STUDY. Intralesional Immunotherapy of Warts With Mumps, Candida, and Trichophyton Skin Test Antigens
|
|
|
- Lesley Adams
- 6 years ago
- Views:
Transcription
1 STUDY Intralesional Immunotherapy of Warts With Mumps, Candida, and Trichophyton Skin Test Antigens A Single-blinded, Randomized, and Controlled Trial Thomas D. Horn, MD; Sandra M. Johnson, MD; Ricki M. Helm, PhD; Paula K. Roberson, PhD Background: Warts occur commonly in humans. Destructive modalities are generally the first physicianadministered therapy. Other treatment options include immunotherapy. Intralesional immunotherapy using mumps, Candida, ortrichophyton skin test antigens has proved efficacy in the treatment of warts. Objectives: To determine rates of wart resolution in response to injection of antigen alone, antigen plus interferon alfa-2b, interferon alfa-2b alone, and normal saline; and to compare response according to viral type, major histocompatibility complex antigens, and peripheral blood mononuclear cell proliferation to autologous human papillomavirus antigen before and after injection. Design: Randomized, single-blinded, placebocontrolled, clinical trial. Setting: Medical school based dermatology department. Patients: Two hundred thirty-three patients clinically diagnosed as having 1 or more warts. Main Outcome Measure: Clinical resolution of warts in response to intralesional immunotherapy. Results: Responders were observed in all treatment arms, but were significantly more likely to have received antigen (P.001). Resolution of distant untreated warts was observed, and was significantly more likely in subjects receiving antigen (P.001). Interferon did not significantly enhance the response rate (P=.20) and did not differ from normal saline (P=.65). No viral type or major histocompatibility complex antigen correlated with response or lack of response (P.99 and P=.86, respectively). A positive peripheral blood mononuclear cell proliferation assay result (2 times pretreatment levels) was significantly more likely among responders (P=.002). While there was no significant difference in response based on sex (P=.56), older subjects ( 40 years) were less likely to respond (P=.01). Conclusions: Intralesional immunotherapy using injection of Candida, mumps, or Trichophyton skin test antigens is an effective treatment for warts, as indicated by significantly higher response rates and distant response rates in subjects receiving antigen. Viral type and major histocompatibility complex antigens did not seem to influence treatment response. Response is accompanied by proliferation of peripheral blood mononuclear cells to human papillomavirus antigens, suggesting that a human papillomavirus directed cell-mediated immune response plays a role in wart resolution. Arch Dermatol. 2005;141: Author Affiliations are listed at the end of this article. Financial Disclosure is listed at the end of this article. WARTS ARE COMMON IN humans and account for 8% of visits to dermatologists. 1 While many warts resolve spontaneously over several years, most patients seek treatment, because the warts are unsightly and often tender or painful. Primary treatment for verruca vulgaris includes destructive therapies, such as topical salicylic acid, cryotherapy with liquid nitrogen, excision, laser vaporization, and bleomycin sulfate injection. Because wart proliferation is controlled by the immune system, 2 various methods have been used to stimulate the immunologic response to the human papillomavirus (HPV). Among these are topically applied inorganic molecules capable of eliciting contact hypersensitivity, imiquimod, and intralesional interferons. CME course available at Researchers 3,4 have shown the effectiveness, in the treatment of common warts, of intralesional injection of antigen preparations of mumps, Candida, or Trichophyton, normally used to assay the status of the cellular immune system via intradermal injection. In an earlier study, 3 74% of subjects receiving immunotherapy experienced reso- 589
2 lution of the treated wart and 78% of subjects with multiple warts experienced resolution of untreated distant warts. Of subjects treated in the cryotherapy arm of the same study, 57% experienced resolution of the treated wart, while no distant wart responses occurred. In a separate report 5 evaluating the effectiveness of this treatment in children, in all of whom 2 treatment modalities had failed, 47% of subjects responded to immunotherapy and 34% cleared untreated distant and anatomically distinct warts. Herein, we extend these observations on intralesional injection of skin test antigens for common warts and report on a single-blinded, randomized, and controlled trial comparing immunotherapy, immunotherapy plus intralesional interferon alfa-2b, intralesional interferon alfa-2b alone, and normal saline. Data correlating treatment success or failure in individual subjects to HPV type, major histocompatibility complex (MHC) antigens, and degree of pretreatment andposttreatmentperipheralbloodmononuclearcell(pbmc) proliferation in response to HPV antigens are presented. METHODS The study protocol received approval from the institutional investigational review boards of the University of Arkansas for Medical Sciences, the Central Arkansas Veterans Healthcare System, Arkansas Children s Hospital, and the General Clinical Research Center of the University of Arkansas for Medical Sciences. PATIENTS AND IMMUNOTHERAPY PROCEDURES Patients (or their guardians) clinically diagnosed as having 1 or multiple warts provided informed consent and were randomized after obtaining a positive intradermal pretest result to 1 of 4 groups: (1) intralesional injection with mumps (Connaught, Swiftwater, Pa), Candida (Bayer, Spokane, Wash), or Trichophyton (Alk-Abello, Round Rock, Tex) skin test antigen preparation (mumps antigen is not currently commercially available); (2) intralesional injection with mumps, Candida, or Trichophyton skin test antigen preparation plus interferon alfa-2b, IU; (3) intralesional injection with interferon alfa-2b, IU; or (4) intralesional injection with saline. Mumps skin test antigen is a concentrated product derived from virus killed with formaldehyde solution and formulated to contain a minimum of 40 colony-forming units per milliliter. Candida and Trichophyton skin test antigens represent allergenic extracts in which crude allergen is added to extracting solution (sodium chloride, sodium bicarbonate, glycerin, and phenol or thimerosal) in specific ratios of allergen to solution, and expressed as protein nitrogen units or weight per volume. We used products of 1000 protein nitrogen units per milliliter and 1:500 wt/vol, respectively. Randomization was computer generated, and the sequence was provided to the investigators in sealed envelopes. All subjects were tested for existing immunity to each antigen preparation by placing 0.1 ml intradermally into the skin of the forearm (pretest). Determination of a positive reaction necessitated induration of at least 5 mm in diameter. The test antigen that induced the greatest response was then used for intralesional injection. If equal reactions occurred, then an arbitrary assignment to Candida first, then Trichophyton, and then mumps was used. If all skin test results were nonreactive, the patient was excluded from the study. Intralesional injection was performed using the following volumes per group: (1) 0.3 ml of antigen preparation, (2) 0.3 ml of antigen preparation plus 0.08 ml of interferon alfa-2b, (3) 0.08 ml of interferon alfa-2b, and (4) 0.3 ml of saline. The effectiveness of this amount of antigen preparation was previously established. 