DENTCA Denture Base II for Carbon printers - Directions for Use
|
|
|
- Timothy Chase
- 5 years ago
- Views:
Transcription
1 P a g e 1 DENTCA Denture Base II for Carbon printers - Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner licensed by the law of the State in which he/she practices to us or order the use of the device. Indications for Use DENTCA Denture Base II for Carbon printers is a light-curable resin indicated for fabrication and repair of full and partial removable dentures and baseplates. The material is an alternative to traditional heatcurable and auto polymerizing resins. Fabrication of dental prosthetics with DENTCA Denture Base II for Carbon printers requires a computeraided design and manufacturing (CAD/CAM) system that includes the following components: digital denture base files based on a digital impression, stereolithographic additive printer, and curing light equipment. Requirements 1. Digital denture base file in STL format 2. Carbon Printer (M1 or M2) and Software 3. Dymax ECE 5000 with Hg Bulb or Dreve PCU LED Basic Material Properties Characteristics/Properties Units Specification Before Curing (Liquid state) Viscosity at 25 ±0.5 o C cps 1000 < X < 2000 Density g/cm < X < 1.20 Surface curing rate second < 2 After Curing Density g/cm < X <1.25 Flexural strength MPa 65 < X Flexural modulus MPa 2000 < X Degree of Conversion % 70 < X
2 P a g e 2 Specific Manufacturing Considerations 1. Digital denture base file 1.1 File format: STL file 1.2 Digital design: Denture base or baseplate 2. Carbon Printer (M1 or M2) and Software 2.1 Hardware a. LED Wavelength: 385 nm b. Slice thickness: 100 micron (standard slicing) or 50 micron (fine slicing) c. Build Volume: M1: 141 x 79 x 326 mm; M2: 189 x 118 x 326 mm d. Pixel size: 75um 2.2 Carbon Printer Software a. STL file import b. Rotation and placement c. View Slices d. Auto and manual generation of supports 2.3 Printing Parameters a. Slice thickness: 100 micron or 50 micron b. Optimal Orientation: degree tilted orientation c. Support point size: varies based on support tip chosen d. Support density: perimeter of denture and palatal region 2.4 Environmental Conditions a. Temperature: o C b. Relative Humidity: % 2.5 Cleaning and Bonding Kit Personal Protective Equipment (PPE), tray, paper towels, foil, part removal tool, hand tools, 2 pairs of silicone tongs, isopropyl alcohol (IPA), labeled wash container, orbital shaker, timer, swabs, pipettes, cup, and soft silicone spatula 2.6 Recommended Printer a. Carbon M1 or M2
3 P a g e 3 3. Recommended curing light equipment (Post curing units) 3.1 UV curing Flood Type System Manufacturer/Model Dymax ECE 5000 with mercury vapor bulb (36970) Dreve PCU LED Supply Voltage VAC +/- 10% Single Phase V Lamp Power 400 watt 18.5 mw/cm 2 Light Intensity >70 mw/cm 2 for Hg bulb in nm nm range Lamp Wavelength UVA ( nm) N/A Curing time 20 min total (10 min on each side) 30 min total (no flipping since both sides cure simultaneously) 4. Notification 3.2 Accessories a. USP Grade glycerol ( 99.5% purity, CAS# ) b. Transparent glass container (recommend Pyrex Basics 2Qt dish: 11.1" x 7.1" x 1.7" when using Dymax or Circleware 23oz dish: 6 x 6 x 2.5 when using Dreve) c. 2 transparent glass plates (recommend McMaster-Carr Borosilicate Sheet - 9" x 9" x 1/8" when using Dymax or Sigma-Aldrich s Corning 75mm x 50 mm slides (CLS294775x50) when using Dreve) d. Large binder clip when using Dymax or electric hot plate, stainless steel pot with pouring spout, and electrical timer when using Dreve e. Thermocouple such as BENETECH GM1312 digital thermometer for K/J/T/E/R/S/N f. Heat-protective gloves g. Water The device specifications have been validated using the software, printer, and process parameters indicated and that any changes to the above will need to be re-validated to determine if design specifications are met. If changes are made that results in outputs that are outside of the design specification, FDA clearance will be needed.
4 P a g e 4 Warnings: 1. DENTCA Denture Base II for Carbon printers contains polymerizable monomers which may cause skin irritation (allergic contact dermatitis) or other allergic reactions in susceptible persons. If resin contacts skin, wash thoroughly with soap and water. If skin sensitization occurs, discontinue use. If dermatitis or other symptoms persist, seek medical assistance. 2. Avoid inhalation or ingestion. High vapor concentration can cause headache, irritation of eyes or respiratory system. Direct contact with eyes may cause possible corneal damage. Long-term excessive exposure to the material may cause more serious health effects. Monitor air quality per OSHA standards. BURN HAZARD: GLYCEROL BATH CAN REACH TEMPERATURES OF 90 o C (~200 o F) AND LEAD TO SEVERE BURNS. Only trained users should perform the glycerol curing step with caution and appropriate PPE. We also recommend placing a warning label on the window of the cure unit to alert all lab users to the potential hazard. Eye Contact: Immediately flush eyes with plenty of clean water for at least 20 minutes, and consult a physician. Wash the contacted area thoroughly with soap and water. Inhalation: In case of exposure to a high concentration of vapor or mist, remove person to fresh air. Give oxygen or artificial respiration as required. Ingestion: Contact your regional poison control center immediately. Precautions: 1. When washing the printed denture bases with solvent or grinding the denture bases, it should be in a properly ventilated environment with proper protective masks and gloves. 2. Store DENTCA Denture Base II for Carbon printers at or below o C (60-77 o F) and avoid direct sunlight. Keep container closed when it is not in use. Product shall not be used after expiration date. 3. Expired or unused DENTCA Denture Base II for Carbon printers shall be disposed in accordance with local regulations. 4. Glycerol and isopropanol shall be disposed in accordance with local regulations. Adverse Reactions: 1. Direct contact with the uncured resin may induce skin sensitization in susceptible individuals. 2. Proper ventilation and personal protective equipment should be used when grinding denture base resins as the particulate generated during grinding may cause respiratory, skin and eye irritation.
5 P a g e 5 Procedure to Fabricate Denture 1. Printing Preparation See attachment for details. a. Select the denture base shade based on prescription. b. Wearing proper PPE, fill cassette with fresh DENTCA Denture Base II for Carbon printers resin. c. Close the printer door. 2. Printing (visit See Carbon for training. a. Upload the denture base model STL file into Carbon s software. b. Orient the denture base so that the tissue side (intaglio surface) faces the build platform. The recommended orientation is a tilted orientation such as space diagonal from 20 o to 40 o. c. Generate fence supports around the perimeter of the denture base. Ensure the regions near the posterior teeth and palate are well-supported. d. Select desired slice thickness. e. Start printing. 3. Cleaning See attachment for details. a. Remove the printed denture base from the build platform. b. Wash the denture base with isopropyl alcohol (IPA) and remove remaining supports. c. Ensure the denture teeth are dry before curing (such as compressed air, paper towels, or additional drying time at room temperature). Denture Fabrication using printed denture teeth and base See attachment for details. 1. Bonding the printed teeth to the printed denture base a. Attention: Do not UV post-cure base or teeth prior to bonding step! b. Prepare the base for bonding by removing any material from the teeth and denture base tooth sockets that impede tooth placement. c. Place the printed teeth into the corresponding tooth sockets on the printed denture base and check teeth fitting. d. Smoothen other support spots on the denture base using a bur or hand tool. e. Apply a small amount of shade-matched light curable adhesive (DENTCA Denture Base II for Carbon printers) into the tooth sockets and bond teeth by exposing to UV light until the teeth set in position. f. If necessary, apply small amount of shade-matched DENTCA Denture Base II for Carbon printers using an applicator to smoothen the edges of the denture base and cure it.
