T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion.
|
|
|
- Marian Potter
- 5 years ago
- Views:
Transcription
1 Processed by MTF, designed and available through.. An allograft spacer for transforaminal posterior lumbar interbody fusion.
2 The has been engineered to meet the specific demands of transforaminal posterior lumbar interbody fusion procedures. Six precise implant heights accommodate individual patient anatomy. Pyramidal teeth on superior and inferior surfaces minimize migration and resist expulsion Precisely machined instrument slot ensures secure fit between implant and implant holder Beveled edge on distal end facilitates implant insertion
3 Spacer Dimensions Dimensions Process Frozen Freeze-Dried Item Item Anterior Number Number Height (mm) mm central AP depth 11 mm total AP depth Height ML width = 26 mm
4 Mechanical Testing Summary Sound engineering principles, extensive anatomical research and mechanical testing are the basis for the T-PLIF design. Figure 1 Compressive Testing Compressive Strength Tests were conducted to ensure that the T-PLIF spacer withstands the compressive loads in the lumbar spine. The ultimate compressive strength of the vertebral body is 8000 N. 1 The design goal for the T-PLIF spacer was to achieve compressive strength of at least 8000 N. Loads above this would result in failure of the vertebral body before failure of the implant. Test results show that the T-PLIF spacer has a compressive strength above 16,000 N (Figure 1). 2 Compressive Strength (N) 20,000 15,000 10,000 5,000 Resistance to Expulsion Resistance to implant expulsion is a major factor in the design of intervertebral spacers. Pyramid-shaped teeth are machined on the superior and inferior surfaces of the implant to increase resistance to pushout. Testing was conducted to ensure that the T-PLIF spacers are capable of resisting expulsion at clinically relevant loads. An axial preload (450 N) and a side (shear) load were applied to the implant to determine the pushout strength. 3 Test results (Figure 2) show that the T-PLIF spacer had pushout strength of 850 N, more than three times the pushout strength of a comparable design without teeth (234 N ± 38 N). 0 Figure Pushout Strength Testing Vertebral Body Pushout Strength (N) Axial Pre-Load Load (N) (with teeth) Allograft Wedge (no teeth) 1. O. Perry. Fracture of the vertebral endplate in the lumbar spine. Acta. Orthop. Scand. 1957; 25 (suppl.) 2. Testing performed at the Mechanical Testing Laboratory,, West Chester, PA A.A. White and M.M. Panjabi. Clinical Biomechanics of the Spine. Philadelphia: Lippincott, William and Wilkins
5 Spacer Instrumentation Synthes instrumentation is designed to make the T-PLIF spacer implantation procedure quick and simple. Sets Instrument Set (not shown) T-PLIF Minimally Invasive Instrument Set T-PLIF Auxiliary Instrument Set
6 Musculoskeletal Transplant Foundation (MTF) Quality is our first priority The Musculoskeletal Transplant Foundation (MTF) is a national consortium of medical schools, academic institutions and recovery organizations involved in the aseptic recovery, processing and distribution of bone and related soft tissue for use in transplant surgery. Its quality and safety standards consistently meet or exceed the requirements of the American Association of Tissue Banks (AATB) and the guidelines for screening and testing of tissue donors set forth by the Food and Drug Administration (FDA). Testing MTF is AATB-accredited and uses the most complete and technically advanced testing available to assure the safety of its allografts. MTF utilizes Nucleic Acid Testing (NAT by TMA) on every donor. NAT is a reliable test to confirm the presence or absence of HIV and HCV early, following exposure. MTF continues its very comprehensive testing for the following: HIV-1*, HIV-2*, HTLV-I, HTLV-II, HB Core, RPR, HCV-Ab, HBs Ag*, NAT, HCV-NAT. Since 1991, over 50,000 donors have been approved and processed, with more than 2.5 million allografts distributed. MTF s testing and processing meet or exceed all American Association of Tissue Banks (AATB) standards and FDA regulations, and MTF maintains an unrivaled safety record. Processing To maintain biological integrity, MTF processes all tissue by using aseptic techniques in class 10 (certified) clean rooms. This eliminates the need for terminal sterilization by high-dose gamma irradiation or ethylene oxide gas, which have been shown to compromise the biological and biomechanical integrity of allograft tissue.