The road to ruin: the formation of disease-associated oral biofilms
|
|
|
- Silas Garrett
- 5 years ago
- Views:
Transcription
1 INVITED MEDICAL REVIEW The road to ruin: the formation of disease-associated oral biofilms NS Jakubovics 1, PE Kolenbrander 2 (2010) 16, doi: /j x Ó 2010 John Wiley & Sons A/S All rights reserved 1 Oral Biology, School of Dental Sciences, Newcastle University, UK; 2 National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA The colonization of oral surfaces by micro-organisms occurs in a characteristic sequence of stages, each of which is potentially amenable to external intervention. The process begins with the adhesion of bacteria to host receptors on epithelial cells or in the salivary pellicle covering tooth surfaces. Interbacterial cell cell binding interactions facilitate the attachment of new species and increase the diversity of the adherent microbial population. Microbial growth in oral biofilms is influenced by the exchange of chemical signals, metabolites and toxic products between neighbouring cells. Bacterial cells on tooth surfaces (dental plaque) produce extracellular polymers such as complex carbohydrates and nucleic acids. These large molecules form a protective matrix that contributes to the development of dental caries and, possibly, to periodontitis. The identification of key microbial factors underlying each step in the formation of oral biofilms will provide new opportunities for preventative or therapeutic measures aimed at controlling oral infectious diseases. (2010) 16, Keywords: antigen I II protein; autoinducer-2; biofilm matrix; coaggregation; dental plaque; oral streptococcus Correspondence: Nicholas S. Jakubovics, Oral Biology, School of Dental Sciences, Newcastle University, Newcastle upon Tyne, NE2 4BW, UK. Tel: , Fax: , nick.jakubovics@ncl.ac.uk Received 8 March 2010; accepted 14 March 2010 Introduction Bacteria like to live at surfaces. Whether it is the air liquid interface at the top of a pond or the solid substratum of a human tooth, surfaces tend to concentrate nutrients and provide a stable habitat that allows the development of organized microbial communities. When it became clear that the behaviour of surfaceassociated bacteria cannot be predicted from observations made on micro-organisms cultured in rich laboratory broths, a new term for describing sessile microbial populations was introduced and the scientific topic of Ôbiofilm research was born. Biofilms are defined as communities of microbial cells and associated extracellular polymeric substances that are present at an interface. In many cases, biofilm bacteria are up to 1000 times more resistant to antimicrobial agents than planktonic cells. Resistance is mediated by tough intercellular matrices, slow growing cells and the up-regulation of antimicrobial systems in biofilm cells (Zhang and Mah, 2008). In addition, mechanisms for the tight adhesion of bacteria to underlying substrata impede the efficient removal of biofilms by physical or chemical means. In the human oral cavity, biofilms form on the surface of hard and soft tissues, and sometimes both (for example, subgingival dental plaque). Bacteria in oral biofilms that are exposed to the mouth are frequently removed, either by shedding of epithelial cells or by mechanical shear from the movement of tongue and cheeks or from toothbrushing. However, oral bacteria are remarkably well adapted to recolonizing host surfaces. Colonization of clean enamel surfaces begins within minutes, and after a few hours, extensive microbial deposition can be observed (Palmer et al, 2006). The development of oral biofilms depends on interactions between bacterial cell-surface adhesins and host receptors. Bacteria bacteria interactions promote further colonization and lead to complex microbial communities, in many cases consisting of 50 or more different species of bacteria at a single location (Aas et al, 2005). Chemical communication between bacteria is critical for the stable coexistence of different species within oral biofilms. Maturation of biofilms is associated with the production of an extracellular matrix composed of polysaccharides and other macromolecules such as nucleic acids. The accumulation of oral biofilms on tooth surfaces (dental plaque) can lead to the development of dental caries or periodontitis, two of the most common diseases in humans. Interactions between different species of bacteria, and between bacteria and the host, are central to the development of oral biofilms. A
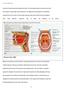
2 730 detailed understanding of the key steps in the formation of these biofilms will provide new opportunities for intervention against oral diseases of microbial origin. Adhesion, coadhesion and coaggregation Adhesion of bacteria to the salivary pellicle represents the first step in the colonization of enamel surfaces in the mouth (Figure 1). Oral streptococci have been consistently shown to be the major primary colonizers of clean enamel surfaces, and these organisms constitute 60 80% of dental plaque bacteria within 4 to 8 h (Nyvad and Kilian, 1987, 1990; Diaz et al, 2006; Dige et al, 2009). Oral viridans streptococci express numerous protein and lipoprotein adhesins on the cell surface. For example, the genome of Streptococcus sanguinis encodes at least 93 polypeptides that are predicted to be anchored on the surface of cells and potentially may be involved in adhesion (Xu et al, 2007). This battery of cell-surface exposed molecules endows streptococci with an unusually broad capacity for binding human or bacterial receptors. For a comprehensive description of adhesion and colonization by streptococci, the reader is referred to an excellent recent review (Nobbs et al, 2009). There is undoubtedly some functional redundancy between different cell-surface adhesins, and not all adhesins are necessarily present in every strain of a given streptococcal species. However, certain adhesin proteins are widely conserved in oral streptococci and these polypeptides are likely to play key roles in adhesion to oral surfaces. For example, the antigen I II (AgI II) family of proteins are expressed by commensal oral streptococci such as S. gordonii, S. oralis and S. sanguinis, by cariogenic mutans streptococci (S. mutans and S. sobrinus) and by extra-oral streptococci such as S. pyogenes (Jakubovics et al, 2005b; Zhang et al, 2006). Polypeptides of the AgI II family are kda and have a number of characteristic structural domains (Figure 2a). AgI II proteins play a central role in binding to salivary agglutinin glycoprotein gp340 (Jakubovics et al, 2005a,b) (Figure 2b). When gp340 is integrated into the acquired enamel pellicle, binding promotes adhesion of streptococci. However, gp340 is also present in the fluid phase and here interactions with streptococcal AgI II proteins result in aggregation of bacterial cells. Large bacterial aggregates do not adhere well to surfaces (Liljemark et al, 1981) and are removed from the mouth by swallowing. AgI II proteins may also influence binding to soft tissues. Streptococcus gordonii produces two AgI II polypeptides, SspA and SspB, and these have been shown to interact with extracellular matrix proteins, collagen and fibronectin (Heddle et al, 2003; Jakubovics et al, 2009). There is strong evidence that adherent streptococci on oral surfaces recruit other bacteria to the biofilm. Streptococci interact with a number of isolated oral bacteria in vitro and are frequently observed in close association with non-streptococcal bacteria in dental plaque. Adhesive interactions between bacteria can be observed in the laboratory by vortex mixing dense suspensions of cells. Within 10 s, cell cell adhesion results in the formation of large clumps, or coaggregates, which are visible to the naked eye. Coaggregation only occurs between compatible partner organisms, and is dependent on the presence of cell-surface adhesins on one cell type and cognate receptors on the other. Similar adhesion-receptor interactions may facilitate the binding of planktonic bacteria to immobilized cells on the tooth surface, and this process is termed coadhesion. Streptococcal AgI II proteins are among a number of bacterial cell-surface molecules that have been shown to mediate coaggregation and coadhesion. Not all AgI II proteins are equivalent in this regard. Thus, S. gordonii SspB protein mediates a strong coaggregation interaction with Actinomyces oris T14V (Figure 2c), whereas SspA polypeptide does not recognize this strain (Egland et al, 2001; Jakubovics et al, 2005b). Conversely, SspA and a number of other AgI II proteins from different species of Streptococcus promote coaggregation with Actinomyces naeslundii PK606. By producing two distinct AgI II proteins, S. gordonii increases its range of potential coaggregation partners. Streptococcus gordonii AgI II proteins also bind to the periodontal pathogen Porphyromonas gingivalis and this interaction enables P. gingivalis to attach to pre-existing S. gordonii biofilms (Lamont et al, 2002). In addition, the AgI II polypeptides of S. gordonii recognize receptors on the surface of Candida albicans hyphae (Bamford et al, 2009) (Figure 2d). Adhesion Communication Matrix-enclosed communities Disease Figure 1 Stages in the formation of dental plaque. Colonization of tooth surfaces is initiated by bacterial adhesion to the salivary pellicle. Coaggregation and coadhesion facilitate the development of multispecies communities. The exchange of chemicals between neighbouring bacteria promotes co-operation or competition. In some cases, communication does not occur until a critical biomass is reached. Adherent bacteria produce a matrix of complex carbohydrates and or extracellular nucleic acids, which helps to bind the biofilm together and to protect the encased cells. Dental caries or periodontitis arises from a shift in the microflora and an accumulation of pathogenic bacteria
3 (a) N N A V P C C 731 LPxTG (b) (c) Figure 2 Adhesive interactions mediated by streptococcal cell-surface antigen I II (AgI II) proteins. (a) AgI II polypeptides are kda and are composed of the following structural domains: An N-terminal region containing a signal for secretion across the cell membrane, three to four repeated Alanine-rich sequences, a central variable region, approximately three repeated prolinerich elements and a C-terminal domain containing an LP TG motif that is a substrate for sortase-mediated cross-linking to cell wall peptidoglycan. (b) Disruption of sspa and sspb genes, encoding AgI II proteins in S. gordonii, reduces adhesion to immobilized salivary agglutinin glycoprotein gp340. Bacteria that are bound to gp340 coated on the surface of microtitre wells were visualized by staining with crystal violet. Note that the AgI II mutant retains some capacity for gp340 binding because of a functionally redundant adhesin, Hsa. (c) AgI II proteins also mediate coaggregation with A. oris. In a test tube, wild-type S. gordonii forms macroscopic coaggregates when mixed with A. oris, whereas an sspab mutant does not. Microscopically, S. gordonii (green) and A. oris (orange) appear well dispersed throughout coaggregates. Bar = 10 lm. (d) S. gordonii AgI II proteins are involved in adhesion to C. albicans hyphae. Some hyphae bear many receptors for streptococci (black arrows), whereas others appear nearly devoid of streptococcal binding (white arrow). Bar = 10 lm. Image D was kindly provided by L.C. Dutton and H.F. Jenkinson, University of Bristol AgI/II + (d) gp340 sspab w-t Coaggregation between oral bacteria has been studied in detail and over a thousand pairwise interactions have been investigated (Kolenbrander et al, 2006). Fusobacterium nucleatum is the most promiscuous coaggregation partner identified to date, and strains of F. nucleatum coaggregate with almost all other oral bacteria. Fusobacterium nucleatum is obligately anaerobic and is not usually found in nascent supragingival dental plaque. However, levels of F. nucleatum increase when plaque grows below the gum line and this organism can make up 20% or more of the bacteria in subgingival dental plaque (Suzuki et al, 2004). Fusobacterium nucleatum appears to act as bridging organism by binding to both early colonizers such as streptococci, and later colonizers including periodontal pathogens. Recently, a large (350 kda) cell-surface protein of F. nucleatum, RadD, has been shown to mediate arginine-inhibitable coaggregation with S. sanguinis (Kaplan et al, 2009). Unlike the wild type, an F. nucleatum radd knockout mutant did not form a structured biofilm with S. sanguinis, indicating that, in this system, coaggregation interactions direct the spatial distribution of the partner organisms in biofilms. In the early stages of biofilm formation, adhesion is the primary interaction between bacterial cells. Adhesinreceptor interactions hold the partner organisms in juxtaposition. Subsequent growth and further coadhesion of bacteria from saliva increase the local cell density and lead to the development of microenvironments within the biofilm. Within these close-knit microbial communities, metabolic products and signalling molecules produced by cells of one species influence neighbouring bacteria. Sometimes, the result is interspecies competition to the detriment of one or both organisms. However, many interactions between oral bacteria are mutually beneficial. It is becoming clear that communities of bacteria are better adapted to growth in saliva than single species of bacteria in isolation. Interbacterial interactions Historically, the analysis of microbial traits has begun with the isolation of bacteria in pure cultures. In dental plaque, however, most bacteria are in close proximity to cells of different species and interbacterial interactions have profound effects on the participating organisms.