6 P a g e 6 2. Post Curing a. Cure the final denture by completely submerging in the glycerol container (glycerol temperature should be greater than 60 o C) for: i. 20 min in the Dymax ECE 5000, flipping the dentures over halfway through the post cure ii. 30 minutes under vacuum at 90% LED intensity in the Dreve PCU LED with no flipping (both sides cure simultaneously). 3. Finishing a. Polish the final denture with wet polishing sand by conventional method. Repair of denture and baseplates Note: This process only applies in temporary repair cases. It is recommended to remake the whole denture using an original design file. 1. Prepare a cast made of a putty using a broken denture. 2. Prepare the fracture area by grinding to open more and roughing the outer side of fracture area. 3. Prime the roughened surfaces of the repair area with DENTCA Denture Base II for Carbon printers. 4. Place the broken denture on the cast. 5. Apply the shade matched DENTCA Denture Base II for Carbon printers resin to cover the roughed and fracture areas and cure the areas by exposing light curing machine until the resin is solidified. 6. Place the denture on the cast in the post-curing machine for 10 min. Carefully remove the denture from the cast and cure the tissue side (intaglio surface) for 10 min. 7. Grind, polish and finish.
DENTCA Denture Teeth for Carbon printers- Directions for Use
 P a g e 1 DENTCA Denture Teeth for Carbon printers- Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner
P a g e 1 DENTCA Denture Teeth for Carbon printers- Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner
DENTCA Denture Base II - Directions for Use
 P a g e 1 DENTCA Denture Base II - Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner licensed by the
P a g e 1 DENTCA Denture Base II - Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner licensed by the
DENTCA Denture Teeth - Directions for Use
 P a g e 1 DENTCA Denture Teeth - Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner licensed by the
P a g e 1 DENTCA Denture Teeth - Directions for Use Prescription Use Statement Caution: Federal law restricts this device to sale by or on the order of dentist or any other practitioner licensed by the
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Kool-Dam Heatless Liquid Dam & Block Out Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
Pulpdent Corporation Revision Date: May 6, 2014 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice OBA Bonding Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
Pulpdent Corporation Revision Date: May 1, 2017 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Orthodontic Bracket Adhesive (OBA) 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Orthodontic Bracket Adhesive (OBA) 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for
Pulpdent Corporation Revision Date: May 1, 2017
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Ortho-Coat 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Ortho-Choice Ortho-Coat 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental professional
Pulpdent Corporation Revision Date: May 1, 2017
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Tuff-Temp Plus Provisional Veneer, Crown & Bridge Resin 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer
VarseoWax CAD/Cast GEBRAUCHSANWEISUNG INSTRUCTIONS FOR USE MODE D EMPLOI INSTRUCCIONES DE USO ISTRUZIONI PER L USO Partners in Progress
 www.bego.com VarseoWax CAD/Cast GEBRAUCHSANWEISUNG INSTRUCTIONS FOR USE MODE D EMPLOI INSTRUCCIONES DE USO ISTRUZIONI PER L USO Partners in Progress EN INSTRUCTION FOR USE VarseoWax CAD/Cast VarseoWax
www.bego.com VarseoWax CAD/Cast GEBRAUCHSANWEISUNG INSTRUCTIONS FOR USE MODE D EMPLOI INSTRUCCIONES DE USO ISTRUZIONI PER L USO Partners in Progress EN INSTRUCTION FOR USE VarseoWax CAD/Cast VarseoWax
3D Printing Full Dentures with the Form 2
 FORMLABS APPLICATION GUIDE: 3D Printing Full Dentures with the Form 2 Today, 50 million dentures are produced globally each year, but only 1 percent are produced using digital tools. Traditional denture
FORMLABS APPLICATION GUIDE: 3D Printing Full Dentures with the Form 2 Today, 50 million dentures are produced globally each year, but only 1 percent are produced using digital tools. Traditional denture
Pulpdent Corporation Revision Date: January 1, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Lime-Lite Light-cured Cavity Liner 1.2 1.2.2 1.2.3 Application / Use SIC Use Category Dental material for use by dental
Dental City - VPS Putty SAFETY DATA SHEET
 Issue Date: 2/5/207. Identification Page of 9 Product identifier Trade name: Dental City - VPS Putty 49-8008 Fast Set / 49-8006 Regular Set Application of the substance or the preparation Addition curing
Issue Date: 2/5/207. Identification Page of 9 Product identifier Trade name: Dental City - VPS Putty 49-8008 Fast Set / 49-8006 Regular Set Application of the substance or the preparation Addition curing
Pulpdent Corporation Revision Date: May 7, 2014
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation 1.2 1.2.2 Application / Use SIC 1.2.3 Use Category 1.3 Manufacturer Pulpdent Corporation 80 Oakland Street, P.O. Box 780
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation 1.2 1.2.2 Application / Use SIC 1.2.3 Use Category 1.3 Manufacturer Pulpdent Corporation 80 Oakland Street, P.O. Box 780
MATERIAL SAFETY DATA SHEET Mercury 5 Min and 15 Min Epoxy. Mercury 5 Min Mercury 15 Min A
 MATERIAL SAFETY DATA SHEET Mercury 5 Min and 15 Min Epoxy COMPANY NAME: Mercury Adhesives Inc ADDRESS: 6110 Parkway North Dr Cumming GA 30040 PHONE: 1 770-886-9566 TRADE NAME: CHEMICAL NAME: Mercury 5