** Some tissues MTF distributes are exposed to a low-dose gamma radiation ( megarads) as a means of reducing bioburden prior to processing. For these tissues the container label will specify PRETREATED WITH LOW-DOSE GAMMA IRRADIATION. This low dose of radiation has been proven to effectively sterilize all nonsystemic bacterial and fungal contaminants. All tissue is computer-tracked from recovery through testing, processing, packaging and distribution. Donor criteria and selection The age criteria for donors is years for males and females. This allows the selection of tissue with denser construction. All potential donors must pass through an extensive quality assurance process. Screening begins at the site of recovery with a comprehensive medical and social history that includes the cause of death. Tissue and blood samples are tested for infectious diseases that include hepatitis, HIV, and syphilis. MTF s testing requirements exceed current AATB and FDA guidelines. A team of medical/technical specialists from the infectious disease and tissue banking fields evaluates all information, including test results. Storage and handling Frozen Frozen tissue has been preserved between -40 C and -90 C until time for shipping and is shipped on dry ice. It is recommended that the graft be stored on dry ice, or in a -40 C to -90 C freezer until the time of surgery. Refer to the tissue information sheet that accompanies the tissue for more detailed information on storage and handling. Freeze-dried It is recommended that freeze-dried tissue be reconstituted in saline prior to use. Refer to the tissue information sheet that accompanies the tissue for more detailed information on storage and handling. The is designed by and available through. was founded in 1991 and is dedicated to designing and developing new spinal instruments and implant systems. To order, call Synthes Customer Service at Fax: * Tested using FDA-required test kits. ** A preliminary study of the use of high-dose gamma irradiation for the inactivation of HIV demonstrates that the commonly applied dose of Mrads does not inactivate the virus. Inactivation would require a higher dose on the order of Mrads. However, doses over 2.5 Mrads have been shown to have a deleterious effect on the structural integrity of the allograft tissue. This information has been prepared by the Musculoskeletal Transplant Foundation based on the most current data and studies available. Full documentation is available on request. To receive a package of documentation materials, please contact MTF at
7 Available through 1302 Wrights Lane East West Chester, PA Telephone: (610) Processed by Musculoskeletal Transplant Foundation 125 May Street Edison, NJ Telephone: (732) Fax: (732) Synthes, Inc. or its affiliates. All rights reserved. Synthes is a trademark of Synthes, Inc. or its affiliates. MTF is a registered trademark of Musculoskeletal Transplant Foundation. Printed in U.S.A. 7/08 J3533-G
Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion.
 Processed by MTF, designed and available through. Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion. Luminary T-PLIF Spacer The Luminary T-PLIF Spacer has
Processed by MTF, designed and available through. Luminary T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion. Luminary T-PLIF Spacer The Luminary T-PLIF Spacer has
Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach.
 Processed by MTF, designed and available through Synthes Spine Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach. Spinal Correction FRA Spacers Two varieties of
Processed by MTF, designed and available through Synthes Spine Spinal Correction FRA Spacers. Angled and parallel spacers designed for a lateral approach. Spinal Correction FRA Spacers Two varieties of
Advanced ACF Spacer. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion.
 Processed by MTF, designed and available through Synthes Spine Advanced. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion. Advanced The Advanced has been engineered
Processed by MTF, designed and available through Synthes Spine Advanced. An allograft spacer with demineralized surfaces for anterior cervical interbody fusion. Advanced The Advanced has been engineered
Oracle Spacer. A PEEK spacer for lateral lumbar interbody fusion.
 Oracle Spacer. A PEEK spacer for lateral lumbar interbody fusion. Anatomic shape Self-distracting nose Large central canal Instruments and implants approved by the AO Foundation Oracle Spacer The Oracle
Oracle Spacer. A PEEK spacer for lateral lumbar interbody fusion. Anatomic shape Self-distracting nose Large central canal Instruments and implants approved by the AO Foundation Oracle Spacer The Oracle
Trephine System. Principle-based anterior lumbar interbody fusion.
 Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
Spinal Correction FRA Spacer System. For use with the Small Stature FRA Spacer and the FRA Spacer.
