Nancy Hailpern, Director, Regulatory Affairs K Street, NW, Suite 1000 Washington, DC 20005
|
|
|
- Ezra Atkinson
- 6 years ago
- Views:
Transcription
1 Summary of Infection Prevention Issues in the Centers for Medicare & Medicaid Services (CMS) FY 2014 Inpatient Prospective Payment System (IPPS) Final Rule Hospital Readmissions Reduction Program-Fiscal Years 2014 and 2015 CMS proposal: to adopt the total hip arthroplasty (THA) and total knee arthroplasty (TKA) (NQF #1551) measure that was adopted for the Hospital Inpatient Quality Reporting (IQR) program in FY 2013 for the Hospital Readmissions Reduction Program beginning in FY APIC recommendation: While APIC supports the addition of THA and TKA to the readmission measure, we do not want to see facilities unfairly penalized if the readmission was a planned part of the care process. Therefore, APIC supports CMS s attempt to develop an algorithm to identify those planned readmissions and exclude them from the measures. CMS is finalizing proposal, without modification, to refine the readmission measures and to adopt the planned readmissions algorithm for the Hospital Readmissions Reduction Program. Hospital Value-Based Purchasing (VBP) Program o Remove for FY 2016 PN-3b: Blood Cultures Performed in the Emergency Department Prior to Initial Antibiotic Received in Hospital (measure no longer endorsed by NQF). o Adopt for FY 2016 Influenza Immunization (IMM-2, NQF #1659) as a chart-abstracted prevention measure that assesses whether patients over six months of age were screened for seasonal influenza immunization status and were vaccinated prior to discharge if appropriate. o Adopt for FY 2016 Catheter-Associated Urinary Tract Infection (CAUTI, NQF #0138) in adult intensive care units (ICU) as a healthcare-associated infection measure reported via the National Healthcare Safety Network (NHSN). o Adopt for FY 2016 Surgical Site Infection (Colon Surgery and Abdominal Hysterectomy) (SSI, NQF #0753) as a healthcare-associated infection measure reported via NHSN. o Adopt for FY 2016 NHSN-based Central Line-Associated Bloodstream Infection (CLABSI) measure in ICU patients in its current state (was previously adopted). o Adopt for FY 2017 Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia standardized infection ratio (SIR). o Adopt for FY 2017 Clostridium difficile SIR. o Align the VBP, IQR and Electronic Health Record (EHR) Incentive Program with the National Quality Strategy (NQS) domains beginning with the FY 2017 Hospital VBP Program. This would place the HAI measures into the Safety domain. o Adopt a Hospital VBP program extraordinary circumstance waiver process that would allow CMS to waive all applicable quality measure data from a performance period, and thus, exclude a hospital from the VBP program for a fiscal year during which the hospital has experienced a disaster or other extraordinary circumstance.
2 o APIC supports the addition of the global measure for influenza immunization, ICU CAUTI, ICU CLABSI and SSI for colon surgery and abdominal hysterectomy into the VBP Program for FY o APIC supports the addition of MRSA bacteremia and C. difficile SIR for FY 2017 VBP, while urging CMS to evaluate the timing of these additions in context with ongoing efforts to improve the risk adjustment and comparison analysis. o APIC supports the alignment of the VBP program with the National Quality Strategy (NQS) domains and specifically supports placement of the HAI measures into the Safety domain. o APIC supports the adoption of an extraordinary circumstance waiver process for the VBP program. o Influenza immunization, CLABSI, CAUTI, SSI measures finalized as proposed. Baseline and performance periods that were mistakenly omitted in the proposed rule have been included in the currently-pending proposed rule for the CY 2014 Hospital Outpatient Prospective Payment system (on which APIC is drafting comments). o Addition of MRSA and C. difficile for FY 2017 has not been finalized in order to allow CMS to further consider the comments it received on this proposal. o Extraordinary circumstance waiver process for VBP finalized, but VBP application process separated from the Hospital IQR Program s waiver process, extending the deadline for Hospital VBP Programspecific exception requests. Hospital-Acquired Condition (HAC) Reduction Program for FY 2015 The HAC Reduction Program, mandated by the Affordable Care Act, requires CMS to reduce hospital payments by 1% for the quartile of hospitals with the highest rates of hospital-acquired conditions (HACs), beginning in FY This proposal identifies the plan for scoring hospitals for the program. o To score hospitals using two separate domains Domain 1 using AHRQ patient safety indicator (PSI) measures, and Domain 2 other using CDC/NHSN measures. o CMS provided a proposed approach for Domain 1 using 6 individual PSI measures, and an alternative approach using one composite of 8 component indicators. o CMS would weigh the 2 domains equally. o APIC supports separating the AHRQ and CDC measures into separate domains. o APIC supports the proposed approach for Domain 1 rather than the alternative approach. o Finalized use of two separate domains to determine Total HAC Score, but decided to use the alternative proposal for Domain 1, including the composite measure. Hospital Inpatient Quality Reporting (IQR) Program o To make the individual patient safety indicators (PSIs) that are part of the PSI-90 composite measure available to the public. o Remove the following measures from the IQR program: PN-3b: Blood Culture Performed in the Emergency Department Prior to First Antibiotic Received in the Hospital (no longer NQF-endorsed). IMM-1: Immunization for Pneumonia (rapidly changing guidelines that are difficult to adapt and update within the system). SCIP-Inf-10: Surgery patients with perioperative temperature management (measure topped out).
3 Continued suspension of SCIP-Inf-6: Appropriate hair removal (measure topped out). o Expansion of CAUTI and CLABSI reporting measures to select non-icu locations (medical, surgical and medical/surgical wards) beginning January o Refinement of SCIP-Inf-4: Controlled 6 am Glucose for Cardiac Surgery Patients (NQF #300), since measure has undergone extensive changes as part of the NQF endorsement maintenance process. o APIC does not support display of the individual PSI indicators under the PSI-90 composite. The PSI- 90 composite includes PSI-7 central venous catheter-related bloodstream infection rate. APIC believes that, since PSI-7 has a similar name but differs significantly from the CDC/NHSN CLABSI measure, this could increase public confusion. o APIC supports the removal or suspension of PN-3b, IMM-1, SCIP-Inf-10, and SCIP-Inf-6, and APIC supports CMS s stance which notes that, despite removal of the pneumonia vaccination measure, hospitals should continue to keep up with vaccination recommendations for various populations. o APIC supports a phased-in approach of expansion with CLABSI and CAUTI beyond the ICUs, specifically recommending that CLABSI expansion be transitioned first, followed by CAUTI after surveillance definitions have been updated and implemented. o APIC supports refinement of SCIP-Inf-4 but recommends the language corrective action be replaced with documentation of clinical attempt of glucose control. o Finalized removal of PN-3b and SCIP-Inf-10 measures from the Hospital IQR Program measure set. Suspended (rather than removed) IMM-1 measure. Finalized continued suspension of SCIP-Inf-6. o Finalized proposal to make publicly available hospital level data for the PSI indicators that are part of the PSI-90 composite in addition to the composite results. o Deferred the implementation date of the CLABSI/CAUTI expansion to non-icu settings to January 1, o Finalized proposed refinement of SCIP-Inf-4 to match refinements made during NQF reendorsement. CMS will consider APIC-suggested language change. Modifications to the Validation Process under the Hospital IQR Program o To better align with NHSN definitions by replacing requirement to note a central venous catheter (CVC) on the CLABSI validation template with central line. o To exclude from the CAUTI validation template all urine cultures with more than two organisms even if they have greater than or equal to 1,000 colony-forming units. o To adopt a sub-regulatory process for handling details of the definition specifications. o To add MRSA bacteremia and C. difficile infection (CDI) to the validation process and randomly assign half the randomly selected hospitals to submit templates for CLABSI and CAUTI and the other half to submit templates for MRSA and CDI for validation. o To reduce the number of validation records from 48 to 36, and to exclude patients with length of stay greater than 120 days. o APIC supports revisions to the CLABSI and CAUTI validation templates to align with NHSN definitions. o APIC supports use of the sub-regulatory process to update measures and validation templates, but encourages CMS to consult with stakeholders, including APIC and CDC/NHSN experts. o APIC supports a split validation of CLABSI, CAUTI, MRSA, and CDI, as well as the exclusion of patients with a length of stay greater than 120 days and the reduction of validation charts from 48 to 36, all efforts which will reduce the validation burden to hospitals and IPs.