3 Only the largest wart, based on surface area, was treated in patients with multiple warts. All subjects received injections every 3 weeks into the same wart until complete clearing of the treated wart was achieved or for a maximum of 5 treatments. Patients were examined at study initiation and at each episode of treatment with notation as to the number and surface area of warts. Surface area was estimated by measurement of diameter using a ruler. At the follow-up visits, presence or absence of response to treatment and approximate decrease in size of warts in responders were recorded. Complete resolution was judged to have occurred when the thickening, hyperkeratosis, and dilated vasculature of the treated wart were no longer evident and normal skin markings returned. No response was judged to have occurred if there was less than 25% decrease in surface area of the treated wart. Partial responses were estimated as follows: 25% to 50%, 51% to 75%, and greater than 75% but less than 100%. For analytic purposes, only those subjects with greater than 75% improvement in the injected or distantly responding warts were defined as responders. Exclusion criteria consisted of prior allergic response to any of the antigen preparations, pregnancy, lactation, infection with human immunodeficiency virus type 1, clinical evidence of epidermodysplasia verruciformis, iatrogenic immunosuppression, primary immunosuppression, or any generalized dermatitis. Only subjects with multiple warts were chosen for viral typing and assay of PBMC proliferation because these procedures required biopsy of 1 wart. Fresh tissue was used for viral typing, as described later. Protein extracted from a portion of the tissue was used in the PBMC stimulation assay, as described later. VIRAL TYPING Sample specimens of wart obtained from consenting patients were received in the laboratory and processed for HPV typing. A section of wart tissue, approximately 2 mm 3, was placed in a sterile microtube and mixed in 200 µl of HPV digestion solution, consisting of a fresh mixture of 1 ml of 1M Tris buffer, ph 8.0; 1 ml of 1M Tris buffer, ph 9.0; 80 µl of 0.5M EDTA, ph 8.0; 200 µl of Tween 20; and 37.1 ml of sterile water and endopeptidase K in a ratio of 197 µl of HPV digestion solution to 3 µl of endopeptidase K. The microtubes were incubated overnight in a 65 C water bath, followed by heating at 95 C to inactivate the endopeptidase K. The microtubes were then centrifuged at 6000 rpm in a microcentrifuge(eppendorf)for5minutes.theresultingdigestsweredecanted andstoredoniceorat 80 C.Viralphenotypingwasthenperformed on the wart digests via polymerase chain reaction (PCR) using sets of PCR primers specific for the HPV genotypes. 6 Template (wart digest or negative control), 2 µl, was added to a PCR master mix consisting of the following components: 45 µl of Supermix(Gibco BRL, Gaithersburg, Md), 2 µl of primer A, and 2 µl of primer B on ice (primers A and B represent primer pairs). The PCR cycling program was run under the following conditions: step 1, 2 minutes at 95 C for the initial melt; step 2, 1 minute at 95 C; step 3, 90 seconds at 50 C; step 4, 2 minutes at 72 C; step 5, repeat steps 2 through 4 a total of 29 times; step 6, 5 minutes at 72 C; and step 7, store at 4 C. The PCR products were then run on a 1.2% agarose gel and identified as to HPV genotype by (1) presence or absence of a specific PCR product and (2) generation of a PCR product of the appropriate band size. 7 PBMC PROLIFERATION ASSAY Venous blood was collected in heparinized test tubes for mononuclear cell isolation before treatment and, on average, 4.5 weeks 590
3 (range, 3-6 weeks) after initiation of the protocol. Sample specimens were immediately transferred to the laboratory for processing. A wart extract from the tissue was prepared by transferring the tissue to a homogenizer (Dounce) containing 1.5 ml of sterile saline or phosphate-buffered saline (PBS). The tissue was homogenized and filtered through a 45-µm filter attached to a 3-mL syringe into sterile cryovials and stored at 20 C. Venous blood, 15 ml, was transferred to a 50-mL centrifuge tube, diluted to a total volume of 30 ml with saline or PBS, underlaid with a density gradient (Fico/Lite-LymphoH; Atlanta Biologicals, Norcross, Ga), and centrifuged for 20 minutes at 2100 rpm (model 5804R centrifuge; Eppendorf). Interface cells were collected and washed twice with saline or PBS, centrifuged, and resuspended in saline or PBS. Cell counts were performed using a particle counter (Z1; Beckman Coulter, Fullerton, Calif), and the cells were resuspended in freezing media (RPMI/20% human AB serum) and stored at 70 C. Prespecimen and postspecimen PBMCs from each patient were stored for proliferation assays. Peripheral blood mononuclear cells were thawed, washed twice in saline or PBS, and resuspended at cells per milliliter in RPMI/10% human AB serum. Cells were plated at 200 µl per well, and wart extract (5, 10, and 15 µl in quadruplicate) was added to desired wells. Concanavalin A, 5 µg/ml, was added as a positive control. Plates were incubated at 37 C, 5% carbon dioxide, for 5 days and then pulsed with 1 µci ( Bq) tritium-thymidine for an additional 6 hours. Cells were harvested and lysed with an automated cell harvester (Filtermate 196; Packard, Meriden, Conn), the filter was air dried, and cells were counted in an automated beta plate counter (Matrix 9600 Direct Beta Counter; Packard). Results were calculated by averaging the groups and dividing by treated wells with media alone (nonspecific incorporation) to obtain a stimulation index. A positive stimulation index was determined if a subject had a 2-fold increase comparing before and after blood draws. MHC DETERMINATION HLA-A, HLA-B, and HLA-DRB typing was performed by a PCR, followed by a sequence-specific oligonucleotide probes method. 8 This method uses locus-specific primers to amplify the polymorphic exons of the gene of interest (exon 2 for HLA-DRB and exons 2 and 3 for HLA-A and HLA-B). Amplified DNA was spotted onto replicate nylon membranes and probed with 30 to 60 different horseradish peroxidase labeled oligonucleotide probes. After a stringency wash, the positive reactions were detected with a chromogenic substrate (tetramethylbenzidine). STATISTICAL ANALYSIS Power/Sample Size The study was originally designed to enroll 100 patients in each of 4 study arms. Assuming 40% clearance of the injected warts in the placebo arm, no effect of granulocyte-macrophage colonystimulating factor (GM-CSF) (later amended to interferon alfa- 2b), and an increase to 60% clearance in each immunotherapy arm, the study had 93% power to detect a difference among treatment arms in the primary comparison of response rates, using 2 3. If GM-CSF or interferon alfa-2b had no effect over placebo but boosted the clearance to 70% when combined with immunotherapy, the power to detect an overall difference among the 4 study arms was 99%. After enrollment of 233 patients, however, an unplanned interim analysis was conducted, for administrative reasons (the supply of mumps antigen was no longer assured), with the intention of stopping the trial if significant treatment effects were evident. By using Gordon Lan DeMets 9 boundaries, it was determined that the stopping boundary for a significant result at this point in the trial should be P.005 (observed). The observed (uncorrected) P value for the primary overall comparison of the 4 groups was less than.001, and enrollment in the trial was stopped. All patients already enrolled in the trial at that point were followed up to planned completion of treatment. Once the decision was made to stop the trial using the Gordon Lan DeMets criteria, further analyses were reported in terms of nominal (uncorrected) P values. Randomization Randomization was conducted by means of sealed envelopes prepared by the Department of Biostatistics at the University of Arkansas for Medical Sciences, using randomized blocks of random block sizes. This ensured approximately equal numbers of patients in each treatment arm at any point in the study. No stratification factors were used for randomization. After a patient who had agreed to participate and had signed informed consent was determined to be reactive to at least 1 of the study antigens, the next sealed envelope was opened in sequence, revealing the assigned study arm. The study was single blind in that the patients, but not the study investigators, were blinded to an individual s treatment assignment. Data Analysis Cross-classified categorical data were analyzed using 2 tests. Characteristics measured on a continuous scale (ie, age) were compared between responders and nonresponders using t tests or Wilcoxon rank sum tests and between treatment groups