4 732 To understand which genes and proteins are critical for the survival and growth of bacteria in oral biofilms, it is necessary to analyse mixed cultures of bacteria. Given that mature dental plaque may contain 100 or more different species of bacteria, the task of identifying relevant interspecies interactions is daunting. Nevertheless, some progress in this area has been made in recent years. The development of high-throughput techniques such as microarrays and next-generation sequencing promises to enhance significantly the pace of research on mixed-species populations. Competition Competitive interactions between oral bacteria can be demonstrated easily in vitro. For example, many oral streptococci including S. mutans and S. salivarius produce antimicrobial peptides (bacteriocins) that have bactericidal activity against a range of oral strains (van der Ploeg, 2005; Hyink et al, 2007). In S. mutans, production of bacteriocins is coordinated with the development of competence, a state in which the cells can take up and incorporate extracellular DNA (edna) from the environment (van der Ploeg, 2005; Kreth et al, 2006). Therefore, bacteriocin secretion potentially benefits the producing organism by providing DNA. The DNA may be incorporated into the chromosome and used as a source of new genetic information, or it may be broken down for energy. Alternatively, edna produced by the action of bacteriocins may remain in the biofilm and act as a stabilizing matrix molecule. In fact, edna is apparently so important to S. mutans that this organism has evolved a mechanism to release DNA from a proportion of its own population when other bacteria are not available. This apparently altruistic process involves the induction of an intracellular bacteriocin, CipB (mutacin V), in 1% of the population in response to competence stimulating peptide (Perry et al, 2009). Disruption of the gene encoding CipB significantly reduced competence for genetic transformation in the population as a whole, indicating that CipB-dependent autolysis is important for the transfer of genetic material between S. mutans cells. Most oral streptococci belong to the Ôviridans group, named from their production of a greenish tinge when cultured on blood agar. This arises from hydrogen peroxide (H 2 O 2 ), which is secreted by streptococci and bleaches the haemoglobin in blood. H 2 O 2 is non-polar and can cross bacterial cell membranes and oxidize intracellular macromolecules including lipids, DNA and proteins, causing stress or cell death. The streptococci that produce H 2 O 2 are relatively resistant to its effects. By contrast, other oral bacteria are killed or prevented from growing by streptococcal H 2 O 2 (Holmberg and Hallander, 1973; Jakubovics et al, 2008b). It is not clear whether sufficient concentrations of H 2 O 2 for antimicrobial activity accumulate in supragingival dental plaque, where small molecules are continually washed out of the biofilm. However, antimicrobial activity of H 2 O 2 may be important during the formation of subgingival dental plaque, which is contained within an enclosed space. The presence of an oxidizing agent will restrict the growth of anaerobic bacteria including periodontal pathogens such as P. gingivalis. Concordantly, levels of the peroxidogenic streptococcus, S. sanguinis are correlated with the absence of periodontal disease (Stingu et al, 2008; Colombo et al, 2009). During the transition from oral health to disease, the microbial composition of dental plaque shifts from predominantly commensal bacteria to a population containing a large proportion of disease-associated organisms (Marsh, 2006). In the case of dental caries, the shift in population involves a reduction in the diversity of micro-organisms at active caries sites (Aas et al, 2008), because of the production of hydrogen ions by acidogenic bacteria. The drop in ph is essentially a competitive factor that favours the survival and growth of aciduric and acidogenic organisms such as mutans streptococci, lactobacilli and bifidobacteria. Although ph-driven competition is most easily seen in mature dental plaque associated with active caries lesions, the onset of competition probably arises much earlier. The early colonizing streptococci such as S. sanguinis, S. gordonii, S. mitis and S. oralis, produce acid from sugars, albeit generally to a lesser extent than S. mutans or S. sobrinus. It has been speculated that low levels of acid production by non-mutans streptococci prepare the ground for the incorporation of more acidogenic organisms into the biofilm (Takahashi and Nyvad, 2008). Mutualism Saliva is the primary nutrient for supragingival dental plaque bacteria. Dietary carbohydrates play an important role in the development of caries but do not have a major impact on health-associated dental plaque (Bowden and Li, 1997). In vitro, very few oral bacteria in monoculture can utilize saliva as the sole source of nutrients. Dental plaque as a whole, however, can degrade host mucins and other salivary components efficiently (Wickstro m and Svensa ter, 2008; Wickstro m et al, 2009). In chemostat studies, microbial consortia composed of five different species were able to grow on hog gastric mucin, a model for human salivary mucins (Bradshaw et al, 1994). Growth was enhanced when additional oral bacteria with novel catabolic capabilities were introduced into the system. These data demonstrate that complex communities are better equipped for growth on saliva than individual species in isolation. During the first few hours of dental plaque accumulation on cleaned enamel surfaces, bacterial cells can be observed in small communities, composed of two or three different species (Chalmers et al, 2008). Is there sufficient microbial diversity in these micro-communities to permit mutualistic growth of the interacting organisms? To address this question, a number of recent studies have investigated the abilities of two- or threespecies partnerships of isolated micro-organisms to grow in saliva-fed biofilms (Periasamy et al, 2009; Periasamy and Kolenbrander, 2009a,b; 2010). Interestingly, many examples of mutualistic communities were identified, where mixed-species populations grew in
5 salivary biofilms, whereas the individual species in monoculture did not. For example, in anaerobic biofilms, growth of the periodontal pathogen P. gingivalis was promoted by pairing with all but one of six species tested (Periasamy and Kolenbrander, 2009b). Only S. oralis was incompatible with P. gingivalis. Even here, inclusion of S. gordonii in addition to S. oralis and P. gingivalis resulted in mutualistic growth of all three organisms. Clearly, there is a degree of specificity in mutualistic associations between oral bacteria. This was further demonstrated when interactions between S. oralis and F. nucleatum were investigated (Periasamy et al, 2009). Streptococcus oralis and F. nucleatum coaggregate, but this interaction does not lead to the growth of F. nucleatum. However, when A. oris (A. naeslundii), which coaggregates with both S. oralis and F. nucleatum, was introduced into the system, F. nucleatum grew. Such studies have shown that there are many productive interactions that occur between oral bacteria, but at present, it is difficult to predict which organisms will form mutually beneficial partnerships. The next challenge will be to identify the molecular basis of these interactions to inform the development of new measures for oral biofilm control. There is evidence that both metabolic interactions and the exchange of signalling molecules (discussed below) contribute to mutualism in oral biofilms. Several nutritional interrelationships have been identified between oral bacteria including, for example, the production and utilization of lactic acid. Lactic acid is a metabolic waste product of streptococci and lactobacilli and, on the other hand, it is a major energy source for Aggregatibacter actinomycetemcomitans and veillonellae. Aggregatibacter actinomycetemcomitans can utilize lactate produced by oral streptococci for growth. Thus, in medium containing sucrose as the sole carbon source, A. actinomycetemcomitans did not grow in monoculture, but was able to grow in the presence of S. gordonii because of the streptococcus-mediated conversion of sucrose to lactate (Brown and Whiteley, 2007). Similarly, Veillonella parvula utilizes lactate produced by Streptococcus salivarius and, in doing so, stimulates glycolysis in the streptococcus (Hamilton and Ng, 1983). In biofilms grown in flowcells with saliva as the sole source of nutrients, Veillonella sp. participates in numerous mutualistic partnerships including with the lactate-producing organism S. oralis. Neither S. oralis nor Veillonella sp. grew in monoculture in this model. However, when inoculated together, both organisms increased 8-fold resulting in a nearly confluent biofilm (Periasamy and Kolenbrander, 2010). In addition, Veillonella sp. overcame the incompatibility of P. gingivalis and S. oralis. In three-species biofilms containing Veillonella sp., P. gingivalis and S. oralis, all organisms grew (Figure 3). Measurements of lactate concentration in the biofilm effluent demonstrated that Veillonella sp. consumed residual lactate in the saliva and also removed lactate produced by S. oralis (Periasamy and Kolenbrander, 2010). These data indicate that lactate consumption is likely to be one key driving force for mutualistic associations between oral biofilm bacteria. Communication Many interspecies interactions between oral bacteria lead to changes in gene expression in one or both of the partner organisms. The identification of genes that are regulated by neighbouring bacteria will help us to understand the microbial factors and processes that become important when bacteria form mixed-species communities. In the case of dual-species interactions between veillonellae and streptococci, gene regulation apparently affects carbohydrate storage in the streptococcus. Thus, the amyb gene of S. gordonii, encoding a-amylase, was up-regulated in batch culture with V. atypica (Egland et al, 2004). Placing a dialysis membrane between the streptococci and the veillonellae did not prevent amyb gene regulation, indicating that regulation occurs in response to a diffusible signal from V. atypica. Using a green fluorescent protein fusion construct, amyb expression was shown to be up-regulated in S. gordonii cells juxtaposed with V. atypica in flowcell biofilms (Egland et al, 2004). Therefore, even in a flowing system where signals are washed out of the biofilm, communication was possible at short range. The signal produced by V. atypica remains to be identified. However, it is likely to be closely linked to carbon metabolism as sensing is mediated by the S. gordonii carbon catabolism regulator CcpA (Johnson et al, 2009). Increased expression of S. gordonii a-amylase likely leads to mobilization of energy reserves, stored as intracellular polysaccharides, and thus increases the availability of lactic acid for V. atypica. In some circumstances, it is the formation of structured communities, rather than the presence of another organism per se, that leads to gene regulation. For example, the expression of 23 genes was changed >3- fold in S. gordonii cells following coaggregation with A. oris (Jakubovics et al, 2008a). Coculture of S. gordonii with A. oris was not sufficient for gene regulation; changes in gene expression were observed only in cultures where coaggregation had been induced by vigorously mixing dense cell suspensions before diluting with growth medium. Nine of the 23 genes that were regulated in this system were involved in arginine metabolism. Actinomyces oris was shown to stabilize S. gordonii arginine biosynthesis and to enable the growth of S. gordonii in low (<0.1 mm) arginine. Coaggregation was essential for this effect. In cocultures without induced coaggregation, growth of S. gordonii was not observed until coaggregates had formed spontaneously >9 h after inoculation. At present, it remains unclear whether S. gordonii responds to cell cell contact directly, or whether a quorum sensing system is involved. There is strong evidence that oral bacteria sense and respond to high cell densities. Thus, autoaggregation of F. nucleatum led to changes in the expression of almost 100 genes, many of which encoded nutrient transporters or carbohydrate metabolism enzymes (Merritt et al, 2009). The artificial induction of high cell densities by centrifugation of cells caused a similar pattern of gene regulation. Centrifugation has also been employed to 733