4 o APIC recognizes that many states have health departments that already provide validation as part of their state reporting requirements. APIC believes State Health Departments are an important stakeholder in prevention and reporting efforts and should lead validation work nationally. This validation work should be coordinated with CMS and their needs to validate NHSN reporting. o APIC supports continued efforts to establish electronic submission of records for the validation process. o Finalized proposals to align CMS validation templates with NHSN definitions. o Finalized proposal to use a sub-regulatory process to update measures and validation templates, and acknowledged APIC s recommendation that the agency consult with IPs, APIC and stakeholder experts on these updates. CMS noted that it will continue to coordinate with CDC, and will consider collaborations with other stakeholders on these updates. o Finalized proposals to add MRSA and CDI validation, as well as efforts that would reduce validation burden on hospitals and IPs. o Recognized the role that many states are taking to prevent HAIs, but noted the agency does not intend to fund state efforts to conduct validation activities. CMS believes state efforts in this area are best viewed as complementary to CMS s efforts to uniformly validate HAI data. CMS notes that, since it has the responsibility to ensure the validity of HAI data reported to its Hospital IQR and VBP programs, its validation process must be consistent nationwide. o Finalized proposals relating to electronic submission of records for the validation process. PPS-Exempt Cancer Hospital Quality Reporting (PCHQR) Program o To adopt the NHSN SSI measure for colon surgeries and abdominal hysterectomies for FY o To adopt six SCIP measures for FY o To defer public reporting of three previously-finalized measures for FY 2014, including the NHSN CLABSI outcome measure and the NHSN CAUTI outcome measure, while CMS is in the process of testing and assessing the data quality. o APIC supports adoption of the NSHN SSI measure and the six SCIP measures. o APIC supports deferral of public reporting of the FY 2014 measures that have not yet been sufficiently tested or assessed for reliability and validity. o Finalized adoption of the NHSN SSI for FY 2015 and subsequent years, and SCIP measures for FY 2016 and subsequent years. o Finalized proposal to defer public display of measures not yet sufficiently tested and assessed. Long-Term Care Hospital Quality Reporting (LTCHQR) Program o To align the reporting period for the Influenza Vaccination Coverage among Healthcare Personnel measure (NQF #0431) with the influenza season, so beginning in FY 2015, the data collection period will be from October 1 (or when the influenza vaccine becomes available) through March 31 of the year in which the flu season ends and submission deadline will be May 15 of the year in which the flu season ends. o To delay the start of the collection and submission of data on the measure Percentage of Residents or Patients who were Assessed and Appropriately Given the Seasonal Influenza Vaccine (NQF #0680) from January 1, 2014 to April 1, 2014 in order to allow time and opportunity for LTCHs and vendors to train, plan for and incorporate changes into their data collection and entry systems.
5 o To add the following measures to the LTCHQR program: NHSN Facility-Wide Inpatient Hospital-Onset MRSA Bacteremia Outcome Measure (NQF #1716) FY NHSN Facility-Wide Inpatient Hospital-onset CDI Outcome Measure (NQF #1717) FY All-cause Unplanned Readmission Measure for 30 Days Post-Discharge from LTCH FY Application of the percent of Residents Experiencing One or More Falls with Major Injury (NQF #0674) FY o Measures under consideration for future years include SSI, Ventilator-Associated Event (VAE), and Ventilator Bundle. o APIC supports alignment of the HCP Influenza Vaccination Coverage measure with the influenza season. o APIC supports delay of the start of the NQF #0680 data collection and submission. o While APIC supports the expansion of reporting of HAI measures within the LTCH setting, we encourage CMS to evaluate the timing of introduction to allow for adequate training and resources for all data collection. APIC expresses concern that the rapid increase of reporting measures over a short period of time could result in limited existing resources being moved from prevention activities to reporting activities. o APIC does not support addition of the SSI measure for a LTCH facility where surgical procedures are not routinely performed. o APIC does not support addition of VAE or the VAP prevention bundle in the LTCH setting, and instead urges CMS to wait for NQF endorsement of the VAE measure as a possibility for future reporting opportunity. o Finalized revision to the data collection and reporting timeline NQF #0431 for FY 2016 and subsequent years. o Finalized revised data collection and reporting timeline for NQF #0680. Starting with the influenza vaccination season, data collection will be required for any patient admitted or discharged between October 1 and April 30. Submission deadlines will be May 15 of the year in which the flu season ends. o Finalized addition of measures for FY 2017 and FY 2018 as proposed. o Did not finalize proposed measures for future years. Change to the Medicare Hospital Conditions of Participation (CoPs) Relating to Administration of Pneumococcal Vaccines CMS proposal: To delete the term polysaccharide from the current CoP standard for the nursing services condition for preparing and administering drugs in reference to the pneumococcal vaccine. APIC recommendation: APIC supports this proposal. Finalized as proposed.
