PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH
|
|
|
- Ross Barrett
- 5 years ago
- Views:
Transcription
1 PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH
2 PRO-STIM Injectable Inductive Graft TECHNICAL MONOGRAPH
3 Headline Contents Headline Appendix Clinical Benefits Self-Forming Porous Scaffold Osteoinductive Potential Healing with PRO-STIM Injectable Graft Compared to Autograft in a Canine Critical Size Defect Model Conclusion References Proper surgical procedures and techniques are the responsibility of the medical professional. The following guidelines are furnished for information purposes only as techniques used by the design surgeons. Each surgeon must evaluate the appropriateness of the procedures based on his or her personal medical training and experience. Prior to use of the system, the surgeon should refer to the product package insert for complete warnings, precautions, indications, contraindications and adverse effects. Package inserts are also available by contacting Wright Medical Technology, Inc. Please contact your local Wright representative for product availability. PRO-STIM Injectable Inductive Bone Graft Substitute 3
4 PRO-STIM Injectable Inductive Bone Graft Substitute Hardening. Resorbable. Osteoinductive. Regenerative.» Accelerated Healing* - Faster healing than autograft at 13 weeks in canine model PRO-STIM Injectable Inductive Graft is a resorbable, hardening, osteoinductive bone graft substitute. Built on the PRO-DENSE material platform as a combination of calcium sulfate and calcium phosphate, PRO-STIM graft adds demineralized bone matrix (DBM) for osteoinductive factors to speed the healing and remodeling process. Autograft remains the gold standard for many surgeons because it includes all three desired features of a bone graft: scaffolding for osteoconduction, proteins for osteoinduction, and stem cells for osteogenesis. Additionally, it is autologous thus eliminating concerns of an immune or foreign body response. 1 Donor morbidity and multiple complications at the site of autograft harvest, however, remain a challenge. 2,3 DBM While many materials have been shown CaSO to perform equivalent to autograft, 4 two products to date- PRO-DENSE graft and now PRO-STIM graft- have CaPO demonstrated superiority to autograft 4 in pre-clinical testing. 4,5 In previous testing in a metaphyseal canine defect model, PRO-DENSE graft demonstrated an ability to regenerate denser and stronger new bone faster than autograft at 13 weeks. Additionally, the new bone regenerate maintained increased density for a period before remodeling (by 26 weeks). As summarized in Section III PRO-STIM graft demonstrated accelerated healing and earlier initiation of remodeling in treated sites compared to autograft in the same canine model. *Canine proximal humerus model: Accelerated healing compared to autograft Compared to autograft, PRO-STIM Graft showed accelerated new bone formation at 13 and 26 weeks. This was evident by the slightly higher average stiffness, the greater amount of average new bone formation, and statistically significantly greater compressive strength shown for PRO-STIM Graft treated defects. By comparing the 26-week clinical and contact radiographs and gross cross-sectional images and histological images, there appeared to be little to no apparent difference between defects f illed with either PRO-STIM Graft or autograft. Comparison of percentage of new bone, compressive strength, and modulus of elasticity showed no statistically signif icant differences between the materials at 26 weeks. It is unknown how results from the canine model compare with clinical results in humans. Data on f ile at Wright. 4 PRO-STIM Injectable Inductive Bone Graft Substitute
5 Self-Forming Porous Scaffold Tri-phasic Resorption Reveals an Interconnected, Porous Scaffold 5 The physical progression of the resorption process is illustrated in FIGURE 1, which shows SEM images of cross sections through embedded PRO-STIM graft after zero, two, four, eight, and twelve days of accelerated in vitro dissolution which has been estimated to occur about six times faster than in vivo. The darker region on the outer edge of the pellet represents the area in which calcium sulfate has largely dissolved, leaving a porous scaffold of brushite (bright white) and DBM particles (very dark spots). Time 0 Days Time 2 Days Time 4 Days Time 8 Days Time 12 Days (approx. 72 days in vivo) FIGURE 1 In vitro accelerated dissolution at 37 C in water (Image analysis via SEM) (Approximately six times faster than in vivo canine model). PRO-STIM Injectable Inductive Bone Graft Substitute 5