 Spinal Correction FRA Spacer System. For use with the Small Stature FRA Spacer and the FRA Spacer. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
Spinal Correction FRA Spacer System. For use with the Small Stature FRA Spacer and the FRA Spacer. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options
 THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
Cervical Solutions. Trinnect. Hydrated Anterior Cervical Spacer System. Surgical Technique Guide
 Cervical Solutions Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide 2 Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide Trinnect Cervical Allograft Spacers
Cervical Solutions Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide 2 Trinnect Hydrated Anterior Cervical Spacer System Surgical Technique Guide Trinnect Cervical Allograft Spacers
Technique Guide. T-PAL. Transforaminal posterior atraumatic lumbar spacer system.
 Technique Guide T-PAL. Transforaminal posterior atraumatic lumbar spacer system. Table of Contents Introduction T-PAL 2 AO Principles 4 Indications and Contraindications 5 Surgical Technique Preparation
Technique Guide T-PAL. Transforaminal posterior atraumatic lumbar spacer system. Table of Contents Introduction T-PAL 2 AO Principles 4 Indications and Contraindications 5 Surgical Technique Preparation
CONFORM SHEET. Demineralized cancellous bone
 CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products.
 End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
Luminary ALIF. Disc preparation and implant insertion instruments.
 Luminary ALIF. Disc preparation and implant insertion instruments. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Luminary ALIF 2 AO Principles 4
Luminary ALIF. Disc preparation and implant insertion instruments. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Luminary ALIF 2 AO Principles 4
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
Basic Trocar System. Basic transbuccal instrumentation system for simplified intraoral plate and screw application.
 Basic Trocar System. Basic transbuccal instrumentation system for simplified intraoral plate and screw application. Lightweight, ergonomic design Allows placement of three screw sizes Size-specific, colorcoded
Basic Trocar System. Basic transbuccal instrumentation system for simplified intraoral plate and screw application. Lightweight, ergonomic design Allows placement of three screw sizes Size-specific, colorcoded
Thoracolumbar Anterior Compression (TAC) System. Allows distraction, compression, and lateral fixation of the lower thoracic and lumbar spine.
 Thoracolumbar Anterior Compression (TAC) System. Allows distraction, compression, and lateral fixation of the lower thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO
Thoracolumbar Anterior Compression (TAC) System. Allows distraction, compression, and lateral fixation of the lower thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the Synthes locking compression plate (LCP) system.
 2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the locking compression plate (LCP) system. Anatomically contoured Multiple screw options for fixedangle support Coaxial holes minimize screw head
2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the locking compression plate (LCP) system. Anatomically contoured Multiple screw options for fixedangle support Coaxial holes minimize screw head
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
Talos -C (HA) and the PEEK-OPTIMA HA Enhanced Technology
 v Talos -C (HA) and the HA Enhanced Technology The innovative Talos -C (HA) IBF Implant made with polymer is fully integrated with the well known material Hydroxyapatite (HA). The Talos -C (HA) IBF Implant
v Talos -C (HA) and the HA Enhanced Technology The innovative Talos -C (HA) IBF Implant made with polymer is fully integrated with the well known material Hydroxyapatite (HA). The Talos -C (HA) IBF Implant
Alamo Tissue Service
 Providing Life Enhancing Donor Tissue San Antonio, TX 78249 Toll free 800.226 9091 or 210.738.2663 www.alamotissueservice.com A) T) S) A Tissue Distribution Network Life Enhancing Donor Tissue To Our
Providing Life Enhancing Donor Tissue San Antonio, TX 78249 Toll free 800.226 9091 or 210.738.2663 www.alamotissueservice.com A) T) S) A Tissue Distribution Network Life Enhancing Donor Tissue To Our
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
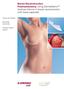 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Technique Guide. SynPOR HD Ocular Spheres. For augmentation or reconstruction.
 Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Interbody fusion cage for the transforaminal approach. Travios. Surgical Technique
 Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
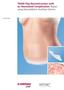 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Module: #15 Lumbar Spine Fusion. Author(s): Jenni Buckley, PhD. Date Created: March 27 th, Last Updated:
 Module: #15 Lumbar Spine Fusion Author(s): Jenni Buckley, PhD Date Created: March 27 th, 2011 Last Updated: Summary: Students will perform a single level lumbar spine fusion to treat lumbar spinal stenosis.