using analysis of variance or Kruskal-Wallis tests. The simultaneous effects of treatment and age on the probability of response were modeled using logistic regression. RESULTS Two hundred thirty-three subjects enrolled in the study. The initial design included treatment arms using GM-CSF insteadofinterferonalfa-2b. Becauseofseriousadverseevents experienced by subjects receiving GM-CSF, these arms were discontinued and the trial proceeded using interferon alfa- 2b in place of GM-CSF. Twenty-three subjects received GM- CSF and are discussed separately, but excluded from analysis, except where expressly noted. Most subjects underwent treatment before enrolling in the study: 49 of 58 in the antigen alone group, 38 of 43 in the antigen plus interferon alfa-2b group, 36 of 48 in the interferon alfa-2b alone group, and 49 of 61 in the saline group. An additional 9 subjects were randomized, but underwent no injections or failed to return for even 1 follow-up visit. Thus, 201 subjects form the main group for analysis. Data on subject age and sex are provided in Table 1. There were no significant differences among the 4 study arms in terms of age (P=.20) or sex (P=.32). Data regarding positive intradermal skin test results (defined as a 5-mm induration) before study initiation for all 233 enrolled subjects are as follows. Positive results to Candida, mumps, and Trichophyton were found in 19 subjects; Candida alone, 53 subjects; mumps alone, 40 subjects; Trichophyton alone, 21 subjects; Candida and mumps, 67 subjects; Candida and Trichophyton, 20 subjects; and mumps and Trichophyton, 13 subjects. Fifty-two of 285 subjects (18.2%) were nonreactive to all antigens. 591
4 Table 1. Immunotherapy Response Data in 201 Subjects Immunotherapy Arm Total Subjects Average Age, y Female-Male Ratio Responder* Nonresponder* Antigen alone :23 29 (54) 25 (46) Antigen plus interferon alfa-2b :12 28 (68) 13 (32) Interferon alfa-2b alone :21 12 (26) 34 (74) Normal saline :28 13 (22) 47 (78) *Data are given as number (percentage) of each group. IMMUNOTHERAPY WITH ANTIGEN VS INTERFERON ALFA-2B OR NORMAL SALINE ALONE ResponsedataineacharmofthestudyareshowninTable1. These are from the 201 subjects with at least 1 follow-up. Compared with injection with interferon alfa-2b or saline alone, subjects receiving antigen, with or without concomitant interferon injection, were statistically more likely to respond(p.001).thesameresultwasobtainedwhenanalyzing the 4 groups separately as when analyzing 2 groups: (1) antigen with or without interferon alfa-2b against (2) interferon alfa-2b alone and saline alone. Response to interferon alfa-2b injection alone was similar to that to saline (P=.65). Adding interferon alfa-2b did not improve response compared with injection of antigen alone(p=.20). There were no differences in response among the individual antigens (Candida, 59%; mumps, 51%; Trichophyton, 62%; P=.48). Fifty-seven subjects injected with antigen were judged to have 100% resolution of warts at study conclusion, 21 of whom had more than 1 wart and experienced 100% study resolution of all distant warts. In the interferon alfa-2b and saline groups, these numbers were 25 and 11, respectively. There was no difference in response based on sex (P=.56), but subjects who did not respond were significantly older (39 vs 34 years) than those who did respond (P=.01, t test; P=.04, Wilcoxon rank sum test). A multivariate logistic regression analysis indicated that age younger than 40 years was positively associated with the probabilityofresponse, aftercontrollingforantigeninjection(p=.01). Across responders in all groups, the average number of injections to complete response was 4.6. The average number of treatments in responding subjects receiving antigen was 5.8. IMMUNOTHERAPY WITH ANTIGEN AND DISTANT RESPONSES Distant response data in each arm of the study are shown in Table 2. One hundred fifty-three subjects had more than 1 wart. Compared with injection with interferon alfa-2b or saline alone, subjects with more than 1 wart receiving antigen, with or without concomitant interferon injection, were statistically more likely to experience resolution of untreated and anatomically distinct warts (P.001). The same result was obtained when analyzing the 4 groups separately as when analyzing the 2 groups, as previously described. The addition of interferon alfa-2b to antigen did not significantly improve response (P=.24). There was no significant difference in response between interferon alfa-2b and saline (P=.23). ADVERSE EVENTS In 1027 episodes of treatment, 47 subjects reported fever and myalgias (4.6% of treatment episodes) and 46 subjects reported edema and erythema at the injection site of acral lesions (4.5% of treatment episodes). Of the 47 subjects experiencing fever and myalgias, 9 received interferon alfa-2b alone, 30 received interferon alfa-2b plus antigen, 7 received mumps antigen alone, and 1 received saline. The 46 subjects experiencing edema and erythema were scattered among all treatment groups. In no case were these adverse events a cause of study discontinuation. Oral antipyretic medications were used to lower temperature. Cool compresses and limb elevation were used to lessen edema and erythema. Signs and symptoms resolved within 24 hours in all subjects. GM-CSF AND SERIOUS ADVERSE EFFECTS Of the 23 subjects receiving GM-CSF, 2 developed hypotension, necessitating medical intervention, within 4 hours of intralesional injection. For this reason, the study arms using GM-CSF were halted, with substitution of interferon alfa-2b for GM-CSF, using the same randomization protocol. Of 10 subjects receiving GM-CSF alone, 2 responded, while 10 of 13 receiving GM-CSF plus antigen responded. These data are excluded from the remainder of the analysis, except as indicated. RESPONDERS VS NONRESPONDERS FOR POSITIVE PBMC PROLIFERATION ASSAY RESULTS Thirty-five subjects consented to the PBMC proliferation assay. Of the 19 who responded to treatment, 17 had significant PBMC proliferation after intralesional injection and 2 did not. Of the 16 who did not respond to treatment, 6 had significant PBMC proliferation after intralesional injection and 10 did not. Because only 35 subjects were studied, the data include subjects receiving GM- CSF and interferon alfa-2b. Nineteen subjects received antigen or antigen plus interferon alfa-2b (or GM-CSF), and 16 received interferon alfa-2b (or GM-CSF) or saline. Responders were more likely to have a positive PBMC proliferation assay result, as previously defined, than nonresponders (P=.002). There was no significant association between use of antigen or antigen plus interferon alfa-2b (or GM-CSF) vs saline or interferon alfa-2b (or GM-CSF) alone and PBMC proliferation assay response 592
5 Table 2. Distant Response Data* Study Arm Responder Nonresponder Antigen alone (n = 37) 15 (41) 22 (59) Antigen plus interferon alfa-2b 20 (57) 15 (43) (n = 35) Interferon alfa-2b alone (n = 34) 3 (9) 31 (91) Normal saline (n = 47) 9 (19) 38 (81) *Response was defined as 100% resolution of at least 1 anatomically distinct distant wart. Data are given as number (percentage) of each group. (P.99). Proliferative responses to Candida, mumps, or Trichophyton antigen were not assayed to conserve PBMCs for study of HPV response. RESPONSE TO IMMUNOTHERAPY AND VIRAL TYPE OR MHC ANTIGEN Viral type was determined in warts from 146 subjects: type 2a, 27, or 57 were found in 120 subjects, type 1a in 13 subjects, type 3 or 10 in 5 subjects, type 32 or 42 in 3 subjects, and another type in 5 subjects. More than 1 type was not identified in any subject. There was no significant association between viral type and response to any injection (P.99). This finding was also true among those receiving antigen (P=.86). Sixty-five subjects underwent MHC determination. There was no association between response and any of the MHC antigens found in this population. COMMENT Manipulating the immune system to achieve a therapeutic or protective response against diseases caused by HPV is an active field of investigation. Interferon alfa-2b is Food and Drug Administration approved for the treatment of condyloma, requiring twice-weekly injection for 3 weeks for optimal results. Topical imiquimod is also reported to have utility in the treatment of nongenital warts 10 and has Food and Drug Administration approval for the treatment of condyloma. Dinitrochlorobenzene, squaric acid dibutyl ester, diphenylcyclopropenone, and Toxicodendron extract have all been successfully used in topical immunotherapy against common warts. 