The Centers for Medicare & Medicaid Services (CMS) Acute Care Hospital Fiscal Year (FY) 2018 Quality Improvement Program Measures
 ID M easure Name NQF # H os pital M easurement Period H os pital H os pital Value-Bas ed Purchas ing M easurement Period H os pital H ealth Record (EH R) Incentive M easurement Period H os pital H os pital-
ID M easure Name NQF # H os pital M easurement Period H os pital H os pital Value-Bas ed Purchas ing M easurement Period H os pital H ealth Record (EH R) Incentive M easurement Period H os pital H os pital-
CMS Hospital Inpatient Quality Reporting (IQR) Program Measures for the FY 2019 Payment Update
 CMS Inpatient Quality Reporting (IQR) Program Measures for the Update Measures Required to Meet IQR Program APU Requirements NHSN Submission CAUTI National Healthcare Safety Network (NHSN) Catheter-Associated
CMS Inpatient Quality Reporting (IQR) Program Measures for the Update Measures Required to Meet IQR Program APU Requirements NHSN Submission CAUTI National Healthcare Safety Network (NHSN) Catheter-Associated
August 29, Dear Dr. Berwick:
 August 29, 2011 Donald Berwick, MD Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services 200 Independence Avenue, SW Room 445-G Washington, DC 20201 Re: Proposed
August 29, 2011 Donald Berwick, MD Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services 200 Independence Avenue, SW Room 445-G Washington, DC 20201 Re: Proposed
CMS Measures - Fiscal Year 2019
 ID Me asure Name NQF # Value- (VBP) - (HACRP) (HRRP) ID Me asure Name NQF # Value- (VBP) - (HACRP) (HRRP) CMS s - Fiscal Year 2019 ID Name NQF # The Centers for Medicare & Medicaid Services (CMS) Improvement
ID Me asure Name NQF # Value- (VBP) - (HACRP) (HRRP) ID Me asure Name NQF # Value- (VBP) - (HACRP) (HRRP) CMS s - Fiscal Year 2019 ID Name NQF # The Centers for Medicare & Medicaid Services (CMS) Improvement
Measure Applications Partnership. Hospital Workgroup In-Person Meeting Follow- Up Call
 Measure Applications Partnership Hospital Workgroup In-Person Meeting Follow- Up Call December 21, 2016 Feedback on Current Measure Sets for IQR, HACs, Readmissions, and VBP 2 Previously Identified Crosscutting
Measure Applications Partnership Hospital Workgroup In-Person Meeting Follow- Up Call December 21, 2016 Feedback on Current Measure Sets for IQR, HACs, Readmissions, and VBP 2 Previously Identified Crosscutting
PPS Exempt Cancer Hospital Quality Reporting (PCHQR) Program Relationship Matrix of Program Measures by Years and Quarters
 PPS Exempt Cancer Quality (PCHQR) Relationship Matrix of Measures by and This reference document for PCHQR participants provides the following: Specific measures with their National Quality Forum (NQF)
PPS Exempt Cancer Quality (PCHQR) Relationship Matrix of Measures by and This reference document for PCHQR participants provides the following: Specific measures with their National Quality Forum (NQF)
CMS Hospital IQR Program Measure Comparison Tables FY 2018 (CY 2016) Measures Required to Meet Hospital IQR APU Requirements NHSN Submission
 CMS IQR Program Measure Comparison Tables (CY 2016) NHSN Submission CLABSI Central Line-Associated Bloodstream Infection (CLABSI) Required NHSN CAUTI Catheter-Associated Urinary Tract Infection (CAUTI)
CMS IQR Program Measure Comparison Tables (CY 2016) NHSN Submission CLABSI Central Line-Associated Bloodstream Infection (CLABSI) Required NHSN CAUTI Catheter-Associated Urinary Tract Infection (CAUTI)
CMS Hospital Inpatient Quality Reporting (IQR) Program Measures for the FY 2020 Payment Update
 CMS Inpatient Quality Reporting (IQR) Program Measures for the Payment Update Measures Required to Meet IQR Program APU Requirements Healthcare-Associated Infection on CAUTI National Healthcare Safety
CMS Inpatient Quality Reporting (IQR) Program Measures for the Payment Update Measures Required to Meet IQR Program APU Requirements Healthcare-Associated Infection on CAUTI National Healthcare Safety
PPS Exempt Cancer Hospital Quality Reporting (PCHQR) Program Relationship Matrix of Program Measures by Years and Quarters
 PPS Exempt Cancer Quality (PCHQR) Relationship Matrix of Measures by and This reference document for PCHQR participants provides the following: Specific measures with their National Quality Forum (NQF)
PPS Exempt Cancer Quality (PCHQR) Relationship Matrix of Measures by and This reference document for PCHQR participants provides the following: Specific measures with their National Quality Forum (NQF)
Medicare Value Based Purchasing Andrew B. Wheeler Vice President of Federal Finance
 Medicare Value Based Purchasing - 101 Andrew B. Wheeler Vice President of Federal Finance What is Medicare s VBP System? Incentive program to improve outcomes, safety, patient satisfaction, and efficiency
Medicare Value Based Purchasing - 101 Andrew B. Wheeler Vice President of Federal Finance What is Medicare s VBP System? Incentive program to improve outcomes, safety, patient satisfaction, and efficiency
Appendix G Explanation/Clarification Summary
 Appendix G Explanation/Clarification Summary Summary of Changes for Recommendations Alignment of measures with VBP by fiscal year Measures and service dates were adjusted to be consistent with the FY2016
Appendix G Explanation/Clarification Summary Summary of Changes for Recommendations Alignment of measures with VBP by fiscal year Measures and service dates were adjusted to be consistent with the FY2016
Performance Measure. Inpatient Clinical Process of Care Measures
 Acute Myocardial Infarction (AMI) 's Maryland Hospital Performance Evaluation System: Inpatient s Quality Based Reimbursement () Measures Highlighted in Green (02/27/2014) Inpatient Clinical Process of
Acute Myocardial Infarction (AMI) 's Maryland Hospital Performance Evaluation System: Inpatient s Quality Based Reimbursement () Measures Highlighted in Green (02/27/2014) Inpatient Clinical Process of
Mandatory Elements of Healthcare Reform Walter Coleman. healthcare consulting
 Mandatory Elements of Healthcare Reform Walter Coleman 1 Agenda ACA Mandatory Elements of Reform Value Based Purchasing Readmission Reduction Program Hospital Acquired Conditions Best practices to analyze
Mandatory Elements of Healthcare Reform Walter Coleman 1 Agenda ACA Mandatory Elements of Reform Value Based Purchasing Readmission Reduction Program Hospital Acquired Conditions Best practices to analyze
Medicare Hospital Acquired Conditions Reduction Program Andrew B. Wheeler Vice President of Federal Finance
 Medicare Hospital Acquired Conditions Reduction Program - 201 Andrew B. Wheeler Vice President of Federal Finance Value-Based Hospital Acquired Purchasing Conditions FFY 2018 FFY -2016 2020 AHRQ Claims
Medicare Hospital Acquired Conditions Reduction Program - 201 Andrew B. Wheeler Vice President of Federal Finance Value-Based Hospital Acquired Purchasing Conditions FFY 2018 FFY -2016 2020 AHRQ Claims
End-Stage Renal Disease Quality Incentive Program (ESRD QIP) Status Type NQF Measure Title
 End-Stage Renal Disease Quality Incentive Program (ESRD QIP) Status Type NQF Measure Title NQF Status ID Implemented Outcome 1454 Proportion of patients with hypercalcemia 0256 Vascular Access Type Catheter
End-Stage Renal Disease Quality Incentive Program (ESRD QIP) Status Type NQF Measure Title NQF Status ID Implemented Outcome 1454 Proportion of patients with hypercalcemia 0256 Vascular Access Type Catheter
2016 Hospital Measures
 2016 Hospital Measures Vicki Tang Olson, Stratis Health Statewide Quality Reporting and Measurement System (SQRMS) Annual Forum June 22, 2015 Objectives Share the process used for 2016 hospital measures
2016 Hospital Measures Vicki Tang Olson, Stratis Health Statewide Quality Reporting and Measurement System (SQRMS) Annual Forum June 22, 2015 Objectives Share the process used for 2016 hospital measures
SCORES FOR 4 TH QUARTER, RD QUARTER, 2014
 SCORES FOR 4 TH QUARTER, 2013 3 RD QUARTER, 2014 PATIENT SATISFACTION SCORES (HCAHPS): 4 STARS OUT OF 5 (ONLY 4 AREA ACUTE CARE HOSPITALS RECEIVED A 4-STAR RATING. NONE ACHIEVED 5-STARS). STRUCTURAL MEASURES:
SCORES FOR 4 TH QUARTER, 2013 3 RD QUARTER, 2014 PATIENT SATISFACTION SCORES (HCAHPS): 4 STARS OUT OF 5 (ONLY 4 AREA ACUTE CARE HOSPITALS RECEIVED A 4-STAR RATING. NONE ACHIEVED 5-STARS). STRUCTURAL MEASURES:
Table of Contents. Claims Based Measures Calculated by CMS (Outpatient) Imaging Efficiency Page 10
 Current Proposed Quality Measures Table of Contents Inpatient Measures Collected Submitted by Hospital Acute Myocardial Infarction/Emergency Department Page2 Immunization/Heart Failure/Pneumonia/Stroke
Current Proposed Quality Measures Table of Contents Inpatient Measures Collected Submitted by Hospital Acute Myocardial Infarction/Emergency Department Page2 Immunization/Heart Failure/Pneumonia/Stroke
FY X Time (48 hrs for cardiac surgery) SCIP-Inf-4 Cardiac Surgery Patients With Controlled 6 A.M. Postoperative Blood
 Valuebased 2013 Hospital Measure Summary Data Collection for Inpatient Quality Reporting FY2015 and Outpatient Reporting CY2014 January 2013 Key: = Required by both CMS and State of Minnesota = Required
Valuebased 2013 Hospital Measure Summary Data Collection for Inpatient Quality Reporting FY2015 and Outpatient Reporting CY2014 January 2013 Key: = Required by both CMS and State of Minnesota = Required
Table of Contents. Current and Proposed CMS Quality Measures for Reporting in 2017 through 2023 Revised 8/8/2017
 Table of Contents Current and Proposed CMS Quality Measures Inpatient Measures Collected and Submitted by Hospital AMI/ED/IMM/Pneumonia/Sepsis/Stroke Page 2 Surgical Care Improvement/VTE/Perinatal Care/Pediatric
Table of Contents Current and Proposed CMS Quality Measures Inpatient Measures Collected and Submitted by Hospital AMI/ED/IMM/Pneumonia/Sepsis/Stroke Page 2 Surgical Care Improvement/VTE/Perinatal Care/Pediatric
50198 Federal Register / Vol. 75, No. 157 / Monday, August 16, 2010 / Rules and Regulations
 50198 Federal Register / Vol. 75, No. 157 / Monday, August 16, 2010 / Rules and Regulations mstockstill on DSKH9S0YB1PROD with RULES2 VerDate Mar2010 17:02 Aug 13, 2010 Jkt 220001 PO 00000 Frm 00158
50198 Federal Register / Vol. 75, No. 157 / Monday, August 16, 2010 / Rules and Regulations mstockstill on DSKH9S0YB1PROD with RULES2 VerDate Mar2010 17:02 Aug 13, 2010 Jkt 220001 PO 00000 Frm 00158
Stratis Health
 2017 Hospital Measure Summary Minnesota Statewide Quality eporting & Measurement System (SQMS) and FY2019 for Center for Medicare & Medicaid Services (CMS) Contents Key... 1 Chart Abstracted Measures...