MATERIAL SAFETY DATA SHEET Mercury 5 Min and 15 Min Epoxy COMPANY NAME: Mercury Adhesives Inc ADDRESS: 6110 Parkway North Dr Cumming GA 30040 PHONE: 1 770-886-9566 TRADE NAME: CHEMICAL NAME: Mercury 5
SAFETY DATA SHEET. Issue Date: 05/25/2015 Version No: 1 1. Identification
 Page 1 of 7 1. Identification Product identifier Application of the substance or the preparation Addition curing vinyl polysiloxane dental impression materials (consisting of Base & Catalyst) Manufacturer/Supplier
Page 1 of 7 1. Identification Product identifier Application of the substance or the preparation Addition curing vinyl polysiloxane dental impression materials (consisting of Base & Catalyst) Manufacturer/Supplier
MATERIAL SAFETY DATA SHEET (MSDS)
 MATERIAL SAFETY DATA SHEET (MSDS) HEALTH: 2 FLAMMABILITY: 1 REACTIVITY: 1 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Trade Name: Codes and colours: Manufacturer name: Ultraviolet light
MATERIAL SAFETY DATA SHEET (MSDS) HEALTH: 2 FLAMMABILITY: 1 REACTIVITY: 1 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Trade Name: Codes and colours: Manufacturer name: Ultraviolet light
MATERIAL SAFETY DATA SHEET SECTION 1 IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING
 MATERIAL SAFETY DATA SHEET SECTION 1 IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Product Name: Eclipse Resin Materials (Baseplate, Set-Up and Contour Resin) Product Number:
MATERIAL SAFETY DATA SHEET SECTION 1 IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Product Name: Eclipse Resin Materials (Baseplate, Set-Up and Contour Resin) Product Number:
EMERGENCY PHONE MATERIAL SAFETY DATA SHEET MP5405/5430/5445 PART A (RESIN)
 9411 Corsair Road Frankfort, IL 60423 1-800 - 552-0299 Phone 1-815 - 464-5650 Fax EMERGENCY PHONE 1-800 - 255-3924 MATERIAL SAFETY DATA SHEET MP5405/5430/5445 PART A (RESIN) HMIS HEALTH 2 FLAMMABILITY
9411 Corsair Road Frankfort, IL 60423 1-800 - 552-0299 Phone 1-815 - 464-5650 Fax EMERGENCY PHONE 1-800 - 255-3924 MATERIAL SAFETY DATA SHEET MP5405/5430/5445 PART A (RESIN) HMIS HEALTH 2 FLAMMABILITY
3D Printing Dentures. Dental Application Print Guide
 Dental Application Print Guide 3D Printing Dentures Denture Base Resin is a highly accurate material specifically formulated for the creation of custom denture bases of all types. Production capabilities
Dental Application Print Guide 3D Printing Dentures Denture Base Resin is a highly accurate material specifically formulated for the creation of custom denture bases of all types. Production capabilities
HEALTH-TEC BITE REGISTRATION SAFETY DATA SHEET
 Issue Date: 05/25/205. Identification Page of 9 Product identifier Trade name: Health-Tec Bite Registration Material HT02B Fast Set Unflavored HT03B Super Fast Unflavored 202B Fast Set Cool Mint, Application
Issue Date: 05/25/205. Identification Page of 9 Product identifier Trade name: Health-Tec Bite Registration Material HT02B Fast Set Unflavored HT03B Super Fast Unflavored 202B Fast Set Cool Mint, Application
STEP-BY-STEP-ANLEITUNG
 STEP-BY-STEP-ANLEITUNG VON TECHNIKERN FÜR TECHNIKER LABORATORY PROCEDURES, STEP BY STEP BY TECHNICIANS FOR TECHNICIANS Eclipse junior Laboratory Procedures, Step by Step* Splints Drilling Stents Temporaries
STEP-BY-STEP-ANLEITUNG VON TECHNIKERN FÜR TECHNIKER LABORATORY PROCEDURES, STEP BY STEP BY TECHNICIANS FOR TECHNICIANS Eclipse junior Laboratory Procedures, Step by Step* Splints Drilling Stents Temporaries
ÆLITE Composites. Bisco. Instructions for Use. Light- Cured. U.S. Patent: 6,709,271
 Bisco ÆLITE Composites 0459 Light- Cured Instructions for Use U.S. Patent: 6,709,271 IN-131R6 Rev. 4/16 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368 Caution:
Bisco ÆLITE Composites 0459 Light- Cured Instructions for Use U.S. Patent: 6,709,271 IN-131R6 Rev. 4/16 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368 Caution:
MATERIAL SAFETY DATA SHEET
 In case of spillage: Absorb with inert material. Collect in closed containers and dispose in accordance with Federal, State and local regulations. Avoid skin contact, wear protective equipment. Waste Disposal:
In case of spillage: Absorb with inert material. Collect in closed containers and dispose in accordance with Federal, State and local regulations. Avoid skin contact, wear protective equipment. Waste Disposal:
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Product Name: 1,6-HEXANEDIOL DIGLYCIDYL ETHER CAS No.: 16096-31-4 Brand: Alfa Chemistry Company:
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Product Name: 1,6-HEXANEDIOL DIGLYCIDYL ETHER CAS No.: 16096-31-4 Brand: Alfa Chemistry Company:
Invisible Retainer with Multi-Tooth Pontic Fabrication Technique (Single-Tooth Pontic Procedure also available)
 Invisible Retainer with Multi-Tooth Pontic Fabrication Technique (Single-Tooth Pontic Procedure also available) Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics.
Invisible Retainer with Multi-Tooth Pontic Fabrication Technique (Single-Tooth Pontic Procedure also available) Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics.
PRO-V IDS Kit. Bisco. Instructions for Use. Provisional Restorative System
 Bisco PRO-V IDS Kit Provisional Restorative System Instructions for Use U.S. Patent: 7,748,980; 8,106,110 IN-199R3 Rev. 5/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000
Bisco PRO-V IDS Kit Provisional Restorative System Instructions for Use U.S. Patent: 7,748,980; 8,106,110 IN-199R3 Rev. 5/16 BISCO, Inc. 1100 W. Irving Park Rd. Schaumburg, IL 60193 U.S.A. 847-534-6000
Pala Digital Dentures. Better Precision. Faster Production. Higher Profitability. Giving a hand to oral health.