11 The inorganic chemicals require an elicitation phase of several weeks. Use of Toxicodendron extract is arguably a viable option only in those persons known to be sensitive to plants in this genus, because the induction of sensitivity would be undesirable in terms of eliciting allergic contact dermatitis on exposure in nature. Few prospective controlled trials of wart therapies exist. We document the clinical efficacy of intralesional immunotherapy using Candida, mumps, or Trichophyton skin test antigens. The response of warts injected with antigen alone was lower in this study than in an earlier publication (60% [combined response data from Table 1, antigen alone and antigen plus interferon alfa-2b groups] vs 74% 3 ). Most subjects described herein were referred for the protocol, whereas in the earlier work, subjects were mainly recruited from a general clinic population. We speculate that a selection bias toward more recalcitrant disease exists in the patients constituting the present study group. Still, injection of antigen, with or without interferon alfa-2b, resulted in a meaningful clinical response when compared with injection of saline or interferon alfa-2b alone (56% vs 23%; P.001). Similarly, resolution of anatomically distinct untreated warts was more likely when antigen was injected compared with injection of saline or interferon alfa-2b alone (49% vs 16%; P.001). That older subjects ( 40 years) were less likely to respond to immunotherapy than younger subjects agrees with other data documenting less robust immunologic responses with increasing age. 12,13 We do not have long-term follow-up results of these subjects to be able to report on relapse rate. In mice vaccinated with viruslike particles to the major capsid protein L1, humoral and cellular responses could be enhanced through coinjection of DNA encoding the costimulatory molecule, B We sought to enhance local immunity through concomitant injection of cytokines. Injection of interferon alfa-2b did not afford a significant clinical benefit, either alone or in combination with antigen. It is possible that a regimen of more frequent injection and a higher dose might enhance outcome, but more frequent visits and larger volumes of injection are inconvenient and uncomfortable for patients. Because we hypothesize that immunologic events in the wart itself contribute to enhanced recognition of epitopes on the HPV, with subsequent expansion of lymphocyte clones targeting the virus, we initially thought to coinject GM-CSF to affect Langerhans cells and trafficking lymphocytes. 15 While the addition of GM-CSF to antigen injection or even injection of GM-CSF alone might provide a therapeutic response and while patients in these arms of the study were observed to respond, the occurrence of hypotension in 2 subjects necessitated discontinuation of GM-CSF in the protocol. Our repeated observation that untreated warts resolve after injection of only 1 wart prompts the speculation that intralesional immunotherapy induces HPV-directed immunity. Indeed, we have observed resolution of hundreds of flat warts in individual patients after injection of only 1 lesion. We found that increased proliferation of PBMCs in response to autologous HPV antigens after initiation of therapy was more likely to be observed among responders than nonresponders regardless of the substance injected (P=.002). It is possible that local and distant responses of warts in subjects who received saline or interferon alfa-2b alone develop by the same mechanism as when antigen is injected and that many triggers of an immune response to HPV exist. While injection of saline is an appropriate control, it is not a true placebo. That noted, local and systemic responses to saline injection in this study were far less likely than when antigen was used. While we found enhanced proliferation of PBMCs, we do not know the subtypes of the responding cells or the responsible effector populations leading to clinical resolution of common warts. In this study, we were unable to identify a correlation between wart response and viral type or any MHC antigen. What factors determine response or resistance to therapy is unclear. 593
6 While little work has been performed with cutaneous wart types in terms of understanding the interplay between viral infection and host immunity, the interest in developing effective vaccines against HPV types causing cervical dysplasia and carcinoma has led to significant knowledge in this area. T lymphocytes responding to HPV-16 can be identified in vivo in infected humans. 2 Lymphocyte proliferative responses are documented in humans in response to vaccination with HPV-18 E6 and E4 fusion proteins. 16 Human papillomavirus 16 L1 viruslike particle vaccination of humans has been shown to result in proliferation of CD4 and CD8 T lymphocytes. 17 In the same study, high neutralizing antibody titers after initial vaccination correlated with strong cytokine responses after a second vaccination 6 months later, suggesting that the cellular and humoral immune responses are linked. Peripheral blood mononuclear cells from patients with cervical cancer may be stimulated by autologous leukocytes pulsed with HPV-16 E7 to generate cytolytic T lymphocytes with appropriate specificity. 18 Rhesus macaques vaccinated with viruslike particles, chimeric viruslike particles, and plasmid DNA against HPV-16 L1 vary in the nature of immune response (humoral vs cellular) and the degree of immune response according to type of vaccination. 19 Intralesional immunotherapy is not a direct vaccination strategy, but may induce an immune response to HPV, perhaps based on mechanisms similar to those documented in the literature. We have mostly treated subjects with common warts but have observed complete responses using intralesional immunotherapy for genital warts as well (unpublished observation, 2004). Intralesional immunotherapy for common warts is effective and safe. It is unique in affording many patients a therapeutic response in untreated warts and may, through stimulation of HPV-directed immunity, provide fewer recurrent warts. While useful in any patient with warts, intralesional immunotherapy may be particularly useful in patients with numerous lesions or lesions covering large surface areas. Accepted for Publication: November 9, Author Affiliations: Departments of Dermatology (Drs Horn and Johnson), Pathology (Dr Horn), Microbiology/Immunology (Dr Helm), and Biostatistics (Dr Roberson), University of Arkansas for Medical Sciences; Central Arkansas Veterans Healthcare System (Drs Horn and Johnson); and Arkansas Children s Hospital Research Institute and Arkansas Children s Nutrition Center (Dr Helm), Little Rock. Financial Disclosure: Drs Horn and Johnson have obtained US patent 6,350,451 and have significant ownership interest in a start-up company formed through the University of Arkansas for Medical Sciences Biomedical Biotechnology Center. This company was created to market a product based on use of the antigens described in this article. Neither author has received payment to perform the work, and the company has not supported any portion of the research described in this article. Correspondence: Thomas D. Horn, MD, Department of Dermatology, University of Arkansas for Medical Sciences, 4301 W Markham, Mail Box 576, Little Rock, AR (hornthomasd@uams.edu). Funding/Support: This study was supported by grant M01RR14288 from the National Center for Research Resources, National Institutes of Health, Bethesda, Md. Acknowledgment: We thank Annette Clemons, PhD, and Charles West, BS, for their assistance in performing the viral typing and PBMC proliferation assays; Uwe Heine, PhD, of LabCorp, Burlington, NC, for methods pertaining to the MHC antigen determination; Schering-Plough, Kenilworth, NJ, for donating some of the interferon alfa-2b used; Immunex Corporation, Seattle, Wash, for donating the GM-CSF used; S. Elizabeth Whitmore, MD, for review of the manuscript; Tracie Baker, BS, for support with the clinical trial; Lucas Moore for secretarial assistance; and the many referring physicians and physicians in training who contributed to the study. REFERENCES 1. Stern R, Johnson M, DeLozier J. Utilization of physician services for dermatologic complaints. Arch Dermatol. 