2017 Hospital Measure Summary Minnesota Statewide Quality eporting & Measurement System (SQMS) and FY2019 for Center for Medicare & Medicaid Services (CMS) Contents Key... 1 Chart Abstracted Measures...
Troubleshooting Audio
 Welcome! Audio for this event is available via ReadyTalk Internet streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Troubleshooting Audio
 Welcome Audio for this event is available via ReadyTalk Internet streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome Audio for this event is available via ReadyTalk Internet streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Final Recommendation for Updating the Quality Based Reimbursement Program
 Final Recommendation for Updating the Quality Based Reimbursement Program for FY 2018 October 14, 2015 Health Services Cost Review Commission 4160 Patterson Avenue Baltimore, Maryland 21215 (410) 764 2605
Final Recommendation for Updating the Quality Based Reimbursement Program for FY 2018 October 14, 2015 Health Services Cost Review Commission 4160 Patterson Avenue Baltimore, Maryland 21215 (410) 764 2605
Table of Contents. Current and Proposed CMS Quality Measures for Reporting in 2017 through 2023 Revised 5/4/2017
 Table of Contents Current and Proposed CMS Quality Measures for Reporting in 2017 through 2023 Inpatient Measures Collected and Submitted by Hospital AMI/ED/IMM/Pneumonia/Sepsis/Stroke Page 2 Surgical
Table of Contents Current and Proposed CMS Quality Measures for Reporting in 2017 through 2023 Inpatient Measures Collected and Submitted by Hospital AMI/ED/IMM/Pneumonia/Sepsis/Stroke Page 2 Surgical
Troubleshooting Audio
 Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
COOK COUNTY HEALTH Meaningful Metrics
 COOK COUNTY HEALTH Meaningful Metrics 2018-2019 Ronald Wyatt MD MHA January 18, 2019 2 Meaningful Measures 3 Meaningful Measures Framework Meaningful Measure Areas Achieve: High quality healthcare Meaningful
COOK COUNTY HEALTH Meaningful Metrics 2018-2019 Ronald Wyatt MD MHA January 18, 2019 2 Meaningful Measures 3 Meaningful Measures Framework Meaningful Measure Areas Achieve: High quality healthcare Meaningful
Core = Core required measures for all CAH nationally r = Required by State of Minnesota X = Additional for MBQIP
 Key: 2016 Hospital Measure Summary Minnesota Statewide Quality eporting and Measurement System (SQMS) and FY2018 for Center for Medicare and Medicaid Services () January 2016 = equired by Core = Core required
Key: 2016 Hospital Measure Summary Minnesota Statewide Quality eporting and Measurement System (SQMS) and FY2018 for Center for Medicare and Medicaid Services () January 2016 = equired by Core = Core required
Troubleshooting Audio
 Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Tennessee s Tenth Report on Healthcare-Associated Infections: Overview of Report, Methodology, and Format
 Tennessee s Tenth Report on Healthcare-Associated Infections: Overview of Report, Methodology, and Format TDH HAI Team September 3, 2015 Acknowledgements THA/TCPS for hosting this webinar TDH HAI Team
Tennessee s Tenth Report on Healthcare-Associated Infections: Overview of Report, Methodology, and Format TDH HAI Team September 3, 2015 Acknowledgements THA/TCPS for hosting this webinar TDH HAI Team
UCLA Health System Apr - Jun 2013 (Q2)
 Denom Observed VBP Standard VBP Benchmark Denom Observed VBP Standard VBP Benchmark N Percent x/n N Percent x/n Value Based Purchasing-Clinical Process of Care Measures (%) SCIP-Inf-9 Urinary catheter
Denom Observed VBP Standard VBP Benchmark Denom Observed VBP Standard VBP Benchmark N Percent x/n N Percent x/n Value Based Purchasing-Clinical Process of Care Measures (%) SCIP-Inf-9 Urinary catheter
SUNY Downstate Medical Center/University Hospital Oct - Dec 2013 (Q4)
 Value Based Purchasing-Clinical Process of Care Measures Denom Observed VBP VBP Benchmark Standard Denom Observed VBP VBP Benchmark Standard N Percent x/n N Percent x/n SCIP-Inf-9 Urinary catheter removed
Value Based Purchasing-Clinical Process of Care Measures Denom Observed VBP VBP Benchmark Standard Denom Observed VBP VBP Benchmark Standard N Percent x/n N Percent x/n SCIP-Inf-9 Urinary catheter removed
What ASMBS Members Need to Know About: New Medicare Payment Policy Governing Bariatric Surgery and Hospital Acquired Conditions (HACs)
 What ASMBS Members Need to Know About: New Medicare Payment Policy Governing Bariatric Surgery and Hospital Acquired Conditions (HACs) Robin Blackstone, MD, FACS, FASMBS Beginning October 1, 2008, Medicare
What ASMBS Members Need to Know About: New Medicare Payment Policy Governing Bariatric Surgery and Hospital Acquired Conditions (HACs) Robin Blackstone, MD, FACS, FASMBS Beginning October 1, 2008, Medicare
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions
 Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
Infection Control: Meeting the Challenge
 22nd Annual Midas+ User Symposium June 2 5, 2013 Tucson, Arizona Infection Control: Meeting the Challenge Wednesday, June 5, 2:30 pm The data demands placed on Infection Control departments have significantly
22nd Annual Midas+ User Symposium June 2 5, 2013 Tucson, Arizona Infection Control: Meeting the Challenge Wednesday, June 5, 2:30 pm The data demands placed on Infection Control departments have significantly
EHs and CAHs have the option of attesting or ereporting CQMs in 2015 through 2017
 CMS-3310-FC & CMS-3311-FC: MU Stage 3 Proposed Reporting on Clinical Quality Measures Using Certified EHR Technology Requirements for Eligible Hospitals & Critical Access Hospitals 2015-2018 Key Takeaways
CMS-3310-FC & CMS-3311-FC: MU Stage 3 Proposed Reporting on Clinical Quality Measures Using Certified EHR Technology Requirements for Eligible Hospitals & Critical Access Hospitals 2015-2018 Key Takeaways
New Mexico Healthcare-associated Infections Annual Report
 New Mexico Healthcare-associated Infections Annual Report Prepared by: New Mexico Healthcare-associated Infections Advisory Committee August 2013 This document and further New Mexico healthcare-associated
New Mexico Healthcare-associated Infections Annual Report Prepared by: New Mexico Healthcare-associated Infections Advisory Committee August 2013 This document and further New Mexico healthcare-associated
Progress Towards Eliminating Healthcare-Associated Infections. November 27, 2012 Washington Marriott Hotel, Washington, D.C.