6 734 (a) S. oralis Veillonella sp. P. gingivalis (b) Veillonella sp. + P. gingivalis Veillonella sp. + P. gingivalis + S. oralis 4 h 18 h 75 mm mm Figure 3 Mutualistic interactions between three species in saliva-fed biofilms. Monocultures of S. oralis, Veillonella sp. or P. gingivalis grew poorly in flowcell biofilms with saliva as the sole source of nutrients. (a) Cocultures of S. oralis and Veillonella sp. or of P. gingivalis and Veillonella sp. exhibited mutualism: within 18 h, the biomass of each partner in these interactions had increased significantly over the initial inoculum (indicated by purple colour). Mutualism was not observed in the dual-species pairing of S. oralis and P. gingivalis (yellow). However, including Veillonella sp. in addition to these organisms resulted in mutualistic growth of all three species. (b) Mutualistic interactions in biofilms observed by confocal microscopy. Species were differentiated by staining with fluorophore-conjugated antisera. In the left panels, Veillonella sp. appears blue and P. gingivalis is red. In the right hand panels, S. oralis appears blue, Veillonella sp. is green and P. gingivalis is red. Strong growth of each organism can be seen between 4 h and 18 h. Images in b were supplied by S. Periasamy create high-density populations of S. mutans. In these cultures, two bacteriocins and at least six other genes were found to be cell density regulated (Merritt et al, 2007). Of note, a two component system, hdrrm, involved in the development of competence and bacteriocin production, was induced in densely packed cells. The coordinated production of bacteriocins and competence genes in dense cell populations represents an efficient mechanism for optimizing DNA acquisition from juxtaposed cells in microbial communities (Okinaga et al, 2010). The species-specific quorum sensing regulators N-acyl homoserine lactones (autoinducer-1) have not been identified in oral bacteria. However, many oral microorganisms produce and or respond to the interspecies signal autoinducer-2 (AI-2). Autoinducer-2 is the collective term given to a number of molecules that spontaneously form an equilibrium when 4,5-dihydroxy-2,3-pentanedione (DPD) is dissolved in water. Bacteria produce AI-2 during amino acid metabolism as a product of the enzyme encoded by the luxs gene. In cocultures of S. oralis and A. oris (A. naeslundii), AI-2 is
7 essential for mutualistic biofilm growth with saliva as the sole source of nutrients (Rickard et al, 2006). Neither S. oralis nor A. oris grew well in monoculture. When inoculated together into a flowcell, however, the organisms formed profuse biofilms. A luxs mutant of S. oralis was unable to grow in monoculture or in coculture with A. oris. Growth was restored by genetic complementation of the luxs mutation or by the addition of exogenous AI-2. Interestingly, AI-2 was only effective over a limited concentration range. The optimal concentration for mutualistic growth was 0.8 nm; concentrations in excess of 8 nm were significantly less effective (Rickard et al, 2006). These data suggest that bacteria have sensitive mechanisms to tune responses to the prevailing concentration of AI-2 surrounding the cell. Recently, techniques have been developed for the sensitive measurement of AI-2 in saliva (Campagna et al, 2009). Whole saliva from eight volunteers contained between 200 nm and 1000 nm AI- 2, concentrations far in excess of those supporting mutualism between S. oralis and A. oris. It is likely that salivary AI-2 concentrations are much lower at the start of plaque accretion, immediately after the bulk of dental plaque has been removed by toothbrushing. However, currently it remains unknown whether the salivary AI-2 concentration is correlated with the extent, or the composition, of dental plaque. The molecular basis of AI-2 sensing by oral bacteria is not well understood at present. The sole exception is A. actinomycetemcomitans, in which two distinct receptors for AI-2, LsrB and RbsB, have been identified (Shao et al, 2007a). In a nutrient-rich medium, the growth of A. actinomycetemcomitans in flowcell biofilms was dependent upon production and sensing of AI-2 (Shao et al, 2007b). Using a microtitre well biofilm model, A. actinomycetemcomitans cells were able to grow attached to surfaces, but growth did not begin until >24 h after inoculation (Periasamy and Kolenbrander, 2009a). It is possible that AI-2 gradually accumulates over time, and that growth is triggered only when a threshold AI-2 concentration is reached. Previous studies have shown that crude preparations of culture-free supernatant from oral bacteria stimulate the growth of other strains (Liljemark et al, 1997). Moreover, the development of natural dental plaque involves a rapid burst of growth following the accumulation of a threshold concentration of bacteria, approximately cells mm )2 (Liljemark et al, 1997). Taken together, the above studies provide strong evidence that bacterial growth in dental plaque is dependent on cell density sensing. The biofilm matrix The secretion of large molecules by adherent bacteria, either by active secretion mechanisms or by cell lysis, leads to the development of a macromolecular scaffold surrounding the cells. This biofilm matrix helps to bind the cells to the surface and acts as an ion exchange resin to restrict the flow of charged or reactive molecules through the biofilm. A great deal of research has been directed towards the insoluble carbohydrate polymers produced by oral streptococci, because these appear to play a major role in the progression of dental caries (reviewed by Banas and Vickerman, 2003; Russell, 2009). More recently, edna has been recognized as an important constituent of biofilm matrices. At present, however, very little remains known about the function of microbial edna in oral biofilms. The production of organic acids from sugars is central to the caries process. Many acidogenic oral bacteria, including mutans and non-mutans streptococci and lactobacilli, also convert sucrose into extracellular glucan or fructan polymers. The water solubility of these polymers is determined by the degree of branching. Streptococcus mutans, for example, synthesizes watersoluble glucans containing primarily a-1,6 linkages and a water-insoluble glucan, known as mutan, with largely a-1,3 linkages. These carbohydrates are produced by glucosyltransferase (GTF) enzymes, secreted proteins of approximately kda that hydrolyse sucrose into the monosaccharides fructose and glucose, and these enzymes polymerize the glucose to form glucans. In S. mutans, water-soluble glucans are synthesized by the product of the gtfd gene, whereas gtfb and gtfc encode enzymes responsible for insoluble glucan production (Banas and Vickerman, 2003). In addition, an inulintype fructan is synthesized by fructosyltransferase (FTF), encoded by the ftf gene. The insoluble glucans are an important component of biofilm matrices and provide attachment sites for planktonic mutans streptococci. Soluble glucans, on the other hand, appear to be dispensable for sucrose-dependent adhesion of S. mutans (Yamashita et al, 1993). Nevertheless, studies of knockout mutants indicate that all GTFs and FTF are required for the cariogenicity of S. mutans in rat models (Munro et al, 1991; Yamashita et al, 1993). There is some evidence that the production of exopolysaccharides by oral streptococci is up-regulated when cells are in biofilms. In S. mutans GS5, for example, the expression of gtfb, gtfc and ftf was, respectively, 22-fold, 15-fold and 12-fold increased in biofilms compared with planktonic cells (Shemesh et al, 2007b). However, up-regulation of gtfb and ftf genes was not observed in biofilm cells of S. mutans UA159 (Shemesh et al, 2007a), indicating that biofilm-mediated regulation of these genes is strain-specific. Further studies are needed using different strains of S. mutans, and with other glucan- and fructan-producing streptococci, to determine whether biofilm-mediated regulation of exopolysaccharide production is an important feature of biofilm development in vivo. The primary nutrient source for subgingival dental plaque is gingival crevicular fluid, an exudate derived from serum. The concentration of simple sugars available to bacteria growing beneath the gum line is very low and consequently glucans and fructans are not major constituents of subgingival biofilms. So what is the biofilm matrix in subgingival dental plaque and is it important in disease? At present, there is very little evidence that directly addresses this question. Extracel- 735
8 736 lular proteins certainly appear to be involved in periodontitis: the cysteine proteases of P. gingivalis, for example, have multifarious effects on host immunity (Pathirana et al, 2010). However, most proteins are too small to form a structural biofilm scaffold without extensive polymerization. Perhaps a more likely candidate for the major subgingival biofilm matrix molecule is DNA. Extracellular DNA was first shown to be a key biofilm matrix component in Pseudomonas aeruginosa biofilms (Whitchurch et al, 2002). Degradation of edna by treatment with DNase I dissolved biofilms and released attached cells. Subsequently, edna has been shown to be an integral component of biofilm matrices in many different bacteria including Grampositive organisms such as Enterococcus faecalis, Gramnegative organisms such as Neisseria meningitides and the fungus C. albicans (Guiton et al, 2009; Martins et al, 2009; Lappann et al, 2010). Oral streptococci release edna during planktonic growth by a mechanism that depends on endogenous production of H 2 O 2, but apparently does not involve extensive cell lysis (Kreth et al, 2009). However, the function of edna in oral bacterial biofilms remains to be elucidated. Treating biofilm-related oral diseases The primary methods for controlling dental plaquerelated diseases at present involve removing as much plaque as possible as often as possible, and protecting enamel with fluoride. These approaches are reasonably effective, yet dental caries and periodontitis remain among the most prevalent diseases in the Western World. Clearly, there is room for improvement. An attractive option is to interfere with plaque accumulation by controlling microbial adhesion, interbacterial communication or establishment of the biofilm matrix. Control measures may be targeted to specific bacteria or aimed more generally at reducing the total accumulation of plaque. One approach that has received a great deal of attention involves preventing adhesion of S. mutans by targeting the AgI II adhesin to reduce the incidence of caries. Early studies in rhesus monkeys using S. mutans AgI II protein as a vaccine demonstrated the potential of this approach (Lehner et al, 1980). Vaccines have been improved by, for example, conjugation of AgI II with the cholera toxin B subunit (Hajishengallis et al, 2005) or priming the immune system with DNA (Li et al, 2009). However, it has proved difficult to raise sufficient support from industry for large-scale clinical trials of a vaccine against a disease that is clearly not life-threatening. Nevertheless, there is still momentum for vaccine development within the scientific community (Taubman and Nash, 2006). An alternative to active immunization is to block adhesins with specific inhibitors such as peptides. A synthetic peptide, P1025, corresponding to a fragment of the S. mutans AgI II, blocks adhesion to gp340 and has been shown to be effective in clinical trials (Younson and Kelly, 2004). Peptides targeting AgI II proteins may also be useful in protection against periodontitis. An SspB adherence region (BAR) peptide has been identified that inhibits the recruitment of P. gingivalis to biofilms containing S. gordonii (Daep et al, 2008). The inhibition of interbacterial binding interactions is an exciting new approach to control the oral microflora. A great advantage of this type of strategy is that bacterial resistance would not be predicted to develop easily, as modification of an adhesin to reduce binding to an applied peptide would also impair adhesion to the natural substrate. The recognition that interspecies communication is important during oral biofilm formation promises to open new avenues for controlling the accumulation of dental plaque. Natural and synthetic compounds have been developed to blockade homoserine lactone-mediated quorum sensing and control infections caused by P. aeruginosa (reviewed by Bjarnsholt and Givskov, 2007; Njoroge and Sperandio, 2009). Similarly, a number of substances have been reported to act as potent antagonists for AI-2 signalling, including the natural furanone fimbrolide, alkyl-dpd compounds and nucleoside analogues (Brackman et al, 2009; Lowery et al, 2009). It is not yet clear which points of the AI-2 signalling pathway are targeted by these compounds or whether they are active against AI-2-dependent communication between oral bacteria. However, in light of the apparent importance of AI-2 in the development of monospecies and dual-species oral bacterial biofilms, AI-2 antagonists (and possibly also AI-2 agonists) would be predicted to bring about major changes in the structure and microbial composition of dental plaque. Removal of plaque bacteria from tooth surfaces may be facilitated by strategies that interfere with the biofilm matrix. Like AgI II polypeptides, GTFs of mutans streptococci have been developed as vaccine candidates (Taubman and Nash, 2006). Peptides against catalytic and glucan-binding domains of GTFs inhibit their function, and a single molecule containing two copies of each peptide was effective at reducing colonization by mutans streptococci in a rodent model of caries (Taubman et al, 2001). Vaccines against GTFs aim to prevent the formation of a tenacious carbohydrate matrix. An alternative approach is to use enzymes to help degrade a preformed matrix. Bacteria produce such enzymes naturally to disperse from biofilms. The biofilm dispersing glycosidase produced by A. (Actinobacillus) actinomycetemcomitans has been identified (Kaplan et al, 2003). This enzyme, known as dispersin B, effectively dissolves matrices of biofilms formed by diverse micro-organisms including Escherichia coli, Staphylococcus epidermidis, Pseudomonas fluorescens and Yersinia pestis (Itoh et al, 2005). However, with the exception of A. actinomycetemcomitans, it is not yet known whether oral bacteria produce biofilm matrix molecules that are sensitive to dispersin B. In fact, given the diversity of extracellular carbohydrates that are produced by bacteria, it seems highly unlikely that dispersin B will be universally effective against oral biofilms. Nucleic acids, by contrast, have a much more uniform structure. If it is found that edna is critical for