Module: #15 Lumbar Spine Fusion Author(s): Jenni Buckley, PhD Date Created: March 27 th, 2011 Last Updated: Summary: Students will perform a single level lumbar spine fusion to treat lumbar spinal stenosis.
Synex System TECHNIQUE GUIDE. An expandable vertebral body replacement device
 Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
Synex System TECHNIQUE GUIDE An expandable vertebral body replacement device Original Instruments and Implants of the Association for the Study of Internal Fixation AO ASIF Synex System Overview The Synex
Technique Guide. Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy.
 Technique Guide Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy. Table of Contents Introduction Cranial Clamps with Application Instrument
Technique Guide Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy. Table of Contents Introduction Cranial Clamps with Application Instrument
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use.
 Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
ProDisc-L Total Disc Replacement. IDE Clinical Study.
 ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
CSLP Quick Lock Screws. Preassembled expansionhead screw and locking screw for use with Cervical Spine Locking Plates (CSLP).
 CSLP Quick Lock Screws. Preassembled expansionhead screw and locking screw for use with Cervical Spine Locking Plates (CSLP). Technique Guide Instruments and implants approved by the AO Foundation Table
CSLP Quick Lock Screws. Preassembled expansionhead screw and locking screw for use with Cervical Spine Locking Plates (CSLP). Technique Guide Instruments and implants approved by the AO Foundation Table
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion
 O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion A new implant for an evolving procedure Unique wedge nose Adaptive longer sizing Large internal graft volume Introduction Stryker Spine enters a new
O.I.C. PEEK UniLIF Unilateral Lumbar Interbody Fusion A new implant for an evolving procedure Unique wedge nose Adaptive longer sizing Large internal graft volume Introduction Stryker Spine enters a new
Dentoalveolar Bone Fixation System. Bone fixation sets for the office.
 Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
AVS ARIA TM Product Overview
 AVS ARIA TM Product Overview Table of Contents Indication 3 Adaptive Sizing 4-5 Wedge Nose Design 6 Graft Volumes 7 Efficient Fixation 8 Lordotic Descriptions 9 Anatomical Descriptions 10 Visualization
AVS ARIA TM Product Overview Table of Contents Indication 3 Adaptive Sizing 4-5 Wedge Nose Design 6 Graft Volumes 7 Efficient Fixation 8 Lordotic Descriptions 9 Anatomical Descriptions 10 Visualization
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device.
 Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device. Effective Date: 2/23/2005 Version 1 ProKera is a corneal-epithelial device consisting of an ophthalmic conformer
Product Insert ProKera is approved by the US FDA (510K Approval) as a class II medical device. Effective Date: 2/23/2005 Version 1 ProKera is a corneal-epithelial device consisting of an ophthalmic conformer
ProDisc PAL (Patient Assistance Line). Assisting patients with obtaining insurance coverage for ProDisc.
 ProDisc PAL (Patient Assistance Line). Assisting patients with obtaining insurance coverage for ProDisc. Patient Assistance Line (PAL) 800-895-7764 Instruments and implants approved by the AO Foundation
ProDisc PAL (Patient Assistance Line). Assisting patients with obtaining insurance coverage for ProDisc. Patient Assistance Line (PAL) 800-895-7764 Instruments and implants approved by the AO Foundation
2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System.
 2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System. 12 anatomically shaped volar plates Multiple screw options for fixedangle support to articular surface Combi holes
2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System. 12 anatomically shaped volar plates Multiple screw options for fixedangle support to articular surface Combi holes
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Demineralized Bone Matrix (DBM) Allografts 22 DBM Chips 23 DBM Cancellous Block 23 DBM Strip 23 H-GENIN Putty 24 H-GENIN Crush-Mix 24
 Allograft Catalog 2013 1 Table of Contents General Allograft Information 4 Welcome Letter 5 Introduction to International Biologics 6 Licenses and Accreditations 6 Donor Screening and Testing 7 Allograft
Allograft Catalog 2013 1 Table of Contents General Allograft Information 4 Welcome Letter 5 Introduction to International Biologics 6 Licenses and Accreditations 6 Donor Screening and Testing 7 Allograft
Case Report. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
 Case Report RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
Case Report RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol. RapidSorb Rapid Resorbable Fixation System. Ridge augmentation in a one-step surgical protocol.