6 Time 0 Days Time 2 Days Time 4 Days Time 7 Days (approx. 42 days in vivo) FIGURE 2 In vitro accelerated dissolution at 37 C in water (Image analysis via SEM) (Approximately six times faster than in vivo canine model). In FIGURE 2, the SEM images of the pellets show that much of the size of the PRO-STIM pellet is maintained at early timepoints. Even at 4 days, the calcium sulfate has started to dissolve revealing the porous calcium phosphate structure containing DBM particles. By 7 days, a large portion of the remaining pellet is the porous structure of calcium phosphate and DBM (large white particles). By contrast, pure calcium sulfate is completely resorbed by 10 days in this same experiment. 6 PRO-STIM Injectable Inductive Bone Graft Substitute
7 Osteoinductive Potential Osteoinductivity of PRO-STIM Injectable Graft in an Athymic Nude Rat Model 5 Objective The objective of this study was to evaluate the osteoinductivity of PRO-STIM graft in an athymic rat muscle pouch model. Materials and Methods Incisions were made through the latissimus dorsi and gluteus superficialis muscles on each side of the midline of an athymic nude rat model. Two pouches were created on each side using blunt dissection. PRO-STIM graft was mixed according to instructions. After aggressively mixing for 30 seconds, samples were manually rolled into small, elliptical balls. The graft specimens were gently implanted into the muscle pouches after they had hardened. Each rat received four implants. After implantation, the muscle incisions were closed. The implants were harvested at 28 days. Specimens were decalcified for ten days, embedded in paraffin, and stained with hematoxylin and eosin (H&E). Results The PRO-STIM graft prepared with human DBM induced measurable new bone growth as shown in FIGURE 3. Conclusion FIGURE 3 Histology image of PRO-STIM graft within athymic nude rat muscle pouch (H&E original mag 20X). PRO-STIM graft was shown to be osteoinductive when implanted in an athymic nude rat muscle pouch model. PRO-STIM Injectable Inductive Bone Graft Substitute 7
8 Healing with PRO-STIM Injectable Graft Compared to Autograft in a Canine Critical Size Defect Model 5 Objective The primary objective of the studies was to evaluate the in vivo performance, including at early timepoints, of PRO-STIM graft in a canine proximal humerus critical size metaphyseal defect model. Defects were created, filled, harvested, and evaluated histologically at 2, 3, 4, 6, 8, 10, 13, and 26 weeks. A second objective was to compare healing in the defects treated with PRO-STIM graft and autograft by compiling data from studies previously conducted in the same model, at the same institute, and by the same research team. Compressive strength of healed defects was also assessed at 13 and 26 weeks. Materials and Methods Cylindrical defects (13mm diameter x 50mm) were created bilaterally in the proximal humerus of adult male, hound-type dogs. Immediately prior to implantation, PRO-STIM graft was prepared according to instructions provided with each kit. One defect per dog was filled with PRO-STIM graft prepared with canine demineralized bone matrix (DBM) and the other defect was filled with autograft. Dogs were sacrificed at 2, 3, 4, 8 and 10 weeks (n=1 per timepoint); 6 weeks (n=3); and 13 and 26 weeks (n=5 per timepoint). Following sacrifice, the proximal humerus was harvested and the length of bone with the implant site was cut into sections. Contact radiographs were taken of sections of the defect. Specimens were processed and embedded in PMMA and slides were stained with basic fuschin and toluidine blue. The percentage of new bone was measured from histology images using a point counting method. Previous data from comparable defects treated with autograft were compared. From specimens harvested at 13 and 26 weeks, samples 8mm diameter x 20mm long were cored from the defect regions and loaded under compression until failure. Results Resorption of calcium sulfate, evident as darkening of the implant, starts at the outer rim of the implant and occurs within two weeks (FIGURE 4). Similar to the progression seen in vitro, the calcium phosphate and DBM initially remain within the resorbing area until surrounded and/ or replaced by bone. Resorption and replacement with mature bone progresses quickly and the bulk of material, particularly the calcium sulfate and calcium phosphate, is gone by 8 weeks (FIGURE 4). In the 8 PRO-STIM Injectable Inductive Bone Graft Substitute
9 autograft implant sites, although a notable amount of new bone is present in the defect sites as early as 2 weeks, the new bone is markedly immature as indicated by thin, small spicules of young bone and remains largely immature past 8 weeks. A. PRO-STIM GRAFT 2 weeks 3 weeks 4 weeks 6 weeks 8 weeks B. AUTOGRAFT 10 weeks 13 weeks 26 weeks 2 weeks 3 weeks 4 weeks 6 weeks 8 weeks 10 weeks 13 weeks 26 weeks FIGURE 4 (a) In contact radiographs of a cross-section of PRO-STIM implant sites, resorption is evident by 2 weeks. By 6 weeks, the bulk of the material is gone and significant bone growth is evident. By 26 weeks, the entire defect site is filled with mature bone resembling adjacent native bone. (b) While new bone growth is evident as early as 2 weeks in the autograft implant sites, the thin bone appears very immature, without the density representative of more mature bone. This immature bone largely persists beyond 8 weeks. PRO-STIM Injectable Inductive Bone Graft Substitute 9