 Progress Towards Eliminating Healthcare-Associated Infections November 27, 2012 Washington Marriott Hotel, Washington, D.C. Welcome Don Wright, MD, MPH Office of the Assistant Secretary for Health (OASH)
Progress Towards Eliminating Healthcare-Associated Infections November 27, 2012 Washington Marriott Hotel, Washington, D.C. Welcome Don Wright, MD, MPH Office of the Assistant Secretary for Health (OASH)
Surgical Care, Pneumonia, Immunizations and Emergency Department Core Measures
 Surgical Care, Pneumonia, Immunizations and Emergency Department Core Measures Audrey Paulman, MD, MMM Principal Clinical Coordinator & Jackie Trojan, RN, BSN Quality Improvement Advisor This material
Surgical Care, Pneumonia, Immunizations and Emergency Department Core Measures Audrey Paulman, MD, MMM Principal Clinical Coordinator & Jackie Trojan, RN, BSN Quality Improvement Advisor This material
Improving Influenza Vaccination Rates in Critical Access Hospitals 10/26/2016
 Improving Influenza Vaccination Rates in Critical Access Hospitals 10/26/2016 Objectives Provide an overview of OP 27 Influenza Vaccination Among Healthcare Personnel (HCP) and IMM 2 Immunization for influenza
Improving Influenza Vaccination Rates in Critical Access Hospitals 10/26/2016 Objectives Provide an overview of OP 27 Influenza Vaccination Among Healthcare Personnel (HCP) and IMM 2 Immunization for influenza
State of the State: Hospital Performance in Pennsylvania September 2012
 State of the State: Hospital Performance in Pennsylvania September 2012 Measuring Progress in PA Hospital Performance: Process Measures 1 PA Hospital Performance: Process Measures We examined the latest
State of the State: Hospital Performance in Pennsylvania September 2012 Measuring Progress in PA Hospital Performance: Process Measures 1 PA Hospital Performance: Process Measures We examined the latest
NHSN and Public Reporting. Linda R. Greene, RN,MPS,CIC Manager Infection Prevention Highland Hospital Rochester, NY linda_
 1 NHSN and Public Reporting Linda R. Greene, RN,MPS,CIC Manager Infection Prevention Highland Hospital Rochester, NY linda_ greene@urmc.rochester.edu 2 Objectives Describe challenges and opportunities
1 NHSN and Public Reporting Linda R. Greene, RN,MPS,CIC Manager Infection Prevention Highland Hospital Rochester, NY linda_ greene@urmc.rochester.edu 2 Objectives Describe challenges and opportunities
CCHHSQualityDashboard-DRAFT
 CCHHSQualityDashboard-DRAFT9..8 Falswith Injury Pressure Injury(Stage I&IV) Aug-7 Nov-7 Feb-8 May-8 Aug-8 Aug-7 Nov-7 Feb-8 May-8 Aug-8 0 4 9 8 5 5 6 5 HospitalAcquiredConditions 07Q 07Q4 08Q 08Q 0.00
CCHHSQualityDashboard-DRAFT9..8 Falswith Injury Pressure Injury(Stage I&IV) Aug-7 Nov-7 Feb-8 May-8 Aug-8 Aug-7 Nov-7 Feb-8 May-8 Aug-8 0 4 9 8 5 5 6 5 HospitalAcquiredConditions 07Q 07Q4 08Q 08Q 0.00
CAH Participation and Quality Measure Results for Hospital Compare 2007 Discharges and Trends: National and North Carolina Results
 January 2009 CAH Participation and Quality Measure Results for Hospital Compare Discharges and - Trends: and Results Michelle Casey, MS 1, Michele Burlew, MS 2, Ira Moscovice, PhD 1 1 University of Minnesota
January 2009 CAH Participation and Quality Measure Results for Hospital Compare Discharges and - Trends: and Results Michelle Casey, MS 1, Michele Burlew, MS 2, Ira Moscovice, PhD 1 1 University of Minnesota
Keeping Up with the Regulatory Requirements and Other Hocus Pocus. Vicky A. Mahn-DiNicola RN, MS, CPHQ Vice President and Product Manager ACS MIDAS+
 Keeping Up with the Regulatory Requirements and Other Hocus Pocus Vicky A. Mahn-DiNicola RN, MS, CPHQ Vice President and Product Manager ACS MIDAS+ Session Objectives Review Medicare s proposed strategies
Keeping Up with the Regulatory Requirements and Other Hocus Pocus Vicky A. Mahn-DiNicola RN, MS, CPHQ Vice President and Product Manager ACS MIDAS+ Session Objectives Review Medicare s proposed strategies
Knowledge to Practice; Applying New Skills
 Knowledge to Practice; Applying New Skills Linda R. Greene, RN, BS, MPS,CIC UR Highland Hospital Rochester, NY linda_greene@urmc.rochester.edu Kim M. Delahanty, RN, BSN, PHN,MBA/HCM,CIC UCSD Health System
Knowledge to Practice; Applying New Skills Linda R. Greene, RN, BS, MPS,CIC UR Highland Hospital Rochester, NY linda_greene@urmc.rochester.edu Kim M. Delahanty, RN, BSN, PHN,MBA/HCM,CIC UCSD Health System
NEW JERSEY 2012 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES
 NEW JERSEY 2012 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES New Jersey Department of Health Health Care Quality Assessment June 2013 NEW JERSEY
NEW JERSEY 2012 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES New Jersey Department of Health Health Care Quality Assessment June 2013 NEW JERSEY
Quality Reporting for CAHs and Rural PPS Hospitals: The Potential Impact of Composite Measures
 UpperMidwest Rural Health Research Center www.uppermidwestrhrc.org July 202 Policy Brief Quality Reporting for CAHs and Rural PPS Hospitals: The Potential Impact of Composite Measures Michelle Casey MS,
UpperMidwest Rural Health Research Center www.uppermidwestrhrc.org July 202 Policy Brief Quality Reporting for CAHs and Rural PPS Hospitals: The Potential Impact of Composite Measures Michelle Casey MS,
Infectious Diseases-HAI Idaho Department of Health and Welfare, Division of Public Health Boise, Idaho. Assignment Description
 Infectious Diseases-HAI Idaho Department of Health and Welfare, Division of Public Health Boise, Idaho Assignment Description The Fellow s assignments will primarily focus on projects within the HAI and
Infectious Diseases-HAI Idaho Department of Health and Welfare, Division of Public Health Boise, Idaho Assignment Description The Fellow s assignments will primarily focus on projects within the HAI and
NHSN Tips for CMS Hospital IQR Program: MRSA Bacteremia and CDI LabID Healthcare Personnel Influenza Vaccination
 NHSN Tips for CMS Hospital IQR Program: MRSA Bacteremia and CDI LabID Healthcare Personnel Influenza Vaccination Maggie Dudeck, MPH, CPH Epidemiologist National Provider Education Webcast May 1, 2013 National
NHSN Tips for CMS Hospital IQR Program: MRSA Bacteremia and CDI LabID Healthcare Personnel Influenza Vaccination Maggie Dudeck, MPH, CPH Epidemiologist National Provider Education Webcast May 1, 2013 National
Infectious Diseases-HAI Hawaii Department of Health, Disease Outbreak Control Division. Honolulu, Hawaii. Assignment Description
 Infectious Diseases-HAI Hawaii Department of Health, Disease Outbreak Control Division Honolulu, Hawaii Assignment Description The CSTE Fellow would be assigned to DOCD under the mentorship of Dr. Park
Infectious Diseases-HAI Hawaii Department of Health, Disease Outbreak Control Division Honolulu, Hawaii Assignment Description The CSTE Fellow would be assigned to DOCD under the mentorship of Dr. Park
REVISIONS: Strategies to Achieve the Healthy People 2020 Annual Goal of 90% Influenza Vaccination Coverage for Health Care Personnel
