/s12894-017-0311-6 CASE REPORT Cone-beam CT findings during prostate artery embolization for benign prostatic hyperplasia-induced lower urinary tract symptoms:
Chen et al. BMC Urology (2017) 17:120 DOI 10.1186/s12894-017-0311-6 CASE REPORT Cone-beam CT findings during prostate artery embolization for benign prostatic hyperplasia-induced lower urinary tract symptoms:
Supplementary Online Content
 Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
National Institute for Health and Care Excellence. Lower Urinary Tract Symptoms Update Addendum Consultation Table 3 rd February 5 pm 3 rd March 2015
 British Association of Urological Surgeons British Association of Urological Surgeons National Institute for Health and Care Excellence Lower Urinary Tract Symptoms Update Addendum Consultation Table 3
British Association of Urological Surgeons British Association of Urological Surgeons National Institute for Health and Care Excellence Lower Urinary Tract Symptoms Update Addendum Consultation Table 3
Index. urologic.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Cochrane Pregnancy and Childbirth Group Methodological Guidelines
 Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
GRADE. Grading of Recommendations Assessment, Development and Evaluation. British Association of Dermatologists April 2018
 GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
UV-2005/01. Chronic Prostatitis and Chronic Pelvic Pain Syndrom (CP/CPPS) Karl-Bickleder-Str. 44C Straubing - Germany
 SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
SYNOPSIS UV-2005/01 Title: Short Title: Indication: Phase: Study Code: Study Director Co-ordinating Investigator: Study Centres: Multicentre, randomised, double-blind, placebo-controlled clinical study
EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT
 Basrah Journal Of Surgery EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT MB, ChB, FIBMS, Assistant Professor
Basrah Journal Of Surgery EVALUATION OF THE EFFICACY OF TADALAFIL IN IMPROVING LOWER URINARY TRACT SYMPTOMS IN PATIENTS WITH SYMPTOMATIC BENIGN PROSTATIC ENLARGEMENT MB, ChB, FIBMS, Assistant Professor
Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist
 Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist Lower Urinary Tract Symptoms Storage Symptoms Frequency, urgency, incontinence, Nocturia Voiding Symptoms Hesitancy, poor flow, intermittency,
Lower Urinary Tract Symptoms K Kuruvilla Zachariah Associate Specialist Lower Urinary Tract Symptoms Storage Symptoms Frequency, urgency, incontinence, Nocturia Voiding Symptoms Hesitancy, poor flow, intermittency,
Association of BPH with OAB: The Plumbing or the Pump?
 Association of BPH with OAB: The Plumbing or the Pump? Ryan P. Terlecki, MD FACS Associate Professor of Urology Director, Men s Health Clinic Director, GURS Fellowship in Reconstructive Urology, Prosthetic
Association of BPH with OAB: The Plumbing or the Pump? Ryan P. Terlecki, MD FACS Associate Professor of Urology Director, Men s Health Clinic Director, GURS Fellowship in Reconstructive Urology, Prosthetic
Prevalence of Benign Prostatic Hyperplasia on Jeju Island: Analysis from a Cross-sectional Community-based Survey
 pissn: 2287-4208 / eissn: 2287-4690 World J Mens Health 2012 August 30(2): 131-137 http://dx.doi.org/10.5534/wjmh.2012.30.2.131 Original Article Prevalence of Benign Prostatic Hyperplasia on Jeju Island:
pissn: 2287-4208 / eissn: 2287-4690 World J Mens Health 2012 August 30(2): 131-137 http://dx.doi.org/10.5534/wjmh.2012.30.2.131 Original Article Prevalence of Benign Prostatic Hyperplasia on Jeju Island:
Male Lower Urinary Tract Symptoms: Management in primary care and beyond. Daniel Cohen PhD FRCS(Urol) Consultant Urological Surgeon
 Male Lower Urinary Tract Symptoms: Management in primary care and beyond Daniel Cohen PhD FRCS(Urol) Consultant Urological Surgeon 1 LUTS Very common: 1/3 men over age of 50 have moderate to severe LUTS
Male Lower Urinary Tract Symptoms: Management in primary care and beyond Daniel Cohen PhD FRCS(Urol) Consultant Urological Surgeon 1 LUTS Very common: 1/3 men over age of 50 have moderate to severe LUTS
GUIDELINES ON NON-NEUROGENIC MALE LUTS INCLUDING BENIGN PROSTATIC OBSTRUCTION
 GUIDELINES ON NON-NEUROGENIC MLE LUTS INCLUDING BENIGN PROSTTIC OBSTRUCTION (Text update March 2015) S. Gravas (Chair), T. Bach,. Bachmann, M. Drake, M. Gacci, C. Gratzke, S. Madersbacher, C. Mamoulakis,
GUIDELINES ON NON-NEUROGENIC MLE LUTS INCLUDING BENIGN PROSTTIC OBSTRUCTION (Text update March 2015) S. Gravas (Chair), T. Bach,. Bachmann, M. Drake, M. Gacci, C. Gratzke, S. Madersbacher, C. Mamoulakis,