10 Native Bone Immature Bone Resorption Front PRO-STIM Implant FIGURE 5 At 3 weeks following implantation of PRO-STIM graft, several regions of healing are distinct. Within the original defect site and immediately adjacent to native bone, there are immature, thin spicules of new bone. Immediately within the new bone is the resorption front region in which much of the calcium sulfate and calcium phosphate material has resorbed, revealing DBM. The area within the resorption region contains PRO-STIM implant material that is primarily intact. Histological images of PRO-STIM implant sites at each timepoint show distinct regions of implant resorption and healing (FIGURE 5) that progress inward over time. Histology images of PRO-STIM graft and autograft implant sites over time (FIGURE 6) show progression of material resorption and new bone growth consistent with views of the contact radiographs. As early as 2 weeks, new bone growth visibly maintains tight contact with the resorption front of the implant material. Consistent with contact radiographs, by 8 weeks, the bolus of implant is gone and the defect site has filled with new bone. This rate of resorption and healing is consistent with the presence of remodeled mature cancellous bone in the defect site that is indistinguishable from surrounding bone at 26 weeks (FIGURE 6). 10 PRO-STIM Injectable Inductive Bone Graft Substitute
11 In the autograft sites, new bone is evident at 2 weeks although the bone is thin and spicule-like representing very immature bone which has little strength due to few, disorganized collagen fibers. This immature bone structure is maintained until 13 weeks at which time the bone has remodeled to more mature bone resembling surrounding bone. A. PRO-STIM GRAFT 2 weeks 3 weeks 4 weeks 6 weeks 8 weeks 10 weeks 13 weeks 26 weeks B. AUTOGRAFT 2 weeks 3 weeks 4 weeks 6 weeks 8 weeks 10 weeks 13 weeks 26 weeks FIGURE 6 (a) Consistent with contact radiographs, at 2 weeks, resorption of PRO-STIM graft with some evidence of new bone growth at the periphery of the implant is observed. Continued material resorption and notable new bone growth is apparent at 3 weeks and continues to progress at a fast rate with the bolus of the implant predominantly gone and replaced with mature bone at 8 weeks. [NOTE: The hole in 6-wk PRO-STIM histology image is due to wash-out during post-explantation processing.] (b) In the autograft sites, new bone observed at 2 weeks is very immature, indicated by thin, small spicules of young bone; remodeling into more mature bone does not occur until after 10 weeks. (Basic fuschin and toluidine blue, 10X) PRO-STIM Injectable Inductive Bone Graft Substitute 11
12 Area Fraction of New Bone PRO-STIM Graft Autograft Percent Normal Bone Time (Weeks) FIGURE 7 A plot of percentage new bone over time in defect sites filled with PRO-STIM graft vs. autograft indicates accelerated healing, increased volume in new bone formed, and remoldeling to normal at 13 weeks with PRO-STIM implants compared to autograft. Compressive Strength (MPa) New Bone Regenerate Autograft PRO-STIM Autograft PRO-STIM Normal Bone 13 Weeks 26 Weeks FIGURE 8 Compressive strength of the new bone regenerate at 13 and 26 weeks. *Friedman Test, p=0.046 Graphical presentation of new bone growth over time (FIGURE 7) demonstrates acceleration of healing and earlier initiation of remodeling in PRO-STIM implant sites compared to autograft sites. At their respective peaks of healing, PRO-STIM graft was associated with greater bone volume than autograft. Mechanical test results indicated compressive strength comparable to that of normal bone in both PRO-STIM graft and autograft-treated sites at both 13 and 26 weeks (FIGURE 8). At 13 weeks, compressive strength of the PRO-STIM specimens was comparable to normal bone and was significantly greater than that of autograft specimens (p=0.046) (FIGURE 8). The lower strength of the autograft sites may be due to decreased maturity of the new bone. By 26 weeks, the compressive strength of both the PRO-STIM graft and autograft-treated sites was comparable to new bone. 12 PRO-STIM Injectable Inductive Bone Graft Substitute