 National Vaccine Advisory Committee Health Care Personnel Influenza Vaccination Subgroup REVISIONS: Strategies to Achieve the Healthy People 2020 Annual Goal of 90% Influenza Vaccination Coverage for Health
National Vaccine Advisory Committee Health Care Personnel Influenza Vaccination Subgroup REVISIONS: Strategies to Achieve the Healthy People 2020 Annual Goal of 90% Influenza Vaccination Coverage for Health
Performance Measures for Adult Immunization
 Performance Measures for Adult Immunization Reva Winkler, MD, MPH Senior Director, Performance Measures May 15, 2012 1 NQF-endorsed consensus standards for adult immunizations Influenza immunization 0431
Performance Measures for Adult Immunization Reva Winkler, MD, MPH Senior Director, Performance Measures May 15, 2012 1 NQF-endorsed consensus standards for adult immunizations Influenza immunization 0431
September 10, To Whom It May Concern:
 September 10, 2018 Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1691-P P.O. Box 8010, Baltimore, MD 21244-8010 RE: CMS-1691-P Medicare Program; End-Stage
September 10, 2018 Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1691-P P.O. Box 8010, Baltimore, MD 21244-8010 RE: CMS-1691-P Medicare Program; End-Stage
Quality Metrics & Immunizations
 Optimizing Patients' Health by Improving the Quality of Medication Use Quality Metrics & Immunizations Hannah Fish, PharmD, CPHQ Discussion Objectives 1. Describe the types and distribution of quality
Optimizing Patients' Health by Improving the Quality of Medication Use Quality Metrics & Immunizations Hannah Fish, PharmD, CPHQ Discussion Objectives 1. Describe the types and distribution of quality
Reporting Options History of NHSN
 The National Healthcare Safety Network (NHSN) and Ambulatory Surgery Centers Ashlie Dowdell HAI Surveillance Coordinator Wisconsin Division of Public Health June 10, 2014 Objectives Provide an overview
The National Healthcare Safety Network (NHSN) and Ambulatory Surgery Centers Ashlie Dowdell HAI Surveillance Coordinator Wisconsin Division of Public Health June 10, 2014 Objectives Provide an overview
Hospice. Hospice Item Set (HIS) Submission Requirements. Quality Reporting Program Provider Training
 Hospice Quality Reporting Program Provider Training Hospice Item Set (HIS) Submission Requirements Presenter: Brenda Karkos, M.S.N./M.B.A., R.N., CHPN Date: January 18, 2017 Objectives Discuss the Hospice
Hospice Quality Reporting Program Provider Training Hospice Item Set (HIS) Submission Requirements Presenter: Brenda Karkos, M.S.N./M.B.A., R.N., CHPN Date: January 18, 2017 Objectives Discuss the Hospice
NEW JERSEY 2011 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES
 NEW JERSEY 2011 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES New Jersey Department of Health and Senior Services Health Care Quality Assessment
NEW JERSEY 2011 HOSPITAL PERFORMANCE REPORT TECHNICAL REPORT: METHODOLOGY RECOMMENDED CARE (PROCESS OF CARE) MEASURES New Jersey Department of Health and Senior Services Health Care Quality Assessment
PfP Quality Metrics: Readmissions, Value-Based Purchasing and Beyond
 PfP Quality Metrics: Readmissions, Value-Based Purchasing and Beyond Presented to ASHNHA Alaska Partnership for Patients Advisory Group February 4, 2015 Gloria Kupferman Readmissions Calculation methods
PfP Quality Metrics: Readmissions, Value-Based Purchasing and Beyond Presented to ASHNHA Alaska Partnership for Patients Advisory Group February 4, 2015 Gloria Kupferman Readmissions Calculation methods
Rapid Response Teams. January 17, Safe Table Webinar
 Rapid Response Teams January 17, 2017 Safe Table Webinar Christin Gordanier, MSN, RN, Inpatient Nursing Director at Virginia Mason Medical Center in Seattle, Washington. Alice Ferguson, BSN, RN, Project
Rapid Response Teams January 17, 2017 Safe Table Webinar Christin Gordanier, MSN, RN, Inpatient Nursing Director at Virginia Mason Medical Center in Seattle, Washington. Alice Ferguson, BSN, RN, Project
Hospital OQR Quality Measures and Timelines for CY 2015 and Subsequent Payment Determinations
 OQR Quality Measures and Timelines for CY 2015 and Subsequent Payment Determinations Data collection, implementation, and public reporting information for each measure are detailed by measure set in the
OQR Quality Measures and Timelines for CY 2015 and Subsequent Payment Determinations Data collection, implementation, and public reporting information for each measure are detailed by measure set in the
Hypoglycemia and Quality Measurement
 Hypoglycemia and Quality Measurement Sam Stolpe Senior Director The Triple Aim Affordable Care Better Care Healthy People/ Communities 1 Comprehensive Overview of CMS Quality Programs Hospital Quality
Hypoglycemia and Quality Measurement Sam Stolpe Senior Director The Triple Aim Affordable Care Better Care Healthy People/ Communities 1 Comprehensive Overview of CMS Quality Programs Hospital Quality
Table 1. Proposed Measures for Use in Establishing Quality Performance Standards that ACOs Must Meet for Shared Savings
 CMS-1345-P 174 Table 1. Proposed Measures for Use in Establishing Quality Performance Standards that ACOs Must Meet for Shared Savings AIM: Better Care for Individuals 1. Patient/Care Giver Experience
CMS-1345-P 174 Table 1. Proposed Measures for Use in Establishing Quality Performance Standards that ACOs Must Meet for Shared Savings AIM: Better Care for Individuals 1. Patient/Care Giver Experience
Breakout: Quality and Performance Measure WG
 Breakout: Quality and Performance Measure WG Maternal Immunization Sub-Group ESRD Sub-Group Adult Composite May 11, 2016 Atlanta, GA Co-Chairs: Amy Groom, IHS Sharon Sprenger, Joint Commission Sam Stolpe,
Breakout: Quality and Performance Measure WG Maternal Immunization Sub-Group ESRD Sub-Group Adult Composite May 11, 2016 Atlanta, GA Co-Chairs: Amy Groom, IHS Sharon Sprenger, Joint Commission Sam Stolpe,
Noel Eldridge, MS. AHRQ Center for Quality Improvement and Patient Safety
 Presentation on: National trends in the frequency of bladder catheterization and physician-diagnosed catheterassociated urinary tract infections: Results from the Medicare Patient Safety Monitoring System
Presentation on: National trends in the frequency of bladder catheterization and physician-diagnosed catheterassociated urinary tract infections: Results from the Medicare Patient Safety Monitoring System
PIN BENCHMARKING DATA DEFINITIONS DICTIONARY
 CORE MEASURES PIN BENCHMARKING DATA DEFINITIONS DICTIONARY 1 Total number of CAH acute care patient admissions. Report all CAH acute care only patient admissions for the quarter. Exclude CAH swing bed,
CORE MEASURES PIN BENCHMARKING DATA DEFINITIONS DICTIONARY 1 Total number of CAH acute care patient admissions. Report all CAH acute care only patient admissions for the quarter. Exclude CAH swing bed,
Improving Quality of Care for Medicare Patients: Accountable Care Organizations
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs MEDICARE FACT SHEET FOR IMMEDIATE RELEASE