Last Review Status/Date: December Summary
 Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
H6D-MC-LVHR Clinical Study Report Synopsis Page LVHR Synopsis (LY450190)
 H6D-MC-LVHR Clinical Study Report Synopsis Page 1 2. LVHR Synopsis H6D-MC-LVHR Clinical Study Report Synopsis Page 2 Clinical Study Report Synopsis: Study H6D-MC-LVHR Title of Study: A Randomized, Double-Blind,
H6D-MC-LVHR Clinical Study Report Synopsis Page 1 2. LVHR Synopsis H6D-MC-LVHR Clinical Study Report Synopsis Page 2 Clinical Study Report Synopsis: Study H6D-MC-LVHR Title of Study: A Randomized, Double-Blind,
Effectiveness of the surgical torque limiter: a model comparing drill- and hand-based screw insertion into locking plates
 Ioannou et al. Journal of Orthopaedic Surgery and Research (2016) 11:118 DOI 10.1186/s13018-016-0458-y RESEARCH ARTICLE Effectiveness of the surgical torque limiter: a model comparing drill- and hand-based
Ioannou et al. Journal of Orthopaedic Surgery and Research (2016) 11:118 DOI 10.1186/s13018-016-0458-y RESEARCH ARTICLE Effectiveness of the surgical torque limiter: a model comparing drill- and hand-based
INVESTIGATION OF LOWER URINARY TRACT SYMPTOMS IN UROLOGICAL OUTPATIENTS USING ORIGINAL IPSS PLUS POST MICTURITION DRIBBLE QUESTIONNAIRE
 INVESTIGATION OF LOWER URINARY TRACT SYMPTOMS IN UROLOGICAL OUTPATIENTS USING ORIGINAL IPSS PLUS POST MICTURITION DRIBBLE QUESTIONNAIRE Tadashi Hanail*, Seiji Matsumotol*, Nobutaka Shimizu, Hirotsugu Uemural
INVESTIGATION OF LOWER URINARY TRACT SYMPTOMS IN UROLOGICAL OUTPATIENTS USING ORIGINAL IPSS PLUS POST MICTURITION DRIBBLE QUESTIONNAIRE Tadashi Hanail*, Seiji Matsumotol*, Nobutaka Shimizu, Hirotsugu Uemural
Predictors of short-term overactive bladder symptom improvement after transurethral resection of prostate in men with benign prostatic obstruction
 bs_bs_banner International Journal of Urology (2014) 21, 1035 1040 doi: 10.1111/iju.12482 Original Article: Clinical Investigation Predictors of short-term overactive bladder symptom improvement after
bs_bs_banner International Journal of Urology (2014) 21, 1035 1040 doi: 10.1111/iju.12482 Original Article: Clinical Investigation Predictors of short-term overactive bladder symptom improvement after
A SURVEY ON LOWER URINARY TRACT SYMPTOMS (LUTS) AMONG PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA (BPH) IN HOSPITAL UNIVERSITI SAINS MALAYSIA (HUSM)
 Malaysian Journal of Medical Sciences, Vol. 14, No. 2, July 2007 (67-71) SHORT COMMUNICATION A SURVEY ON LOWER URINARY TRACT SYMPTOMS (LUTS) AMONG PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA (BPH) IN HOSPITAL
Malaysian Journal of Medical Sciences, Vol. 14, No. 2, July 2007 (67-71) SHORT COMMUNICATION A SURVEY ON LOWER URINARY TRACT SYMPTOMS (LUTS) AMONG PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA (BPH) IN HOSPITAL
Issues to Consider in the Design of Randomized Controlled Trials
 Issues to Consider in the Design of Randomized Controlled Trials Jay Wilkinson, MD, MPH Professor of Pediatrics & Epidemiology Miller School of Medicine Seminar Purpose To facilitate an interactive discussion
Issues to Consider in the Design of Randomized Controlled Trials Jay Wilkinson, MD, MPH Professor of Pediatrics & Epidemiology Miller School of Medicine Seminar Purpose To facilitate an interactive discussion
Understanding test accuracy research: a test consequence graphic
 Whiting and Davenport Diagnostic and Prognostic Research (2018) 2:2 DOI 10.1186/s41512-017-0023-0 Diagnostic and Prognostic Research METHODOLOGY Understanding test accuracy research: a test consequence
Whiting and Davenport Diagnostic and Prognostic Research (2018) 2:2 DOI 10.1186/s41512-017-0023-0 Diagnostic and Prognostic Research METHODOLOGY Understanding test accuracy research: a test consequence
Statistical Analysis Plan
 Effectiveness of nutritional supplementation (RUTF and multi micronutrient) in preventing malnutrition in children 6-59 months with infection (malaria, pneumonia, diarrhoea), a randomized controlled trial
Effectiveness of nutritional supplementation (RUTF and multi micronutrient) in preventing malnutrition in children 6-59 months with infection (malaria, pneumonia, diarrhoea), a randomized controlled trial
Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods
 Jakobsen et al. BMC Medical Research Methodology 2014, 14:120 CORRESPONDENCE Open Access Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods Janus Christian
Jakobsen et al. BMC Medical Research Methodology 2014, 14:120 CORRESPONDENCE Open Access Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods Janus Christian