13 Conclusion PRO-STIM graft has demonstrated accelerated healing, increased volume in new bone formed, and remodeling to normal bone at 13 weeks vs. autograft in the canine critical sized defect model. PRO-DENSE and PRO-STIM grafts have been shown to be superior to autograft in the canine proximal humerus model at the 13 and 26 week time points. 5,6 PRO-STIM graft demonstrates a resorption pattern that reveals the osteoinductive DBM over a defined period of time. The osteoinductivity was clearly demonstrated in the athymic nude rat muscle pouch model in which the composite graft was implanted and new bone formation was evident. PRO-STIM graft has demonstrated accelerated healing, increased volume in new bone formed, and remodeling to normal at 13 weeks vs. autograft in the canine critical sized defect model. PRO-STIM Injectable Inductive Bone Graft Substitute 13
14 References 1. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury Nov;36 Suppl 3:S Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3): Fowler BL, Dall BE, Rowe DE. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop (Belle Mead NJ) Dec;24(12): Turner TM, Urban RM, Hall DJ, Cheema N, Lim TH. Restoration of large bone defects using a hard-setting, injectable putty containing demineralized bone particles compared to cancellous autograft bone. Orthopedics May;26(5 Suppl):s PRO-STIM Graft Data on file at Wright Medical Technology. 6. Urban RM, Turner TM, Hall DJ, Inoue N, Gitelis S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res Jun;459: PRO-STIM Injectable Inductive Bone Graft Substitute
15 Sizing and Ordering Information Product Name PRO-STIM EXTREMITY 4CC PRO-STIM 10CC PRO-STIM 20CC PRO-STIM CORE DECOMPRESSION KIT* Part Number 86SR SR SR SR-CK15 *Contains all necessary instruments for a standard core decompression technique. PRO-STIM Injectable Inductive Bone Graft Substitute 15
16 16 PRO-STIM Injectable Inductive Bone Graft Substitute A_30-June-2014
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
PRO-DENSE. Injectable Regenerative Graft T ECHNIC A L MONO GR A PH
 PRO-DENSE Injectable Regenerative Graft T ECHNIC A L MONO GR A PH Headline Contents Headline Appendix 4 4 4 5 6 8 8 10 13 13 Abstract Background In Situ Reaction Mechanisms and Kinetics of PRO-DENSE Graft
PRO-DENSE Injectable Regenerative Graft T ECHNIC A L MONO GR A PH Headline Contents Headline Appendix 4 4 4 5 6 8 8 10 13 13 Abstract Background In Situ Reaction Mechanisms and Kinetics of PRO-DENSE Graft
GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS
 GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS Based Upon the Published Source: Martin GJ, Jr., Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix
GRAFTON DEMINERALIZED BONE: FIBER TECHNOLOGY AND PERFORMANCE IMPLICATIONS Based Upon the Published Source: Martin GJ, Jr., Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix
In Vivo Evaluation of BioSphere Bioactive Bone Graft Putty: Improved Bone Formation
 In Vivo Evaluation of BioSphere Bioactive Bone Graft : Improved Bone Formation ABSTRACT BioSphere is a novel bone graft product that was developed using spherical particles of bioactive glass with a narrow,
In Vivo Evaluation of BioSphere Bioactive Bone Graft : Improved Bone Formation ABSTRACT BioSphere is a novel bone graft product that was developed using spherical particles of bioactive glass with a narrow,
Increased Bone Formation Using a Calcium Sulfate and Calcium Phosphate Composite Graft
 CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 000, pp. 000 000 2007 Lippincott Williams & Wilkins Increased Bone Formation Using a Calcium Sulfate and Calcium Phosphate Composite Graft Robert M. Urban;
CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 000, pp. 000 000 2007 Lippincott Williams & Wilkins Increased Bone Formation Using a Calcium Sulfate and Calcium Phosphate Composite Graft Robert M. Urban;
BIOACTIVE SYNTHETIC GRAFT
 B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D.
 C E L L P L E X TCP synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D. TECHNICAL MONOGRAPH CELLPLEX TCP SYNTHETIC CANCELLOUS BONE CELLPLEX TCP synthetic CANCELLOUS BONE introduction
C E L L P L E X TCP synthetic CANCELLOUS BONE technical monograph Presented by Barbara Blum, Ph.D. TECHNICAL MONOGRAPH CELLPLEX TCP SYNTHETIC CANCELLOUS BONE CELLPLEX TCP synthetic CANCELLOUS BONE introduction
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Calcium Phosphate Cement
 Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Calcium Phosphate Cement Fast-Setting Bone Graft and AutoGraft Extender. * Ossilix is a high performance next generation calcium phosphate cement indicated for filling bony defects in cancellous bone.
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
B U I L D S T R O N G B O N E F A S T
 B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Radiology Case Reports. Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia. Penelope J. Galbraith, M.D., and Felix S. Chew, M.D.