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs MEDICARE FACT SHEET FOR IMMEDIATE RELEASE
Healthcare Associated Infection Report February 2016 data
 Healthcare Associated Infection Report February 2016 data Section 1 Board Wide Issues Section 1 of the HAIRT covers Board wide infection prevention and control activity and actions. For reports on individual
Healthcare Associated Infection Report February 2016 data Section 1 Board Wide Issues Section 1 of the HAIRT covers Board wide infection prevention and control activity and actions. For reports on individual
Inpatient Psychiatric Facility Quality Reporting (IPFQR) Program Measures for Fiscal Year (FY) 2017 and FY 2018
 IPFQR Program s for FY 2017 Payment Determination HBIPS-2: Hours of Physical Restraint Use HBIPS-3: Hours of Seclusion Use HBIPS-5: Patients Discharged on Multiple Antipsychotic Medications with Appropriate
IPFQR Program s for FY 2017 Payment Determination HBIPS-2: Hours of Physical Restraint Use HBIPS-3: Hours of Seclusion Use HBIPS-5: Patients Discharged on Multiple Antipsychotic Medications with Appropriate
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions
 Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
2018 CNISP HAI Surveillance Case definitions
 2018 CNISP HAI Surveillance Case definitions The following case definitions for the surveillance of healthcare-associated infections (HAIs) are used by all acute-care hospitals that participate in the
2018 CNISP HAI Surveillance Case definitions The following case definitions for the surveillance of healthcare-associated infections (HAIs) are used by all acute-care hospitals that participate in the
Validation of HAI Reporting in New Hampshire Hospitals: Data from
 Validation of HAI Reporting in New Hampshire Hospitals: Data from 2014-15 Nancy Reinhalter, RN CCRC JSI Research & Training Institute, Inc. February 22, 2017 ACKNOWLEDGEMENTS JSI Team Priscilla Davis Paddy
Validation of HAI Reporting in New Hampshire Hospitals: Data from 2014-15 Nancy Reinhalter, RN CCRC JSI Research & Training Institute, Inc. February 22, 2017 ACKNOWLEDGEMENTS JSI Team Priscilla Davis Paddy
APIC NHSN Webinar 9/8/2015. Topic Overview. Overall Learning Objectives
 APIC NHSN Webinar Janet Brooks, Cindy Gross, Denise Leaptrot, & Eileen Scalise Subject Matter Experts September 9, 2015 National Center for Emerging and Zoonotic Infectious Diseases Place Descriptor Here
APIC NHSN Webinar Janet Brooks, Cindy Gross, Denise Leaptrot, & Eileen Scalise Subject Matter Experts September 9, 2015 National Center for Emerging and Zoonotic Infectious Diseases Place Descriptor Here
Quality & Hospital Acquired Conditions
 Quality & Hospital Acquired Conditions Rebecca Armbruster, DO, MS, FACOI Medical Director Resource Management Patricia Heys, BS Director of Infection Prevention & Control Sally Hinkle, DNP, MPA, RN Director
Quality & Hospital Acquired Conditions Rebecca Armbruster, DO, MS, FACOI Medical Director Resource Management Patricia Heys, BS Director of Infection Prevention & Control Sally Hinkle, DNP, MPA, RN Director
Medicare & Medicaid EHR Incentive Programs
 Medicare & Medicaid EHR Incentive Programs Stage 2 NPRM Overview Robert Anthony March 6, 2012 Proposed Rule Everything discussed in this presentation is part of a notice of proposed rulemaking (NPRM).
Medicare & Medicaid EHR Incentive Programs Stage 2 NPRM Overview Robert Anthony March 6, 2012 Proposed Rule Everything discussed in this presentation is part of a notice of proposed rulemaking (NPRM).
This Core Measure Report shows performance to date. CAVEAT: Data collection is still in progress for the current and immediate past quarter!
 This Core Measure Report shows performance to date. CAVEAT: Data collection is still in progress for the current and immediate past quarter! AMI-1 -- Aspirin at Arrival 9 8 7 6 5 4 3 2 1 AMI-2 -- Aspirin
This Core Measure Report shows performance to date. CAVEAT: Data collection is still in progress for the current and immediate past quarter! AMI-1 -- Aspirin at Arrival 9 8 7 6 5 4 3 2 1 AMI-2 -- Aspirin
State of New Hampshire HEALTHCARE-ASSOCIATED INFECTIONS 2013 HOSPITAL REPORT
 of New Hampshire HEALTHCARE-ASSOCIATED INFECTIONS 2013 HOSPITAL REPORT Prepared by New Hampshire Department of Health and Human Services Division of Public Health Services Infectious Disease Surveillance
of New Hampshire HEALTHCARE-ASSOCIATED INFECTIONS 2013 HOSPITAL REPORT Prepared by New Hampshire Department of Health and Human Services Division of Public Health Services Infectious Disease Surveillance
COMMUNICABLE DISEASE REPORT Quarterly Report
 COMMUNICABLE DISEASE REPORT Quarterly Report Volume 31, Number 3 December 2014 Healthcare-Associated Infections In past issues of the Communicable Disease Report the focus has been on antibiotic-resistant
COMMUNICABLE DISEASE REPORT Quarterly Report Volume 31, Number 3 December 2014 Healthcare-Associated Infections In past issues of the Communicable Disease Report the focus has been on antibiotic-resistant
Publicly Reported Quality Measures
 Publicly Reported Quality Measures Five-Star Quality Rating System As part of the initiative to add five-star quality ratings to its Compare Web sites, the Centers for Medicare & Medicaid Services (CMS)
Publicly Reported Quality Measures Five-Star Quality Rating System As part of the initiative to add five-star quality ratings to its Compare Web sites, the Centers for Medicare & Medicaid Services (CMS)
Absent: Director Layla P. Suleiman Gonzalez, PhD, JD (1)
 Minutes of the meeting of the Quality and Patient Safety Committee of the Board of Directors of the Cook County Health and Hospitals System held Friday, January 18, 2019 at the hour of 10:00 A.M. at 1950
Minutes of the meeting of the Quality and Patient Safety Committee of the Board of Directors of the Cook County Health and Hospitals System held Friday, January 18, 2019 at the hour of 10:00 A.M. at 1950
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions
 Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
Great Lakes Partners for Patients: Hospital Improvement Innovation Network - Encyclopedia of Measures Frequently Asked Questions Contents Great Lakes Partners for Patients: Hospital Improvement Innovation
2012 Core Measures. Acute Myocardial Infarction (AMI)
 2012 Core Measures Acute Myocardial Infarction (AMI) Aspirin at Arrival Aspirin Prescribed at Discharge Angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for left ventricular
2012 Core Measures Acute Myocardial Infarction (AMI) Aspirin at Arrival Aspirin Prescribed at Discharge Angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for left ventricular
Improving Prevention and Control of Infection Quarter 2 Report: April 2009 September 2009
 Improving Prevention and Control of Infection Quarter 2 Report: April 2009 September 2009 1. Introduction This Quarter 2 updates the Health Board on infection prevention and control issues within the BCUHB.