 Brochure Get great results with fully 3D printed options designed to save time and cost! Pala Digital Dentures Better Precision. Faster Production. Higher Profitability. Giving a hand to oral health. The
Brochure Get great results with fully 3D printed options designed to save time and cost! Pala Digital Dentures Better Precision. Faster Production. Higher Profitability. Giving a hand to oral health. The
Safety Data Sheet. Section 1: Identification. Section 2: Hazard Identification. Down To Earth Distributors, Inc Judkins Road Eugene, OR 97403
 Section 1: Identification Product Name: Down To Earth Bone Meal 3-15-0 Common Name(s): bone meal Product Code: CAS #: Recommended Use: Fertilizer Manufacturer: Physical Address: Down To Earth Distributors,
Section 1: Identification Product Name: Down To Earth Bone Meal 3-15-0 Common Name(s): bone meal Product Code: CAS #: Recommended Use: Fertilizer Manufacturer: Physical Address: Down To Earth Distributors,
SECTION 1 - IDENTIFICATION
 1 of 5 11/25/2009 9:53 AM TARKSOL GREEN Tarksol Aqua Kleen C Material Safety Data Sheet Trade Name: Tarksol Aqua Kleen C Manufacturer: Tarksol, Inc. 3400 Ridge Road West, S-300 Rochester, New York 14626
1 of 5 11/25/2009 9:53 AM TARKSOL GREEN Tarksol Aqua Kleen C Material Safety Data Sheet Trade Name: Tarksol Aqua Kleen C Manufacturer: Tarksol, Inc. 3400 Ridge Road West, S-300 Rochester, New York 14626
MATERIAL SAFETY DATA SHEET (MSDS)
 MATERIAL SAFETY DATA SHEET (MSDS) HEALTH: 2 FLAMMABILITY: 1 REACTIVITY: 1 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Trade Name, Codes Manufacturer name: Barnices para tinta de curado
MATERIAL SAFETY DATA SHEET (MSDS) HEALTH: 2 FLAMMABILITY: 1 REACTIVITY: 1 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Trade Name, Codes Manufacturer name: Barnices para tinta de curado
Pulpdent Corporation Revision Date: September 1, 2015
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name Codes 1.2 1.2.2 1.2.3 ACTIVA BioACTIVE-BASE / LINER ACTIVA BioACTIVE-CEMENT ACTIVA BioACTIVE-RESTORATIVE ACTIVA KIDS BioACTIVE-RESTORATIVE
1.0 Commercial Product Name and Supplier 1.1 Commercial product name Codes 1.2 1.2.2 1.2.3 ACTIVA BioACTIVE-BASE / LINER ACTIVA BioACTIVE-CEMENT ACTIVA BioACTIVE-RESTORATIVE ACTIVA KIDS BioACTIVE-RESTORATIVE
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DURATEMP Temporary Crown & Bridge Material. Temrex Corporation October 2009
 DURATEMP Temporary Crown & Bridge Material Temrex Corporation October 2009 DURATEMP Temporary Crown & Bridge Material Overview Product Characteristics Physical Properties Case Report Instructions Ordering
DURATEMP Temporary Crown & Bridge Material Temrex Corporation October 2009 DURATEMP Temporary Crown & Bridge Material Overview Product Characteristics Physical Properties Case Report Instructions Ordering
Essential oils,sandalwood (cas ) MSDS
 Click http//www.guidechem.com/cas-800/8006-87-9.html for suppliers of this product Essential oils,sandalwood (cas 8006-87-9) MSDS 1. 1.1 IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING
Click http//www.guidechem.com/cas-800/8006-87-9.html for suppliers of this product Essential oils,sandalwood (cas 8006-87-9) MSDS 1. 1.1 IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING
Section 1: Identification. Product Name: Plan B Organics Manufacturer: Plan B Organics Glacial Rock Dust
 Section 1: Identification Product Name: Plan B Organics Manufacturer: Plan B Organics Glacial Rock Dust Common Name(s): rock dust Physical Address: 50 P.H 10 Product Code: Castle Rock, WA, 98611 CAS #:
Section 1: Identification Product Name: Plan B Organics Manufacturer: Plan B Organics Glacial Rock Dust Common Name(s): rock dust Physical Address: 50 P.H 10 Product Code: Castle Rock, WA, 98611 CAS #:
SIGMA-ALDRICH. Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 08/24/2011
 SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 08/24/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : D206539 Brand
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 08/24/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : D206539 Brand
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION REVISION DATE: 01/14/2010 PRODUCT NUMBER HMIS CODES 800014 Health 2 Flammability 1 Reactivity 1 PRODUCT NAME PPE X CA-40 LDI (US)
MATERIAL SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION REVISION DATE: 01/14/2010 PRODUCT NUMBER HMIS CODES 800014 Health 2 Flammability 1 Reactivity 1 PRODUCT NAME PPE X CA-40 LDI (US)
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET SECTION 1 - IDENTIFICATION OF PRODUCT AND COMPANY Pfizer Inc Pfizer Animal Health 235 East 42nd Street New York, NY 10017 Product name Therapeutic use Description Emergency telephone
MATERIAL SAFETY DATA SHEET SECTION 1 - IDENTIFICATION OF PRODUCT AND COMPANY Pfizer Inc Pfizer Animal Health 235 East 42nd Street New York, NY 10017 Product name Therapeutic use Description Emergency telephone
1. IDENTIFICATION OF SUBSTANCE PODOPHYLLUM RESIN 25-50% IN BENZOIN Name: TINCTURE
 According to ISO 11014-1 Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE PODOPHYLLUM RESIN 25-50% IN BENZOIN Name: TINCTURE Manufacturer: Information Department: Department of Pharmacy Duke University Medical
According to ISO 11014-1 Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE PODOPHYLLUM RESIN 25-50% IN BENZOIN Name: TINCTURE Manufacturer: Information Department: Department of Pharmacy Duke University Medical
Introducing the Pala 3D-printed Denture
 ebook Create your best workflow and optimize production with cara 3D printing technology. Introducing the Pala 3D-printed Denture Powered by cara Print 4.0 and dima Print materials. < 2 hrs. Giving a hand
ebook Create your best workflow and optimize production with cara 3D printing technology. Introducing the Pala 3D-printed Denture Powered by cara Print 4.0 and dima Print materials. < 2 hrs. Giving a hand
MATERIAL SAFETY DATA SHEET Gantz Road Grove City, OH Shoreline Court, Suite 101 South San Francisco, CA 94080
 MATERIAL SAFETY DATA SHEET MANUFACTURER: DISTRIBUTOR: Tosoh Bioscience, Inc. 3600 Gantz Road Grove City, OH 43123 Tosoh Bioscience, Inc. 6000 Shoreline Court, Suite 101 South San Francisco, CA 94080 PRODUCT
MATERIAL SAFETY DATA SHEET MANUFACTURER: DISTRIBUTOR: Tosoh Bioscience, Inc. 3600 Gantz Road Grove City, OH 43123 Tosoh Bioscience, Inc. 6000 Shoreline Court, Suite 101 South San Francisco, CA 94080 PRODUCT
Material Safety Data Sheet:
 Date Updated: 05-2013 Material Safety Data Sheet: sspions PDMS 1. Product and Company Information PRODUCT NAME: sspions PDMS CHEMICAL NAME: single Superparamagnetic Iron Oxide Nanoparticles (sspions) dispersed
Date Updated: 05-2013 Material Safety Data Sheet: sspions PDMS 1. Product and Company Information PRODUCT NAME: sspions PDMS CHEMICAL NAME: single Superparamagnetic Iron Oxide Nanoparticles (sspions) dispersed
May be harmful if inhaled. Causes respiratory tract irritation. Skin. May be harmful if absorbed through skin. Causes skin irritation.
 SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 07/25/2010 Print Date 02/14/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Lauryl methacrylate Product Number
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 07/25/2010 Print Date 02/14/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Lauryl methacrylate Product Number
Pulpdent Corporation Revision Date: July 1, 2013 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name Code 1.2 1.2.2 1.2.3 1.3 1.3.1 Embrace WetBond Class V Embrace WetBond First Coat Embrace Esthetic Opaquer Embrace WetBond Pit & Fissure
1.0 Commercial Product Name and Supplier 1.1 Commercial product name Code 1.2 1.2.2 1.2.3 1.3 1.3.1 Embrace WetBond Class V Embrace WetBond First Coat Embrace Esthetic Opaquer Embrace WetBond Pit & Fissure
SAFETY DATA SHEET MOSO BAMBOO INDUSTRIALE (HD) MOSO Bamboo Industriale (Industrial flooring), High Density version
 SAFETY DATA SHEET MOSO BAMBOO INDUSTRIALE (HD) 1) CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: MOSO Bamboo Industriale (Industrial flooring), High Density version Supplier: Moso International
SAFETY DATA SHEET MOSO BAMBOO INDUSTRIALE (HD) 1) CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product name: MOSO Bamboo Industriale (Industrial flooring), High Density version Supplier: Moso International
SAFETY DATA SHEET. Allyl phenoxyacetate
 SAFETY DATA SHEET SECTION 1 Identification of the substance/mixture and of the company/undertaking Product identifiers Product name Allyl phenoxyacetate W203807 REACH No. CAS-No. 7493-74-5 Product Number
SAFETY DATA SHEET SECTION 1 Identification of the substance/mixture and of the company/undertaking Product identifiers Product name Allyl phenoxyacetate W203807 REACH No. CAS-No. 7493-74-5 Product Number
DRYDEN AQUA LTD Safety Data Sheet 18
 DRYDEN AQUA LTD Safety Data Sheet 18 According to Regulation (EC) No. 1907/2006 Date of compilation: 04/12/12 Print date: 03/02/13 Section 1 Identification of the substance/mixture and of the company/undertaking
DRYDEN AQUA LTD Safety Data Sheet 18 According to Regulation (EC) No. 1907/2006 Date of compilation: 04/12/12 Print date: 03/02/13 Section 1 Identification of the substance/mixture and of the company/undertaking
Invisible Retainer with Single-Tooth Pontic Fabrication Technique (Multi-Tooth Pontic Procedure also available)
 Invisible Retainer with Single-Tooth Pontic Fabrication Technique (Multi-Tooth Pontic Procedure also available) Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics.
Invisible Retainer with Single-Tooth Pontic Fabrication Technique (Multi-Tooth Pontic Procedure also available) Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics.
Material Safety Data Sheet
 Material Safety Data Sheet Version 3.0 Revision Date 08/21/2009 Print Date 09/07/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : D94208 Brand : Aldrich Company : Sigma-Aldrich
Material Safety Data Sheet Version 3.0 Revision Date 08/21/2009 Print Date 09/07/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : D94208 Brand : Aldrich Company : Sigma-Aldrich
 Page 1 of 5 MATERIAL SAFETY DATA SHEET NOA 81 For information or emergency: Telephone 609-395-1966 Date prepared November 1, 2009 TRADE NAME: Norland Optical Adhesive 81 I. PRODUCT INFORMATION SYNONYMS:
Page 1 of 5 MATERIAL SAFETY DATA SHEET NOA 81 For information or emergency: Telephone 609-395-1966 Date prepared November 1, 2009 TRADE NAME: Norland Optical Adhesive 81 I. PRODUCT INFORMATION SYNONYMS:
th Ave., Kenosha WI, Phone:
 ABATRON, INC. 5501 95th Ave., Kenosha, WI 53144 U.S.A (262) 653-2000 Fax (262) 653-2019 www.abatron.com e-mail: info@abatron.com 24 Hour Emergency No: (800) 424-9300 Safety Data Sheet SECTION 1: Product
ABATRON, INC. 5501 95th Ave., Kenosha, WI 53144 U.S.A (262) 653-2000 Fax (262) 653-2019 www.abatron.com e-mail: info@abatron.com 24 Hour Emergency No: (800) 424-9300 Safety Data Sheet SECTION 1: Product
1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer:
 Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer: Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information Department:
Page 1 of 5 1. IDENTIFICATION OF SUBSTANCE Name: ALUMINUM CHLORIDE SOLUTION 30% Manufacturer: Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information Department:
Medescan Nebuliser Med - S600A Instructions Manual
 Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. PRODUCT IDENTIFICATION Manufacturer Country Russia Product Identification Birch Plywood Synonyms Bietula Birch White Birch Exterior, WBP (Phenolic) Trade Name Russian Birch
MATERIAL SAFETY DATA SHEET 1. PRODUCT IDENTIFICATION Manufacturer Country Russia Product Identification Birch Plywood Synonyms Bietula Birch White Birch Exterior, WBP (Phenolic) Trade Name Russian Birch
Health & Safety in the Darkroom
 Health & Safety in the Darkroom While the majority of the black and white chemicals are in the range of non-toxic to slightly toxic to most people, black and white developers are in the range of slightly
Health & Safety in the Darkroom While the majority of the black and white chemicals are in the range of non-toxic to slightly toxic to most people, black and white developers are in the range of slightly
Penicillin G Procaine in Sesame Oil
 SECTION 1 IDENTIFICATION SAFETY DATA SHEET Product Name: Amount: 100,000 units/10ml syringe Company Identification: 304 Oneida Street Syracuse, NY 13201 (315) 476-7418 Emergency Contact Info: CHEMTREC
SECTION 1 IDENTIFICATION SAFETY DATA SHEET Product Name: Amount: 100,000 units/10ml syringe Company Identification: 304 Oneida Street Syracuse, NY 13201 (315) 476-7418 Emergency Contact Info: CHEMTREC
Safety Data Sheet HARD-ROK ANCHORING CEMENT Created On: 03/10/2015 Revision Date: 03/01/16 Version: 3.0
 1. Product and Company Identification Product Name: Product Use: Anchoring Company Identification: Emergency Phone: ADHESIVES TECHNOLOGY CORP. Chem-Tel: 450 East Copans Road 1.800.255.3924 (24hrs) Pompano