9 the stability of oral biofilms, then the use of DNA degrading enzymes to help disrupt biofilms would become a very real prospect. Summary The fundamental steps in the development of oral biofilms are the adhesion of bacteria present in saliva to hard or soft tissues, followed by the growth of attached cells. Attachment and colonization are influenced by several other processes including coadhesion, cell cell communication and the development of a biofilm matrix. Ultimately, disease arises from the production of acid by cariogenic bacteria (in the case of dental caries) or from the triggering of an immune response that leads to gingivitis and or periodontitis. There is an urgent need for improved measures to control dental plaque-related diseases. The development of new treatments will be greatly enhanced by a detailed understanding, at the molecular level, of the processes that lead to the formation of dental plaque. An idea of the complexity of the microbiota of the human mouth can be gleaned from analysis of the gut microflora, which contains similar numbers of bacterial species to the oral cavity. A recent large-scale metagenomic analysis indicates that each individual carries > prevalent bacterial or archaeal genes in the gut (Qin et al, 2010). Similar metagenomic analyses of the oral microflora are in progress. Such studies will greatly enhance the rate at which new genes are discovered and characterized. It is hoped that the subsequent translation of these discoveries into new clinically useful agents will, in due course, revolutionize our approach to controlling oral diseases. Acknowledgements We are very grateful to Howard Jenkinson, Lindsay Dutton and Saravanan Periasamy for generously providing images. References Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005). Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: Aas JA, Griffen AL, Dardis SR et al (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46: Bamford CV, d Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF (2009). Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun 77: Banas JA, Vickerman MM (2003). Glucan-binding proteins of the oral streptococci. Crit Rev Oral Biol Med 14: Bjarnsholt T, Givskov M (2007). Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Philos Trans R Soc Lond B Biol Sci 362: Bowden GH, Li YH (1997). Nutritional influences on biofilm development. Adv Dent Res 11: Brackman G, Celen S, Baruah K et al (2009). AI-2 quorumsensing inhibitors affect the starvation response and reduce virulence in several Vibrio species, most likely by interfering with LuxPQ. Microbiology 155: Bradshaw DJ, Homer KA, Marsh PD, Beighton D (1994). Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140: Brown SA, Whiteley M (2007). A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J Bacteriol 189: Campagna SR, Gooding JR, May AL (2009). Direct quantitation of the quorum sensing signal, autoinducer-2, in clinically relevant samples by liquid chromatography-tandem mass spectrometry. Anal Chem 81: Chalmers NI, Palmer RJ Jr, Cisar JO, Kolenbrander PE (2008). Characterization of a Streptococcus sp.-veillonella sp. community micromanipulated from dental plaque. J Bacteriol 190: Colombo AP, Boches SK, Cotton SL et al (2009). Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol 80: Daep CA, Lamont RJ, Demuth DR (2008). Interaction of Porphyromonas gingivalis with oral streptococci requires a motif that resembles the eukaryotic nuclear receptor box protein-protein interaction domain. Infect Immun 76: Diaz PI, Chalmers NI, Rickard AH et al (2006). Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol 72: Dige I, Nyengaard JR, Kilian M, Nyvad B (2009). Application of stereological principles for quantification of bacteria in intact dental biofilms. Oral Microbiol Immunol 24: Egland PG, Duˆ LD, Kolenbrander PE (2001). Identification of independent Streptococcus gordonii SspA and SspB functions in coaggregation with Actinomyces naeslundii. Infect Immun 69: Egland PG, Palmer RJ Jr, Kolenbrander PE (2004). Interspecies communication in Streptococcus gordonii-veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA 101: Guiton PS, Hung CS, Kline KA et al (2009). Contribution of autolysin and sortase A during Enterococcus faecalis DNAdependent biofilm development. Infect Immun 77: Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW (2005). Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res 84: Hamilton IR, Ng SKC (1983). Stimulation of glycolysis through lactate consumption in a resting cell mixture of Streptococcus salivarius and Veillonella parvula. FEMS Microbiol Lett 20: Heddle C, Nobbs AH, Jakubovics NS et al (2003). Host collagen signal induces antigen I II adhesin and invasin gene expression in oral Streptococcus gordonii. Mol Microbiol 50: Holmberg K, Hallander HO (1973). Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol 18: Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR (2007). Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol 73:
10 738 Itoh Y, Wang X, Hinnebusch BJ, Preston JF III, Romeo T (2005). Depolymerization of b-1,6-n-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 187: Jakubovics NS, Kerrigan SW, Nobbs AH et al (2005a). Functions of cell surface-anchored antigen I II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors. Infect Immun 73: Jakubovics NS, Stro mberg N, van Dolleweerd CJ, Kelly CG, Jenkinson HF (2005b). Differential binding specificities of oral streptococcal antigen I II family adhesins for human or bacterial ligands. Mol Microbiol 55: Jakubovics NS, Gill SR, Iobst SE, Vickerman MM, Kolenbrander PE (2008a). Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J Bacteriol 190: Jakubovics NS, Gill SR, Vickerman MM, Kolenbrander PE (2008b). Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol Ecol 66: Jakubovics NS, Brittan JL, Dutton LC, Jenkinson HF (2009). Multiple adhesin proteins on the cell surface of Streptococcus gordonii are involved in adhesion to human fibronectin. Microbiology 155: Johnson BP, Jensen BJ, Ransom EM et al (2009). Interspecies signaling between Veillonella atypica and Streptococcus gordonii requires the transcription factor CcpA. J Bacteriol 191: Kaplan JB, Ragunath C, Ramasubbu N, Fine DH (2003). Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous b-hexosaminidase activity. J Bacteriol 185: Kaplan CW, Lux R, Haake SK, Shi W (2009). The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol 71: Kolenbrander PE, Palmer RJ Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI (2006). Bacterial interactions and successions during plaque development. Periodontol : Kreth J, Merritt J, Zhu L, Shi W, Qi F (2006). Cell densityand ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol Lett 265: Kreth J, Vu H, Zhang Y, Herzberg MC (2009). Characterization of hydrogen peroxide-induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J Bacteriol 191: Lamont RJ, El-Sabaeny A, Park Y, Cook GS, Costerton JW, Demuth DR (2002). Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology 148: Lappann M, Claus H, van Alen T et al (2010). A dual role of extracellular DNA during biofilm formation of Neisseria meningitidis. Mol Microbiol 75: Lehner T, Russell MW, Caldwell J (1980). Immunisation with a purified protein from Streptococcus mutans against dental caries in rhesus monkeys. Lancet 1: Li Y, Jin J, Yang Y, Bian Z, Chen Z, Fan M (2009). Enhanced immunogenicity of an anti-caries vaccine encoding a cellsurface protein antigen of Streptococcus mutans by intranasal DNA prime-protein boost immunization. J Gene Med 11: Liljemark WF, Bloomquist CG, Germaine GR (1981). Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun 31: Liljemark WF, Bloomquist CG, Reilly BE et al (1997). Growth dynamics in a natural biofilm and its impact on oral disease management. Adv Dent Res 11: Lowery CA, Abe T, Park J et al (2009). Revisiting AI-2 quorum sensing inhibitors: direct comparison of alkyl-dpd analogues and a natural product fimbrolide. J Am Chem Soc 131: Marsh PD (2006). Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health 6(Suppl 1): S14. Martins M, Uppuluri P, Thomas DP et al (2009). Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia In Press (DOI /s y). Merritt J, Zheng L, Shi W, Qi F (2007). Genetic characterization of the hdrrm operon: a novel high-cell-densityresponsive regulator in Streptococcus mutans. Microbiology 153: Merritt J, Niu G, Okinaga T, Qi F (2009). Autoaggregation response of Fusobacterium nucleatum. Appl Environ Microbiol 75: Munro C, Michalek SM, Macrina FL (1991). Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun 59: Njoroge J, Sperandio V (2009). Jamming bacterial communication: new approaches for the treatment of infectious diseases. EMBO Mol Med 1: Nobbs AH, Lamont RJ, Jenkinson HF (2009). Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73: Nyvad B, Kilian M (1987). Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res 95: Nyvad B, Kilian M (1990). Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res 24: Okinaga T, Niu G, Xie Z, Qi F, Merritt J (2010). The hdrrm operon of Streptococcus mutans encodes a novel regulatory system for coordinated competence development and bacteriocin production. J Bacteriol 192: Palmer RJ Jr, Diaz PI, Kolenbrander PE (2006). Rapid succession within the Veillonella population of a developing human oral biofilm in situ. J Bacteriol 188: Pathirana RD, O Brien-Simpson NM, Reynolds EC (2010). Host immune responses to Porphyromonas gingivalis antigens. Periodontol : Periasamy S, Kolenbrander PE (2009a). Aggregatibacter actinomycetemcomitans builds mutualistic biofilm communities with Fusobacterium nucleatum and Veillonella species in saliva. Infect Immun 77: Periasamy S, Kolenbrander PE (2009b). Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol 191: Periasamy S, Kolenbrander PE (2010). Central role of early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle and late colonizers of enamel. J Bacteriol In Press (doi: /jb ). Periasamy S, Chalmers NI, Duˆ -Thumm L, Kolenbrander PE (2009). Fusobacterium nucleatum ATCC requires Actinomyces naeslundii ATCC for growth on saliva in a three-species community that includes Streptococcus oralis 34. Appl Environ Microbiol 75:
VIRULENCE SCHEME OVERVIEW
 VIRULENCE SCHEME OVERVIEW Sucrose Stronger ADHESION; Greater Accumulation Bacterial factors that promote biofilm development ADHESION ACIDOGENICITY Fermentable Carbohydrates Selection for S. mutans and
VIRULENCE SCHEME OVERVIEW Sucrose Stronger ADHESION; Greater Accumulation Bacterial factors that promote biofilm development ADHESION ACIDOGENICITY Fermentable Carbohydrates Selection for S. mutans and
Oral Health Applications for Probiotics
 Oral Health Applications for Probiotics Andrew McBain Biofilm Research Group Manchester Pharmacy School University of Manchester Overview Oral microbiology introduction Dental probiotics and replacement
Oral Health Applications for Probiotics Andrew McBain Biofilm Research Group Manchester Pharmacy School University of Manchester Overview Oral microbiology introduction Dental probiotics and replacement
Interspecies Communication In Oral Biofilm
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 6 Ver. II (Jun. 2015), PP 65-69 www.iosrjournals.org Interspecies Communication In Oral Biofilm
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 6 Ver. II (Jun. 2015), PP 65-69 www.iosrjournals.org Interspecies Communication In Oral Biofilm
Prophylactic effect of yogurt on Fusobacterium nucleatum in the mouth. Brigham Yang
 Prophylactic effect of yogurt on Fusobacterium nucleatum in the mouth Brigham Yang BIOL 494 Mentor: Dr. R. Shane Gold 8 th April 2014 Abstract Periodontal diseases are closely related to the activity of
Prophylactic effect of yogurt on Fusobacterium nucleatum in the mouth Brigham Yang BIOL 494 Mentor: Dr. R. Shane Gold 8 th April 2014 Abstract Periodontal diseases are closely related to the activity of
Dental plaque. Lectuer (4) Dr. Baha, H.AL-Amiedi Ph.D.Microbiology
 Dental plaque Lectuer (4) Dr. Baha, H.AL-Amiedi Ph.D.Microbiology it is now well established that caries & periodontal disease are infectious disease associated with resident microorganisms of dental plaque
Dental plaque Lectuer (4) Dr. Baha, H.AL-Amiedi Ph.D.Microbiology it is now well established that caries & periodontal disease are infectious disease associated with resident microorganisms of dental plaque
Microbiota and Oral Disease Prof. Dennis Cvitkovitch
 1 Professor Dennis Cvitkovitch Faculty of Dentistry Dental Research Institute University of Toronto The human microbiome We are a composite species: eukaryotic, bacterial, archeal Every human harbors over
1 Professor Dennis Cvitkovitch Faculty of Dentistry Dental Research Institute University of Toronto The human microbiome We are a composite species: eukaryotic, bacterial, archeal Every human harbors over
Interspecies Interactions within Oral Microbial Communities
 MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Dec. 2007, p. 653 670 Vol. 71, No. 4 1092-2172/07/$08.00 0 doi:10.1128/mmbr.00024-07 Copyright 2007, American Society for Microbiology. All Rights Reserved.
MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Dec. 2007, p. 653 670 Vol. 71, No. 4 1092-2172/07/$08.00 0 doi:10.1128/mmbr.00024-07 Copyright 2007, American Society for Microbiology. All Rights Reserved.
Mutualistic Biofilm Communities Develop with Porphyromonas gingivalis and Initial, Early, and Late Colonizers of Enamel
 JOURNAL OF BACTERIOLOGY, Nov. 2009, p. 6804 6811 Vol. 191, No. 22 0021-9193/09/$12.00 doi:10.1128/jb.01006-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Mutualistic Biofilm
JOURNAL OF BACTERIOLOGY, Nov. 2009, p. 6804 6811 Vol. 191, No. 22 0021-9193/09/$12.00 doi:10.1128/jb.01006-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Mutualistic Biofilm
Microbial biofilms are
 Managing the complexity of a dynamic biofilm John G. Thomas, MS, PhD; Lindsay A. Nakaishi, BS Microbial biofilms are common in nature. Virtually any fluid environment in which microorganisms are subject
Managing the complexity of a dynamic biofilm John G. Thomas, MS, PhD; Lindsay A. Nakaishi, BS Microbial biofilms are common in nature. Virtually any fluid environment in which microorganisms are subject
Periodontal Microbiology (Dental Plaque)
 Periodontal Microbiology (Dental Plaque) Dental plaque Dental plaque is soft deposits that form the biofilm adhering to the tooth surface of other hard surface in the oral cavity, including removable and
Periodontal Microbiology (Dental Plaque) Dental plaque Dental plaque is soft deposits that form the biofilm adhering to the tooth surface of other hard surface in the oral cavity, including removable and
THE EFFECT OF CIGARETTE SMOKING ON THE VIRULENCE OF STREPTOCOCCUS MUTANS CARIES AND CARDIOVASCULAR
 THE EFFECT OF CIGARETTE SMOKING ON THE VIRULENCE OF STREPTOCOCCUS MUTANS CARIES AND CARDIOVASCULAR DISEASES-EPIDEMIOLOGICAL ANALYSIS AND IN VITRO STUDIES Cunge Zheng Submitted to the faculty of the University
THE EFFECT OF CIGARETTE SMOKING ON THE VIRULENCE OF STREPTOCOCCUS MUTANS CARIES AND CARDIOVASCULAR DISEASES-EPIDEMIOLOGICAL ANALYSIS AND IN VITRO STUDIES Cunge Zheng Submitted to the faculty of the University
Evidence Based Management of Dental Caries
 Evidence Based Management of Dental Caries Understanding the etiology of dental caries Dr. Wenyuan Shi Professor and Chair, Oral Biology, UCLA SOD Professor, Microbiology & Immunology, UCLA SOM Disruptive
Evidence Based Management of Dental Caries Understanding the etiology of dental caries Dr. Wenyuan Shi Professor and Chair, Oral Biology, UCLA SOD Professor, Microbiology & Immunology, UCLA SOM Disruptive
Extracellular Polysaccharides Matrix An Often Forgotten Virulence Factor in Oral Biofilm Research
 http://www.ijos.org.cn Koo et al. Extracellular Polysaccharides Matrix doi: 10.4248/IJOS.09086 LETTER TO EDITOR Extracellular Polysaccharides Matrix An Often Forgotten Virulence Factor in Oral Biofilm
http://www.ijos.org.cn Koo et al. Extracellular Polysaccharides Matrix doi: 10.4248/IJOS.09086 LETTER TO EDITOR Extracellular Polysaccharides Matrix An Often Forgotten Virulence Factor in Oral Biofilm
Molecular Principles of Adhesion and Biofilm Formation
 Molecular Principles of Adhesion and Biofilm Formation Jens Kreth and Mark C. Herzberg Abstract Oral bacteria are responsible for oral health and disease, including caries, periodontal disease, and endodontic
Molecular Principles of Adhesion and Biofilm Formation Jens Kreth and Mark C. Herzberg Abstract Oral bacteria are responsible for oral health and disease, including caries, periodontal disease, and endodontic
Biofilm Journal Biofilm, Volume 2, Paper 1 (BF97001) 1997 Online Journal - URL:
 http://www.bioline.org.br/request?bf97001 JOURNALS REPORTS NEWSLETTERS BOOKS registration prices about help Biofilm Journal Biofilm, Volume 2, Paper 1 (BF97001) 1997 Online Journal - URL: http://bioline.bdt.org.br/bf
http://www.bioline.org.br/request?bf97001 JOURNALS REPORTS NEWSLETTERS BOOKS registration prices about help Biofilm Journal Biofilm, Volume 2, Paper 1 (BF97001) 1997 Online Journal - URL: http://bioline.bdt.org.br/bf
EFFECT OF NICOTINE ON BIOFILM FORMATION OF STREPTOCOCCUS MUTANS ISOLATES FROM SMOKING VERSUS
 i EFFECT OF NICOTINE ON BIOFILM FORMATION OF STREPTOCOCCUS MUTANS ISOLATES FROM SMOKING VERSUS NON-SMOKING SUBJECTS by Nasreen Farouk El-ezmerli Submitted to the Graduate Faculty of the School of Dentistry
i EFFECT OF NICOTINE ON BIOFILM FORMATION OF STREPTOCOCCUS MUTANS ISOLATES FROM SMOKING VERSUS NON-SMOKING SUBJECTS by Nasreen Farouk El-ezmerli Submitted to the Graduate Faculty of the School of Dentistry
DEPOSITS. Dentalelle Tutoring 1
 DEPOSITS Dentalelle Tutoring WWW.DENTALELLE.COM 1 PH SCALE WWW.DENTALELLE.COM 2 DENTAL CARIES Dental caries is a dynamic process that involves a susceptible tooth, cariogenic bacteria in dental plaque
DEPOSITS Dentalelle Tutoring WWW.DENTALELLE.COM 1 PH SCALE WWW.DENTALELLE.COM 2 DENTAL CARIES Dental caries is a dynamic process that involves a susceptible tooth, cariogenic bacteria in dental plaque
Overview. Dental biofilm bacteria. Biofilm. Multi-species dental biofilms. Biofilm work group meeting Marts 15th Irene Dige
 Overview Multi-species dental biofilms Introduction to oral and dental biofilms Previous studies of dental biofilms Recent studies of multispecies dental biofilms Methodological considerations Irene Dige
Overview Multi-species dental biofilms Introduction to oral and dental biofilms Previous studies of dental biofilms Recent studies of multispecies dental biofilms Methodological considerations Irene Dige
Communication among Oral Bacteria
 MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Sept. 2002, p. 486 505 Vol. 66, No. 3 1092-2172/02/$04.00 0 DOI: 10.1128/MMBR.66.3.486 505.2002 Communication among Oral Bacteria Paul E. Kolenbrander,* Roxanna
MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Sept. 2002, p. 486 505 Vol. 66, No. 3 1092-2172/02/$04.00 0 DOI: 10.1128/MMBR.66.3.486 505.2002 Communication among Oral Bacteria Paul E. Kolenbrander,* Roxanna
Normal Human Flora. (Human Microbiome) Dr.Sarmad M.H. Zeiny Baghdad College of Medicine
 Normal Human Flora (Human Microbiome) Dr.Sarmad M.H. Zeiny Baghdad College of Medicine 2014-2015 Objectives Describe important human normal flora. Demonstrate the epidemiology of human normal flora. Determine
Normal Human Flora (Human Microbiome) Dr.Sarmad M.H. Zeiny Baghdad College of Medicine 2014-2015 Objectives Describe important human normal flora. Demonstrate the epidemiology of human normal flora. Determine
Research and Reviews: Journal of Dental Sciences
 Research and Reviews: Journal of Dental Sciences A Review on the Human Oral Microflora Sowmya Y * Department of Microbiology, Andhra University, Visakhapatnam, Andhra Pradesh, India Review Article Received:
Research and Reviews: Journal of Dental Sciences A Review on the Human Oral Microflora Sowmya Y * Department of Microbiology, Andhra University, Visakhapatnam, Andhra Pradesh, India Review Article Received:
DHR International Journal Of Medical Sciences (DHR-IJMS) ISSN: X, Vol. 3(1), 2012 Available online
 DHR International Journal Of Medical Sciences (DHR-IJMS) ISSN: 2278-831X, Vol. 3(1), 2012 Available online http://www.doublehelixresearch.com/dhrijms Double Helix Research Oral probiotics: the beneficial
DHR International Journal Of Medical Sciences (DHR-IJMS) ISSN: 2278-831X, Vol. 3(1), 2012 Available online http://www.doublehelixresearch.com/dhrijms Double Helix Research Oral probiotics: the beneficial
A periodontitis-associated multispecies model of an oral biofilm
 A periodontitis-associated multispecies model of an oral biofilm Jong Hwa Park 1, Jae-Kwan Lee 1, Heung-Sik Um 1, Beom-Seok Chang 1, *, Si-Young Lee 2 1 Department of Periodontology, Research Institute
A periodontitis-associated multispecies model of an oral biofilm Jong Hwa Park 1, Jae-Kwan Lee 1, Heung-Sik Um 1, Beom-Seok Chang 1, *, Si-Young Lee 2 1 Department of Periodontology, Research Institute
Interbacterial Adherence Between Actinomyces viscosus and Strains
 INFECTION AND IMMUNITY, Apr. 1984, p. 86-90 0019-9567/84/040086-05$02.00/0 Copyright 1984, American Society for Microbiology Vol. 44, No. 1 Interbacterial Adherence Between Actinomyces viscosus and Strains
INFECTION AND IMMUNITY, Apr. 1984, p. 86-90 0019-9567/84/040086-05$02.00/0 Copyright 1984, American Society for Microbiology Vol. 44, No. 1 Interbacterial Adherence Between Actinomyces viscosus and Strains
Bacterial composition in the supragingival plaques of children with and without dental caries
 182 Ro kiewicz D, et al. Advances in Medical Sciences Vol. 51 2006 Suppl. 1 Bacterial composition in the supragingival plaques of children with and without dental caries Ro kiewicz D 1, Daniluk T 2, Zaremba
182 Ro kiewicz D, et al. Advances in Medical Sciences Vol. 51 2006 Suppl. 1 Bacterial composition in the supragingival plaques of children with and without dental caries Ro kiewicz D 1, Daniluk T 2, Zaremba
Hundreds of bacterial species populate the body. The oral cavity provides a unique environment
 1.0 Introduction Hundreds of bacterial species populate the body. The oral cavity provides a unique environment that supports a wide range of bacterial species. The highly diverse flora grows in the different
1.0 Introduction Hundreds of bacterial species populate the body. The oral cavity provides a unique environment that supports a wide range of bacterial species. The highly diverse flora grows in the different
Soeherwin Mangundjaja., Abdul Muthalib., Ariadna Djais Department of Oral Biology Faculty of Dentistry Universitas Indonesia
 THE EFFECT OF DENTIFRICE CONTAINING ENZYME ON SALIVARY MUTANS STREPTOCOCCAL LEVEL IN ORTHODONTIC PATIENTS Soeherwin Mangundjaja., Abdul Muthalib., Ariadna Djais Department of Oral Biology Faculty of Dentistry
THE EFFECT OF DENTIFRICE CONTAINING ENZYME ON SALIVARY MUTANS STREPTOCOCCAL LEVEL IN ORTHODONTIC PATIENTS Soeherwin Mangundjaja., Abdul Muthalib., Ariadna Djais Department of Oral Biology Faculty of Dentistry
Bacterial Plaque and Its Relation to Dental Diseases. As a hygienist it is important to stress the importance of good oral hygiene and
 Melissa Rudzinski Preventive Dentistry Shaunda Clark November 2013 Bacterial Plaque and Its Relation to Dental Diseases As a hygienist it is important to stress the importance of good oral hygiene and
Melissa Rudzinski Preventive Dentistry Shaunda Clark November 2013 Bacterial Plaque and Its Relation to Dental Diseases As a hygienist it is important to stress the importance of good oral hygiene and
Comparative Evaluation of 0.2 percent Chlorhexidine and Magnetized Water as a Mouth Rinse on Streptococcus mutans in Children
 10.5005/jp-journals-10005-1108 Nidhi ORIGINAL Gupta, Manohar ARTICLE Bhat Comparative Evaluation of 0.2 percent Chlorhexidine and Magnetized Water as a Mouth Rinse on Streptococcus mutans in Children 1
10.5005/jp-journals-10005-1108 Nidhi ORIGINAL Gupta, Manohar ARTICLE Bhat Comparative Evaluation of 0.2 percent Chlorhexidine and Magnetized Water as a Mouth Rinse on Streptococcus mutans in Children 1
The effect of Piper betle and Psidium guajava extracts on the cell-surface hydrophobicity of selected early settlers of dental plaque
 71 Journal of Oral Science, Vol. 48, No. 2, 71-75, 2006 Original The effect of Piper betle and Psidium guajava extracts on the cell-surface hydrophobicity of selected early settlers of dental plaque Fathilah
71 Journal of Oral Science, Vol. 48, No. 2, 71-75, 2006 Original The effect of Piper betle and Psidium guajava extracts on the cell-surface hydrophobicity of selected early settlers of dental plaque Fathilah
Oral bacterial interactions in periodontal health and disease
 Vol. 6(5), pp. 51-57, August 2014 DOI: 10.5897/JDOH2014.0127 Article Number: 84910BB46956 ISSN 2006-9871 Copyright 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/jdoh
Vol. 6(5), pp. 51-57, August 2014 DOI: 10.5897/JDOH2014.0127 Article Number: 84910BB46956 ISSN 2006-9871 Copyright 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/jdoh
Dental biofilms: difficult therapeutic targets
 Periodontology 2000, Vol. 28, 2002, 12 55 Copyright C Munksgaard 2002 Printed in Denmark All rights reserved PERIODONTOLOGY 2000 ISSN 0906-6713 Dental biofilms: difficult therapeutic targets SIGMUND S.