1977;113: Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8: Johnson S, Roberson P, Horn T. Intralesional injection of mumps or Candida skin test antigens. Arch Dermatol. 2001;137: Phillips R, Ruhl T, Pfenninger J, et al. Treatment of warts with Candida antigen injection. Arch Dermatol. 2000;136: Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20: Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82: Harwood CA, Spink PJ, Surentheran T, et al. Degenerate and nested PCR. J Clin Microbiol. 1999;37: Scharf SJ, Griffith RL, Erlich HA. Rapid typing of DNA sequence polymorphism at the HLA-DRB1 locus using the polymerase chain reaction and nonradioactive oligonucleotide probes. Hum Immunol. 1991;30: Gordon Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70: Micali G, Dall Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungal cutaneous warts. J Dermatolog Treat. 2003;14: Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145: Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100: Mittler JN, Lee WT. Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment, I: the primary T cell response is unaffected. Mech Ageing Dev. 2004;125: Zheng J, Si L, Song J, Sun X, Yu J, Wang Y. Enhanced immune response to DNAbased HPV16L1 vaccination by costimulatory molecule B7-2. Antiviral Res. 2003; 59: Prignano F, Gerlini G, Fossombroni V, et al. Control of the differentiation state and function of human epidermal Langerhans cells by cytokines in vitro. J Eur Acad Dermatol Venereol. 2001;15: Stanley MA. Progress in prophylactic and therapeutic vaccines for human papillomavirus infection. Expert Rev Vaccines. 2003;2: Pinto LA, Edwards J, Castle PE, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188: Alexander M, Salgaller ML, Celis E, et al. Generation of tumor-specific cytolytic T lymphocytes from peripheral blood of cervical cancer patients by in vitro stimulation with a synthetic human papillomavirus type 16 E7 epitope. Am J Obstet Gynecol. 1996;175: Tobery TW, Smith JF, Kuklin N, et al. Effect of vaccine delivery system on the induction of HPV16L1-specific humoral and cell-mediated immune responses in immunized rhesus macaques. Vaccine. 2003;21:
STUDY. Sandra Marchese Johnson, MD; Paula K. Roberson, PhD; Thomas D. Horn, MD. (HPV) causes the common
 Intralesional Injection of Mumps or Candida Skin Test Antigens A Novel for Warts STUDY Sandra Marchese Johnson, MD; Paula K. Roberson, PhD; Thomas D. Horn, MD Background: Warts are common and induce physical
Intralesional Injection of Mumps or Candida Skin Test Antigens A Novel for Warts STUDY Sandra Marchese Johnson, MD; Paula K. Roberson, PhD; Thomas D. Horn, MD Background: Warts are common and induce physical
Original Article Role of Candida antigen in treatment of viral warts: a placebo-controlled study Khawar Khurshid, Sabrina Suhail Pal
 Original Article Role of Candida antigen in treatment of viral warts: a placebo-controlled study Khawar Khurshid, Sabrina Suhail Pal Department of Dermatology Unit-II, King Edward Medical University/Mayo
Original Article Role of Candida antigen in treatment of viral warts: a placebo-controlled study Khawar Khurshid, Sabrina Suhail Pal Department of Dermatology Unit-II, King Edward Medical University/Mayo
Intradermal vs intralesional purified protein derivatives in treatment of warts
 ORIGINAL ARTICLE Intradermal vs intralesional purified protein derivatives in treatment of warts Ibraheem M Abo Elela, MD, Ahmed R Elshahid, MD, Al-Sadat Mosbeh, MD Department of Dermatology, Venereology
ORIGINAL ARTICLE Intradermal vs intralesional purified protein derivatives in treatment of warts Ibraheem M Abo Elela, MD, Ahmed R Elshahid, MD, Al-Sadat Mosbeh, MD Department of Dermatology, Venereology
Dr. Ashish Jagati, Dr. Shreya Agarwal, Dr. Kirti S Parmar, Dr. Bela J Shah, Dr. Jaydip Tank
 2017; 3(2): 37-41 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 2017; 3(2): 37-41 www.allresearchjournal.com Received: 09-12-2016 Accepted: 10-01-2017 Dr. Ashish Jagati Assistant
2017; 3(2): 37-41 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 2017; 3(2): 37-41 www.allresearchjournal.com Received: 09-12-2016 Accepted: 10-01-2017 Dr. Ashish Jagati Assistant
The no t-so-simple cutaneous wart
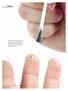 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart
 Original Article Efficacy of intralesional injection of mumps-measles-rubella vaccine in with wart Abbas Zamanian 1, Pezhman Mobasher 2, Ghazaleh Ahmadi Jazi 3 1 Skin and Stem Cell Research Center, Department
Original Article Efficacy of intralesional injection of mumps-measles-rubella vaccine in with wart Abbas Zamanian 1, Pezhman Mobasher 2, Ghazaleh Ahmadi Jazi 3 1 Skin and Stem Cell Research Center, Department
HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual)
 HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual) BACKGROUND Human Immunodeficiency Virus ( HIV ) can be divided into two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to
HIV-1 p24 ELISA Pair Set Cat#: orb54951 (ELISA Manual) BACKGROUND Human Immunodeficiency Virus ( HIV ) can be divided into two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
Research Article An Open Randomized Comparative Study of Various Intralesional Immunotherapeutic Agents in Cutaneous Warts
 International Journal of Medical Science and Clinical Invention 5(06): 3852-3859, 2018 DOI:10.18535/ijmsci/v5i6.01 ICV 2016: 77.2 e-issn:2348-991x, p-issn: 2454-9576 2018,IJMSCI Research Article An Open
International Journal of Medical Science and Clinical Invention 5(06): 3852-3859, 2018 DOI:10.18535/ijmsci/v5i6.01 ICV 2016: 77.2 e-issn:2348-991x, p-issn: 2454-9576 2018,IJMSCI Research Article An Open
Comparative study of therapeutic efficacy of intralesional vitamin D3 versus intralesional purified protein derivative in the treatment of warts
 Original Research Article DOI: 10.18231/2581-4729.2018.0048 Comparative study of therapeutic efficacy of intralesional vitamin D3 versus intralesional purified protein derivative in the treatment of warts
Original Research Article DOI: 10.18231/2581-4729.2018.0048 Comparative study of therapeutic efficacy of intralesional vitamin D3 versus intralesional purified protein derivative in the treatment of warts
Human Immunodeficiency Virus type 1 (HIV-1) gp120 / Glycoprotein 120 ELISA Pair Set
 Human Immunodeficiency Virus type 1 (HIV-1) gp120 / Glycoprotein 120 ELISA Pair Set Catalog Number : SEK11233 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) gp120 / Glycoprotein 120 ELISA Pair Set Catalog Number : SEK11233 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set
 Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Treatment of Warts with Candida Antigen Injection
 Home Up Treatment of Warts with Candida Antigen Injection Vignette published in Archives of Dermatology, October, 2001 This full report published with the permission of the Archives of Dermatology. Rebecca
Home Up Treatment of Warts with Candida Antigen Injection Vignette published in Archives of Dermatology, October, 2001 This full report published with the permission of the Archives of Dermatology. Rebecca
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2*
 Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Human Papillomavirus
 Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
Human Papillomavirus Dawn Palaszewski, MD Assistant Professor of Obstetrics and Gynecology University of February 18, 2018 9:40 am Dawn Palaszewski, MD Assistant Professor Department of Obstetrics and
Transfection of Sf9 cells with recombinant Bacmid DNA
 Transposition Bacmid DNA Mini Culturing baculo cells Transfection of Sf9 cells with recombinant Bacmid DNA Amplification of the virus Titration of baculo stocks Testing the expression Transposition 1.