1. Product and Company Identification Product Name: Product Use: Anchoring Company Identification: Emergency Phone: ADHESIVES TECHNOLOGY CORP. Chem-Tel: 450 East Copans Road 1.800.255.3924 (24hrs) Pompano
GHS SAFETY DATA SHEET
 GHS SAFETY DATA SHEET 1. Identification Product Name: ECO-100 Teak Cleaner Powder Synonyms: None CAS Number: Mixture Product Use: Marine Deck Cleaning Manufacturer/Supplier: Teakdecking Systems, Inc. th
GHS SAFETY DATA SHEET 1. Identification Product Name: ECO-100 Teak Cleaner Powder Synonyms: None CAS Number: Mixture Product Use: Marine Deck Cleaning Manufacturer/Supplier: Teakdecking Systems, Inc. th
Safety Data Sheet. Product Name: DetectX Palladium Screening Fluorescent Detection Kit. Section 1: Identification. Section 2: Hazard(s) Identification
 Safety Data Sheet Revision Date: 17 October 2016 Product Name: DetectX Palladium Screening Fluorescent Detection Kit Section 1: Identification Product Name: DetectX Palladium Screening Fluorescent Detection
Safety Data Sheet Revision Date: 17 October 2016 Product Name: DetectX Palladium Screening Fluorescent Detection Kit Section 1: Identification Product Name: DetectX Palladium Screening Fluorescent Detection
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT(S) AND COMPANY IDENTIFICATION Supplier Name and address: Genlantis, A division of Gene Therapy Systems, Inc. 10190 Telesis Court San Diego, CA 92121
MATERIAL SAFETY DATA SHEET SECTION 1 - CHEMICAL PRODUCT(S) AND COMPANY IDENTIFICATION Supplier Name and address: Genlantis, A division of Gene Therapy Systems, Inc. 10190 Telesis Court San Diego, CA 92121
Occlusal Splint Fabrication Technique
 Occlusal Splint Fabrication Technique Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics. Items featured in this technique are found on the last page. 1 Mount
Occlusal Splint Fabrication Technique Safety glasses should be worn for all lab procedures as well as gloves when handling acrylics. Items featured in this technique are found on the last page. 1 Mount
Pulpdent Corporation Revision Date: May 1, 2017 Safety Data Sheet
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Etch-All 10% Phosphoric Acid Etching Gel 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer Pulpdent
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Etch-All 10% Phosphoric Acid Etching Gel 1.2 1.2.2 1.2.3 Application / Use SIC Use Category 1.3 Manufacturer Pulpdent
CAS-No. EC-No. Index-No. Concentration [%] Paraformaldehyde
![CAS-No. EC-No. Index-No. Concentration [%] Paraformaldehyde CAS-No. EC-No. Index-No. Concentration [%] Paraformaldehyde](/thumbs/94/120851104.jpg) SIGMA-ALDRICH Material Safety Data Sheet Version 3.0 Revision Date 07/20/2007 Print Date 08/13/2007 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Paraformaldehyde Product Number : P6148 Brand :
SIGMA-ALDRICH Material Safety Data Sheet Version 3.0 Revision Date 07/20/2007 Print Date 08/13/2007 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Paraformaldehyde Product Number : P6148 Brand :
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 REVISION DATE: 11-01-2005 SECTION 1: CHEMICAL PRODUCT AND COMPANY IDENTIFICATION COMPANY INFORMATION Advanced Protective Technologies LLC 2502 Beech Street Unit 100 Valparaiso, IN 46383 PRODUCT
Page 1 of 6 REVISION DATE: 11-01-2005 SECTION 1: CHEMICAL PRODUCT AND COMPANY IDENTIFICATION COMPANY INFORMATION Advanced Protective Technologies LLC 2502 Beech Street Unit 100 Valparaiso, IN 46383 PRODUCT
Safety Data Sheet. Product Name: Product Number: Product Identity. 2. Hazardous Ingredients. Formaldehyde: CAS: Methanol: CAS:
 Olathe, KS Tel: 913-390-6184 Safety Data Sheet Solution (Formalin, Formol, Methanol, Formaldehyde containing solutions, Formalith) Emergency phone: 800 424 9300 (Chemtrec) NFPA Rating: Health 3, Flammability
Olathe, KS Tel: 913-390-6184 Safety Data Sheet Solution (Formalin, Formol, Methanol, Formaldehyde containing solutions, Formalith) Emergency phone: 800 424 9300 (Chemtrec) NFPA Rating: Health 3, Flammability
MSDS Material Safety Data Sheet
 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NUMBER PRODUCT NAME PERMABOND LH 051 MANUFACTURER PERMABOND LLC 14 Robinson Street Pottstown, PA 19464 EMERGENCY PHONES: MEDICAL 1 866 827 6282 TRANSPORT
1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NUMBER PRODUCT NAME PERMABOND LH 051 MANUFACTURER PERMABOND LLC 14 Robinson Street Pottstown, PA 19464 EMERGENCY PHONES: MEDICAL 1 866 827 6282 TRANSPORT
MATERIAL SAFETY DATA SHEET DATE: FEB 21, 2011 NAIL GLUE Page 1 of 4
 MATERIAL SAFETY DATA SHEET DATE: FEB 21, 2011 NAIL GLUE Page 1 of 4 SECTION 1:. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: NAIL GLUE PRODUCT TYPE: cyanoacrylate adhesive PRODUCT USE: fingernail adhesive
MATERIAL SAFETY DATA SHEET DATE: FEB 21, 2011 NAIL GLUE Page 1 of 4 SECTION 1:. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: NAIL GLUE PRODUCT TYPE: cyanoacrylate adhesive PRODUCT USE: fingernail adhesive
Page Number: 1 of
 Page Number: 1 of 6 SECTION 1. PRODUCT AND COMPANY INFORMATION Product Name: THINNER CAS Number N/A Hazard Rating: Health: 2 Fire: 3 Reactivity: 0 PPI: Company Identification: DAMPNEY CO INC. 85 PARIS
Page Number: 1 of 6 SECTION 1. PRODUCT AND COMPANY INFORMATION Product Name: THINNER CAS Number N/A Hazard Rating: Health: 2 Fire: 3 Reactivity: 0 PPI: Company Identification: DAMPNEY CO INC. 85 PARIS
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET Issued: 03/14/95 Prepared by: Gary Wong Revised: 01/29/02 Manager EHS Revision: 01 Core No. 092 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Erythromycin Ophthalmic Ointment
MATERIAL SAFETY DATA SHEET Issued: 03/14/95 Prepared by: Gary Wong Revised: 01/29/02 Manager EHS Revision: 01 Core No. 092 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Erythromycin Ophthalmic Ointment
Triad Visible Light Cure System
 Triad Visible Light Cure System Triad Visible Light Cure System Denture Relines & Repairs For Complete Control from Design to Delivery Dentsply Sirona s unique Visible Light Cure Technology delivers maximum