Periodontology 2000, Vol. 28, 2002, 12 55 Copyright C Munksgaard 2002 Printed in Denmark All rights reserved PERIODONTOLOGY 2000 ISSN 0906-6713 Dental biofilms: difficult therapeutic targets SIGMUND S.
Bacterial Mechanisms of Pathogenicity. 2 nd Lecture
 Bacterial Mechanisms of Pathogenicity 2 nd Lecture Preferred Portal of Entry Just because a pathogen enters your body it does not mean it s going to cause disease. pathogens - preferred portal of entry
Bacterial Mechanisms of Pathogenicity 2 nd Lecture Preferred Portal of Entry Just because a pathogen enters your body it does not mean it s going to cause disease. pathogens - preferred portal of entry
The virulence of Streptococcus mutans and the ability to form biofilms
 Eur J Clin Microbiol Infect Dis (2014) 33:499 515 DOI 10.1007/s10096-013-1993-7 REVIEW The virulence of Streptococcus mutans and the ability to form biofilms W. Krzyściak & A. Jurczak & D. Kościelniak
Eur J Clin Microbiol Infect Dis (2014) 33:499 515 DOI 10.1007/s10096-013-1993-7 REVIEW The virulence of Streptococcus mutans and the ability to form biofilms W. Krzyściak & A. Jurczak & D. Kościelniak
Saliva. Introduction. Salivary Flow. Saliva and the Plaque Biofilm. The Minerals in Saliva
 Saliva Introduction Saliva is like a bloodstream to the mouth. As does blood, saliva helps build and maintain the health of the soft and hard tissues. Saliva removes waste products and provides disease-fighting
Saliva Introduction Saliva is like a bloodstream to the mouth. As does blood, saliva helps build and maintain the health of the soft and hard tissues. Saliva removes waste products and provides disease-fighting
Linking Research to Clinical Practice
 Probiotics and Oral Health Denise M. Bowen, RDH, MS Linking Research to Clinical Practice The purpose of Linking Research to Clinical Practice is to present evidence based information to clinical dental
Probiotics and Oral Health Denise M. Bowen, RDH, MS Linking Research to Clinical Practice The purpose of Linking Research to Clinical Practice is to present evidence based information to clinical dental
Bio Microbiology - Spring 2013 Study Guide 20 Normal Flora - Normal Responses - Disease
 Bio 230 - Microbiology - Spring 2013 Study Guide 20 Normal Flora - Normal Responses - Disease It has been calculated that the normal human houses about 10 12 bacteria on the skin, 10 10 in the mouth, and
Bio 230 - Microbiology - Spring 2013 Study Guide 20 Normal Flora - Normal Responses - Disease It has been calculated that the normal human houses about 10 12 bacteria on the skin, 10 10 in the mouth, and
LANTIBIOTIC SMB: CHARACTERIZATION OF THE IMMUNITY PROTEIN, IDENTIFICATION OF A NOVEL RECEPTOR-LIKE PROTEIN, AND A NEW PERSPECTIVE ON REGULATION
 LANTIBIOTIC SMB: CHARACTERIZATION OF THE IMMUNITY PROTEIN, IDENTIFICATION OF A NOVEL RECEPTOR-LIKE PROTEIN, AND A NEW PERSPECTIVE ON REGULATION By SASWATI BISWAS March 13, 2014 Submitted to the graduate
LANTIBIOTIC SMB: CHARACTERIZATION OF THE IMMUNITY PROTEIN, IDENTIFICATION OF A NOVEL RECEPTOR-LIKE PROTEIN, AND A NEW PERSPECTIVE ON REGULATION By SASWATI BISWAS March 13, 2014 Submitted to the graduate
PATHOGENICITY OF MICROORGANISMS
 PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
BACTERIAL PATHOGENESIS
 BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
actinomycetemcornitans and
 Periodontology 2000, Vol. 20, 1999, 341-362 Printed in Denmark. All rights reserved Copyright 8 Munksgaard 1999 PERIODONTOLOGY 2000 ISSN 0906-6713 Ecological considerations in the treatment of Actinobacillus
Periodontology 2000, Vol. 20, 1999, 341-362 Printed in Denmark. All rights reserved Copyright 8 Munksgaard 1999 PERIODONTOLOGY 2000 ISSN 0906-6713 Ecological considerations in the treatment of Actinobacillus
Competition and Coexistence between Streptococcus mutans and Streptococcus sanguinis in the Dental Biofilm
 JOURNAL OF BACTERIOLOGY, Nov. 2005, p. 7193 7203 Vol. 187, No. 21 0021-9193/05/$08.00 0 doi:10.1128/jb.187.21.7193 7203.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Competition
JOURNAL OF BACTERIOLOGY, Nov. 2005, p. 7193 7203 Vol. 187, No. 21 0021-9193/05/$08.00 0 doi:10.1128/jb.187.21.7193 7203.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Competition
The Effect of Anchovy Stelophorus commersonii on Salivary Mutans Streptococci.
 The Effect of Anchovy Stelophorus commersonii on Salivary Mutans Streptococci. Mangundjaja S., Djais A., Harun AG Department of Oral Biology Faculty of Dentistry Universitas Indonesia Jakarta Indonesia
The Effect of Anchovy Stelophorus commersonii on Salivary Mutans Streptococci. Mangundjaja S., Djais A., Harun AG Department of Oral Biology Faculty of Dentistry Universitas Indonesia Jakarta Indonesia
Microbes as Agents of Infectious Disease
 Microbes as Agents of Infectious Disease Normal Flora Virulence and Pathogenicity Toxicity vs. Invasiveness WE ARE NOT ALONE! We are outnumbered. The average human contains about 10 trillion cells. On
Microbes as Agents of Infectious Disease Normal Flora Virulence and Pathogenicity Toxicity vs. Invasiveness WE ARE NOT ALONE! We are outnumbered. The average human contains about 10 trillion cells. On
Can an ecological approach control or prevent dental disease? P. D. Marsh
 Can an ecological approach control or prevent dental disease? P. D. Marsh Health Protection Agency, Centre for Emergency Preparedness & Response (CEPR), Salisbury, & Leeds Dental Institute. Can an ecological
Can an ecological approach control or prevent dental disease? P. D. Marsh Health Protection Agency, Centre for Emergency Preparedness & Response (CEPR), Salisbury, & Leeds Dental Institute. Can an ecological
Acquired Enamel Pellicle (AEP) - A Film with New Futuristic, Diagnostic and Therapeutic Perspectives.
 RESEARCH AND REVIEWS: JOURNAL OF DENTAL SCIENCES Acquired Enamel Pellicle (AEP) - A Film with New Futuristic, Diagnostic and Therapeutic Perspectives. Spiti Datta*, and Devaraj CG. Department of Periodontology
RESEARCH AND REVIEWS: JOURNAL OF DENTAL SCIENCES Acquired Enamel Pellicle (AEP) - A Film with New Futuristic, Diagnostic and Therapeutic Perspectives. Spiti Datta*, and Devaraj CG. Department of Periodontology
Microbiota Transplantation Workshop: Oral Cavity
 Microbiota Transplantation Workshop: Oral Cavity December 3, 2015 Floyd E. Dewhirst, DDS, PhD Department of Microbiology The Forsyth Institute Outline Microbiota of the oral cavity Diseases of oral dysbiosis
Microbiota Transplantation Workshop: Oral Cavity December 3, 2015 Floyd E. Dewhirst, DDS, PhD Department of Microbiology The Forsyth Institute Outline Microbiota of the oral cavity Diseases of oral dysbiosis
Controlling the oral biofilm with antimicrobials
 journal of dentistry 38, S1 (2010) S11 S15 available at www.sciencedirect.com journal homepage: www.intl.elsevierhealth.com/journals/jden Controlling the oral biofilm with antimicrobials P.D. Marsh* Department
journal of dentistry 38, S1 (2010) S11 S15 available at www.sciencedirect.com journal homepage: www.intl.elsevierhealth.com/journals/jden Controlling the oral biofilm with antimicrobials P.D. Marsh* Department
Microbiology - Problem Drill 21: Microbial Diseases of the Digestive System
 Microbiology - Problem Drill 21: Microbial Diseases of the Digestive System No. 1 of 10 1. Which of the following organs is not part of the gastrointestinal tract (GI)? (A) Esophagus (B) Small intestine
Microbiology - Problem Drill 21: Microbial Diseases of the Digestive System No. 1 of 10 1. Which of the following organs is not part of the gastrointestinal tract (GI)? (A) Esophagus (B) Small intestine
ROLE OF PROBIOTICS IN ORAL HEALTH: A REVIEW
 Review Article International Journal of Dental and Health Sciences Volume 03,Issue 02 ROLE OF PROBIOTICS IN ORAL HEALTH: A REVIEW Yadav Pramod Kumar 1, Mujeeb Juhi 2, Hasan Junaidul 3, Neelofar 4 1.Assistant
Review Article International Journal of Dental and Health Sciences Volume 03,Issue 02 ROLE OF PROBIOTICS IN ORAL HEALTH: A REVIEW Yadav Pramod Kumar 1, Mujeeb Juhi 2, Hasan Junaidul 3, Neelofar 4 1.Assistant
ORAL HEALTH COMPLICATIONS?