 Radiology Case Reports Volume 2, Issue 4, 2007 Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia Penelope J. Galbraith, M.D., and Felix S. Chew, M.D. A 33-year-old man presented for follow
Radiology Case Reports Volume 2, Issue 4, 2007 Bone Graft Extruded From an Intramedullary Nail Tract in the Tibia Penelope J. Galbraith, M.D., and Felix S. Chew, M.D. A 33-year-old man presented for follow
SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary
 SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary Summary of In Vivo Use Of Bioresorbable Xenograft Bone Graft Materials In The Treatment Of One-Walled Intrabony Defects In A Canine Model
SalvinOss Xenograft Bone Graft Material In Vivo Testing Summary Summary of In Vivo Use Of Bioresorbable Xenograft Bone Graft Materials In The Treatment Of One-Walled Intrabony Defects In A Canine Model
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
Bone Grafting and Bone Graft Substitutes. Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010
 Bone Grafting and Bone Graft Substitutes Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010 Bone Graft Function Structural support of articular fracture Tibial plateau
Bone Grafting and Bone Graft Substitutes Original Author: James Krieg, MD Revision Author: David Hak, MD Last Revision May 2010 Bone Graft Function Structural support of articular fracture Tibial plateau
Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs
 Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Developments in bone grafting in veterinary orthopaedics part one
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Surgical Technique Guide. Impact & Inject Formulations
 Surgical Technique Guide Impact & Inject Formulations System Overview OsteoVation QWIK is a bone void filler consisting of a proprietary composite of calcium phosphate and calcium sulfate granules. The
Surgical Technique Guide Impact & Inject Formulations System Overview OsteoVation QWIK is a bone void filler consisting of a proprietary composite of calcium phosphate and calcium sulfate granules. The
Bone Void Filler. Callos. The Next Generation in Calcium Phosphate Cement A COLSON ASSOCIATE
 Callos Bone Void Filler The Next Generation in Calcium Phosphate Cement A COLSON ASSOCIATE Callos Calcium Phosphate Cement Callos is a high performance next generation calcium phosphate cement indicated
Callos Bone Void Filler The Next Generation in Calcium Phosphate Cement A COLSON ASSOCIATE Callos Calcium Phosphate Cement Callos is a high performance next generation calcium phosphate cement indicated
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
Indications. Neuro Surgery. Plastic Surgery. ENT Surgery. Oral Maxillofacial Surgery
 Indications Neuro Surgery Cranioplasty/Craniectomy Bur Hole Filling Cranial Cuts Onlay & Inlay grafting Plastic Surgery Cranial Reconstruction Orbital Reconstruction Onlay Grafting ENT Surgery Frontal
Indications Neuro Surgery Cranioplasty/Craniectomy Bur Hole Filling Cranial Cuts Onlay & Inlay grafting Plastic Surgery Cranial Reconstruction Orbital Reconstruction Onlay Grafting ENT Surgery Frontal
BEGO BIOMATERIALS When the result counts
 BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
OssiMend Bone Graft Matrix
 OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
botiss dental bone & tissue regeneration biomaterials collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
Void Fillers: White Paste or Bone
 J. TRACY WATSON M.D. PROFESSOR OF ORTHOPAEDIC SURGERY CHIEF ORTHOPAEDIC TRAUMA SERVICE ST. LOUIS UNIVERSITY SCHOOL OF MEDICINE Void Fillers: White Paste or Bone Void Fillers: White Paste or Bone INJECTABLE
J. TRACY WATSON M.D. PROFESSOR OF ORTHOPAEDIC SURGERY CHIEF ORTHOPAEDIC TRAUMA SERVICE ST. LOUIS UNIVERSITY SCHOOL OF MEDICINE Void Fillers: White Paste or Bone Void Fillers: White Paste or Bone INJECTABLE
ORS 2015 Annual Meeting (Orthopedic Research Society)
 ORS 2015 Annual Meeting (Orthopedic Research Society) Poster No: 1045 CERAMENT Bone Void Filler Impregnated with Gentamicin Increases Bone Formation and Decreases the Rate of Detectable Infection after
ORS 2015 Annual Meeting (Orthopedic Research Society) Poster No: 1045 CERAMENT Bone Void Filler Impregnated with Gentamicin Increases Bone Formation and Decreases the Rate of Detectable Infection after
NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE
 NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE THE PROBLEMS Fixation failure Malunion F 83 yrs 2 Months 6 Months F 81 yrs 3 Months FIXATION AUGMENTATION TECHNIQUES (FATs) Surgical procedures aimed at increasing
NEW FIXATION STRATEGIES FOR OSTEOPOROTIC BONE THE PROBLEMS Fixation failure Malunion F 83 yrs 2 Months 6 Months F 81 yrs 3 Months FIXATION AUGMENTATION TECHNIQUES (FATs) Surgical procedures aimed at increasing
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Trephine System. Principle-based anterior lumbar interbody fusion.
 Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
botiss biomaterials bone & tissue regeneration collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
BONE void FILLER. Beta-tricalcium phosphate ( b-tcp) bone graft substitute. OvERvIEW BROCHURE
 chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
ctive Bone Bonding Bone Regeneration Osteostimulation*
 www.bonalive.com ctive Bone Bonding Bone Regeneration Osteostimulation* A revolution in bone grafting Osteoconductive bone graft substitute with osteostimulative* properties *non-osteoinductive Before
www.bonalive.com ctive Bone Bonding Bone Regeneration Osteostimulation* A revolution in bone grafting Osteoconductive bone graft substitute with osteostimulative* properties *non-osteoinductive Before
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
Bone augmentation with biomaterials
 Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
DEMINERALIZED BONE MATRIX
 DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE
 Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
REASONS TO USE R.T.R.
 3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
The injectable, self-setting calcium phosphate bone graft substitute.
 The injectable, self-setting calcium phosphate bone graft substitute. With a porous architecture similar to natural bone. STRUCSURE CP Bone Graft Substitute Because simplicity matters STRUCSURE CP Bone
The injectable, self-setting calcium phosphate bone graft substitute. With a porous architecture similar to natural bone. STRUCSURE CP Bone Graft Substitute Because simplicity matters STRUCSURE CP Bone
Ankle and subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
Solitary Bone Cyst of the Lunate: A Case Report
 Cronicon OPEN ACCESS ORTHOPAEDICS Case Report Solitary Bone Cyst of the Lunate: A Case Report MihirDesai* and Shivanand Bandekar Department of Orthopedics, Goa Medical College, Goa, India *Corresponding
Cronicon OPEN ACCESS ORTHOPAEDICS Case Report Solitary Bone Cyst of the Lunate: A Case Report MihirDesai* and Shivanand Bandekar Department of Orthopedics, Goa Medical College, Goa, India *Corresponding
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION M.
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION M.
Technique Guide. chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite.
 Technique Guide chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite. Table of Contents Introduction chronos Strip 2 Indications and Contraindications 3 Surgical Technique Preparation
Technique Guide chronos Strip. Osteoconductive betatricalcium phosphate ( -TCP) composite. Table of Contents Introduction chronos Strip 2 Indications and Contraindications 3 Surgical Technique Preparation
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Bone modeling and remodeling How does a bone form without augmentation?
 Overview on Bone formation,bone grafts materials, and Augma Biomaterials bone cements Bone modeling and remodeling How does a bone form without augmentation? Bone Modeling Initial formation of a bone A
Overview on Bone formation,bone grafts materials, and Augma Biomaterials bone cements Bone modeling and remodeling How does a bone form without augmentation? Bone Modeling Initial formation of a bone A
Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI Procedure. Subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
C-THRU Anterior Spinal System
 C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
C-THRU Anterior Spinal System Surgical Technique Manufactured From Contents Introduction... Page 1 Design Features... Page 2 Instruments... Page 3 Surgical Technique... Page 4 Product Information... Page
CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE
 CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE Bruce M. Frankel, MD and Susan G. Capps, PhD CLINICAL EXPERIENCES
CLINICAL EXPERIENCES OF SINGLE AND MULTI-LEVEL LUMBAR SPINE FUSIONS USING A DBM/CALCIUM SULFATE/BMA COMPOSITE GRAFT TO EXTEND LOCAL BONE Bruce M. Frankel, MD and Susan G. Capps, PhD CLINICAL EXPERIENCES
Orthobiologics In Orthopaedic Trauma
 Orthobiologics In Orthopaedic Trauma Aaron Nauth, MD, MSc Chair in Fracture Care Research St. Michael s Hospital, University of Toronto Created August 2017 Previous Versions J. Tracy Watson (2007, 2010)
Orthobiologics In Orthopaedic Trauma Aaron Nauth, MD, MSc Chair in Fracture Care Research St. Michael s Hospital, University of Toronto Created August 2017 Previous Versions J. Tracy Watson (2007, 2010)