Transposition Bacmid DNA Mini Culturing baculo cells Transfection of Sf9 cells with recombinant Bacmid DNA Amplification of the virus Titration of baculo stocks Testing the expression Transposition 1.
Tumor Immunology. Wirsma Arif Harahap Surgical Oncology Consultant
 Tumor Immunology Wirsma Arif Harahap Surgical Oncology Consultant 1) Immune responses that develop to cancer cells 2) Escape of cancer cells 3) Therapies: clinical and experimental Cancer cells can be
Tumor Immunology Wirsma Arif Harahap Surgical Oncology Consultant 1) Immune responses that develop to cancer cells 2) Escape of cancer cells 3) Therapies: clinical and experimental Cancer cells can be
What is the immune system? Types of Immunity. Pasteur and rabies vaccine. Historical Role of smallpox. Recognition Response
 Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human
 Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Human HBcAb IgM ELISA kit
 Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
The humoral immune responses to IBV proteins.
 The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
Phosphate buffered saline (PBS) for washing the cells TE buffer (nuclease-free) ph 7.5 for use with the PrimePCR Reverse Transcription Control Assay
 Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
5- Assistant Professor of Dermatology, Department of Dermatology, Afzalipour School of Medicine, Kerman University of Medical Sciences,
 JKMU Journal of Kerman University of Medical Sciences, 2018; 25 (1): 1-8 A Randomized Clinical Trial of Using Niosomal Zinc Sulfate Plus Cryotherapy in Comparison with Placebo Along with Cryotherapy in
JKMU Journal of Kerman University of Medical Sciences, 2018; 25 (1): 1-8 A Randomized Clinical Trial of Using Niosomal Zinc Sulfate Plus Cryotherapy in Comparison with Placebo Along with Cryotherapy in
Tumors arise from accumulated genetic mutations. Tumor Immunology (Cancer)
 Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment of Periungual Warts
 Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Immunotherapy of Warts
 Immunotherapy of Warts Jacob Mashiah, MD, MHA Dermatology and Pediatric Dermatology Dana-Dowek children s hospital Sourasky Medical Center Tel-Aviv, Israel No conflict of interest to disclose Human Papillomavirus
Immunotherapy of Warts Jacob Mashiah, MD, MHA Dermatology and Pediatric Dermatology Dana-Dowek children s hospital Sourasky Medical Center Tel-Aviv, Israel No conflict of interest to disclose Human Papillomavirus
Can HPV, cervical neoplasia or. HIV transmission?
 Interactions between HPV and HIV: STIs and HIV shedding, regulation of HPV by HIV, and HPV VLP influence upon HIV Jennifer S. Smith Department of Epidemiology pd University of North Carolina Can HPV, cervical
Interactions between HPV and HIV: STIs and HIV shedding, regulation of HPV by HIV, and HPV VLP influence upon HIV Jennifer S. Smith Department of Epidemiology pd University of North Carolina Can HPV, cervical
10/18/2012. A primer in HLA: The who, what, how and why. What?
 A primer in HLA: The who, what, how and why What? 1 First recognized in mice during 1930 s and 1940 s. Mouse (murine) experiments with tumors Independent observations were made in humans with leukoagglutinating
A primer in HLA: The who, what, how and why What? 1 First recognized in mice during 1930 s and 1940 s. Mouse (murine) experiments with tumors Independent observations were made in humans with leukoagglutinating
Lecture 11. Immunology and disease: parasite antigenic diversity
 Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
CHAPTER 3 LABORATORY PROCEDURES
 CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CELL MEDIATED IMMUNE RESPONSE
 CELL MEDIATED IMMUNE RESPONSE Chapter IV - CELL MEDIATED IMMUNE RESPONSE Sujatha, M. 2013. Evaluation of Immunological changes in Fish, Catla catla administered with bacterial pathogen, Aeromonas hydrophila,
CELL MEDIATED IMMUNE RESPONSE Chapter IV - CELL MEDIATED IMMUNE RESPONSE Sujatha, M. 2013. Evaluation of Immunological changes in Fish, Catla catla administered with bacterial pathogen, Aeromonas hydrophila,
A comparative study between intralesional PPD and vitamin D3 in treatment of viral warts
 International Journal of Research in Dermatology Singh SK et al. Int J Res Dermatol. 18 May;4(2):xxx-xxx http://www.ijord.com Original Research Article DOI: http://dx.doi.org/.183/issn.24-429.intjresdermatol1893
International Journal of Research in Dermatology Singh SK et al. Int J Res Dermatol. 18 May;4(2):xxx-xxx http://www.ijord.com Original Research Article DOI: http://dx.doi.org/.183/issn.24-429.intjresdermatol1893
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Third line of Defense. Topic 8 Specific Immunity (adaptive) (18) 3 rd Line = Prophylaxis via Immunization!
 Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in
 Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity
 1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
PRODUCT INFORMATION GARDASIL 9. [Human Papillomavirus 9-valent (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine, Recombinant]
![PRODUCT INFORMATION GARDASIL 9. [Human Papillomavirus 9-valent (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine, Recombinant] PRODUCT INFORMATION GARDASIL 9. [Human Papillomavirus 9-valent (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine, Recombinant]](/thumbs/80/82176955.jpg) PRODUCT INFORMATION GARDASIL 9 [Human Papillomavirus 9-valent (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine, Recombinant] DESCRIPTION GARDASIL 9 *, Human Papillomavirus 9-valent Vaccine, Recombinant,
PRODUCT INFORMATION GARDASIL 9 [Human Papillomavirus 9-valent (Types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine, Recombinant] DESCRIPTION GARDASIL 9 *, Human Papillomavirus 9-valent Vaccine, Recombinant,
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
In vitro human regulatory T cell suppression assay
 Human CD4 + CD25 + regulatory T cell isolation, in vitro suppression assay and analysis In vitro human regulatory T cell suppression assay Introduction Regulatory T (Treg) cells are a subpopulation of
Human CD4 + CD25 + regulatory T cell isolation, in vitro suppression assay and analysis In vitro human regulatory T cell suppression assay Introduction Regulatory T (Treg) cells are a subpopulation of
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent
Things that go bump: Wart & Molluscum
 Things that go bump: Wart & Molluscum Raegan Hunt, MD, PhD Chief of Section, Pediatric Dermatology Texas Children s Hospital Disclosures Off label use of products may be discussed No relevant financial
Things that go bump: Wart & Molluscum Raegan Hunt, MD, PhD Chief of Section, Pediatric Dermatology Texas Children s Hospital Disclosures Off label use of products may be discussed No relevant financial
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man Coulter HIV-1 p24 ELISA May 21, 2004
 Coulter HIV p24 1. PRINCIPLE The Human Immunodeficiency Virus Type 1 (HIV-1) is recognized as the etiologic agent of acquired immunodeficiency syndrome (AIDS). The virus is transmitted by sexual contact,
Coulter HIV p24 1. PRINCIPLE The Human Immunodeficiency Virus Type 1 (HIV-1) is recognized as the etiologic agent of acquired immunodeficiency syndrome (AIDS). The virus is transmitted by sexual contact,
Unit 5 The Human Immune Response to Infection
 Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
ACTG Laboratory Technologist Committee Revised Version 1.0 ACTG Lab Man Qualitative CSF/PBMC Microculture Assay 22 April 2004
 1. PRINCIPLE QUALITATIVE CSF/PBMC MICROCULTURE ASSAY 1.1 Human immunodeficiency virus (HIV) has been shown to be the etiologic agent of Acquired Immunodeficiency Syndrome (AIDS). Isolation of HIV-1 from
1. PRINCIPLE QUALITATIVE CSF/PBMC MICROCULTURE ASSAY 1.1 Human immunodeficiency virus (HIV) has been shown to be the etiologic agent of Acquired Immunodeficiency Syndrome (AIDS). Isolation of HIV-1 from
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
Practice Problems 8. a) What do we define as a beneficial or advantageous mutation to the virus? Why?