 ORAL HEALTH COMPLICATIONS? Your pathway to a better oral health ORAL HEALTH AND PERIODONTAL (GUM) DISEASE Dental cavities are a multifactorial disease of bacterial origin that is characterized by acid
ORAL HEALTH COMPLICATIONS? Your pathway to a better oral health ORAL HEALTH AND PERIODONTAL (GUM) DISEASE Dental cavities are a multifactorial disease of bacterial origin that is characterized by acid
Chapter 14 Outline. Chapter 14: Hygiene-Related Oral Disorders. Dental Caries. Dental Caries. Prevention. Hygiene-Related Oral Disorders
 Chapter 14 Outline Chapter 14: Hygiene-Related Oral Disorders Hygiene-Related Oral Disorders Dental caries Prevention Gingivitis Prevention Tooth hypersensitivity Pathophysiology Treatment 2 Hygiene-Related
Chapter 14 Outline Chapter 14: Hygiene-Related Oral Disorders Hygiene-Related Oral Disorders Dental caries Prevention Gingivitis Prevention Tooth hypersensitivity Pathophysiology Treatment 2 Hygiene-Related
Dental biofilm: ecological interactions in health and disease
 J Clin Periodontol 2017; 44 (Suppl. 18): S12 S22 doi: 10.1111/jcpe.12679 Dental biofilm: ecological interactions in health and disease P. D. Marsh 1 and Egija Zaura 2 1 Department of Oral Biology, School
J Clin Periodontol 2017; 44 (Suppl. 18): S12 S22 doi: 10.1111/jcpe.12679 Dental biofilm: ecological interactions in health and disease P. D. Marsh 1 and Egija Zaura 2 1 Department of Oral Biology, School
Beyond Mucosal Infection: a Role for C. albicans-streptococcal Interactions in the Pathogenesis of Dental Caries
 Curr Oral Health Rep (2014) 1:86 93 DOI 10.1007/s40496-013-0011-6 MICROBIOLOGY (H KOO, SECTION EDITOR) Beyond Mucosal Infection: a Role for C. albicans-streptococcal Interactions in the Pathogenesis of
Curr Oral Health Rep (2014) 1:86 93 DOI 10.1007/s40496-013-0011-6 MICROBIOLOGY (H KOO, SECTION EDITOR) Beyond Mucosal Infection: a Role for C. albicans-streptococcal Interactions in the Pathogenesis of
Determination of a Second Substitution, [alpha]-glycerophosphate, Common to C311 Pilins
![Determination of a Second Substitution, [alpha]-glycerophosphate, Common to C311 Pilins Determination of a Second Substitution, [alpha]-glycerophosphate, Common to C311 Pilins](/thumbs/82/86025176.jpg) Non-S-Layer Glycoproteins: A Review Introduction p. 1 Flagellar Glycoproteins p. 2 Bacteria p. 2 Archaeal Flagella p. 5 Glycosylated Flagellar Proteins: What Are the Sugar Chains For? p. 7 Glycoproteins
Non-S-Layer Glycoproteins: A Review Introduction p. 1 Flagellar Glycoproteins p. 2 Bacteria p. 2 Archaeal Flagella p. 5 Glycosylated Flagellar Proteins: What Are the Sugar Chains For? p. 7 Glycoproteins
The Streptococci. Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens
 The Streptococci Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens Strong fermenters Facultative anaerobes Non-motile Catalase Negative 1 Classification 1 2 Classification
The Streptococci Diverse collection of cocci. Gram-positive Chains or pairs significant pathogens Strong fermenters Facultative anaerobes Non-motile Catalase Negative 1 Classification 1 2 Classification
Biomarkers into saliva
 Biomarkers into saliva Saliva used as biomarker for risk for caries Cario-Analyse saliva testing strategies Saliva composition? Saliva comes from blood serum associated with oral microflora and viruses
Biomarkers into saliva Saliva used as biomarker for risk for caries Cario-Analyse saliva testing strategies Saliva composition? Saliva comes from blood serum associated with oral microflora and viruses
Correlation of Dental Plaque Acidogenicity and Acidurance with Caries Activity Perspectives of the Ecological Plaque Hypothesis
 DOI 10.7603/s40782-014-0009-6 GSTF International Journal of Advances in Medical Research (JAMR) Vol.1 No.1, May 2014 Correlation of Dental Plaque Acidogenicity and Acidurance with Caries Activity Perspectives
DOI 10.7603/s40782-014-0009-6 GSTF International Journal of Advances in Medical Research (JAMR) Vol.1 No.1, May 2014 Correlation of Dental Plaque Acidogenicity and Acidurance with Caries Activity Perspectives
This is a repository copy of Dental biofilm: ecological interactions in health and disease..
 This is a repository copy of Dental biofilm: ecological interactions in health and disease.. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/113767/ Version: Accepted Version
This is a repository copy of Dental biofilm: ecological interactions in health and disease.. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/113767/ Version: Accepted Version
Fig. LPS in Gram negative bacteria
 Structure of bacterial cell Dentistry college - first class Medical biology- Lec.3 Lecturer D. Hanan S A- Cell wall ***Chemical composition of the cell wall Bacteria are divided into two separated groups
Structure of bacterial cell Dentistry college - first class Medical biology- Lec.3 Lecturer D. Hanan S A- Cell wall ***Chemical composition of the cell wall Bacteria are divided into two separated groups
More Chocolate! The FUN Biochemistry of Cavity Prevention
 More Chocolate! The FUN Biochemistry of Cavity Prevention Dr. Roger Lucas, DDS Board Diplomate of American Board of Pediatric Dentistry Biochemist Email: Roger@TheDentistDad.com Website: TheDentistDad.com
More Chocolate! The FUN Biochemistry of Cavity Prevention Dr. Roger Lucas, DDS Board Diplomate of American Board of Pediatric Dentistry Biochemist Email: Roger@TheDentistDad.com Website: TheDentistDad.com
Peptide pheromone induced cell death of Streptococcus mutans
 FEMS Microbiology Letters 251 (2005) 321 326 www.fems-microbiology.org Peptide pheromone induced cell death of Streptococcus mutans Fengxia Qi a, J. Kreth a, Celine M. Lévesque b, Olga Kay c, Richard W.
FEMS Microbiology Letters 251 (2005) 321 326 www.fems-microbiology.org Peptide pheromone induced cell death of Streptococcus mutans Fengxia Qi a, J. Kreth a, Celine M. Lévesque b, Olga Kay c, Richard W.
Microbes as Agents of Infectious Disease
 Microbes as Agents of Infectious Disease Normal Flora Virulence and Pathogenicity Toxicity vs. Invasiveness WE ARE NOT ALONE! We are outnumbered. The average human contains about 10 trillion cells. On
Microbes as Agents of Infectious Disease Normal Flora Virulence and Pathogenicity Toxicity vs. Invasiveness WE ARE NOT ALONE! We are outnumbered. The average human contains about 10 trillion cells. On
Effect of temperature change of 0.2% chlorhexidine rinse on matured human plaque: an in vivo study.
 ISSN: 2278 0211 (Online) Effect of temperature change of 0.2% chlorhexidine rinse on matured human plaque: an in vivo study. Dr. Yashika Jain Senior Lecturer, Institution: SGT Dental College & Hospital,
ISSN: 2278 0211 (Online) Effect of temperature change of 0.2% chlorhexidine rinse on matured human plaque: an in vivo study. Dr. Yashika Jain Senior Lecturer, Institution: SGT Dental College & Hospital,
Ecological Control: In Vitro Inhibition of Anaerobic Bacteria by Oral Streptococci
 æoriginal ARTICLE æ Ecological Control: In Vitro Inhibition of Anaerobic Bacteria by Oral Streptococci A. Doran 1, S. Kneist 2 and J. Verran 1 From the 1 Department of Biological Sciences, Manchester Metropolitan
æoriginal ARTICLE æ Ecological Control: In Vitro Inhibition of Anaerobic Bacteria by Oral Streptococci A. Doran 1, S. Kneist 2 and J. Verran 1 From the 1 Department of Biological Sciences, Manchester Metropolitan
37? A! 61 /to. ~7S2.6
 37? A! 61 /to. ~7S2.6 ORAL MICROBIOLOGY THESIS Presented to the Graduate Council of the University of North Texas in Partial Fulfillment of The Requirements For the Degree of MASTER OF SCIENCE By Shaiesta
37? A! 61 /to. ~7S2.6 ORAL MICROBIOLOGY THESIS Presented to the Graduate Council of the University of North Texas in Partial Fulfillment of The Requirements For the Degree of MASTER OF SCIENCE By Shaiesta
Oral Candida biofilm model and Candida Staph interactions
 Oral Candida biofilm model and Candida Staph interactions Mark Shirtliff, PhD Associate Professor Department of Microbial Pathogenesis, School of Dentistry Department of Microbiology and Immunology, School
Oral Candida biofilm model and Candida Staph interactions Mark Shirtliff, PhD Associate Professor Department of Microbial Pathogenesis, School of Dentistry Department of Microbiology and Immunology, School
The role of fructans on dental bio lm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces viscosus
 FEMS Microbiology Letters 195 (2001) 205^210 www.fems-microbiology.org The role of fructans on dental bio lm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces
FEMS Microbiology Letters 195 (2001) 205^210 www.fems-microbiology.org The role of fructans on dental bio lm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces
Biology of the Salivary Glands 513 (KEY) FINAL Examination June 26, 1997 To see correct answers, show hidden text (C) (B)
 Biology of the Salivary Glands 513 (KEY) FINAL Examination June 26, 1997 To see correct answers, show hidden text 1. With increased age, the following event does not occur in the major salivary glands:
Biology of the Salivary Glands 513 (KEY) FINAL Examination June 26, 1997 To see correct answers, show hidden text 1. With increased age, the following event does not occur in the major salivary glands:
Influence of Different Prebiotics and Probiotics on Selective Intestinal Pathogens
 ISSN: 2319-7706 Volume 3 Number 10 (2014) pp. 657-663 http://www.ijcmas.com Original Research Article Influence of Different Prebiotics and Probiotics on Selective Intestinal Pathogens Anayata Sharma 1*
ISSN: 2319-7706 Volume 3 Number 10 (2014) pp. 657-663 http://www.ijcmas.com Original Research Article Influence of Different Prebiotics and Probiotics on Selective Intestinal Pathogens Anayata Sharma 1*
Molecular Modulation of Single and Multi-species Biofilm. Formation by Orally-associated Bacteria. An honors thesis presented to the
 Molecular Modulation of Single and Multi-species Biofilm Formation by Orally-associated Bacteria An honors thesis presented to the Department of Biological Sciences University at Albany State University
Molecular Modulation of Single and Multi-species Biofilm Formation by Orally-associated Bacteria An honors thesis presented to the Department of Biological Sciences University at Albany State University
NEW. Block plaque, calculus, and halitosis with the science of prevention
 NEW Block plaque, calculus, and halitosis with the science of prevention OraVet Dental Hygiene Chews Backed by Science You Can Believe In A New Way to Combat Plaque, Calculus, and Halitosis Where They
NEW Block plaque, calculus, and halitosis with the science of prevention OraVet Dental Hygiene Chews Backed by Science You Can Believe In A New Way to Combat Plaque, Calculus, and Halitosis Where They
Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology. Robin Lorenz
 Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology Robin Lorenz rlorenz@uab.edu Why do we Need to Understand How the Mucosal Immune System Works? The mucosa is the major
Mucosal Immunology Sophomore Dental and Optometry Microbiology Section I: Immunology Robin Lorenz rlorenz@uab.edu Why do we Need to Understand How the Mucosal Immune System Works? The mucosa is the major