PARADIGM SPINE. Anterior Cervical Fusion Cage. Cervical Interbody Fusion
 PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
The Implant. (Klappen außen sind nur 192 mm breit!)
 SEMIAL product information The Implant SEMIAL implants are designed to restore interbody vertebral disc height and maintain solid bony fusion when used in conjunction with bone graft material and supportive
SEMIAL product information The Implant SEMIAL implants are designed to restore interbody vertebral disc height and maintain solid bony fusion when used in conjunction with bone graft material and supportive
3.5 mm LCP Tibial Plateau Sets. Configuration options.
 3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Simply Complete. VA-LCP Elbow Plating System.
 Simply Complete. VA-LCP Elbow Plating System. Building on success Founded upon the established principles of Synthes LCP technology and the first generation elbow plates, the Variable Angle LCP Elbow Plating
Simply Complete. VA-LCP Elbow Plating System. Building on success Founded upon the established principles of Synthes LCP technology and the first generation elbow plates, the Variable Angle LCP Elbow Plating
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
SURGICAL TECHNIQUE ROI-C TM ANTERIOR CERVICAL CAGE
 SURGICAL TECHNIQUE ROI-C TM ANTERIOR CERVICAL CAGE SURGICAL TECHNIQUE ROI-C TM Table of Contents page 1 - Disc location............................................................................................
SURGICAL TECHNIQUE ROI-C TM ANTERIOR CERVICAL CAGE SURGICAL TECHNIQUE ROI-C TM Table of Contents page 1 - Disc location............................................................................................
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
Posterior Lumbar Interbody Fusion System
 Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Px Posterior Lumbar Interbody Fusion System Px PEEK INTERBODY FUSION SYSTEM INDICATIONS FOR USE The Innovasis Px PEEK IBF System is an intervertebral body fusion device for use in patients with degenerative
Products and Services Catalogue
 Products and Services Catalogue Solid and Flexible Bio-Processed Allograft Implants Advancing Tissue Innovations Since 1972 Updated June 2011 A History of Providing Highest Quality and Innovation Mount
Products and Services Catalogue Solid and Flexible Bio-Processed Allograft Implants Advancing Tissue Innovations Since 1972 Updated June 2011 A History of Providing Highest Quality and Innovation Mount
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems
 Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
Surgical technique. SynCage-C short
 Surgical technique SynCage-C short Table of contents Implants 2 Indications/contra-indications 3 Surgical technique 4 Image intensifier control Warning This description is not sufficient for immediate
Surgical technique SynCage-C short Table of contents Implants 2 Indications/contra-indications 3 Surgical technique 4 Image intensifier control Warning This description is not sufficient for immediate
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps.
 Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Pediatric Femoral Shaft Frame. Using medium multi-pin clamps. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
A Fusion of Versatility, Performance and Ease.
 MATRIX Spine System. Degenerative and Deformity. System Guide A Fusion of Versatility, Performance and Ease. Instruments and implants approved by the AO Foundation The MATRIX Spine System is a universal
MATRIX Spine System. Degenerative and Deformity. System Guide A Fusion of Versatility, Performance and Ease. Instruments and implants approved by the AO Foundation The MATRIX Spine System is a universal
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
Technique Guide. SureLock Distal Targeting Device. C-arm guided targeting for trochanteric fixation nail.
 Technique Guide SureLock Distal Targeting Device. C-arm guided targeting for trochanteric fixation nail. Table of Contents Introduction SureLock Distal Targeting Device 2 Surgical Technique Preoperative
Technique Guide SureLock Distal Targeting Device. C-arm guided targeting for trochanteric fixation nail. Table of Contents Introduction SureLock Distal Targeting Device 2 Surgical Technique Preoperative
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
INNOVATE INVOLVE INVENT. Innovasis Bone Products
 INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF)
 Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Royal Oak IBFD System Surgical Technique Posterior Lumbar Interbody Fusion (PLIF) Preoperative Planning Preoperative planning is necessary for the correct selection of lumbar interbody fusion devices.
Occipital Cervical Fusion System. Implants and instruments designed to optimize fixation to the occiput.