Triad Visible Light Cure System Triad Visible Light Cure System Denture Relines & Repairs For Complete Control from Design to Delivery Dentsply Sirona s unique Visible Light Cure Technology delivers maximum
Safety Data Sheet Section 1 Product and Company Identification
 Section 1 Product and Company Identification Product Name Product Identifier Product Use Spectrum Quartz Quartz surfacing, engineered stone Quartz surfacing applications for indoor applications such as
Section 1 Product and Company Identification Product Name Product Identifier Product Use Spectrum Quartz Quartz surfacing, engineered stone Quartz surfacing applications for indoor applications such as
Date Printed: 03/31/2009 Date Updated: 02/06/2006 Version 1.3 ELLAGIC ACID FROM TREE BARK. Substance Name CAS # SARA 313 ELLAGIC ACID No
 SIGMA-ALDRICH MATERIAL SAFETY DATA SHEET Date Printed: 03/31/2009 Date Updated: 02/06/2006 Version 1.3 Section 1 - Product and Company Information Product Name Product Number Brand ELLAGIC ACID FROM TREE
SIGMA-ALDRICH MATERIAL SAFETY DATA SHEET Date Printed: 03/31/2009 Date Updated: 02/06/2006 Version 1.3 Section 1 - Product and Company Information Product Name Product Number Brand ELLAGIC ACID FROM TREE
GINGI-PAK I S O 9001:2008 CERTIFIED
 Chemical Name: Trade Name: Identification Number(s): Ethyl p-amino benzoate Benzocaine 20100, 20103, 20108, 20013, 20017, 2001B, 2001P, 20111, 20113, 2011B, 20114, 2011C, 20117, 2011P, 20118, 2011S, 20119,
Chemical Name: Trade Name: Identification Number(s): Ethyl p-amino benzoate Benzocaine 20100, 20103, 20108, 20013, 20017, 2001B, 2001P, 20111, 20113, 2011B, 20114, 2011C, 20117, 2011P, 20118, 2011S, 20119,
SAFETY DATA SHEET. Soy Cleaner/Degreaser. 1. Product and Company Identification. 2. Hazards Identification
 Product Code: Product Name: Company Name: Web site address: 0490 Genlabs 5568 Schaefer Ave. Chino, CA 970 SAFETY DATA SHEET. Product and Company Identification www.genlabscorp.com Phone Number: (909)59-845
Product Code: Product Name: Company Name: Web site address: 0490 Genlabs 5568 Schaefer Ave. Chino, CA 970 SAFETY DATA SHEET. Product and Company Identification www.genlabscorp.com Phone Number: (909)59-845
Emergency telephone Gorilla Glue Europe A/S. Tel: The Gorilla Glue Company. Tel: 001 (513)
 SAFETY DATA SHEET Gorilla Super Glue 1. IDENTIFICATION OF THE PREPARATION AND OF THE COMPANY Product name Product type Intended use Supplier Gorilla Super Glue Cyanoacrylate adhesive Adhesive for wood,
SAFETY DATA SHEET Gorilla Super Glue 1. IDENTIFICATION OF THE PREPARATION AND OF THE COMPANY Product name Product type Intended use Supplier Gorilla Super Glue Cyanoacrylate adhesive Adhesive for wood,
Pulpdent Corporation Revision Date: May 21, 2014 Safety Data Sheet. 1.1 Commercial product name / designation Root Canal Sealer Powder and Liquid
 1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Root Canal Sealer Powder and Liquid 1. 1.. 1..3 Application / Use SIC Use Category Dental material for use by dental professional
1.0 Commercial Product Name and Supplier 1.1 Commercial product name / designation Root Canal Sealer Powder and Liquid 1. 1.. 1..3 Application / Use SIC Use Category Dental material for use by dental professional
Concentrated Liquid Soap and Release Agent Industrial Water Treatment Not Applicable (CHEMTEL)
 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Description: Suggested Use: Restrictions on Use: Concentrated Liquid Soap and Release Agent Industrial Water Treatment Supplier: Chemicator Brand 8728
1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Description: Suggested Use: Restrictions on Use: Concentrated Liquid Soap and Release Agent Industrial Water Treatment Supplier: Chemicator Brand 8728
Safety Data Sheet Product Name: Heparin / PF4 Serum Panel - For In Vitro Diagnostic Use Only
 Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Greenline Plywood Products Ltd.
 Greenline Plywood Products Ltd. MATERIAL SAFTY DATA SHEET SECTION #1 COMPANY IDENTIFICATION Name: Fineline Reconstituted Wood Veneer Date: August 15, 2007 Wood: Fineline Company Information Greenline Plywood
Greenline Plywood Products Ltd. MATERIAL SAFTY DATA SHEET SECTION #1 COMPANY IDENTIFICATION Name: Fineline Reconstituted Wood Veneer Date: August 15, 2007 Wood: Fineline Company Information Greenline Plywood
HELENA LABORATORIES 1530 Lindbergh Dr. / P.O. Box 752 Beaumont, TX USA Toll Free SPIFE 4000 IFE Kit
 SDS: 2300 Page 1 of 11 SAFETY DATA SHEET HELENA LABORATORIES 1530 Lindbergh Dr. / P.O. Box 752 Beaumont, TX 77704-0752 USA Toll Free 800-231-5663 DATE PREPARED: 3/8/2018 REVISION: 4 1. IDENTIFICATION Product
SDS: 2300 Page 1 of 11 SAFETY DATA SHEET HELENA LABORATORIES 1530 Lindbergh Dr. / P.O. Box 752 Beaumont, TX 77704-0752 USA Toll Free 800-231-5663 DATE PREPARED: 3/8/2018 REVISION: 4 1. IDENTIFICATION Product
GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION
 Product Identifier: Material Name: Recommended Use: Restrictions on Use: Manufacturer: SAFETY DATA SHEET GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION Betagen Topical
Product Identifier: Material Name: Recommended Use: Restrictions on Use: Manufacturer: SAFETY DATA SHEET GENTAMICIN SULFATE WITH BETAMETHASONE VALERATE TOPICAL SPRAY SECTION 1: IDENTIFICATION Betagen Topical
NextDent, the leading manufacturer of dental materials for 3D printing
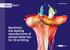 NextDent, the leading manufacturer of dental materials for 3D printing Biocompatible 3D printing materials Company NextDent B.V. was founded in 2012 in The Netherlands as an independent subsidiary company
NextDent, the leading manufacturer of dental materials for 3D printing Biocompatible 3D printing materials Company NextDent B.V. was founded in 2012 in The Netherlands as an independent subsidiary company
CAS-No. EC-No. Index-No. Concentration Chloroform-d May be harmful if inhaled. May cause respiratory tract irritation.