 Life Sciences 1a Practice Problems 8 1. You have two strains of HIV one is a wild type strain of HIV and the second has acquired a mutation in the gene encoding the protease. This mutation has a dual effect
Life Sciences 1a Practice Problems 8 1. You have two strains of HIV one is a wild type strain of HIV and the second has acquired a mutation in the gene encoding the protease. This mutation has a dual effect
Influenza A H1N1 HA ELISA Pair Set
 Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
attomol HLA-B*27-Realtime LT 2 Assay for the detection of the human HLA-B*27-locus using LightCycler (Do not use for tissue typing!
 attomol HLA-B*27-Realtime LT 2 Assay for the detection of the human HLA-B*27-locus using LightCycler (Do not use for tissue typing!) For in vitro diagnostic use only! 50 determinations Order number: 95
attomol HLA-B*27-Realtime LT 2 Assay for the detection of the human HLA-B*27-locus using LightCycler (Do not use for tissue typing!) For in vitro diagnostic use only! 50 determinations Order number: 95
Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central
Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man HIV Qualitative PBMC Micrococulture 1 June 2004
 HIV QUALITATIVE PBMC MICROCOCULTURE ASSAY 1. PRINCIPLE: 1.1 A co-culture of patient peripheral blood mononuclear cells (PBMC) and uninfected PHA-stimulated PBMCs is maintained under ideal conditions to
HIV QUALITATIVE PBMC MICROCOCULTURE ASSAY 1. PRINCIPLE: 1.1 A co-culture of patient peripheral blood mononuclear cells (PBMC) and uninfected PHA-stimulated PBMCs is maintained under ideal conditions to
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/35908 holds various files of this Leiden University dissertation Author: Soema, Peter Title: Formulation of influenza T cell peptides : in search of a universal
Cover Page The handle http://hdl.handle.net/1887/35908 holds various files of this Leiden University dissertation Author: Soema, Peter Title: Formulation of influenza T cell peptides : in search of a universal
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab)
 Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab) Updated 12/3/02 Reagents: ChIP sonication Buffer (1% Triton X-100, 0.1% Deoxycholate, 50 mm Tris 8.1, 150 mm NaCl, 5 mm EDTA): 10 ml 10 % Triton
Chromatin Immunoprecipitation (ChIPs) Protocol (Mirmira Lab) Updated 12/3/02 Reagents: ChIP sonication Buffer (1% Triton X-100, 0.1% Deoxycholate, 50 mm Tris 8.1, 150 mm NaCl, 5 mm EDTA): 10 ml 10 % Triton
Make Love Not Warts Genital Warts
 Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Patricia Wong, MD 735 Cowper Street Palo Alto, CA 94301 650-473-3173 www.patriciawongmd.com Make Love Not Warts Genital Warts Genital warts are the most common sexually transmitted disease. The lifetime
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16
 COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
Rapid antigen-specific T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata
 Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
Original Article Efficacy of Topical Diphencyprone in the Treatment of Alopecia Areata Maryam Akhiani, MD Hasan Seyrafi, MD Farshad Farnaghi, MD Parastoo Banan, MD Vahideh Lajvardi, MD Department of Dermatology,
CONTENTS. STUDY DESIGN METHODS ELISA protocol for quantitation of mite (Dermatophagoides spp.) Der p 1 or Der f 1
 CONTENTS STUDY DESIGN METHODS ELISA protocol for quantitation of mite (Dermatophagoides spp.) Der p 1 or Der f 1 ELISA protocol for mite (Dermatophagoides spp.) Group 2 ALLERGENS RESULTS (SUMMARY) TABLE
CONTENTS STUDY DESIGN METHODS ELISA protocol for quantitation of mite (Dermatophagoides spp.) Der p 1 or Der f 1 ELISA protocol for mite (Dermatophagoides spp.) Group 2 ALLERGENS RESULTS (SUMMARY) TABLE
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
AM. Massing, WL. Epstein, Natural History of Warts. A Two-Year Study Arch Dermatol. 1963;87(3):
 Recommended Reading: Warts and their treatment AM. Massing, WL. Epstein, Natural History of Warts. A Two-Year Study Arch Dermatol. 1963;87(3):306-310. Christina Boull and David Groth. Update: Treatment
Recommended Reading: Warts and their treatment AM. Massing, WL. Epstein, Natural History of Warts. A Two-Year Study Arch Dermatol. 1963;87(3):306-310. Christina Boull and David Groth. Update: Treatment
Recognition of a cervical cancer derived tumor cell line by a human papillomavirus type 16 E specific CD8 T cell clone
 Cancer Immun 1424-9634Academy of Cancer Immunology Cancer Immunity (30 June 2006) Vol. 6, p. 9 Submitted: 2 September 2005. Resubmitted: 28 April 2006. Accepted: 2 June 2006. Copyright 2006 by Mayumi Nakagawa
Cancer Immun 1424-9634Academy of Cancer Immunology Cancer Immunity (30 June 2006) Vol. 6, p. 9 Submitted: 2 September 2005. Resubmitted: 28 April 2006. Accepted: 2 June 2006. Copyright 2006 by Mayumi Nakagawa
The Immune System. A macrophage. ! Functions of the Immune System. ! Types of Immune Responses. ! Organization of the Immune System
 The Immune System! Functions of the Immune System! Types of Immune Responses! Organization of the Immune System! Innate Defense Mechanisms! Acquired Defense Mechanisms! Applied Immunology A macrophage
The Immune System! Functions of the Immune System! Types of Immune Responses! Organization of the Immune System! Innate Defense Mechanisms! Acquired Defense Mechanisms! Applied Immunology A macrophage
Multi-clonal origin of macrolide-resistant Mycoplasma pneumoniae isolates. determined by multiple-locus variable-number tandem-repeat analysis
 JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
JCM Accepts, published online ahead of print on 30 May 2012 J. Clin. Microbiol. doi:10.1128/jcm.00678-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 2 Multi-clonal origin
Adaptive Immunity: Humoral Immune Responses
 MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric)
 Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human Papillomavirus
 Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Infectious Diseases in Obstetrics and Gynecology 7:186-189 (1999) (C) 1999 Wiley-Liss, Inc. A Randomized, Double-Blind, Placebo-Controlled Trial of 5-Fluorouracil for the Treatment of Cervicovaginal Human
Detailed step-by-step operating procedures for NK cell and CTL degranulation assays
 Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
CHAPTER 18: Immune System