 Occipital Cervical Fusion System. Implants and instruments designed to optimize fixation to the occiput. Technique Guide and implants approved by the AO Foundation Table of Contents Introduction Occipital
Occipital Cervical Fusion System. Implants and instruments designed to optimize fixation to the occiput. Technique Guide and implants approved by the AO Foundation Table of Contents Introduction Occipital
Solitaire Anterior Spinal System
 Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Surgical Technique Solitaire Anterior Spinal System Independent Stabilization for the Anterior Column Available in Titanium and Contents Introduction... Page 1 Design Features... Page 2 Instruments...
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius.
 Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
Low-Profile Wrist Fixator. For stabilization of fractures of the distal radius. Technique Guide Part of the External Fixation System Low-Profile Wrist Fixator Indications Intended for stabilization of
DEMINERALIZED BONE MATRIX
 DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
product overview Implant heights range from 8mm-20mm in 2mm increments, with two lordocic angle options of 6 and 12.
 ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
ETHOS A-Spacer PEEK System Surgical Technique Guide Synchronizing Medical Innovation with Global Markets product overview The SyncMedical Ethos PEEK IBF System is an intervertebral body fusion device for
SURGICAL TECHNIQUE ROI-T TM TLIF INTERSOMATIC IMPLANT TRANS-FORAMINAL APPROACH
 TLIF INTERSOMATIC IMPLANT TRANS-FORAMINAL APPROACH Table of Contents page Step 1 - Articular resection.................................................................................. 3 Step 2 - Pedicle
TLIF INTERSOMATIC IMPLANT TRANS-FORAMINAL APPROACH Table of Contents page Step 1 - Articular resection.................................................................................. 3 Step 2 - Pedicle
Technique Guide. 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide. TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee.
 Technique Guide TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee. Table of Contents Introduction TomoFix Osteotomy System 2 AO Principles 4 Indications
Technique Guide TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee. Table of Contents Introduction TomoFix Osteotomy System 2 AO Principles 4 Indications
PILLAR AL. Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) PEEK Spacer System OPERATIVE TECHNIQUE
 PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
PILLAR AL PEEK Spacer System Anterior Lumbar Interbody Fusion (ALIF) and Partial Vertebral Body Replacement (pvbr) OPERATIVE TECHNIQUE Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE TECHNIQUE 3 OPERATIVE
TABLE OF CONTENTS. Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview. Implants 3. Instruments 5. Surgical Technique 10
 Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical Technique TABLE OF CONTENTS Vault C Anterior Cervical Discectomy 2 and Fusion (ACDF) System Overview Indications 2 Implants 3 Instruments 5 Surgical Technique 10 1. Preoperative planning 10 2.
Surgical technique. synex. The vertebral body replacement with ratchet mechanism.
 Surgical technique synex. The vertebral body replacement with ratchet mechanism. Table of contents Indications and contraindications 2 Implants 3 Surgical technique 4 Cleaning of instruments 10 Optional
Surgical technique synex. The vertebral body replacement with ratchet mechanism. Table of contents Indications and contraindications 2 Implants 3 Surgical technique 4 Cleaning of instruments 10 Optional
Commander Cervical Cage - SURGICAL TECHNIQUE
 Commander Cervical Cage - SURGICAL TECHNIQUE D e s i g n e d to c l o s e l y f i t yo u! Commander Cervical Cage - SURGICAL TECHNIQUE Indications Commander cervical cages are designed primarly for restoring
Commander Cervical Cage - SURGICAL TECHNIQUE D e s i g n e d to c l o s e l y f i t yo u! Commander Cervical Cage - SURGICAL TECHNIQUE Indications Commander cervical cages are designed primarly for restoring
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Zero-P Instruments and Implants. Zero-profile anterior cervical interbody fusion (ACIF) device.
 Zero-P Instruments and Implants. Zero-profile anterior cervical interbody fusion (ACIF) device. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Zero-P
Zero-P Instruments and Implants. Zero-profile anterior cervical interbody fusion (ACIF) device. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Zero-P
Osteocel. An introduction to
 An introduction to Osteocel This booklet provides general information on Osteocel cellular allografts as a bone graft substitute in your spinal fusion surgery. It is not meant to replace any personal conversations
An introduction to Osteocel This booklet provides general information on Osteocel cellular allografts as a bone graft substitute in your spinal fusion surgery. It is not meant to replace any personal conversations
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System.