CONFORM SHEET. Demineralized cancellous bone
 CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Stem cells, growth factors and scaffolds in craniofacial regenerative medicine
 Stem cells, growth factors and scaffolds in craniofacial regenerative medicine Viktor Tollemar, Zach J. Collier, Maryam K. Mohammed, Michael J. Lee, Guillermo A. Ameer, and Russell R. Reid Genes Dis. 2016
Stem cells, growth factors and scaffolds in craniofacial regenerative medicine Viktor Tollemar, Zach J. Collier, Maryam K. Mohammed, Michael J. Lee, Guillermo A. Ameer, and Russell R. Reid Genes Dis. 2016
POWER TO RESTORE WITHOUT LEAVING A TRACE. The only remaining evidence of the trauma
 POWER TO RESTORE WITHOUT LEAVING A TRACE The only remaining evidence of the trauma Perfect partner for your trauma and non-unions Your choice of synthetic bone graft not only influences the efficiency
POWER TO RESTORE WITHOUT LEAVING A TRACE The only remaining evidence of the trauma Perfect partner for your trauma and non-unions Your choice of synthetic bone graft not only influences the efficiency
Bone Regeneration in a Bilateral Sinus Lift
 2 A comparative pilot case study between irradiated human cancellous bone and a synthetic analogue Bone Regeneration in a Bilateral Sinus Lift Anthony McGee, BDS, Staines, and Dr J. A. Hunt, Liverpool/England
2 A comparative pilot case study between irradiated human cancellous bone and a synthetic analogue Bone Regeneration in a Bilateral Sinus Lift Anthony McGee, BDS, Staines, and Dr J. A. Hunt, Liverpool/England
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
Advice sheet for Surgeons and Radiologists. Radiographic appearance of CERAMENT
 Advice sheet for Surgeons and Radiologists Radiographic appearance of CERAMENT The structure and function of CERAMENT CERAMENT is an osteoconductive bone graft substitute which consists of hydroxyapatite
Advice sheet for Surgeons and Radiologists Radiographic appearance of CERAMENT The structure and function of CERAMENT CERAMENT is an osteoconductive bone graft substitute which consists of hydroxyapatite
Choice of spacer material for HTO! P. Landreau, MD Chief of Surgery Aspetar, Orthopaedic and Sports Medicine Hospital Doha, Qatar
 Choice of spacer material for HTO! P. Landreau, MD Chief of Surgery Aspetar, Orthopaedic and Sports Medicine Hospital Doha, Qatar High Tibial Osteotomy: HTO! Valgisation HTO Intended to transfer the mechanical
Choice of spacer material for HTO! P. Landreau, MD Chief of Surgery Aspetar, Orthopaedic and Sports Medicine Hospital Doha, Qatar High Tibial Osteotomy: HTO! Valgisation HTO Intended to transfer the mechanical
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26
 P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
Allograft Bone and Super Critical Fluid (SCF) Technology
 Allograft Bone and Super Critical Fluid (SCF) Technology Summary: 1. Supercritical Fluids (SCF) have the properties of a gas and a liquid when it is in its supercritical state; 2. SCF treatment provides
Allograft Bone and Super Critical Fluid (SCF) Technology Summary: 1. Supercritical Fluids (SCF) have the properties of a gas and a liquid when it is in its supercritical state; 2. SCF treatment provides
AUGMENT. Bone Graft. rhpdgf-bb/β-tcp SURGICAL TECHNIQUE
 rhpdgf-bb/β-tcp SURGICAL TECHNIQUE AUGMENT Bone Graft T HE F I R S T A ND O N LY PROV EN A LT ERN AT I V E TO AU TO GR A F T IN A NK LE A ND HINDFOOT A RT HRODESIS The Time Has Come to Augment Your Fusion.
rhpdgf-bb/β-tcp SURGICAL TECHNIQUE AUGMENT Bone Graft T HE F I R S T A ND O N LY PROV EN A LT ERN AT I V E TO AU TO GR A F T IN A NK LE A ND HINDFOOT A RT HRODESIS The Time Has Come to Augment Your Fusion.
Derma S O F T T I S S U E A U G M E N TAT I O N. Acellular dermal matrix
 Derma A XENOGENIC GRAFT FOR S O F T T I S S U E A U G M E N TAT I O N Acellular dermal matrix A xenogenic graft for soft tissue augmentation CHARACTERISTICS Obtained from derma of porcine origin, using
Derma A XENOGENIC GRAFT FOR S O F T T I S S U E A U G M E N TAT I O N Acellular dermal matrix A xenogenic graft for soft tissue augmentation CHARACTERISTICS Obtained from derma of porcine origin, using
Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells,
 What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
CALCIUM-BASED NEUTRAL AND BIORESORBABLE SELF-SETTING INJECTABLE BONE PUTTY. Ping Luo and Kenneth Trauner* Berkeley Advanced Biomaterials, Inc.