Improving Prevention and Control of Infection Quarter 2 Report: April 2009 September 2009 1. Introduction This Quarter 2 updates the Health Board on infection prevention and control issues within the BCUHB.
Troubleshooting Audio
 Welcome! Audio for this event is available via ReadyTalk Internet Streaming. telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet Streaming. telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Quality Committee Core Measures Report AMI. Acute Myocardial Infarction
 AMI 2011 Acute Myocardial Infarction ASPIRIN AT ARRIVAL: A higher number is better. This measure shows the percentage of heart attack patients who receive aspirin within 24 hrs of arrival at hospital.
AMI 2011 Acute Myocardial Infarction ASPIRIN AT ARRIVAL: A higher number is better. This measure shows the percentage of heart attack patients who receive aspirin within 24 hrs of arrival at hospital.
Antisepsis Bath and Oral.. Should We Change Practice? DR AZMIN HUDA ABDUL RAHIM
 Antisepsis Bath and Oral.. Should We Change Practice? DR AZMIN HUDA ABDUL RAHIM Chlorhexidine Exposure in ICU Chlorhexidine gluconate Long acting topical antiseptic In use since 1954 Water soluble Remains
Antisepsis Bath and Oral.. Should We Change Practice? DR AZMIN HUDA ABDUL RAHIM Chlorhexidine Exposure in ICU Chlorhexidine gluconate Long acting topical antiseptic In use since 1954 Water soluble Remains
Update on Healthcare Personnel Influenza Vaccination
 Update on Healthcare Personnel Influenza Vaccination Raymond A. St rikas, MD, MPH Immunization Services Division National Center for Immunization and Respiratory Diseases October 23, 2012 National Center
Update on Healthcare Personnel Influenza Vaccination Raymond A. St rikas, MD, MPH Immunization Services Division National Center for Immunization and Respiratory Diseases October 23, 2012 National Center
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management
 Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
June 13, of 10: SHEA/IDSA Comment Letter
 June 13, 2008 Mr. Kerry Weems Acting Administrator, Centers for Medicare & Medicaid Services Attention: CMS 1390 P, Mail Stop C4 26 05 7500 Security Boulevard, Baltimore, MD 21244 1850 Re: Medicare Program;
June 13, 2008 Mr. Kerry Weems Acting Administrator, Centers for Medicare & Medicaid Services Attention: CMS 1390 P, Mail Stop C4 26 05 7500 Security Boulevard, Baltimore, MD 21244 1850 Re: Medicare Program;
Leveraging Technology to Prevent Catheter- Associated Urinary Tract Infections (CAUTI): Disrupting the Lifecycle of the Urinary Catheter
 Leveraging Technology to Prevent Catheter- Associated Urinary Tract Infections (CAUTI): Disrupting the Lifecycle of the Urinary Catheter Zachary N Gordon, MD ABMS Annual Conference September 28, 2016 CAUTIs:
Leveraging Technology to Prevent Catheter- Associated Urinary Tract Infections (CAUTI): Disrupting the Lifecycle of the Urinary Catheter Zachary N Gordon, MD ABMS Annual Conference September 28, 2016 CAUTIs:
Publicly Reported Quality Measures
 Publicly Reported Quality Measures Five-Star Quality Rating System As part of the initiative to add five-star quality ratings to its Compare Web sites, the Centers for Medicare & Medicaid Services (CMS)
Publicly Reported Quality Measures Five-Star Quality Rating System As part of the initiative to add five-star quality ratings to its Compare Web sites, the Centers for Medicare & Medicaid Services (CMS)
2016 AMC Quality and Accountability Performance Scorecard Vidant Medical Center. Overall Rank. Overall Score 63.4% Efficiency 7.
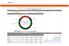 2016 AMC Quality and Accountability Performance Scorecard Vidant Medical Center Star Rating Mortality 12.66% of 25% Domain Performance Overall Rank 27 Overall Score 63.4% Equity 5.00% of 5% Efficiency
2016 AMC Quality and Accountability Performance Scorecard Vidant Medical Center Star Rating Mortality 12.66% of 25% Domain Performance Overall Rank 27 Overall Score 63.4% Equity 5.00% of 5% Efficiency
Quality Improvement Updates Foley Discontinuation Protocol Surgical Care Improvement Project
 Quality Improvement Updates Foley Discontinuation Protocol Surgical Care Improvement Project Barbara J Martin, RN, MBA Quality Consultant, Center for Clinical Improvement Indwelling Urinary Catheters Insertion,
Quality Improvement Updates Foley Discontinuation Protocol Surgical Care Improvement Project Barbara J Martin, RN, MBA Quality Consultant, Center for Clinical Improvement Indwelling Urinary Catheters Insertion,
USING THE WEBEX Q&A FEATURE
 USING THE WEBEX Q&A FEATURE All lines are placed on mute to block out background noises. However, you can send in questions to the panelists via the Q&A button. Follow the directions below to use the Q&A
USING THE WEBEX Q&A FEATURE All lines are placed on mute to block out background noises. However, you can send in questions to the panelists via the Q&A button. Follow the directions below to use the Q&A
Healthcare-Associated Infections Across the Spectrum of Care
 MODULE 9: HEALTHCARE-ASSOCIATED INFECTIONS ACROSS THE SPECTRUM OF CARE Healthcare-Associated Infections Across the Spectrum of Care Susan E. Coffin, MD, MPH UPENN School of Medicine, Department of Pediatrics
MODULE 9: HEALTHCARE-ASSOCIATED INFECTIONS ACROSS THE SPECTRUM OF CARE Healthcare-Associated Infections Across the Spectrum of Care Susan E. Coffin, MD, MPH UPENN School of Medicine, Department of Pediatrics
NHS GRAMPIAN. Healthcare Associated Infection (HAI) Bimonthly Report January 2016
 NHS GRAMPIAN Healthcare Associated Infection (HAI) Bimonthly Report uary 2016 1. Actions Recommended The Board is requested to note the content of this summary bimonthly HAI Report, as directed by the
NHS GRAMPIAN Healthcare Associated Infection (HAI) Bimonthly Report uary 2016 1. Actions Recommended The Board is requested to note the content of this summary bimonthly HAI Report, as directed by the
On behalf of the Infectious Diseases Society of America (IDSA), I am pleased to provide
 Transmitted by Jonathan Nurse, Director of Government Relations, IDSA The Infectious Diseases Society of America s (IDSA) Fiscal Year 2015 Funding Statement Submitted to the House Appropriations Subcommittee
Transmitted by Jonathan Nurse, Director of Government Relations, IDSA The Infectious Diseases Society of America s (IDSA) Fiscal Year 2015 Funding Statement Submitted to the House Appropriations Subcommittee
Quality Innovation Network - Quality Improvement Organization Adult Immunization Task. May 14, Agenda
 Quality Innovation Network - Quality Improvement Organization Adult Immunization Task National Adult and Influenza Immunization Summit Centers for Medicare & Medicaid Services 1 May 14, 2015 Agenda Quick
Quality Innovation Network - Quality Improvement Organization Adult Immunization Task National Adult and Influenza Immunization Summit Centers for Medicare & Medicaid Services 1 May 14, 2015 Agenda Quick
Advancing Care Coordination through Episode Payment Models (EPMs): Summary of the Proposed Rule
 Advancing Care Coordination through Episode Payment Models (EPMs): Summary of the Proposed Rule Overview Three new mandatory Episode Payment Models (EPMs) Cardiac Rehabilitation (CR) Incentive Payment
Advancing Care Coordination through Episode Payment Models (EPMs): Summary of the Proposed Rule Overview Three new mandatory Episode Payment Models (EPMs) Cardiac Rehabilitation (CR) Incentive Payment