 CHAPTER 18: Immune System 1. What are four characteristics of the specific immune system? a. b. c. d. 2. List the two main types of defense mechanisms and briefly describe features of each. 3. Give examples
CHAPTER 18: Immune System 1. What are four characteristics of the specific immune system? a. b. c. d. 2. List the two main types of defense mechanisms and briefly describe features of each. 3. Give examples
08/02/59. Tumor Immunotherapy. Development of Tumor Vaccines. Types of Tumor Vaccines. Immunotherapy w/ Cytokine Gene-Transfected Tumor Cells
 Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
The Swarm: Causes and consequences of HIV quasispecies diversity
 The Swarm: Causes and consequences of HIV quasispecies diversity Julian Wolfson Dept. of Biostatistics - Biology Project August 14, 2008 Mutation, mutation, mutation Success of HIV largely due to its ability
The Swarm: Causes and consequences of HIV quasispecies diversity Julian Wolfson Dept. of Biostatistics - Biology Project August 14, 2008 Mutation, mutation, mutation Success of HIV largely due to its ability
Fluid movement in capillaries. Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system
 Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1
 [Gann, 66, 167-174; April, 1975] IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1 Tsuyoshi AKIYOSHI, Akira HATA, and Hideo TSUJI Department of Surgery,
[Gann, 66, 167-174; April, 1975] IN VITRO CELLULAR RESPONSES TO AUTOLOGOUS TUMOR EXTRACT DETECTED BY INHIBITION OF MACROPHAGE MIGRATION*1 Tsuyoshi AKIYOSHI, Akira HATA, and Hideo TSUJI Department of Surgery,
Product Datasheet. HLA ABC Antibody (W6/32) NB Unit Size: 0.25 mg. Store at -20C. Avoid freeze-thaw cycles. Reviews: 1 Publications: 22
 Product Datasheet HLA ABC Antibody (W6/32) NB100-64775 Unit Size: 0.25 mg Store at -20C. Avoid freeze-thaw cycles. Reviews: 1 Publications: 22 Protocols, Publications, Related Products, Reviews, Research
Product Datasheet HLA ABC Antibody (W6/32) NB100-64775 Unit Size: 0.25 mg Store at -20C. Avoid freeze-thaw cycles. Reviews: 1 Publications: 22 Protocols, Publications, Related Products, Reviews, Research
Cytotoxicity assays. Rory D. de Vries, PhD 1. Viroscience lab, Erasmus MC, Rotterdam, the Netherlands
 Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
Woo Dae Kang, Ho Sun Choi, Seok Mo Kim
 Is vaccination with quadrivalent HPV vaccine after Loop Electrosurgical Excision Procedure effective in preventing recurrence in patients with High-grade Cervical Intraepithelial Neoplasia (CIN2-3)? Chonnam
Is vaccination with quadrivalent HPV vaccine after Loop Electrosurgical Excision Procedure effective in preventing recurrence in patients with High-grade Cervical Intraepithelial Neoplasia (CIN2-3)? Chonnam
Exploring Immunotherapies: Beyond Checkpoint Inhibitors
 Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
ANTIBODY IMMUNITY TO PROSTATE CANCER ASSOCIATED ANTIGENS CAN BE DETECTED IN THE SERUM OF PATIENTS WITH PROSTATE CANCER
 0022-5347/00/1645-1825/0 THE JOURNAL OF UROLOGY Vol. 164, 1825 1829, November 2000 Copyright 2000 by AMERICAN UROLOGICAL ASSOCIATION, INC. Printed in U.S.A. ANTIBODY IMMUNITY TO PROSTATE CANCER ASSOCIATED
0022-5347/00/1645-1825/0 THE JOURNAL OF UROLOGY Vol. 164, 1825 1829, November 2000 Copyright 2000 by AMERICAN UROLOGICAL ASSOCIATION, INC. Printed in U.S.A. ANTIBODY IMMUNITY TO PROSTATE CANCER ASSOCIATED
Therapeutic Immunization with Autologous DC Pulsed with Autologous Inactivated HIV-1 Infected Apoptotic Cells
 Therapeutic Immunization with Autologous DC Pulsed with Autologous Inactivated HIV-1 Infected Apoptotic Cells Sharon A. Riddler, MD, MPH University of Pittsburgh May 2008 Slide 1 HIV and DC Vaccines During
Therapeutic Immunization with Autologous DC Pulsed with Autologous Inactivated HIV-1 Infected Apoptotic Cells Sharon A. Riddler, MD, MPH University of Pittsburgh May 2008 Slide 1 HIV and DC Vaccines During
STUDY. Duct Tape for the Treatment of Common Warts in Adults
 STUDY Duct Tape for the Treatment of Common Warts in Adults A Double-blind Randomized Controlled Trial Rachel Wenner, MD; Sharone K. Askari, MD; Peter M. H. Cham, MD; Deborah A. Kedrowski, RN; An Liu,
STUDY Duct Tape for the Treatment of Common Warts in Adults A Double-blind Randomized Controlled Trial Rachel Wenner, MD; Sharone K. Askari, MD; Peter M. H. Cham, MD; Deborah A. Kedrowski, RN; An Liu,
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Hosts. New Treatments for Cancer
 Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
Role of Paired Box9 (PAX9) (rs ) and Muscle Segment Homeobox1 (MSX1) (581C>T) Gene Polymorphisms in Tooth Agenesis
 EC Dental Science Special Issue - 2017 Role of Paired Box9 (PAX9) (rs2073245) and Muscle Segment Homeobox1 (MSX1) (581C>T) Gene Polymorphisms in Tooth Agenesis Research Article Dr. Sonam Sethi 1, Dr. Anmol
EC Dental Science Special Issue - 2017 Role of Paired Box9 (PAX9) (rs2073245) and Muscle Segment Homeobox1 (MSX1) (581C>T) Gene Polymorphisms in Tooth Agenesis Research Article Dr. Sonam Sethi 1, Dr. Anmol
Warts. Dr. Ghassan Salah
 Warts Dr. Ghassan Salah Background Information Warts are small harmless lesions of the skin caused by a virus: the human papilloma virus. The appearance of warts can differ based on the type of wart and
Warts Dr. Ghassan Salah Background Information Warts are small harmless lesions of the skin caused by a virus: the human papilloma virus. The appearance of warts can differ based on the type of wart and
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
LAB 1, Immunology. Laboratory manual Immunology and Infection Biology Biomedicine course Autumn 2007
 LAB 1, Immunology Laboratory manual Immunology and Infection Biology Biomedicine course Autumn 2007 QUANTIFICATION OF CYTOTOXIC T LYMPHOCYTE ACTIVITY AND DETECTION OF FAS/TRAILR INDUCED APOPTOSIS RESPONSIBLE:
LAB 1, Immunology Laboratory manual Immunology and Infection Biology Biomedicine course Autumn 2007 QUANTIFICATION OF CYTOTOXIC T LYMPHOCYTE ACTIVITY AND DETECTION OF FAS/TRAILR INDUCED APOPTOSIS RESPONSIBLE:
2.0 Synopsis. ABT-333 M Clinical Study Report R&D/09/956
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
What is the Safety and Efficacy of Vaccinating the Male Gender to Prevent HPV Related Neoplastic Disorders in Both the Male and Female Genders
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 What is the Safety and Efficacy of Vaccinating
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 What is the Safety and Efficacy of Vaccinating
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