Bacterial Diseases IMMUNITY TO BACTERIAL INFECTIONS. Gram Positive Bacteria. Gram Negative Bacteria. Many Infectious agents and many diseases
 IMMUNITY TO BACTERIAL INFECTIONS Chapter 18 Bacterial Diseases Many Infectious agents and many diseases Bacteria can Infect any part of the body Cause disease due to Growth of the microbe in a tissue Produce
IMMUNITY TO BACTERIAL INFECTIONS Chapter 18 Bacterial Diseases Many Infectious agents and many diseases Bacteria can Infect any part of the body Cause disease due to Growth of the microbe in a tissue Produce
Chapter 29 Lecture Notes: Parasitism, pathogenicity and resistance
 Chapter 29 Lecture Notes: Parasitism, pathogenicity and resistance I. Symbiosis relationship in which 2 organisms spend a portion or all of their lifecycles associated with one another A. Commensalism
Chapter 29 Lecture Notes: Parasitism, pathogenicity and resistance I. Symbiosis relationship in which 2 organisms spend a portion or all of their lifecycles associated with one another A. Commensalism
SockIt Oral Pain Gel
 SockIt Oral Pain Gel Protects Wounds, Controls Pain, Prevents Microbial Infection, and Inhibits the of Microbes Required for the Preservative Challenge Test and Microorganisms Found in the Mouth Implicated
SockIt Oral Pain Gel Protects Wounds, Controls Pain, Prevents Microbial Infection, and Inhibits the of Microbes Required for the Preservative Challenge Test and Microorganisms Found in the Mouth Implicated
Unit 1: Biochemistry
 Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Abdullah zurayqat. Bahaa Najjar. Mamoun Ahram
 9 Abdullah zurayqat Bahaa Najjar Mamoun Ahram Polysaccharides Polysaccharides Definition and Structure [Greek poly = many; sacchar = sugar] are complex carbohydrates, composed of 10 to up to several thousand
9 Abdullah zurayqat Bahaa Najjar Mamoun Ahram Polysaccharides Polysaccharides Definition and Structure [Greek poly = many; sacchar = sugar] are complex carbohydrates, composed of 10 to up to several thousand
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units
 Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
Glycosaminoglycans: Anionic polysaccharide chains made of repeating disaccharide units Glycosaminoglycans present on the animal cell surface and in the extracellular matrix. Glycoseaminoglycans (mucopolysaccharides)
Targeted Antimicrobial Therapy Against Streptococcus mutans Establishes Protective Non-cariogenic Oral Biofilms and Reduces Subsequent Infection
 Targeted Antimicrobial Therapy Reduces S. mutans Subsequent Colonization Li et al. http://www.ijos.org.cn doi: 10.4248/IJOS10024 ORIGINAL SCIENTIFIC ARTICLES Targeted Antimicrobial Therapy Against Streptococcus
Targeted Antimicrobial Therapy Reduces S. mutans Subsequent Colonization Li et al. http://www.ijos.org.cn doi: 10.4248/IJOS10024 ORIGINAL SCIENTIFIC ARTICLES Targeted Antimicrobial Therapy Against Streptococcus
ZINC EFFECTS ON OXIDATIVE PHYSIOLOGY OF ORAL BACTERIA
 Advances in Natural Sciences, Vol. 7, No. 1 & 2 (2006) (131 138) Biotechnology ZINC EFFECTS ON OXIDATIVE PHYSIOLOGY OF ORAL BACTERIA Nguyen Thi Mai Phuong Institute of Biotechnology, VAST Phan Tuan Nghia
Advances in Natural Sciences, Vol. 7, No. 1 & 2 (2006) (131 138) Biotechnology ZINC EFFECTS ON OXIDATIVE PHYSIOLOGY OF ORAL BACTERIA Nguyen Thi Mai Phuong Institute of Biotechnology, VAST Phan Tuan Nghia
Probiotics In Gingivitis Management: A Randomized Clinical Trial. Jordi Espadaler, PhD. Director of Innovation
 Probiotics In Gingivitis Management: A Randomized Clinical Trial Jordi Espadaler, PhD. Director of Innovation espadaler@ab-biotics.com Improving oral care Mechanical plaque removal, brushing and rinsing
Probiotics In Gingivitis Management: A Randomized Clinical Trial Jordi Espadaler, PhD. Director of Innovation espadaler@ab-biotics.com Improving oral care Mechanical plaque removal, brushing and rinsing
Materials and Methods: Literature review and Authors opinion.
 Haffajee AD, Bogren A, Hasturk H et al. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol 2004; 31:996-1002. Purpose: To compare the subgingival
Haffajee AD, Bogren A, Hasturk H et al. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol 2004; 31:996-1002. Purpose: To compare the subgingival
Understanding probiotics and health
 Understanding probiotics and health Gemma Laws MSc Student Microbiology and Immunology Department The gut microbiota The name given to the total microbial population living in our intestine Bacteria, fungi,
Understanding probiotics and health Gemma Laws MSc Student Microbiology and Immunology Department The gut microbiota The name given to the total microbial population living in our intestine Bacteria, fungi,
Fathilah Abdul Razak and Zubaidah Haji Abd Rahim. Department of Oral Biology, Faculty of Dentistry, University of Malaya, Kuala Lumpur, Malaysia
 201 Journal of Oral Science, Vol. 45, No. 4, 201-206, 2003 Original The anti-adherence effect of Piper betle and Psidium guajava extracts on the adhesion of early settlers in dental plaque to saliva-coated
201 Journal of Oral Science, Vol. 45, No. 4, 201-206, 2003 Original The anti-adherence effect of Piper betle and Psidium guajava extracts on the adhesion of early settlers in dental plaque to saliva-coated
I. Polymers & Macromolecules Figure 1: Polymers. Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis
 I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
Concluding remarks 239
 Concluding remarks Impetus for oral microbiological research has come from the finding of an association between dental plaque and two of the most prevalent infections known to man - caries and periodontal
Concluding remarks Impetus for oral microbiological research has come from the finding of an association between dental plaque and two of the most prevalent infections known to man - caries and periodontal
Role of Interbacterial Adherence in Colonization of the Oral
 INFECTION AND IMMUNITY, Aug. 1981, p. 467472 0019-9567/81/080467-06$02.00/0 Vol. 33, No. 2 Role of Interbacterial Adherence in Colonization of the Oral Cavities of Gnotobiotic Rats Infected with Streptococcus
INFECTION AND IMMUNITY, Aug. 1981, p. 467472 0019-9567/81/080467-06$02.00/0 Vol. 33, No. 2 Role of Interbacterial Adherence in Colonization of the Oral Cavities of Gnotobiotic Rats Infected with Streptococcus
Characteristics of Biofilm Formation by Streptococcus mutans in the Presence of Saliva
 INFECTION AND IMMUNITY, Sept. 2008, p. 4259 4268 Vol. 76, No. 9 0019-9567/08/$08.000 doi:10.1128/iai.00422-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Characteristics of
INFECTION AND IMMUNITY, Sept. 2008, p. 4259 4268 Vol. 76, No. 9 0019-9567/08/$08.000 doi:10.1128/iai.00422-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Characteristics of
Chapter 1: Introduction
 1.1 Biofilms John Donne (1572 1631) famously wrote No man is an island, entire of itself ; words that summarise an absolute truth of humanity- it is impossible to thrive and flourish in isolation. An idea
1.1 Biofilms John Donne (1572 1631) famously wrote No man is an island, entire of itself ; words that summarise an absolute truth of humanity- it is impossible to thrive and flourish in isolation. An idea
Epithelial IL-8 Responses to Oral Bacterial Biofilms. R. Peyyala, S. Kirakodu, K. F. Novak*, and J. L. Ebersole
 CVI Accepts, published online ahead of print on 10 August 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05162-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
CVI Accepts, published online ahead of print on 10 August 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05162-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
The Human Microbiome Christine Rodriguez, Ph.D. Harvard Outreach 2012
 The Human Microbiome Christine Rodriguez, Ph.D. Harvard Outreach 2012 Microbes are all over us Page 1 of 22 There are millions of microbes per square inch on your body Thousands of different species on
The Human Microbiome Christine Rodriguez, Ph.D. Harvard Outreach 2012 Microbes are all over us Page 1 of 22 There are millions of microbes per square inch on your body Thousands of different species on
Coaggregation-Mediated Interactions of Streptococci and Actinomyces Detected in Initial Human Dental Plaque
 JOURNAL OF BACTERIOLOGY, June 2003, p. 3400 3409 Vol. 185, No. 11 0021-9193/03/$08.00 0 DOI: 10.1128/JB.185.11.3400 3409.2003 Coaggregation-Mediated Interactions of Streptococci and Actinomyces Detected
JOURNAL OF BACTERIOLOGY, June 2003, p. 3400 3409 Vol. 185, No. 11 0021-9193/03/$08.00 0 DOI: 10.1128/JB.185.11.3400 3409.2003 Coaggregation-Mediated Interactions of Streptococci and Actinomyces Detected
2012 Ph.D. APPLIED EXAM Department of Biostatistics University of Washington
 2012 Ph.D. APPLIED EXAM Department of Biostatistics University of Washington Background Dental caries is an infectious, transmissible bacterial disease that is a common childhood condition in the United
2012 Ph.D. APPLIED EXAM Department of Biostatistics University of Washington Background Dental caries is an infectious, transmissible bacterial disease that is a common childhood condition in the United
The Adhesion of Streptococcus sa/ivarius and Staphylococcus aureus to Five Dental Composite Resins
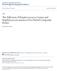 Southern Adventist Univeristy KnowledgeExchange@Southern Senior Research Projects Southern Scholars 1998 The Adhesion of Streptococcus sa/ivarius and Staphylococcus aureus to Five Dental Composite Resins
Southern Adventist Univeristy KnowledgeExchange@Southern Senior Research Projects Southern Scholars 1998 The Adhesion of Streptococcus sa/ivarius and Staphylococcus aureus to Five Dental Composite Resins
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Mechanisms of Pathogenicity
 Mechanisms of Pathogenicity The Microbes Fight Back Medically important bacteria Salmonella Bacillus anthracis Shigella dysenteriae Campylobacter Shigella sonnei Clostridium botulinum Staphylococcus aureus
Mechanisms of Pathogenicity The Microbes Fight Back Medically important bacteria Salmonella Bacillus anthracis Shigella dysenteriae Campylobacter Shigella sonnei Clostridium botulinum Staphylococcus aureus
Normal Flora PROF. HANAN HABIB DEPARTMENT OF PATHOLOGY COLLEGE OF MEDICINE, KSU
 Normal Flora PROF. HANAN HABIB DEPARTMENT OF PATHOLOGY COLLEGE OF MEDICINE, KSU Objectives 1. Define the terms: Normal Flora, Resident flora, Transient flora and carrier state 2. Know the origin of normal
Normal Flora PROF. HANAN HABIB DEPARTMENT OF PATHOLOGY COLLEGE OF MEDICINE, KSU Objectives 1. Define the terms: Normal Flora, Resident flora, Transient flora and carrier state 2. Know the origin of normal
BACTERIAL GROWTH. Refers to an increase in bacterial cell number (multiplication). Results from bacterial reproduction (binary fission)
 BACTERIAL GROWTH Refers to an increase in bacterial cell number (multiplication). Results from bacterial reproduction (binary fission) parameter called generation time (the average time required for cell
BACTERIAL GROWTH Refers to an increase in bacterial cell number (multiplication). Results from bacterial reproduction (binary fission) parameter called generation time (the average time required for cell