 SIGMA-ALDRICH Material Safety Data Sheet Version 3.3 01/11/2008 Print Date 08/31/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : C8513 Brand : Sigma Company : Sigma-Aldrich 3050
SIGMA-ALDRICH Material Safety Data Sheet Version 3.3 01/11/2008 Print Date 08/31/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Product Number : C8513 Brand : Sigma Company : Sigma-Aldrich 3050
MATERIAL SAFETY DATA SHEET CHEMICAL SUBSTANCES AND PRODUCTS ACCORDING DIRECTIVE 91/155/EEC
 MATERIAL SAFETY DATA SHEET CHEMICAL SUBSTANCES AND PRODUCTS ACCORDING DIRECTIVE 91/155/EEC date: March 2007 version: 7 author: GM 1 IDENTIFICATION OF THE PRODUCT AND COMPANY 1.1 product name: VERTEX Rapid-Simplified
MATERIAL SAFETY DATA SHEET CHEMICAL SUBSTANCES AND PRODUCTS ACCORDING DIRECTIVE 91/155/EEC date: March 2007 version: 7 author: GM 1 IDENTIFICATION OF THE PRODUCT AND COMPANY 1.1 product name: VERTEX Rapid-Simplified
NEPTUNE'S HARVEST ROSE FLOWERING FORMULA 2 6 4
 Safety Data Sheet Section 1: Identification Product Name Neptune's Harvest Rose & Flowering Formula Fertilizer 2 6 4 Relevant identified uses of the substance or mixture and uses advised against Recommended
Safety Data Sheet Section 1: Identification Product Name Neptune's Harvest Rose & Flowering Formula Fertilizer 2 6 4 Relevant identified uses of the substance or mixture and uses advised against Recommended
SIGMA-ALDRICH. Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 07/08/2010
 SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 07/08/2010 1. PRODUCT AND COMPANY IDENTIFICATION Product name : e Product Number : P5669 Brand
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/28/2010 Print Date 07/08/2010 1. PRODUCT AND COMPANY IDENTIFICATION Product name : e Product Number : P5669 Brand
Safety Data Sheet. Product Name: DetectX Glutathione Colorimetric Detection Kit. Section 1: Identification. Section 2: Hazard(s) Identification
 Safety Data Sheet Revision Date: 14 October 2016 Product Name: DetectX Glutathione Colorimetric Detection Kit Section 1: Identification Product Name: DetectX Glutathione Colorimetric Detection Kit Also
Safety Data Sheet Revision Date: 14 October 2016 Product Name: DetectX Glutathione Colorimetric Detection Kit Section 1: Identification Product Name: DetectX Glutathione Colorimetric Detection Kit Also
SIGMA-ALDRICH. Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 02/14/2011
 SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 02/14/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Oleamide Product Number : O2136
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.0 Revision Date 02/27/2010 Print Date 02/14/2011 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Oleamide Product Number : O2136
Date Printed: 04/24/2008 Date Updated: 01/29/2006 Version 1.4
 SIGMA-ALDRICH MATERIAL SAFETY DATA SHEET Date Printed: 04/24/2008 Date Updated: 01/29/2006 Version 1.4 Section 1 - Product and Company Information Product Name (DIACETOXYIODO)BENZENE Product Number 31490
SIGMA-ALDRICH MATERIAL SAFETY DATA SHEET Date Printed: 04/24/2008 Date Updated: 01/29/2006 Version 1.4 Section 1 - Product and Company Information Product Name (DIACETOXYIODO)BENZENE Product Number 31490
Material Safety Data Sheet According to ISO Page 1 of 6
 According to ISO 11014-1 Page 1 of 6 1. IDENTIFICATION OF SUBSTANCE Name: Manufacturer: Information Department: SMOG ENEMAS Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710
According to ISO 11014-1 Page 1 of 6 1. IDENTIFICATION OF SUBSTANCE Name: Manufacturer: Information Department: SMOG ENEMAS Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710
bis(diphenylphosphino)butane
 SIGMA-ALDRICH Material Safety Data Sheet Version 3.0 Revision Date 08/23/2008 Print Date 01/20/2009 1. PRODUCT AND COMPANY IDENTIFICATION Product name : (+)-2,3-O-Isopropylidene-2,3-dihydroxy-1,4- bis(diphenylphosphino)butane
SIGMA-ALDRICH Material Safety Data Sheet Version 3.0 Revision Date 08/23/2008 Print Date 01/20/2009 1. PRODUCT AND COMPANY IDENTIFICATION Product name : (+)-2,3-O-Isopropylidene-2,3-dihydroxy-1,4- bis(diphenylphosphino)butane
Kovanaze TM Nasal Spray (6 mg Tetracaine Hydrochloride and 0.1 mg Oxymetazoline Hydrochloride per 0.2 ml spray)
 SAFETY DATA SHEET Page 1 of 7 1. IDENTIFICATION OF SUBSTANCE Name: Product Code: NDC # 69803 100 10 (6 mg tetracaine and 0.1 mg oxymetazoline ) Recommended use: Restrictions on use: Manufacturer: Emergency
SAFETY DATA SHEET Page 1 of 7 1. IDENTIFICATION OF SUBSTANCE Name: Product Code: NDC # 69803 100 10 (6 mg tetracaine and 0.1 mg oxymetazoline ) Recommended use: Restrictions on use: Manufacturer: Emergency
1.1 Commercial name: Bicaseal Putty (Activator) 1.2 Type of product: Sealing compound for power cable accessories
 MATERIAL SAFETY DATA SHEET BICASEAL PUTTY (ACTIVATOR) ISSUE DATE: 31 ST July 2008 1 IDENTIFICATION OF SUBSTANCE 1.1 Commercial name: Bicaseal Putty (Activator) 1.2 Type of product: Sealing compound for
MATERIAL SAFETY DATA SHEET BICASEAL PUTTY (ACTIVATOR) ISSUE DATE: 31 ST July 2008 1 IDENTIFICATION OF SUBSTANCE 1.1 Commercial name: Bicaseal Putty (Activator) 1.2 Type of product: Sealing compound for
SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING PRODUCT DESCRIPTION: Z-Poxy Finishing resin - B (hardener) - PT40 MANUFACTURER Pacer Technology 9420 Santa Anita
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING PRODUCT DESCRIPTION: Z-Poxy Finishing resin - B (hardener) - PT40 MANUFACTURER Pacer Technology 9420 Santa Anita
Safety Data Sheet THE CRACKER Created On: 03/13/2015 Revision Date: 02/29/16 Version: 3.0
 1. Product and Company Identification Product Name: THE CRACKER Product Use: Concrete Demolition Company Identification: Emergency Phone: ADHESIVES TECHNOLOGY CORP. Chem-Tel: 450 East Copans Road 1.800.255.3924
1. Product and Company Identification Product Name: THE CRACKER Product Use: Concrete Demolition Company Identification: Emergency Phone: ADHESIVES TECHNOLOGY CORP. Chem-Tel: 450 East Copans Road 1.800.255.3924
Safety Data Sheet according to (EC) No 1907/ ISO
 Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 6 3880 Adhesive Electrically Conductive SDS no. : 168456 Revision: 12.06.2008 printing date: 04.05.2009 1. Identification of the
Safety Data Sheet according to (EC) No 1907/2006 - ISO 11014-1 Page 1 of 6 3880 Adhesive Electrically Conductive SDS no. : 168456 Revision: 12.06.2008 printing date: 04.05.2009 1. Identification of the
Designer Molecules, Inc. Safety Data Sheet Product: BMI-689 Revised Date: 09/17/15 MSDS ID: R1155 Replaces Date: 06/10/15
 1. General Information Trade Name: BMI-689 Product Code: R1155 Product Type: Maleimide Terminated Polyimide Resin Company: Designer Molecules, Inc. 10080 Willow Creek Rd. San Diego, CA 92131 Telephone:
1. General Information Trade Name: BMI-689 Product Code: R1155 Product Type: Maleimide Terminated Polyimide Resin Company: Designer Molecules, Inc. 10080 Willow Creek Rd. San Diego, CA 92131 Telephone:
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET ELAN CHEMICAL COMPANY, INC Date Printed: October 23, 2007 Date Updated: November 9, 2005 1. Product /Company Identification Product Name: NATURAL ETHYL BUTYRATE Manufacturer:
MATERIAL SAFETY DATA SHEET ELAN CHEMICAL COMPANY, INC Date Printed: October 23, 2007 Date Updated: November 9, 2005 1. Product /Company Identification Product Name: NATURAL ETHYL BUTYRATE Manufacturer:
MMC International. MSDS Material Safety Data Sheet. 1. Identification of the substance/mixture and of the company
 1. Identification of the substance/mixture and of the company 1.1 Product identifiers Product name : Product number : Brand : Crystal meth/xtc Test METAM0110 MMC International 1.2 Relevant identified uses
1. Identification of the substance/mixture and of the company 1.1 Product identifiers Product name : Product number : Brand : Crystal meth/xtc Test METAM0110 MMC International 1.2 Relevant identified uses