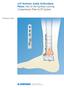 LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
15. Procuring, processing and transporting gametes and
 15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
ALLOPURE. Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE
 ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system.
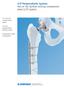 LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
2.4 mm Variable Angle LCP Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis.
 2.4 mm Variable Angle LCP Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis. Technique Guide Instruments and implants approved by the AO Foundation Table
2.4 mm Variable Angle LCP Intercarpal Fusion System. Variable angle locking technology for mediocarpal partial arthrodesis. Technique Guide Instruments and implants approved by the AO Foundation Table
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
Quality of Life. Quality of Motion.
 Quality of Life. Quality of Motion. Lateral Bend Vertical Translation Flexion Extension Lateral Translation Axial Rotation Anterior Posterior Translation Motion in all Directions Kinematics is the study
Quality of Life. Quality of Motion. Lateral Bend Vertical Translation Flexion Extension Lateral Translation Axial Rotation Anterior Posterior Translation Motion in all Directions Kinematics is the study
CROSS -FUSE P E E K V B R / I B F SYST E M
 S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
S U R G I C A L T E C H N I Q U E CROSS -FUSE P E E K V B R / I B F SYST E M S U R G I C A L S Y S T E M O V E R V I E W 2 CROSS-FUSE P E E K V B R / I B F S Y S T E M S U R G I C A L T E C H N I Q U E
SynCage. Surgical Technique. This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
 SynCage Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier control Warning This description alone
SynCage Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Image intensifier control Warning This description alone
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Large External Fixator Modular Knee Bridge. Using multi-pin clamps.
 Large External Fixator Modular Knee Bridge. Using multi-pin clamps. Technique Guide Part of the Large External Fixation System Large External Fixator Modular Knee Bridge Technique Overview Insert Schanz
Large External Fixator Modular Knee Bridge. Using multi-pin clamps. Technique Guide Part of the Large External Fixation System Large External Fixator Modular Knee Bridge Technique Overview Insert Schanz
POSTERIOR SPINE TRUSS SYSTEM
 POSTERIOR SPINE TRUSS SYSTEM ORDERING & SHIPPING ALL FIELDS ARE REQUIRED ORDER FORM PLEASE PRINT LEGIBLY RECEIVING YOUR PSTS TRAYS & IMPLANTS All implant bins and trays have a warehouse number on them
POSTERIOR SPINE TRUSS SYSTEM ORDERING & SHIPPING ALL FIELDS ARE REQUIRED ORDER FORM PLEASE PRINT LEGIBLY RECEIVING YOUR PSTS TRAYS & IMPLANTS All implant bins and trays have a warehouse number on them
Allograft Tissue Catalog
 Allograft Tissue Catalog ConMed provides safe, high-quality tissue from the Musculoskeletal Transplant Foundation (MTF) to meet the needs of physicians and their patients. OSTEOCHONDRAL GRAFTS BIOLOGIC
Allograft Tissue Catalog ConMed provides safe, high-quality tissue from the Musculoskeletal Transplant Foundation (MTF) to meet the needs of physicians and their patients. OSTEOCHONDRAL GRAFTS BIOLOGIC
Technique Guide. 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology.
 Technique Guide 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Table of Contents Introduction 2.4 mm Variable Angle LCP
Technique Guide 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Table of Contents Introduction 2.4 mm Variable Angle LCP
Products and Services Catalogue
 Products and Services Catalogue 2009-2010 Solid and Flexible Bio-Processed Allograft Implants Advancing Tissue Innovations Since 1972 A History of Providing Highest Quality and Innovation Mount Sinai Allograft
Products and Services Catalogue 2009-2010 Solid and Flexible Bio-Processed Allograft Implants Advancing Tissue Innovations Since 1972 A History of Providing Highest Quality and Innovation Mount Sinai Allograft
Surgical Technique INTERSOMATIC CERVICAL CAGE
 R INTERSOMATIC CERVICAL CAGE NEOCIF IMPLANTS NEOCIF is an implant designed to make anterior cervical interbody fusion (ACIF) easier and to remove the need for structural autologous graft. The cage is made
R INTERSOMATIC CERVICAL CAGE NEOCIF IMPLANTS NEOCIF is an implant designed to make anterior cervical interbody fusion (ACIF) easier and to remove the need for structural autologous graft. The cage is made