 CALCIUM-BASED NEUTRAL AND BIORESORBABLE SELF-SETTING INJECTABLE BONE PUTTY Ping Luo and Kenneth Trauner* Berkeley Advanced Biomaterials, Inc. San Leandro, CA 94577 *Presenting Author Phone: (510) 883-1644
CALCIUM-BASED NEUTRAL AND BIORESORBABLE SELF-SETTING INJECTABLE BONE PUTTY Ping Luo and Kenneth Trauner* Berkeley Advanced Biomaterials, Inc. San Leandro, CA 94577 *Presenting Author Phone: (510) 883-1644
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER. Magnifuse. Bone Graft
 POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
Metha Short Hip Stem System
 Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
chronos Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use
 Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use Granules, blocks, wedges, cylinders Safe Synthetic origin provides unsurpassed safety
Synthetic cancellous bone graft substitute ( -tricalcium phosphate) Remodels Replaced by bone in 6 18 months Easy to use Granules, blocks, wedges, cylinders Safe Synthetic origin provides unsurpassed safety
AttraX. Portfolio. Surface Drives Performance
 Portfolio Surface Drives Performance Advanced Biomaterial Microarchitecture Drives Bone Formation Traditional calcium phosphate materials generally do not give rise to bone formation when implanted in
Portfolio Surface Drives Performance Advanced Biomaterial Microarchitecture Drives Bone Formation Traditional calcium phosphate materials generally do not give rise to bone formation when implanted in
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
BAK/C Cervical Anterior Interbody Fusion System
 Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
Surgical Technique BAK/C Cervical Anterior Interbody Fusion System The Comfortable Choice for Cervical Fusion BAK/C Cervical Surgical Technique 1 The BAK/C Cervical Fusion System is an alternative to conventional
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products.
 End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
End-To-End Solution Posterior Lumbar. A comprehensive offering of complementary products. End-To-End Solution Posterior Lumbar From access through fixation, provides a comprehensive solution for posterior
Periimplant Regeneration Fenestration
 Indication Sheet PIR-1 Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Indication Sheet PIR-1 Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Bringing you Geistlich biocompatibility with improved application and handling benefits. Your combination for success
 Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
Step-by-Step Step-by-Step. Internal Hex. Implant System MAKE IT SIMLE
 4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
BONE TISSUE. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
CRANIAL RECONSTRUCTION SOLUTIONS
 CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
Pre op Failed endodontic treatment with sinus involvement.
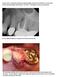 Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Clinical Policy Title: Bone graft substitutes
 Clinical Policy Title: Bone graft substitutes Clinical Policy Number: 14.02.09 Effective Date: July 1, 2016 Initial Review Date: May 18, 2016 Most Recent Review Date: May 19, 2017 Next Review Date: May
Clinical Policy Title: Bone graft substitutes Clinical Policy Number: 14.02.09 Effective Date: July 1, 2016 Initial Review Date: May 18, 2016 Most Recent Review Date: May 19, 2017 Next Review Date: May
Periimplant Regeneration Fenestration
 Indication Sheet PIR Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Indication Sheet PIR Periimplant Regeneration Fenestration Treatment concept of Dr. Jean-Pierre Gardella (surgeon) and Dr. Christian Richelme (prosthodontist), Marseille, France > Filling of a peri-implant
Comparative Animal Study of OssiMend and Healos 13,14
 Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
Bone Grafting for Socket Preservation
 Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Procedure Manual and Catalog
 Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
ORTHOPEDICS BONE Recalcitrant nonunions In total hip replacement total knee surgery increased callus volume
 ORTHOPEDICS Orthopedics has to do with a variety of tissue: bone, cartilage, tendon, ligament, muscle. In this regard orthopedic and sports medicine share the same tissue targets. Orthopedics is mostly
ORTHOPEDICS Orthopedics has to do with a variety of tissue: bone, cartilage, tendon, ligament, muscle. In this regard orthopedic and sports medicine share the same tissue targets. Orthopedics is mostly
In Vivo Heat-stimulus Triggered Osteogenesis
 In Vivo Heat-stimulus Triggered Osteogenesis Kunihiro Ikuta 1, Hiroshi Urakawa 1, Eiji Kozawa 1, Shunsuke Hamada 1, Naoki Ishiguro 2, Yoshihiro Nishida 1. 1 Nagoya University, Nagoya, Japan, 2 Nagoya Graduated
In Vivo Heat-stimulus Triggered Osteogenesis Kunihiro Ikuta 1, Hiroshi Urakawa 1, Eiji Kozawa 1, Shunsuke Hamada 1, Naoki Ishiguro 2, Yoshihiro Nishida 1. 1 Nagoya University, Nagoya, Japan, 2 Nagoya Graduated
BIOFIBER. Absorbable Biologic Scaffolding Solutions
 BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
34 th Annual Meeting of the European Bone and Joint Infection Society (EBJIS)
 34 th Annual Meeting of the European Bone and Joint Infection Society (EBJIS) 10-12 September 2015 Estoril, Portugal Free Paper: #135 A COMPARATIVE STUDY OF THREE BIOABSORBABLE ANTIBIOTIC CARRIERS IN CHRONIC
34 th Annual Meeting of the European Bone and Joint Infection Society (EBJIS) 10-12 September 2015 Estoril, Portugal Free Paper: #135 A COMPARATIVE STUDY OF THREE BIOABSORBABLE ANTIBIOTIC CARRIERS IN CHRONIC
Surgical Solutions USA Regenerative Product Catalog USA
 RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
